術後感染予防を目的とした、帝王切開術前の消毒液を用いた腟洗浄
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT. | |
| Participants | Inclusion: pregnant women schedule for term elective cesarean section ‐ indications were prior cesarean, abnormal presentation, maternal request, prior cystocele repair or prior perineal tear Exclusion: emergency cesarean, premature ruptured membranes, placenta previa, immunocompromised status. Setting: Saudi Arabia | |
| Interventions | Intervention: chlorhexidine 0.25% antiseptic wipes in vagina (3 10 cm x 10 cm pieces used from apex to introitus including fornices for approximately 1 minute total time). Control: no vaginal cleansing. Intention‐to‐treat analysis. | |
| Outcomes | Outcomes: infectious morbidities – endometritis, fever, wound infection. Endometritis ‐ fever with tenderness and offensive lochia. Febrile morbidity ‐ fever of 38 degrees C or more without infectious clinical findings. Wound infection ‐ erythema or wound edge separation with purulent discharge requiring antibiotics and wound care. Side effects | |
| Notes | All outcomes are summed for overall results. Apparently no one with endometritis also had a wound infection. These are not necessarily mutually exclusive. October 2014 to end of December 2015. Funding source: not stated Author declarations of interest: no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization method used. |
| Allocation concealment (selection bias) | Unclear risk | No other information provided beside the use of sealed envelopes. |
| Blinding of participants and personnel (performance bias) | High risk | Vaginal scrub was performed while the surgeon was in the room. |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical care team was blinded to either arm. |
| Incomplete outcome data (attrition bias) | Low risk | 7 in intervention and 11 in control arm lost to follow‐up. Otherwise, complete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | None |
| Methods | RCT. | |
| Participants | Inclusion: 434 women undergoing emergency cesarean with labor duration > 6 hours regardless of membrane rupture. Exclusion: diabetes, anemia, obstructed labor, any febrile condition. Setting: Islamabad, Pakistan | |
| Interventions | Intervention: vaginal cleansing with povidone‐iodine (n = 217 randomized). Control: no vaginal cleansing (n = 217 randomized). | |
| Outcomes | Fever, wound infection, endometritis. | |
| Notes | February 1 to July 31, 2016. Funding source: not stated Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Population randomized, but not clearly stated how it was accomplished. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None |
| Methods | Double blind quasi‐RCT. | |
| Participants | Inclusion: women undergoing non‐emergent or laboring cesarean delivery. Exclusion: iodine sensitivity, chorioamnionitis, gestational herpes, abnormal vaginal discharge, emergency cesarean (due to fetal distress, placenta previa). Setting: Iran. | |
| Interventions | Intervention: 2 4 x 4 gauze sponges soaked in 10% povidone ‐iodine solutions rotated 360 degrees for 30 seconds from vault to introitus (n = 284). Control: no vaginal cleansing (n = 284). Intention‐to‐treat analysis. | |
| Outcomes | Febrile morbidity, endometritis, wound infection. | |
| Notes | May 2007‐April 2008. Funding source: not stated Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomized, alternating sequence. |
| Allocation concealment (selection bias) | High risk | Quasi‐randomized, alternating sequence. |
| Blinding of participants and personnel (performance bias) | Low risk | Participants: unclear but stated "double blind". |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded ‐ all data reviewed by 1 physician without knowledge of patient assignment. |
| Incomplete outcome data (attrition bias) | Low risk | Complete outcome data. 10 withdrawals from intervention group, 7 from control group. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | High risk | Large differences in baseline characteristics ‐ more examinations, longer labor, more preterm, longer surgery, longer duration of PROM in vaginal cleansing group. |
| Methods | RCT. | |
| Participants | Inclusion: 120 pregnant women undergoing elective cesarean delivery, no active infection, completion of week 37 of gestation. Exclusion: preterm labor, PROM, emergency cesarean, body temperature above 38 degrees celsius, severe anemia, allergic reaction to agents. Setting: Sanko University. | |
| Interventions | Intervention group 1: povidone‐iodine vaginal cleansing for 30 seconds (n = 41). Intervention group 2: benzalkonium chloride vaginal cleansing for 30 seconds (n = 39). Control: no vaginal cleansing (n = 40). Intention‐to‐treat analysis. | |
| Outcomes | Postoperative pain evaluation, time to flatulence and defecation, and Hb, WBC, Plt, CRP in 24 hours. | |
