成人危重症患者的低热量营养支持处方
Abstract
研究背景
一直以来,危重病人应该给予的卡路里量和营养支持类型存在着争议。少数研究者主张低热量营养支持的潜在益处,但证据尚无定论。
研究目的
本研究旨在评估低热量营养支持处方与标准营养支持相比对成人危重症患者的影响。
检索策略
我们检索了Cochrane对照试验中央注册库(CENTRAL,Cochrane图书馆)、MEDLINE、Embase和Lilacs(从建库到2017年6月20日),针对每个数据库制定了相应的策略。我们还评估了三个网站、会议论文和参考文献列表;对于未检测出的或未发表的研究,我们联系了该领域和制药公司的专家以获取全文。不限定日期、语言或发表状态。
标准/纳入排除标准
我们纳入了随机和半随机对照试验,对比在重症监护病房(ICU)住院的成人低热量营养支持与正常或高热量营养支持或无营养支持(例如禁食)的相关研究。
数据收集与分析
我们使用了Cochrane推荐的标准方法流程。我们对临床异质性较低的数据进行了Meta分析。我们进行了预先指定的亚组分析和敏感性分析以及包括Meta回归在内的事后比较分析。主要结局包括:死亡率(在ICU和普通病房住院期间发生的死亡,或28至30天的全因死亡率);住院时长(在ICU和普通病房住院的天数)以及感染并发症。次要结局包括机械通气的时长。我们用GRADE分级评价了证据的质量。
主要结果
我们确定纳入了15项试验,涉及3129名来自美国、哥伦比亚、沙特阿拉伯、加拿大、希腊、德国和伊朗的大学附属医院ICU的受试者。有两项研究目前正在进行。受试者受到医疗和外科手术条件的影响、涉及多种不同的纳入标准。4项研究采用肠外营养支持,9项研究仅采用肠内营养支持,尚不清楚其余2项研究是否采用肠外营养支持。大部分研究未能达到预设的热量目标,导致干预组和对照组之间热量摄入的差别很小。大多数研究是由美国政府或非政府机构资助的,但3项研究获得该行业的资助。5项研究没有具体说明其资金来源。
纳入的研究很大程度上受到临床和统计学异质性的影响。这种异质性使得我们无法汇总估计主要和次要结局,因此我们对其进行了叙述性描述。
当对比低热量营养支持与对照组营养支持受试者时发现,其住院死亡率(9项研究,1775名受试者)的RR值为0.23‐5.54;ICU死亡率(4项研究,1291名受试者)的RR值为0.81‐5.54;30天内死亡率(7项研究,2611名受试者)的RR值为0.79‐3.00。这些估计中的大部分包括空值。由于不明确或高风险的偏倚、各研究的不一致性和不精确性,该证据的质量极低。
与对照营养支持组相比,低热量营养支持组受试者的平均住院时长缩短10.70‐15.70天(10项研究,1677名受试者),平均ICU住院时长缩短5.40‐11.00天(11项研究,2942名受试者),平均机械通气时长缩短8.36‐13.20天(12项研究,3000名受试者)。由于不明确或高风险的偏倚、各研究的不一致性和不精确性,该证据的质量极低。
每项研究的感染并发症(10项研究,2804名受试者)RR值为0.54‐2.54。由于不明确或高风险的偏倚、各研究的不一致性和不精确性,该证据的质量极低。
我们无法用亚组分析和敏感性分析或Meta回归来解释观察到的异质性的原因。
作者结论
纳入的研究有相当大的临床异质性。我们发现证据的质量极低,证明了低热量营养支持对在普通病房、ICU和入院30天的死亡率的影响,以及对普通病房住院时长和ICU住院时长,感染并发症和机械通气时长的影响。从这些结局来看,低热量营养支持处方的疗效是不确定的,因为估计值的范围明显同时包括了益处和危害。
鉴于这些限制,考虑到这种干预措施带来的风险和危害尚不清楚,临床上必须谨慎地对待这些结果。今后的研究要解决受试者和干预措施的临床异质性,研究的限制和样本大小可以阐明这种干预措施的影响。
PICO
Plain language summary
低热量营养支持处方是否有助于成人危重病症患者的康复?
综述问题
与标准热量营养支持相比,低热量营养支持是否能够通过胃或小肠(肠内)或静脉(肠外)吸收,改善重症监护病房(ICU)的成人危重症患者的临床结局?
主要结局是死亡(在医院、ICU,30天内);在ICU和普通病房住院的时长;感染出现并发症和机械通气(ICU用来辅助患者呼吸的机器)的时长。
背景
重症患者在损伤或败血症期间(一种危及生命的状况,该状况下机体对感染的反应导致其自身器官损伤)会经历重大的代谢变化(一种化学物质通过一系列步骤转化为另一种化学物质)。他们接受营养支持以防止或尽量减少一些不良反应。然而,过度进食和饥饿都是有害的。
目前对于重症患者应给予的卡路里量还未达成共识。正常的热量摄入可用来估计热量的需求。低热量喂养提供了一种有意降低的卡路里量。
研究特点
我们纳入了来自大学附属医院的3129名ICU外科或医学受试者的15项试验。其中4项研究使用肠外营养支持,9项研究仅使用肠内营养支持。其余2项研究中的营养支持方案尚不清楚。虽然这些研究计划在试验组和对照组间提供不同的卡路里量,但实际差异很小。大多数研究是由美国政府或非政府机构资助,但有3项研究得到了该行业的资助。5项研究没有说明它们是如何获得资助的。
主要结局
营养类型和受试者类型的差异使得我们无法将研究结果合并起来,因此我们描述了各个研究结果的变化。
在普通病房或ICU住院30天内,接受低热量营养支持患者的死亡人数与对照组相近。与对照组相比,普通病房住院时长、ICU住院时长以及机械通气时长因研究而异,有的更短、有的更长。感染病例的数量也因研究而异。我们试图分析受试者的亚组以阐明这种差异,但结果并不一致。
证据质量
根据GRADE证据分级,证据的总体质量从极低到低不等。这是由于研究设计和实施、研究结果的变化(研究之间的不一致)和广泛的可能结果(不精确)的问题所导致的。
Authors' conclusions
Summary of findings
| Hypocaloric nutrition compared to control for critically‐ill adults | |||
| Patient or population: critically‐ill adults Comparison: control nutritional support with a higher caloric intake than the 'intervention' group | |||
| Outcomes | Effect estimate (range of results of individual studies) | N of Participants | Quality of the evidence |
| Mortality in hospital: death occurring during the hospital stay | Range of risk ratios from 0.23 to 5.54a | 1775 (9 studies) | ⊕⊕⊝⊝ |
| Mortality in ICU: death occurred during the ICU stay | Range of risk ratios from 0.81 to 5.54a | 1291 | ⊕⊕⊝⊝ |
| Mortality at 30 days: 28 to 30 days all‐cause mortality | Range of risk ratios from 0.79 to 3.00a | 2611 | ⊕⊕⊝⊝ |
| Length of hospital stay: days stayed in the hospital | Range of length of hospital stay from 15.70 days lower to 10.70 days highera | 1677 | ⊕⊝⊝⊝ |
| Length of ICU stay: days stayed in the ICU | Range of length of ICU stay from 11.00 days lower to 5.40 days highera | 2942 | ⊕⊝⊝⊝ |
| Infectious complications: events of any type of infectious complications occurred during the hospital stay, registered by the study authors according to their diagnostic criteria of infections. | Range of risk ratios from 0.54 to 2.54a | 2804 | ⊕⊝⊝⊝ |
| Length of mechanical ventilation: days on mechanical ventilation during ICU stay | Range of mean differences: 13.20 days lower to 8.36 days highera | 3000 (12 studies) | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| aResults were not combined due to clinical heterogeneity. | |||
Background
Description of the condition
Most critically‐ill people treated for injury or sepsis have some degree of hypermetabolism and hypercatabolism and are also unable to feed themselves. For these reasons, it was recommended to provide them with nutrition support by enteral or parenteral routes, in order to prevent or minimize depletion of protein and caloric stores; to enhance protein synthesis; and to avoid deficiencies in essential and semi‐essential nutrients (Cerra 1997). However, there are several aspects of nutrition support for the critically‐ill that are still under debate, such as: the time at which to initiate nutrition support; the route (enteral, parenteral or combined); the caloric and protein requirements; the amount and type of protein to give; the composition of lipids; the supplementation of some amino acids and micronutrients; and the occurrence and type of some complications. Several of these topics were recently discussed (Berger 2012; Biolo 2002; Bost 2014; Heyland 2003; Kreymann 2006;Preiser 2015; Wischmeyer 2012; Wischmeyer 2013), and some of these aspects are included in Cochrane Reviews on adults (Alkhawaja 2015; Allingstrup 2015; Fuentes Padilla 2016; Lewis 2016; Tao 2014), and children (Joffe 2016), as well as in a Cochrane protocol (Dushianthan 2016). This current review focuses on the prescription of hypocaloric versus normocaloric feeding debate in nutrition support for critically‐ill adults.
During the 1970s, the proposed goal of nutrition support was to provide sufficient calories to match the measured increased resting energy expenditure (hypermetabolism) in order to prevent protein depletion. As indirect calorimetry (the gold standard) is not available in most intensive care units (ICUs) or it is not possible to perform it in certain patients, it is usual to estimate the daily caloric requirements using formulae. For years the most frequently used one was the Long equation (resting metabolic expenditure calculated by the Harris‐Benedict equation with the addition of an injury factor and an activity factor; Long 1979). This approach often led to overestimation of caloric requirements (compared with the values obtained by indirect calorimetry), mainly in ventilated and sedated patients (McClave 1992). It also induced some degree of overfeeding with nutrition support, which was associated with several metabolic complications (Klein 1998), such as hypertriglyceridaemia, increased production of CO2, hepatic steatosis and hyperglycaemia, which also behaves as an independent factor for increased mortality in critically‐ill patients (Badawi 2012; Krinsley 2003).
It is currently known that the caloric requirements for nutrition support of a critically‐ill person could differ from the estimated resting or total energy expenditure (Reid 2004). We must take into account variability due to several factors: the presence of injury or sepsis (type, severity and metabolic response of the host) (Hoffer 2003); the time course of the disease or the elapsed time in the ICU (Monk 1996; Uehara 1999); current ICU care and treatments (Boulanger 1994); the nutrition state or the fat‐free mass (Zauner 2006); the complications and some factors associated with the disease states (Magnuson 2011; Stahel 2010), and comorbidities. This variability contributes to the difficulty in estimating energy needs for the nutrition support of these patients (Frankenfield 2011). The use of predictive equations (Cooney 2012) could be one of the causes of underfeeding or overfeeding in some critically‐ill people (Reid 2006).
There is consensus about some aspects of caloric and protein requirements for nutrition support of the critically‐ill ventilated person:
a) the degree of hypermetabolism due to injury or sepsis is lower than that reported at the beginning of the 1970s (Liggett 1990), particularly during the first days in the ICU (Biolo 2002; Heyland 2003; Kreymann 2006);
b) positive or neutral energy balance failed to decrease the protein catabolic rate or nitrogen loss and did not prevent negative nitrogen balance and protein depletion (Frankenfield 1997; Plank 2003);
c) positive energy balance is associated with increased fat mass, without changes in lean body mass (Hart 2002; Streat 1987);
d) the main determinant of a positive, or less negative, nitrogen balance during nutrition support seems to be the nitrogen intake (Iapichino 1984; Weijs 2013);
e) nutrition support did not modify the rate of protein catabolism, but was able to preserve some nitrogen loss (less negative nitrogen balance) by promoting whole‐body protein synthesis, with protein intake of up to 1.5 g/kg/day (Shaw 1987).
The well‐known clinical guidelines for the nutrition support of critically‐ill people (ASPEN / SCCM guidelines 2016; ASPEN / SCCM guidelines 2009; ESPEN guidelines 2009) sometimes disagreed with each other, and in the literature there are some open debates. For example, when and how to initiate the nutrition support; when to begin lipid administration by the parenteral route and the type of lipids to be used; the role and timing for supplemental parenteral nutrition; the amount of protein or the non‐protein calories/nitrogen ratio to prescribe; the dose and type of supplemental trace elements and antioxidant vitamins; the best way to estimate the caloric requirements; if caloric provision should be optimized to prevent a caloric deficit during the first days of ICU in order to minimize the initial or delayed complications associated with undernutrition (Dvir 2006; Heidegger 2013; Rubinson 2004; Wischmeyer 2013), or if it is better to give hypocaloric nutrition during the first days of intense inflammatory response (and metabolic changes) induced by injury or sepsis (Berger 2007; Berger 2012; Casaer 2014; Cooney 2012; Dickerson 2011; Kreymann 2012; Singer 2010; Weijs 2013; Wischmeyer 2012). This review focuses only on the clinical results of providing hypocaloric nutrition support compared to normocaloric nutrition to critically‐ill adults.
Description of the intervention
More than 20 years ago, Zaloga 1994 proposed a short period of dietary restriction during the first few days of acute injury or sepsis, originally designated "permissive underfeeding" and later "hypocaloric nutrition support" (Patiño 1999). The provision of hypocaloric nutrition support with high‐protein content was successfully used in a group of obese stressed patients (Dickerson 1986). This approach was first reviewed (Kushner 2011), suggested by a group of experts for critically‐ill people (McClave 2011), and recommended in some clinical guidelines (ASPEN guidelines 2013; ASPEN / SCCM guidelines 2016). The use of hypocaloric nutrition support in critically‐ill people, mainly during the first days of ICU stay, has been frequently mentioned in the literature; some evidence and opinions were reported in several narrative reviews ( Boitano 2006; Berger 2007; Jeejeebhoy 2004; Malone 2007; Stapleton 2007).
How the intervention might work
Severely critically‐ill people experiencing major metabolic changes during the acute phase of systemic inflammatory response induced by injury or sepsis could benefit from this approach. This may be explained by: avoidance of the well‐known deleterious effects of overfeeding or the consequences of starvation; diminishing metabolic disturbances, especially hyperglycaemia, and the level of inflammatory cytokines. In certain animal models, hypocaloric nutrition during acute stress seemed to lower morbidity and mortality. This could also be possible in critically‐ill people, but the available data are not conclusive about the potential benefits of hypocaloric feeding. On the contrary, there is some evidence that underfeeding could be associated with complications and worse outcomes for critically‐ill people (Dvir 2006; Villet 2005), and that the effect of hypocaloric nutrition support could be different in malnourished and well‐nourished people (Braunschweig 2001). The possible role of starvation‐induced autophagy is currently under consideration (Marik 2016a).
Why it is important to do this review
We do not so far have conclusive evidence for how many calories we should give to critically‐ill people in order to improve outcomes and diminish complications. However, today we certainly know that caloric requirements are rather less than that proposed in the 1970s or 1980s (Krishnan 2003; Rubinson 2004). Currently, in several countries there are intensive care or nutrition support specialists providing hypocaloric nutrition support to most of their critically‐ill patients during the first few days of illness, or tolerating the administration of less than prescribed enteral nutrition (fewer calories than the estimated ones) for their patients. This is based more on observational evidence or expert opinions than on scientific data.
Several authors consider it important to optimize the energy provision, targeting measured or estimated requirements, in order to avoid caloric deficits ("caloric debt") during the first days of ICU stay (Faysy 2008; Singer 2010; Singer 2011; Wischmeyer 2013; Wischmeyer 2015), or, even more importantly, to also target the protein supply (Weijs 2012; Weijs 2014; Hoffer 2012; Nicolo 2016), or give some supplementary protein (Alberda 2009).
