Préparation vaginale avec une solution antiseptique avant une césarienne en prévention des infections postopératoires
Résumé scientifique
Contexte
La césarienne est l'une des interventions chirurgicales les plus fréquemment pratiquées par les obstétriciens. La morbidité infectieuse après un accouchement par césarienne a un impact considérable sur la rémission post‐partum de la mère et sa capacité à s'occuper de son bébé. Malgré l'utilisation très répandue d'antibiotiques prophylactiques, la morbidité infectieuse postopératoire continue d'entraîner des complications après une césarienne.
Objectifs
Déterminer si le nettoyage du vagin à l'aide d'une solution antiseptique avant une césarienne diminue le risque de morbidités infectieuses chez la mère, notamment l'endométrite et les complications au niveau de la cicatrice.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre des essais du groupe Cochrane sur la grossesse et l'accouchement (10 décembre 2014).
Critères de sélection
Nous avons inclus des essais randomisés et quasi randomisés évaluant l'impact du nettoyage vaginal juste avant la césarienne avec tout type de solution antiseptique par rapport à une solution placebo ou à la norme de soins, en termes de morbidité infectieuse post‐césarienne.
Recueil et analyse des données
Nous avons évalué de façon indépendante l'éligibilité et la qualité des études.
Résultats principaux
Sept essais impliquant 2 816 femmes randomisées (dont 2 635 ont été analysées) ont évalué les effets du nettoyage vaginal (tous avec de la povidone iodée) sur la morbidité infectieuse post‐césarienne. Le risque de biais était généralement faible et les études de bonne qualité. La préparation vaginale juste avant la césarienne a réduit de façon significative l'incidence d'endométrite post‐césarienne, de 8,3 % dans les groupes de contrôle à 4,3 % dans les groupes avec nettoyage vaginal (risque relatif (RR) moyen 0,45, intervalle de confiance (IC) à 95 % de 0,25 à 0,81, sept essais, 2 635 femmes). La réduction du risque a été particulièrement importante pour les femmes qui étaient déjà en travail au moment de l'accouchement par césarienne (7,4 % dans le groupe avec nettoyage vaginal contre 13,0 % dans le groupe de contrôle ; RR 0,56, IC à 95 % de 0,34 à 0,95, trois essais, 523 femmes) et pour les femmes aux membranes rompues (4,3 % dans le groupe avec nettoyage vaginal contre 17,9 % dans le groupe de contrôle ; RR 0,24, IC à 95 % de 0,10 à 0,55, trois essais, 272 femmes). Aucun autre résultat clinique n'a obtenu une différence significative entre les groupes avec nettoyage vaginal et les groupes de contrôle. Aucun événement indésirable n'a été rapporté avec le nettoyage à la povidone iodée.
La qualité des preuves selon l'approche GRADE était faible pour l'endométrite post‐césarienne, modérée pour la fièvre post‐opératoire et faible pour l'infection de la plaie.
Conclusions des auteurs
La préparation vaginale avec une solution de povidone iodée juste avant la césarienne réduit le risque d'endométrite postopératoire. Ce bénéfice est particulièrement marqué pour les femmes qui accouchent par césarienne après l'entrée en travail ou la rupture des membranes. Les professionnels de santé doivent envisager la mise en œuvre du nettoyage vaginal préopératoire avec de la povidone iodée, qui constitue une intervention simple et généralement peu coûteuse, avant de pratiquer une césarienne.
PICO
Résumé simplifié
Nettoyage vaginal avant une césarienne pour réduire les infections post‐césariennes
Les accouchements par césarienne sont très fréquents aujourd'hui, représentant jusqu'à une naissance sur trois dans certains pays. Les antibiotiques sont administrés en routine avant ou pendant l'intervention pour réduire le risque d'infection, mais certaines femmes développent quand même ces complications. Entre une femme sur quatre et une femme sur 10 développe une infection de l'utérus (endométrite) ou un problème au niveau de la cicatrice, respectivement. Ces complications entraînent une rémission plus lente suite à la chirurgie et peuvent altérer la capacité de la mère à s'occuper de son bébé. D'autres interventions sont nécessaires pour réduire davantage le risque d'infection de l'utérus et les problèmes de cicatrisation après une césarienne.