| Notes | July to August 2014. Funding source: not stated Author declarations of interest: no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomization method. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | High risk | Operating physician applied cleansing agents. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) | Low risk | Complete outcome data, all WOMEN were in hospital so none lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | None. |
| Methods | RCT. | |
| Participants | Inclusion: 160 women undergoing cesarean delivery. Exclusion: medical contraindications to vaginal preparation ‐ emergency cesarean, allergy, placenta previa. Setting: University Medical Center in TX, USA. | |
| Interventions | Intervention: povidone‐iodine vaginal wash (concentration not specified) (n = 80). Control: saline vaginal wash (n = 80). | |
| Outcomes | Endometritis (temperature > 100.4 degrees F at least twice > 24 hours after surgery or of 101 degrees F any time after surgery, with abdominal/uterine tenderness). Cellulitis (advancing erythema around the incision). | |
| Notes | March 2000 to July 2001. Funding source: not stated Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified, simply states "randomized into one of two arms". |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | Cleansing done by nurse while providers outside and thus providers were blinded to the intervention. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessors blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Inclusion: all women undergoing cesarean delivery, age ≥ 18 years. Exclusion: emergency cesarean delivery, allergy to iodine. Setting: academic medical center in Indiana, USA. | |
| Interventions | Intervention: preoperative vaginal cleansing with 1% povidone‐iodine scrubs. 3 sponge sticks soaked in 1% povidone‐iodine in a prepackaged sterile pouch. The vaginal scrub encompassed the vaginal apex to the introitus with attention to the anterior, posterior, and lateral walls including all fornices (n = 155). Control: no preoperative vaginal cleansing (n = 145). Intention‐to‐treat analysis. | |
| Outcomes | Post‐cesarean endometritis (uterine tenderness plus postoperative fever requiring antibiotics). Postoperative fever (> 38 degrees Celcius, > 24 hours after surgery). Wound infection requiring antibiotics. Wound separation, seroma, hematoma, or need for debridement. Composite infectious morbidity outcome: either endometritis, fever, sepsis, hospital readmission, wound infection, or wound complication. | |
| Notes | The trial was stopped early due to difficulty recruiting. September 2006 to January 2009. Funding source: Internally funded. Author declarations of interest: no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table, replacement randomization. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque security envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Not specifically blinded but after anesthesia care providers did not necessarily know group. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessor blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Appeared to be complete data on all participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Trial stopped early at safety analysis due to difficulty recruiting and effect seen. |
| Methods | RCT. | |
| Participants | Inclusion: women > 18 years of age undergoing cesarean section. Exclusion: allergy to iodine solution, bleeding placenta previa. Setting: Hyderabad, Pakistan. | |
| Interventions | Intervention: 10% pyodine soaked pieces of gauze (3) used for vaginal scrub immediately before cesarean from vaginal apex to introitus with attention to vaginal walls (n = 100). Control: no vaginal cleansing (n = 100). Intention to treat ‐ unclear. | |
| Outcomes | Postoperative febrile morbidity (oral temperature of 38 degrees C after 1st 24 hours of surgery). Endometritis (postoperative fever with uterine tenderness and foul smelling lochia requiring broad spectrum antibiotic therapy). Wound complications (infection at surgical site ‐ seroma, hematoma, and disruption of abdominal incision ‐ that required parenteral antibiotics and wound care. Composite infectious morbidity ‐ a sum of the 3 outcomes above. | |
| Notes | February to July 2010. Funding source: not stated Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "randomly assigned" with no other details. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) | Low risk | Stated that physician evaluating the data was unaware of any woman's participation. |
| Incomplete outcome data (attrition bias) | Low risk | Appeared to be complete data on all participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. Poorly defined composite infectious morbidity overall outcome appears to be the sum of endometritis, fever, and wound infection. |
| Methods | RCT. | |
| Participants | Inclusion: women admitted and mentally competent to consent for a cesarean delivery. Exclusion: medical contraindications to the cleansing ‐ highly emergent cesarean, bleeding placenta previa, allergy to iodine or shellfish, active genital herpes. Setting: University of North Carolina Women's Hospital, North Carolina, USA. | |
| Interventions | Intervention: 10% povidone‐iodine surgical scrub solution vaginally immediately before cesarean (n = 247). Control: no vaginal cleansing (n = 251). Intention‐to‐treat analysis. | |
| Outcomes | Fever (38 degrees C or greater after the day of surgery). Febrile morbidity (postoperative fever on 2 or more calendar days, excluding the day of surgery). Endometritis (postoperative fever, with a physician's note indicating uterine or abdominal pain or tenderness, preceding an order for antibiotics and a statement indicating that the antibiotics were for uterine or pelvic infection and laboratory studies did not indicate other source for the infection). Wound separation (defined by chart note reporting separation of the operative incision requiring intervention). Number of postoperative days with fever. Average duration of antibiotic administration. Length of hospitalization. | |
| Notes | Chorioamnionitis participants excluded from analysis. May 1996 to September 1998. Funding source: not stated Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted block randomization schedule. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed and numbered envelopes taped to abdominal prep packs. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specifically stated. Cleansing done by residents during routine prep. These may have been the same surgeons who did the surgery and postoperative care. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessor masked. |
| Incomplete outcome data (attrition bias) | Low risk | 3 withdrawals lacked necessary charting information |
| Selective reporting (reporting bias) | High risk | Large number of participants excluded after randomization who had chorioamnionitis (a known risk factor for postoperative infectious morbidity) because their inclusion "distorted the absolute rates of fever and infectious morbidity." That trial states that when the 68 participants with antepartum infection were included, the estimates of effect of vaginal preparation were not meaningfully different. Thus they planned to exclude those participants from reports of outcomes. As this represented 13.5% of the originally randomized sample, however, there is a risk that this introduced selective reporting bias into the trial. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Inclusion: women admitted for delivery > 24 weeks' gestation. Exclusion: contraindications to digital examinations, placenta previa, active herpes, chorioamnionitis before randomization or allergy to chlorhexidine. Setting: University of Alabama ‐ Birmingham, USA. | |
| Interventions | Intervention: 200 mL irrigation of 0.2% chlorhexidine solution in labor or if a planned cesarean then immediately before surgery. Control: 200 mL sterile water placebo solution. Intention‐to‐treat analysis. | |
| Outcomes | Endometritis. | |
| Notes | February 1994 to January 1996. 1024 women enrolled and trial designed for vaginal irrigation during labor. Trial did report on 14 women who had elective cesarean before labor and thus just got the irrigation before the procedure, thus qualifying the study for inclusion in the analysis for those 14 women only. Funding source: Agency for Health Care Policy Research Contract DHHS No. 290‐92‐0055 Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered study labels on identical bottles prepared by Investigational Drug Service at the site. |
| Blinding of participants and personnel (performance bias) | Low risk | Active and placebo solutions were clinically indistinguishable. |
| Blinding of outcome assessment (detection bias) | Low risk | Data collection done before the assignment was known. |
| Incomplete outcome data (attrition bias) | Low risk | 10 total withdrawals, allocation not determined. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Inclusion: women to undergo non‐emergency cesarean delivery. Exclusion: placenta previa, chorioamnionitis. Setting: Chicago Lying‐In Hospital, Illinois, USA. | |
| Interventions | Intervention: pre‐packaged povidone‐iodine solution (EZ Prep 200, 5%) vaginal preparation for 30 seconds (n = 142). Control: no preoperative vaginal cleansing (n = 166). | |
| Outcomes | Febrile morbidity (any postoperative temperature > 38 degrees C). Endometritis (temperature elevation > 38 degrees C beyond the first postoperative day, in association with uterine tenderness and foul lochia, in the absence of evidence of other infection; given at the time of clinical evaluation). Wound infection (clinical diagnosis evidenced by erythema or wound edge separation with purulent drainage; including wound dehiscence and necrotizing fasciitis and excluding skin separation without evidence of cellulitis). | |
| Notes | November 1997 to March 2000. Funding source: University of Chicago Hospitals Resident Research Fund Author declarations of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random digit table. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Not stated for participants but treating providers at the time of fever were unaware of participation status. |
| Blinding of outcome assessment (detection bias) | Low risk | Chart reviewer unaware of group. |
| Incomplete outcome data (attrition bias) | Unclear risk | Ultimately 92 participants excluded from analysis posts‐randomization (400 originally randomized), reasons explained: 33 due to lost envelopes, 6 for violations of inclusion criteria, and 53 because their hospital charts could not be located. Of all the women excluded, 54 were in the vaginal cleansing group and 38 were in the control group. Only outcomes for women for whom all data were available were reported. The large number of women excluded also makes this trial subject to an unclear risk of bias, however as there is no outcome data for the excluded participants, the potential impact is unclear. Unclear if exclusions impacted data. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | RCT. | |
| Participants | Inclusion: women undergoing either a scheduled or emergency cesarean delivery. Exclusion: umbilical cord prolapse, placenta previa, or known allergy to povidone‐iodine Setting: Istanbul, Turkey | |
| Interventions | Intervention: 30 second vaginal cleansing with 2 prepackaged povidone‐iodine solution‐soaked foam sponges preoperatively performed in conjunction with the abdominal preparation with 2 prepackaged foam sponges that contained the solution, rotated 360 degrees (n = 335). Control: no preoperative vaginal preparation (n = 335). | |
| Outcomes | Postpartum endometritis (primary outcome) body temperature > 38.5 degrees C with concomitant foul‐smelling discharge or abnormally tender uterus on bimanual examination). Wound infection (partial or total separation of the incision, as well as the presence of purulent or serous wound discharge, with induration, warmth, and tenderness). Fever (elevated temperature of 38 degrees C or higher for a minimum of 24 hours following surgery not associated with signs of infection). | |
| Notes | January to August 2011. Funding source: not stated Author declarations of interest: no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated randomization process." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing random numbers. Assignment based on those numbers. |
| Blinding of participants and personnel (performance bias) | High risk | The researchers in the study were not blinded and the assignment was written in the medical record. |
| Blinding of outcome assessment (detection bias) | High risk | The researchers in the study were not blinded and the assignment was written in the medical record. |
| Incomplete outcome data (attrition bias) | Low risk | Only 1 participant withdrew. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. |
| Other bias | Low risk | No evidence of other bias. |
CRP: C‐reactive protein
Hb: hemoglobin
Plt: platelets
PROM: premature rupture of membranes
RCT: randomized controlled trial
WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Study retracted. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT |
| Participants | 400 women getting elective cesarean delivery at term, Iran |
| Interventions | Vaginal washing with 2 gauze with 10% povidone‐iodine for 30 seconds Control no vaginal preparation |
| Outcomes | Primary: fever, uterine tenderness, tachycardia, foul‐smelling lochia |
| Notes | Iranian trial registry says complete. Emailed study contact 7/12/2017, no response. |
| Methods | RCT |
| Participants | 526 women getting cesarean at term, excluding chorioamnionitis |
| Interventions | Vaginal irrigation with povidone‐iodine Control: no vaginal preparation |
| Outcomes | Primary‐ fever (body temperature) |
| Notes | Iranian trial registry says complete. Emailed study contact 7/12/2017, no response. |
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Vaginal antimicrobacterial preparation before cesarean section for endometritis prevention |
| Methods | RCT |
| Participants | 1040 women getting a cesarean delivery |
| Interventions | Vaginal preparation with septal soap before cesarean Control: no vaginal preparation |
| Outcomes | Primary‐ endometritis |
| Starting date | April 2017, anticipated completion April 2020 |
| Contact information | Hila Ben‐Asher, Rambam Health Care |
| Notes | Not yet recruiting, verified in clinicaltrials.gov by PI April 2017. |
| Trial name or title | Preoperative application of chlorhexidine to reduce infection with cesarean section after labor (PRACTICAL) |
| Methods | RCT |
| Participants | 800 women getting a cesarean delivery in labor |
| Interventions | 4% chlorhexidine gluconate vaginal scrub prior to cesarean ControlL no vaginal cleansing |
| Outcomes | Primary: rate of surgical site infection up to 6 weeks postpartum: composite of wound infection and postpartum endometritis, defined as fever of 100.4 degrees F or more 24 hours after delivery associated with uterine tenderness and persistent foul‐smelling lochia requiring broad spectrum intravenous antibiotic administration |
| Starting date | March 2018, anticipated completion March 2020 |
| Contact information | Angela Bianco at Icahn School of Medicine at Mount Sinai, New York |
| Notes | Not yet recruiting as of posting February 6, 2017 |
| Trial name or title | Chlorhexidine gluconate versus povidone‐iodine as vaginal preparation antiseptics prior to cesarean delivery |
| Methods | RCT |
| Participants | 100 women getting a scheduled cesarean delivery at least 37 weeks' gestation (not in labor or with ruptured membranes) |
| Interventions | Group 1: 4% chlorhexidine gluconate preoperative vaginal preparation Group 2: 10% povidone‐iodine preoperative vaginal preparation with scrub and paint |
| Outcomes | Primary: bacterial load immediately postoperative prior to exit from operating room‐ outcome is change in total bacterial load from preoperative sampling Secondary outcomes include length of hospital stay and postoperative infections including endometritis, pelvic abscesses, and skin/wound infection |
| Starting date | May 2017, anticipated completion December 2019 |
| Contact information | Lauryn Przeslawski at Metro Health in Michigan |
| Notes | Currently recruiting as of August 30, 2017 |
| Trial name or title | Chlorhexidine gluconate vs povidone‐iodine vaginal cleansing solution prior to cesarean delivery |
| Methods | RCT |
| Participants | 1500 women getting non‐emergent cesarean delivery, chorioamnionitis excluded |
| Interventions | Group 1: 10% povidone‐iodine solution for vaginal cleansing with 4 minutes of drying time before draping Group 2: 4% chlorhexidine gluconate solution for vaginal cleansing |
| Outcomes | Primary outcome: postpartum endometritis 0‐3 days postpartum with diagnosis involving fever, uterine fundal tenderness, or purulent lochia requiring antibiotic therapy |
| Starting date | December 2016, anticipated completion May 2018 |
| Contact information | Nisha Lakhi, MD at Richmond University Medical Center, New York |
| Notes | Currently recruiting as of February 16, 2018 |
| Trial name or title | Preoperative vaginal cleansing with povidone iodine and the risk of post‐cesarean endometritis |
| Methods | RCT |
| Participants | 306 women undergoing cesarean |
| Interventions | Vaginal cleansing with 3 gauze pieces soaked in 10% povidone‐iodine from vaginal apex to introitus Control: no vaginal cleansing |
| Outcomes | Primary outcome: postcesarean endometritis diagnosed by fever 38.4 degrees C or greater in first 48 hours with either uterine tenderness, foul smelling lochia or positive C‐reactive protein |
| Starting date | April 2015 |
| Contact information | Amer Ahmed Mahmoud Riad, Ain Shams Maternity Hospital |
| Notes | Currently recruiting as of February 2016. |
| Trial name or title | Vaginal cleansing before cesarean delivery to reduce infection: a randomized trial |
| Methods | RCT |
| Participants | 608 women undergoing cesarean |
| Interventions | Vaginal cleansing with 2 sponge sticks soaked in 1% povidone‐iodine Control: no cleansing All will receive standard abdominal cleansing using chlorhexidine or Betadine per provider preference |
| Outcomes | Primary: composite postoperative infectious morbidity up to 30 days‐ fever, endometritis, infection or abscess, wound complications or infection |
| Starting date | August 2015 |
| Contact information | Lorene Temming, Washington University, St. Louis |
| Notes | Anticipated completion August 2018, verified 2/22/18‐ NCT02495753 |
RCT: randomized controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 10 | 3283 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.63] |
| Analysis 1.1  Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 1 Post‐cesarean endometritis. | ||||
| 1.1 Iodine‐based solution | 8 | 3069 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.69] |
| 1.2 Chlorhexidine‐based solution | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.75] |
| 2 Postoperative fever Show forest plot | 8 | 3109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| Analysis 1.2  Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 2 Postoperative fever. | ||||
| 2.1 Iodine‐based solution | 7 | 2909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 2.2 Chlorhexidine‐based solution | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.56] |
| 3 Postoperative wound infection Show forest plot | 8 | 2839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.11] |
| Analysis 1.3  Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 3 Postoperative wound infection. | ||||
| 3.1 Iodine‐based solution | 7 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 3.2 Chlorhexidine‐based solution | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.82] |
| 4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.07] |
| Analysis 1.4  Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 4 Composite wound complication. | ||||
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.82] |
| Analysis 1.5  Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 5 Composite wound complication or endometritis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.32, 1.06] |
| Analysis 2.1 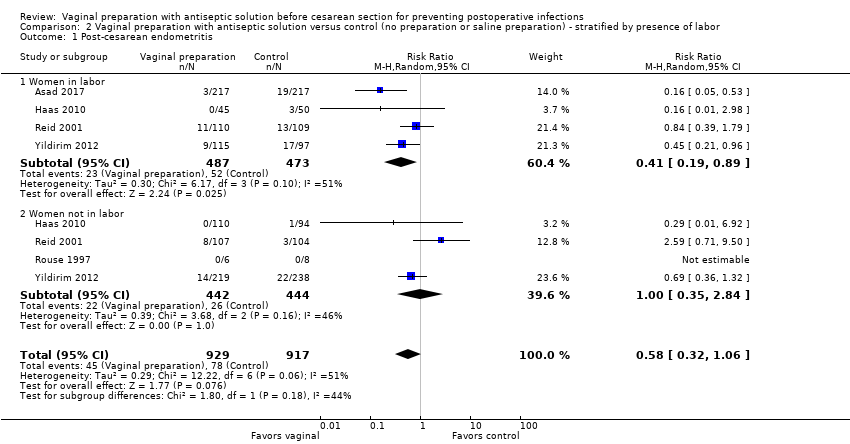 Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 1 Post‐cesarean endometritis. | ||||
| 1.1 Women in labor | 4 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.19, 0.89] |
| 1.2 Women not in labor | 4 | 886 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.84] |
| 2 Postoperative fever Show forest plot | 3 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.57, 1.03] |
| Analysis 2.2  Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 2 Postoperative fever. | ||||
| 2.1 Women in labor | 3 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.96] |
| 2.2 Women not in labor | 2 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.49] |
| 3 Postoperative wound infection Show forest plot | 3 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.08] |
| Analysis 2.3  Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 3 Postoperative wound infection. | ||||
| 3.1 Women in labor | 3 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.24] |
| 3.2 Women not in labor | 2 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.27, 1.57] |
| 4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.09] |
| Analysis 2.4 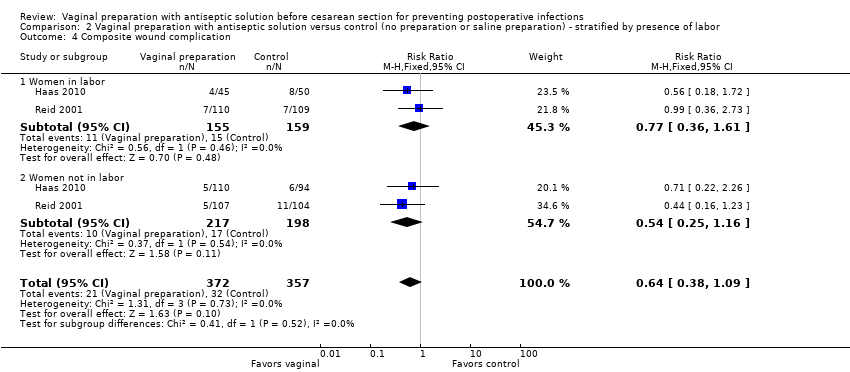 Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 4 Composite wound complication. | ||||
| 4.1 Women in labor | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.61] |
| 4.2 Women not in labor | 2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.85] |
| Analysis 2.5 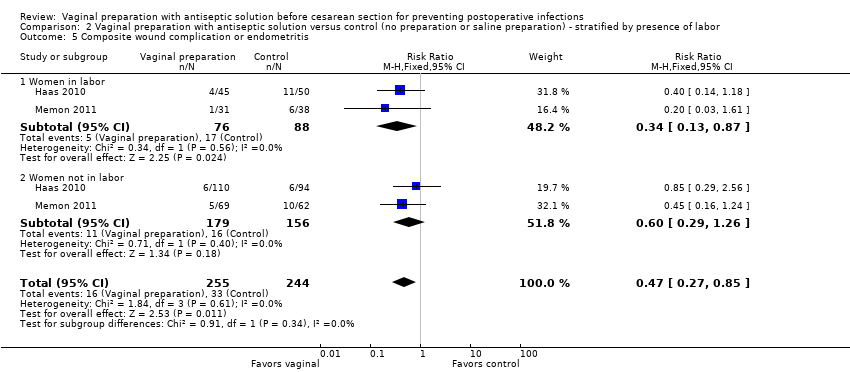 Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 5 Composite wound complication or endometritis. | ||||
| 5.1 Women in labor | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.87] |
| 5.2 Women not in labor | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 4 | 1329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.27, 0.62] |
| Analysis 3.1  Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 1 Post‐cesarean endometritis. | ||||
| 1.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 1.2 Women with intact membranes | 4 | 1057 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.82] |
| 2 Postoperative fever Show forest plot | 3 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.11] |
| Analysis 3.2  Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 2 Postoperative fever. | ||||
| 2.1 Women with ruptured membranes | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.12] |
| 2.2 Women with intact membranes | 3 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| 3 Postoperative wound infection Show forest plot | 4 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.43, 1.30] |
| Analysis 3.3  Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 3 Postoperative wound infection. | ||||
| 3.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.16, 6.70] |
| 3.2 Women with intact membranes | 4 | 1057 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.28] |
| 4 Composite wound complication Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.44] |
| Analysis 3.4  Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 4 Composite wound complication. | ||||
| 4.1 Women with ruptured membranes | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.89] |
| 4.2 Women with intact membranes | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.10] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.85] |
| Analysis 3.5  Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 5 Composite wound complication or endometritis. | ||||
| 5.1 Women with ruptured membranes | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.13] |
| 5.2 Women with intact membranes | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
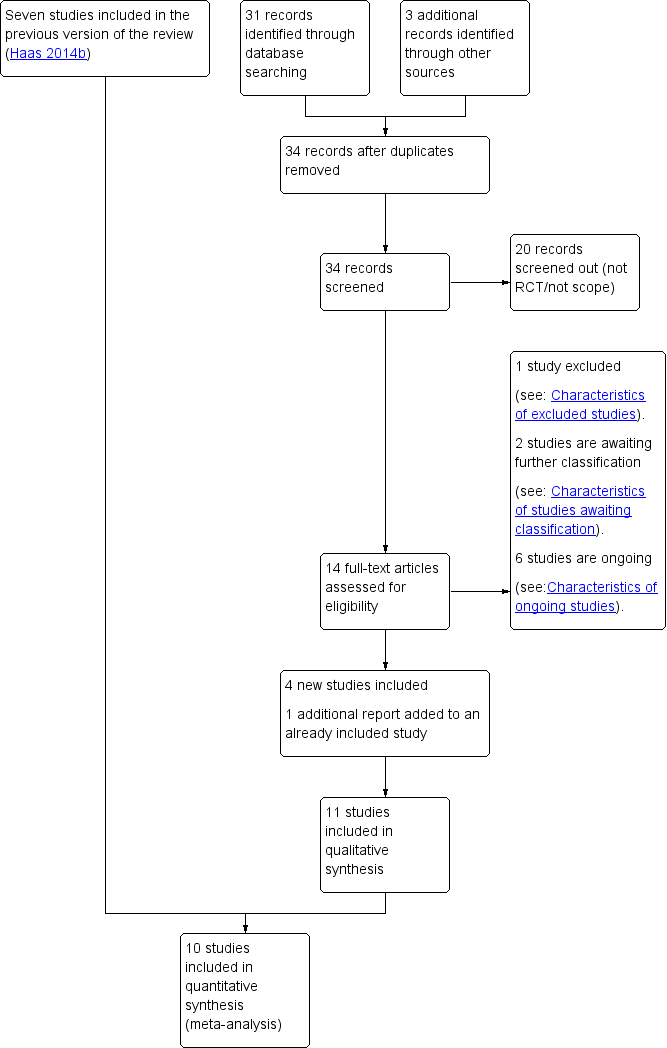
Study flow diagram.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 1 Post‐cesarean endometritis.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 2 Postoperative fever.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 3 Postoperative wound infection.

Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 4 Composite wound complication.
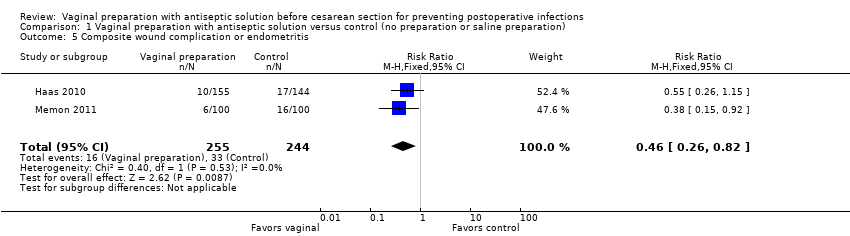
Comparison 1 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation), Outcome 5 Composite wound complication or endometritis.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 1 Post‐cesarean endometritis.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 2 Postoperative fever.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 3 Postoperative wound infection.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 4 Composite wound complication.

Comparison 2 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of labor, Outcome 5 Composite wound complication or endometritis.
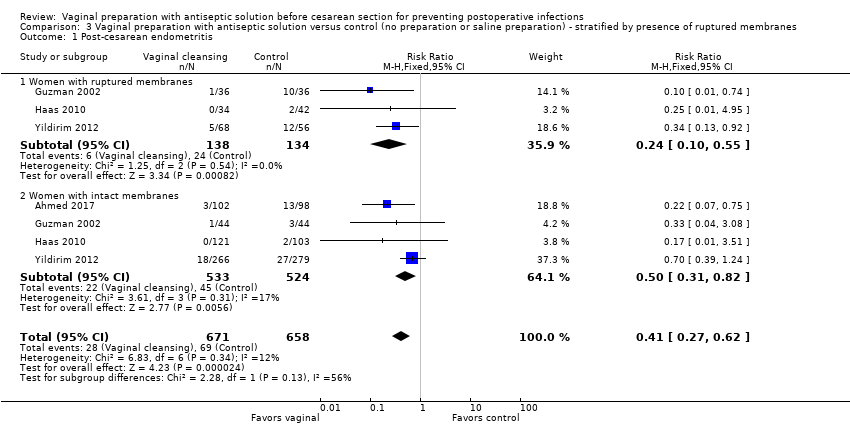
Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 1 Post‐cesarean endometritis.
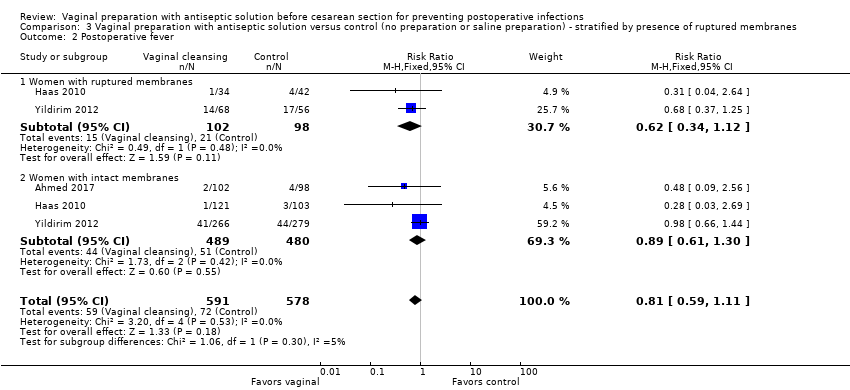
Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 2 Postoperative fever.

Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 3 Postoperative wound infection.
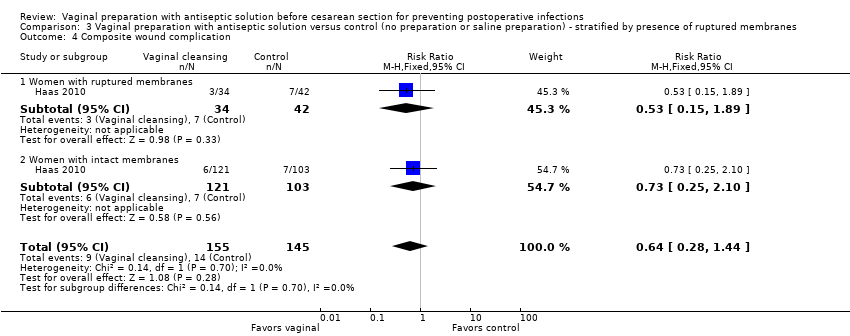
Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 4 Composite wound complication.

Comparison 3 Vaginal preparation with antiseptic solution versus control (no preparation or saline preparation) ‐ stratified by presence of ruptured membranes, Outcome 5 Composite wound complication or endometritis.
| Vaginal preparation with antiseptic solution compared to control (no preparation or saline preparation) for preventing postoperative infections | ||||||
| Patient or population: pregnant women who were about to receive a cesarean delivery. This included women receiving elective, laboring, or urgent cesareans | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with control | Risk with vaginal preparation | |||||
| Post‐cesarean endometritis | Study population | Average RR 0.36 | 3283 | ⊕⊕⊕⊝ | ||
| 86 per 1000 | 31 per 1000 | |||||
| Postoperative fever | Study population | RR 0.87 | 3109 | ⊕⊕⊕⊝ | ||
| 125 per 1000 | 109 per 1000 | |||||
| Postoperative wound infection | Study population | RR 0.74 | 2839 | ⊕⊕⊕⊝ | ||
| 36 per 1000 | 27 per 1000 | |||||
| Composite wound complication or endometritis | Study population | RR 0.46 | 499 | ⊕⊕⊕⊝ | ||
| 135 per 1000 | 62 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Over 40% of included studies had some design limitations. 2 Wide confidence intervals in included studies. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 10 | 3283 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.63] |
| 1.1 Iodine‐based solution | 8 | 3069 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.21, 0.69] |
| 1.2 Chlorhexidine‐based solution | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.75] |
| 2 Postoperative fever Show forest plot | 8 | 3109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.05] |
| 2.1 Iodine‐based solution | 7 | 2909 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 2.2 Chlorhexidine‐based solution | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.56] |
| 3 Postoperative wound infection Show forest plot | 8 | 2839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.49, 1.11] |
| 3.1 Iodine‐based solution | 7 | 2639 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.19] |
| 3.2 Chlorhexidine‐based solution | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.17, 1.82] |
| 4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.07] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 5 | 1846 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.32, 1.06] |
| 1.1 Women in labor | 4 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.19, 0.89] |
| 1.2 Women not in labor | 4 | 886 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.35, 2.84] |
| 2 Postoperative fever Show forest plot | 3 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.57, 1.03] |
| 2.1 Women in labor | 3 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.43, 0.96] |
| 2.2 Women not in labor | 2 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.49] |
| 3 Postoperative wound infection Show forest plot | 3 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.08] |
| 3.1 Women in labor | 3 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.24] |
| 3.2 Women not in labor | 2 | 661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.27, 1.57] |
| 4 Composite wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.09] |
| 4.1 Women in labor | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.61] |
| 4.2 Women not in labor | 2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.85] |
| 5.1 Women in labor | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.87] |
| 5.2 Women not in labor | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 4 | 1329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.27, 0.62] |
| 1.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 1.2 Women with intact membranes | 4 | 1057 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.82] |
| 2 Postoperative fever Show forest plot | 3 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.11] |
| 2.1 Women with ruptured membranes | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.12] |
| 2.2 Women with intact membranes | 3 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.30] |
| 3 Postoperative wound infection Show forest plot | 4 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.43, 1.30] |
| 3.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.16, 6.70] |
| 3.2 Women with intact membranes | 4 | 1057 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.28] |
| 4 Composite wound complication Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.44] |
| 4.1 Women with ruptured membranes | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.89] |
| 4.2 Women with intact membranes | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.10] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.85] |
| 5.1 Women with ruptured membranes | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.13] |
| 5.2 Women with intact membranes | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |

