Antioxidantes para la subfertilidad femenina
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Prospective randomised trial | |
| Participants | Women attending a teaching hospital fertility clinic undergoing ovulation induction for timed intercourse (N = 58). Mean age 32.2 years (range 19 to 40) Inclusion criteria: anovulatory infertility, at least 12 months of unexplained infertility, PCOS, hypothyroidism or minimal endometriosis Exclusion criteria: women whose partners had semen abnormalities and those who had been on multivitamins (except folate) 6 weeks before recruitment Women with tubal disease, moderate and severe endometriosis, medical disorders or haemoglobinopathies; smokers, those with excessive alcohol intake or BMI < 19 or > 34 kg/m2 | |
| Interventions | 1. Multiple micronutrients (MMN): (n = 30) 1 tablet a day until completion of study (3 treatment cycles). Women who became pregnant could continue if they wished. These micronutrients consist of thiamine, riboflavin, niacin B3, vitamins B6 and B12, folate, vitamins C, A and D, calcium, phosphorus, magnesium, sodium, potassium, chloride, iron, zinc, copper, selenium, iodine, vitamin E, vitamin K, L‐arginine, inositol, N‐acetyl‐cysteine, biotin, pantothenic acid 2. Folic acid (n = 28): 1 tablet a day. Mean age = 32.5 ± 0.83 Women underwent ovulation induction with clomiphene citrate or human menopausal gonadotropin approximately 4 weeks after starting MMN or folic acid and continued until end of study, which was 3 cycles even if pregnancy was attained. | |
| Outcomes | Clinical pregnancy Ongoing pregnancy Miscarriage Ectopic pregnancy | |
| Notes | 2 women did not complete the study-1 from each group. Reasons given: 1 woman in the control group stopped because she wanted to take the micronutrients, and 1 in the treatment group stopped because of nausea Trial is self‐funded. Author stated in an email received 13th February that the trial was not funded. Recruited between Febuary and August 2009 Location: London UK Informed consent Ethical approval Sample size power calculation performed ITT performed Emailed author 12th January 2012about whether the women had IUI or timed intercourse. Author replied on 7th February 2012 saying that all women underwent timed intercourse, not IUI. This email also gave adverse event data (miscarriage and ectopic pregnancy data) for the first cycle. Dr Agrawal is also currently recruiting for a new trial. Emailed author on 9th August 2012 asking about any live birth data. Author replied saying that live birth data were unavailable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Third party randomization ... was carried out through the research and development department of the University College London and the Royal Free Hospitals using stratification..." "Participants were randomly allocated". |
| Allocation concealment (selection bias) | Low risk | "Third party randomisation and allocation concealment was carried out through the research and development department of the University College London and the Royal Free Hospitals using stratification and numbered envelopes". |
| Blinding (performance bias and detection bias) | Low risk | "Women, caregivers and investigators were blinded to the treatment allocation". |
| Incomplete outcome data (attrition bias) | Low risk | ITT was performed and explanations given for the 2 dropouts (1 from each group) |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in the text are reported. |
| Other bias | Low risk | No other bias found |
| Methods | Randomised placebo‐controlled trial | |
| Participants | Women with infertility (n = 88) Mean age: Treatment: 29.7 years; Control: 28.3 years Inclusion criteria: Infertility for at least 12 months with endometriosis (different stages) diagnosed by laparoscopy Exclusion criteria: women with other infertility factors including tubal obstruction | |
| Interventions | 1. Pentoxifylline 400 mg: 1 tablet twice a day for 12 months (n = 43) 2. Placebo (n = 45) Duration: 12 months, 1‐year follow‐up | |
| Outcomes | Cumulative pregnancy rate Recurrence of endometriosis | |
| Notes | Study approved by the Shiraz University of Medical Sciences Institutional Review Board Trial conducted in Shiraz, Iran, from January 2002 to December 2003 Funding source not reported Tried to contact the author regarding clinical pregnancy rate and live birth 12th February 2013, but no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "They were assigned into 1 of 2 groups by simple randomisation. An independent pharmacist generated the allocation and assigned the patients to their groups. To do so, he gave each patient a number on the basis of the order of her being referred to him. For example, the first patient was enlisted as number 1 and the second as number 2 and so on. He then assigned patients with odd numbers into one group and patients with even numbers into another. He decided which one should be the control group by flipping a coin". There is a query as to whether this trial is adequately randomised. It could be seen as block randomisation (cluster) or as alternate (in which case this study would have been excluded). After discussion with the statistician, we decided to include this study because of the double‐blind concealment-if double‐blinding was truly successful and nobody involved in recruitment affected the sequence, then it is a third‐party concealed allocation system that is protecting against selection bias, despite the lack of proper randomisation. |
| Allocation concealment (selection bias) | Low risk | An independent pharmacist generated the allocation and assigned the participants to their groups |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded: "During this period, neither the clinicians nor the patients knew who received the medication and who received the placebo. The only person who knew this was the pharmacist". |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Low risk | Both outcomes stated in Methods and reported on |
| Other bias | Low risk | No other bias found |
| Methods | Randomised clinical trial | |
| Participants | "Infertile women undergoing standardised controlled ovarian hyperstimulation for ICSI‐ZIFT [zygote intrafallopian transfer" (n = 112) Table 1 p. 177 mentions cause of infertility to be male in 51 of 112. Participants aged from 20 ‐ 39 years; mean age 29.69, (treatment group mean age: 29.96; control group mean age 29.41) with no previous history of IVF or ZIFT failure. Infertility duration from 1 ‐ 20 years Exclusion criteria: hypothalamic amenorrhoea, drug reactions, endometriosis and fibroids | |
| Interventions | 1. Pentoxifylline 400 mg and vitamin E 400 mg: 1 tablet of each twice a day (n = 56). Administered for 2 cycles before ICSI‐ZIFT 2. No treatment (n = 56). Duration: 2 cycles. | |
| Outcomes | Term delivery Clinical pregnancy rate confirmed by beta human chorionic gonadotropin (hCG) at 14 days after embryo transfer and transvaginal ultrasound 14 days after this Miscarriage rate Multiple pregnancy | |
| Notes | Conducted in 1 centre in Tehran, Iran; ethical approval gained and written consents obtained Trial was carried out between April 2006 and April 2007 Funded by the institution For sensitivity analysis performed because more than half (41/56) of women had male subfertile partners or because both partners had fertility problems | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables were used |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered envelopes |
| Blinding (performance bias and detection bias) | High risk | Comparison group received no treatment. Authors stated "study not blinded" (p. 176) |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | High risk | Cause of infertility is male in 51 of 112 participants (see Table 1 p. 177), although this is not mentioned in the text, where it says that the participants were 112 infertile women |
| Other bias | Low risk | No other bias found |
| Methods | Prospective randomised double‐blind controlled trial | |
| Participants | Women attending a fertility outpatient clinic for management of unexplained fertility problems (n = 804) Mean age: Treatment group: 27.9 years; Control group 28.1 years Inclusion criteria: All women had at least 1 year of marriage without conception, unexplained subfertility and normal ovulating cycles; tubes were patent Exclusion criteria: any known reason for subfertility Timed intercourse | |
| Interventions | 1. N‐acetyl‐cysteine 1200 mg: 1 tablet a day plus clomiphene citrate 50 mg: 1 tablet twice a day for 5 days, starting on day 2 of the cycle (n = 404) 2. Placebo plus clomiphene citrate: 50 mg 1 tablet twice a day (n = 400) Duration of treatment: 1 cycle Timed intercourse | |
| Outcomes | Number and size of follicles Hormonal profiles Endometrial thickness Clinical Pregnancy Miscarriage Multiple pregnancy No loss to follow‐up | |
| Notes | Conducted in 1 centre in Mansoura, Egypt. Ethical approval and informed consents obtained. Trial ran from October 2003 to April 2005 Funding source not reported Contacted author 13th February 2013 regarding methods of randomisation, but no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation apart from: "Patients were allocated randomly to either the trial group". |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially‐numbered, identical envelopes were used |
| Blinding (performance bias and detection bias) | Low risk | Participants, investigators, outcome assessor and clinicians were blinded |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | Unclear risk | Outcomes stated in the text-multiple pregnancy and miscarriage-reported on, although not initially stated as outcomes of interest |
| Other bias | Low risk | No other bias found |
| Methods | Prospective randomised controlled trial. Pilot study | |
| Participants | Infertile women with asymptomatic minimal or mild endometriosis (n = 60). Mean age: Treatment: 31.2 ± 3.8; Control: 32.4 ± 3.1 Inclusion criteria: at least 12 months of primary infertility, no previous pelvic surgery, minimal or mild endometriosis confirmed by laparoscopy Exclusion criteria: any previous pelvic surgery, pelvic disorders such as adhesions and tubal obstructions, in addition to endometriosis | |
| Interventions | 1. Pentoxifylline 400 mg: 1 tablet twice a day for 12 months (n = 30) 2. Placebo (n = 30). 12‐month duration and 12‐month follow‐up. During this time, participants received treatment for infertility problems (i.e. male problems, ovulatory problems, cervical mucus abnormalities, IUI, ovulation induction) | |
| Outcomes | Pregnancy rates confirmed by ultrasound Miscarriage rate | |
| Notes | 1 dropout from the treatment group and 3 from the control group-all due to refusal to start treatment after randomisation. Number reported is 56. ITT is used for meta‐analysis Trial held from November 1993 to December 1995 Single‐centre study conducted in Spain Ethical approval and all consents obtained Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process that was using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Allocation described as being "designated". Authors contacted regarding this and confirmed concealment "computerised allocation" |
| Blinding (performance bias and detection bias) | Low risk | Women are described as being blinded. Authors contacted regarding other blinded persons. They confirmed that participants were blinded, but investigators, outcome assessors and clinicians were not |
| Incomplete outcome data (attrition bias) | Low risk | Only a small number of dropouts-4 participants lost; 1 in treatment, 3 in control. All explained-1 due to refusal and 3 due to failure to continue taking the medication. No ITT carried out |
| Selective reporting (reporting bias) | Unclear risk | Pregnancy rates were stated as the outcome of interest in the Methods section of the paper. However, miscarriage rates were given in the Results and were not mentioned in the Methods. 1 participant in each study group became pregnant, then miscarried, then became pregnant again. The first 2 pregnancies were not included in the analysis. Live births not reported |
| Other bias | High risk | Some women with other fertility issues apart from endometriosis were treated for these additional conditions (i.e. male factor (receiving bromocriptine), oligo‐ovulation (receiving ovulation induction and some additional IUI) poor post‐coital test, hyperprolactinaemia). Numbers of women in treatment and numbers of controls in each of these categories are given. However, these treatments may bias the results, as nearly double the control women in the additional treatment group received IUI compared with the treatment group |
| Methods | Randomised controlled trial | |
| Participants | Women with primary infertility between 20 and 40 years undergoing IVF (n = 85) Inclusion criteria: regular menstruation, no hormonal or non‐hormonal drug therapy for less than 3 months and no systemic illness Exclusion criteria: serious endometriosis, serious male factor (azoospermia) hypogonadism with an FSH level < 13. Also participants with cycles cancelled were excluded. | |
| Interventions | 1. Melatonin 3 mg: 1 tablet a day (n = 40) 2. No treatment (n = 45). | |
| Outcomes | Primary outcome: number of morphologically mature oocytes retrieved (Mll oocytes) Secondary outcome: fertilisation rate, embryo quality and pregnancy rate | |
| Notes | No information on miscarriage numbers Trial held in Turkey, study dates not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation computer‐assisted 1:1. |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | Embyologist was the only person blinded |
| Incomplete outcome data (attrition bias) | Low risk | ITT used. No dropouts were reported |
| Selective reporting (reporting bias) | High risk | Unclear why chemical pregnancy numbers are lower than clinical pregnancy numbers |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women attending fertility clinic having failed an IVF cycle (poor responders) (n = 34). Mean age: 40 ± 2.1 years, range 37 ‐ 44 years. Undergoing IVF Inclusion: Infertile women with tubal infertility who had not taken hormonal treatments 4 months prior to 1st IVF treatment Exclusion: Intercurrent illness, BMI > 30, endometriosis, ovarian functional cyst or ovariectomy, regular exercise, heavy smokers (> 10 a day), diastolic blood pressure > 90 mmHg | |
| Interventions | 1. Oral L arginine 16 g: 1 tablet a day (n = 17) 2. No treatment (n = 17) Duration: from day 1 of the menstrual cycle to end of the IVF cycle | |
| Outcomes | Hormonal and biochemical evaluation IVF cancellation rates Oocyte and embryo number Clinical pregnancy rates | |
| Notes | Conducted in Italy, study dates not reported Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered sealed envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women attending Modena University Infertility Clinic (n = 37) Exclusion criteria: participants with intercurrent illness, BMI ≥ 30, endometriosis, ovarian functional cyst, PCOS, unilateral ovarian resection or ovariectomy, participants who took regular exercise, heavy smokers (> 10 cigarettes a day), those with hypertension (systolic blood pressure > 140 mm Hg and/or diastolic pressure > 90 mm Hg) and women who had received hormonal treatments in the 4 months before the first IVF attempt | |
| Interventions | 1. L‐arginine 4 grams: 4 times a day (n = 18) 2. Placebo (n = 19). Both groups were undergoing IVF with long gonadotropin‐releasing hormone (GnRH) agonist protocol and pure FSH Duration: 10, 12 days | |
| Outcomes | Clinical pregnancy rates Side effects Endometrial thickness Live birth | |
| Notes | Consent and ethical approval were obtained, and the trial was conducted in Modena, Italy, study dates not reported 32 participants completed the trial, with 5 dropouts due to poor response. Funding source not reported Author was emailed 16th August 2012 and 12th Febrary 2013 with request for the number of live births for each group. Author replied on 14th Febrary 2013, providing data for live birth and miscarriage | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number table". |
| Allocation concealment (selection bias) | Low risk | "opening sequentially numbered sealed envelopes containing treatment allocation". |
| Blinding (performance bias and detection bias) | Low risk | Investigtors, participants and outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | 37 women were enrolled, and investigators stated "All 34 patients completed the trial". Numbers given for dropouts from each group. We contacted the authors regarding this ITT not used. Five were said to be cancelled because of "poor response". |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported, including live birth |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind placebo‐controlled randomised trial | |
| Participants | IVF/ICSI patients (n = 39) Inclusion criteria: infertility requiring IVF–ICSI and age 35 – 43, mean age; CoQ10 39.0 ± 0.79 and placebo 39.1 ± 0.52 Exclusion criteria: body mass index (BMI) >38 kg/m2; early follicular phase (day 2 – 4) serum FSH level 20 mIU/mL; abnormal uterine cavity as evidenced by sonohysterogram or hysterosalpingography; any current use of systemic steroid medication or any infertility treatment within 3 months of study enrolment; any contraindication to being pregnant and carrying a pregnancy to term; contraindication for the use of CoQ10, superfact, menopur, hCG, estrase, and progesterone suppositories; any ovarian or abdominal abnormality that may interfere with adequate TVS evaluation; absence of 1 or both ovaries; clinically relevant systemic disease (e.g. insulin‐dependent diabetes, adrenal dysfunction, organic intracranial lesion, PCOS, hyperprolactinemia, or hypothalamic tumor) or serious illness (neoplasia); history (within past 12 months) or current abuse of alcohol or drugs; administration of any investigational drugs within 3 months before the study enrolment; any medical condition that may interfere with the absorption, distribution, metabolism, or excretion of the study drugs; gastrointestinal diseases; malabsorption syndromes; and liver dysfunction; unexplained gynaecological bleeding; ejaculated sperm not sufficient for ICSI; abnormal controlled ovarian hyperstimulation (COH) screening blood done for both partners, including prolactin, thyroid stimulating hormone, HIV serology, hepatitis B and C serology, rubella, group and screen, and syphilis serology before participation in the study; the concurrent use of any of the following drugs: daunorubicin, doxorubicin, blood pressure medications, warfarin, timolol, atorvastatin, cerivastatin, lovastatin, pravastatin, simvastatin, gemfibrozil, tricyclic antidepressant medications (including amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, and trimipramine), multivitamins, or any vitamin supplementation except folic acid | |
| Interventions | 1. CoQ10 600 mg: 1 tablet a day with breakfast (n = 17) 2. Placebo ‐ identical capsules containing rice oil and starch (n = 22) Duration of treatment up to 3 cycles if pregnancy did not occur. All participants took either CoQ10 or placebo for 2 months. On day 3 of the following cycle, they started ovarian stimulation for IVF while continuing the consumption of the supplements. | |
| Outcomes | Primary outcome: number of euploid eggs per retrieval Secondary outcome: cumulative pregnancy rate per retrieval and cumulative livebirth rate per retrieval | |
| Notes | 12 (5 dropouts) CoQ10 group and 15 (7 dropouts) in the placebo completed the study and 10 in the CoQ10 and 14 of the placebo group completed an IVF/ICSI cycle. Overall there were 15 dropouts from recruitment until the end of the study; 6 women withdrew from the study for personal reasons, 3 for conceiving spontaneously, 2 for poor compliance, 1 for failing to achieve the target BMI, and 3 because of poor ovarian response Participant enrolment to the study began in 2010 and was terminated in June 2012 before sample size reached, due to a paper published in May 2012 by Levin et al describing the negative effects of polar body biopsy on embryogenesis. In the CoQ10 group, 30.8% of the women were treated with the long luteal GnRH agonist protocol, compared with 7.7% in the placebo group. The rest of the participants in both groups were treated with the short microdose flare protocol 2 centres Toronto, Canada Trial registration no: NCT01048385 Informed consent Ethics approval Funding; Ferring Pharmaceuticals provided Menopur Conflict of interests; one of the authors has a consultancy role with Fertility Neutraceuticals involved in the manufacturing and distribution of CoQ10 Email sent to author regarding live birth, clinical pregnancy, dropouts and allocation concealment on 24th November 2015 '[email protected]', no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned in chronological order according to the day of study enrolment to a computer‐generated randomization" |
| Allocation concealment (selection bias) | Unclear risk | "Each enrolled participant received a pre‐assigned package containing either placebo or CoQ10 for the duration of the study". |
| Blinding (performance bias and detection bias) | Low risk | "The study was a double blind, placebo‐controlled, randomized trial". "Both the physician and the patient were blinded regarding assignment of the patients". |
| Incomplete outcome data (attrition bias) | High risk | "At the point the study was terminated (June 2012), we had recruited a total of 39 patients who were evaluated and randomized (17 to the CoQ10 group and 22 to the placebo group). Only 27 had started the treatment with the supplements (12 of the CoQ10 group and 15 of the placebo group). In all, 24 patients completed the treatment cycle and had a polar body biopsy (PBBX) and embryo transfer done (10 of the CoQ10 group and 14 of the placebo group). Six patients withdrew from the study for personal reasons, three for conceiving spontaneously, two for poor compliance, one for failing to achieve the target BMI, and three because of poor ovarian response." |
| Selective reporting (reporting bias) | Low risk | Both primary and secondary outcomes reported in the Methods were reported in the results. Protocol available. |
| Other bias | High risk | "However, because of the premature termination of the study, the CoQ10 group had only one‐third and the control group half of the target number". Early termination of trial for embryo safety reasons may cause an overestimation of the effect of the intervention |
| Methods | Open‐label RCT Patients divided, according to a controlled randomized pattern, into two groups | |
| Participants | Women undergoing ICSI (n = 149) Inclusion criteria: Age between 37 ‐ 40 years; "The recruitment criteria include being under 40 years old, at least one previous failed attempt with ICSI with low‐quality oocyte recovery, diagnosis of PCOS (i.e., with oligomenorrhea, hyperandrogenism and pelvic ultrasonographic appearance characterized by multiple anechoic areas) 8, diagnosis of “poor responders” (i.e., with poor ovarian response to hormonal stimulation, an age greater than 37 years and the need for high doses of FSH stimulation in previous cycles). Only ICSI treatments arrived to the transfer of embryos in the uterus (Embryo‐Transfer) and carried out on Day +2/3 are included in the study". Exclusion criteria: "Patients with a partner with a diagnosis of severe male infertility such as cryptozoospermia (i.e., retrieval of sperm in the semen after centrifugation) and azoospermia (i.e., eventual retrieval of sperm from the testicle or epididymis) were excluded from the study." | |
