剖宫产前阴道消毒预防术后感染
摘要
研究背景
剖宫产是妇产科医生最常用的手术方法之一。剖宫产产后感染的发病率可能对产后妇女恢复正常功能及其照顾婴儿的能力产生巨大影响。尽管广泛使用预防性抗生素,但术后感染的发病率仍使剖宫产复杂化。这是一个Cochrane 系统综述的更新,该综述首次发表于2010年,并于2012年、2014年两次、2017年和2018年进行了更新。
研究目的
为了确定在剖宫产前用抗菌溶液清洗阴道是否能降低孕产妇感染性疾病的风险,包括子宫内膜炎和伤口并发症。我们还评价了阴道清洁液的副作用,以确定与干预措施相关的不良事件。
检索策略
我们检索了Cochrane 妊娠和分娩试验注册库(Cochrane Pregnancy and Childbirth’s Trials Register),ClinicalTrials.gov 世界卫生组织(World Health Organization, WHO)国际临床试验注册平台( International Clinical Trials Registry Platform, ICTRP)(2019年7月7日)并且检索了相关文章的参考文献。
纳入排除标准
我们纳入了随机对照试验( randomized controlled trials, RCTs)和准RCT,用于评价剖宫产分娩前立即使用任何类型的抗菌液与安慰剂溶液/护理标准对剖宫产术后感染率的影响。
整群随机对照试验均符合纳入标准,但没有发现相关研究。我们排除了在自然分娩过程中进行阴道准备或未使用抗生素进行术前准备的试验。我们还排除了任何使用交叉设计的试验。仅当摘要中含有足够的有关分析方法和结局信息时,我们才纳入只发表了摘要的试验。
资料收集与分析
至少三位位综述作者独立筛选评评价了所有可能符合纳入标准的研究。指派了两名综述作者从符合条件的研究中提取研究特征和资料,并进行质量评价。
主要结果
我们纳入了21项试验,包含7038名妇女,报告了有关阴道清洁对剖宫产后感染发病率影响的结果(17项研究使用了聚维酮碘,3项使用氯己定,1项使用苯扎氯铵)。试验使用阴道棒,海绵或纱布湿巾进行阴道准备。对照组通常不进行阴道准备(17项试验)或使用生理盐水进行阴道准备(4项试验)。一项试验没有报告任何我们感兴趣的结局。研究在10个国家开展(沙特阿拉伯、巴基斯坦、伊朗、泰国、土耳其、美国、埃及、英国、肯尼亚和印度)。在磨损偏倚,报告和其他偏倚方面,总体偏倚风险较低。大约一半试验的选择性偏倚被评为低风险,其余的大多数研究的选择性偏倚被评为不清楚。由于缺乏盲法,我们将近三分之一的试验中的实施偏倚评为高风险,三分之一为低风险,三分之一为不明确。
剖宫产前立即用抗菌液进行阴道准备可能将剖宫产后子宫内膜炎的发生率从对照组的7.1%降低到阴道清洗组的3.1%(平均风险比(average risk ratio, (aRR) =0.41, 95%置信区间(confidence interval, (CI) [0.29, 0.58]; 20项试验,共6918名妇女;中等质量证据)。使用含碘溶液和含氯己定的溶液都可以降低感染风险。阴道抗菌液的使用也可能降低术后发烧和术后伤口感染的风险(发热:aRR =0.64, 95%CI [0.50, 0.82]; 16项试验,6163名妇女;以及伤口感染:RR=0.62, 95%CI [0.50, 0.77]; 18项试验,6385名妇女;均为中等质量证据)。两项试验发现,接受术前阴道准备的妇女面临更小的发生伤口并发症或子宫内膜炎复合结局的风险(RR=0.46, 95%CI [0.26, 0.82]; 2项试验,499名妇女;低质量证据)。聚维酮碘或氯已定阴道清洁均未见不良反应 。
亚组分析表明,在接受检查的五分之四的结局中(剖宫产后子宫内膜炎;术后发烧;术后伤口感染;复合伤口并发症或子宫内膜炎),自然分娩的产妇与非自然分娩的产妇相比,前者阴道用药效果更好。这种明显的差异需要在以后的试验中进一步研究。 我们没有观察到胎膜破裂的产妇和胎膜完整的产妇的任何差异。
作者结论
(相比于使用生理盐水或不进行术前清洁)剖宫产分娩前立即用聚维酮碘或氯已定溶液进行阴道准备可能会降低术后子宫内膜炎,发烧和手术伤口感染的风险。亚组分析发现,无论使用含碘溶液还是含氯已定的溶液,也无论剖宫产前妇女何时分娩,通常都存在这些益处。对自然分娩产妇的益处需要在未来的试验中进一步研究。
对于所有报告的结局,都有使用GRADE 进行评价,均为中度确定性证据,并根据研究设计的局限性或不精确性对证据进行了降级。
作为一种简单的干预措施,施术者可以考虑在进行剖宫产之前,用聚维酮碘或氯已定进行术前阴道清洗。需要对该干预措施进行进一步的研究,将其纳入针对剖宫产产妇的护理计划系列里。
PICO
简语概要
剖宫产前用抗菌液清洗阴道以减少术后感染风险
我们着手从随机对照试验中探寻,在剖宫产前用抗菌溶液清洁阴道是否可以安全地降低产妇感染的风险。
本综述的研究问题是什么?
剖宫产前清洁阴道可以减少阴道中天然存在的细菌数量。阴道和子宫颈中的这些细菌可在外科手术过程中向上移入子宫,并引起子宫内膜和手术伤口的感染。手术前常规给予抗生素可降低感染的风险,但是一些女性仍然遭受这些并发症的困扰。某些抗生素并不总是能够根除所有细菌,并且可能存在抗药性细菌。为减少手术后的感染,可能不对妇女提供阴道术前准备。诸如氯己定和聚维酮碘之类的阴道清洁溶液价格便宜,且副作用极少。
为什么这很重要?
剖宫产是很常见的,在美国等一些国家,剖宫产所生的婴儿中几乎有三分之一。剖宫产的妇女发生子宫感染(子宫内膜炎)或皮肤切口问题的情况并不少见。如果孕妇的羊水已破裂或在剖宫产之前正在分娩,则感染的风险会更大。这些并发症可能会减慢产妇的术后恢复速度,并可能影响她们照顾婴儿的能力。本文是首次发表于2010年,分别更新于2012, 2014, 2017年的Cochrane 系统综述。
我们发现了什么证据?
我们于2019年7月7日检索了相关证据。在本次更新中,我们纳入了21项随机对照研究,涉及7038名接受剖宫产的妇女。本研究在10个国家(沙特阿拉伯,巴基斯坦,伊朗,泰国,土耳其,美国,埃及,英国,肯尼亚和印度)进行。对照组在18项研究中没有使用阴道制剂,在三项研究中,受试者使用了生理盐水阴道制剂。我们不纳入手术前或手术中不使用抗生素的试验,也不包括分娩时进行阴道准备的试验。十七项研究使用聚维酮碘进行阴道清洁,三项研究使用洗必泰,一项研究使用苯扎氯铵。
剖宫产分娩前立即用抗菌液清洗阴道可能会降低剖宫产术后子宫感染的发生率( 20例试验,6918名妇女;中等质量证据)。含碘溶液和含氯己定的溶液的使用都可以减少感染机会。阴道清洁还可能降低术后发烧的风险(16项试验,6163名女性)和术后伤口感染的风险(18个试验,6385名女性);均为中等质量证据)。接受术前阴道消毒液清洗的女性,出现伤口并发症或子宫感染的风险可能更低(2项试验,499名女性)。没有研究报告任何不良事件,例如对清洁溶液的过敏反应或刺激。
进一步的分析表明,在接受检查的五分之四的结果中(剖宫产术后子宫感染;术后发烧;术后伤口感染;伤口并发症或子宫感染),自然分娩产妇与剖宫产产妇相比,在前者体现除了更好的效果。但这一差异需要在以后的试验中进一步研究。 我们没有观察到胎膜破裂的产妇和胎膜完整的产妇之间的任何差异。
这意味着什么?
剖宫产分娩前立即用聚维酮碘或洗必泰溶液清洁阴道可能会降低子宫感染,发烧和手术伤口感染的风险(相比于使用生理盐水或不清洁)。进一步的分析发现,无论使用含碘溶液还是含氯已定的溶液,也无论剖宫产产妇何时分娩,这些益处通常都存在。
阴道准备是一种简单且耐受良好的方法,可降低因剖宫产后发生感染的几率。
Authors' conclusions
Summary of findings
| Vaginal preparation with antiseptic solution compared to control (no preparation or saline preparation) for preventing postoperative infections | ||||||
| Patient or population: pregnant women undergoing cesarean section | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with control (no preparation or saline preparation) | Risk with vaginal preparation with antiseptic solution | |||||
| Post‐cesarean endometritis | Study population | RR 0.41 | 6918 | ⊕⊕⊕⊝ | ||
| 72 per 1000 | 30 per 1000 | |||||
| Postoperative fever | Study population | RR 0.64 | 6163 | ⊕⊕⊕⊝ | ||
| 120 per 1000 | 77 per 1000 | |||||
| Postoperative wound infection | Study population | RR 0.62 | 6385 | ⊕⊕⊕⊝ | ||
| 61 per 1000 | 38 per 1000 | |||||
| Composite wound complication or endometritis | Study population | RR 0.46 | 499 | ⊕⊕⊕⊝ | ||
| 135 per 1000 | 62 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThere is some funnel plot asymmetry. Having conducted sensitivity analyses to investigate the contribution of small studies and studies at high risk of bias, we do not believe that the effect estimate has been biased by possible missing results due to non‐publication. We did not downgrade. | ||||||
Background
Cesarean section delivery rates are increasing worldwide, with rates in Latin America and North America of 40.5% and 32.3%, respectively (Betran 2016). Cesarean section deliveries are often complicated by infections occurring after surgery (Zuarez‐Easton 2017).
Description of the condition
Endometritis, an infection of the uterus in the postpartum period, can complicate the postoperative course of a cesarean delivery 6% to 27% of the time (Guzman 2002; Smaill 2014). This complication, up to 10 times more frequent after a cesarean delivery than after vaginal delivery, can lead to serious complications of bacterial infection in the blood (10% to 20%), peritonitis (general infection in the abdominal cavity), intra‐abdominal abscess (cavity filled with infected material), and sepsis (Mackeen 2015; Yokoe 2001). Additionally, cesarean deliveries are frequently complicated by maternal fever and wound complications, including seroma (fluid collection under the skin), hematoma (blood clots under the skin), infection, and separation (Zuarez‐Easton 2017). These morbidities could lead to a delay in return to normal function.
Fevers and infections after cesarean deliveries are associated with the length of ruptured membranes, length of labor, and number of vaginal examinations (Disgupta 1988; Yonekura 1985). Post‐cesarean endometritis and infectious morbidity are the result often of the presence of bacteria in the vagina and cervix that move higher in the genital tract to infect the uterus (Martens 1991). These bacteria have been shown to be responsible for failure of antibiotic prophylaxis during cesarean deliveries (Watts 1991). Additionally, some antibiotics do not consistently eradicate some bacteria (such as Enterococcus spp), and the vagina has been shown to become colonized with antibiotic resistant bacteria after preoperative surgical antibiotic prophylaxis (Gibbs 1982; Graham 1993; Stiver 1984). Currently, it is standard care to give preoperative antibiotics to women receiving a cesarean delivery (Smaill 2014), but the rate of post‐cesarean infections remains a problem.