Cette revue a révélé que le nettoyage du vagin à l'aide d'une solution antiseptique juste avant la césarienne réduisait le risque d'infection post‐césarienne de l'utérus (preuves de faible qualité). Les bénéfices étaient plus importants si la mère avait déjà perdu les eaux (c.‐à‐d. si les membranes étaient déjà rompues) ou si elle était déjà en travail au moment de la césarienne. Cette revue n'a pas trouvé que le nettoyage vaginal réduisait le nombre de femmes qui développent de la fièvre ou des complications au niveau de la cicatrice après une césarienne. L'antiseptique était de la povidone iodée et aucun événement indésirable dû à la solution de préparation vaginale, tel qu'une allergie ou une irritation, n'a été observé dans aucun des sept essais randomisés, impliquant 2 635 femmes.
Authors' conclusions
Summary of findings
| Vaginal preparation versus control for preventing postoperative infections | ||||||
| Population: Pregnant women who received a cesarean delivery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vaginal preparation versus control | |||||
| Post‐cesarean endometritis | Study population | RR 0.45 | 2635 | ⊕⊕⊝⊝ | ||
| 83 per 1000 | 37 per 1000 | |||||
| Moderate | ||||||
| 75 per 1000 | 34 per 1000 | |||||
| Postoperative fever | Study population | RR 0.9 | 2475 | ⊕⊕⊕⊝ | ||
| 141 per 1000 | 127 per 1000 | |||||
| Moderate | ||||||
| 117 per 1000 | 105 per 1000 | |||||
| Wound infection | Study population | RR 0.86 | 2205 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 29 per 1000 | |||||
| Moderate | ||||||
| 31 per 1000 | 27 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations. | ||||||
Background
Cesarean sections currently account for approximately one‐third of the babies born in the United States. Cesarean section deliveries are often complicated by infections occurring after the surgery.
Description of the condition
Endometritis, an infection of the uterus in the postpartum period, can complicate the postoperative course of a cesarean delivery 6% to 27% of the time (Guzman 2002; Hofmeyr 2002). This complication, up to 10 times more frequent after a cesarean delivery than after vaginal delivery, can lead to serious complications of bacterial infection in the blood (10% to 20%), peritonitis (general infection in the abdominal cavity), intra‐abdominal abscess (cavity filled with infected material), and sepsis (French 2004; Yokoe 2001). Additionally, cesarean deliveries are frequently complicated by maternal fever and wound complications including seroma (fluid collection under the skin), hematoma (blood clots under the skin), infection, and separation. These morbidities can lead to significant delay in a return to normal function.
Fevers and infections after cesarean deliveries are associated with the length of ruptured membranes, length of labor, and number of vaginal examinations (Disgupta 1988; Yonekura 1985). Post‐cesarean endometritis and infectious morbidity are the result often of the presence of bacteria in the vagina and cervix that move higher in the genital tract to infect the uterus (Martens 1991). These bacteria have been shown to be responsible for failure of antibiotic prophylaxis during cesarean deliveries (Watts 1991). Additionally, some antibiotics do not consistently eradicate some bacteria (such as enterococcus) and the vagina has been shown to become colonized with antibiotic‐resistant bacteria after preoperative surgical antibiotic prophylaxis (Gibbs 1982; Graham 1993; Stiver 1984). Currently, it is standard care to give antibiotics to women receiving a cesarean delivery, but the rate of post‐cesarean infections remains a problem.
Description of the intervention
Previous studies have evaluated whether vaginal cleansing before a cesarean delivery with various solutions can reduce the incidence of febrile morbidity (endometritis and wound infections). Povidone iodine, chlorhexidine, and vaginal metronidazole have been reported with varying results. Older data comparing iodine with chlorhexidine before hysterectomy showed lower morbidity in the iodine group, with improved activity against anaerobic pathogens (Duignan 1975; Haeri 1984). Currently, it is not standard care in the United States to prepare the vagina with an antiseptic solution before cesarean delivery. Vaginal cleansing solutions such as chlorhexidine and povidone iodine have very few side effects in general, with low rates of noted allergies or irritation symptoms.
How the intervention might work
By cleansing the vagina of bacteria before the cesarean delivery occurs, there may be less of a bacterial load in the vagina that might cause infectious morbidity postoperatively. As ascending infection is thought to be a major etiology of postoperative endometritis, this could logically reduce that risk.
Why it is important to do this review
Cesarean delivery is increasing, particularly in the developed world. Postoperative infectious morbidity after cesarean delivery impacts the woman's return to normal function and potentially her bonding with the newborn. It can also cause major medical problems and sequelae. Finding an easy, inexpensive method to reduce this risk could have major public health impact in both developed and developing countries.