| Interventions | 1. Myo‐inositol 2000 mg, D‐chiro‐inositol 400 mg, and folic acid 400 mg: 1 of each tablet a day (n = 58) 2. Folic acid 400 mg: 1 tablet a day (n = 91) Treatment duration 3 months before the ICSI cycle | |
| Outcomes | Oocyte quality Embryo quality Biochemical pregnancy Clinical pregnancy "Each patient was included only once; therefore, the results for each patient refer to a single treatment cycle" | |
| Notes | Conducted in Perugia Italy Trial duration between June 2012 and May 2013 Email sent to author regarding randomised pattern, allocation concealment and whether there were any dropouts; [email protected]; no reply. No mention of ethics approval, consent or funding Authors state "no conflicts of interest" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "According to a randomized pattern, the total number of patients was divided into two groups". "The two groups were homogeneous within the parameters of inclusion adopted for the study". Numbers are very unequal between the groups, with 58 in the intervention group and 91 in control |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | "An open study" |
| Incomplete outcome data (attrition bias) | Unclear risk | No dropouts mentioned and groups are unequal |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the Results |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind RCT | |
| Participants | Women undergoing ICSI (n not stated) | |
| Interventions | 1. Myo‐inostol 4 g and folic acid 400 μg: 1 tablet of each a day (n not stated) 2. Folic acid 400 μg: 1 tablet a day (n not stated) Taken for 3 months before ICSI and throughout pregnancy | |
| Outcomes | Total rFSH units Number of stimulation days Fertilisation and cleavage rate Embryo quality Biochemical pregnancy rate Clinical pregnancy rate | |
| Notes | Conducted in Italy, study dates not reported Conference abstract; percentages given but no total participant numbers available Funding by an institutional grant. An author was an employee of a pharmaceutical company Email sent to author 24th November 2015 [email protected]; no reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to two groups; MI treated or placebo" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | "Double blind" |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Unclear risk | Unknown |
| Methods | Prospective randomised placebo‐controlled pilot trial | |
| Participants | Infertile Iranian women with PCOS, aged from 25 ‐ 35 years, undergoing ICSI treatment (N = 80) Inclusion criteria: Women who met the Rotterdam criteria for PCOS Exclusion criteria: Hypersensitivity to either MET (metformin) or NAC, infertility factors other than anovulation, male infertility, pelvic organic pathologies, congenital adrenal hyperplasia, thyroid dysfunction, Cushing’s syndrome, hyperprolactinaemia, androgen‐secreting neoplasia, diabetes mellitus, consumption of medications affecting carbohydrate metabolism and hormonal analogues other than progesterone 2 months prior to enrolment in the study and severe hepatic or kidney disease | |
| Interventions | 4 groups (n = 20 in each, 5 dropouts from each) 1. Placebo oral rehydration salts; 3 times a day 2. Metformin 500 mg: 1 tablet 3 times a day 3. NAC 600 mg: 1 tablet 3 times a day 4. Metformin 500 mg: 1 tablet 3 times a day + NAC 600 mg: 1 tablet 3 times a day All treatments were administered for 6 weeks | |
| Outcomes | Oocyte and embryo quality Endocrine parameters Clinical pregnancy Side effects | |
| Notes | Iran, study ran from July 2012 to February 2013 Need to clarify primary and secondary outcomes Author emailed regarding RoB, live birth and miscarriage information; no reply Funded by institutional grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | State "random" but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Double‐blinded placebo‐controlled, but the placebo group received oral rehydration salts, which are usually in solution, while the treatments were tablets |
| Incomplete outcome data (attrition bias) | High risk | Dropouts accounted for, but > 25% dropout |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomised controlled trial | |
| Participants | Infertile women with PCOS (n = 100) undergoing IVF/ICSI | |
| Interventions | 1. Calcium 400 mg + vitamin D 1000 IU: 1 of each tablet a day (n = 50) 2. Placebo (n = 50) Given on the starting day of OC pretreatment, followed by controlled ovarian stimulation (COS) using GnRH antagonist for IVF/ICSI. Calcium 400 mg/day with vitamin D 1000 IU/d or placebo was administered once daily from the starting day of OC to the day of human chorionic gonadotropin (hCG) injection | |
| Outcomes | Total dose and days of rhFSH administered Numbers of retrieved, mature and fertilised oocytes, and grade 1 or 2 embryos Miscarriage rate | |
| Notes | Korea, study dates not reported Conference abstract Funding source not reported No data for clinical pregnancy or live birth stated in the conference abstract; emailed co‐author CH Kim; [email protected], asking about risk of bias, any full publication of the trial and whether they had any clinical pregnancy and miscarriage data. No reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "infertile patients with PCOS were randomized" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with a diagnosis of unexplained infertility undergoing ovulation induction and IUI (n = 107) Inclusion criteria: no ovulatory problems, normal hysterosalpingography and laparoscopy. Normal semen sample Exclusion criteria: endometriosis, hypertension, diabetes, uterine myoma, ovarian cyst, excessive alcohol, caffeine, chronic illness and smoking | |
| Interventions | 1. Vitamin E: 400 IU: one tablet per day from 3rd to 5th day of the menstrual cycle until the hCG injection. (n = 53) 2. No treatment (n = 50) 4 women were lost to follow‐up as a result of incorrect dose consumption (n = 3) and cycle cancellation (n = 1). ITT not used in the trial | |
| Outcomes | Primary outcome: ongoing pregnancy rate Secondary outcomes: biochemical and clinical pregnancy rate, number of follicles, endometrium thickness, implantation rate | |
| Notes | Study was conducted between June 2011 and December 2011 in Turkey Sample size calculated Ethics approved and written consent obtained Funding not reported, but authors say they have no conflict of interest Emailed author 9th August 2012 regarding the number of women lost from treatment and/or control group. Data added. Will perform sensitivity analysis for quality if we do not hear back from the author regarding ITT. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned according to a randomisation table |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | Not blinded as the control was no treatment |
| Incomplete outcome data (attrition bias) | High risk | Reasons and numbers for attrition were given but unclear whether from treatment or control groups. ITT not used |
| Selective reporting (reporting bias) | Low risk | Nil known |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS attending a fertility clinic-Gynaecological Endocrinology Clinics and Human Reproduction Pathophysiology Centre (n = 34) Inclusion criteria: women with PCOS younger than 40 years Exclusion criteria: concomitant endocrine and metabolic pathologies, such as hypothyroidism, hyperthyroidism, diabetes mellitus, androgen‐secreting cancers, adrenal hyperplasia, Cushing's syndrome Women received IVF or ICSI after evaluation of sperm analysis | |
| Interventions | 1. Myo‐inisitol 2 g + folic acid 200 µg: 1 tablet of each twice a day (n = 16) 2. Folic acid 200 µg: 1 tablet twice a day (n = 18) Treatment was given over 3 months | |
| Outcomes | Number of follicles Number of oocytes retrieved Number of embryos transferred Embryo quality Study states that there was "no difference in the total number of biochemical pregnancies detected", but no data were provided. Author replied, giving the data for chemical pregnancies and stating that no adverse events were reported | |
| Notes | Trial held in Catania, Italy Contacted authors 21st November 2011 via letter and email regarding pregnancy data, allocation concealment and who was blinded. Author responded 28th November 2011 Emailed the author 5th February 2012 requesting data on clinical pregnancies and whether the sealed envelopes were numbered. No reply Funding, ethics approval and power calculation not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a randomisation table, patients were divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Author states that allocation was in "white sealed envelopes". |
| Blinding (performance bias and detection bias) | Low risk | "the investigation was performed in a double‐blind design". Author states, "clinicians and patients were blinded". |
| Incomplete outcome data (attrition bias) | Low risk | No women were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS attending an IVF clinic (n = 100) Inclusion criteria: BMI <28 and FSH <10 IU/L with a diagnosis of PCOS according to Rotterdam 2003 and a normal uterine cavity Exclusion criteria: advanced stage (III or IV) endometriosis and those classified as poor responders or as suffering from premature ovarian failure | |
| Interventions | 1. Myo‐inositol 550 mg and DCI 13.8 mg: 1 tablet of each twice a day (INOFOLIC combi, soft gel, Lo.Li. Pharma Roma, Italy; patented) (n = 47) Treatment was given for 12 weeks before rFSH administration and throughout pregnancy | |
| Outcomes | Primary outcomes: number of morphologically mature oocytes, total IU of rFSH administered and the number of grade 1 embryos | |
| Notes | The trial was registered on clinicaltrials.org (NCT1338844) Conducted in Italy No reproductive outcomes given in the data but pregnancy referred to in the text; emailed author regarding pregnancy outcomes 14th September 2016. Author replied on the 27th September 2016, saying that there are no pregnancy data available but that the trial was double‐blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to a block of ten by a computer‐generated program" |
| Allocation concealment (selection bias) | Low risk | "The key to the coding of the treatments was kept by the Lo.Li. Pharma. Both the participants and the research team were blinded. The randomization code was not broken until the completion of the study" |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | "One of the patients who was enrolled and assigned to the MI‐DCI treated group decided to quit the IVF procedure due to personal reasons" |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with mild to moderate endometriosis (n = 104) post‐laparoscopic surgery Inclusion criteria: at least 12 months with asymptomatic primary infertility, regular menstruation, aged between 23 and 37 years with normal BMI. Women with other infertility factors were included if those factors were correctable and were non‐contributory Exclusion criteria: women with other pelvic disorders such as adhesions and tubal obstructions in addition to endometriosis | |
| Interventions | 1. Pentoxifylline 400 mg: 1 tablet twice a day (n = 51) 2. Placebo (n = 53) Other procedures given post‐laparoscopy included biopsies, tubal dye perfusion and destruction of endometriotic implants by cautery Treatment was started with the first menses after laparoscopic surgery; then participants were observed for 6 months. During this time, participants with other infertility factors were treated (e.g. male problems or ovulatory defects). Treatments included IUI or ovulation induction, or both. Not all participants were treated or received the same treatment, thus the potential for bias. | |
| Outcomes | Pregnancy Miscarriage | |
| Notes | 6 women dropped out: 4 from the treatment group and 2 from the control group. Reasons explained. No ITT. Trial held in Barcelona, Spain Work supported in part by the Comissionat per Universitat i Recerca‐Generalitat de Catalunya Authors were also involved in Balasch 1997 Author emailed 23rd November 2011 regarding live birth data. No reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list generated using the method of simple randomisation". |
| Allocation concealment (selection bias) | Low risk | "Concealment of treatment allocation was achieved with the use of sealed opaque envelopes, each containing a unique study number and prepared independently by a secretary". |
| Blinding (performance bias and detection bias) | Unclear risk | Trial was blinded, not stated whether single, double or triple; "randomised controlled blind trial". |
| Incomplete outcome data (attrition bias) | Low risk | Small numbers of dropouts and reasons explained, no ITT |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Unclear risk | Some women with other fertility issues apart from endometriosis were treated for these additional conditions (i.e. male factor (receiving bromocriptine), oligo‐ovulation (receiving ovulation induction and some additional IUI), poor post‐coital test, hyperprolactinaemia). Numbers of women in treatment and control in each of these categories are given. However, treatments may bias the results, as nearly double the control women in the additional treatment group received IUI compared with the treatment group |
| Methods | Randomised controlled trial | |
| Participants | Overweight and obese women with PCOS, aged 20 ‐ 40 years, (n = 84) diagnosed depending on the Rotterdam Criteria | |
| Interventions | 1. Oral omega 3, 3 g: 1 tablet a day 2. Placebo Duration of treatment 8 weeks | |
| Outcomes | Biochemical markers (FSH, LH, Prolactin) | |
| Notes | Conference proceeding Conducted in Iran, study dates not reported Funding source not reported Author emailed regarding RoB and pregnancy data. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | Double‐blinded (placebo control) |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Unclear risk | Unknown |
| Methods | Randomised controlled trial | |
| Participants | Infertile women at Bangladesh fertility unit (n = 156) undergoing ovulation induction with clomiphene citrate | |
| Interventions | 1. Multinutrient supplementation; unknown included antioxidants and dosage 2. Folic acid; unknown dosage | |
| Outcomes | Chemical pregnancy Clinical pregnancy Ovulation rate | |
| Notes | Conference abstract only, limited details Set in Bangladesh, study dates not reported Funding source not reported Author emailed for RoB information and data. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Clinical pregnancy reported in Methods but not in the Results; this is a conference abstract, so they may not have the data yet |
| Other bias | Unclear risk | Unknown |
| Methods | Prospective randomised controlled trial | |
| Participants | Women with clomiphene‐citrate‐resistant PCOS attending a fertility outpatient clinic (n = 110) Inclusion criteria: diagnosis of PCOS. All women were previously treated with 150 mg clomiphene citrate daily for 5 days per cycle, for 2 or 3 cycles with persistent anovulation or ovulate with very thin endometrium (< 5 mm). All women had patent fallopian tubes Exclusion criteria: women with hyperprolactinaemia, hypercorticism and thyroid dysfunction and women receiving medications such as cholesterol‐lowering drugs, beta‐blockers and tricyclic antidepressants | |
| Interventions | 1. CoQ10 60 mg: 3 capsules a day + clomiphene citrate 150 mg: 1 tablet a day, from cycle days 2 – 6 starting on cycle day 2 and until the day of hCG administration (n = 55) 2. Clomiphene citrate 150 mg: 1 tablet a day from cycle day 2 for 5 days (n = 55) The mean duration of CoQ10 treatment in the 1st cycle was 16.2 ± 2.1 days and in the 2nd cycle 17.1 ± 2.9 days | |
| Outcomes | Primary outcomes: number of growing and mature follicles, serum oestradiol, serum progesterone, ovulation rate, endometrial thickness Secondary outcomes: clinical pregnancy (ultrasound visualisation of gestational sac with pulsating fetal pole) and miscarriage (spontaneous termination of a clinical pregnancy before 20 weeks of gestation) | |
| Notes | Timed intercourse Sample size calculation done 4 dropouts from the intervention and 5 from the control group Egypt Trial duration January 2010 to January 2013 The study was approved by the departmental ethical committee and all participants gave informed consent before inclusion in the trial (committee reference no. 231, approved December, 12 2009) This trial is registered at ClinicalTrials.gov (ID NCT01910766) Email to author regarding live birth data and allocation concealment sent 26th November 2015. No reply. Endometrial thickness; significant difference in favour of the treatment group vs control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated using a computer generated random table" |
| Allocation concealment (selection bias) | Low risk | "sealed envelopes" "Allocation process was done by a third party (nurse)" |
| Blinding (performance bias and detection bias) | High risk | "The physicians monitoring the cycles were blinded to the protocol of each group" |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts accounted for from each arm |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as stated in the Methods |
| Other bias | Low risk | No other bias found |
| Methods | Randomised single‐centre controlled clinical trial | |
| Participants | Women undergoing IVF with sleep disturbances (n = 63) from 24 ‐ 38 years Inclusion criteria: unexplained infertility, no ovulatory problems, normal hysterosalpingogram or laparoscopy and normal semen sample Exclusion criteria: chronic drug usage, history of > 1 fertilisation failure, hypertension, diabetes, uterine myoma, ovarian cyst and smoking | |
| Interventions | 1. Melatonin 3 mg; 1 tablet a day, taken at 22:00 to 23:00 from 3rd to 5th day of the menstrual cycle until the hCG injection (n = 30) 2. No treatment (n = 30) | |
| Outcomes | Primary outcome: oocyte quality Secondary outcomes: fertilisation failure rate, number of follicles, number of oocytes retrieved, number of Mll oocytes, fertilisation rate, number of embryos transferred, embryo quality, implantation rate and clinical pregnancy rate | |
| Notes | Trial held in Turkey Ethics approved, written informed consent gained. Authors declare no conflicts of interest Power calculation performed Emailed author 9th August 2012 regarding which group or groups lost the 3 women. Data added. Tried to contact the author again regarding live birth data 5th February 2013 Author replied on 7th February 2013 , saying that the 3 dropouts were from the treatment group, and that no allocation concealment was performed and no live birth data were available because participants were mainly from rural sites | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned according to a randomisation table |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding (performance bias and detection bias) | High risk | No blinding as control is no treatment |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts explained |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind randomised trial | |
| Participants | Women with oligomenorrhoea or amenorrhoea and PCOS were recruited from gynaecology, endocrine and fertility clinics. Women were < 35 years of age, mean age 29.7 (n = 92) "Infertility was an ailment in only half of the patients in each group. There was no difference in the proportions of infertile women with the groups". Exclsion criteria: hyperprolactinaemia, hormone treatment, abnormal thyroid function, congenital adrenal hyperplasia | |
| Interventions | 1. InfolicⓇ, a combination of myo‐inositol 2g plus folic acid 400 μg: 1 tablet twice a day (n = 45). Mean age 29.0 2. Folic acid 400 μg: 1 tablet twice a day (n = 47). Mean age 29.7 Duration: 16 weeks | |
| Outcomes | Ovulation frequency Hormonal levels Pregnancy | |
| Notes | "Ethical committee approval was obtained before the study, and written informed consent was given by each patient". Trial carried out in Italy, study dates not reported Power calculation carried out High dropouts, > 30% in the treatment group. Included, but data not used, as half the participants did not want to conceive. Study is included on the basis that half the participants were from a subfertility clinic Funding source not reported Authors contacted (May 2010) to request any pregnancy outcomes considered and to ask whether the authors of the paper have the individual data on which women in each group were infertile. No reply as of 12th June 2013 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was effected in a double blind fashion; patients received either MYO combined with folic acid (Inofolic®) or only folic acid as placebo, according to the code provided by computer‐generated randomization." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Described as "double‐blind fashion" ("placebo control" however control is folic acid therefore considered to be no treatment) |
| Incomplete outcome data (attrition bias) | High risk | "The high dropout rate in the myo‐inositol arm (more than 30%) is notable". |
| Selective reporting (reporting bias) | High risk | Only half the participants declared before the study that they wanted to conceive. No ITT for the pregnancy outcome. 1 miscarriage was reported but no details of whether this occurred in the treatment or the control group. Miscarriage not prespecified as an outcome of interest |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomised, placebo‐controlled, group comparative, double‐blind study | |
| Participants | Subfertile women having 1st IVF cycle aged < 40 years with mean age of 31.73 ± 4.4 years (n = 620) 10% described as male factor infertility, and associated data were not presented separately Inclusion criteria: tubal, idiopathic and male infertility were included Exclusion criteria: women with repeated IVF cycles and women with renal or gastrointestinal disease | |
| Interventions | 1. Ascorbic acid 1 g: 1 tablet a day (n = 172) 2. Ascorbic acid 5 g: 1 tablet a day (n = 153) 3. Ascorbic acid 10 g: 1 tablet a day (n = 136) 4. Placebo (n = 158) Duration 1 cycle | |
| Outcomes | Clinical pregnancy rate confirmed by fetal heartbeat at 8 weeks Implantation rate per embryo transfer | |
| Notes | 1 woman lost to follow‐up-no explanation. Tried to contact author. No reply 10% of women had partners with male infertility Trial conducted in 2 clinics in Budapest, Hungary (n = 237) and Vienna, Austria (n = 383) No power calculation performed Pregnancies were confirmed at 8 weeks with no further follow‐up; authors contacted regarding this. No reply as of 12th June 2013 No clarity regarding the number of treatment cycles involved in this study Ethics approval not gained as "a study on vitamin supplementation is not subject to IRB approval". Consent forms were signed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "This prospective randomised double‐blind study". Method not described |
| Allocation concealment (selection bias) | Low risk | By an independent pharmacy in Vienna "prepared and coded by number". |
| Blinding (performance bias and detection bias) | Low risk | Women and clinicians were blinded: "double‐blind study". |