Due to these unanswered questions, the controversial data and the different interpretations of it, it is necessary to perform systematic reviews of each contentious topic and to analyse the clinical significance of each nutritional approach. We have therefore conducted this systematic review to explore the effects of prescribed hypocaloric enteral or parenteral nutrition on clinical and metabolic outcomes in critically‐ill adults.
Objectives
To assess the effects of prescribed hypocaloric nutrition support in comparison with standard nutrition support for critically‐ill adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized controlled trials. We considered the inclusion of quasi‐randomized controlled trials in order to enlarge the evidence about the efficacy and safety of hypocaloric nutrition support (Schneider 2007; Shadish 2002).
Types of participants
We included all adult participants (aged 18 years or more) hospitalized for different diseases and severity at medical, surgical or disease‐specific (burns, trauma, neurological, etc.) intensive care units (ICUs) and requiring any type of nutrition support.
Exclusion criteria: none.
Types of interventions
The experimental intervention evaluated was: hypocaloric nutrition support with fewer total calories than measured resting energy expenditure (REE) by indirect calorimetry or, if not measured, less than 25 kcal/kg/day. This could be done through restricted doses of carbohydrates or lipids, or both, with either normal or increased protein dose. The control intervention was:
-
Normo‐ or hypercaloric nutrition support: equal to or more than the measured REE or than 25 kcal/kg/day (with the same characteristics as above); or
-
No nutrition support at all: fasting or dextrose solutions.
We evaluated the results of trials designed to compare prescribed hypocaloric enteral or parenteral nutrition support (or permissive underfeeding) with standard nutrition support, or with no nutrition, even if those trials did not reach their caloric goals in the intervention or control groups (intention‐to‐treat analysis). Furthermore, we did not include trials that planned to provide full nutrition support but resulted in unintended hypocaloric provision (for any reason).
Types of outcome measures
Primary outcomes
The primary outcomes were the following clinical outcomes:
-
Mortality. Death occurring during the ICU and hospital stay, or 28‐ to 30‐day all‐cause mortality.
-
Length of stay. Days stayed in the ICU and in the hospital.
-
Infectious complications. Events of any type of infectious complications occurring during the hospital stay, registered by the study authors according to their diagnostic criteria of infections.
Secondary outcomes
The secondary outcomes were one or more of the following outcomes:
-
Length of mechanical ventilation. Days on mechanical ventilation during ICU stay.
-
Non‐infectious complications. Events of any non‐infectious complication during the hospital stay, potentially associated with the nutrition status or the nutrition support, according the criteria of the study authors (for example: wound dehiscence, decubitus ulcers, etc.)
-
Carbohydrate metabolic outcomes. Events of hyperglycaemia (glycaemia higher than 150 mg/dl) during ICU stay. Events of hypoglycaemia (glycaemia lower than 70 mg/dl) during ICU stay.
-
Lipid metabolic outcomes. Events of hypertriglyceridaemia (higher than 200 mg/dl) or any lipid metabolic complication associated with the nutrition support according to the criteria of the study authors.
-
Protein metabolic outcomes. Nitrogen balance (positive or negative in grams/day) or any protein metabolic complication associated with the nutrition support according to the criteria of the study authors.
-
Nutrition status or clinical condition at ICU discharge. Nutrition or functional evaluation, made at the time of ICU discharge with any method of assessment used by the study authors.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library Issue 5, 2017); MEDLINE/Ovid (1946 to 20 June 2017); Embase (1980 to 20 June 2017), and LILACS (1992 to 20 June 2017). We developed a specific strategy for each database (see Appendix 1 for CENTRAL, Appendix 2 for MEDLINE, Appendix 3 for Embase and Appendix 4 for LILACS).
We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy phases one and two, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The filter used to identify randomized controlled trials (RCTs) in the search strategy for MEDLINE was from Glanville 2006. For Embase we applied the trial filter for therapy maximizing sensitivity developed by Health Information Research Unit (HIRU) at McMaster University: hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx).
We did not apply restrictions by language or by publication status.
We also searched (up to 20 June 2017) for relevant ongoing trials in specific trial registries:
-
ClinicalTrials.Gov: clinicaltrials.gov/
-
International Clinical Trials Registry Platform: apps.who.int/trialsearch/
-
ISRCTN Registry: www.isrctn.com/
Searching other resources
We searched the Conference Proceedings of the annual congresses of the following four societies, as published in their respective journals, in order to find papers presented at different meetings:
-
American Society for Parenteral and Enteral Nutrition (ASPEN), through the Journal of Parenteral and Enteral Nutrition (1990 to 30 June 2017).
-
European Society for Clinical Nutrition and Metabolism (ESPEN), through the journal Clinical Nutrition (1990 to 30 June 2017).
-
Society of Critical Care Medicine (SCCM), through the journal Critical Care Medicine (1990 to 30 June 2017).
-
European Society of Intensive Care Medicine (ESICM), through the journal Intensive Care Medicine (1997 to 30 June 2017).
We also handsearched the original papers published in the following journals:
-
Journal of Parenteral and Enteral Nutrition (1990 to 30 June, 2017).
-
Clinical Nutrition (1990 to 30 June 2017).
-
Nutrition (1990 to 30 June 2017).
-
Nutrition Clinique et Métabolisme (1994 to 30 June 2017).
We also checked the reference list and citations of the relevant articles and reviews related to hypocaloric feeding and to caloric and protein requirements of critically‐ill people (1970 to 30 June 2017).
Correspondence
We contacted main authors of relevant trials and reviews to identify any additional studies, and relevant pharmaceutical companies for published and unpublished reports.
Data collection and analysis
Selection of studies
Three review authors (MP, ACr and CL) independently scanned the titles and abstracts of reports identified by electronic searching, manual searching, snowballing and by contacts with clinical experts and the pharmaceutical industry. We retrieved and evaluated potentially relevant studies, chosen by at least one review author, in full‐text versions. These review authors independently selected trials that met the inclusion criteria using a checklist designed in advance for that purpose. We resolved any disagreement through consultation with a fourth review author (GP). We rejected articles at the initial screening only if we could determine from the title and abstract that the study was not a report of a randomized or quasi‐randomized controlled trial; or that it did not address enteral and/or parenteral nutrition in critically‐ill adults. When we could not reject a study with certainty, we obtained the full text of the article for further evaluation.
Data extraction and management
Two review authors (ACr and CL) independently extracted data using a standardized checklist. We registered it in the data extraction form. We resolved any disagreement through consultation with a third review author (MP).
Assessment of risk of bias in included studies
Two review authors (GP and CL) independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement through discussion and consultation with a third assessor (ACi).
(1) Sequence generation (checking for possible selection bias)
We looked for the description of methods used in each included study to generate the allocation sequence, and assessed if they were adequate to produce comparable groups (unbiased selection). We classified methods as being at low, high or unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We looked for the description of methods used in each included study to conceal the allocation sequence and assessed if they were adequate to avoid the intervention allocation being foreseen or changed. We classified methods as being at low, high or unclear risk of bias.
(3) Blinding (checking for possible performance bias)
We looked for the description of methods used, if any, in each included study to blind study participants and personnel from knowledge of which intervention a participant received. We also considered partial blinding (e.g. where it had not been feasible to blind participants but outcome assessment was carried out without knowledge of group assignment). Where blinding was not possible we assessed whether the lack of blinding was likely to have introduced bias. We classified methods as being at low, high or unclear risk of bias.
We also assessed any information about whether the intended blinding was effective.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We looked for the completeness of outcome data in each included study, for each main outcome, including attrition and exclusions from the analysis. We assessed whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition/exclusion, and any re‐inclusions in analyses. We classified methods as being at low, high or unclear risk of bias.
(5) Selective reporting bias
We assessed this by comparing the study protocol, when available, and all of the study's pre‐specified outcomes that are of interest in the review. We classified methods as being at low, high or unclear risk of bias.
(6) Other sources of bias
We looked for any important concerns about other possible sources of bias in each included study. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Has the study been claimed to be fraudulent? Has the researcher gained sponsorship from agencies with a vested interest in the findings? We assessed whether each study was free of other problems that could put it at risk of bias. We classified methods as being at low, high or unclear risk of bias.
(7) Overall risk of bias
We made an explicit judgement about whether studies were at an overall high, low or unclear risk of bias, according to the following criteria: low risk if all six 'Risk of bias' domains were rated low for that study; unclear risk if at least one domain was rated at unclear risk; high risk if at least one domain was rated at high risk of bias.
We assessed the likely magnitude and direction of identified risks of bias, and whether we considered this could have a significant effect on the findings. We explored the impact of the level of bias through sensitivity analyses.
Measures of treatment effect
For dichotomous outcomes we calculated risk ratios (RRs) and 95% confidence intervals (95% CIs). We calculated the mean difference (MD) for continuous outcomes measured using the same scales, or the standardized mean difference (SMD) if they used different scales.
Unit of analysis issues
The unit of analysis was the participant in each trial arm. All included studies had a parallel‐group design, so there was no need for adjustment for a cluster or cross‐over design.
Dealing with missing data
We obtained missing data from study authors, if feasible, and performed intention‐to‐treat analyses if data were available; otherwise, we performed available‐case analyses. We investigated attrition rates, such as dropouts, losses to follow‐up and withdrawals, and we critically appraised issues of missing data. We did not impute missing data.
We contacted by email the first authors of the following included and ongoing trials:
-
Ahrens 2005. The first author sent the estimates of continuous outcomes as means and standard deviations for length of hospital and ICU stay and for length of mechanical ventilation.
-
Arabi 2015 The first author sent us the length of hospital and ICU stay and of mechanical ventilation in means and standard deviation.
-
Charles 2014 The first author sent us mean and standard deviation of days on mechanical ventilation, and additional information to complete the 'Risk of bias' table.
-
NHLBI 2012 and Rice 2011 The corresponding author sent us all the information required to render their data compatible, and also some additional unpublished results: length of hospital stay, ICU and mechanical ventilation in means and standard deviations, number of participants with infections and hyperglycaemic episodes, and amount of calories received by participants in both groups.
-
Ochoa 2017 We contacted the lead author. He replied that he would try to recover and send the requested study results, but we have not received them yet.
-
Petros 2016. The study was initially published only in abstract form. The first author sent us all the information we required from its finished but unpublished pilot study. The full paper of the pilot trial was recently published (Petros 2016).
-
Rugeles 2013 We initially identified the study before publication. The first author sent us the full paper ready to be published in advance of publication, and some additional considerations to better assess the risk of bias and the number of participants with hyperglycaemia.
-
Rugeles 2016 The first author sent us the full paper of this clinical trial before it was indexed in MEDLINE (It was registered in clinicaltrials.gov as NCT02577211). The second author gave us the means and standard deviations for length of ICU stay and of mechanical ventilation, and also some additional information to complete the 'Risk of bias' table.
-
Theodorakopoulou 2016 We did not received an answer to several questions about the abstract of the trial.
Assessment of heterogeneity
In cases of statistical heterogeneity, i.e. a Chi2 test with a P value less than 0.10 or an I2 greater than 30% (Higgins 2002), we examined the potential causes of the heterogeneity by prespecified subgroup and sensitivity analyses. We followed the suggestions in Section 9.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions and interpreted and rated heterogeneity according to the I2 value as follows: 'not important' if 40% or less, 'moderate' with I2 between 30% and 60%, 'substantial' with I2 between 50% and 90%, and 'considerable' if I2 is higher than 75% (see Data synthesis section for levels of I2 that allowed us to report numerical results or not) (Higgins 2002).
We assessed clinical heterogeneity by considering different parameters of clinical practice. We considered the objectives and methodology of the trials, the type/severity of the participants (surgical, medical and others), and several aspects of the nutrition support, such as time to initiation, route, duration and amount of calories and protein received by the intervention and the control groups. The most important parameters of this pragmatic and subjective assessment were the amount of calories received by each group of participants, and the difference in calories received by the intervention and control groups. We defined clinical heterogeneity as 'low', 'moderate' or 'important', according to a clinical judgement about the possibility of comparing trials with small, moderate or important differences according to the above parameters.
Some of the parameters we used to define clinical heterogeneity were also used for subgroup and sensitivity analyses, to investigate the heterogeneity (Subgroup analysis and investigation of heterogeneity). In addition, where we identified important statistical or clinical heterogeneity we performed meta‐regression in order to explore the possible causes.
Assessment of reporting biases
The search strategy included consultation with leaders in the field, the pharmaceutical industry, conference and congress proceedings and snowballing techniques to maximize the possibility of finding unpublished studies. We performed funnel plot analyses when eight or more studies were included in each outcome analysis.
Data synthesis
We first reviewed the data from included studies qualitatively. Then, if possible, we combined them quantitatively by population, intervention and outcome, using Cochrane statistical software (Review Manager 2014). We based the quantitative analyses of outcomes on intention‐to‐treat (ITT) results.
In case of unimportant statistical heterogeneity between studies (I2 of 30% or less), we performed meta‐analyses using the fixed‐effect model. In case of I2 between 30% and 50%, we used a random‐effects model to produce more conservative confidence intervals. If the I2 was above 50%, we did not report pooled estimates of the meta‐analysis. In cases of important clinical heterogeneity we did not report pooled estimates of the meta‐analyses, even in the absence of statistical heterogeneity.
In the subgroup analyses we reported results using a random‐effects model if one or more of the subgroups had an I2 between 30% and 50%, for a more conservative analysis. If the total statistical heterogeneity test showed I2 above 50% or if the clinical heterogeneity was important, we did not report summary estimates of the meta‐analysis.
In all cases where it was not possible to perform or report total or subtotal analyses, we produced a short descriptive comment about the results of the studies for each outcome.
Subgroup analysis and investigation of heterogeneity
The prespecified possible causes of heterogeneity were the following:
-
Age: 18 to 65 years old, 66 to 75 years old, and more than 75 years old.
-
Primary disease of the participants: major surgery, trauma, sepsis, medical diseases.
-
Disease severity with or without organ failure: acute physiology and chronic health evaluation II (APACHE II); simplified acute physiology score II (SAPS II); sequential organ failure assessment (SOFA); multiple organ dysfunction score (MODS); logistic organ dysfunction system (LODS), other scores.
-
With or without comorbidities: assessed by the Charlson score or similar.
-
Nutrition status: obese, malnourished or well‐nourished.
-
Level of inflammation (by determination of plasma level of C reactive protein or other acute phase reactants) or hypermetabolism (by indirect calorimetry) or hypercatabolism (by measured or estimated total urinary nitrogen).
-
Amount of calories in the intervention group: low versus very low amount of calories.
After retrieval of studies, we acknowledged that there were important differences among them that we should consider in the assessment of clinical heterogeneity. We therefore added other non‐prespecified explorations of heterogeneity:
-
Subgroup analysis by route of nutrition support: enteral or parenteral nutrition.
-
Meta‐regressions (using STATA 14.1; Stata), to explore the effect of the following variables on the main outcomes: type of participants, the calories received, and the difference in calories received by the intervention and control groups.
To investigate differences between two or more subgroups we used the test for heterogeneity across subgroup results rather than across individual study results. We also calculated an I2 statistic for subgroup differences (Higgins 2011). We considered a P value less than 0.05 as statistically significant.
Sensitivity analysis
-
Trial design: we performed three prespecified sensitivity analyses: 1) excluding the quasi‐randomized trials: 2) excluding those studies with at least one high 'Risk of bias' criterion; and 3) in all the outcomes performed with the fixed‐effect model, we also conducted the analysis with the random‐effects model.