Objectives
Our objective was to determine if cleansing the vagina with an antiseptic solution before a cesarean delivery decreases the risk of maternal morbidities, including endometritis and wound complications. We also assessed the side effects of vaginal cleansing solutions to determine adverse events associated with the intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized studies.
Types of participants
Pregnant women who received a cesarean delivery.
Types of interventions
Any method of vaginal cleansing (including douches, wipes, sponges, etc.) with any type of antiseptic solution (povidone iodine, chlorhexidine, etc.) versus a placebo solution/standard care (no vaginal preparation).
We included only studies where vaginal preparation was performed no more than one hour before surgery. This review addressed the use of preoperative vaginal cleansing after the decision to perform a cesarean had been made. This review did not address the use of vaginal preparation during labor. Thus, we excluded trials utilizing vaginal cleansing solutions during labor. We also excluded studies where prophylactic surgical antibiotics were explicitly not used. Surgical prophylaxis with intravenous antibiotics before or during cesarean deliveries has been clearly demonstrated as beneficial in reducing postoperative infectious morbidities. Thus, it is the standard of care. Inclusion of trials not utilizing general surgical antibiotic prophylaxis would not represent the current standard of care and the results would not be translatable into current practice.
Types of outcome measures
Primary outcomes
Postpartum endometritis: defined as a clinical diagnosis, usually involving fever, uterine fundal tenderness, or purulent lochia requiring antibiotic therapy.
Secondary outcomes
-
Postoperative wound infection: defined as erythema, tenderness, purulent drainage from the incision site, with or without fever, requiring antibiotic therapy.
-
Postoperative fever: defined as greater than 38 degrees C or 100.4 degrees F.
-
Postoperative wound seroma or hematoma: defined as collection of serous fluid or blood/clot in the subcutaneous area of the incision.
-
Composite wound complications: defined as the presence of any one of the following: wound infection, seroma, hematoma, separation.
-
Composite wound complications or endometritis.
-
Side effects of vaginal preparation (allergy, irritation). As these solutions are applied gently and not absorbed, there should be no adverse fetal/neonatal effects. We did not anticipate or find mention of adverse neonatal effects from the vaginal cleansing.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (10 December 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language or date restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeHaas 2014.
For this update, we used the following methods for assessing the two new reports that were identified as a result of the updated search. The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
All three review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, all three review authors extracted the data using the agreed form. We resolved discrepancies through discussion. We entered data into Review Manager software (RevMan 2014).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Three review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
-
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
-
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk of bias (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
-
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
-
low, high or unclear risk of bias for participants;
-
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
-
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses.
We assessed methods as:
-
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
-
high risk of bias (e.g. numbers (> 20%) or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomization);
-
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
-
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
-
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
-
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk of other bias;
-
high risk of other bias;
-
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. In future updates, if more studies are included, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update, the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
-
Postpartum endometritis.
-
Postoperative wound infection.
-
Postoperative fever.
GRADEprofiler (Grade 2008) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We planned to use the standardized mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomized trials
There were no cluster‐randomized trials. If in future updates some are identified, we will include cluster‐randomized trials in the analyses along with individually‐randomized trials. We will adjust their sample sizes using the methods described in the Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomized trials and individually‐randomized trials, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomization unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomization unit and perform a sensitivity analysis to investigate the effects of the randomization unit.
Cross‐over trials
Cross‐over trials are not possible for this intervention and are not included.
Other unit of analysis issues
We included quasi‐randomized trials but noted their increased risk of bias in this design.
Dealing with missing data
For included studies, we noted levels of attrition. We did not encounter large levels of attrition. In future updates, if we do encounter large levels of attrition, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomized to each group in the analyses, and all participants were analyzed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomized minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
There are only five studies included. If there are 10 or more studies in the meta‐analysis at a future update, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
For this update, we carried out the following subgroup analyses.
-
Women in labor versus women not in labor.
-
Women with ruptured membranes versus women with intact membranes.
-
Women with chorioamnionitis preoperatively versus women without chorioamnionitis.
-
Women undergoing emergency cesarean versus those undergoing unscheduled cesarean versus those undergoing scheduled cesarean.
-
Women with internal fetal or uterine monitors in place versus those with only external monitors in place before the cesarean.
All reported outcomes in the primary analysis were used in the subgroup analyses.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We did not perform any sensitivity analyses due to a lack of studies included within analyses. In future updates, we plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates (> 20%), or both, with poor quality studies being excluded from the analyses, in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
The original search yielded six reports of four studies. The search of the Pregnancy and Childbirth Group's Trials Register conducted in August 2012 resulted in three further trial reports. One was the published report of Haas 2010 and the other two were reports of Asghania 2011. We obtained full data from the authors. The December 2014 updated search identified two more trial reports (Memon 2011; Yildirim 2012).