| Incomplete outcome data (attrition bias) | Low risk | 1 set of participant data noted as missing but not explained; authors contacted regarding this |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Unclear risk | Unequal baseline group numbers |
| Methods | Randomised double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | Infertile women with PCOS. Mean age: Treatment group: 24.6 ± 3.2; Control group: 24.8 ± 2.7.Timed intercourse (n = 170) Inclusion criteria; < 35 years of age, presenting with primary or secondary infertility following regular intercourse for at least 1 year and diagnosed with PCOS with no other abnormalities Exclusion criteria; FSH values ≥ 10IU/ml | |
| Interventions | 1. Clomiphene citrate 250 mg: 1 tablet a day from day 3 to day 7 of the cycle plus oral‐carnitine 3 g: 1 tablet a day from day 3 until the day of the first positive pregnancy test (n = 85) 2. Clomiphene citrate 250 mg: 1 tablet a day plus placebo (n = 85) All participants received clomiphene citrate from day 3 until day 7 of the cycle.Timed intercourse | |
| Outcomes | Clinical pregnancy rate Miscarriage Multiple pregnancies Ovulation rate Days until hCG injection Endometrial thickness Number of follicles Number of pregnancies Laboratory parameters | |
| Notes | All participants were counselled about their participation in the study. A signed informed consent was obtained. Participants had the right to refuse to participate or to withdraw from the study at any time without being denied their regular full clinical care. Personal information and medical data collected were subject to confidentiality and were not made available to a third party. Women’s Health Hospital, Assiut University, Assiut, Egypt The study was conducted between January 2010 and March 2012 Sample size calculation done "The authors have no conflict to disclose" Funding source not reported Email sent to author on 26th November 2015 regarding live birth data; author replied on 7th December 2015 saying there are no live birth data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated numbers" "randomized according to computer‐generated randomization tables to ensure an equal number of patients in each arm (1:1 ratio)". |
| Allocation concealment (selection bias) | Low risk | "using previously prepared sealed envelopes with computer‐generated numbers" "Throughout the trial, access to the randomization code was available only to the pharmacist who manufactured the placebo and packed the envelopes and was not available to any of the treating physicians or patients". "The capsules were placed in sacks and then stored in envelopes numbered from 1 to 170. The envelopes were numbered" |
| Blinding (performance bias and detection bias) | Low risk | "double blind" "The placebo capsules were specially manufactured to look identical to the L‐carnitine capsules". |
| Incomplete outcome data (attrition bias) | Low risk | 18/170 dropouts with numbers per group and reasons given |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the Methods were reported |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blinded randomised control trial | |
| Participants | Women aged 18 ‐ 41 with PCOS which was clomiphene‐resistant who attended fertility clinic in Iran (n = 93) | |
| Interventions | 1. Oral NAC 1.2 g: 1 tablet a day (n = 53) 2. Vitamin C (?dose) (n = 40) | |
| Outcomes | Oestradiol levels Number of follicles > 18 mm Endometrial thickness | |
| Notes | Conducted in Iran, study began in 2010 (end unknown) Unknown trial duration Funding source not reported Tried to contact author regarding pregnancy data, uneven number in each group. No reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blinded (another antioxidant, not placebo) |
| Incomplete outcome data (attrition bias) | High risk | Uneven number in each group |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women aged 25 ‐ 39 years with PCOS undergoing IVF (n = 58) | |
| Interventions | 1. NAC 400 mg: 1 tablet twice a day (n = unknown) 2. Placebo (n = unknown) Duration 13 ‐ 15 weeks. | |
| Outcomes | Insulin sensitivity Endocrine levels Ovarian stimulation Number and size of follicles Number of retrieved oocytes Number and quality of embryos transferred Pregnancy rate Miscarriage Ovarian hyperstimulation syndrome rates | |
| Notes | Conference abstract only Trial held in Korea, study dates not reported Funding source not reported The authors contacted to request pregnancy outcome data and study protocol to appraise risk of bias elements. No reply as of 14th September 2011 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients randomly assigned..." No description of method of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Unclear risk | Unknown |
| Methods | Prospective randomised controlled study | |
| Participants | Infertile women with a history of unexplained total fertilisation failure undergoing ICSI (n = 98). Ages not given Inclusion criteria: unknown Exclusion criteria: unknown | |
| Interventions | 1. Omega‐3‐polyunsaturated fatty acids (o‐3 PUFAs) 1000 mg: 1 tablet a day (n = unknown) 2. Unknown control (n = unknown) | |
| Outcomes | Total recombinant human (rh)FSH dose and days required Numbers of oocytes retrieved Number of oocytes fertilised Embryo quality Embryo implantation Clinical pregnancy rate | |
| Notes | Conference abstract only Trial held in Korea, study dates not reported Funding source not reported Authors emailed 22nd November 2011regarding risk of bias, pregnancy data per woman, numbers in intervention and control groups and inclusion/exclusion criteria. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Prospective randomised controlled study"-no explanation given. |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Unclear risk | Unknownn. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Unclear risk | Unknown |
| Other bias | Unclear risk | Unknown |
| Methods | vRandomised study | |
| Participants | Women with PCOS indicated by oligomenorrhoea and/or hyperandrogenism and/or hyperandrogaenemia and/or typical features of ovaries on ultrasound scan were enrolled in this study. At least 2 of the above‐mentioned criteria were present in all the participants Women were undergoing IVF and aged < 40 years (n = 29) Exclusion criteria: any other medical conditions causing ovulatory disorders such as hyperprolactinaemia or thyroidal disorders or Cushing syndrome | |
| Interventions | 1. Myo‐inositol 4000 mg plus folic acid 400ug: 1 tablet per day (n = 14) 2. Placebo (n = 15) Treatment was for 2 months prior to the IVF cycle and the trial ran for 4 months | |
| Outcomes | Number of retrieved oocytes Ratio of follicles to retrieved oocytes Fertilisation rate Oocyte quality Amount of FSH units used Days of stimulation | |
| Notes | Conducted in Germany, study dates not reported Has an author who was an employee of a pharmaceutical company Funding source not reported Contact details; Pedro‐Antonio Regidor (pedro‐[email protected]) email sent on 13th October 2016 asking whether the placebo group received folic acid, methods of randomisation, allocation concealment, clinical pregnancy, live birth data and the length of the trial. Author replied 17th October 2016, no outcomes yet "but this is ongoing" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The method of randomization was a manual one. After fulfilling the including criteria the patients were allocated to the previously defined randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Unclear risk | Single‐blinded. "The biologist which carried out the fertilization was the blinded person. He did not know if the women were treated with myo‐Inositol or not" (placebo used) |
| Incomplete outcome data (attrition bias) | Low risk | All randomised women were analysed |
| Selective reporting (reporting bias) | Low risk | No apparent reporting bias |
| Other bias | Low risk | No other bias found |
| Methods | Randomised open‐label, multicentre pilot study | |
| Participants | Infertile women undergoing IVF/ICSI, mean age 34.4 ± 3.4 (n = 100) Exclusion criteria: women with PCOS, with any endocrine or metabolic disease, taking any hormonal treatment, with BMI > 30 kg/m2 | |
| Interventions | 1. Myo‐inositiol 4000 mg: "into two administrations per day" + folic acid 400 µg: 1 tablet a day (n = 50) 2. Folic acid 400 µg: 1 tablet a day (n = 50) Duration of treatment 3 months, duration of trial 12 months | |
| Outcomes | Length of stimulation Total quantity of gonadotropins required Number of oocytes retrieved Implantation rate Clinical pregnancy | |
| Notes | Center for Reproductive Medicine Research, Clinica Villa Mafalda, Rome, Italy, study held from January 2011 to January 2012 Funding source not reported Emailed author 13th February 2013 regarding randomisation, allocation concealment and live birth data. Professor Lisi replied, clarifying these questions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation in a computer‐generated sequence" is written in the paper and in further correspondence from the author ..."About randomization, a computer software generated 100 numbers from 1 to 10,000, and the numbers were stored in sealed envelopes and opened on the day of preparation and explanation of the stimulation protocol to patients. Patients with odd number were assigned to folic acid, myo‐inositol and rhFSH; patients with even number were assigned to folic acid and rFSH". Unsure whether this may be quasi‐randomised. We sought further information from the author. Author replied, "The envelope outside had 100 numbers in order and opened in that order; numbers outside were different from numbers inside". |
| Allocation concealment (selection bias) | Low risk | Envelopes were numbered sequentially and were opaque |
| Blinding (performance bias and detection bias) | High risk | Open‐label, although outcome assessors were blinded participants were not |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised study | |
| Participants | Women with PCOS (based on Rotterdam criteria, ESHRE/ASRM 2004), the diagnosis of PCOS is determined by the presence of 2 of the following conditions: oligo‐ovulation or anovulation, hyperandrogenism and polycystic ovaries detected by ultrasonography with the presence of 12 or more follicles measuring 2 – 9mm in diameter, and/or at least 1 enlarged ovary (410 cm). None of the participants had history of clomiphene citrate resistance (n = 120) Timed intercourse Mean age was 26 years for all 3 groups | |
| Interventions | 1. Clomiphene citrate 100 mg orally in 2 divided doses a day. No treatment (n = 40) 2. NAC 1200 mg in 2 divided doses a day (in the form of powder inserted in small pockets to be diluted into a standard glass of water from day 3 until day 7 of the menstrual cycle) (n = 40) 3. Metformin 500 mg: 1 tablet 3 times a day (n = 40) Treatment period; from day 3 to day 7 of the menstrual cycle, treatment was repeated in non‐pregnant cases for 3 successive cycles | |
| Outcomes | Clinical pregnancy (defined as the presence of gestational sac containing fetal hearts on ultrasound scan) Endometrial thickness and pattern Number and size of follicles | |
| Notes | Conducted in Egypt Trial period; September 2012 to March 2014. Funding source not reported Ahmed Mohamed Maged, Obstetrics and Gynecology Department, Kasr Aini Hospital Cairo University, 135 King Faisal Street Haram Giza, Cairo, Egypt. Tel: 0105227404. Fax:35873103. E‐mail [email protected]. Email sent 13th October 2016 regarding live birth and any dropouts. No reply. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised at the beginning of each cycle by sealed opaque envelopes containing randomly generated numbers into 3 groups |
| Allocation concealment (selection bias) | Low risk | Patients were randomized at the beginning of each cycle by sealed opaque envelopes containing randomly generated numbers into 3 groups |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | Numbers analysed in each group are not given |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with peritoneal endometriosis stage 1 or 2 diagnosed by laparoscopy (n = 36). All participants had fulguration of endometrial implants. Mean age: Treatment group 32.7 ± 2.36; Placebo group 32.7 ± 2.36 Inclusion criteria: women between 25 and 35 years old who had been diagnosed as having peritoneal endometriosis on exploratory laparoscopy, with fertile male partner Exclusion criteria: women who reported having used nutritional supplements during the previous year; who had pelvic inflammatory disease or autoimmune, endocrine or metabolic disorders; or who did not agree to participate or missed a medical visit | |
| Interventions | 1. Vitamins C 343 mg + Vitamin E 84 mg: in a bar form, 1 bar a day (n = 18) 2. Placebo (n = 18) Duration of trial was 6 months Follow‐up for up to 9 months after the trial | |
| Outcomes | Live birth (no data available) Pregnancy (no explanation of whether pregnancies were biochemical, clinical or ongoing). "None of the patients became pregnant during the trial. Once the trial ended, patients were followed up for 9 months for a possible pregnancy". The pregnancy rate was 19% (3 of 16) in the supplementation group and 12% (2 of 18) in the placebo MDA, oxidative stress markers obtained during the exploratory laparoscopy | |
| Notes | Consent signed Ethics was approved The study was conducted at the National Institute of Perinatology “Isidro Espinosa de los Reyes” in Mexico City, study dates not reported Funding given as a grant from Consejo Nacional de Ciencia Tecnologia Mexico. Power calculation done. Tried to contact author. Contacted author again 12th February 2013 to ask about clinical pregnancy and live birth. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reference was made to the use of 'randomisation codes', and investigators stated, "Thirty‐six participants were randomly assigned". Authors contacted regarding this |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the paper. Authors contacted regarding this. The response was, "women were randomly allocated depending on the colour of a ball they took out from a container" |
| Blinding (performance bias and detection bias) | Low risk | Women were blinded. The bars were "identical‐looking and tasting bars". Authors contacted regarding this and confirmed that investigators, outcome assessors and clinicians were blinded also. "Randomization codes were unlocked at the end of the study". |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 women in the treatment arm dropped out "for personal reasons". ITT not applied |
| Selective reporting (reporting bias) | High risk | Investigators stated they would collect live birth rates but reported only pregnancy rates |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with PCOS (n = 44). Natural or timed intercourse Mean age: Treatment group: 26.5 yr (20 ‐ 43); Control group: 29 yrs (23 ‐ 26) Inclusion criteria: primary or secondary infertility due to PCOS according to Rotterdam criteria including oligomenorrhoea, amenorrhoea, clinical or laboratory evidence of increase androgen level or polycystic ovaries in sonography Exclusion criteria: any definite gland disorders such as kaohsiung hypothyroid, hypothyroidism, diabetics and increase in blood prolactin levels | |
| Interventions | 1. Clomiphene 50 mg: 1 tablet a day + 400 units of Vitamin D + 1000 mg calcium: 1 tablet a day (n = 22) 2. Clomiphene 50 mg + placebo: 1 tablet a day (n = 22) Duration: 3 menstruation cycles (3 months) | |
| Outcomes | Follicle size Pregnancy (unknown whether this is clinical or biochemical ‐ sonography had been done for all participants up to 3 months but this could be to assess follicle size) | |
| Notes | Conducted in Iran Trial was run between 2010 and 2011. Funding source not reported Email was sent to author on the 30th November 2015 regarding data and risk of bias [email protected] ‐ no reply. Dr Vahid Seyfoddin helped translate key points from the paper. New email found [email protected], email sent 27th September 2016 regarding block size, allocation concealment and clinical pregnancy data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were divided into two groups (22 Intervention and 22 controls) using block randomization method". Unknown process of selection of blocks |
| Allocation concealment (selection bias) | Unclear risk | Unknown block number |
| Blinding (performance bias and detection bias) | Low risk | "Specialists did the randomisation only and the residents managed the study, the radiologists was blinded while using the same instrument and only one practitioner" (placebo control) |
| Incomplete outcome data (attrition bias) | Low risk | "All participants completed the study" |
| Selective reporting (reporting bias) | Unclear risk | Unknown whether the reported pregnancies were biochemical or clinical. Protocol available |
| Other bias | Low risk | No other bias found |
| Methods | Randomised, double‐blind, placebo‐controlled pilot study | |
| Participants | Women undergoing unilateral laparoscopic ovarian drilling (LOD) for clomiphene‐resistant PCOS (n = 60) Aged 18 ‐ 38 years; mean age: treatment group 28.4 ± 4.2; placebo group 29.2 ± 3.7, with at least 2 years of infertility due to anovulation, patent fallopian tubes, normal semen analysis Exclusion criteria included no hormonal treatment for 3 months before enrolment and any contraindications to anaesthesia or laparoscopy | |
| Interventions | 1. NAC 1.2 grams: 1 sachet a day for 5 days, starting at day 3 of the cycle (immediately after LOD) for 12 consecutive cycles (n = 30) 2. Placebo (n = 30) Both groups also had LOD Follow‐up by cycle monitoring and timed intercourse for a year. No women were lost to follow‐up. | |
| Outcomes | Primary outcome: biochemical pregnancy Secondary outcomes: ovulation, number of follicles, endometrial thickness, clinical pregnancy, miscarriage, multiple pregnancies, ongoing pregnancy, number of preterm deliveries, live birth | |
| Notes | Trial took place in Egypt between January 2005 and June 2007 Ethics obtained. Informed written consent. Endometrial thickness; significant difference in favour of the treatment group Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised double‐blind placebo‐controlled pilot study", "computer‐generated random numbers". |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes". |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. "The placebo sachets were specially manufactured to look identical to the NAC sachets". "Throughout the study, access to the randomisation code was available only to the pharmacist and was not available to the treating gynaecologist or patients". |
| Incomplete outcome data (attrition bias) | Low risk | No women lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised trial | |
| Participants | Women undergoing IVF aged 22 ‐ 43 years. Mean age treatment group: 30.7 ± 4.5; placebo group: 28.8 ± 3.2) (n = 56) Inclusion criteria: non‐smokers, free from major illness including hypertension, all interested in becoming pregnant Exclusion criteria: myoma, adenomyosis, congenital abnormality, ovarian tumours, hormone or long‐term medication use | |
| Interventions | 1. Multi‐vitamin/mineral (containing vitamins A, B, C, D, E and H, calcium, folic acid, nicotinic acid, iron, magnesium, phosphor copper, manganese and zinc): 1 tablet a day (n = 26) 2. Placebo (candy) (n = 30). for 45 days | |
| Outcomes | Follicular fluid | |
| Notes | Conducted in Turkey 3 groups were used in the study. The 1st group consisted of age‐matched controls, so we did not use these data in this review. The 2nd and 3rd groups were randomly assigned Author emailed on 1st August 2012 to ask for any data on pregnancy, live birth or adverse events. Author replied on 13th August 2012. No outcomes appropriate to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | High risk | Placebo used was candy |
| Incomplete outcome data (attrition bias) | Unclear risk | None mentioned |
| Selective reporting (reporting bias) | Low risk | None known |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled double‐blind trial | |
| Participants | Women with PCOS undergoing ICSI aged between 27 ‐ 38 years (n = 569) Inclusion criteria: absence of tubal, uterine, genetics and male causes of infertility; serum levels of FSH on day 3 of the ovarian cycle 512 IU/L; Rotterdam criteria for PCOS; normal uterine cavity; BMI of 20 to 26 kg/m2; first IVF treatment. Only women undergoing 1st‐time ICSI procedure fulfilling inclusion criteria were enrolled in the study in order to limit their heterogeneity | |
| Interventions | 1. Myo‐inositol 4000 mg + folic acid 400 mcg (Inofolic®): 1 tablet twice a day and Melatonin 3 mg: 1 tablet twice a day (n = 178) 2. Myo‐inositol 4000 mg + folic acid 400 mcg (Inofolic®): 1 tablet twice a day (n = 180) 3. Folic acid 400 mcg: 1 tablet twice a day (n = 211) Treatment was from the first day of the cycle until 14 days after embryo transfer | |
| Outcomes | Primary end points were: oocyte and embryo quality, clinical pregnancy (identified by the presence of a gestational sac on ultrasonography 5 weeks after oocyte retrieval) and implantation rates. Secondary outcomes were: gonadotropin IU administered, days of stimulation, serum estradiol(E2) levels and endometrial thickness on the day of human chorionic gonadotropin (hCG) administration. | |
| Notes | Conducted in Italy Trial ran from July 2009 to December 2011 43 women dropped out, 16 from the control, 13 from the intervention group A and 14 from group B; reasons provided Clinical Trial registration Number: NCT01540747 (ClinicalTrials.gov registry) Has an author who was an employee of a pharmaceutical company Funding source not reported Emailed Dr Pacchiarotti 18th October 2016 to ask about allocation concealment and live birth data and asked about trial details from included study Valeri 2015. Contact details; [email protected]. Dr Pacchiarotti replied 20th March 2017 saying that the clinical pregnancy was per woman as they have 80% of the live birth data. We replied asking whether we could include these data. No reply yet. We will need to follow up on this for next review update. Power calculation performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a computer based random assignment schedule for each patient" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Unclear risk | "double‐blinded" but not placebo‐controlled |
| Incomplete outcome data (attrition bias) | Low risk | Dropout reasons were described and numbers given for each group |
| Selective reporting (reporting bias) | Low risk | Outcomes in the Methods were reported as per protocol |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Women with PCOS having clomiphene citrate for ovulation induction (timed intercourse) (n = 200) | |
| Interventions | 1. Combined antioxidant supplementation; vitacap which contains vitamin A (Palmitate) 5000 iu, vitamin B1 (thiamine mononitrate) 5 mg, vitamin B6 (pyridoxine HCL) 2 mg, vitamin B12 (cyanocobalamin 5 mg, vitamin C 75 mg, vitamin D3 (cholecalciferol) 400 iu, Vitamin E (d‐alpha tecopheryl acetate ) 15 mg, nicotinamide 45 mg, folic acid 1000 mcg, ferrous fumerate 50 mg, dibasic calcium phosphate 70 mg, copper sulphate 0.1 mg, manganese sulphate 0.01 mg, zinc sulphate 50 mg, potassium iodide 0.025 mg and magnesium oxide 0.5 mg: 1 vitacap a day (n = 100) 2. Placebo; containing folic acid and fersolate. 1 tablet a day (n = 100) Treatment given for 6 months | |