Description of the intervention
As many pelvic organ infections after surgeries, such as cesarean deliveries, contain organisms from the vagina, cleansing the vagina with antiseptic solutions before surgeries, such as hysterectomies, has been performed for years (ACOG 2018; Haeri 1976; Osborne 1977). As it has been used to reduce infections after hysterectomies, it is logical that after a cesarean delivery, where the uterus remains potentially exposed to the vagina through the cervix, reducing the bacterial content before a cesarean delivery could reduce post‐cesarean infections of the uterus. Previous studies have evaluated whether vaginal cleansing before a cesarean delivery with various solutions can reduce the incidence of febrile morbidity (endometritis and wound infections). Povidone‐iodine, chlorhexidine, and vaginal metronidazole have been reported with varying results (Pitt 2001; Zuarez‐Easton 2017). Older data comparing iodine with chlorhexidine before hysterectomy showed lower morbidity in the iodine group, with improved activity against anaerobic pathogens (Duignan 1975; Haeri 1976). Vaginal preparation has not typically been included in evidence‐based bundles to reduce post‐cesarean infectious morbidity (Carter 2017; Hsu 2016; NICE 2011). Vaginal cleansing solutions, such as chlorhexidine and povidone‐iodine, have very few side effects in general, with low rates of noted allergies or irritation symptoms. Thus, the intervention is now appearing in recommendations about post‐cesarean recovery protocols (Caughey 2018).
How the intervention might work
By cleansing the vagina of bacteria before the cesarean delivery occurs, there may be less of a bacterial load in the vagina that might cause infectious morbidity postoperatively. As ascending infection is thought to be a major etiology of postoperative endometritis, this could logically reduce that risk (Martens 1991).
Why it is important to do this review
Rates of cesarean delivery are increasing, particularly in high‐income countries (Betran 2016). Postoperative infectious morbidity after cesarean delivery may impact the woman's return to normal function, and potentially her bonding with the newborn, as she is dealing with additional healthcare needs to treat the infection (Zuarez‐Easton 2017). It can also cause major medical problems and sequelae and increase healthcare costs (Olsen 2010). Finding an easy, inexpensive method to reduce this risk could have a major public health impact in high‐, middle‐, and low‐income countries.
Objectives
To determine if cleansing the vagina with an antiseptic solution before a cesarean delivery decreases the risk of maternal morbidities, including endometritis and wound complications. We also assessed the side effects of vaginal cleansing solutions to determine adverse events associated with the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) and two quasi‐RCTs. Cluster‐RCTs were eligible for inclusion, but we did not identify any.
Types of participants
Pregnant women who were about to receive a cesarean delivery. This included women receiving elective, laboring, or urgent cesareans.
Types of interventions
Any method of vaginal cleansing (including douches, wipes, sponges, etc.) with any type of antiseptic solution (povidone‐iodine, chlorhexidine, etc.) versus a placebo solution/standard care (no vaginal preparation).
We included only studies where vaginal preparation was performed no more than one hour before surgery. This review addressed the use of preoperative vaginal cleansing after the decision to perform a cesarean had been made. This review did not address the use of vaginal preparation during labor. Thus, we excluded trials utilizing vaginal cleansing solutions during labor. We also excluded studies where prophylactic surgical antibiotics were explicitly not used. Surgical prophylaxis with intravenous antibiotics before or during cesarean deliveries has been clearly demonstrated as beneficial in reducing postoperative infectious morbidities (Smaill 2014). Thus, it is the standard of care. Inclusion of trials not utilizing general surgical antibiotic prophylaxis would not represent the current standard of care and the results would not be translatable into current practice. We did not discriminate trials on the basis of when the antibiotics were administered (before or after infant umbilical cord clamping), as this practice has changed over time (Mackeen 2014).
Types of outcome measures
Primary outcomes
-
Post‐cesarean endometritis: defined as a clinical diagnosis, usually involving fever, uterine fundal tenderness, or purulent lochia requiring antibiotic therapy.
Secondary outcomes
-
Postoperative fever: defined as greater than 38 °C or 100.4 °F.
-
Postoperative wound infection: defined as erythema, tenderness, purulent drainage from the incision site, with or without fever, requiring antibiotic therapy.
-
Postoperative wound seroma or hematoma: defined as collection of serous fluid or blood/clot in the subcutaneous area of the incision.
-
Composite wound complications: defined as the presence of any one of the following: wound infection, seroma, hematoma, separation.
-
Composite wound complications or endometritis.
-
Side effects of vaginal preparation (maternal allergy, irritation). As these solutions are applied gently and not absorbed, there should be no adverse fetal/neonatal events. We did not anticipate or find mention of adverse neonatal events from the vaginal cleansing.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (7 July 2019).
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
monthly searches of CINAHL (EBSCO);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (7 July 2019) using the search methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies. We attempted to contact trialists for further information (September 2019). We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, see Haas 2018.
For this update, we used the following methods for assessing the 14 new reports that were identified as a result of the updated search.
Selection of studies