Included studies
All seven studies qualified for inclusion in this review. All studies compared preoperative vaginal povidone‐iodine solution preparation with a control group. In one trial (Guzman 2002), the control group was a saline vaginal wash. The other six trials compared the vaginal cleansing with no vaginal cleansing (Asghania 2011; Haas 2010; Memon 2011; Reid 2001; Starr 2005; Yildirim 2012).
Excluded studies
No studies were excluded.
Risk of bias in included studies
See 'Risk of bias' tables for the included studies in Characteristics of included studies and Figure 1; and Figure 2, for summaries of 'Risk of bias' assessments. Overall, the quality of these five studies was generally high as defined by Higgins 2008. Most of the information for the review is derived from studies at low risk of bias. All the studies reported on the outcome of endometritis, while four reported on postoperative fever and wound infection. Two studies reported any wound complication and only one study reported a composite of endometritis or any wound complication. One trial excluded women with chorioamnionitis (Starr 2005). Two trials excluded women undergoing emergency cesarean deliveries (Guzman 2002; Reid 2001).

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Only the Guzman 2002 and Memon 2011 studies were unclear about the randomization sequence generation and allocation concealment.The Guzman 2002 trial, however, appeared free of any other biases. One study (Asghania 2011) was a quasi‐randomized trial with alternate allocation.
Blinding
Six trials blinded outcomes assessors and most had some mechanism for blinding of providers or were unclear about whether participants were blinded. One trial (Yildirim 2012) stated that the researchers were not blinded and that the assignment was written in the medical records so outcomes assessors were unlikely to be blinded either.
Incomplete outcome data
Only the Reid 2001 study was felt to potentially have incomplete outcome bias. This stems from the post‐hoc exclusion of women with chorioamnionitis.
Selective reporting
One trial (Reid 2001) had a large number of participants excluded after randomization who had chorioamnionitis (a known risk factor for postoperative infectious morbidity) because their inclusion "distorted the absolute rates of fever and infectious morbidity." That trial states that when the 68 participants with antepartum infection were included, the estimates of effect of vaginal preparation were not meaningfully different. Thus they planned to exclude those participants from reports of outcomes. As this represented 13.5% of the originally randomized sample, however, there is a risk that this introduced selective reporting bias into the trial (Reid 2001). One other trial had a potential selective reporting bias (Starr 2005). Of 400 participants randomized, 92 (23%) were excluded after randomization: 33 due to lost envelopes, six for violations of inclusion criteria, and 53 because their hospital charts could not be located. Of all the women excluded, 54 were in the vaginal cleansing group and 38 were in the control group. Only outcomes for women for whom all data were available were reported. The large number of women excluded also makes this trial subject to an unclear risk of bias, however as there is no outcome data for the excluded participants, the potential impact is unclear (Starr 2005).
Other potential sources of bias
One trial (Haas 2010) was stopped early at a planned safety analysis due to difficulty recruiting participants. The Asghania 2011 trial excluded women with potential infection before the surgery, including chorioamnionitis, but this was done before enrollment.
Effects of interventions
We included seven trials involving 2816 randomized women (2635 analyzed). One trial (Guzman 2002) compared vaginal povidone‐iodine with a saline vaginal preparation. The remaining six trials compared vaginal povidone‐iodine with no vaginal cleansing (Asghania 2011; Haas 2010; Reid 2001; Starr 2005; Memon 2011; Yildirim 2012).
Vaginal cleansing with povidone iodine solution reduces the risk of post‐cesarean endometritis from 8.3% in control groups to 4.3% in vaginal cleansing groups (risk ratio (RR) 0.45, 95% confidence interval (CI) 0.25 to 0.81, seven trials, 2635 women). Random‐effects meta‐analysis was utilized for this outcome because of high heterogeneity (I² = 55% and Tau² = 0.28), seeAnalysis 1.1. The substantial heterogeneity indicates that treatment effects vary between studies, so we investigated the factors affecting treatment effects by the prespecified subgroup analyses (see below). As all of the trials did not include all subgroups, it is unclear if the subgroup analyses were able to account for all of the heterogeneity. However, we considered that the trials were similar enough clinically that the average treatment effect would be clinically meaningful. Vaginal cleansing did not lead to a statistically significant reduction in the outcomes of postoperative fever (RR 0.90, 95% CI 0.74 to 1.10, six trials, 2475 women, Analysis 1.2), wound infection (RR 0.86, 95% CI 0.54 to 1.36, six trials, 2205 women, Analysis 1.3), or any wound complication (RR 0.63, 95% CI 0.37 to 1.07, two trials, 729 women, Analysis 1.4), but did reduce the composite of endometritis or wound complication (RR 0.46, 95% CI 0.26 to 0.82, two trials, 499 women, Analysis 1.5).