| Outcomes | Live birth Clinical pregnancy Menstrual regularisation | |
| Notes | Conducted in Nigeria, study dates not reported Conference abstract Funding source not reported [email protected], email sent 18th October 2016. Author replied 20th October 201 with live birth data. Emailed author re allocation concealment of odd and even envelopes 25th January 2017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The women were randomized into two groups by picking one of the two closed envelops. within the envelop is written odd or even number. The odd number for intervention and even number for control. The selection of odd and even number for intervention and control group was done by toss of coin" |
| Allocation concealment (selection bias) | Low risk | "The women were randomized into two groups by picking one of the two closed envelopes, within the envelope is written odd or even number". |
| Blinding (performance bias and detection bias) | Low risk | "The trial was single blinded. The patient did not know" a placebo was used |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts accounted for |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile women with PCOS undergoing ovulation induction for ICSI (n = 60) Mean age (years) treatment: 36.2 ± 2.4; non‐treatment 35.4 ± 2.5 Inclusion criteria: women aged < 40 years with PCOS, indicated by oligomenorrhoea (6 or fewer menstrual cycles during a period of 1 year), hyperandrogenism (hirsutism, acne or alopecia) or hyperandrogenaemia (elevated levels of total or free T) and typical features of ovaries on ultrasound scan. All women had been treated at the IVF clinic for > 12 months. Exclusion criteria: other medical conditions causing ovulatory disorders such as hyperinsulinaemia, hyperprolactinaemia, androgen excess such as adrenal hyperplasia or Cushing's syndrome Duration of infertility (months) treatment: 46.1 ± 18.5, non‐treatment: 37.7 ± 9.6 | |
| Interventions | 1. Myo‐inositol 2 g + folic acid 400 µg (Inofolic®): 2 tablets a day (n = 30) 2. Folic acid 400 µg (n = 30) Duration: for 1 cycle of ICSI. Treatment starting on the day of GnRH administration | |
| Outcomes | Number of mature oocytes retrieved Embryo quality Pregnancy Implantation rates Total number of days of FSH stimulation Total dose of gonadotropin administered Oestrogen levels Fertilisation rate Number of retrieved oocytes Embryo cleavage rate Live births Miscarriage rates Cancellation rate Incidence of moderate or severe ovarian hyperstimulation syndrome | |
| Notes | The Institutional Review Board approved the protocol, and all participants gave written informed consent before entering into the trial Source of funding not stated Power calculation performed Authors contacted. Trial conducted in Italy, start date March 2004 (unknown end date) Authors could not supply live birth data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to "computerised allocation". Authors have since confirmed this |
| Allocation concealment (selection bias) | Unclear risk | "Computerised allocation" |
| Blinding (performance bias and detection bias) | High risk | Authors confirmed in correspondence that participants and investigators were not blinded; however, outcome assessors and clinicians were blinded. Not a placebo control |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | High risk | Data on live birth were not reported in the paper, even though it was listed as an outcome in the abstract of the paper |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomized, double‐blind placebo‐controlled trial | |
| Participants | Infertile women undergoing IVF/ICSI, 34 women with ICSI cycles and 18 oocyte donation (n = 52) | |
| Interventions | 1. Vitamin D 100.000 IU: 1 tablet per month (n = 26) 2. Placebo (n = 26) Trial duration; 5 consecutive months | |
| Outcomes | Endometrial thickness Number of oocytes retrieved Cancellation rate Number of embryos transferred Implantation rate Clinical pregnancy rate Live birth | |
| Notes | Conducted in Argentina,study dates not reported conference abstract Funding source not reported Author email; [email protected]. Author contacted on the 20th November 2015 regarding risk of bias factors and live birth data. Author replied 14th December 2015 regarding dropouts, miscarriage, adverse effects and live birth. Trial not yet published as a full text | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Fifty two patients were computer randomized" |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Low risk | "Double blind" (placebo control) |
| Incomplete outcome data (attrition bias) | Low risk | "no dropouts" email from author |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Randomised clinical trial | |
| Participants | Infertile women with PCOS (n = 60). Mean age Treatment group: 24.95 ± 3.56, Control groups 25.8 ± 4.61 and 26.9 ± 4.44 respectively Inclusion criteria: oligomenorrhoea/amenorrhoea, hyperandrogenism, polycystic ovaries on transvaginal ultrasound. Exclusion criteria: women with systemic disease, coexisting male factor infertility or abnormal hysterosalpingography. Natural conception | |
| Interventions | 1. Calcium 1000 mg + vitamin D 400 IU (n = 20) This arm is not used in the analysis. 2. Calcium 1000 mg + vitamin D 400 IU + metformin 1500 mg: 1 tablet of each a day (n = 20) 3. Metformin 1500 mg: 1 tablet a day (n = 20) Trial lasted 3 months with a 3‐month follow‐up. | |
| Outcomes | Follicular response Frequency of menstrual cycle Chemical pregnancy Clinical pregnancy No pregnancy occurred in any of the groups | |
| Notes | Trial held in Iran, study ran from February 2004 (unknown end date) Funding source not reported Tried to contact authors regarding allocation concealment and blinding 13th February 2013. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided into 3 groups with the use of a random number table |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts from the trial |
| Selective reporting (reporting bias) | Unclear risk | Reported only chemical pregnancy |
| Other bias | Low risk | No other bias found |
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | Infertile women with PCOS 18 ‐ 40 years old (n = 64) Mean age: 25.1 ± 4.5 vs 25.4 ± 4.9 years, P = 0.85 Inclusion criteria: age between 18 and 40 years with PCOS according to Rotterdam criteria Exclusion criteria: elevated levels of prolactin, thyroid disorder, or Type 2 diabetes and congenital adrenal hyperplasia. In addition, all PCOS women had normal baseline renal function tests, bilirubin, and aminotransferases | |
| Interventions | 1. Selenium 200 ug: 1 tablet a day + metformin 500 mg: which was elevated in a stepwise manner during the first 3 weeks to incorporate the side effects until the participants were taking a total of 1500 mg a day (n = 32) 2. Placebo plus metformin: same dosage as above (n = 32) Treatment was for 8 weeks The trial ran from October 2014 to December 2014 | |
| Outcomes | Pregnancy rates (biochemical) Hormone levels | |
| Notes | Conducted in Iran Trial was supported by an institutional grant. Clinical trial number: IRCT201412295623N33 Contact details: Dr Z Asemi; [email protected]. Email sent 18th October 2016 regarding clinical pregnancy data and block size. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients with PCOS were randomly divided into 2 groups" "Patient allocation and block size were obtained using random number tables". |
| Allocation concealment (selection bias) | Low risk | "At the time of randomization,sequentially numbered, sealed envelopes were opened. Allocation to study group was concealed until the main analyses were completed". |
| Blinding (performance bias and detection bias) | Low risk | Supplements and placebos were in the same form of package and the participants and researcher were not conscious of the content of the pack until the end of trial |
| Incomplete outcome data (attrition bias) | Low risk | Reasons and numbers for dropouts from each group were provided. ITT used in the analysis |
| Selective reporting (reporting bias) | Low risk | None noted |
| Other bias | Low risk | No other bias found |
| Methods | Placebo‐controlled, double‐blind, randomised trial | |
| Participants | Women diagnosed with clomiphene citrate‐resistant PCOS (n = 150) aged 18 ‐ 39 years, undergoing therapy for infertility. Timed intercourse. Mean age Treatment group: 28.9 ± 4.7, Placebo group 28.4 ± 5.7. Inclusion criteria: clomiphene citrate‐resistant, at least 1 patent tube, adequate semen analysis according to WHO guidelines, no hormonal treatment Exclusion criteria: hormonal treatment within 2 months of the study, no participants had taken medication to affect carbohydrate metabolism, hyperprolactinaemia, hypercorticism or thyroid dysfunction | |
| Interventions | 1. NAC 1.2 g: 1 tablet a day + clomiphene citrate 100 mg: 1 tablet a day for 5 days, starting at day 3 of the cycle for 1 cycle (n = 75) 2. Placebo + clomiphene citrate 100 mg: 1 tablet a day (n = 75) | |
| Outcomes | Ovulation rate Ongoing pregnancy rate, however only pregnancy rate reported Number of follicles of 18 mm Hormone levels Endometrial thickness Ovarian hyperstimulation syndrome (OHSS) Multiple gestations | |
| Notes | Single‐centre university‐based hospital and private infertility practice in Eygpt Trial conducted from March 2002 to November 2003. Informed consent. No mention of funding source Data for miscarriage and multiple pregnancy not in meta‐analysis, as they appear to skew data because of the fact that there were no pregnancies or live birth events in the control group, so no miscarriages. The intervention appears worse in terms of miscarriage when it is simply due to the intervention group having pregnancy and live birth. Emailed author 7th September 2012 regarding the pregnancy rate in the control group and asking for live birth data. Author replied on 10.09.12, confirming that there were no pregnancies in the control group and no live birth data Endometrial thickness; significant difference in favour of the treatment group vs control; see conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to receive CC and either NAC or placebo". Method not described |
| Allocation concealment (selection bias) | Low risk | "Allocation was done by a third party (nurse)". "Using sealed envelopes". |
| Blinding (performance bias and detection bias) | Low risk | "The NAC and placebo were supplied in identical sachets. The patients and the physician monitoring the cycles were blinded to the identity of each medication". |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts were reported |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Prospective, randomised clinical trial | |
| Participants | Women with low oocyte quality detected in previous IVF cycles (n = 65). Aged 35 ‐ 42 years. Mean age Treatment group 37.81 ± 2.61, Placebo 38.09 ± 1.97 IVF | |
| Interventions | 1. Myo‐inositol 2 g + folic acid 200 mg + melatonin 3 mg: each tablet twice a day (n = 32) 2. Myo‐inositol 2 g + folic acid 200 mg: each tablet twice a day (n = 33) Administered continuously from the day of GnRH administration | |
| Outcomes | Embryo quality Pregnancy rate, biochemical and clinical Total number of oocytes retrieved (immature and mature oocytes) Fertilisation rate per number of retrieved oocytes and embryo cleavage rate Spontaneous abortion defined as a pregnancy loss from 5 ‐ 12 weeks pregnancy | |
| Notes | Setting: Messina, Italy, study dates not reported All participants gave written informed consent for the procedure, and the study was approved by the local ethics committee. Source of funding unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised: "According to a randomisation table, patients were assigned to receive either 2 g..." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Data given for all outcomes reported in the text |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled study | |
| Participants | Women aged < 40 years with PCOS undergoing ICSI (n = 54). Mean age Group 1: 36.8; Group 2: 36.9; Group 3 36.7; Group 4: 37.0; Placebo: 36.9 Exclusion criteria: Women with insulin resistance and/or hyperglycaemia | |
| Interventions | 1. D‐chiro‐inositiol 300 mg: 1 tablet a day (n = 10) 2. D‐chiro‐inositol 600 mg: 1 tablet a day (n = 11) 3. D‐chiro‐inositol 1200 mg: 1 tablet a day (n = 10) 4. D‐chiro‐inositol 2400 mg: 1 tablet a day (n = 12) 5. Placebo (n = 11) Treatment given 8 weeks before ICSI | |
| Outcomes | Number of oocytes retrieved Total rFSH 17B‐E2 levels on hCG administration Stimulation days Number of cycles cancelled | |
| Notes | Conducted in Italy, study dates not reported Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization procedure was performed using a computer‐based program" |
| Allocation concealment (selection bias) | Unclear risk | Unknown allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised, controlled, double‐blind trial | |
| Participants | Women with PCOS attending IVF clinic (n = 46); Mean age Treatment group: 27; Control group 28 Exclusion criteria: infertility factors apart from anovulation, other pathologies, hormone consumption for less than 2 months before enrolment. | |
| Interventions | 1. NAC 200 mg: 1 tablet 3 times a day (n = 23) 2. Placebo (n = 23) 7 women lost to follow‐up. Reasons described were intolerance to the smell of medications and blood samples inappropriate for the study Treatment 6 weeks' duration Follow‐up 6 weeks | |
| Outcomes | Ovulation Weight Endocrine Metabolic and hormonal factors | |
| Notes | Trial carried out in Teheran, Iran, from February 2007 and February 2008 Informed consent. Power calculation. Ethics approved. Funding source stated, "research is supported by Shahid Beheshti Medical University" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "In order to minimise the effects of confounding factors through a randomised method". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | Low risk | "Medication was provided to patients by a midwife. Both patient and physician were blinded to the type of treatment regimen". |
| Incomplete outcome data (attrition bias) | Unclear risk | 14 dropouts; 7 from each arm, reasons generally described but not for each woman |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the text were reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | Infertile women with PCOS undergoing timed intercourse (n = 180) Women were aged 20 – 35 years Inclusion criteria: infertility duration < 10 years, BMI < 35 kg/m2, both participant tubes confirmed by hysterosalpingography or laparoscopy and with partner’s normal semen analysis results Exclusion criteria: thyroid dysfunction, hyperprolactinaemia, hypercorticism, history of large ovarian cyst formation (> 6 cm), history of visual disturbance caused by clomiphene citrate and finally history of asthma and or allergy to medications. Women who had received any hormonal medications (except progesterone for withdrawal bleeding) or medications affecting glucose metabolism for at least 3 months before the study were also excluded; also no sexual dysfunction | |
| Interventions | 1. NAC 1.2 g: 1 sachet a day (divided into 2 doses per day) + clomiphene citrate 100 mg: 1 tablet a day (n = 90) 2. Placebo + clomiphene citrate 100 mg/day divided and given in 2 doses a day given for 5 days starting at day 3 of the cycle. Timed intercourse occurred after an hCG trigger (n = 90) 8 women in the 1st group and 4 in the 2nd group left the study due to inappropriate drug intake or discontinued cycle; also 1 woman dropped out of the placebo group due to developing an ovarian cyst | |
| Outcomes | Number of follicles > 18 mm Endometrial thickness Ovulation rates Pregnancy rates Adverse effects including multiple pregnancy Ovarian hyperstimulation syndrome | |
| Notes | Trial carried out in the Shahid Beheshti University of Medical Sciences IVF Center, Taleghani Hospital, Iran between January 2008 and December 2009 Informed consent Power calculation Ethics approved Reprint request to: Dr Azadeh Akbari Sene, IVF Center, Infertility and Reproductive Health Research Center, Taleghani Hospital, Velenjak st, Tehran, Iran. Email: [email protected]. RM‐P sent an email 12th December 2015. No reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Then patients were randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Low risk | Double‐blind. In the 2nd group in addition to 100 mg daily CC, participants received a placebo (oral rehydration solution powder) from day 3 until day 7. ORS powder was given to the participants in the same packets as NAC |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts were explained |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the text were reported |
| Other bias | Low risk | No other bias found |
| Methods | Randomised trial | |
| Participants | Infertile women diagnosed with insulin‐resistant PCOS (n = 102) 18 women were scheduled for ovulation induction and 84 for IVF/ICSI Mean age: 28.8 ± 0.4 years | |
| Interventions | 1. Folic acid 0.4 mg a day (n = 23) 2. Metformin 1700 mg a day (2 divided doses of 850 mg tablets) + folic acid 0.4 mg: 1 tablet a day (n = 28) 3. Vitamin B complex (50 mg B6, 400 ug folic acid, 500 ug B12, 1 g trimethylglycine and 6 mg pyridoxal‐5‐phosphate): 1 tablet a day (n = 24) 4. Metformin 1700 mg a day (2 divided doses of 850 mg tablets) + vitamin B complex: 1 tablet a day (n = 27) Women were recruited over 14 months and outcomes were measured over 3 cycles All groups were given folic acid | |
| Outcomes | Homocysteine levels Cumulative clinical pregnancy rate over 3 cycles Ongoing pregnancy rate | |
| Notes | Israel Tel Aviv, study dates not reported Dr Morey Schachter [email protected]. Email sent 8th December 2015, no reply Laboratory costs were partially funded by a company producing vitamins and supplements | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "These 102 patients were randomized before treatment, and after giving informed consent, assigned to one of four groups by opening sealed envelopes containing computer generated random assignation numbers" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing computer‐generated random assignation numbers" |
| Blinding (performance bias and detection bias) | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Prospective randomised trial | |
| Participants | Euglycemic (normal levels of serum glucose) women with PCOS undergoing ovulation induction for ICSI (n = 84). Mean age Treatment group: 35.5 ± 3.2; D‐chiro‐inositol group: 36.5 ± 2.5 Inclusion criteria: women who have attended IVF department for > 12 months, younger than 40 years, diagnosed with PCOS according to the Rotterdam criteria Exclusion criteria: women with hyperglycaemia or insulin resistance, or both | |
| Interventions | 1. Myo‐inositol 2 g: 1 tablet twice a day (n = 43) 2. D‐chiro‐inositol (member of vitamin B family) 0.6 g: 1 tablet twice a day (n = 41) Twice a day for 8 weeks | |
| Outcomes | Total number of oocytes Number of mature oocytes Embryo quality Pregnancy, divided into biochemical and clinical pregnancies Miscarriage | |
| Notes | Trial held in Messina, Italy, study dates not reported Funding source not mentioned. Emailed and posted letter to Dr Unfer 28th November 2011, requesting information on risk of bias. A colleague of the author, Gianfranco Carlomagno ([email protected]), replied 5th December 2011 with risk of bias information and offering to find data on live birth. Emailed back asking for live birth data 12th December 2011. Emailed again 10th August 2012 asking about live birth data. Author replied 16th August 2012. Live birth data added to the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to receive..." In email correspondence, author replied "The randomisation was computer based". |
| Allocation concealment (selection bias) | Unclear risk | In email correspondence, author replied, "the treatments were provided in opaque envelopes identified by A or B". |
| Blinding (performance bias and detection bias) | Unclear risk | In email correspondence, author replied, "both patients and clinicians were blinded". However not placebo‐controlled |
| Incomplete outcome data (attrition bias) | Low risk | All losses were accounted for: 4 women in the control group had cancelled cycles and nil in the treatment group |
| Selective reporting (reporting bias) | Low risk | Key outcomes reported, including live birth. Protocol available |
| Other bias | Low risk | No other bias found |
| Methods | Double‐blind randomised controlled trial | |
| Participants | Infertile women aged > 40 years undergoing IVF (n = 358) | |
| Interventions | 1. Melatonin 5 mg: 1 tablet a day (n = 178) 2. No treatment (n = 180) Trial ran from July 2009 to December 2013 | |
| Outcomes | Oocyte maturity Oxidative stress Antioxidative capacity Progesterone concentration in follicular fluid Embryo grade | |
| Notes | Conducted in Italy, trial ran from July 2009 to Decenber 2013 Trial funded by pharmaceutical company Trial registration no: NCT01540747 Email sent to Dr Pacchiarotti regarding the methods of this trial. Dr Pacchiarotti replied 20th March 2017 giving some methods information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blind but control is no treatment |
| Incomplete outcome data (attrition bias) | Unclear risk | Unknown |
| Selective reporting (reporting bias) | Low risk | No known selective reporting |
| Other bias | Low risk | No other bias found |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Infertile women (n = 93). Mean age Treatment group: 35.4; Placebo group 34.8 Inclusion criteria: women aged 24 ‐ 42 years, unsuccessfully trying to conceive for 6 to 36 months Exclusion criteria: any woman taking any pharmacological treatment for infertility for 2 months before start of the trial. | |
| Interventions | 1. Fertility blend: capsules containing chaste berry, green tea amino acid, L‐arginine, vitamins E, B6 and B12 and folate, iron, magnesium, zinc and selenium. 3 capsules a day for 3 menstrual cycles (n = 53) 2. Placebo (n = 40) Duration of treatment: 3 menstrual cycles, then women received an additional 3 months of open‐label fertility blend after completion of the study, with monitoring only of pregnancy and side effects Duration of trial: 4 months | |
| Outcomes | Basal body temperature changes Length of menstrual cycle Pregnancy rates Side effects Mid‐luteal phase progesterone levels Miscarriage | |
| Notes | No power calculation performed Institutional review board approval was obtained for the trial Conducted in the USA, study dates not reported Funding stated: David Sen Lin Foundation No loss to follow‐up. 14 pregnant in treatment group in first 3 months, then 17 in 6 months, but the second 3 months was unblinded; therefore, only first 3 months' data used. Not all women in the trial received the extra 3 months of treatment or placebo Miscarriage and side effect data cannot be used as they include data from the later 3 months when not all women received treatment or placebo in this phase. Tried to contact author 25th November 2009 with email, mail and fax, with no reply. Tried to contact author again regarding live birth, no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; mechanism not stated. "Fertilty Blend5 (FB), administered in a randomised, double‐blind, placebo‐controlled fashion". |