-
We undertook two more non‐prespecified sensitivity analyses, excluding trials with a primary goal different from prescribed hypocaloric enteral or parenteral nutrition.
'Summary of findings' table and GRADE
We present the overall quality of the evidence for selected outcomes using the GRADE approach (Schünemann 2011). This approach takes into account five criteria:
-
Risk of bias
-
Inconsistency
-
Imprecision
-
Directness
-
Publication bias
For each comparison, two review authors (JVAF, ACi) independently rated the quality of evidence for each outcome as 'high', 'moderate', 'low', or 'very low', using GRADEpro GDT software. We resolved any discrepancies by consensus, or, if needed, by arbitration by a third review author (MP).
We present the results for the comparison of hypocaloric nutrition versus control for the following outcomes:
-
Mortality in hospital
-
Mortality in ICU
-
Mortality at 30 days
-
Length of hospital stay (days)
-
Length of ICU stay (days)
-
Infectious complications
-
Length of mechanical ventilation (days)
Since meta‐analysis was not possible in most cases due to both statistical and clinical heterogeneity, we present the range of effect estimates of the individual studies along with the number of participants, number of included studies and confidence in the effect estimates (Guyatt 2011; Schünemann 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy from electronic databases, updated to 20 June 2017, retrieved 5055 records. We found four more studies by handsearching. One full paper was sent by the first author before it was indexed in MEDLINE (original reference in clinicaltrials.gov, with the identifier NCT02577211). After removing duplicates we screened the remaining 4880 records. After title and abstract evaluation, we eliminated 4840 records as irrelevant. We found two ongoing trials. We assessed 47 full‐text reports for eligibility and excluded 20 of them for different reasons (see Characteristics of excluded studies). We therefore included the remaining 15 studies (18 reports, Characteristics of included studies). See the updated flow diagram of the studies in Figure 1.
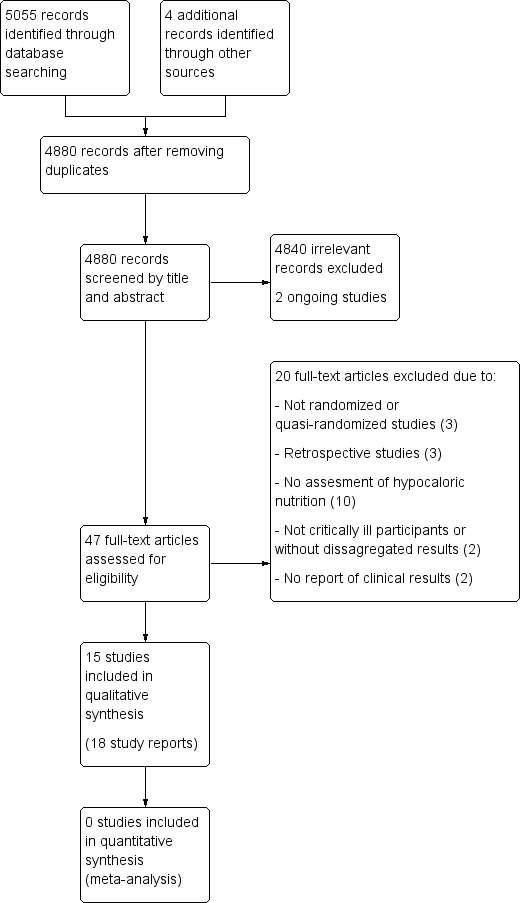
Updated study flow diagram, 20 June 2017
Included studies
Fifteen studies fulfilled the inclusion criteria (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Choban 1997; Ibrahim 2002; McCowen 2000; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016; Theodorakopoulou 2016). Two studies (Norouzy 2013; Theodorakopoulou 2016), were available as abstract only, so some of the study characteristics are missing.
Sample sizes
The total number of ICU participants included was 3129. The range of number of ICU participants included in the trials varied from 13 to 1000.
Setting
Eight included studies were performed in the USA, two in Colombia, one in Saudi Arabia and Canada, and one in each of the following countries: Saudi Arabia, Greece, Germany and Iran. Fourteen of the included studies were RCTs and one was a quasi‐randomized trial (Ibrahim 2002). The setting was mostly university‐associated hospitals.
Participants
Two studies (Ahrens 2005; Choban 1997), reported data of participants in the ICU and on a regular patient care floor. In those studies we only included the data of the ICU participants. The rest of the trials only included ICU participants. The type of ICU was reported in the studies as medical, medical‐surgical or trauma ICU, but after evaluating the reported diagnoses of the included participants, we considered only two categories: surgical participants in five trials (Ahrens 2005; Battistella 1997; Charles 2014; Choban 1997; McCowen 2000;), and medical participants in 10 trials (Arabi 2011; Arabi 2015, Ibrahim 2002; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016; Theodorakopoulou 2016). Some inclusion criteria considered participants with specific conditions, such as hyperglycaemia (Arabi 20111), obesity (Choban 1997), sepsis (Theodorakopoulou 2016), or mechanical ventilation for at least 24 hours (Ibrahim 2002; Rice 2011). In four studies the participants received parenteral nutrition (Ahrens 2005; Battistella 1997; Choban 1997; McCowen 2000). Nine studies used only enteral nutrition (Arabi 2011; Arabi 2015; Ibrahim 2002; NHLBI 2012; Norouzy 2013; Rice 2011; Rugeles 2013; Rugeles 2016; Theodorakopoulou 2016). In two studies the indication was enteral nutrition, but if this was not possible they used parenteral nutrition (Petros 2016; Charles 2014), (see Table 1).
| Study ID | Type of participants Primary outcomes | Arm | Number of ICU participants | APACHE II score mean ± SD | Route (enteral or parenteral) | Duration of PN or EN (days) | Mechanical ventilation (% of participants) | ICU mortality % | Hospital mortality % |
| Surgical participants with PN requirement Incidence/severity hyperglycaemia and insulin received by the participants | Hypoc. | 8 (other 12 non‐ICU) | 20 ± 9 | Parenteral | 6 (4 to 10) | 100 | Not reported | Not reported | |
| Control | 10 (other 10 non‐ICU) | 19 ± 11 | 7 (5 to 10) | 80 | |||||
| Medical (mainly) and surgical participants with EN. 2 x 2 factorial trial with Intensive Insuline therapy 28 days all‐cause mortality | Hypoc. | 120 | 25 ± 8 | Enteral | Not reported | 99 | 18 | 30 | |
| Control | 120 | 25 ± 8 | 99 | 22 | 43 | ||||
| Critically‐ill participants (75% medical) 90‐day all‐cause mortality | Hypoc. | 448 | 21 ± 7.9 | Enteral | 9.1 ± 4.6 | 97.3 | 16.1 | 24.2 | |
| Control | 446 | 21 ± 8.2 | 9.4 ± 4.4 | 96.2 | 19.1 | 27.6 | |||
| Trauma participants with PN requirement Length of hospital stay, length of stay in the ICU, number of days on mechanical ventilation and infectious complications. | Hypoc. | 27 | 22 ± 5 | Parenteral | 10 | Not reported | 7.4 | Not reported | |
| Control | 30 | 23 ± 6 | 10 | 0 | |||||
| Critically‐ill surgical participants Hospital‐acquired infection | Hypoc. | 41 | 16.6 ± 0.9 | Enteral & parenteral | 12.6 ± 2.8 | 68 | N/A | 7.3 | |
| Control | 42 | 17.3 ± 0.8 | 10.4 ± 1.1 | 57 | N/A | 9.5 | |||
| Obese participants with PN requirement. Predominantly surgical diseases Achievement of nitrogen balance | Hypoc. | 6 (other 10 non‐ICU) | 13 ± 5 | Parenteral | 10 ± 3 | Not reported | Not reported | 0 | |
| Control | 7 (other 7 non‐ICU) | 15 ± 5 | 11 ± 2 | 28.6 | |||||
| Medical ICU participants with EN Incidence of ventilator‐associated pneumonia | Hypoc. | 75 | 26 ± 8 | Enteral | 5 ± 6 | 100 | Not reported | 27 | |
| Control | 75 | 25 ± 8 | 10 ± 12 | 100 | 20 | ||||
| Participants with predominantly surgical diseases requiring PN Glycaemic control and Infections | Hypoc. | 21 | not reported | Parenteral | ≥ 5 | 50 | 10 | Not reported | |
| Control | 19 | not reported | ≥ 5 | 33 | 16 | ||||
| Acute lung injury predominantly due to medical diseases (61% and 63% of participants) with EN Ventilator‐free days at study day 28 | Hypoc. | 508 | APACHE III 92 ± 28 | Enteral | 6 | 100 | Not reported | 22.4 | |
| Control | 492 | APACHE III 90 ± 27 | Enteral | 6 | 100 | 19.6 | |||
| Critically‐ill head trauma participants 28 days of all‐cause mortality | Hypoc. | 30 | Not reported | Enteral | 7 | Not reported | Not reported | 10.7a | |
| Control | 30 | 7 | 3.8a | ||||||
| Medical ICU with EN and/or PN requirement Glycaemic control and mortality | Hypoc. | 46 | 31 ± 9 | Enteral & parenteral | 7 | not reported | 22 | 37 | |
| Control | 54 | 28 ± 8 | 7 | 22 | 31 | ||||
| Acute lung injury, predominantly due to medical diseases with EN Ventilator‐free days at study day 28 | Hypoc. | 98 | 27 ± 8 | Enteral | 6 ± 4 | 100 | Not reported | 22 | |
| Control | 102 | 27 ± 7 | 5 ± 3 | 100 | 20 | ||||
| Medical ICU participants with EN requirement Change in SOFA score at 48 hours | Hypoc. | 40 | 14 ± 5 | Enteral | 7 | Not reported | Not reported | Not reported | |
| Control | 40 | 15 ± 6 | |||||||
| Medical ICU participants with EN requirement Change in SOFA score at 48 hours | Hypoc. | 60 | 13.5 ± 6.4 | Enteral | 7 | Not reported | Not reported | 30a | |
| Control | 60 | 13.7 ± 6.8 | 27a | ||||||
| Septic, mechanically ventilated critically‐ill participants 28‐day mortality | Hypocal. | Total sample of 74 participants | Total sample 22 ± 4 | Enteral | Not reported | Not reported | Not reported | Not reported | |
| Control |
a28‐day mortality.
EN = Enteral nutrition; ICU = Intensive Care Unit; N/A: not available; PN = Parenteral nutrition; SOFA = Sequential Organ Failure Assessment
Interventions and study design
All studies had a parallel‐group design, except for two (Arabi 2011; NHLBI 2012) which had a factorial design. These also evaluated, respectively, intensive insulin treatment versus standard insulin treatment, and a nutritional supplement containing omega‐3 fatty acids and antioxidants versus an isocaloric formula. The 15 included studies had a control group with prescribed normocaloric nutrition support. None of the included studies had fasting or only hydration as a comparator. See Table 1; Table 2. Most of the included studies did not achieve the proposed caloric target, with a difference in calories between the intervention and control groups in the range of 2 to 14 kcal/kg/day.
| Studies | How data was reported | Hypocaloric (intervention) group | Control group | Calories received by the "hypocaloric" intervention group (kcal/kg/day) | Calories received by the "normocaloric" control group (kcal/kg/day) | Categories denominated by the calories really received in the intervention and the control groups a |
| Total calories/kg/day (median (IQ))b | 26.6 (26.2 to 27.5) | 37 (36.0 to 38.4) | 26.60 (median) | 37.00 (median) | Normocaloric vs hypercaloric | |
| Protein g/kg/day (mean± SD) | 1.61 ± 0.13 | 1.53 ± 0.26 | ||||
| Calories/day (mean ± SD) | 1066.6 ± 306.1 | 1251.7 ± 432.5 | 13.85 | 16.40 | Hypocaloric vs hypocaloric | |
| Protein g/day (mean ± SD) | 47.5 ± 21.2 | 43.6 ± 18.9 | ||||
| Calories/day (mean ± SD) | 835 ± 297 | 1299 ± 2470 | 10.56 | 16.04 | Hypocaloric vs hypocaloric | |
| Protein g/day (mean ± SD) | 57 ± 24 | 59 ± 25 | ||||
| Calories/kg ideal body weight/day (mean ± SD) | 27.4 ± 2 | 34.4 ± 2 | 27.4 (of ideal body weight) | 34.4 (of ideal body weight) | Normocaloric vs. normocaloric | |
| Protein g/kg ideal body weight/day (mean± SD) | 1.6 ± 0.1 | 1.6 ± 0.2 | ||||
| Calories/kg/day (mean ± SD) | 12.3 ± 0.7 | 17.1 ± 1.1 | 12 | 17 | Hypocaloric vs hypocaloric | |
| Protein g/kg/day (mean ± SD) | 1.1 ± 0.1 | 1.1 ± 0.1 | ||||
| Kcal/kg actual body weight/day (mean ± SD) Kcal/kg ideal body weight/day (mean ± SD) | 8.6 ± 2.39 13.88 ± 2.87 | 17.45 ± 4.06 27.99 ± 3.83 | 14.00 (of ideal body weight) | 28.00 (of ideal body weight) | Hypocaloric vs normocaloric | |
| Protein g/kg actual body weight/day (mean ± SD) Protein g/kg ideal body weight/day (mean ± SD) | 1.2 ± 0.2 2.0 ± 0.1 | 1.2 ± 1.2 2.0 ± 0.1 | ||||
| Calories/day (mean ± SD) | 126 ± 115 | 474 ± 400 | 1.53 | 5.81 | Very hypocaloric vs very hypocaloric | |
| Proteins g/day (mean) (mean ± SD) | 5.3 ± 5.3 | 18.7 ± 15.4 | ||||
| Calories/kg/day (mean ± SD) | 14 ± 3 | 18 ± 4 | 14.30 | 18.40 | Hypocaloric vs hypocaloric | |
| Proteins g/kg/day (mean ± SD) | 1.1 ± 0.2 | 1.3 ± 0.2 | ||||
| Calories/day (mean ± SD) | 399 ± 225 | 1365 ± 596 | 4.64 (estimated by kcal/day divided by weight from the baseline table) | 15.69 (estimated by kcal/day divided by weight from the baseline table) | Very hypocaloric vs hypocaloric | |
| Proteins: information not collected | ‐ | ‐ | ||||
| Calories/kg/day (mean ± SD) | Not reported | Not reported | N/A | N/A | N/A | |
| Protein g/kg/day (mean ± SD) | Not reported | Not reported | ||||
| Calories/kg/day (mean ± SD) | 11.3 ± 3.1 | 19.7 ± 5.7 | 11.30 | 19.70 | Hypocaloric vs hypocaloric | |
| Protein | Data not reported | Data not reported | ||||
| Calories/day (mean ± SD of study days 1 to 5) | 300 ± 149 | 1418 ± 686 | 3.60 | 17.31 | Very hypocaloric vs hypocaloric | |
| Proteins g/day (mean ± SD of study days 1 to 5) | 10.9 ± 6.8 | 54.4 ± 33.2 | ||||
| Calories/kg/day (mean ± SD) | 12 ± 3.9 | 14 ± 6.2 | 12.00 | 14.00 | Hypocaloric vs hypocaloric | |
| Protein g/kg/day (mean ± SD) | 1.4 ± 0.44 | 0.76 ± 0.32 | ||||
| Total calories/kg ideal body weight/day (mean ± SD) | 12.6 ± 3.4 | 20.5 ± 5.1 | 13 | 21 | Hypocaloric vs hypocaloric | |
| Protein g/kgIBW/day (mean ± SD) | 1.4 ± 0.4 | 1.4 ± 0.3 | ||||
| Calories/day (mean ± SD) | 962 ± 314 | 1308 ± 513 | Not reported Estimatedc 16.63 kcal/kg/day | Not reported Estimatedc 22.62 kcal/kg/day | Estimatedc Hypocaloric vs normocaloric | |
| Protein g/day (mean ± SD) | 57 ± 24 | 59 ± 25 | Not reported Estimatedc 0.99 g/kg/day | Not reported Estimatedc 1.02 g/kg/day |
aCategories denominated by the amount of calories really received by both study groups, according to the following: very hypocaloric = < 10 kcal/kg/day; hypocaloric = ≥ 10 to < 25 kcal/kg/day; normocaloric = ≥ 25 to < 35 kcal/kg/day; hypercaloric = ≥ 35 kcal/kg/day.
bIQ: interquartile range ‐ Median total calories received by all 20 participants (ICU and non‐ICU participants) in each group (the total calories received by the 8 and 10 ICU participants in each group were not reported).
cNot reported in the abstract. The numbers are a crude estimation of kcal and grams of protein/kg/day from the whole sample data of height and BMI.