At least three review authors (DH, SM, KC, SE) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. We extracted trial information and dates, outcomes, sources of trial funding, and trial authors' declarations of interest (if available). For eligible studies, at least two review authors extracted the data using the agreed form. Assignments for data extraction were distributed among the four review authors equitably. We resolved discrepancies through discussion. We entered data into Review Manager software (Review Manager 2014).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Three review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
For each included study we assessed the method as being at:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study we described the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as being at:
-
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as being at:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as being at:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
For each included study, and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to reinclude missing data in the analyses that we undertook.
We assessed methods as being at:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as being at:
-
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study we described any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgments about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to have an impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios (RRs) with 95% confidence intervals (CIs).
Continuous data
None of our outcomes are continuous in nature. If an outcome is added in the future that contains continuous data, we plan to use the mean difference (MD) if outcomes were measured in the same way between trials. We plan to use the standardized mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
We did not identify any cluster‐randomized trials. If, in future updates we identify cluster‐randomized trials, we will include them in the analyses along with individually‐randomized trials. We will adjust their sample sizes using the methods described in the Handbook (Higgins 2011), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Cross‐over trials
Cross‐over trials are not relevant for this intervention and are not included.
Other unit of analysis issues
We found one trial that compared three groups. It did not contribute outcome data (Goymen 2017). However, if it had or we encounter three‐armed trials in future updates, we would utilize the methods in the Handbook to decide the optimal way to include them in the meta‐analysis. One trial used a "no wash" and a saline wash control group (Hassan 2016). In our analysis, we combined these as controls, as some other trials used a saline wash for the control group.
Dealing with missing data
For included studies, we noted levels of attrition. We did not encounter large levels of attrition. In future updates, if we do encounter large levels of attrition, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analyses.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat (ITT) basis, i.e. we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
There were 21 included studies. Since there were 10 or more studies in the meta‐analysis contributing data to the primary outcome, we investigated reporting biases (such as publication bias) using funnel plots. We assessed for reporting bias by inspecting the funnel plot asymmetry visually. Because potential asymmetry was found visually, we tested to see if the results were different when limiting to small (< 300 participants) or large trial effects or if the results were different when excluding trials at high risk of bias in multiple domains.
Data synthesis
We carried out statistical analysis using the Review Manager software (Review Manager 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, we presented the results as the average treatment effect with 95% CIs, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
For this update, we carried out the following subgroup analyses.
-
Women in labor versus women not in labor.
-
Women with ruptured membranes versus women with intact membranes.
We used all reported outcomes in the primary analysis in the subgroup analyses.
We assessed subgroup differences by interaction tests available within RevMan 5 (Review Manager 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value. Where we identified significant heterogeneity, we used a random‐effects analysis to produce the summaries of effect.
We were unable to carry out the following subgroup analyses because this information was not reported in the included studies.
-
Women with chorioamnionitis preoperatively versus women without chorioamnionitis.
-
Women undergoing emergency cesarean versus those undergoing unscheduled cesarean versus those undergoing scheduled cesarean.
-
Women with internal fetal or uterine monitors in place versus those with only external monitors in place before the cesarean.
Sensitivity analysis
We did not perform any sensitivity analyses due to a lack of studies included within the analyses. In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (> 20%), or both, and exclude poor quality studies from the analyses, in order to assess whether this makes any difference to the overall result.
Summary of findings and assessment of the certainty of the evidence
For this update, we assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE Handbook in order to assess the certainty of the body of evidence relating to the following outcomes for the main comparisons (Schünemann 2013).
-
Post‐cesarean endometritis: defined as a clinical diagnosis, usually involving fever, uterine fundal tenderness, or purulent lochia requiring antibiotic therapy.
-
Postoperative fever: defined as greater than 38 °C or 100.4 °F.
-
Postoperative wound infection: defined as erythema, tenderness, purulent drainage from the incision site, with or without fever, requiring antibiotic therapy.
-
Composite wound complications or endometritis.
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5 (GRADEpro GDT 2015; Review Manager 2014), in order to create a ’Summary of findings’ table. Using the GRADE approach, we produced a summary of the intervention effect and a measure of certainty for each of the above outcomes. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Results
Description of studies
Results of the search
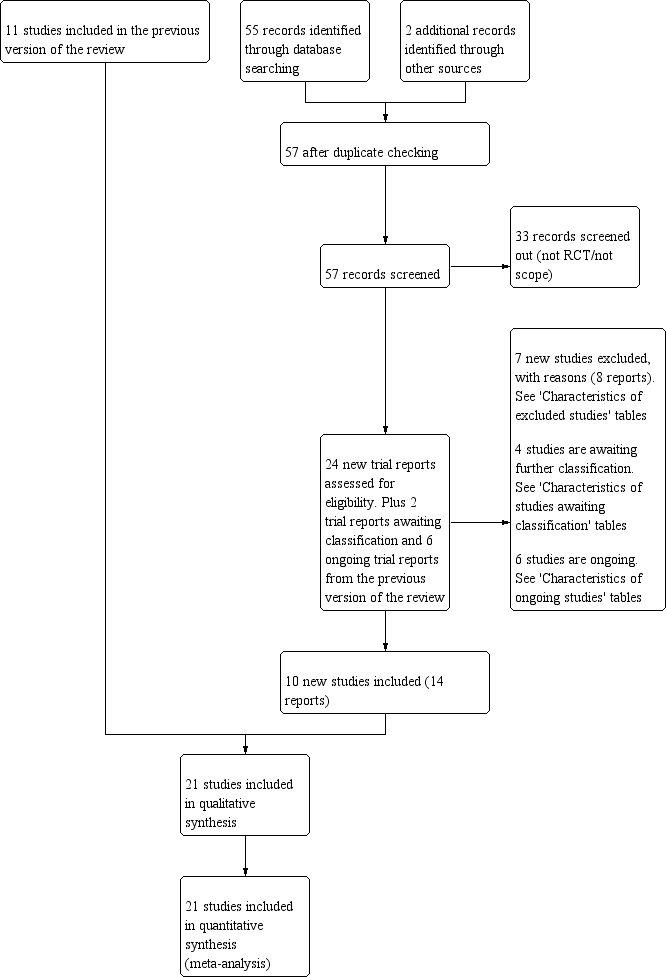
See Figure 1.