| Allocation concealment (selection bias) | Unclear risk | Mechanism not stated. Authors contacted May 2010 regarding this |
| Blinding (performance bias and detection bias) | Low risk | Stated as being double‐blinded, no clear explanation. Authors contacted regarding this. Placebo control |
| Incomplete outcome data (attrition bias) | Low risk | No withdrawals or dropouts |
| Selective reporting (reporting bias) | High risk | Data on miscarriage and side effects cannot be used in analysis, as these data were combined with the extra open‐label 3‐month data. Not all women received treatment or placebo in this phase |
| Other bias | Low risk | No other bias found |
| Methods | Randomised controlled trial | |
| Participants | Infertile couples with unexplained infertility seeking ICSI/IVF treatment following at least 3 failed previous IUI cycles (n = 218). Mean age Treatment group: 30.9 years; Control group: 30.6 Inclusion criteria: women aged < 40 years with normal ovulatory cycles, normal baseline; FSH 12 IU/l, thyroid‐stimulating hormone, prolactin levels, tubal patency at hysterosalpingography, normal transvaginal ultrasound scan, presence of both ovaries and normal findings at laparoscopy. All male partners had a normal semen analysis by WHO criteria Exclusion criteria: Couples who had received any form of vitamin supplementation for a period of 3 months before start of treatment | |
| Interventions | Women in both groups received a daily dose of 2.5 mg of folic acid. 1. OCTATRON ® NERHADOU INTERNATIONAL (composition; vitamin A 3000 IU; d‐alpha tocopheryl acid; (vitamin E) 15 IU; ascorbic acid (vitamin C) 90 mg; Zinc (amino acid‐chelated) 11 mg; molybdenum (amino acid chelated) 45 μg; selenium (amino acid chelated) 55 μg, biotin 10 μg and mixed bioflavonoid 100 mg): 1 capsule a day (n= 112) 2. Folic acid 2.5 mg: 1 tablet a day (n = 106) Treatment was for 2½ months 7 women lost from each arm with explanation | |
| Outcomes | The primary outcome was the number of mature oocytes Secondary outcomes were clinical pregnancy rate, defined as appearance of intrauterine gestational sac with fetal heart pulsation at 7 weeks Fertilisation rate Number of embryos transferred and cryopreserved Multiple pregnancy rate Duration of stimulation Amount of FSH | |
| Notes | Cairo Egypt Trial ran from February 2011 to March 2013 "On pregnancy confirmation, both groups received antioxidant and folic acid supplementation during the first trimester with follow‐up in accordance with this canter ’s policy. Participants ’ compliance with treatment, that is, the intake of supplements was confirmed and recorded on each visit by the caring physicians". This paper is the published version of Aboulfoutouh 2011 in first version of the review Email and letter sent to authors 9th August 2012asking about types of antioxidants used and ITT in the pregnancy outcome. Authors replied with data information. Participants were followed up only to clinical pregnancy, so no live birth data are provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We developed computer generated list for randomization" from an email received 12th December 2015 |
| Allocation concealment (selection bias) | Low risk | "used closed opaque envelops for concealment by third party nurse" from an email received 12th December 2015 |
| Blinding (performance bias and detection bias) | High risk | No blinding, control is no treatment |
| Incomplete outcome data (attrition bias) | Low risk | Attrition explained |
| Selective reporting (reporting bias) | Low risk | Outcomes reported. Protocol available |
| Other bias | Low risk | No other bias found |
17B‐E2: 17B estradiol
ASRM: American Society for Reproductive Medicine
BMI: body mass index
CC: clomiphene citrate
ESHRE: European Society for Human Reproduction and Embryology
FSH: follicle stimulating hormone
GnRH: gonadotropin releasing hormone
hCG: human chorionic gonadotropin
HSG: hysterosalpingography
ICSI: intracytoplasmic sperm injection
ITT: intention‐to‐treat
IVF: in vitro fertilisation
MDA: malondialdehyde
NAC: N‐acetylcysteine
OC: oral contraceptive
OHSS: ovarian hyperstimulation syndrome
PCOS: poly cystic ovarian syndrome
rFSH: recombinant follicle stimulating hormone
SD: standard deviation
WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Population were vitamin D‐deficient | |
| Not a randomised study | |
| Non‐randomised trial. "Forty‐two infertile PCOS were divided into three groups". | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review. | |
| Vitamin D not given orally but by injection into the muscle | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| Not an RCT. "patients for the supplemented and control sets were selected by the case‐control method according to their age and smoking or non‐smoking habits." | |
| Participants were fertile women with infertile male partners. | |
| Interventions N‐acetyl‐cysteine versus metformin | |
| The intervention versus control used in this trial was metformin versus placebo | |
| 83% of the women enrolled were vitamin D‐deficient | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is inappropriate for inclusion in this review | |
| Interventions N‐acetyl‐cysteine plus clomiphene citrate versus metformin plus clomiphene citrate | |
| Enrolled women who attended infertility clinic due to male factor issues | |
| Described as randomised, but authors confirmed the process of allocation as "alternative treatments". Additionally, 28/46 in the placebo arm withdrew because of travel difficulties and movement out of the study area. No withdrawals from the treatment arm were reported. There was no Intention‐to‐treat. | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| Wrong comparison; Inositol vs metformin | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review. | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with endometriosis, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| Participants were not subfertile women; they were partners of subfertile men | |
| Not randomised. Attempted to contact authors regarding sequence allocation via email 10 November 2011 | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| Lead investigator confirmed (May 2010). Stated that the trial was abandoned before recruitment because of lack of funding | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| A secondary analysis of an RCT measuring ovulation induction (OI) outcomes in women with polycystic ovary syndrome (PCOS). | |
| Not a randomised controlled trial | |
| Interventions myo‐inositol plus folic acid versus clomiphene citrate | |
| Inappropriate population | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| Interventions myo‐inositol plus folic acid versus metformin | |
| A secondary analysis of an RCT on the cost‐effectiveness of fast track to IVF | |
| Different doses of clomiphene in each arm, i.e. L‐carnitine 3 gm plus clomiphene 100 mg (n = 85) versus clomiphene 150 mg (n = 85) | |
| The population included here were women with endometriosis, and the trial aimed to show differences in inflammatory markers. These women were not attending a fertility clinic | |
| Population is Vitamin D‐deficient | |
| A quasi‐randomised trial. "Patients were divided into two groups". Email sent asking about randomisation but undeliverable. Letter sent to University of Texas 12 January 2012. Letter returned to sender 17 February 2012. | |
| Participants were randomly assigned to different stimulation protocols and not to folic acid. All participants took folic acid | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review | |
| This trial included women with PCOS, but they were not intending to become pregnant. Therefore, the population is not suitable for inclusion in this review |
PCOS: poly cystic ovarian syndrome
RCT: random controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Nutritional supplement for women With polycystic ovary syndrome or subfertilty |
| Methods | Randomised, parallel‐group, placebo‐controlled trial Method of allocation concealment: pre‐numbered or coded identical Containers blinding and masking: participant‐ and investigator‐blinded |
| Participants | Inclusion criteria: Women between 18 and 38 years of age with PCOS Presence of any 2 of the following parameters: (Roterdam criteria 2003)
OR
|
| Interventions | Intervention 1: multiple micronutrients supplementation: 1 Formula A tablet + 1 Formula B tablet together after main meal twice a day for 4 months |
| Outcomes | Improvement in overall status of PCOS or infertility.
Improvement in different parameters defining the status of PCOS or infertility‐like hormonal levels, insulin resistance, weight and safety of the therapy
|
| Starting date | 31 August 2012 |
| Contact information | Dr Yashwant Mane Dr Yashwant Mane Atharva Infertilty and Test Tube Baby Center Jagir Complex Dwarka, Nasik, India 422011 Nashik, MAHARASHTRA India ph 02532598953 email [email protected] |
| Notes |
| Trial name or title | A pilot double‐blind randomised placebo‐controlled dose–response trial assessing the effects of melatonin on infertility treatment (MIART): study protocol |
| Methods | Pilot phase II cross‐over double‐blinded randomised placebo‐controlled dose–response trial Each treatment arm will be randomly allocated a letter (A, B, C or D) by way of opaque sealed envelope Randomisation will be computerised and participants will be randomised at a ratio of 1:1:1:1 to 1 of |
| Participants | We plan to recruit a total of 160 infertile women undergoing IVF/ICSI INCLUSION CRITERIA; 1. Undergoing first cycle of IVF or ICSI; EXCLUSION CRITERIA |
| Interventions | 1. Placebo capsule taken twice a day; |
| Outcomes | Primary outcome: Clinical pregnancy rate, defined as the presence of a live pregnancy in the uterine cavity at a transvaginal ultrasound at 6 – 8 weeks’ gestation Secondary outcomes: Live birth, miscarriage, adverse events, melatonin serum levels |
| Starting date | Recruitment will start in July 2014. This will occur over 2 years. Analysis and dissemination will occur after this period of time. The study is expected to be completed by February 2017. |
| Contact information | Dr Shavi Fernando; [email protected] |
| Notes | Participants will be recruited from Monash IVF infertility clinics in Melbourne, Australia over a period of 2 years Ethics and dissemination: Ethical approval has been obtained from Monash Health HREC (Ref:13402B), Monash University HREC (Ref: CF14/523‐2014000181) and Monash Surgical Private Hospital HREC (Ref: 14107). Data analysis, interpretation and conclusions will be presented at national and international conferences and published in peer reviewed journals. |
| Trial name or title | Evaluation of the effects of Calcium‐ Vitamine D supplementary on ovulation and fertility outcomes of patients with poly cystic ovary syndrome referring to Infertility Clinic of Imam Khomeini Hospital in 2011 and 2012 for in vitro fertilization |
| Methods | Randomisation: randomised. Blinding: double‐blind. Placebo: used |
| Participants | Abnormal menstrual cycles (oligomenorrhoea or amenorrhoea); sonographically‐confirmed polycystic ovary; hyperandrogenism |
| Interventions | Intervention 1: In control group, participants do not receive routine administration of calcium‐D combinations Intervention 2: In intervention group, supplementary tablets of 1000 mg calcium combined with 400 IU vitamin D are administered (orally) twice a day for 3 months |
| Outcomes | Pregnancy. Timepoint: 2 and 12 weeks. Method of measurement: sonography and biochemistry |
| Starting date | 21 January 2012 |
| Contact information | Azadeh Mahdian Department of Obstetrics and Gynecology, Vali‐e‐ Asr Hospital, Imam Khomeini Hospital Complex, Keshavarz Blv Tehran Iran, Islamic Republic of |
| Notes |
| Trial name or title | Vitamin D during In Vitro Fertilization (IVF) ‐ a prospective randomised trial delivery |
| Methods | Randomised double‐blind trial |
| Participants | Target sample size: 1000 women older than 18 years of age initiating IVF treatment in Sweden |
| Interventions | Dietary supplementation: ergocalciferol (vitamin D), either high 100,000 U once or low‐dose 500 U once |
| Outcomes | Biochemical pregnancy, live birth, take‐home baby rate, OHSS and pregnancy complication rate (pregnancy, hypertension, SGA, diabetes) |
| Starting date | November 2009 |
| Contact information | Pelle G. Lindqvist Karolinska University Hospital Huddinge ClinicalTrials.gov identifier NCT01019785. |
| Notes | clinicaltrials.gov/ct2/show/NCT01019785?term=NCT01019785&rank=1 |
| Trial name or title | Improving oocyte retrieval using a combined therapy of recombinant follicle‐stimulating hormone (rFSH) and inositol and melatonin |
| Methods | Randomised double‐blinded (participant, investigator) controlled trial. |
| Participants | Women 18 ‐ 39 years undergoing assisted reproductive techniques (ART) because of male infertility BMI 18 ‐ 30 kg/m2 Fewer than 3 prior oocyte retrievals No fertility problems |
| Interventions | Recombinant FSH: 225 IU rFSH Drug: recombinant FSH (rFSH) 225 IU Experimental: recombinant FSH inositol melatonin 225 IU rFSH, 4 g inositol and 3 mg melatonin dietary supplement: rFSH + inositol + melatonin 225 IU rFSH, 4 g inositol, 3 mg melatonin |
| Outcomes | Primary: total number of oocytes, number of clinical pregnancies, live birth rate |
| Starting date | December 2010 |
| Contact information | Vittorio Unfer, MD +39 0640500835 Gianfranco Carlomagno, PhD University of Modena and Reggio Emilia Recruiting Reggio Emilia, Italy, 42100 Contact: Giovanni Battista La Sala, MD +39 0522 296464 Principal investigator: Giovanni Battista La Sala, MD Research Center for Reproductive Medicine Villa Mafalda Recruiting Roma, Italy, 00199 |
| Notes | May not become an included study because all women are fertile, but they have subfertile male partners ClinicalTrials.gov Identifier: NCT01267604. Recruiting. Trial found on clinicaltrials.gov on 7th August 2012 |
| Trial name or title | Effect of resveratrol on metabolic parameters and oocyte quality in PCOS patients (RES‐IVF) |
| Methods | Randomised |
| Participants | Women with PCOS |
| Interventions | Resveratrol versus placebo |
| Outcomes | Implantation Pregnancy rates |
| Starting date | February 2013 |
| Contact information | Israel Ortega, Medical doctor 91 180 2900 |
| Notes | Not yet recruiting. Madrid |
| Trial name or title | Use of antioxidant in endometriotic women to improve intracytoplasmic sperm injection (ICSI) (ROS) Official title: Does antioxidant supplementation to endometriotic women undergoing ICSI alter reactive oxygen species (ROS) levels and affect pregnancy outcome |
| Methods | Randomised, parallel‐assignment, single‐blind (participant) |
| Participants | Women with endometriosis undergoing IVF, 20 ‐ 40 years |
| Interventions | Drug: ascorbate 1000 mg, vitamin E 400, zinc and selenium |
| Outcomes | Pregnancy |
| Starting date | March 2013 |
| Contact information | Olfat Nouh Riad, Assisstant Professor, Cairo University Egyption centre for IVF Maadi, Egypt, 11451 |
| Notes | ClinicalTrials.gov Identifier: NCT02058212 |
| Trial name or title | Lipoic acid supplementation in IVF |
| Methods | Allocation: randomised |
| Participants | Women infertility ‐ 30 to 50 years |
| Interventions | Dietary supplement: oral lipoic acid|drug: vaginal progesterone |
| Outcomes | Number of implants per cycle Number of biochemical pregnancies per group Number of clinical pregnancies per group Number of live birth per group Number of miscarriage per group |
| Starting date | 2015 |
| Contact information | Lo.Li.Pharma s.r.l |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 8 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.45, 3.12] |
| Analysis 1.1 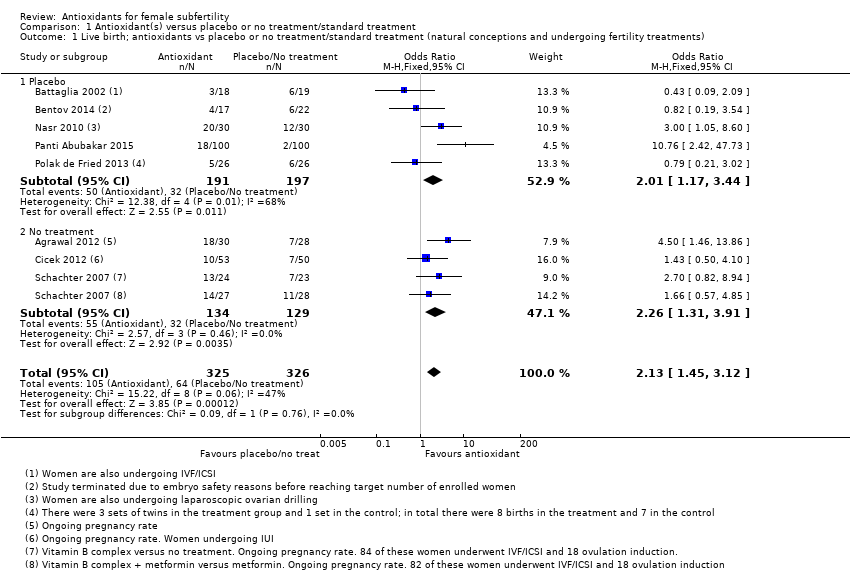 Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments). | ||||
| 1.1 Placebo | 5 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.17, 3.44] |
| 1.2 No treatment | 3 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.31, 3.91] |
| 2 Live birth; type of antioxidant Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 2 Live birth; type of antioxidant. | ||||
| 2.1 N‐acetyl‐cysteine | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.05, 8.60] |
| 2.2 L‐arginine | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.09] |
| 2.3 CoQ10 | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.19, 3.54] |
| 2.4 Vitamin D | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 3.02] |
| 2.5 Vitamin B complex | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.93, 4.57] |
| 2.6 Combined antioxidants | 2 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.76 [2.79, 16.41] |
| 2.7 Vitamin E | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 3 Live birth; indications for subfertility Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 3 Live birth; indications for subfertility. | ||||
| 3.1 Polycystic ovary syndrome | 3 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.90, 5.86] |
| 3.2 Tubal subfertility | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.09] |
| 3.3 Varying indications | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.46, 13.86] |
| 3.4 Unexplained subfertility | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 4 Live birth; IVF/ICSI Show forest plot | 4 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| Analysis 1.4  Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 4 Live birth; IVF/ICSI. | ||||
| 5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 26 | 4271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.31, 1.76] |
| Analysis 1.5 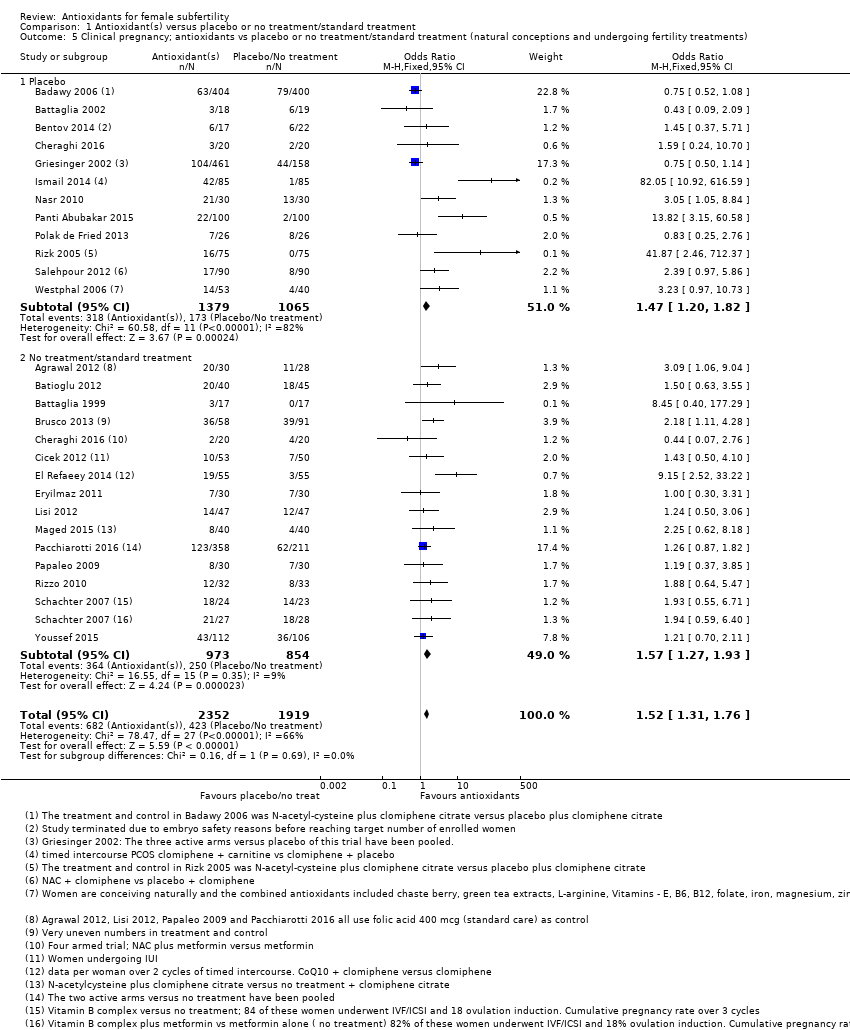 Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments). | ||||
| 5.1 Placebo | 12 | 2444 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.82] |
| 5.2 No treatment/standard treatment | 15 | 1827 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.93] |
| 6 Clinical pregnancy; type of antioxidant Show forest plot | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 6 Clinical pregnancy; type of antioxidant. | ||||
| 6.1 N‐acetyl‐cysteine | 6 | 1354 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.63] |
| 6.2 Combined antioxidants | 4 | 569 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.51, 3.43] |
| 6.3 Melatonin | 4 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.91, 1.83] |
| 6.4 Vitamin E | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 6.5 Ascorbic acid | 1 | 619 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.14] |
| 6.6 L‐arginine | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.46] |
| 6.7 Myo‐inositol plus folic acid | 4 | 694 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.98, 1.86] |
| 6.8 CoQ10 | 2 | 149 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.28 [1.79, 10.26] |
| 6.9 L‐carnitine | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 82.05 [10.92, 616.59] |
| 6.10 Vitamin D | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.76] |
| 6.11 Vitamin B complex | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.82, 4.58] |
| 6.12 Myo‐inositol plus melatonin plus folic acid | 1 | 389 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.90, 2.12] |
| 7 Clinical pregnancy; indications for subfertility Show forest plot | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 7 Clinical pregnancy; indications for subfertility. | ||||
| 7.1 Polycystic ovary syndrome | 11 | 1721 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [2.06, 3.36] |
| 7.2 Unexplained | 3 | 967 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.14] |
| 7.3 Tubal subfertility | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.46] |
| 7.4 Varying indications | 5 | 1004 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.94, 1.71] |
| 7.5 Poor responders | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.64, 5.47] |
| 8 Clinical pregnancy; IVF/ICSI Show forest plot | 15 | 2263 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.43] |
| Analysis 1.8 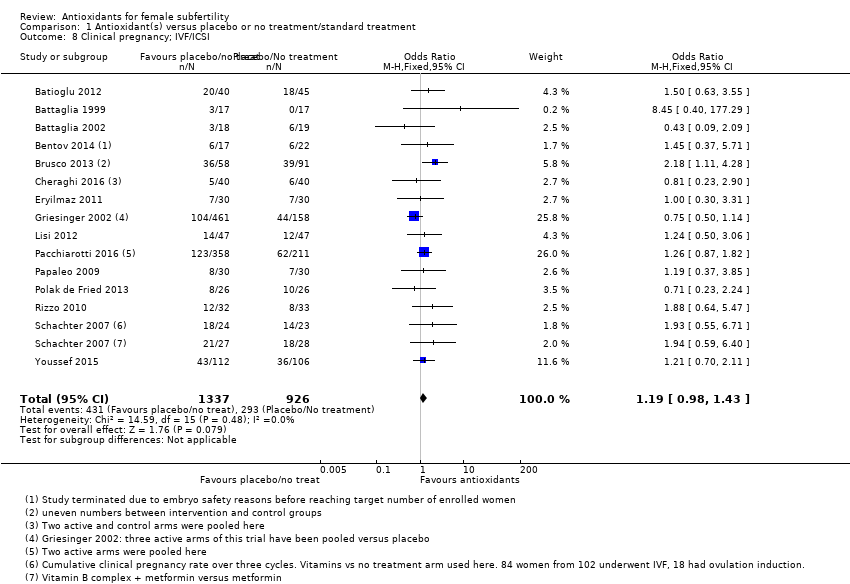 Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 8 Clinical pregnancy; IVF/ICSI. | ||||
| 9 Adverse events Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 9 Adverse events. | ||||
| 9.1 Miscarriage | 18 | 2834 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.08] |
| 9.2 Multiple pregnancy | 8 | 2163 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.38] |
| 9.3 Gastrointestinal disturbances | 3 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.47, 5.10] |
| 9.4 Ectopic pregnancy | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.11, 74.13] |
| 9.5 Headache | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; type of antioxidant (natural conceptions and undergoing fertility treatments) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Head‐to‐head antioxidants, Outcome 1 Live birth; type of antioxidant (natural conceptions and undergoing fertility treatments). | ||||
| 1.1 Myo‐Inositol versus d‐chiro‐inositol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy; type of antioxidant (natural conceptions and undergoing fertility treatments) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Head‐to‐head antioxidants, Outcome 2 Clinical pregnancy; type of antioxidant (natural conceptions and undergoing fertility treatments). | ||||
| 2.1 Myo‐Inositol versus d‐chiro‐inositol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Head‐to‐head antioxidants, Outcome 3 Adverse events. | ||||
| 3.1 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.68, 3.50] |
| Analysis 3.1 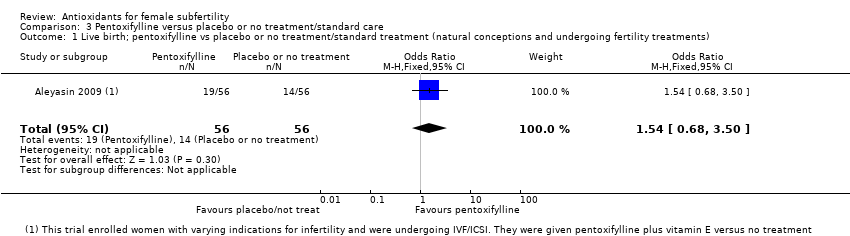 Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 1 Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments). | ||||
| 2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.20, 3.56] |
| Analysis 3.2  Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments). | ||||
| 2.1 Placebo | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 2.2 No treatment | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 3 Clinical pregnancy; type of antioxidant Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 3 Clinical pregnancy; type of antioxidant. | ||||
| 3.1 Pentoxifylline | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 3.2 Pentoxifylline plus vitamin E | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 4 Clinical pregnancy; indications for subfertility Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.4  Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 4 Clinical pregnancy; indications for subfertility. | ||||
| 4.1 Endometriosis | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 4.2 Varying indications | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 5 Clinical pregnancy; IVF/ICSI Show forest plot | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| Analysis 3.5 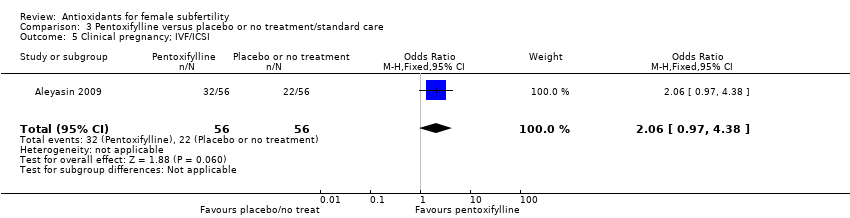 Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 5 Clinical pregnancy; IVF/ICSI. | ||||
| 6 Adverse events Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.6  Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 6 Adverse events. | ||||
| 6.1 Miscarriage | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.90] |
| 6.2 Multiple pregnancy | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.20, 3.09] |
| 6.3 Ectopic pregnancy | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.18, 23.13] |