BMI = Body Mass Index; g = gram; ICU = Intensive Care Unit; kcal = kilocalories; N/A: not available; SD = standard deviation; vs = versus
Outcomes
For full details of the reported outcomes see Table 3 and Characteristics of included studies.
| Study | Difference in calories between groups (kcal/kg/day) | Hospital mortality (%) IG vs CG | ICU mortality (%) IG vs CG | Mortality at 30 days (%) IG vs CG | Infectious complications (%) IG vs CG | Length of hospital stay (days)a IG vs CG | ICU length of stay (days)a IG vs CG | Length of mechanical ventilation (days)a IG vs CG | Categories denominated by the calories really received in the intervention and the control groupsb |
| 2.00 | N/A | N/A | N/A | N/A | N/A | 9.5 vs 10.4 | 8.5 vs 9.7 | Hypocaloric vs hypocaloric | |
| 2.55 | 30% vs 42.5% | 17.5% vs 21.7% | 18.3% vs 23.3% | 44.2% vs 46.7% | 70.2 vs 67.2 | 11.7 vs 14.5 | 10.6 vs 13.2 | Hypocaloric vs hypocaloric | |
| 4.10 | 9.5% vs 15.8% | N/A | N/A | 28.6% vs 52.6% | 19 vs 17 | N/A | N/A | Hypocaloric vs hypocaloric | |
| 4.28 | 26.7% vs 20% | N/A | N/A | 30.7% vs 49.3% | 16.7 vs 22.9 | 9.8 vs 13.6 | 8.1 vs 12.9 | Very hypocaloric vs very hypocaloric | |
| 5.00 | 7.3% vs 9.5% | N/A | N/A | 56.1% vs 57.1% | 35.2 vs 31 | 16.7 vs 13.6 | 10.8 vs 8.3 | Hypocaloric vs hypocaloric | |
| 5.48 | 24.2% vs 27.6% | 16.1% vs 19.1% | 20.8% vs 21.8% | 35.9% vs 37.9% | 48.3 vs 54.4 | 15.8 vs 16.4 | 11.3 vs 13.5 | Hypocaloric vs hypocaloric | |
| 7.00 | 7.4% vs 0% | 7.4% vs 0% | N/A | 48.2% vs 73.3% | 27 vs 39 | 18 vs 29 | 15 vs 27 | Normocaloric vs normocaloric | |
| 7.90 | N/A | N/A | 30% vs 26.7% | N/A | N/A | 13.2 vs 13.5 | 10.8 vs 10.8 | Hypocaloric vs hypocaloric | |
| 8.40 | 37% vs 31.5% | 21.7% vs 22.2% | 39.1% vs 33.3% | 28.3% vs 11.1% | 38.1 vs 27.4 | 22.4 vs 17 | 20.7 vs 12.4 | Hypocaloric vs hypocaloric | |
| 10.40 | N/A | N/A | N/A | 25% vs 10% | 23.4 vs 27.8 | 16.8 vs 23 | 11.1 vs 20.3 | Normocaloric vs hypercaloric | |
| 11.05 | N/A | N/A | 19.5% vs 19.3% | 18.9% vs 16.1% | N/A | 11.5 vs 11 | 10.5 vs 10.2 | Very hypocaloric vs hypocaloric | |
| 13.71 | 22.4% vs 19.6% | N/A | 22.4% vs 19.6% | 30.6% vs 32.4% | N/A | 8.1 vs 7.6 | 5.7 vs 6.2 | Very hypocaloric vs hypocaloric | |
| 14.00 | 0% vs 29% | N/A | N/A | N/A | 48 vs 45 | N/A | N/A | Hypocaloric vs normocaloric | |
| N/A | N/A | N/A | 10% vs 3.3% | N/A | 19.9 vs 35.6 | N/A | 4.7 vs 17.9 | N/A | |
| N/A | N/A | N/A | 18.4% vs 28.9% | N/A | N/A | N/A | N/A | Hypocaloric vs normocaloric |
aLengths of hospital, ICU stays and of mechanical ventilation presented in mean days.
bCategories denominated by the amount of calories really received by both study groups, according to the following: very hypocaloric = < 10 kcal/kg/day; hypocaloric = ≥ 10 to < 25 kcal/kg/day; normocaloric = ≥ 25 to < 35 kcal/kg/day; hypercaloric = ≥ 35 kcal/kg/day.
IG = Intervention Group; CG = Control Group; N/A = Not available; vs = versus
Funding
Studies were funded by non‐governmental associations or foundations (Arabi 2015; Choban 1997; Ibrahim 2002), or by the US government (Battistella 1997; Charles 2014; NHLBI 2012; Rice 2011). Three studies received funding from the industry (Arabi 2011; Rugeles 2013; Rugeles 2016), and five studies did not specified their sources of funding (Ahrens 2005; McCowen 2000; Norouzy 2013; Petros 2016; Theodorakopoulou 2016).
Excluded studies
Out of the 47 full papers we initially assessed for eligibility, we finally excluded 20 for the following reasons:
-
Three were not randomized or quasi‐randomized controlled trials (Alberda 2009; Arabi 2010; Müller 1995).
-
Three were retrospective studies (Casadei 2006; Dickerson 2002; Lau 2010).
-
Ten studies did not assess hypocaloric nutrition (Desachy 2008; Dissanaike 2007; Doig 2013; Fiaccadori 2005; Garrel 1995; Mackenzie 2005; Moses 2009; Rodríguez 2005; Esterle 2010; Wewalka 2010).
-
Two studies did not include critically‐ill participants or only some of them without disaggregated results (Owais 2014; Schricker 2005).
-
Two studies did not report clinical results (Berg 2013; Iapichino 1990).
Refer to the Characteristics of excluded studies for further details.
Studies awaiting classification
There are no studies awaiting classification.
Ongoing studies
There are two ongoing studies.
We identified one study (NCT01665664) through clinical trial registries. It is set in Israel, and plans to include adult participants with mechanical ventilation and to compare hypocaloric nutrition to normocaloric nutrition. The study outcomes include all‐cause mortality, ICU mortality, hospital mortality, length of stay (hospital and ICU), length of mechanical ventilation, rate of infections, ventilator‐free days and rate of ventilator‐associated pneumonia. This study was last verified in 2012 in ClinicalTrials.gov and was "not recruiting". We were unsuccessful in contacting the study author.
We identified the second ongoing study in a conference proceeding (Ochoa 2017). This multicentre RCT includes adult, obese, critically‐ill and mechanically ventilated participants requiring enteral nutrition, and compares hypocaloric versus normocaloric enteral nutrition support. The study outcomes include events of hyperglycaemia and hypoglycaemia. Since the abstract included limited information about a preliminary interim analysis we contacted the study author for further information. This study is funded by Nestlé Health Science.
Refer to the Characteristics of ongoing studies
Risk of bias in included studies
We assessed seven domains of possible biases, according to prespecified criteria. Details for each included study are provided in their corresponding 'Risk of bias' table in the Characteristics of included studies. A graphical summary can be seen in Figure 2 and Figure 3 (showing overall percentages of risk level for each domain, and levels of risk of bias for each study, respectively).
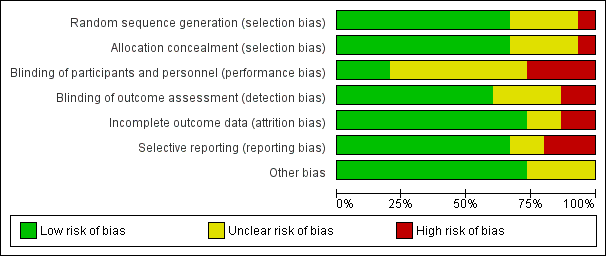
Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study. Red colour represents high risk of bias; green, low risk of bias; and yellow, unclear risk of bias.
Overall, only one study had low risk of bias in all the evaluated domains (Ahrens 2005). Six studies had at least one high 'Risk of bias' criterion (Arabi 2015; Charles 2014; Ibrahim 2002; McCowen 2000; Rugeles 2013; Rugeles 2016). The eight remaining studies had at least one unclear 'Risk of bias' criterion. In six of them (Arabi 2011; Battistella 1997, NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011), this was attributable to an unblinded study design. In these cases, although most outcomes were objective or well‐defined with low risk of detection bias, the descriptions of the processes of care by clinical personnel did not have enough detail to assess whether this could have led to a performance bias.
For publication bias, the funnel plots for the outcomes with at least eight trials did not show significant asymmetry.
Allocation
The random sequence generation and the allocation concealment were appropriately performed in 10 studies (Ahrens 2005; Arabi 2011; Arabi 2015; Charles 2014; Choban 1997; NHLBI 2012; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016). One study was quasi‐randomized (Ibrahim 2002), and therefore had a high risk of bias. Four studies (Battistella 1997; McCowen 2000; Norouzy 2013; Theodorakopoulou 2016) did not clearly describe these processes, and we classified them as being at unclear risk of bias.
Blinding
Lack of blinding (open‐label or blinding only participants) was the main driver of the high or unclear risks of bias in most studies (Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; McCowen 2000; NHLBI 2012; Petros 2016; Rice 2011; Norouzy 2013; Rugeles 2016; Theodorakopoulou 2016). The inherent difficulty of blinding a nutrition support strategy in critically‐ill people explains the fact that 80% of the studies could not blind the healthcare personnel. Nevertheless, three studies managed to do it (Ahrens 2005; Choban 1997; Rugeles 2013).
Incomplete outcome data
Only two studies had a high risk of attrition bias (McCowen 2000; Rugeles 2013). They excluded participants because they did not fulfil the prespecified follow‐up criteria. Nevertheless, they should have reported all included participants in an intention‐to‐treat analysis. We classified two studies as being at unclear risk, due to a lack of information in these trials which were only published as conference abstracts (Norouzy 2013; Theodorakopoulou 2016). The other 11 studies reported outcomes for all included participants .
Selective reporting
Three studies had a high risk of reporting bias (Ibrahim 2002; McCowen 2000; Rugeles 2013). For Ibrahim 2002, some prespecified secondary outcomes (duration of mechanical ventilation, need for gastrostomy tube) were not reported. For McCowen 2000, "nitrogen balance was only measured in 12 participants (57%) in the hypocaloric and 10 (53%) of the control group, usually because of an error during collection". Rugeles 2013 did not report mortality. The authors justified this by explaining that they excluded participants who did not fulfil the 96 hours of enteral nutrition requirement. They therefore did not report mortality because this result would have been biased (they only measured mortality in participants who completed the 96 hours). A better approach would have been to perform an intention‐to‐treat analysis and also to report premature deaths. In Norouzy 2013 and Theodorakopoulou 2016, the information was not provided, so we classified them as being at unclear risk. We rated all the other studies at low risk of reporting bias.
Other potential sources of bias
Choban 1997 was partially funded by a corporation. Since we could not guarantee that this sponsorship had no material interest in the findings of the study, we classified it as being at unclear risk of bias.
The lack of detail in the description of the methods section of McCowen 2000 could not warrant a 'low risk' rating for Other sources of bias. We therefore classified it as being at unclear risk of bias. Due to the lack of information in the abstracts of Norouzy 2013 and Theodorakopoulou 2016 we also classified them as being at unclear risk of Other potential sources of bias.
Effects of interventions
The 15 included studies showed significant clinical heterogeneity between them, mainly related to the amount of calories provided to the intervention and control groups (Table 2), and also to some differences in trials methodology, the target participants and the feeding strategies. As stated in Assessment of heterogeneity and in Data synthesis, the degree of clinical or statistical heterogeneity precluded us from reporting the numerical summary results of the meta‐analysis for all the primary and secondary outcomes (Analysis 1.1 to Analysis 1.11). We used similar criteria to report the sensitivity or subgroup analyses.
When we could not report results due to clinical or statistical heterogeneity or both, we did a qualitative synthesis of the trial results. We also reported trial results of the included studies in tabular form: percentages and means of the hypocaloric and the control group of the seven main outcomes (Table 3).
Primary outcomes
1.1 Mortality in hospital
For this outcome we found nine relevant trials (1775 participants) (Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Choban 1997; Ibrahim 2002; McCowen 2000; Petros 2016; Rice 2011). We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to the differences in the underlying diagnoses of the medical or surgical ICU participants and the route/characteristics of administration of enteral or parenteral nutrition or both, but mainly to the wide differences in calories and protein received by the participants in the included trials (Table 1; Table 2). We therefore did not pool the point estimates in meta‐analysis (Analysis 1.1). There were 210 deaths in the 881 participants who received hypocaloric nutrition, and 235 deaths in the 894 participants who received the control intervention. All studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.1). The central estimates of risk ratios for hospital mortality of each individual studies ranged from 0.23 to 5.54. When we excluded Battistella 1997, the range of risk ratio estimates was narrower, since this study has a more extreme estimate due to small sample size and zero events in the control group. The quality of the evidence for this outcome was very low, due to high risk of attrition bias, imprecision and inconsistency (wide variance of point estimates across studies) (Summary of findings table 1).
1.2 Mortality in the intensive care unit (ICU)
We found four relevant trials for this outcome (1291 participants) (Arabi 2011; Arabi 2015; Battistella 1997; Petros 2016). We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to the type of participants, the nutrition methodology and the amount of calories received by the participants (Table 1; Table 2). We therefore have not pooled the point estimates (Analysis 1.2). There were 105 deaths in the 641 participants who received hypocaloric nutrition, and 123 deaths in the 650 participants who received the control intervention. All studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.2). The central estimates of risk ratios for ICU mortality of each individual studies ranged from 0.81 to 5.54. When we excluded Battistella 1997, the range of risk ratio estimates was narrower, since this study has a more extreme estimate due to small sample size and zero events in the control group. The quality of the evidence for this outcome was very low, due to a high risk of attrition bias, imprecision and inconsistency (wide variance of point estimates across studies) (Summary of findings table 1).