Study flow diagram.
An updated search in July 2019 retrieved 22 new trial reports to assess and we also reassessed two trials 'awaiting classification' and the six trials listed as 'ongoing' in the previous version of the review. We also assessed and subsequently excluded two additional trial reports (Pitt 2001; Sweeten 1997). After title evaluation, and review of the full‐text manuscripts and trial registry reports, we included 10 new trials (14 reports) and excluded seven additional trials (eight reports). Six trials are ongoing (see Characteristics of ongoing studies). Four trials are awaiting further classification (see Characteristics of studies awaiting classification).
Included studies
Methods
In this updated review we included 21 studies, reporting results for 7038 women. Nineteen trials were randomized controlled trials (RCTs) and two were quasi‐RCTs.
Settings
All trials were either in academic centers or large hospitals. Five trials were performed in the USA (Guzman 2002; Haas 2010; Reid 2001; Rouse 1997; Starr 2005), three in Pakistan (Asad 2017; Kiani 2018; Memon 2011), three in Turkey (Goymen 2017; Olmez 2013; Yildirim 2012), two in Iran (Asghania 2011; Barat 2016), two in Egypt (Hassan 2016; Mohamed 2015), two in Saudi Arabia (Ahmed 2017; Aref 2019), one in Thailand (Charoenviboonphan 2011), one in the UK (Hodgetts 2019), one in Kenya (Mwangi 2013), and one in India (Nandi 2015). The Charoenviboonphan 2011 trial was written in Thai, with the abstract and results tables in English. We were able to secure a translation of the methods of the trial for abstraction.
Trial dates
The trials were reported as being conducted during the following periods.
-
Ahmed 2017 ‐ October 2014 to December 2015
-
Aref 2019 ‐ September 2016 to December 2017
-
Asad 2017 ‐ 1 February 2016 to 31 July 2016
-
Asghania 2011 ‐ May 2007 to April 2008
-
Barat 2016 ‐ 2013 to 2014 (months not stated)
-
Charoenviboonphan 2011 ‐ September 2010 to January 2011
-
Goymen 2017 ‐ July 2014 to August 2014
-
Guzman 2002 ‐ March 2000 to July 2001
-
Haas 2010 ‐ September 2006 to January 2009
-
Hassan 2016 ‐ September 2015 to March 2016
-
Hodgetts 2019 ‐ 13 November 2017 to 3 March 2018
-
Kiani 2018 ‐ September 2014 to January 2015
-
Memon 2011 ‐ February 2010 to July 2010
-
Mohamed 2015 ‐ May 2014 to August 2014
-
Mwangi 2013 ‐ July 2016 to October 2016
-
Nandi 2015 ‐ January 2013 to July 2014
-
Olmez 2013 September 2009 to July 2010
-
Reid 2001 ‐ May 1996 to September 1998
-
Rouse 1997 ‐ February 1994 to January 1996
-
Starr 2005 ‐ November 1997 to March 2000
-
Yildirim 2012 ‐ January 2011 to August 2011
Participants
Six trials only included women for scheduled or elective cesareans (Ahmed 2017; Aref 2019; Barat 2016; Goymen 2017; Hassan 2016; Mohamed 2015). Two trials only included women who were in labor (Asad 2017; Kiani 2018), and the remainder of the studies included women both in labor and for scheduled cesareans (Asghania 2011; Guzman 2002; Haas 2010; Hodgetts 2019; Memon 2011; Mwangi 2013; Nandi 2015; Olmez 2013; Reid 2001; Rouse 1997; Starr 2005; Yildirim 2012). Two trials specifically excluded women with ruptured membranes (Ahmed 2017; Goymen 2017). One of the trials that included only women for elective cesareans excluded women with premature ruptured membranes (Barat 2016). By consensus, we did not believe we could judge if women presenting for elective cesareans might have been in labor. However, we judged that all women presenting for an elective cesarean would have been likely to have had intact membranes to be included. Thus, we counted trials including women for elective cesareans as having women with intact membranes as well. Seven trials specifically excluded women with chorioamnionitis (Asad 2017; Goymen 2017; Kiani 2018; Mwangi 2013; Reid 2001; Rouse 1997; Starr 2005). Three trials excluded women undergoing emergency cesarean deliveries (Aref 2019; Guzman 2002; Reid 2001).
Interventions and comparisons
Three studies compared chlorhexidine cleansing versus no cleansing (Ahmed 2017; Hodgetts 2019; Mohamed 2015). One study compared chlorhexidine solution versus a saline solution (Rouse 1997). One trial used cetrimide, which the authors noted contained chlorhexidine and thus we included with the chlorhexidine subgroup (Mohamed 2015). One report had two intervention groups compared with controls without cleansing ‐ one group received povidone‐iodine cleansing and one group received benzalkonium chloride cleansing (Goymen 2017). All other studies compared preoperative vaginal povidone‐iodine solution preparation with a control group. In one trial (Guzman 2002), the control group was a saline vaginal wash. Hassan 2016 used two intervention groups, one a saline washing and one a povidone‐iodine wash, while the control group had no washing. We combined the saline group and no washing groups as the control group, per the protocol definitions for the review. The other 14 trials compared vaginal cleansing with an iodine‐based solution to no vaginal cleansing (Aref 2019; Asad 2017; Asghania 2011; Barat 2016; Charoenviboonphan 2011; Haas 2010; Kiani 2018; Memon 2011; Mwangi 2013; Nandi 2015; Olmez 2013; Reid 2001; Starr 2005; Yildirim 2012).
Outcomes
All but one trial (Goymen 2017), reported on various infectious morbidity outcomes specified in this review (see Characteristics of included studies).
The Goymen 2017 study did not report on any of the primary or secondary outcomes prespecified for this review. The reported outcomes for that study were associated with postoperative recovery of bowel function and pain scores. Thus, it did not contribute any data to the analyses.
All the studies contributing data reported on the outcome of endometritis, while 16 studies reported on postoperative fever, and 17 reported on wound infection (see Characteristics of included studies). Two studies reported any wound complication and a composite of endometritis or any wound complication.
Sources of trial funding
Five trials reported sources of funding. Haas 2010, Mwangi 2013, and Starr 2005 reported internal institutional or hospital funding. Rouse 1997 received federal funding from the United States Department of Health and Human Services. Hodgetts 2019 stated funding from the Birmingham Women's and Children's National Health Service Foundation Trust. One trial specifically listed no sources of support (Aref 2019). All other reports did not list any sources of funding.
Declarations of interest
Ten trials specified no conflicts of interest from the authors (Ahmed 2017; Aref 2019; Barat 2016; Goymen 2017; Haas 2010; Hassan 2016; Hodgetts 2019; Mohamed 2015; Mwangi 2013; Yildirim 2012). The remainder of the trials did not mention declarations of interest.
Excluded studies
We excluded one trial as the journal retracted the publication (Abdallah 2015). Seven other trials were excluded due to the wrong comparisons or intervention timing (Pitt 2001; Sweeten 1997; Tewfik 2015; NCT03925155; Dudko 2018; NCT03133312; Lakhi 2016).
Risk of bias in included studies
See 'Risk of bias' tables for the included studies in Characteristics of included studies and Figure 2; and Figure 3, for summaries of 'Risk of bias' assessments.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Overall, the quality of these 21 studies was generally moderate, as defined by Higgins 2011.
Allocation
Random sequence generation
