Intervenciones diferentes de los anticoagulantes y antibióticos sistémicos para la prevención de las infecciones relacionadas con el catéter venoso central en niños con cáncer
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double blind randomisation | |
| Participants | 103 newly diagnosed patients with haematological and non‐haematological malignancies enrolled between August 1994 and July 1998; ages 1 to 21 years (mean age 9.2 ± 0.8 years in study group and 8.6 ± 0.8 years in control group); implanted catheters only; only patients whose catheter placement was anticipated to be > 6 months were enrolled. | |
| Interventions | Intervention: Monthly catheter flushes with 3ml of Urokinase‐Heparin which had 5000 IU of urokinase (Abbokinase) Control: Monthly catheter flushes with 3ml of Heparin which had 300 units of heparin Only those participants who received catheter flushes on at least six occasions at one month intervals were included in the analysis. | |
| Outcomes | Bacteraemia (CAI); premature catheter removal for infection Outcomes assessed on 74/103 patients as 7 did not receive first flush within 1 month of study entry and 22 received fewer than the mandated 6 flushes | |
| Notes | Funding: Abbott Laboratories, National Institute of Health, Children's Cancer Fund for Dallas and Wipe Out Kids' Cancer Premature closure of trial due to removal of study drug from market. Planned target of enrolling 120 participants not met. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "Patients were randomised using a single 1:1 permuted block of length 120" |
| Allocation concealment? | Unclear risk | Allocation concealment not described |
| Blinding? | Low risk | Quote: "The pharmacist was the only unblinded person involved in this study" Comment ‐ Probably done |
| Incomplete outcome data addressed? | Unclear risk | 29/103 of participants enrolled in the study excluded from analysis. Insufficient reporting of exclusions within control and study group to permit judgement. ‘As‐treated’ analysis done |
| Free of selective reporting? | Unclear risk | The study did not report on CRBSI or pocket infection |
| Free of other bias? | Unclear risk | Trial stopped early when the study drug urokinase was removed from the market by US Food and Drug Administration. However, bias for study drug is unlikely as the study reported no difference in outcomes between control and study groups |
| Methods | Randomisation. Blinding of participants and personnel not feasible. Blinding of outcome assessors not described | |
| Participants | 113 children with malignancy (mainly non‐haematological) who were candidates for high‐dose chemo/radiotherapy followed by bone marrow transplantation enrolled between July 1990 and April 1993; median age 5 years (range 1 to 22 years) in study group and 7 years (range 2 to 19 years) in control group; external catheters only | |
| Interventions | Intervention: Catheter dressing changed every 15 days Control: Catheter dressing changed every 4 days Three types of dressings used depending on the condition of the underlying skin (grade 0/1 cutaneous toxicity ‐ tegaderm; grade 2/3 ‐ mefix type; grade 4 ‐ sterile gauze and tape) | |
| Outcomes | Skin toxicity at dressing site; local pain; bacteraemia; premature catheter removal for infection | |
| Notes | Only 17% of those in the intervention group compared to 76% of those in the control group had dressing changed at pre‐specified frequency. This was mainly due to loose dressing in the intervention group necessitating earlier dressing changes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Quote: "A computer generated list was used" "Randomisation was stratified by the type of HDC (with or without busulfan)" |
| Allocation concealment? | Unclear risk | Allocation concealment not described |
| Blinding? | Unclear risk | Blinding of participants and personnel not feasible. Blinding of outcome assessors not described |
| Incomplete outcome data addressed? | Low risk | Quote: "One patient relapsed after randomisation and did not receive HDC (15‐day Group)" Comment ‐ Outcome reported for 112/113 randomised patients. Missing outcome data are unlikely to have a clinically meaningful impact on the intervention effect estimate. |
| Free of selective reporting? | Unclear risk | The study did not report on CRBSI or CAI |
| Free of other bias? | High risk | The frequency of dressing affects cutaneous toxicity which in turn affects material used for dressing. So any effect attributed to dressing frequency might actually be confounding for dressing material |
| Methods | Randomisation stratified by type of CVC | |
| Participants | 577 patients with haematological and non‐haematological malignancies enrolled between July 1997 and December 1998; ages 3 months to 21 years (> 95% were < 18 years age); median age 4.5 years; implanted and external catheters | |
| Interventions | Intervention: CVC flushed every two weeks with urokinase (Abbokinase 5000 IU/ml) in volume sufficient to fill the entire catheter Control: CVC flushed every two weeks with heparin (100 units/ml) in volume sufficient to fill the entire catheter The drug was required to remain in the catheter lumen for a minimum of 1 hour and a maximum of 14 days | |
| Outcomes | Complete catheter occlusion; partial catheter occlusion; catheter‐related infection; time to first catheter occlusion; time to first catheter‐related infection | |
| Notes | Funding: In part by Abbott Laboratories Premature closure of trial due to removal of study drug from market. Planned target of enrolling 680 participants not met. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Sequence generation not described |