Study flow diagram.
Methodological risk of bias summary: review authors' judgements about each methodological bias item for each included study.

Methodological risk of bias graph: review authors' judgements about each methodological bias item presented as percentages across all included trials.

Funnel plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).

Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).

Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
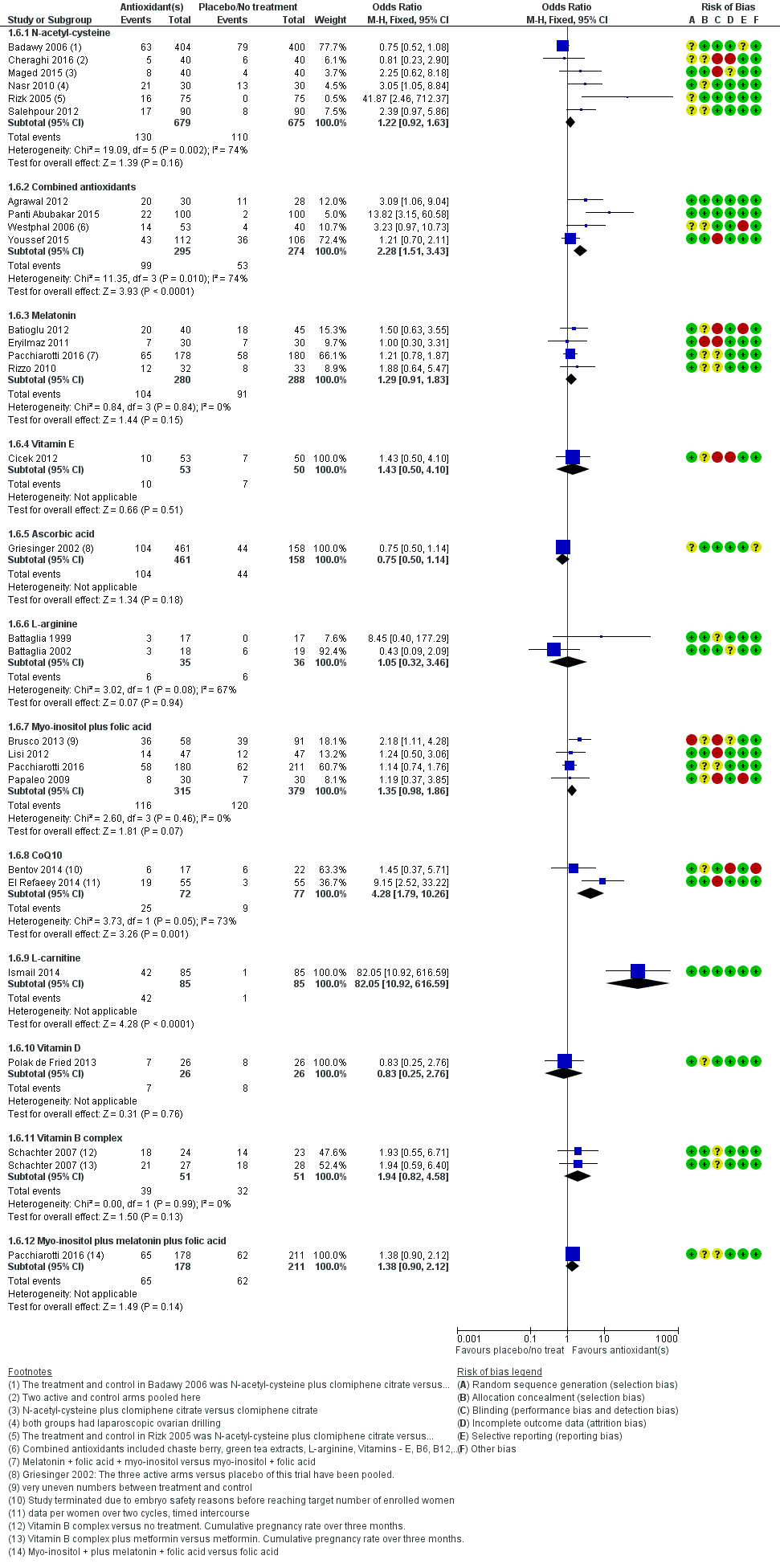
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.6 Clinical pregnancy; type of antioxidant.
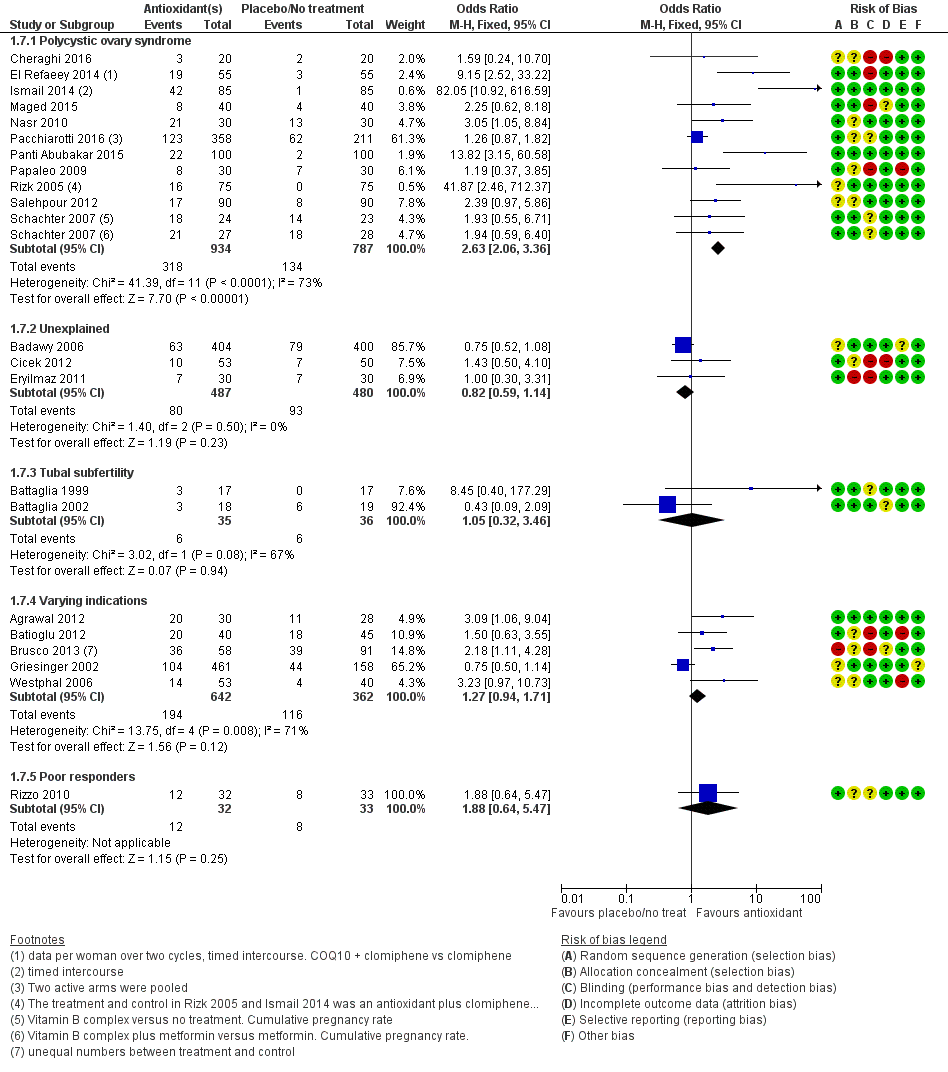
Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.7 Clinical pregnancy; indications for subfertility.