Subgroup analysis ‐ women in labor versus women not in labor
Four trials (Haas 2010; Memon 2011; Reid 2001; Yildirim 2012) stratified data for women in labor versus not in labor. Two trials (Haas 2010; Reid 2001) reported on the outcomes of endometritis and any wound complication. One study reported on stratified outcomes for endometritis, febrile morbidity, and wound infection (Yildirim 2012). One trial only reported stratified results for composite infectious morbidity (Memon 2011). There was a reduction in endometritis for women in labor who received vaginal preparation from 13.0% in the control group to 7.4% in the vaginal preparation group (RR 0.56, 95% CI 0.34 to 0.95, three trials, 523 women; Analysis 2.1) and the composite outcome for infectious morbidity for women in labor (RR 0.34, 95% CI 0.13 to 0.87, two trials, 164 women), see Analysis 2.5, but the small number of women in these groups limit this conclusion. All confidence intervals for the other outcomes overlapped 1.0 for the outcomes for women in labor (postoperative fever: RR 0.82, 95% CI 0.59 to 1.13, two trials, 307 women; wound infection: RR 0.72, 95% CI 0.24 to 2.21, two trials, 307 women; any wound complication: RR 0.77, 95% CI 0.36 to 1.61, two trials, 314 women), seeAnalysis 2.2; Analysis 2.3; Analysis 2.4. The subgroup analysis for women who were not in labor before the cesarean delivery failed to demonstrate any statistically significant differences in outcomes (endometritis: RR 0.89, 95% CI 0.52 to 1.54, three trials, 871 women; postoperative fever: RR 0.96, 95% CI 0.61 to 1.49, two trials, 658 women; wound infection: RR 0.64, 95% CI 0.27 to 1.56, two trials, 652 women; any wound complication: RR 0.54, 95% CI 0.25 to 1.16, two trials, 415 women; composite endometritis or wound complication: RR 0.60, 95% CI 0.29 to 1.26, two trials, 335 women), seeAnalysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5.
There was also no evidence of any difference between subgroups according to the test for subgroup differences performed: Test for subgroup differences: Chi² = 1.41, df = 1 (P = 0.23), I² = 29.2%, Analysis 2.1; Chi² = 1.11, df = 1 (P = 0.29), I² = 10.2%, Analysis 2.2; Chi² = 0.03, df = 1 (P = 0.87), I² = 0%, Analysis 2.3; Chi² = 0.41, df = 1 (P = 0.52), I² = 0%, Analysis 2.4; Chi² = 0.91, df = 1 (P = 0.34), I² = 0%, Analysis 2.5.
Subgroup analysis ‐ women with ruptured membranes versus women with intact membranes
Four trials (Guzman 2002; Haas 2010; Memon 2011; Yildirim 2012) stratified data for women with ruptured membranes versus women without ruptured membranes. Two trials (Guzman 2002; Haas 2010) reported on the outcomes of endometritis and postoperative fever. One study reported on stratified outcomes for endometritis, febrile morbidity, and wound infection (Yildirim 2012). One trial only reported stratified results for composite infectious morbidity (Memon 2011). There was a statistically significant reduction in the rate of endometritis for women receiving vaginal preparation with povidone‐iodine solution preoperatively with ruptured membranes (4.3% in the vaginal cleansing group versus 17.9% in the control group; RR 0.24, 95% CI 0.10 to 0.55, three trials, 272 women), seeAnalysis 3.1. There were no statistically significant differences between the vaginal preparation and control groups in the other outcomes for women with ruptured membranes (postoperative fever: RR 0.62, 95% CI 0.34 to 1.12, two trials, 200 women; wound infection: RR 1.22, 95% CI 0.46 to 3.20, two trials, 272 women; any wound complication: RR 0.53, 95% CI 0.15 to 1.89, 1 trial, 76 women; composite endometritis or wound complication: RR 0.39, 95% CI 0.13 to 1.13, two trials, 134 women), seeAnalysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5. For women with intact membranes, the rate of postoperative endometritis was not significantly reduced in the vaginal preparation group (4.4% in the vaginal cleansing group versus 7.5% in the control group; RR 0.62, 95% CI 0.36 to 1.06, three trials, 857 women), although a trend to reduced rates was seen, seeAnalysis 3.1. All of the reported outcomes for women without ruptured membranes were not statistically significantly different between the vaginal preparation and control groups (postoperative fever: RR 0.93, 95% CI 0.63 to 1.36, two trials, 769 women; wound infection: RR 0.72, 95% CI 0.35 to 1.52, three trials, 857 women; any wound complication: RR 0.73, 95% CI 0.25 to 2.10, one trial, 224 women; composite endometritis or wound complication: RR 0.52, 95% CI 0.26 to 1.04, two trials, 336 women), seeAnalysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5.