| Allocation concealment? | Unclear risk | Allocation concealment not described |
| Blinding? | Unclear risk | Blinding not described |
| Incomplete outcome data addressed? | Unclear risk | 8/577 of participants enrolled in the study excluded from analysis. Insufficient reporting of exclusions within control and study group to permit judgement. ‘As‐treated’ analysis done. |
| Free of selective reporting? | High risk | The study did not report on CRBSI. Also outcomes of interest (premature catheter removal for infection) are reported incompletely. |
| Free of other bias? | Unclear risk | Trial stopped early when the study drug urokinase was removed from the market by US Food and Drug Administration. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Earlier published abstract of Freiberger 1992 which was excluded for reasons mentioned below. | |
| The study compared different antiseptics used to clean the skin as well as different dressings used to cover the exit site. No information available on number of participants randomised to each arm. The study also did not report any outcomes of interest. | |
| This study compared non‐tunnelled catheters with tunnelled catheters. | |
| The study randomised blood sampling from CVC and reinfusion of blood following the sampling in the usual clean way versus an exaggerated unclean way. It did not report any outcomes of interest. | |
| Earlier published abstract of Dillon 2004 which has been included in this review. | |
| In this study children with malignancies who were not malnourished were randomised to receive intravenous hyperalimentation or regular diet. Although the study looked at catheter‐related infections as an outcome, the intervention was not prophylactic. | |
| The study compared flushing CVC with bacteriostatic saline (sterile saline mixed with 1% benzyl alcohol) versus sterile saline alone. The outcomes were bacterial colonisation and clinical sepsis. It did not report outcomes of interest. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised study of Taurolock for the locking of tunnelled central venous catheters in children with malignant diseases |
| Methods | Randomised |
| Participants | Children aged 0 to 17 years with malignant disease requiring a tunnelled CVC |
| Interventions | When not in use the children's tunnelled CVC are locked with the liquid Taurolock or heparin |
| Outcomes | Primary Outcome Measures: Number of CRBSI/1000 CVC days in the Taurolock group versus the heparin group. Number of CVCs removed in the Taurolock group versus the heparin group. |
| Starting date | April 2008 |
| Contact information | Mette M Handrup, MD. +45 8949 6749. [email protected] |
| Notes | This study is currently recruiting participants. Outcomes are expected in November 2010. |
| Trial name or title | Ethanol lock therapy for the prevention of catheter‐related blood stream infections |
| Methods | Double‐blind randomised |
| Participants | Children aged 6 months to 21 years, central venous access and a history of three or more catheter‐related blood stream infections in the prior 6 months |
| Interventions | Experimental arm ‐ 25% ethanol lock, Control arm ‐ heparin lock |
| Outcomes | Number of episodes of catheter‐related blood stream infections |
| Starting date | August 2008 |
| Contact information | Judith M Martin, MD. University of Pittsburgh |
| Notes | This study is currently recruiting participants. Outcomes are expected in December 2010. |
| Trial name or title | Ethanol lock solution for the prevention of tunnelled central venous catheter infections in paediatric oncology patients, a randomised controlled trial |
| Methods | Double‐blind randomised |
| Participants | Paediatric oncology patients between 1 and 18 years of age with a newly inserted tunnelled central venous catheter (both internal and external devices) |
| Interventions | After insertion of the catheter an ethanol (70%)‐lock solution will be administered (3 ml) for a duration of 2 hours, once weekly or longer if the catheter is not locked in between. The control group will be locked with the standard heparin (100U/ml) solution (3 ml). |
| Outcomes | Primary outcome measures ‐ First catheter‐related bacteraemia, death of the patient, or removal of the catheter, whatever comes first. Secondary outcome measures ‐ fever, antibiotic use, days of hospital admission and thrombosis. |
| Starting date | October 2007 |
| Contact information | Dr MD Wetering. +31 (0)20 5663050. [email protected] |
| Notes | This study is currently recruiting participants. Outcomes are expected in October 2010. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐Associated Infection Show forest plot | 2 | Rate Ratio (Fixed, 95% CI) | 0.72 [0.12, 4.41] | |
| Analysis 1.1  Comparison 1 Urokinase (with or without heparin) versus heparin, Outcome 1 Catheter‐Associated Infection. | ||||

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
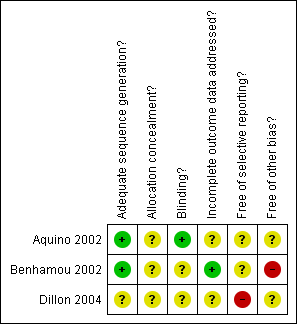
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Forest plot of comparison: 1 Urokinase (with or without Heparin) versus heparin, outcome: 1.1 Catheter‐associated infection.
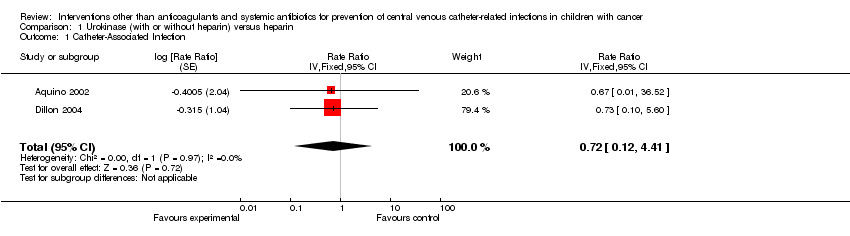
Comparison 1 Urokinase (with or without heparin) versus heparin, Outcome 1 Catheter‐Associated Infection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Catheter‐Associated Infection Show forest plot | 2 | Rate Ratio (Fixed, 95% CI) | 0.72 [0.12, 4.41] | |