Five trials were unclear about the randomization sequence generation (Aref 2019; Asad 2017; Guzman 2002; Memon 2011; Olmez 2013). We judged three studies to be at potentially high risk of bias for random sequence generation. Asghania 2011 was a quasi‐randomized trial with alternate allocation, earning a high risk of bias rating. Hassan 2016 used number patient name lists, assigning evens to control group and odds to one of two intervention groups. Mohamed 2015 also used an odd‐even alternating randomization. The remaining trials were at a low risk of bias due to random sequence generation (Ahmed 2017; Barat 2016; Charoenviboonphan 2011; Goymen 2017; Haas 2010; Hodgetts 2019; Kiani 2018; Mwangi 2013; Nandi 2015; Reid 2001; Rouse 1997; Starr 2005; Yildirim 2012).
Allocation concealment
Eleven of the reports were unclear about allocation concealment (Ahmed 2017; Asad 2017; Barat 2016; Charoenviboonphan 2011; Goymen 2017; Guzman 2002; Hassan 2016; Kiani 2018; Memon 2011; Mohamed 2015; Nandi 2015), mainly due to no mention of that in the publication. One trial had a high risk of bias due to alternating sequence (Asghania 2011). The other trials had low risk of allocation bias (Aref 2019; Haas 2010; Hodgetts 2019; Mwangi 2013; Olmez 2013; Reid 2001; Rouse 1997; Starr 2005; Yildirim 2012).
Blinding
Blinding of participants and personnel (performance bias)
Six trials had a high risk of bias regarding blinding of the participants and care providers (Ahmed 2017; Aref 2019; Asad 2017; Goymen 2017; Nandi 2015; Yildirim 2012). As the intervention involved vaginal cleansing or not, it is understandable that in some clinical scenarios, blinding of this step might be difficult. Eight trials were at unclear risk of bias because it was not stated (Barat 2016; Charoenviboonphan 2011; Hassan 2016; Kiani 2018; Memon 2011; Mohamed 2015; Olmez 2013; Reid 2001).
Seven trials specifically noted ways they attempted to blind participants and/or care providers, or noted how it was unlikely for them to know the group assignment (i.e. participant had regional anesthesia and was behind a drape, surgeons were not in the room during surgical preparation) (Asghania 2011; Guzman 2002; Haas 2010; Hodgetts 2019; Mwangi 2013; Rouse 1997; Starr 2005). We assessed these trials as having a low risk of performance bias.
Blinding of outcome assessment (detection bias)
Twelve trials blinded outcomes assessors (Ahmed 2017; Asghania 2011; Barat 2016; Guzman 2002; Haas 2010; Hodgetts 2019; Kiani 2018; Memon 2011; Mwangi 2013; Reid 2001; Rouse 1997; Starr 2005), and we assessed them at low risk of detection bias. One trial stated that the researchers were not blinded and that the assignment was written in the medical records (Yildirim 2012), so outcome assessors were unlikely to be blinded either; we assessed this trial as having a high risk of detection bias. The remaining studies did not state blinding of outcomes assessors and we judged them to have a low risk of detection bias (Aref 2019; Asad 2017; Charoenviboonphan 2011; Goymen 2017; Hassan 2016; Mohamed 2015; Nandi 2015; Olmez 2013).
Incomplete outcome data
One report did not describe attrition fully as it was a published abstract, earning it an unclear 'Risk of bias' assessment (Asad 2017). We also rated other trial as unclear for attrition bias (Starr 2005); of 400 participants randomized, 92 (23%) were excluded after randomization: 33 due to lost envelopes, six for violations of inclusion criteria, and 53 because their hospital charts could not be located. Of all the women excluded, 54 were in the vaginal cleansing group and 38 were in the control group. Only outcomes for women for whom all data were available were reported. The large number of women excluded also makes this trial subject to an unclear risk of bias, however as there are no outcome data for the excluded participants, the potential impact is unclear (Starr 2005). The remaining 19 studies had a low risk of attrition bias.
Selective reporting
One trial had a large number of participants excluded after randomization who had chorioamnionitis (a known risk factor for postoperative infectious morbidity) because their inclusion "distorted the absolute rates of fever and infectious morbidity" (Reid 2001). That trial states that when the 68 participants with antepartum infection were included, the estimates of effect of vaginal preparation were not meaningfully different. Thus, they planned to exclude those participants from reports of outcomes. As this represented 13.5% of the originally randomized sample, however, there is a risk that this introduced selective reporting bias into the trial. We assessed this trial as having a high risk of reporting bias (Reid 2001). The other 20 trials were at low risk of reporting bias.
Other potential sources of bias
One trial was stopped early at a planned safety analysis due to difficulty recruiting participants (Haas 2010); we assessed this trial at unclear risk of other bias. The Asghania 2011 trial had large differences in the baseline and labor characteristics between the groups, including more examinations, longer labors, more preterm deliveries, longer surgery times, and longer duration of membrane rupture in the cleansing group. We assessed this trial as having a high risk of potential bias. The other 19 trials were at low risk of other sources of bias.
Effects of interventions
Vaginal preparation with antiseptic solution before cesarean section versus control (no preparation or saline preparation) (comparison 1)
Primary outcome: post‐cesarean endometritis
Vaginal cleansing with povidone‐iodine solution reduced the risk of post‐cesarean endometritis from 7.2% in control groups to 3.1% in vaginal cleansing groups (average risk ratio (aRR) 0.41, 95% confidence interval (CI) 0.29 to 0.58; 20 trials, 6918 women; moderate‐certainty evidence). We used a random‐effects meta‐analysis for this outcome because of high heterogeneity (I² = 44% and Tau² = 0.22; Analysis 1.1). The substantial heterogeneity indicates that treatment effects vary between studies, so we investigated the factors affecting treatment effects by the prespecified subgroup analyses (see below). As all of the trials did not include all subgroups, it is unclear if the subgroup analyses were able to account for all of the heterogeneity. However, we considered that the trials were similar enough clinically that the average treatment effect would be clinically meaningful. Stratifying these findings by solution yielded similar results for iodine‐based solution and chlorhexidine‐based solution (aRR 0.41, 95% CI 0.28 to 0.60; 16 trials, 6197 women for iodine; aRR 0.38, 95% CI 0.28 to 0.89; 4 trials, 721 women for chlorhexidine; Analysis 1.1).
Assessment of reporting (publication) biases for the primary outcome
Since our primary outcome analysis included more than 10 studies, we investigated reporting bias. We prepared a funnel plot (Figure 4), and this shows signs of visual asymmetry. To determine if this potential publication bias influenced the results, we then carried out a number of tests as to whether this made a difference to the results. In these analyses, we restricted the analysis to the larger trials (> 300 total participants), only small trials, and trials deemed of lower risk of bias by having no domains judged as high risk of bias. Limiting the results by trial size or quality did not change the overall findings of benefit for the primary outcome. Thus, we do not believe that selective reporting (publication) biased these findings. It is possible that some of the funnel plot asymmetry is present due to the wide variation in apparent population risk among the trials. The rates of endometritis in the control groups varies greatly. These different baseline population risk differences may have contributed to the asymmetry.

Funnel plot of comparison: 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), outcome: 1.1 Post‐cesarean endometritis.
Secondary outcomes
Vaginal cleansing also led to a clear reduction in the outcomes of postoperative fever (aRR 0.64, 95% CI 0.50 to 0.82; 16 trials, 6163 women; moderate‐certainty evidence; Analysis 1.2) and postoperative wound infection (RR 0.62, 95% CI 0.50 to 0.77; 18 trials, 6385 women; moderate‐certainty evidence; Analysis 1.3). There was more uncertainty around the estimate of vaginal cleansing's impact on composite wound complications (RR 0.63, 95% CI 0.37 to 1.07; 2 trials, 729 women; moderate‐certainty evidence; Analysis 1.4). However, based mainly on the results for endometritis, vaginal cleansing may lead to a reduction in the composite wound complication or endometritis outcome (RR 0.46, 95% CI 0.26 to 0.82; 2 trials, 499 women; low‐certainty evidence; Analysis 1.5). The improved outcomes for women receiving vaginal cleansing were noted for subgroups receiving both iodine‐based solutions and chlorhexidine‐based solutions for postoperative fever and postoperative wound infection (Analysis 1.2; Analysis 1.3). We did not note any side effectsof vaginal preparation in the four trials commenting on possible adverse events from the vaginal preparation solution (Ahmed 2017; Goymen 2017; Haas 2010; Rouse 1997). None of the other trials mentioned any adverse events, but did not specifically discuss the topic.
We did not find any evidence of differences between subgroups according to the subgroup differences test we performed.
Subgroup analysis: women in labor versus women not in labor (comparison 2)
Five trials stratified data for women in labor versus not in labor (Haas 2010; Memon 2011; Mwangi 2013; Reid 2001; Yildirim 2012), while two trials only included women in labor (Asad 2017; Kiani 2018). One trial included 14 women receiving irrigation before elective cesareans not in labor and only reported on the endometritis outcome for the group (Rouse 1997). Two trials reported on the outcomes of post‐cesarean endometritis and composite wound complication (Haas 2010; Reid 2001). Four studies reported on stratified outcomes for post‐cesarean endometritis, postoperative fever, and postoperative wound infection (Asad 2017; Kiani 2018; Mwangi 2013; Yildirim 2012). One trial only reported stratified results for composite infectious morbidity (Memon 2011).
Primary outcome: post‐cesarean endometritis
There was a reduction in rates of post‐cesarean endometritis for women undergoing a cesarean after being in labor who received vaginal preparation from 9.3% in the control group to 3.4% in the vaginal preparation group (aRR 0.35, 95% CI 0.19 to 0.67; 6 trials, 1634 women; Analysis 2.1). There was no clear difference in rates of post‐cesarean endometritis for women who were not in labor (7.8% in control group versus 3.7% in vaginal preparation group (aRR 0.86, 95% CI 0.33 to 2.21; 5 trials, 1043 women; Analysis 2.1). However, there were no clear differences between these two subgroups, as indicated by the subgroup interaction test (test for subgroup differences: Chi² = 2.37, df = 1 (P = 0.12), I² = 57.8%).
Secondary outcomes
Women in labor reported a reduction in rates of postoperative fever (RR 0.61, 95% CI 0.42 to 0.87; 5 trials, 1415 women; Analysis 2.2), postoperative wound infection (RR 0.52, 95% CI 0.30 to 0.90; 5 trials, 1415 women; Analysis 2.3), and the composite wound complication or endometritis outcome (RR 0.34, 95% CI 0.13 to 0.87; 2 trials, 164 women; Analysis 2.5). The small number of women in these groups limits this conclusion. There were no clear differences in rates of composite wound complications for women receiving vaginal preparation (RR 0.77, 95% CI 0.36 to 1.61; 2 trials, 314 women; Analysis 2.4).
The subgroup analyses, specifically for women who were not in labor before the cesarean delivery, failed to demonstrate any clear differences in any secondary outcomes: postoperative fever (RR 0.93, 95% CI 0.60 to 1.43; 3 trials, 818 women; Analysis 2.2); postoperative wound infection (RR 0.67, 95% CI 0.35 to 1.31; 3 trials, 818 women; Analysis 2.3); composite wound complication (RR 0.54, 95% CI 0.25 to 1.16; 2 trials, 415 women; Analysis 2.4); composite wound complication or endometritis (RR 0.60, 95% CI 0.29 to 1.26; 2 trials, 335 women; Analysis 2.5).
We did not find any evidence of differences between subgroups according to the subgroup differences test we performed.
Subgroup analysis: women with ruptured membranes versus women with intact membranes (comparison 3)
Five trials stratified data for women with ruptured membranes versus women without ruptured membranes (Guzman 2002; Haas 2010; Memon 2011; Mwangi 2013; Yildirim 2012). Five trials excluded women with premature ruptured membranes in women only undergoing elective cesarean (Ahmed 2017; Aref 2019; Barat 2016; Kiani 2018; Mohamed 2015). These trials only contributed data to the intact membranes subgroup. Two trials reported on the outcomes of post‐cesarean endometritis and postoperative fever (Guzman 2002; Haas 2010). Most other studies reported on stratified outcomes for post‐cesarean endometritis, postoperative fever, and postoperative wound infection (Ahmed 2017; Aref 2019; Barat 2016; Haas 2010; Kiani 2018; Mohamed 2015; Mwangi 2013; Yildirim 2012). One trial only reported stratified results for composite wound complications or endometritis (Memon 2011).
Primary outcome postpartum endometritis
For women with ruptured membranes, there was a reduction in the rates of post‐cesarean endometritis for women receiving vaginal preparation preoperatively (3.4% in the vaginal cleansing group versus 13.7% in the control group; RR 0.23, 95% CI 0.12 to 0.45; 5 trials, 552 women; Analysis 3.1). There was also a reduction in the rate of post‐cesarean endometritis for women with intact membranes who received vaginal cleansing before cesarean delivery (rate of 4.1% in the vaginal cleansing group versus 8.7% in the control group; RR 0.48, 95% CI 0.34 to 0.68; 8 trials, 2082 women) and the subgroup interaction test indicated that there may be a suggestion of a difference between these two subgroups (test for subgroup differences: Chi² = 3.59, df = 1 (P = 0.06), I² = 72.2%).
Secondary outcomes
There was a reduction in postoperative fever for women with ruptured membranes receiving vaginal preparation (aRR 0.42, 95% CI 0.22 to 0.80; 4 trials, 480 women; Analysis 3.2), but not for other secondary outcomes: postoperative wound infection (aRR 0.54, 95% CI 0.19 to 1.50; 5 trials, 552 women; Analysis 3.3); composite wound complication (RR 0.53, 95% CI 0.15 to 1.89; 1 trial, 76 women; Analysis 3.4); composite wound complication or endometritis (RR 0.39, 95% CI 0.13 to 1.13; 2 trials, 134 women; Analysis 3.5).