Forest plot of comparison: 1 Antioxidant(s) versus placebo or no treatment/standard treatment, outcome: 1.9 Adverse events.

Forest plot of comparison: 3 Pentoxifylline versus placebo or no treatment/standard care, outcome: 3.2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
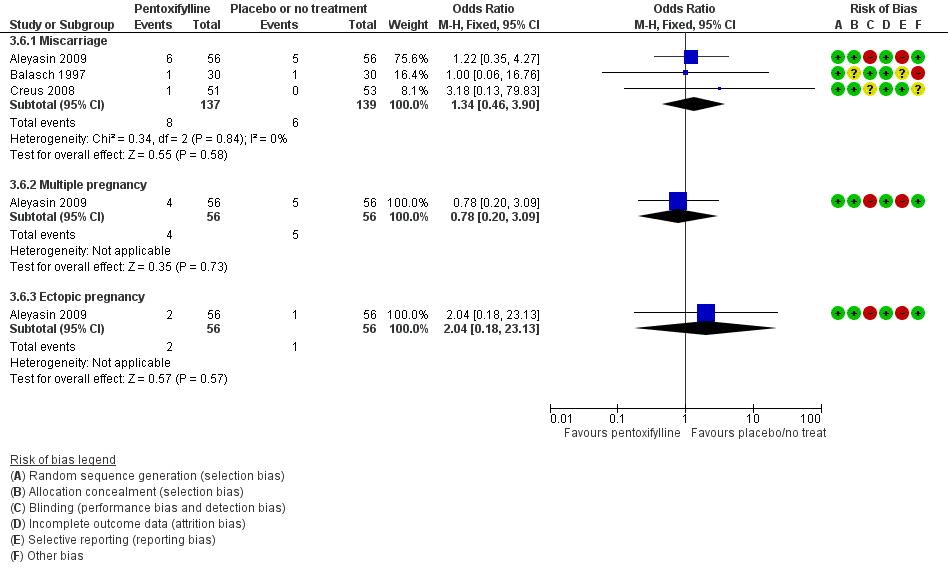
Forest plot of comparison: 3 Pentoxifylline versus placebo or no treatment/standard care, outcome: 3.6 Adverse events.

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 2 Live birth; type of antioxidant.

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 3 Live birth; indications for subfertility.