1.3 Mortality at 30 days
For this outcome we found seven relevant trials (2611 participants) (Arabi 2011; Arabi 2015; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2016). We found the abstract of an additional trial (Theodorakopoulou 2016), with mortality reported narratively for 38 participants. We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to participants' diagnoses, type and characteristics of the nutrition support, the amount of calories and the differences in calories received by the participants of both groups in the analysed trials (Table 1; Table 2). We therefore did not pool the point estimates (Analysis 1.3). There were 275 deaths in the 1309 participants who received hypocaloric nutrition, and 275 deaths in the 1302 participants who received the control intervention. All studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.3). The central estimates of risk ratios for mortality at 30 days of the individual studies ranged from 0.79 to 3.00. The quality of the evidence for this outcome was very low, due to a high risk of attrition bias, imprecision and inconsistency (wide variance of point estimates across studies) (Summary of findings table 1).
2. 1 Length of hospital stay (days)
We found 10 relevant trials for this outcome (1677 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Choban 1997; Ibrahim 2002; McCowen 2000; Norouzy 2013; Petros 2016). We found considerable statistical heterogeneity (I2 = 78%) and important clinical heterogeneity due to differences in participants, nutrition methodology, and calories received by the participants of the intervention and control groups (Table 1; Table 2). We therefore did not pool the estimates (Analysis 1.4). Participants who received hypocaloric nutrition support had a mean length of stay of 15.70 days lower to 10.70 days higher compared to those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Summary of findings table 1).
2. 2 Length of ICU stay (days)
For this outcome we found 11 relevant trials (2942 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; NHLBI 2012; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016). We found considerable statistical heterogeneity (I2 = 81%) and important clinical heterogeneity due to differences in the type of participants, nutrition methodology and the differences in total amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial ( Table 1; Table 2). We therefore have not pooled the effect estimates (Analysis 1.5). Participants who received hypocaloric nutrition support had a mean length of stay 11.00 days lower to 5.40 days higher compared to those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Summary of findings table 1).
3. Infectious complications. Events of any type of infectious complications occurring during the hospital stay, registered by the study authors according to their diagnostic criteria of infections
Ten studies reported this outcome (2804 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; McCowen 2000; NHLBI 2012; Petros 2016; Rice 2011). We found moderate statistical heterogeneity (I2 = 49%) and important clinical heterogeneity due to the type of participants, study methodology and amount of calories and protein received by the participants (Table 1; Table 2). We therefore have not pooled the estimates. There were 423 participants with infections in the 1404 participants who received hypocaloric nutrition, and 438 infections in the 1400 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value (Analysis 1.6). The range of the central estimate of risk ratios for infectious complications of the individual studies ranged from 0.54 to 2.54. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias, inconsistency and imprecision (Summary of findings table 1).
Secondary outcomes
1. Length of mechanical ventilation. Days on mechanical ventilation during ICU stay
For this outcome we found 12 relevant trials (3000 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Battistella 1997; Charles 2014; Ibrahim 2002; NHLBI 2012; Norouzy 2013; Petros 2016; Rice 2011; Rugeles 2013; Rugeles 2016). We found substantial statistical heterogeneity (I2 = 69%) and important clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 1; Table 2). We therefore did not pool the effect estimates. Participants who received hypocaloric nutrition support had a mean length of mechanical ventilation of 13.20 days lower to 8.36 days higher compared with those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Analysis 1.7; summary of findings Table for the main comparison).
2. Non‐infectious complications. Events of any non‐infectious complication during the hospital stay, potentially associated with the nutrition status or the nutrition support, according to the criteria of the study authors (diarrhoea)
Three studies reported this outcome (1994 participants) (Arabi 2015; NHLBI 2012; Petros 2016). We found considerable statistical heterogeneity (I2 = 76%) and important clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 1, Table 2). We therefore did not pool the effect estimates. There were 187 participants with non‐infectious complications (diarrhoea) in the 1002 participants who received hypocaloric nutrition, and 242 participants with non‐infectious complications in the 992 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value. The range of the central estimate of risk ratios for non‐infectious complications of the individual studies ranged from 0.32 to 0.85. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias, inconsistency and imprecision (Analysis 1.8).
3.1 Carbohydrate metabolic outcomes: hyperglycaemia (glycaemia higher than 150 mg/dl) during ICU stay
For this outcome we found six relevant trials (1380 participants) (Ahrens 2005; McCowen 2000; NHLBI 2012; Petros 2016; Rugeles 2013; Rugeles 2016). We found substantial statistical heterogeneity (I2 = 62%) with moderate clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 1; Table 2). We therefore did not pool the effect estimates. There were 205 participants who suffered hyperglycaemia in the 695 participants who received hypocaloric nutrition, and 279 participants who suffered hyperglycaemia in the 685 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value. The central estimate of risk ratios for hyperglycaemia of the individual studies ranged from 0.36 to 0.93. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias, inconsistency and imprecision (Analysis 1.9).
3.2 Carbohydrate metabolic outcomes: events of hypoglycaemia (glycaemia lower than 70 mg/dl) during ICU stay
We found five relevant trials for this outcome (1394 participants) (Ahrens 2005; Arabi 2011; Arabi 2015; Petros 2016; Rugeles 2016). We found no statistical heterogeneity (I2 = 0%) but important clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants , as well as the caloric difference between the groups in each trial (Table 1; Table 2). We therefore did not pool the effect estimates. There were 46 participants who suffered hypoglycaemia in the 694 participants who received hypocaloric nutrition, and 38 participants who suffered hypoglycaemia in the 700 participants who received the control intervention. Most studies suffered from imprecision and their confidence intervals included the null value. The central estimate of risk ratios for hypoglycaemia of the individual studies ranged from 0.85 to 1.76. In Rugeles 2016, a risk ratio was not estimable due to no events in either group. The quality of the evidence for this outcome was low, due to unclear or high risk of bias and imprecision (Analysis 1.10).
4. Lipid metabolic outcomes. Events of hypertriglyceridaemia (higher than 200 mg/dl) or any lipid metabolic complication associated with the nutrition support according to the criteria of the study authors
None of the included trials reported this outcome
5. Protein metabolic outcomes: nitrogen balance
For this outcome we found three relevant trials (92 participants) (Battistella 1997; Choban 1997; McCowen 2000). We found substantial statistical heterogeneity (I2 = 72%) with moderate clinical heterogeneity due to the type of participants, nutrition methodology and the differences in the amount of calories and protein received by the participants, as well as the caloric difference between the groups in each trial (Table 1; Table 2). We therefore did not pool the effect estimates (Analysis 1.11). Participants who received hypocaloric nutrition support had a mean nitrogen balance of −7.70 g/day to +2.00 g/day compared to those with normocaloric nutrition support. The quality of the evidence for this outcome was very low, due to unclear or high risk of bias in most studies, inconsistency and imprecision (Analysis 1.11).
6. Nutrition status or clinical condition at ICU discharge. Nutrition or functional evaluation, made at the time of ICU discharge with any method of assessment used by the study authors.
None of the included trials reported this outcome
Subgroup analyses
We focused our subgroup analyses on the seven outcomes reported in summary of findings Table for the main comparison. Out of these seven outcomes only four had considerable statistical heterogeneity: length of hospital and ICU stay, infectious complications and length of mechanical ventilation. We explored sources of statistical heterogeneity and assessed whether meta‐analysis was possible, considering clinical heterogeneity in the predefined subgroups.
Due to insufficient information available, we were unable to perform subgroup analysis by: age, disease severity, presence of comorbidities, nutrition status (malnourished or well‐nourished), level of inflammation, hypermetabolism or hypercatabolism. It was only possible to perform prespecified subgroup analyses by: obesity status (as a condition of nutrition status), type of underlying medical condition (surgical or medical), amount of calories actually received by participants in the intervention and control groups. During the process of data extraction we realized that the included trials had several methodological differences between them. At this time (before any analysis of results), we decided to perform two additional analyses not prespecified in the protocol (Subgroup analysis and investigation of heterogeneity): subgroup analysis by route of nutrition support (enteral or parenteral) and meta‐regression (see below). The I2 values for these subgroup analyses are shown in Table 4. Most of the subgroup analyses were unable to explain the statistical heterogeneity of the results across studies:
| Subgroup | N participants (n studies) | Subgroup testing |
| 1. Nutrition status | ||
| 1.1. Length of hospital stay | ||
| Obese | 13 (1 RCT) | I2 = 0%, P = 0.76 |
| General | 1664 (9 RCTs) | |
| 2. Route of nutrition support | ||
| 2.1. Length of hospital stay | ||
| Parenteral | 150 (4 RCTs) | I2 = 0%, P = 0.72 |
| Enteral | 1725 (6 RCTs) | |
| 2.2. Length of ICU stay | ||
| Parenteral | 75 (2 RCTs) | I2 = 83.3%, P < 0.01 |
| Enteral | 2867 (9 RCTs) | |
| 2.3. Infectious complications | ||
| Parenteral | 137 (3 RCTs) | I2 = 0%, P = 0.35 |
| Enteral | 2667 (7 RCTs) | |
| 2.4. Length of mechanical ventilation | ||
| Parenteral | 73 (2 RCTs) | I2 = 85.4%, P < 0.01 |
| Enteral | 2927 (10 RCTs) | |
| 3. Type of participant | ||
| 3.1. Length of hospital stay | ||
| Surgical participants | 223 (5 RCTs) | I2 = 0%, P = 0.55 |
| Medical participants | 1354 (5 RCTs) | |
| 3.2. Length of ICU stay | ||
| Surgical participants | 158 (3 RCTs) | I2 = 0%, P = 0.52 |
| Medical participants | 2784 (8 RCTs) | |
| 3.3. Infectious complications | ||
| Surgical participants | 220 (4 RCTs) | I2 = 0%, P = 0.45 |
| Medical participants | 2584 (6 RCTs) | |
| 3.4. Length of mechanical ventilation | ||
| Surgical participants | 156 (3 RCTs) | I2 = 0%, P = 0.45 |
| Medical participants | 2854 (9 RCTs) | |
| 4. Amount of calories received by each study group | ||
| 4.1. Length of hospital stay | ||
| Normo‐hypercaloric | 97 (2 RCTs) | I2 = 84.1%, P < 0.01 |
| Hypocaloric | 1370 (6 RCT) | |
| Very hypocaloric | 150 ( RCT) | |
| 4.2. Length of ICU stay | ||
| Normo‐hypercaloric | 75 (2 RCTs) | I2 = 0%, P = 0.42 |
| Hypocaloric | 1517 (6 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| 4.3. Infectious complications | ||
| Normo‐hypercaloric | 97 (2 RCTs) | I2 = 0%, P = 0.94 |
| Hypocaloric | 1357 (5 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| 4.4. Length of mechanical ventilation | ||
| Normo‐hypercaloric | 73 (2 RCTs) | I2 = 73.1%, P = 0.02 |
| Hypocaloric | 1517 (6 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
RCT = randomized controlled trial; ICU = Intensive care unit
-
In the subgroup analysis by nutrition status, limited to obesity, we did not observe subgroup differences in length of hospital stay.
-
In the subgroup analysis by route of nutrition support we found considerable subgroup differences in length of stay in ICU and in duration of mechanical ventilation.
-
In the subgroup analysis by the type of participant we did not find subgroup differences between the surgical or medical participants in any of the outcomes analysed
-
In the subgroup analysis by the amount of calories received by each study group we found considerable subgroup differences in length of hospital stay and in duration of mechanical ventilation.
Sensitivity analyses
-
Excluding quasi‐randomized trials. The sensitivity analysis after excluding the only quasi‐randomized trial (Ibrahim 2002) did not show major changes in the overall results for the outcomes of mortality in hospital, length of hospital stay, length of ICU stay and infectious complications. We only observed a change in the outcome of duration of mechanical ventilation. After excluding this trial, the statistically significant difference in favour of the hypocaloric group disappeared. However, this result should be interpreted with caution due to the substantial statistical heterogeneity (I2 = 69%) and important clinical heterogeneity.
-
Excluding trials with at least one high 'Risk of bias' criterion. The sensitivity analysis after excluding the six trials with at least one high 'Risk of bias' criterion ( Arabi 2015; Charles 2014; Ibrahim 2002; McCowen 2000; Rugeles 2013; Rugeles 2016) did not show major changes in the results of the primary and secondary outcomes analysed, nor in the subgroup analyses. We only observed some minor changes in the statistical heterogeneity of several subgroups analysed.
-
By fixed‐effect or random‐effects models. We analysed the primary and secondary outcomes by fixed‐effect or random‐effects models according to the value of I2, as stated in Data synthesis. In all the analyses where the pooled estimates were done with the fixed‐effect model, we conducted a sensitivity analysis with the random‐effects model to explore the robustness of results. No primary or secondary outcomes or subgroup analyses showed a major change in their statistical significance or heterogeneity.
-
By different primary goal of enteral nutrition trials. We performed a post hoc sensitivity analysis (see Sensitivity analysis), excluding the three studies (Ibrahim 2002; NHLBI 2012; Rice 2011) with a primary goal to assess the effects of early initiation of trophic (hypocaloric) enteral nutrition or standard (normocaloric) enteral feeding from the beginning. We did not find major differences in the primary or secondary outcomes, except for minor changes in two unreported outcomes due to the heterogeneity: length of mechanical ventilation and hyperglycaemia.
-
By different primary goal of parenteral nutrition trial. Another post hoc sensitivity analysis was the exclusion of the Battistella 1997 trial, because its primary goal was the evaluation of parenteral nutrition with or without lipids (equivalent to normocaloric or hypocaloric nutrition, respectively). After excluding this trial, we did not see major changes in the primary and secondary outcomes evaluated, with the exception of the length of mechanical ventilation: loss of statistical significance in favour of the hypocaloric group (result not reported due to the substantial statistical heterogeneity).
Meta‐regression
Considering that we found high levels of clinical and statistical heterogeneity, we performed non‐prespecified meta‐regressions using STATA 14.1 to explore the effect of covariates for which we had data (Table 2; Table 3):
-
Type of participants (medical or surgical participants)
-
The calories received in the hypocaloric group, based on the three aforementioned categories (see Subgroup analysis by the amount of calories received by each study group): normo‐hypercaloric, hypocaloric or very low hypocaloric.
-
The difference in calories received between study groups (control minus intervention groups).
We performed meta‐regression on the primary outcomes with results of nine or more trials: hospital mortality, infectious complications, hospital length of stay and ICU length of stay. We did not find significant results explaining sources of heterogeneity using this analysis. None of the analysed explanatory variables influenced the size of the intervention effect of the outcome variables. The details on the definition of variables, dataset and outcome measures are available in Appendix 5 and Appendix 6.
Table 3: In order to show some aspects of the heterogeneous results, in Table 3 we present crude results of the primary outcomes and of length of mechanical ventilation for the 15 included studies. The files of the table were ordered from top to bottom by the difference in the amount of calories receive by the control groups minus those received by the hypocaloric groups (second column from the left).
Assessment of reporting bias
We performed a funnel plot for the outcomes with more than eight included studies (Analysis 1.1; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7). We did not see important asymmetries in the funnel plots, suggesting publication bias (we give one example in Figure 4).
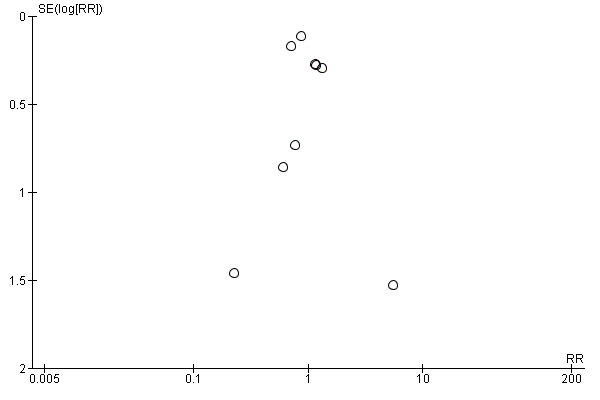
Funnel plot of comparison: 1 Hypocaloric nutrition (intervention) vs. Control, outcome: 1.1 Mortality in hospital.
Discussion
Summary of main results
We identified 15 trials including a total of 3129 ICU participants. The included trials had different objectives, participant characteristics and methodology for the administration of the nutrition support. The consequence of this was important methodological diversity between the included trials (Table 1). Due to the high clinical and statistical heterogeneity, we did not report summary estimates for the primary and secondary outcome analyses. Of all the causes of clinical heterogeneity, the most relevant ones precluding the report of summary estimates were the disparity in the amount of calories/protein received by the intervention and control groups, and the disparity in the differences in the calories received between the study groups in the included trials (Table 2; Table 3). For the same reason, we did not report total estimates of subgroup analyses (See Table 4).
In a descriptive analysis of the results of the included trials for the main outcomes (See summary of findings Table for the main comparison), we can summarize the following:
-
We found very low‐quality evidence for the outcomes related to mortality (in hospital, in ICU and at 30 days), with no statistical but important clinical heterogeneity. Most studies did not find differences in the incidence of mortality between hypocaloric and control groups. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
-
We found very low‐quality evidence for the outcome length of hospital and ICU stay, with both clinical and statistical heterogeneity. In smaller studies, there was a tendency towards a shorter length of stay in participants in the hypocaloric group, but the results across studies were inconsistent, some favouring hypocaloric nutrition support and some control. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
-
We found very low‐quality evidence for the outcome infectious complications, with moderate statistical and important clinical heterogeneity. The results across studies were inconsistent, some favouring hypocaloric nutrition support and some control. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
-
We found very low‐quality evidence for the outcome length of mechanical ventilation, with both clinical and statistical heterogeneity. The results across studies were inconsistent, some favouring hypocaloric nutrition support and some control. The reasons for downgrading the evidence were unclear or high risk of bias in the included studies, inconsistency and imprecision.
Other outcomes
-
For diarrhoea (non‐infectious complications) the statistical heterogeneity was considerable and the clinical heterogeneity important. The central estimates of the individual studies favoured hypocaloric nutrition support, but the quality of this evidence was very low, due to unclear or high risk of bias in the included studies, inconsistency and imprecision.
-
For hyperglycaemia the statistical heterogeneity was substantial and the clinical heterogeneity moderate. The central estimates of the individual studies favoured hypocaloric nutrition support, but the quality of this evidence was very low, due to unclear or high risk of bias in the included studies, inconsistency and imprecision.
-
For hypoglycaemia, the clinical heterogeneity was important, but with no statistical heterogeneity. The individual studies did not find differences in the incidence of hypoglycaemia between hypocaloric and control groups, but the quality of this evidence was low, due to unclear or high risk of bias in the included studies and imprecision.
-
For nitrogen balance, the statistical heterogeneity was substantial and the clinical heterogeneity moderate. The results were inconsistent, some favouring hypocaloric nutrition support and some control; the quality of evidence was very low, due to unclear or high risk of bias in the included studies, inconsistency and imprecision.
We did not find data to perform several of the subgroup analyses proposed in the review protocol. We performed subgroup analyses for the main outcomes, but these could not comprehensively explain the statistical heterogeneity (See Table 4).
In the three prespecified sensitivity analyses (excluding the quasi‐randomized trial; the three trials with at least one high 'Risk of bias' domain; or the change of results from fixed‐effect to random‐effects model) we did not see major changes in the results, nor in the post hoc sensitivity analysis excluding three studies with a primary goal to assess hypocaloric trophic enteral nutrition versus standard enteral feeding. In the other post hoc sensitivity analysis excluding Battistella 1997, in the primary and secondary outcomes we only observed the loss of an unreported significant difference in length of mechanical ventilation.
As we established in the subgroup and sensitivity analyses, Ahrens 2005 and Battistella 1997 had very dissimilar results compared to the other included studies. In both trials, the control groups received a high caloric dose, a median 37 total kcal/kg/day in Ahrens 2005, and 34.4 total kcal/kg ideal body weight/day in Battistella 1997. Moreover, in Ahrens 2005, the control participants not only received hypercaloric parenteral nutrition but also more dextrose than currently recommended for critically‐ill participants (ASPEN / SCCM guidelines 2009; ESPEN guidelines 2009), with a median (interquartile (IQ) range) of 4.9 (4.79 to 5.07) mg dextrose/kg/min. (The authors also reported that the administration of more dextrose than 4 mg/kg/min behaved as a predictor of hyperglycaemia). Finally, It is also important to remember that Battistella 1997 compared parenteral nutrition with and without lipid emulsions. It is therefore difficult to discriminate whether the observed results were due to the amount of calories administered, to the withholding of soy‐derived lipid emulsions in the parenteral nutrition, or both.
In order to evaluate causes of heterogeneity or to formulate hypotheses about them, we performed a non‐prespecified meta‐regression with the available covariates for the primary outcomes with nine or more trials. We did not find significant results explaining sources of heterogeneity (Appendix 5; Appendix 6). However, the results of the meta‐regression should be considered cautiously, due to the fact of post hoc analysis, and to the limited number of studies for the number of covariates in the model.
Overall completeness and applicability of evidence
The research strategy was comprehensive and inclusive. Given the scarcity of evidence, it sought to include all possible trials with a design and goal to evaluate hypocaloric versus normocaloric nutrition support in critically‐ill people. This is why we also included quasi‐randomized controlled trials, different types of ICU settings (medical, surgical, mixed), types of participants (age, medical condition, etc.), administration routes (enteral, parenteral or both), and also considered trials with a different primary goal or methodology to achieve prescribed hypocaloric feeding. We therefore believe that the included studies represent a complete set of up‐to‐date evidence on hypocaloric nutrition support in critically‐ill adults.
Nevertheless, breadth of scope was at the expense of clinical and statistical heterogeneity. Some of these differences in participants, interventions and outcomes of the included trials can be seen in Table 1 and Table 2. In addition, all studies were performed at university‐associated or teaching hospitals, which are probably different from other clinical settings. It is therefore arguable whether our results could be generalized. The clinical and statistical heterogeneity precluded a quantitative synthesis of all the outcomes and most of the subgroup analyses. In the clinical field, the results should be interpreted with caution, considering all these issues.
When we analyse the amount of calories actually received by the groups in each trial (many of them different from those prespecified in their study protocols), we find that most of the included studies did not really evaluate the administration of hypocaloric versus normocaloric nutrition support, but a wide range of calories administered (Table 2). Moreover, the difference in calories received by the control minus the hypocaloric group was quite small in some of the included trials (Table 3). Both factors not only contributed to the clinical heterogeneity, but could also have been associated with the lack of statistically significant differences (if any) between the study groups.
Most of the included trials did not analyse the role of protein administration in the outcomes evaluation. The amount of protein administered to the intervention and control groups was reported in 12 trials: quite diverse in three of them, rather similar in four, and more or less the same in the other five (Table 2). There is wide consensus that obese critically‐ill people should receive hyperproteic hypocaloric feeding (ASPEN / SCCM guidelines 2016; Choban 2013; Dickerson 1986), but there is a current debate about the best protein dose for the non‐obese people: higher doses of proteins seemed to be associated with better outcomes in the critically‐ill people (Dickerson 2012; Weijs 2012; Weijs 2013; Van Zanten 2016). The different daily protein administered to the study groups in the included trials should be considered as another component of the clinical heterogeneity.
Even though our results did not find conclusive significant evidence in favour of the hypocaloric nutrition support, it is also interesting to note that we did not find high‐quality evidence for harms. This is in contrast to two observational studies that reported some poorer clinical outcomes or complications when certain levels of calories were not achieved (Rubinson 2004; Villet 2005).
Quality of the evidence
According to GRADE, the quality of evidence for the primary outcomes was very low (see summary of findings Table for the main comparison).
Six out of 15 included studies presented one or more high 'Risk of bias' criteria and eight studies had one or more unclear 'Risk of bias' criteria. Given the complexity of nutrition support in critically‐ill people, blinding the personnel (the major driver for high risk of bias in this systematic review) is challenging, although some studies were able to do it. Only one included trial (Ahrens 2005) had low risk of bias in all predefined criteria (Figure 3). The quality of evidence according to GRADE was low to very low for all the primary outcomes.
Another reason for downgrading the quality of evidence was inconsistency. We explored the qualitative characteristics that could explain inconsistency, but we were unable to identify them in subgroup analyses (Table 4), sensitivity analyses and meta‐regression. Inconsistency was also evident in the wide variance of point estimates for mortality.
Imprecision affected the quality of all main outcomes, especially mortality, due to the low number of events. The confidence intervals were wide and we could not improve precision by pooling results in most cases, due to the clinical heterogeneity.
For publication bias, the funnel plots for the outcomes with at least eight trials did not show significant asymmetry.
Potential biases in the review process
We followed the procedures of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), in order to minimize biases in the review process. The search strategy was defined by a senior librarian and evaluated by another independent expert. Our search strategy was comprehensive, including consultation with opinion leaders, the pharmaceutical industry, conference and congress proceedings and snowballing techniques to maximize the chances of retrieving all existing studies, published or unpublished. Three review authors independently screened the trials, and data extraction and assessment of risks of bias were also done by two independent review authors. We resolved any disagreement through consultation with a third review author.
We strictly followed the inclusion‐exclusion criteria of our protocol (Perman 2009). Our included trials are non‐homogeneous, with different objectives and methodologies. The main differences related to the type and conditions of the participants and the methodology for the administration of the nutrition support (goals, time of initiation, route, strategy of delivery and calories administered, among others) (Table 1). This clinical and methodological heterogeneity added complexity to the analysis of data and the interpretation of results. It was not possible to report summary estimates due to the clinical or statistical heterogeneity or both.
We acknowledge that the multiplicity of subgroup analyses and post hoc analyses could have yielded false positive results. We added these post hoc analyses in order to explore the clinical heterogeneity found in the included studies. We tried to reduce the risk of false‐positive results by restricting the exploration of subgroups to those outcomes in which we found statistical heterogeneity, as we had defined in our protocol.
Agreements and disagreements with other studies or reviews
There are eight previous systematic reviews and meta‐analyses directly or indirectly related to the topic of this review. They have similar purposes, but different review questions and inclusion criteria.
The first review (Jiang 2011), evaluated randomized controlled trials comparing hypocaloric parenteral nutrition (≤ 20 non‐protein kcal/kg/day) versus standard or high‐energy parenteral nutrition (≥ 25 or > 30 non‐protein kcal/kg/day, respectively) in surgical or trauma participants. According to their inclusion‐exclusion criteria, they included five trials, two of which (Ahrens 2005; Battistella 1997) we also include. The other three studies (in the Chinese language) were trials of postoperative, not critically‐ill participants (according to the titles and one abstract of the studies) (Jiang 2003; Mao 2015; Zhan 2007). They reported a statistically significant reduction in infectious complications and length of hospital stay, with moderate heterogeneity, in the surgical participants receiving hypocaloric parenteral nutrition. Those results were more consistent and with less heterogeneity when they excluded the small‐sample size trials. The calories administered to the participants seemed to be more homogeneous, but the authors did the analysis with administered non‐protein calories. If we add the caloric content of the administered protein, the intervention group received an average of 24.0 (range 20.5 to 27.0) total kcal/kg/day and the control group 34.5 (range 32.5 to 36.0) total kcal/kg/day. This means that the study compared almost normocaloric versus normo‐ to hypercaloric parenteral nutrition. The favourable effects of the lower‐caloric parenteral nutrition on infectious complications and length of hospital stay reported in this meta‐analysis should therefore be limited to surgical participants receiving parenteral nutrition with higher than recommended caloric dose. In our subgroup analyses we observed similar results with the analyses of Ahrens 2005 and Battistella 1997.
In 2015 the Canadian Critical Care Nutrition Clinical Practice Guidelines Committee updated the Canadian Clinical Practice Guidelines for nutrition support for critically‐ill adults. They produced three different but related systematic reviews and meta‐analyses, with the following titles: Intentional Underfeeding: Trophic Feeds vs. Full Feeds (Canadian Guideline 3.3a 2015); Intentional Underfeeding: Hypocaloric Enteral Nutrition (Canadian Guideline 3.3b 2015); and Strategies to Optimize Parenteral Nutrition and Minimize Risks: Dose of PN (Canadian Guideline 10.1 2015). This approach was guidelines‐oriented, but also served to diminish the clinical heterogeneity of the included trials and the statistical heterogeneity in some analyses.
For the evaluation of trophic (hypocaloric) versus full (normocaloric) feeding (Canadian Guideline 3.3a 2015), the Canadian group evaluated two studies, also included in our review (NHLBI 2012; Rice 2011), but they did not include a quasi‐randomized trial with a similar goal and methodology (Ibrahim 2002). The meta‐analysis did not show statistical differences in mortality or ventilator‐associated pneumonia between the study groups. They did not report results of length of hospital stay, of ICU stay or of mechanical ventilation, due to the way the data were reported in the trials. However, we could analyse these results after receiving the information from the first author of each trial. We did not find statistically significant differences between the study groups.
To update the 2015 guideline Intentional Underfeeding: Hypocaloric Enteral Nutrition (Canadian Guideline 3.3b 2015), the Canadian Committee included four trials in the meta‐analysis. We included all four of them in our review (Arabi 2011; Arabi 2015; Charles 2014; Petros 2016). They found that hypocaloric enteral nutrition was associated with a trend towards lower hospital and ICU mortality, and a statistically significant reduction in the length of mechanical ventilation. They did not find significant differences for infectious complications or length of hospital and ICU stay. In the enteral nutrition subgroup analysis we were prevented from reporting summary estimates of the six outcomes evaluated, due to the high clinical or statistical heterogeneity. When we did the same analysis as they did, the results were almost the same (some minor numerical differences).
The Canadian group included four trials in their meta‐analysis of parenteral nutrition (Canadian Guideline 10.1 2015), which we also included in our review (Ahrens 2005; Battistella 1997; Choban 1997; McCowen 2000). (They included results from an "unpublished Ahrens 2003" trial, which were the same as our included Ahrens 2005 trial). They did not find statistically significant differences between the intervention and control groups for hospital mortality or infectious complications. In our subgroup analysis of parenteral nutrition, we found some minor numerical differences from the Canadian Guideline in the same two outcomes, but the results were essentially the same. They also reported some additional results (sensitivity analysis and results of individual studies), but not significant ones.
Another systematic review and meta‐analysis (Choi 2015), compared the effect of initial enteral nutrition with an underfeeding dose versus initial full‐feeding dose of enteral nutrition in critically‐ill adults. They included four trials, three of which we also included in our review (Arabi 2011; NHLBI 2012; Rice 2011), and one which we excluded due to a different primary objective (Desachy 2008). They did not find significant differences in overall mortality and other clinical outcomes between the underfeeding and the full‐feeding groups. In the subgroup analysis, the underfeeding subgroup that received 33.3% or more of the standard caloric requirement showed a significantly lower overall mortality, compared with the full‐feeding group. This was not seen in the underfeeding subgroup that received less than a 33.3% dose of enteral nutrition. This suggests the possibility that a moderate underfeeding enteral nutrition, but not a minimal intake, could be associated with a better prognosis. Nevertheless, the included trials showed clinical heterogeneity, as well as our subgroup analysis of enteral nutrition, where we did not see differences in hospital mortality.
The Tian 2015 meta‐analysis included eight randomized trials showing significantly different calories administered by the enteral route. We included four of these trials in our review (Arabi 2011; Charles 2014; NHLBI 2012; Rice 2011). They did not find significant differences between the low‐ and high‐energy groups for mortality; infectious complications; pneumonia; gastrointestinal intolerance and the length of hospital stay, of ICU stay and of mechanical ventilation. In the subgroup analysis, the low‐energy groups who received between 33.3% and 66.6% of the caloric goal had a significantly lower mortality compared with the high‐energy group. In the subgroup analysis with different amount of protein administration, they found that high protein administration (more than 0.85 g/kg/day) plus high energy could decrease the rate of infectious complication. In our subgroup analysis with three categories of calories administered, we did not find any significant result, but it is necessary to keep in mind the results of the Choi 2015 and Tian 2015 meta‐analyses for the dose of calories with enteral nutrition, as well as the dose of protein (Tian 2015).
The Marik 2016b systematic review and meta‐analysis compared normocaloric (80% to 100% of daily energy expenditure) with intentional hypocaloric enteral nutrition, dividing it into two different strategies: 'permissive underfeeding' (less than 70% of daily energy expenditure) and 'trophic' (20% of the dose during the first week). They included six trials, which we also include in our review, but analysed them separately in the subgroup 'trophic' (NHLBI 2012; Rice 2011), and 'permissive underfeeding' (Arabi 2011; Arabi 2015; Charles 2014; Petros 2016). In the meta‐analysis the statistical heterogeneity was low and they did not find significant differences between the study groups for infectious complications, length of ICU stay and hospital mortality (only a trend towards a lower mortality in the permissive underfeeding subgroup) and ventilator‐free days. In line with our protocol, we performed different subgroup and sensitivity analyses, with conceptually similar results. However, their subgroups approach should be considered in future systematic reviews.
In a second systematic review and meta‐analysis (Tian 2017), the authors included 11 studies comparing low‐ and high‐energy enteral nutrition (in two studies also enteral plus supplemental parenteral nutrition), administered to adults who were critically‐ill but not malnourished. We also included five of these studies (Arabi 2011; Arabi 2015; Charles 2014; NHLBI 2012; Rice 2011). In the meta‐analysis, they did not find statistically significant differences between low‐ and high‐energy groups for mortality, infectious complications, pneumonia, length of hospital and ICU stay, and length of mechanical ventilation. They found significantly less gastrointestinal intolerance in the low‐energy group. In the subgroup analysis for mortality, they observed significantly less mortality in the low‐energy group but only within the range of 33.3% to 66.6% of the goal calories. In another subgroup analysis, the incidence of infectious complications was significantly lower in the high‐energy group, but only when the enteral nutrition also provided higher amounts of protein. Even though one might question their decision to perform meta‐analysis with such high clinical heterogeneity, we should consider in future studies the role of the enteral nutrition dose between 33.3% and 66.6% of the caloric goal and the amount of protein administered.
It is important to emphasize that, in line with our protocol (Perman 2009), we only included trials comparing any type of 'prescribed' hypocaloric nutrition support with different control groups in critically‐ill adults. Although there are several reports in the literature of critically‐ill people receiving hypocaloric enteral nutrition due to difficulties, intolerance or complications during the administration (occurring in 59% of the cases, as reported in the international survey done in 158 ICUs by Cahill 2010), we did not include any study assessing this 'non‐ prescribed', unintentional hypocaloric nutrition support. Nevertheless, we point out that many trials included in our study could not achieve their prespecified caloric goals.
We included three studies that indirectly assessed our review question by the intentional administration of trophic (hypocaloric) enteral feeding during the first five or six days in ICU versus full enteral (normocaloric) feeding from the beginning of the ICU stay (Ibrahim 2002; NHLBI 2012; Rice 2011). In order to be as inclusive as possible, we also included Battistella 1997 (primary objective to evaluate parenteral nutrition with or without soy‐lipid emulsions). It is important to highlight that the results of the latter trial could be associated with the less‐caloric parenteral nutrition, with the lack of lipids, or a combination of both factors (ASPEN 2012; Ren 2013). When we did a sensitivity analysis excluding those trials, we found only minor differences in the results.
We did not include studies evaluating enteral nutrition optimized with supplemental parenteral nutrition to reach the 'target energy' (measured by indirect calorimetry or estimated by formulae) to avoid “caloric debts” (Heidegger 2013), or to assess when to initiate supplemental parenteral nutrition (Doig 2013). Both topics are the subject of currently debate.

Updated study flow diagram, 20 June 2017
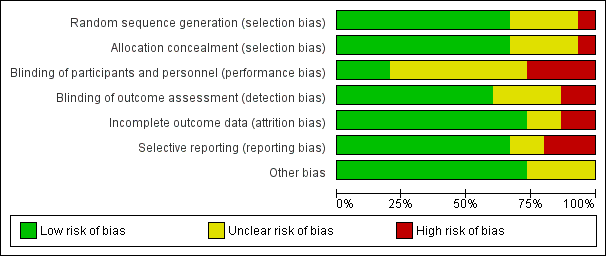
Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias domain for each included study. Red colour represents high risk of bias; green, low risk of bias; and yellow, unclear risk of bias.

Funnel plot of comparison: 1 Hypocaloric nutrition (intervention) vs. Control, outcome: 1.1 Mortality in hospital.
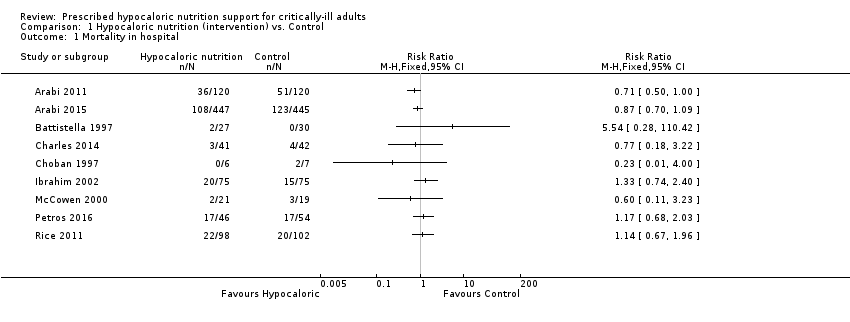
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 1 Mortality in hospital.
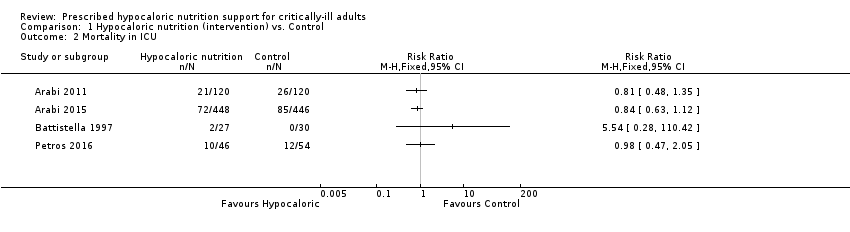
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 2 Mortality in ICU.
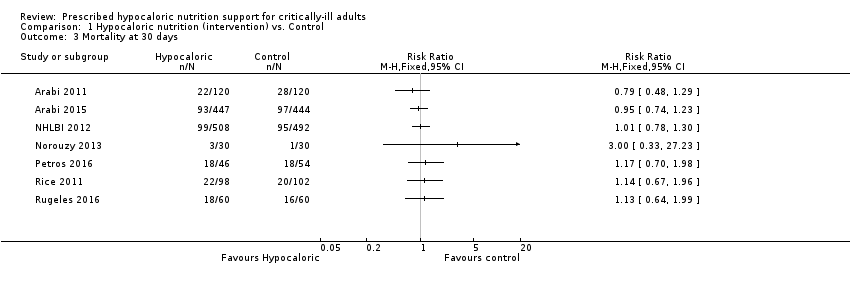
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 3 Mortality at 30 days.
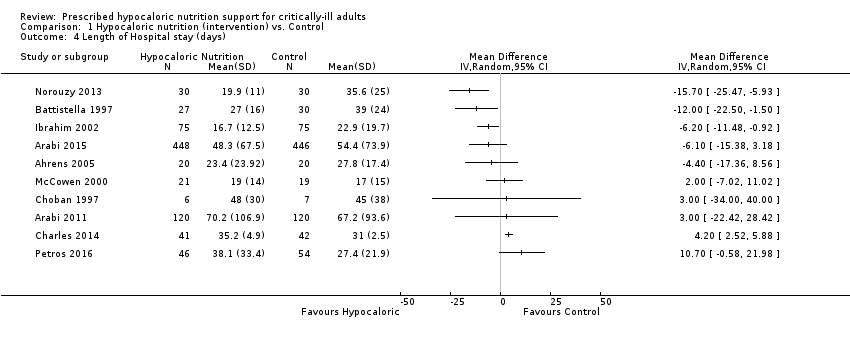
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 4 Length of Hospital stay (days).
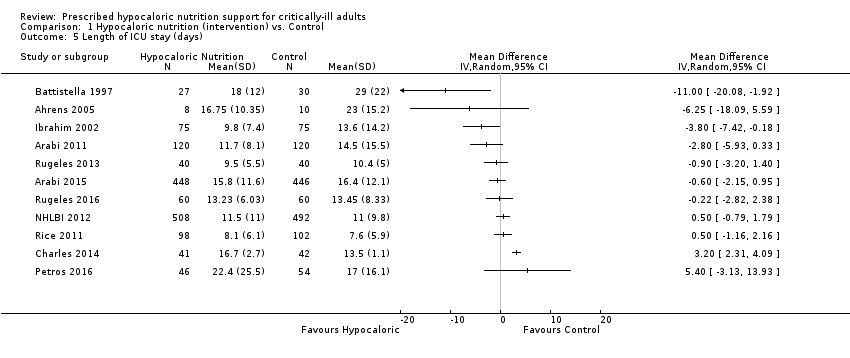
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 5 Length of ICU stay (days).

Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 6 Infectious complications.

Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 7 Length of mechanical ventilation (days).
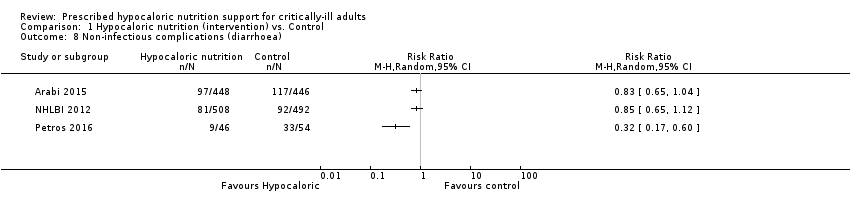
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 8 Non‐infectious complications (diarrhoea).

Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 9 Hyperglycaemia.
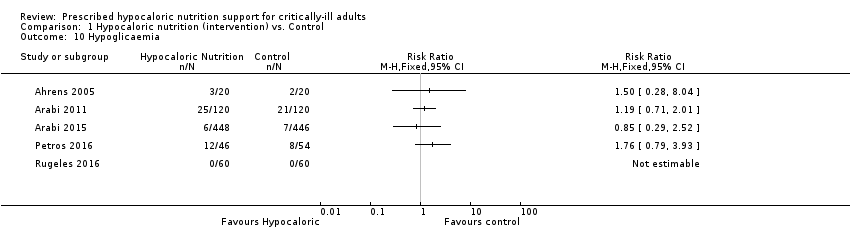
Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 10 Hypoglicaemia.

Comparison 1 Hypocaloric nutrition (intervention) vs. Control, Outcome 11 Nitrogen balance (g/day).