There was also only evidence of subgroup differences in the trials for the post‐cesarean endometritis outcome (Chi² = 3.52, df = 1 (P = 0.06), I² = 71.6%, Analysis 3.1). No evidence of any other differences between subgroups according to the test for subgroup differences performed were found: Test for subgroup differences: Chi² = 1.28, df = 1 (P = 0.26), I² = 21.8%, Analysis 3.2; Chi² = 0.70, df = 1 (P = 0.40), I² = 0%, Analysis 3.3; Chi² = 0.41, df = 1 (P = 0.52), I² = 0%, Analysis 3.4; Chi² = 0.91, df = 1 (P = 0.34), I² = 0%, Analysis 3.5.
Other subgroups ‐ women with chorioamnionitis preoperatively versus women without chorioamnionitis; women undergoing emergency cesarean versus those undergoing unscheduled cesarean versus those undergoing scheduled cesarean; women with internal fetal or uterine monitors in place versus those with only external monitors in place before the cesarean
Neither of the two trials that included women diagnosed with chorioamnionitis stratified their data based on the presence or absence of chorioamnionitis. Neither of the two trials that did not exclude women undergoing emergency cesarean stratified their data based on emergency cesarean versus unscheduled versus scheduled cesarean. In addition, while three trials reported on the presence of internal monitoring (Haas 2010; Starr 2005; Yildirim 2012), none of them stratified their outcome data based on this variable. Thus we did not perform these three subgroup analyses.
No adverse events were noted in any of the trials from the vaginal preparation solution.
Discussion
Summary of main results
Vaginal cleansing with povidone‐iodine solutions before cesarean delivery can reduce the incidence of post‐cesarean endometritis. The heterogeneity in the results for this variable may be explainable by the study design and patient populations. The Guzman 2002 study used a placebo vaginal saline wash. This may have led to a lower baseline incidence of postoperative morbidity. The Haas 2010 study contained a majority of women who were obtaining planned repeat cesarean deliveries, a group known to be at lower risk for postoperative infectious morbidities. Additionally, vaginal preparation before cesarean delivery reduced the rate of a composite outcome of febrile morbidity, endometritis, and wound complications together. The subgroup analyses demonstrated that the reduction in postoperative endometritis is most pronounced for women with ruptured membranes and those women who undergo a cesarean delivery after already being in labor. These subgroup analyses should be interpreted with caution, however, as the number of participants and events is low. Thus, the intervention may be particularly useful for cesareans performed for women who have ruptured membranes. Ruptured membranes are a known risk factor for post‐cesarean infectious morbidity. The use of vaginal preparation in women with ruptured membranes thus makes particular sense.
Overall completeness and applicability of evidence
The evidence is relatively complete, consistent, and highly applicable to clinical care.
Quality of the evidence
The risk of bias of the seven included trials is reasonably low, with very few areas being identified as potential sources of bias (Figure 1; Figure 2). The agreement of the trial data in general and the large number of participants represented lend validity to the results of the meta‐analysis. The clinical heterogeneity was essentially eliminated in the subgroup analyses, the results of which were consistent with the overall group results. Thus, fixed‐effect modeling was retained in the overall results. The quality of the evidence using GRADE was low for post‐cesarean endometritis due to design limitations and statistical heterogeneity, moderate for postoperative fever due to design limitations, and low for wound infection due to design limitations and wide confidence interval crossing the line of no effect (summary of findings Table for the main comparison).
Potential biases in the review process
There is always potential that the review process was biased. However, the updated trial search yielded several additional studies. The study evaluation and data extraction were performed by three review authors with almost no discrepancies that needed to be resolved by consensus. Thus there is a minimal risk of bias in the review process. The studies were carried out in both developed and developing countries.