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 4 Live birth; IVF/ICSI.
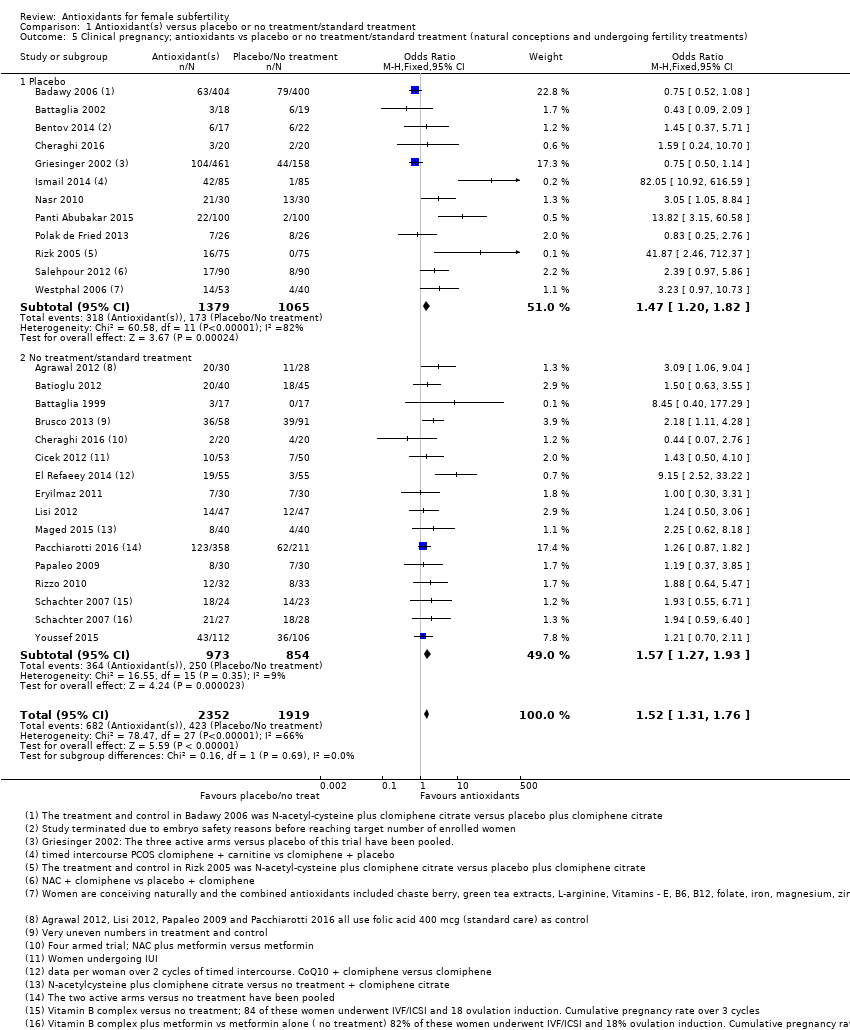
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).
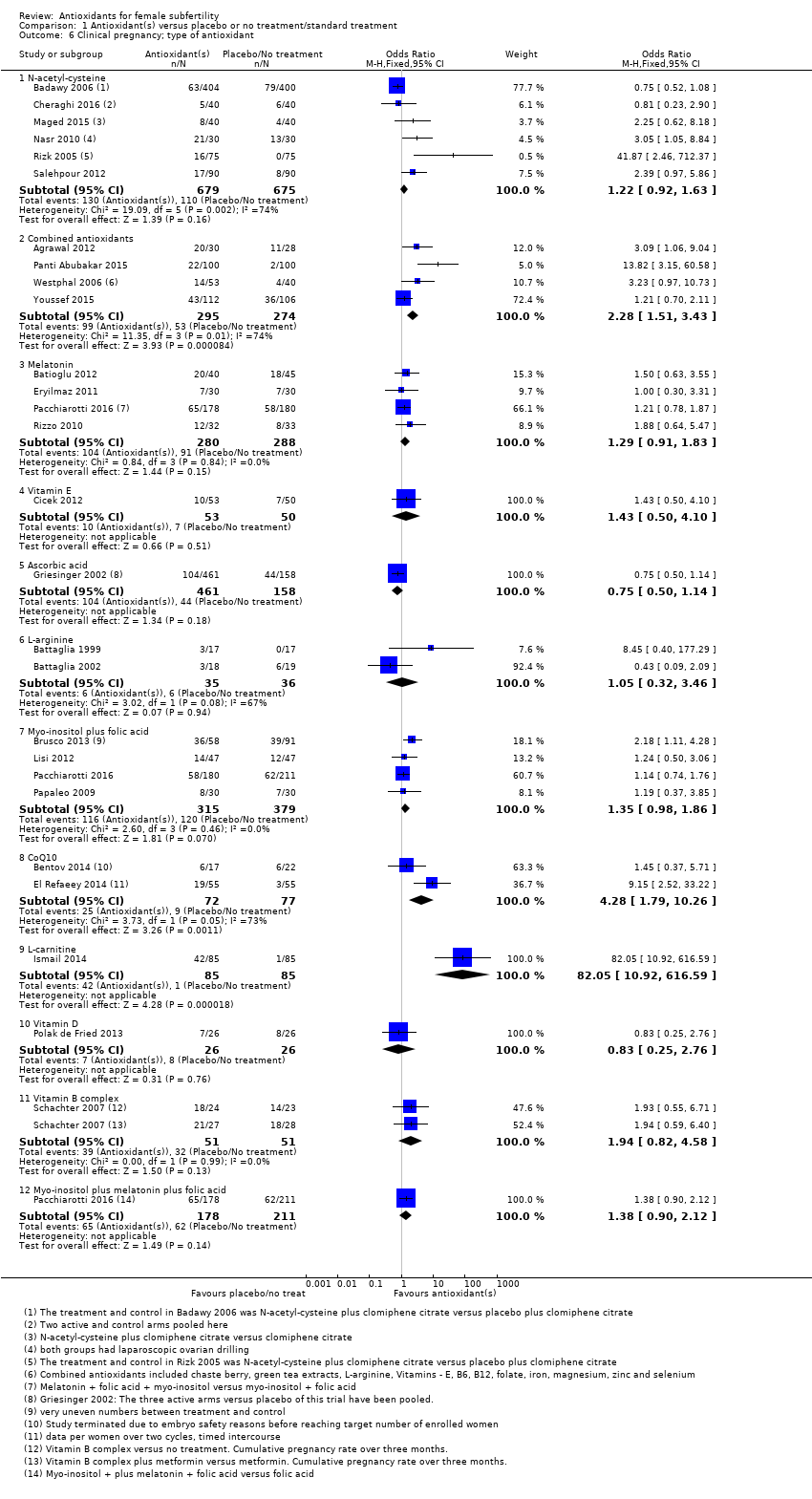
Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 6 Clinical pregnancy; type of antioxidant.

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 7 Clinical pregnancy; indications for subfertility.

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 8 Clinical pregnancy; IVF/ICSI.

Comparison 1 Antioxidant(s) versus placebo or no treatment/standard treatment, Outcome 9 Adverse events.

Comparison 2 Head‐to‐head antioxidants, Outcome 1 Live birth; type of antioxidant (natural conceptions and undergoing fertility treatments).
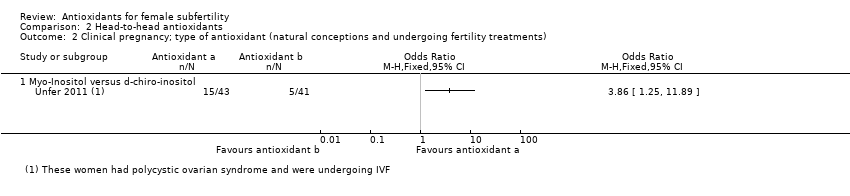
Comparison 2 Head‐to‐head antioxidants, Outcome 2 Clinical pregnancy; type of antioxidant (natural conceptions and undergoing fertility treatments).

Comparison 2 Head‐to‐head antioxidants, Outcome 3 Adverse events.
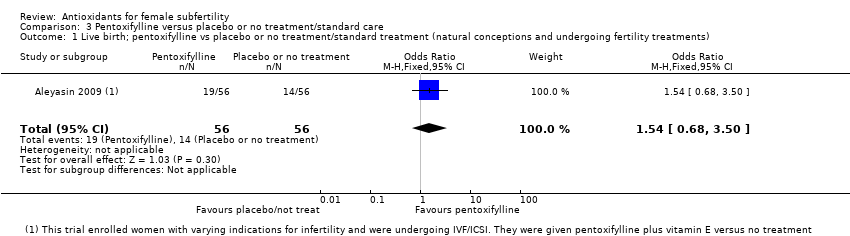
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 1 Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).

Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments).

Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 3 Clinical pregnancy; type of antioxidant.

Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 4 Clinical pregnancy; indications for subfertility.

Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 5 Clinical pregnancy; IVF/ICSI.
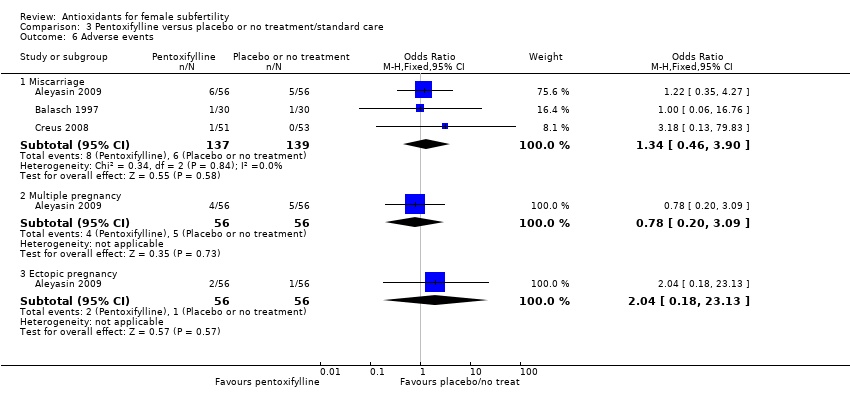
Comparison 3 Pentoxifylline versus placebo or no treatment/standard care, Outcome 6 Adverse events.
| Antioxidant(s) compared to placebo or no treatment/standard treatment for female subfertility | ||||||
| Patient or population: subfertile women who had been referred to a fertility clinic and might or might not be undergoing assisted reproductive techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment/standard treatment | Risk with Antioxidant(s) | |||||
| Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 196 per 1,000 | 342 per 1,000 | OR 2.13 | 651 | ⊕⊝⊝⊝ | |
| Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 220 per 1,000 | 301 per 1,000 | OR 1.52 | 4271 | ⊕⊝⊝⊝ | |
| Adverse events ‐ Miscarriage | 68 per 1,000 | 55 per 1,000 | OR 0.79 | 2834 | ⊕⊝⊝⊝ | |
| Adverse events ‐ Multiple pregnancy | 80 per 1,000 | 80 per 1,000 | OR 1.00 | 2163 | ⊕⊝⊝⊝ | |
| Adverse events ‐ Gastrointestinal disturbances | 24 per 1,000 | 37 per 1,000 | OR 1.55 | 343 | ⊕⊝⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to very serious risk of bias; at high risk of bias in two domains. 2Downgraded one level due to serious imprecision; the event rate is low (< 300). 3Downgraded two levels due to very serious inconsistency (I2 = 81%) with differing directions of effect. 4Downgraded two levels due to very serious imprecision; the event rate is very low (n = 11). 5In practice, full downgrading not possible as evidence already graded as very low quality. | ||||||
| Pentoxifylline compared to placebo or no treatment/standard care for female subfertility | ||||||
| Patient or population: subfertile women who had been referred to a fertility clinic and might or might not be undergoing assisted reproductive techniques | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with placebo or no treatment/standard care | Risk with Pentoxifylline | |||||
| Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 250 per 1,000 | 339 per 1,000 | OR 1.54 | 112 | ⊕⊝⊝⊝ | |
| Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) | 245 per 1,000 | 401 per 1,000 | OR 2.07 | 276 | ⊕⊝⊝⊝ | |
| Adverse events ‐ Miscarriage | 43 per 1,000 | 57 per 1,000 | OR 1.34 | 276 | ⊕⊝⊝⊝ | |
| Adverse events ‐ Multiple pregnancy | 89 per 1,000 | 71 per 1,000 (19 to 233) | OR 0.78 (0.20 to 3.09) | 112 | ⊕⊝⊝⊝ | |
| Adverse events ‐ Gastrointestinal disturbances | Not reported in any included study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to questionable applicability: study table states that cause of infertility is male in 51 of 112 participants, although text states that the participants were 112 infertile women. 2Downgraded two levels due to very serious imprecision; the event rate is very low (n = 33 for live birth, n = 14 for miscarriage, n=9 for multiple pregnancy), wide confidence intervals. 3Downgraded two levels due to questionable applicability of one study (see footnote 1) and very serious risk of bias: all studies at unclear or high risk of bias in one or more domains. 4Downgraded one level due to serious imprecision; the event rate is low (n = 87). | ||||||
| Outcome | Data | Notes |
| Clinical pregnancy rate; myo‐inositol + folic acid | 4/23 | Only 42 of the 92 women enrolled in this trial declared a desire to become pregnant |
| Clinical pregnancy rate; folic acid + placebo | 1/19 | ‐ |
| Miscarriage rate; myo‐inositol + folic acid | Miscarriage reported, but unknown whether from treatment or control | 1 miscarriage occurred in the first trimester, but it is unknown from which group |
| Miscarriage rate; folic acid + placebo | Unknown | ‐ |
| Trial | Pregnancy in antioxidant group | Pregnancy in control group |
| 4/16 (myo‐inositol + folic acid) | 5/18 (folic acid) | |
| 17/43 (pentoxifylline) | 16/45 (placebo) | |
| 0/16 (vitamins C + E), at follow‐up over 9 months 3/16 | 0/18 (placebo), at follow‐up over 9 months 2/18 | |
| 9/22 (vitamin D) | 7/22 (placebo) | |
| 6/32 (selenium) | 1/32 (placebo) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 8 | 651 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.45, 3.12] |
| 1.1 Placebo | 5 | 388 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [1.17, 3.44] |
| 1.2 No treatment | 3 | 263 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.31, 3.91] |
| 2 Live birth; type of antioxidant Show forest plot | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 N‐acetyl‐cysteine | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [1.05, 8.60] |
| 2.2 L‐arginine | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.09] |
| 2.3 CoQ10 | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.19, 3.54] |
| 2.4 Vitamin D | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.21, 3.02] |
| 2.5 Vitamin B complex | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.93, 4.57] |
| 2.6 Combined antioxidants | 2 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.76 [2.79, 16.41] |
| 2.7 Vitamin E | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 3 Live birth; indications for subfertility Show forest plot | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Polycystic ovary syndrome | 3 | 362 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.90, 5.86] |
| 3.2 Tubal subfertility | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.09, 2.09] |
| 3.3 Varying indications | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.46, 13.86] |
| 3.4 Unexplained subfertility | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 4 Live birth; IVF/ICSI Show forest plot | 4 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.69, 2.11] |
| 5 Clinical pregnancy; antioxidants vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 26 | 4271 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.31, 1.76] |
| 5.1 Placebo | 12 | 2444 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.20, 1.82] |
| 5.2 No treatment/standard treatment | 15 | 1827 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.93] |
| 6 Clinical pregnancy; type of antioxidant Show forest plot | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 N‐acetyl‐cysteine | 6 | 1354 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.63] |
| 6.2 Combined antioxidants | 4 | 569 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.28 [1.51, 3.43] |
| 6.3 Melatonin | 4 | 568 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.91, 1.83] |
| 6.4 Vitamin E | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.10] |
| 6.5 Ascorbic acid | 1 | 619 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.14] |
| 6.6 L‐arginine | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.46] |
| 6.7 Myo‐inositol plus folic acid | 4 | 694 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.98, 1.86] |
| 6.8 CoQ10 | 2 | 149 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.28 [1.79, 10.26] |
| 6.9 L‐carnitine | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 82.05 [10.92, 616.59] |
| 6.10 Vitamin D | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.25, 2.76] |
| 6.11 Vitamin B complex | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.82, 4.58] |
| 6.12 Myo‐inositol plus melatonin plus folic acid | 1 | 389 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.90, 2.12] |
| 7 Clinical pregnancy; indications for subfertility Show forest plot | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Polycystic ovary syndrome | 11 | 1721 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.63 [2.06, 3.36] |
| 7.2 Unexplained | 3 | 967 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.14] |
| 7.3 Tubal subfertility | 2 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.32, 3.46] |
| 7.4 Varying indications | 5 | 1004 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.94, 1.71] |
| 7.5 Poor responders | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.64, 5.47] |
| 8 Clinical pregnancy; IVF/ICSI Show forest plot | 15 | 2263 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.98, 1.43] |
| 9 Adverse events Show forest plot | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Miscarriage | 18 | 2834 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.08] |
| 9.2 Multiple pregnancy | 8 | 2163 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.38] |
| 9.3 Gastrointestinal disturbances | 3 | 343 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.47, 5.10] |
| 9.4 Ectopic pregnancy | 1 | 58 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.11, 74.13] |
| 9.5 Headache | 1 | 170 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.18, 22.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; type of antioxidant (natural conceptions and undergoing fertility treatments) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Myo‐Inositol versus d‐chiro‐inositol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical pregnancy; type of antioxidant (natural conceptions and undergoing fertility treatments) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Myo‐Inositol versus d‐chiro‐inositol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Miscarriage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.68, 3.50] |
| 2 Clinical pregnancy; pentoxifylline vs placebo or no treatment/standard treatment (natural conceptions and undergoing fertility treatments) Show forest plot | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.20, 3.56] |
| 2.1 Placebo | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 2.2 No treatment | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 3 Clinical pregnancy; type of antioxidant Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pentoxifylline | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 3.2 Pentoxifylline plus vitamin E | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 4 Clinical pregnancy; indications for subfertility Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Endometriosis | 2 | 164 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.94, 4.56] |
| 4.2 Varying indications | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 5 Clinical pregnancy; IVF/ICSI Show forest plot | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.97, 4.38] |
| 6 Adverse events Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Miscarriage | 3 | 276 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.46, 3.90] |
| 6.2 Multiple pregnancy | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.20, 3.09] |
| 6.3 Ectopic pregnancy | 1 | 112 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.18, 23.13] |