| Hypocaloric nutrition compared to control for critically‐ill adults | |||
| Patient or population: critically‐ill adults Comparison: control nutritional support with a higher caloric intake than the 'intervention' group | |||
| Outcomes | Effect estimate (range of results of individual studies) | N of Participants | Quality of the evidence |
| Mortality in hospital: death occurring during the hospital stay | Range of risk ratios from 0.23 to 5.54a | 1775 (9 studies) | ⊕⊕⊝⊝ |
| Mortality in ICU: death occurred during the ICU stay | Range of risk ratios from 0.81 to 5.54a | 1291 | ⊕⊕⊝⊝ |
| Mortality at 30 days: 28 to 30 days all‐cause mortality | Range of risk ratios from 0.79 to 3.00a | 2611 | ⊕⊕⊝⊝ |
| Length of hospital stay: days stayed in the hospital | Range of length of hospital stay from 15.70 days lower to 10.70 days highera | 1677 | ⊕⊝⊝⊝ |
| Length of ICU stay: days stayed in the ICU | Range of length of ICU stay from 11.00 days lower to 5.40 days highera | 2942 | ⊕⊝⊝⊝ |
| Infectious complications: events of any type of infectious complications occurred during the hospital stay, registered by the study authors according to their diagnostic criteria of infections. | Range of risk ratios from 0.54 to 2.54a | 2804 | ⊕⊝⊝⊝ |
| Length of mechanical ventilation: days on mechanical ventilation during ICU stay | Range of mean differences: 13.20 days lower to 8.36 days highera | 3000 (12 studies) | ⊕⊝⊝⊝ |
| GRADE Working Group grades of evidence | |||
| aResults were not combined due to clinical heterogeneity. | |||
| Study ID | Type of participants Primary outcomes | Arm | Number of ICU participants | APACHE II score mean ± SD | Route (enteral or parenteral) | Duration of PN or EN (days) | Mechanical ventilation (% of participants) | ICU mortality % | Hospital mortality % |
| Surgical participants with PN requirement Incidence/severity hyperglycaemia and insulin received by the participants | Hypoc. | 8 (other 12 non‐ICU) | 20 ± 9 | Parenteral | 6 (4 to 10) | 100 | Not reported | Not reported | |
| Control | 10 (other 10 non‐ICU) | 19 ± 11 | 7 (5 to 10) | 80 | |||||
| Medical (mainly) and surgical participants with EN. 2 x 2 factorial trial with Intensive Insuline therapy 28 days all‐cause mortality | Hypoc. | 120 | 25 ± 8 | Enteral | Not reported | 99 | 18 | 30 | |
| Control | 120 | 25 ± 8 | 99 | 22 | 43 | ||||
| Critically‐ill participants (75% medical) 90‐day all‐cause mortality | Hypoc. | 448 | 21 ± 7.9 | Enteral | 9.1 ± 4.6 | 97.3 | 16.1 | 24.2 | |
| Control | 446 | 21 ± 8.2 | 9.4 ± 4.4 | 96.2 | 19.1 | 27.6 | |||
| Trauma participants with PN requirement Length of hospital stay, length of stay in the ICU, number of days on mechanical ventilation and infectious complications. | Hypoc. | 27 | 22 ± 5 | Parenteral | 10 | Not reported | 7.4 | Not reported | |
| Control | 30 | 23 ± 6 | 10 | 0 | |||||
| Critically‐ill surgical participants Hospital‐acquired infection | Hypoc. | 41 | 16.6 ± 0.9 | Enteral & parenteral | 12.6 ± 2.8 | 68 | N/A | 7.3 | |
| Control | 42 | 17.3 ± 0.8 | 10.4 ± 1.1 | 57 | N/A | 9.5 | |||
| Obese participants with PN requirement. Predominantly surgical diseases Achievement of nitrogen balance | Hypoc. | 6 (other 10 non‐ICU) | 13 ± 5 | Parenteral | 10 ± 3 | Not reported | Not reported | 0 | |
| Control | 7 (other 7 non‐ICU) | 15 ± 5 | 11 ± 2 | 28.6 | |||||
| Medical ICU participants with EN Incidence of ventilator‐associated pneumonia | Hypoc. | 75 | 26 ± 8 | Enteral | 5 ± 6 | 100 | Not reported | 27 | |
| Control | 75 | 25 ± 8 | 10 ± 12 | 100 | 20 | ||||
| Participants with predominantly surgical diseases requiring PN Glycaemic control and Infections | Hypoc. | 21 | not reported | Parenteral | ≥ 5 | 50 | 10 | Not reported | |
| Control | 19 | not reported | ≥ 5 | 33 | 16 | ||||
| Acute lung injury predominantly due to medical diseases (61% and 63% of participants) with EN Ventilator‐free days at study day 28 | Hypoc. | 508 | APACHE III 92 ± 28 | Enteral | 6 | 100 | Not reported | 22.4 | |
| Control | 492 | APACHE III 90 ± 27 | Enteral | 6 | 100 | 19.6 | |||
| Critically‐ill head trauma participants 28 days of all‐cause mortality | Hypoc. | 30 | Not reported | Enteral | 7 | Not reported | Not reported | 10.7a | |
| Control | 30 | 7 | 3.8a | ||||||
| Medical ICU with EN and/or PN requirement Glycaemic control and mortality | Hypoc. | 46 | 31 ± 9 | Enteral & parenteral | 7 | not reported | 22 | 37 | |
| Control | 54 | 28 ± 8 | 7 | 22 | 31 | ||||
| Acute lung injury, predominantly due to medical diseases with EN Ventilator‐free days at study day 28 | Hypoc. | 98 | 27 ± 8 | Enteral | 6 ± 4 | 100 | Not reported | 22 | |
| Control | 102 | 27 ± 7 | 5 ± 3 | 100 | 20 | ||||
| Medical ICU participants with EN requirement Change in SOFA score at 48 hours | Hypoc. | 40 | 14 ± 5 | Enteral | 7 | Not reported | Not reported | Not reported | |
| Control | 40 | 15 ± 6 | |||||||
| Medical ICU participants with EN requirement Change in SOFA score at 48 hours | Hypoc. | 60 | 13.5 ± 6.4 | Enteral | 7 | Not reported | Not reported | 30a | |
| Control | 60 | 13.7 ± 6.8 | 27a | ||||||
| Septic, mechanically ventilated critically‐ill participants 28‐day mortality | Hypocal. | Total sample of 74 participants | Total sample 22 ± 4 | Enteral | Not reported | Not reported | Not reported | Not reported | |
| Control | |||||||||
| a28‐day mortality. EN = Enteral nutrition; ICU = Intensive Care Unit; N/A: not available; PN = Parenteral nutrition; SOFA = Sequential Organ Failure Assessment | |||||||||
| Studies | How data was reported | Hypocaloric (intervention) group | Control group | Calories received by the "hypocaloric" intervention group (kcal/kg/day) | Calories received by the "normocaloric" control group (kcal/kg/day) | Categories denominated by the calories really received in the intervention and the control groups a |
| Total calories/kg/day (median (IQ))b | 26.6 (26.2 to 27.5) | 37 (36.0 to 38.4) | 26.60 (median) | 37.00 (median) | Normocaloric vs hypercaloric | |
| Protein g/kg/day (mean± SD) | 1.61 ± 0.13 | 1.53 ± 0.26 | ||||
| Calories/day (mean ± SD) | 1066.6 ± 306.1 | 1251.7 ± 432.5 | 13.85 | 16.40 | Hypocaloric vs hypocaloric | |
| Protein g/day (mean ± SD) | 47.5 ± 21.2 | 43.6 ± 18.9 | ||||
| Calories/day (mean ± SD) | 835 ± 297 | 1299 ± 2470 | 10.56 | 16.04 | Hypocaloric vs hypocaloric | |
| Protein g/day (mean ± SD) | 57 ± 24 | 59 ± 25 | ||||
| Calories/kg ideal body weight/day (mean ± SD) | 27.4 ± 2 | 34.4 ± 2 | 27.4 (of ideal body weight) | 34.4 (of ideal body weight) | Normocaloric vs. normocaloric | |
| Protein g/kg ideal body weight/day (mean± SD) | 1.6 ± 0.1 | 1.6 ± 0.2 | ||||
| Calories/kg/day (mean ± SD) | 12.3 ± 0.7 | 17.1 ± 1.1 | 12 | 17 | Hypocaloric vs hypocaloric | |
| Protein g/kg/day (mean ± SD) | 1.1 ± 0.1 | 1.1 ± 0.1 | ||||
| Kcal/kg actual body weight/day (mean ± SD) Kcal/kg ideal body weight/day (mean ± SD) | 8.6 ± 2.39 13.88 ± 2.87 | 17.45 ± 4.06 27.99 ± 3.83 | 14.00 (of ideal body weight) | 28.00 (of ideal body weight) | Hypocaloric vs normocaloric | |
| Protein g/kg actual body weight/day (mean ± SD) Protein g/kg ideal body weight/day (mean ± SD) | 1.2 ± 0.2 2.0 ± 0.1 | 1.2 ± 1.2 2.0 ± 0.1 | ||||
| Calories/day (mean ± SD) | 126 ± 115 | 474 ± 400 | 1.53 | 5.81 | Very hypocaloric vs very hypocaloric | |
| Proteins g/day (mean) (mean ± SD) | 5.3 ± 5.3 | 18.7 ± 15.4 | ||||
| Calories/kg/day (mean ± SD) | 14 ± 3 | 18 ± 4 | 14.30 | 18.40 | Hypocaloric vs hypocaloric | |
| Proteins g/kg/day (mean ± SD) | 1.1 ± 0.2 | 1.3 ± 0.2 | ||||
| Calories/day (mean ± SD) | 399 ± 225 | 1365 ± 596 | 4.64 (estimated by kcal/day divided by weight from the baseline table) | 15.69 (estimated by kcal/day divided by weight from the baseline table) | Very hypocaloric vs hypocaloric | |
| Proteins: information not collected | ‐ | ‐ | ||||
| Calories/kg/day (mean ± SD) | Not reported | Not reported | N/A | N/A | N/A | |
| Protein g/kg/day (mean ± SD) | Not reported | Not reported | ||||
| Calories/kg/day (mean ± SD) | 11.3 ± 3.1 | 19.7 ± 5.7 | 11.30 | 19.70 | Hypocaloric vs hypocaloric | |
| Protein | Data not reported | Data not reported | ||||
| Calories/day (mean ± SD of study days 1 to 5) | 300 ± 149 | 1418 ± 686 | 3.60 | 17.31 | Very hypocaloric vs hypocaloric | |
| Proteins g/day (mean ± SD of study days 1 to 5) | 10.9 ± 6.8 | 54.4 ± 33.2 | ||||
| Calories/kg/day (mean ± SD) | 12 ± 3.9 | 14 ± 6.2 | 12.00 | 14.00 | Hypocaloric vs hypocaloric | |
| Protein g/kg/day (mean ± SD) | 1.4 ± 0.44 | 0.76 ± 0.32 | ||||
| Total calories/kg ideal body weight/day (mean ± SD) | 12.6 ± 3.4 | 20.5 ± 5.1 | 13 | 21 | Hypocaloric vs hypocaloric | |
| Protein g/kgIBW/day (mean ± SD) | 1.4 ± 0.4 | 1.4 ± 0.3 | ||||
| Calories/day (mean ± SD) | 962 ± 314 | 1308 ± 513 | Not reported Estimatedc 16.63 kcal/kg/day | Not reported Estimatedc 22.62 kcal/kg/day | Estimatedc Hypocaloric vs normocaloric | |
| Protein g/day (mean ± SD) | 57 ± 24 | 59 ± 25 | Not reported Estimatedc 0.99 g/kg/day | Not reported Estimatedc 1.02 g/kg/day | ||
| aCategories denominated by the amount of calories really received by both study groups, according to the following: very hypocaloric = < 10 kcal/kg/day; hypocaloric = ≥ 10 to < 25 kcal/kg/day; normocaloric = ≥ 25 to < 35 kcal/kg/day; hypercaloric = ≥ 35 kcal/kg/day. BMI = Body Mass Index; g = gram; ICU = Intensive Care Unit; kcal = kilocalories; N/A: not available; SD = standard deviation; vs = versus | ||||||
| Study | Difference in calories between groups (kcal/kg/day) | Hospital mortality (%) IG vs CG | ICU mortality (%) IG vs CG | Mortality at 30 days (%) IG vs CG | Infectious complications (%) IG vs CG | Length of hospital stay (days)a IG vs CG | ICU length of stay (days)a IG vs CG | Length of mechanical ventilation (days)a IG vs CG | Categories denominated by the calories really received in the intervention and the control groupsb |
| 2.00 | N/A | N/A | N/A | N/A | N/A | 9.5 vs 10.4 | 8.5 vs 9.7 | Hypocaloric vs hypocaloric | |
| 2.55 | 30% vs 42.5% | 17.5% vs 21.7% | 18.3% vs 23.3% | 44.2% vs 46.7% | 70.2 vs 67.2 | 11.7 vs 14.5 | 10.6 vs 13.2 | Hypocaloric vs hypocaloric | |
| 4.10 | 9.5% vs 15.8% | N/A | N/A | 28.6% vs 52.6% | 19 vs 17 | N/A | N/A | Hypocaloric vs hypocaloric | |
| 4.28 | 26.7% vs 20% | N/A | N/A | 30.7% vs 49.3% | 16.7 vs 22.9 | 9.8 vs 13.6 | 8.1 vs 12.9 | Very hypocaloric vs very hypocaloric | |
| 5.00 | 7.3% vs 9.5% | N/A | N/A | 56.1% vs 57.1% | 35.2 vs 31 | 16.7 vs 13.6 | 10.8 vs 8.3 | Hypocaloric vs hypocaloric | |
| 5.48 | 24.2% vs 27.6% | 16.1% vs 19.1% | 20.8% vs 21.8% | 35.9% vs 37.9% | 48.3 vs 54.4 | 15.8 vs 16.4 | 11.3 vs 13.5 | Hypocaloric vs hypocaloric | |
| 7.00 | 7.4% vs 0% | 7.4% vs 0% | N/A | 48.2% vs 73.3% | 27 vs 39 | 18 vs 29 | 15 vs 27 | Normocaloric vs normocaloric | |
| 7.90 | N/A | N/A | 30% vs 26.7% | N/A | N/A | 13.2 vs 13.5 | 10.8 vs 10.8 | Hypocaloric vs hypocaloric | |
| 8.40 | 37% vs 31.5% | 21.7% vs 22.2% | 39.1% vs 33.3% | 28.3% vs 11.1% | 38.1 vs 27.4 | 22.4 vs 17 | 20.7 vs 12.4 | Hypocaloric vs hypocaloric | |
| 10.40 | N/A | N/A | N/A | 25% vs 10% | 23.4 vs 27.8 | 16.8 vs 23 | 11.1 vs 20.3 | Normocaloric vs hypercaloric | |
| 11.05 | N/A | N/A | 19.5% vs 19.3% | 18.9% vs 16.1% | N/A | 11.5 vs 11 | 10.5 vs 10.2 | Very hypocaloric vs hypocaloric | |
| 13.71 | 22.4% vs 19.6% | N/A | 22.4% vs 19.6% | 30.6% vs 32.4% | N/A | 8.1 vs 7.6 | 5.7 vs 6.2 | Very hypocaloric vs hypocaloric | |
| 14.00 | 0% vs 29% | N/A | N/A | N/A | 48 vs 45 | N/A | N/A | Hypocaloric vs normocaloric | |
| N/A | N/A | N/A | 10% vs 3.3% | N/A | 19.9 vs 35.6 | N/A | 4.7 vs 17.9 | N/A | |
| N/A | N/A | N/A | 18.4% vs 28.9% | N/A | N/A | N/A | N/A | Hypocaloric vs normocaloric | |
| aLengths of hospital, ICU stays and of mechanical ventilation presented in mean days. IG = Intervention Group; CG = Control Group; N/A = Not available; vs = versus | |||||||||
| Subgroup | N participants (n studies) | Subgroup testing |
| 1. Nutrition status | ||
| 1.1. Length of hospital stay | ||
| Obese | 13 (1 RCT) | I2 = 0%, P = 0.76 |
| General | 1664 (9 RCTs) | |
| 2. Route of nutrition support | ||
| 2.1. Length of hospital stay | ||
| Parenteral | 150 (4 RCTs) | I2 = 0%, P = 0.72 |
| Enteral | 1725 (6 RCTs) | |
| 2.2. Length of ICU stay | ||
| Parenteral | 75 (2 RCTs) | I2 = 83.3%, P < 0.01 |
| Enteral | 2867 (9 RCTs) | |
| 2.3. Infectious complications | ||
| Parenteral | 137 (3 RCTs) | I2 = 0%, P = 0.35 |
| Enteral | 2667 (7 RCTs) | |
| 2.4. Length of mechanical ventilation | ||
| Parenteral | 73 (2 RCTs) | I2 = 85.4%, P < 0.01 |
| Enteral | 2927 (10 RCTs) | |
| 3. Type of participant | ||
| 3.1. Length of hospital stay | ||
| Surgical participants | 223 (5 RCTs) | I2 = 0%, P = 0.55 |
| Medical participants | 1354 (5 RCTs) | |
| 3.2. Length of ICU stay | ||
| Surgical participants | 158 (3 RCTs) | I2 = 0%, P = 0.52 |
| Medical participants | 2784 (8 RCTs) | |
| 3.3. Infectious complications | ||
| Surgical participants | 220 (4 RCTs) | I2 = 0%, P = 0.45 |
| Medical participants | 2584 (6 RCTs) | |
| 3.4. Length of mechanical ventilation | ||
| Surgical participants | 156 (3 RCTs) | I2 = 0%, P = 0.45 |
| Medical participants | 2854 (9 RCTs) | |
| 4. Amount of calories received by each study group | ||
| 4.1. Length of hospital stay | ||
| Normo‐hypercaloric | 97 (2 RCTs) | I2 = 84.1%, P < 0.01 |
| Hypocaloric | 1370 (6 RCT) | |
| Very hypocaloric | 150 ( RCT) | |
| 4.2. Length of ICU stay | ||
| Normo‐hypercaloric | 75 (2 RCTs) | I2 = 0%, P = 0.42 |
| Hypocaloric | 1517 (6 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| 4.3. Infectious complications | ||
| Normo‐hypercaloric | 97 (2 RCTs) | I2 = 0%, P = 0.94 |
| Hypocaloric | 1357 (5 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| 4.4. Length of mechanical ventilation | ||
| Normo‐hypercaloric | 73 (2 RCTs) | I2 = 73.1%, P = 0.02 |
| Hypocaloric | 1517 (6 RCTs) | |
| Very hypocaloric | 1350 (3 RCTs) | |
| RCT = randomized controlled trial; ICU = Intensive care unit | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality in hospital Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mortality in ICU Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Mortality at 30 days Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Length of Hospital stay (days) Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Length of ICU stay (days) Show forest plot | 11 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Infectious complications Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Length of mechanical ventilation (days) Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Non‐infectious complications (diarrhoea) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Hyperglycaemia Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 Hypoglicaemia Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Nitrogen balance (g/day) Show forest plot | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