For women with intact membranes, there was also a reduction in postoperative fever for women receiving vaginal preparation (aRR 0.70, 95% CI 0.49 to 0.99; 7 trials, 1994 women; Analysis 3.2). All of the other reported secondary outcomes for women without ruptured membranes were not clearly different between the vaginal preparation and control groups: postoperative wound infection (aRR 0.73, 95% CI 0.50 to 1.07; 8 trials, 2082 women; Analysis 3.3); composite wound complication (RR 0.73, 95% CI 0.25 to 2.10; 1 trial, 224 women; Analysis 3.4); composite wound complication or endometritis (RR 0.52, 95% CI 0.26 to 1.04; 2 trials, 336 women; Analysis 3.5).
We did not find any evidence of differences between subgroups according to the subgroup differences test we performed.
Other planned subgroup analyses: women with chorioamnionitis preoperatively versus women without chorioamnionitis; women undergoing emergency cesarean versus those undergoing unscheduled cesarean versus those undergoing scheduled cesarean; women with internal fetal or uterine monitors in place versus those with only external monitors in place before the cesarean
Neither of the two trials that specifically included women diagnosed with chorioamnionitis stratified their data based on the presence or absence of chorioamnionitis. Neither of the two trials that did not exclude women undergoing emergency cesarean stratified their data based on emergency cesarean versus unscheduled versus scheduled cesarean. In addition, while three trials reported on the presence of internal monitoring (Haas 2010; Starr 2005; Yildirim 2012), none of them stratified their outcome data based on this variable. Thus we did not perform these three subgroup analyses.
Discussion
Summary of main results
Vaginal cleansing with either povidone‐iodine or chlorhexidine solutions before cesarean delivery can reduce the incidence of post‐cesarean endometritis, postoperative fever, and postoperative wound infections. A clear reduction in the rate of endometritis from 7.2% to 3.4% was seen. The heterogeneity in the results for these outcomes may be explainable by the study design and patient populations. The Guzman 2002, Hassan 2016, and Rouse 1997 studies used a placebo vaginal saline or water wash. This may have led to a lower baseline incidence of postoperative morbidity. Haas 2010 and many of the trials added in this update contained a majority of women or only women who were obtaining planned repeat cesarean deliveries, a group known to be at lower risk for postoperative infectious morbidities. Additionally, vaginal preparation before cesarean delivery reduced the rate of a composite outcome of the presence of wound complication or endometritis. These results are summarized in summary of findings Table 1.
Interestingly, the benefits of vaginal preparation were seen with both iodine‐based and chlorhexidine‐based solutions for both post‐cesarean endometritis and postoperative fever. The effects of the intervention seemed bigger in some subgroups although the interaction tests for subgroup differences were not statistically significant. The subgroup analyses demonstrated that the reduction in postoperative endometritis is most pronounced for women with ruptured membranes and those women who undergo a cesarean delivery after already being in labor. These subgroup analyses should be interpreted with caution, however, as the number of participants and events is relatively low. Ruptured membranes and being in labor are known risk factors for post‐cesarean infectious morbidity. The use of vaginal preparation in women in labor or with ruptured membranes thus makes particular sense.
Overall completeness and applicability of evidence
While there is heterogeneity in study design, the evidence is relatively complete, consistent, and highly applicable to clinical care. Currently, there are six ongoing trials (NCT02495753; NCT02693483; NCT03093194; NCT03397615; NCT03423147; PACTR201709002597110).
Certainty of the evidence
The risk of bias of the 21 included trials is reasonably low to moderate, with only a few areas being identified as potential sources of high risk of bias (Figure 2; Figure 3). The most common area found to have high risk of bias was in the area of blinding. This is because the control groups in most trials did not receive vaginal cleansing and often the participant and providers may have known who received the vaginal preparation as it would be obvious to anyone standing in the operating room. There were also some areas at unclear risk of bias, often in allocation concealment due to lack of commenting on that factor in the trial report. The agreement of the trial data in general, and the large number of participants represented, lend validity to the results of the meta‐analysis. The clinical heterogeneity was essentially eliminated in the subgroup analyses, the results of which were consistent with the overall group results.
The certainty of the evidence using GRADE was moderate for post‐cesarean endometritis, postoperative fever and postoperative wound infection with downgrading decisions based on limitations in study design (risk of bias). The certainty of the evidence was low for the composite outcome of wound complication or endometritis, with downgrading decisions based on both limitations in study design (risk of bias) and imprecision (summary of findings Table 1).
Potential biases in the review process
There is always potential that the review process was biased. However, the updated trial search yielded several additional studies. The study evaluation and data extraction were performed by four review authors, with almost no discrepancies that needed to be resolved by consensus. Thus, there is a minimal risk of bias in the review process. The studies were carried out in a wide variety of low‐, middle‐, and high‐income countries.
Agreements and disagreements with other studies or reviews
We added 10 new trials to this update, giving a total of 21 included studies. The addition of the new trials strengthens the conclusions of the earlier versions of this review (Haas 2010a; Haas 2013; Haas 2014a; Haas 2014b; Haas 2018). The additional evidence changes the conclusions by also showing that vaginal preparation lowers the rates of both postoperative fever and postoperative wound infection. The findings of lower risk of post‐cesarean endometritis is consistent with a recently published meta‐analysis (Caissutti 2017). We plan to include data from ongoing trials in future updates of this review. Uniformity in the reporting of the data outcomes and the subgroup data stratification would have also aided this review.
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Funnel plot of comparison: 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), outcome: 1.1 Post‐cesarean endometritis.