Agreements and disagreements with other studies or reviews
This review is limited by the somewhat small number of trials of preoperative vaginal preparation immediately before cesarean delivery. Because cesarean deliveries are so commonly performed and this intervention would seem to be an inexpensive, simple intervention to reduce post‐cesarean infectious morbidities, it was surprising to find such a paucity of randomized trial data. While the data point to a reduction in post‐cesarean endometritis with the intervention, it is possible that with more trial data, the trends towards other reduced infectious morbidity would also become statistically significant. Uniformity in the reporting of the data outcomes and the subgroup data stratification would have also aided this review.
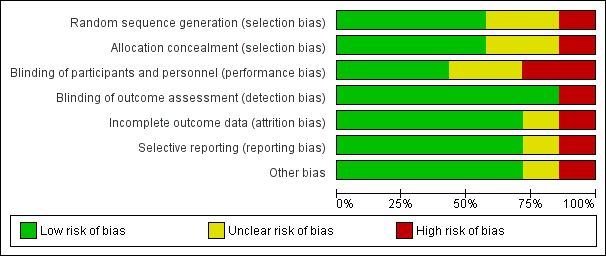
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Vaginal preparation versus control, Outcome 1 Post‐cesarean endometritis.

Comparison 1 Vaginal preparation versus control, Outcome 2 Postoperative fever.
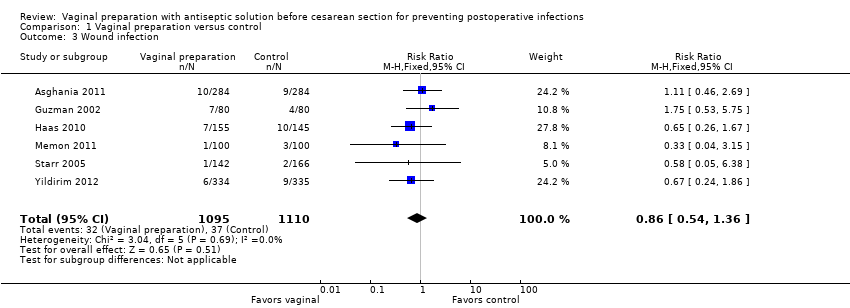
Comparison 1 Vaginal preparation versus control, Outcome 3 Wound infection.

Comparison 1 Vaginal preparation versus control, Outcome 4 Any wound complication.

Comparison 1 Vaginal preparation versus control, Outcome 5 Composite wound complication or endometritis.

Comparison 2 Vaginal preparation versus control ‐ stratified by presence of labor, Outcome 1 Post‐cesarean endometritis.

Comparison 2 Vaginal preparation versus control ‐ stratified by presence of labor, Outcome 2 Postoperative fever.

Comparison 2 Vaginal preparation versus control ‐ stratified by presence of labor, Outcome 3 Wound infection.
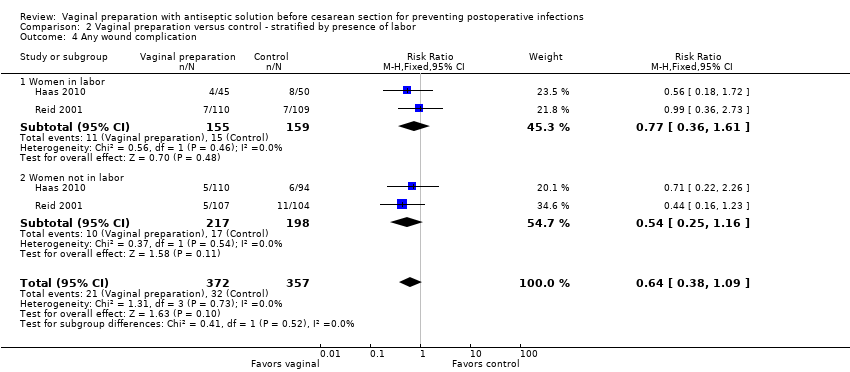
Comparison 2 Vaginal preparation versus control ‐ stratified by presence of labor, Outcome 4 Any wound complication.
