Gabapentina para el dolor neuropático crónico en adultos
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and longer trials provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. To summarise some of the recent insights that must be considered in this new review:
-
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011b), back pain (Moore 2010d), and arthritis (Moore 2010e), as well as in fibromyalgia (Straube 2010); in all cases average results usually describe the experience of almost no‐one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
-
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks' duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010d); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
-
The proportion of people with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010d; Moore 2010e; Moore 2013b; Moore 2014b; Straube 2008; Sultan 2008). One Cochrane Review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good reasons for doing so.
-
Individual patient analyses indicate that people who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010c; Moore 2014b).
-
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012a).
Appendix 2. CENTRAL search strategy
-
(gabapentin* or neurontin* or neurotonin*):TI,AB,KY (1184)
-
MESH DESCRIPTOR Neuralgia EXPLODE ALL TREES (718)
-
MESH DESCRIPTOR Peripheral Nervous System Diseases EXPLODE ALL TREES (2963)
-
MESH DESCRIPTOR Somatosensory Disorders EXPLODE ALL TREES (796)
-
((pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)):TI,AB,KY (3931)
-
((neur* or nerv*) adj6 (compress* or damag*)):TI,AB,KY (732)
-
2 OR 3 OR 4 OR 5 OR 6 (7377)
-
1 AND 7 (215)
-
01/01/2014 TO 16/01/2017:CD (269940)
-
8 AND 9 (107)
Appendix 3. MEDLINE (via OVID) search strategy
-
(gabapentin* or neurontin* or neurotonin*).mp. (5327)
-
exp NEURALGIA/ (18298)
-
exp PERIPHERAL NERVOUS SYSTEM DISEASES/ (142504)
-
exp SOMATOSENSORY DISORDERS/ (20859)
-
((pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)).mp. (50119)
-
((neur* or nerv*) adj6 (compress* or damag*)).mp. (59288)
-
2 or 3 or 4 or 5 or 6
-
randomized controlled trial.pt. (484826)
-
controlled clinical trial.pt. (97360)
-
randomized.ab. (371343)
-
placebo.ab. (182076)
-
drug therapy.fs (2092559)
-
randomly.ab. (255301)
-
trial.ti. (167136)
-
groups.ab (1572017)
-
8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 (3937255)
-
1 and 7 and 16 (1363)
-
limit 17 to yr="2014 ‐Current" (237)
Appendix 4. Embase (via OVID) search strategy
-
gabapentin/ (25201)
-
(gabapentin* or neurontin* or neurotonin*).mp. (35892)
-
1 or 2 (25892)
-
exp neuralgia/ (93025)
-
exp peripheral neuropathy/ (62561)
-
exp somatosensory disorder/ (83900)
-
((pain* or discomfort*) adj10 (central or complex or nerv* or neuralg* or neuropath*)).mp. (94901)
-
((neur* or nerv*) adj6 (compress* or damag*)).mp. (81121)
-
4 or 5 or 6 or 7 or 8 (330939)
-
clinical trial/ (1025277)
-
controlled clinical trial/ (468659)
-
randomized controlled trial/ (469523)
-
double‐blind procedure/ (140238)
-
(clin* adj25 trial*).mp. (1445057)
-
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)). mp. (222270)
-
placebo*.ti.ab. (252536)
-
random*.ti,ab. (1172190)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 (2247471)
-
3 and 9 and 18 (2998)
-
limit 19 to yr="2014 ‐Current" (484)
Appendix 5. Potential sources of bias in studies of chronic pain used in the 'Risk of bias' table
| Item | High | Unclear | Low |
| Randomisation | Not randomised | Claims randomisation, but no method given | Randomised by adequate method |
| Allocation concealment | Not reported | Reported but not described | Allocation undertaken independently and blind to investigator |
| Blinding | Not double‐blind | Claims double‐blind, but no method | Convincingly double‐blind |
| Duration | 2 weeks or less | 3 to 6 weeks | 7 weeks or more |
| Outcome | Anything less than 30% pain intensity reduction | Responder: pain intensity reduction of ≥ 30% from baseline | Responder: pain intensity reduction of ≥ 50% from baseline |
| Incomplete outcome assessment | Average results only | Responder or state with last observation carried forward or imputation method for missing data or after withdrawal not stated | Responder or state response, using baseline observation carried forward (zero improvement after withdrawal) |
| Size | < 50 participants per treatment arm | 50 to 199 participants per treatment arm | ≥ 200 participants per treatment arm |
Appendix 6. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Cochrane Handbook of Systematic Reviews of Interventions, Chapter 12, Schünemann 2011b).
-
High: randomised trials; or double‐upgraded observational studies
-
Moderate: downgraded randomised trials; or upgraded observational studies
-
Low: double‐downgraded randomised trials; or observational studies
-
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports
Factors that may decrease the quality level of a body of evidence are:
-
limitations in the design and implementation of available studies suggesting high likelihood of bias;
-
indirectness of evidence (indirect population, intervention, control, outcomes);
-
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
-
imprecision of results (wide confidence intervals);
-
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
-
large magnitude of effect;
-
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
-
dose‐response gradient.
Appendix 7. Summary of outcomes in individual studies
| Study | Withdrawals | Efficacy | Adverse events | Adverse events |
| Postherpetic neuralgia | ||||
| All‐cause withdrawal AE withdrawal No LoE withdrawals in double‐blind phase | At least 30% reduction in pain | At least one AE | Dizziness | |
| All‐cause withdrawal | At least 50% improvement over baseline pain (Likert) | No serious AE reported | Sleepiness | |
| 16 participants withdrew because of AEs, lack of | 25% to ≤ 50% pain relief | At least one AE Gabapentin 78/109 Most appeared in the first week, and symptoms relieved gradually as the treatment continued | Dizziness Gabapentin 27/109 Placebo 10/106 Somnolence Gabapentin 19/109 Placebo 3/106 Peripheral oedema Gabapentin 6/109 Placebo 1/106 | |
| All‐cause | ≥ 50% red in PI | Overall incidence of AEs and changes in safety parameters were small and similar between doses | Dizziness Somnolence Peripheral oedema | |
| All‐cause withdrawal 15 total | At least 50% reduction in pain score | Serious AE | Somnolence | |
| All‐cause | Reduction in PI from baseline | At least one AE | Vertigo | |
| Gabapentin 1800 mg | At least 50% reduction in mean pain score | At least one AE | Somnolence | |
| Gabapentin | PGIC moderate or much improved | At least one AE | Somnolence | |
| All‐cause withdrawal | At least 50% reduction in pain | At least one AE | Dizziness | |
| y | All‐cause withdrawal | At least 50% improvement over baseline pain (Likert) Much or very much improved on PGIC Gabapentin 99/269 | At least one AE | Dizziness Somnolence Peripheral oedema |
| To end of maintenance phase | At least 50% reduction in pain by end maintenance | At least 1 AE | Dizziness Somnolence Peripheral oedema | |
| Painful diabetic neuropathy | ||||
| All‐cause withdrawal | PGIC much or moderately improved | At least one AE | Dizziness | |
| All‐cause withdrawal LoE withdrawal | At least 50% reduction in pain score | At least 1 AE | Somnolence | |
| CTR 945‐1008 | All‐cause withdrawal | At least 30% reduction in pain | At least one AE | Somnolence |
| Gorson et al. J Neurol, Neurosurg Psych 1999 66:251‐252 | Moderate or excellent pain relief (both phases) | At least one AE | ||
| All‐cause withdrawal/early cross‐over | No significant difference at end of treatment | At least one AE | Sedation | |
| No withdrawals apparent | At least 50% reduction in pain by 4 weeks | No major side effects reported for gabapentin group | No data | |
| All‐cause withdrawal | At least 50% reduction in pain by end M week 12 | At least 1 AE | Dizziness Somnolence Peripheral oedema | |
| All‐cause withdrawal | At least 50% reduction in pain from baseline to week 4 (BOCF) | At least one AE | Dizziness Somnolence Nausea Headache | |
| All‐cause withdrawal | PGIC moderate or much improved | No deaths reported, and no serious adverse events reported | Somnolence | |
| Mixed neuropathic pain | ||||
| AE withdrawals | No statistically significant differences between groups for any efficacy parameter % pain relief (mean change at end of study): Satisfaction with treatment (satisfied and very satisfied): | Overall 61/62 participants reported at least one AE | Most frequent AEs were fatigue, dizziness (excluding vertigo), weakness, somnolence, decreased appetite, nausea, confusion, vision blurred | |
| 16 withdrawals during treatment | At least moderate pain relief (5‐point scale) for those completing a given treatment: | Not interpretable | Not interpretable | |
| All‐cause withdrawals | Pain significantly lower with combination than either drug alone, by < 1/10 points | No serious AE recorded | Individual AE reporting showed higher incidence during titration than at maximum tolerated dose | |
| All‐cause withdrawals | At least 50% reduction in pain | At least one AE | Somnolence | |
| Radicular leg pain | ||||
| All‐cause withdrawal | ≥ 30% decrease in pain intensity ≥ 30% ≥ 50% | At least one AE (possibly or probably attributed) | Dizziness | |
| All‐cause withdrawal | Decrease in average leg pain at 3 months Decrease in worst leg pain at 3 months | At least one AE (injection‐related) At least one AE (drug‐related) | Sedation/fatigue | |
| Spinal cord injury | ||||
| Discontinuations | Not interpretable | No data | No data | |
| All completed | Average fall in pain 62% with gabapentin, 13% with placebo | All‐cause AE | Sedation | |
| 16/38 withdrew | No dichotomous data. | No dichotomous data | No dichotomous data | |
| Nerve injury pain | ||||
| All‐cause withdrawal | Marked pain relief | Serious AE | Dizziness | |
| Phantom | ||||
| No apparent withdrawals | "Meaningful decrease in pain" (top of 5‐point scale) | No data | No data | |
| No data on where withdrawals occurred | No dichotomous data | No data | Somnolence | |
| Cancer‐associated neuropathic pain | ||||
| All‐cause withdrawal | Somewhat better pain responses with gabapentin than placebo | No data | Somnolence | |
| All‐cause withdrawal | No significant difference between gabapentin and placebo, but pain scores were low and the study may have lacked sensitivity | No data | Dizziness | |
| HIV | ||||
| All‐cause withdrawal | Improvement in pain and sleep interference with gabapentin and placebo, with sustained difference in sleep but not pain | No serious AE or deaths reported | Somnolence | |
| AE: adverse event; CTR: clinical trial report; GabaEn: gabapentin encarbil; GabaEr: gabapentin extended‐release; LoE: lack of efficacy; PGIC: Patient Global Impression of Change; QoL: quality of life; SAE: serious adverse event; VAS: visual analogue scale; | ||||

Study flow diagram

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.1 At least 50% pain reduction over baseline.
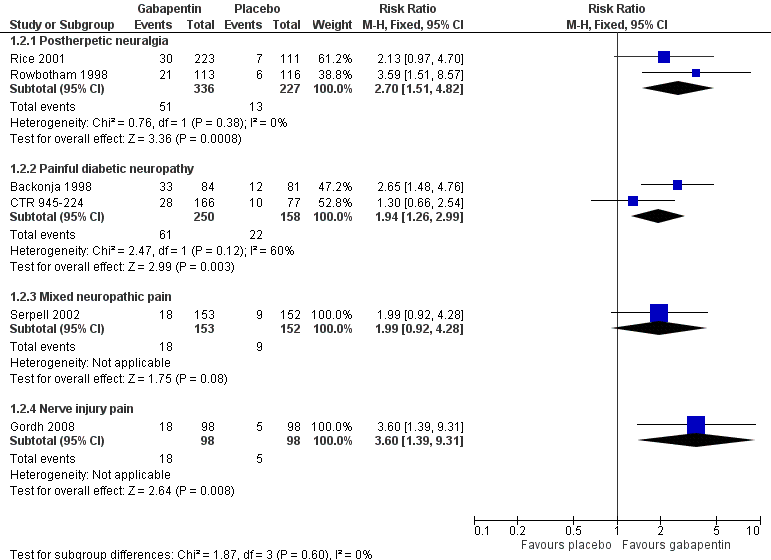
Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.2 Very much improved.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.3 Much or very much improved.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.4 IMMPACT outcome of substantial improvement.

Forest plot of comparison: 1 All placebo‐controlled studies, outcome: 1.5 IMMPACT outcome of at least moderate improvement.

Postherpetic neuralgia: percentage of participants achieving at least 50% pain intensity reduction (PIR) over baseline with gabapentin 1200 mg‐3600 mg daily, or placebo

Painful diabetic neuropathy: percentage of participants achieving at least 50% pain intensity reduction (PIR) over baseline with gabapentin 1200‐3600 mg daily, or placebo

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 1 At least 50% pain reduction over baseline.

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 2 Very much improved.
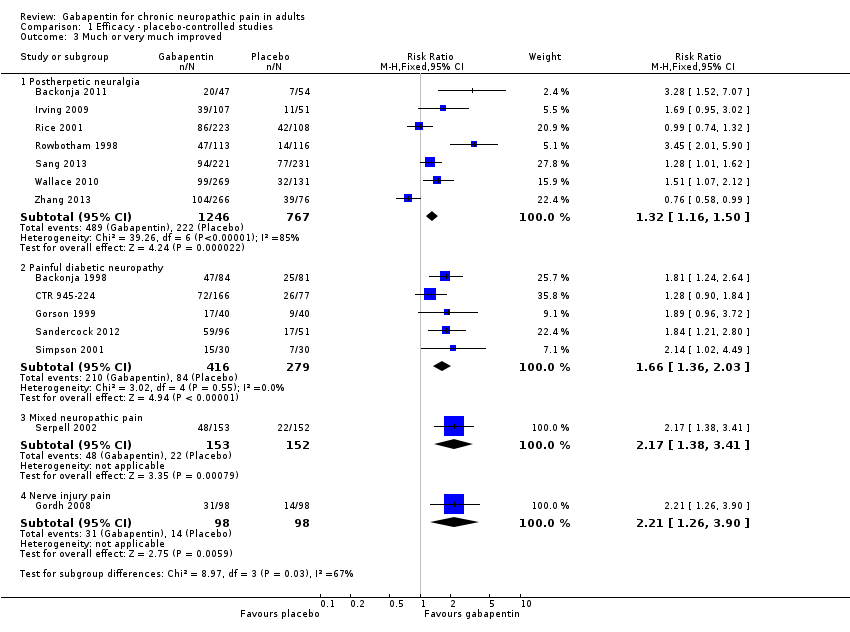
Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 3 Much or very much improved.
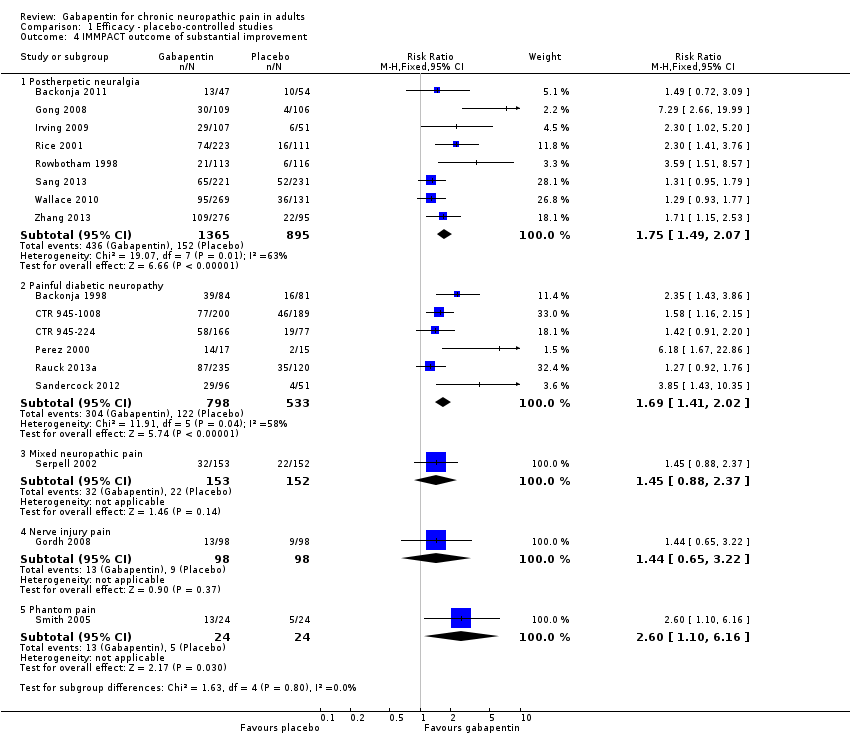
Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 4 IMMPACT outcome of substantial improvement.

Comparison 1 Efficacy ‐ placebo‐controlled studies, Outcome 5 IMMPACT outcome of at least moderate improvement.

Comparison 2 Withdrawals ‐ placebo‐controlled studies, Outcome 1 All‐cause withdrawal.
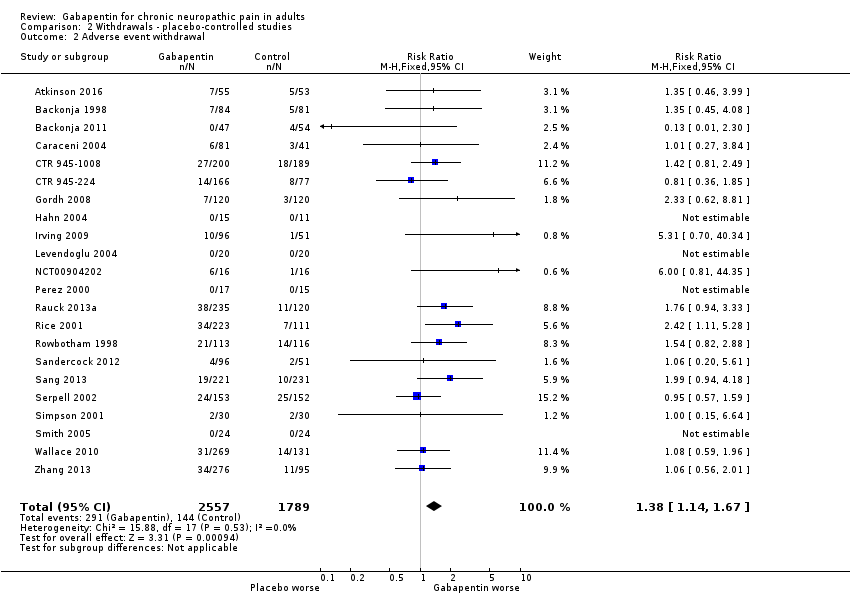
Comparison 2 Withdrawals ‐ placebo‐controlled studies, Outcome 2 Adverse event withdrawal.
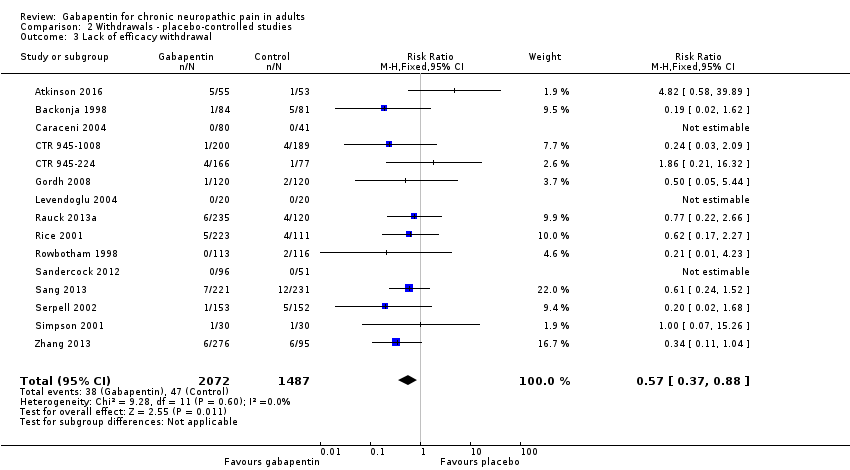
Comparison 2 Withdrawals ‐ placebo‐controlled studies, Outcome 3 Lack of efficacy withdrawal.

Comparison 3 Adverse events, Outcome 1 At least one adverse event.

Comparison 3 Adverse events, Outcome 2 Serious adverse events.

Comparison 3 Adverse events, Outcome 3 Somnolence.

Comparison 3 Adverse events, Outcome 4 Dizziness.
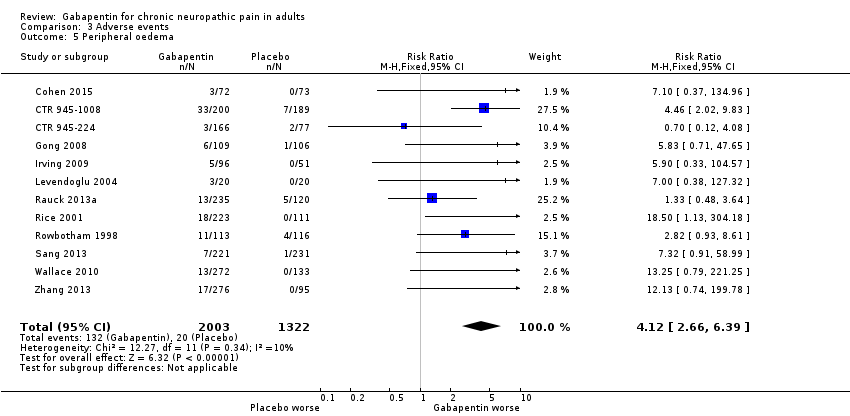
Comparison 3 Adverse events, Outcome 5 Peripheral oedema.

Comparison 3 Adverse events, Outcome 6 Ataxia or gait disturbance.
| Gabapentin compared with placebo for postherpetic neuralgia: efficacy | ||||||
| Patient or population: adults with postherpetic neuralgia Settings: community Intervention: gabapentin ≥ 1800 mg daily or gabapentin encarbil 1200 mg daily Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNT (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| At least 50% reduction in pain or equivalent | 330 per 1000 | 190 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.9 (5.5 to 9.4) | 7 studies 2031 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| IMMPACT definition ‐ any substantial pain benefit | 320 per 1000 | 170 per 1000 | RR 1.8 (1.5 to 2.1) NNT 6.7 (5.4 to 8.7) | 8 studies 2260 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Patient Global Impression of Change much or very much improved | 390 per 1000 | 290 per 1000 | RR1.3 (1.2 to 1.5) NNT 9.7 (6.9 to 16) | 7 studies 2013 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| IMMPACT definition ‐ any at least moderate pain benefit (includes Gong 2008 at 25% pain relief) | 46 per 1000 | 25 per 1000 | RR 1.8 (1.6 to 2.0) NNT 4.8 (4.1 to 6.0) | 8 studies 2260 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| CI: confidence interval; IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): †Substantially different: a large enough difference that it might affect a decision. | ||||||
| Gabapentin compared with placebo for peripheral diabetic neuropathy: efficacy | ||||||
| Patient or population: adults with peripheral diabetic neuropathy Settings: community Intervention: ≥ 1800 mg daily or gabapentin encarbil 1200 mg daily Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNT (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| At least 50% pain intensity reduction | 380 per 1000 | 230 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.6 (5.0 to 9.7) | 6 studies 1331 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Any definition of substantial benefit (at least 50% pain intensity reduction or PGIC very much improved) | 380 per 1000 | 230 per 1000 | RR 1.7 (1.4 to 2.0) NNT 6.6 (5.0 to 9.7) | 6 studies 1331 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| PGIC much or very much improved | 500 per 1000 | 300 per 1000 | RR 1.7 (1.4 to 2.0) NNT 4.9 (3.6 to 7.6) | 5 studies 695 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| Any definition of moderate benefit (at least 30% pain intensity reduction or PGIC much or very much improved) | 520 per 1000 | 370 per 1000 | RR 1.4 (1.3 to 1.6) NNT 6.6 (4.9 to 9.9) | 7 studies 1439 participants | Moderate | Downgraded because of issues around dosing, formulation, and imputation |
| CI: confidence interval; IMMPACT: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials; NNT: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Gabapentin compared with placebo for neuropathic pain (all conditions pooled): adverse events and withdrawals | ||||||
| Patient or population: adults with neuropathic pain Settings: community Intervention: gabapentin 1800 mg to 3600 mg daily (gabapentin encarbil 1200 mg to 3600 mg daily) Comparison: placebo | ||||||
| Outcome | Probable outcome with gabapentin | Probable outcome with placebo | RR and NNH (95% CI) | Number of studies, participants | Certainty of the evidence | Comments |
| Participants experiencing at least one adverse event | 630 per 1000 | 490 per 1000 | RR 1.3 (1.2 to 1.4) NNH 7.5 (6.1 to 9.6) | 18 studies 4279 participants | Moderate | Many events. Unlikely new research would change this finding |
| Adverse event withdrawals | 110 in 1000 | 82 in 1000 | RR 1.4 (1.1 to 1.7) NNH 30 (20 to 66) | 22 studies 4346 participants | High | Unlikely new research would change this finding |
| Serious adverse events | 32 in 1000 | 28 in 1000 | RR 1.2 (0.83 to 1.7) NNH not calculated | 19 studies 3948 participants | Moderate | Small number of events but no suggestion of difference |
| Death | 3 in max 3603 exposed | 5 in max 2377 exposed | Not calculated | Not calculated | Very low | Few events, relatively short duration for drug possibly taken over periods of years |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| Descriptors for levels of evidence (EPOC 2015): † Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least 50% pain reduction over baseline Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Postherpetic neuralgia | 7 | 2031 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.43, 2.00] |
| 1.2 Painful diabetic neuropathy | 6 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.41, 2.02] |
| 1.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.88, 2.37] |
| 1.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.65, 3.22] |
| 2 Very much improved Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Postherpetic neuralgia | 2 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.51, 4.82] |
| 2.2 Painful diabetic neuropathy | 2 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 2.99] |
| 2.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.92, 4.28] |
| 2.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.6 [1.39, 9.31] |
| 3 Much or very much improved Show forest plot | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Postherpetic neuralgia | 7 | 2013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.16, 1.50] |
| 3.2 Painful diabetic neuropathy | 5 | 695 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.36, 2.03] |
| 3.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [1.38, 3.41] |
| 3.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.26, 3.90] |
| 4 IMMPACT outcome of substantial improvement Show forest plot | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postherpetic neuralgia | 8 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.49, 2.07] |
| 4.2 Painful diabetic neuropathy | 6 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.41, 2.02] |
| 4.3 Mixed neuropathic pain | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.88, 2.37] |
| 4.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.65, 3.22] |
| 4.5 Phantom pain | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [1.10, 6.16] |
| 5 IMMPACT outcome of at least moderate improvement Show forest plot | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postherpetic neuralgia | 8 | 2260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.56, 2.00] |
| 5.2 Painful diabetic neuropathy | 7 | 1439 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.24, 1.59] |
| 5.3 Mixed neuropathic pain | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.49, 2.95] |
| 5.4 Nerve injury pain | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.92, 2.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause withdrawal Show forest plot | 22 | 4617 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.16] |
| 2 Adverse event withdrawal Show forest plot | 22 | 4346 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.14, 1.67] |
| 3 Lack of efficacy withdrawal Show forest plot | 15 | 3559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one adverse event Show forest plot | 18 | 4279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.22, 1.36] |
| 2 Serious adverse events Show forest plot | 19 | 3948 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.71] |
| 3 Somnolence Show forest plot | 20 | 4288 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [2.27, 3.50] |
| 4 Dizziness Show forest plot | 21 | 4739 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [2.40, 3.44] |
| 5 Peripheral oedema Show forest plot | 12 | 3325 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [2.66, 6.39] |
| 6 Ataxia or gait disturbance Show forest plot | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.53 [2.49, 12.28] |