Comparison 1: Vaginal preparation with antiseptic solution before cesarean section versus control (no preparation or saline preparation), Outcome 1: Post‐cesarean endometritis

Comparison 1: Vaginal preparation with antiseptic solution before cesarean section versus control (no preparation or saline preparation), Outcome 2: Postoperative fever

Comparison 1: Vaginal preparation with antiseptic solution before cesarean section versus control (no preparation or saline preparation), Outcome 3: Postoperative wound infection

Comparison 1: Vaginal preparation with antiseptic solution before cesarean section versus control (no preparation or saline preparation), Outcome 4: Composite wound complication

Comparison 1: Vaginal preparation with antiseptic solution before cesarean section versus control (no preparation or saline preparation), Outcome 5: Composite wound complication or endometritis

Comparison 2: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 1: Post‐cesarean endometritis

Comparison 2: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 2: Postoperative fever

Comparison 2: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 3: Postoperative wound infection

Comparison 2: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 4: Composite wound complication

Comparison 2: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 5: Composite wound complication or endometritis

Comparison 3: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 1: Post‐cesarean endometritis

Comparison 3: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 2: Postoperative fever

Comparison 3: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 3: Postoperative wound infection

Comparison 3: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 4: Composite wound complication

Comparison 3: Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 5: Composite wound complication or endometritis
| Vaginal preparation with antiseptic solution compared to control (no preparation or saline preparation) for preventing postoperative infections | ||||||
| Patient or population: pregnant women undergoing cesarean section | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with control (no preparation or saline preparation) | Risk with vaginal preparation with antiseptic solution | |||||
| Post‐cesarean endometritis | Study population | RR 0.41 | 6918 | ⊕⊕⊕⊝ | ||
| 72 per 1000 | 30 per 1000 | |||||
| Postoperative fever | Study population | RR 0.64 | 6163 | ⊕⊕⊕⊝ | ||
| 120 per 1000 | 77 per 1000 | |||||
| Postoperative wound infection | Study population | RR 0.62 | 6385 | ⊕⊕⊕⊝ | ||
| 61 per 1000 | 38 per 1000 | |||||
| Composite wound complication or endometritis | Study population | RR 0.46 | 499 | ⊕⊕⊕⊝ | ||
| 135 per 1000 | 62 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aThere is some funnel plot asymmetry. Having conducted sensitivity analyses to investigate the contribution of small studies and studies at high risk of bias, we do not believe that the effect estimate has been biased by possible missing results due to non‐publication. We did not downgrade. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Post‐cesarean endometritis Show forest plot | 20 | 6918 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.29, 0.58] |
| 1.1.1 Iodine‐based solution | 16 | 6197 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.60] |
| 1.1.2 Chlorhexidine‐based solution | 4 | 721 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.16, 0.89] |
| 1.2 Postoperative fever Show forest plot | 16 | 6163 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.50, 0.82] |
| 1.2.1 Iodine‐based solution | 14 | 5763 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.50, 0.87] |
| 1.2.2 Chlorhexidine‐based solution | 2 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.83] |
| 1.3 Postoperative wound infection Show forest plot | 18 | 6385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.50, 0.77] |
| 1.3.1 Iodine‐based solution | 15 | 5767 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.50, 0.81] |
| 1.3.2 Chlorhexidine‐based solution | 3 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.90] |
| 1.4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.07] |
| 1.5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Post‐cesarean endometritis Show forest plot | 7 | 2677 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.81] |
| 2.1.1 Women in labor | 6 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.19, 0.67] |
| 2.1.2 Women not in labor | 5 | 1043 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.33, 2.21] |
| 2.2 Postoperative fever Show forest plot | 5 | 2233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.55, 0.95] |
| 2.2.1 Women in labor | 5 | 1415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.87] |
| 2.2.2 Women not in labor | 3 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.60, 1.43] |
| 2.3 Postoperative wound infection Show forest plot | 5 | 2233 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.88] |
| 2.3.1 Women in labor | 5 | 1415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.90] |
| 2.3.2 Women not in labor | 3 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.35, 1.31] |
| 2.4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.09] |
| 2.4.1 Women in labor | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.61] |
| 2.4.2 Women not in labor | 2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| 2.5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.85] |
| 2.5.1 Women in labor | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.87] |
| 2.5.2 Women not in labor | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 3.1 Post‐cesarean endometritis Show forest plot | 9 | 2634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.30, 0.55] |
| 3.1.1 Women with ruptured membranes | 5 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.12, 0.45] |
| 3.1.2 Women with intact membranes | 8 | 2082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.68] |
| 3.2 Postoperative fever Show forest plot | 8 | 2474 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.38, 0.78] |
| 3.2.1 Women with ruptured membranes | 4 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.80] |
| 3.2.2 Women with intact membranes | 7 | 1994 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.49, 0.99] |
| 3.3 Postoperative wound infection Show forest plot | 9 | 2634 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.47, 0.91] |
| 3.3.1 Women with ruptured membranes | 5 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.19, 1.50] |
| 3.3.2 Women with intact membranes | 8 | 2082 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.07] |
| 3.4 Composite wound complication Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.44] |
| 3.4.1 Women with ruptured membranes | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.89] |
| 3.4.2 Women with intact membranes | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.10] |
| 3.5 Composite wound complication or endometritis Show forest plot | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.85] |
| 3.5.1 Women with ruptured membranes | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.13] |
| 3.5.2 Women with intact membranes | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |