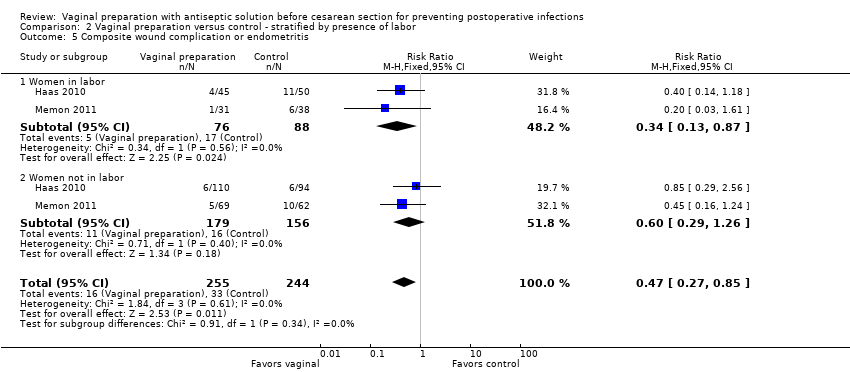
Comparison 2 Vaginal preparation versus control ‐ stratified by presence of labor, Outcome 5 Composite wound complication or endometritis.

Comparison 3 Vaginal preparation versus control ‐ stratified by presence of ruptured membranes, Outcome 1 Post‐cesarean endometritis.

Comparison 3 Vaginal preparation versus control ‐ stratified by presence of ruptured membranes, Outcome 2 Postoperative fever.

Comparison 3 Vaginal preparation versus control ‐ stratified by presence of ruptured membranes, Outcome 3 Wound infection.

Comparison 3 Vaginal preparation versus control ‐ stratified by presence of ruptured membranes, Outcome 4 Any wound complication.

Comparison 3 Vaginal preparation versus control ‐ stratified by presence of ruptured membranes, Outcome 5 Composite wound complication or endometritis.
| Vaginal preparation versus control for preventing postoperative infections | ||||||
| Population: Pregnant women who received a cesarean delivery | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Vaginal preparation versus control | |||||
| Post‐cesarean endometritis | Study population | RR 0.45 | 2635 | ⊕⊕⊝⊝ | ||
| 83 per 1000 | 37 per 1000 | |||||
| Moderate | ||||||
| 75 per 1000 | 34 per 1000 | |||||
| Postoperative fever | Study population | RR 0.9 | 2475 | ⊕⊕⊕⊝ | ||
| 141 per 1000 | 127 per 1000 | |||||
| Moderate | ||||||
| 117 per 1000 | 105 per 1000 | |||||
| Wound infection | Study population | RR 0.86 | 2205 | ⊕⊕⊝⊝ | ||
| 33 per 1000 | 29 per 1000 | |||||
| Moderate | ||||||
| 31 per 1000 | 27 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Most studies contributing data had design limitations. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 7 | 2635 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.25, 0.81] |
| 2 Postoperative fever Show forest plot | 6 | 2475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 3 Wound infection Show forest plot | 6 | 2205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.36] |
| 4 Any wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.07] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.26, 0.82] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 3 | 1394 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.02] |
| 1.1 Women in labor | 3 | 523 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.34, 0.95] |
| 1.2 Women not in labor | 3 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.52, 1.54] |
| 2 Postoperative fever Show forest plot | 2 | 965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
| 2.1 Women in labor | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.08] |
| 2.2 Women not in labor | 2 | 658 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.61, 1.49] |
| 3 Wound infection Show forest plot | 2 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.34] |
| 3.1 Women in labor | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.24, 2.21] |
| 3.2 Women not in labor | 2 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.27, 1.56] |
| 4 Any wound complication Show forest plot | 2 | 729 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.09] |
| 4.1 Women in labor | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.36, 1.61] |
| 4.2 Women not in labor | 2 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.25, 1.16] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.27, 0.85] |
| 5.1 Women in labor | 2 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.87] |
| 5.2 Women not in labor | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Post‐cesarean endometritis Show forest plot | 3 | 1129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.29, 0.70] |
| 1.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 1.2 Women with intact membranes | 3 | 857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.06] |
| 2 Postoperative fever Show forest plot | 2 | 969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.14] |
| 2.1 Women with ruptured membranes | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.12] |
| 2.2 Women with intact membranes | 2 | 769 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.63, 1.36] |
| 3 Wound infection Show forest plot | 3 | 1129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.57] |
| 3.1 Women with ruptured membranes | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.46, 3.20] |
| 3.2 Women with intact membranes | 3 | 857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.35, 1.52] |
| 4 Any wound complication Show forest plot | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.44] |
| 4.1 Women with ruptured membranes | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.15, 1.89] |
| 4.2 Women with intact membranes | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.10] |
| 5 Composite wound complication or endometritis Show forest plot | 2 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.85] |
| 5.1 Women with ruptured membranes | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.13] |
| 5.2 Women with intact membranes | 2 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |

