Dosis de clorpromazina en pacientes con esquizofrenia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007778.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 13 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Esquizofrenia
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Katharine Dudley ‐ data re‐extraction, 'Risk of bias' tables, 'Summary of findings' tables, analyses, re‐writing the report (2014, 2016 searches).
Saskia de Haan ‐ development of the protocol, data extraction, analyses, writing the report (2009 search).
Xiaomeng Liu ‐ development of the protocol, data extraction, analyses, writing the report (2009 search).
Sources of support
Internal sources
-
University of Nottingham, UK.
-
Utrecht University, Netherlands.
External sources
-
No sources of support supplied
Declarations of interest
None known.
Acknowledgements
We would like to thank the staff at the Cochrane Schizophrenia Group for their help and assistance, in particular Hirsto Girgorov for translating a Russian paper, Jun Xia for her help with Chinese papers and Farhad Shokraneh the Information Scientist. Special thanks to Professor Clive Adams for his guidance and support. We have used the generic text supplied by the Cochrane Schizophrenia Group for the Methods section and adapted it to our needs.
We would like to thank Lorna Lawrence for peer reviewing this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 13 | Chlorpromazine dose for people with schizophrenia | Review | Katharine Dudley, Xiaomeng Liu, Saskia De Haan | |
| 2009 Apr 15 | Chlorpromazine dose for people with schizophrenia | Review | Xiaomeng Liu, Saskia De Haan | |
Differences between protocol and review
For the 2014 update, we included data from Wode‐Helgodt 1978 for extrapyramidal adverse effects that had been reported using a modification of a previously published scale (Simpson 1970). We realise that this is not entirely in keeping with our previous methods where we stated that we should not use any modified scales ‐ citing Marshall 2000. However, we felt these outcomes to be important although they must carry high risk of bias.
We have moved the outcome of leaving the study early to below global and mental state, moved 'death' to adverse effects.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antipsychotic Agents [*administration & dosage, adverse effects];
- Barbiturates [administration & dosage];
- Chloral Hydrate [administration & dosage];
- Chlorpromazine [*administration & dosage, adverse effects];
- Drug Administration Schedule;
- Hypnotics and Sedatives [administration & dosage];
- Patient Dropouts [statistics & numerical data];
- Randomized Controlled Trials as Topic;
- Schizophrenia [*drug therapy];
Medical Subject Headings Check Words
Humans;
PICO
Chlorpromazine structure
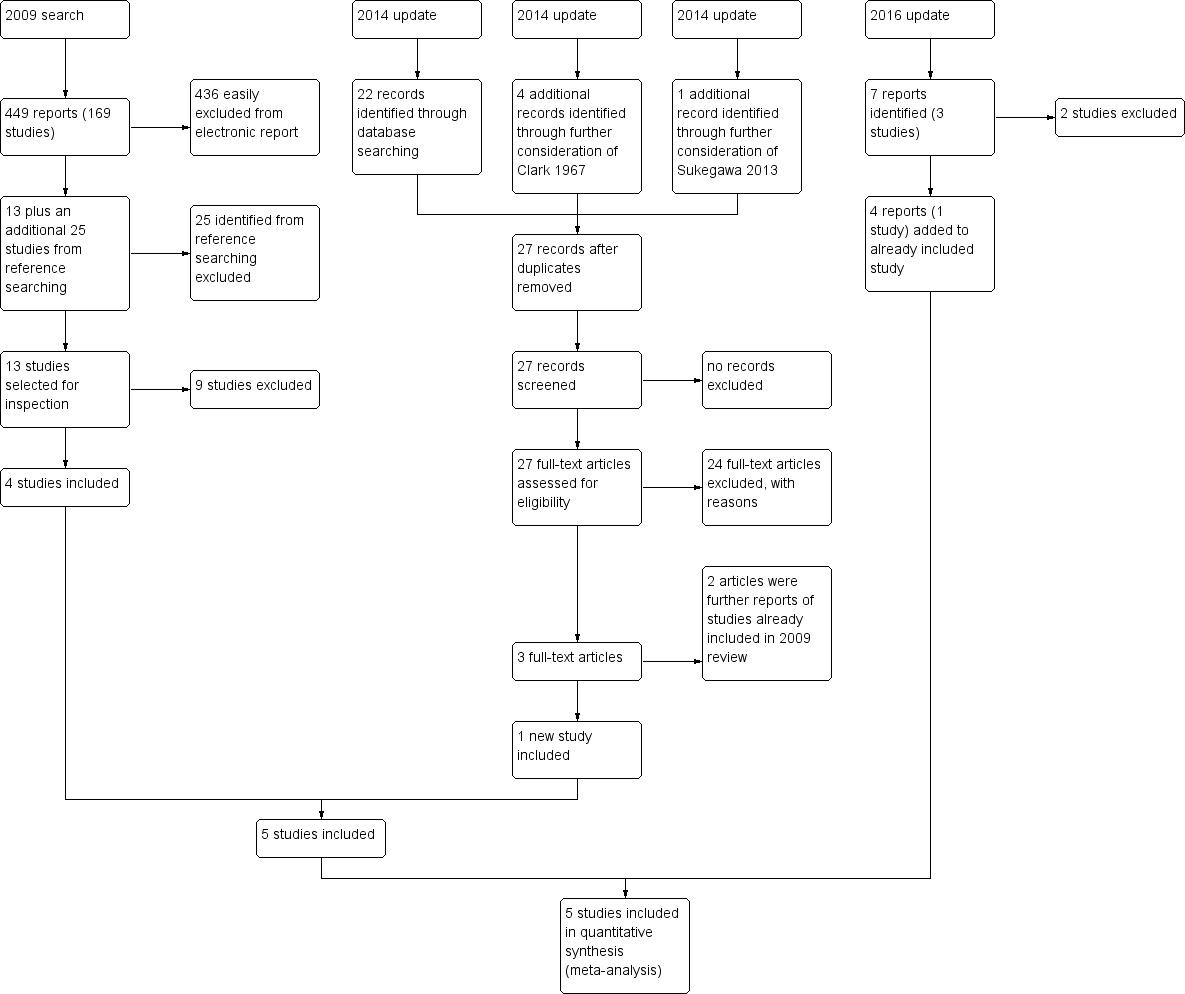
Study flow diagram
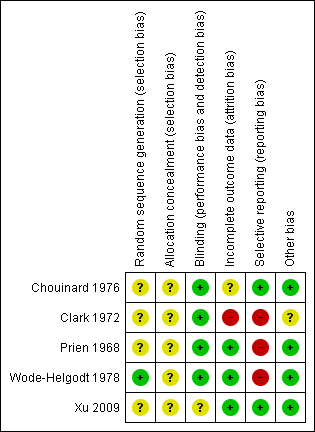
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
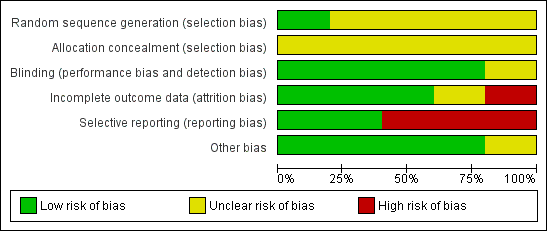
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
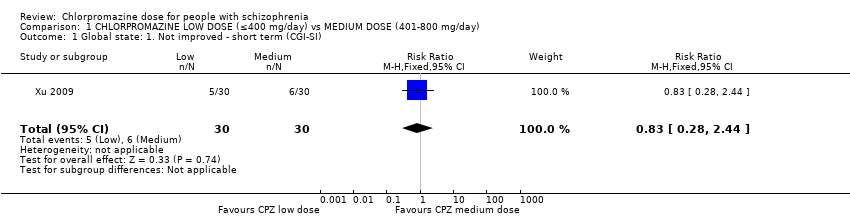
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 1 Global state: 1. Not improved ‐ short term (CGI‐SI).
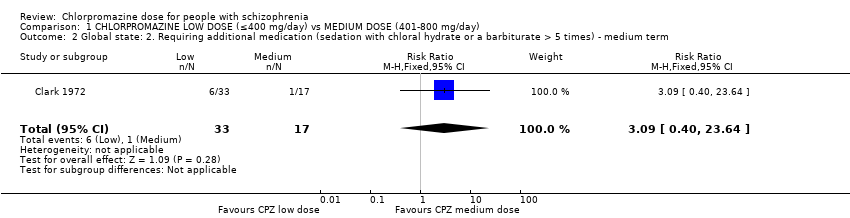
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 2 Global state: 2. Requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term.
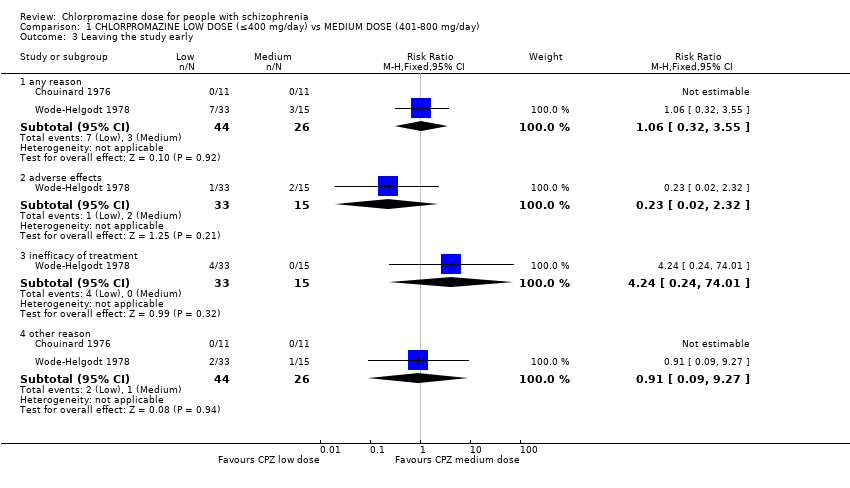
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 3 Leaving the study early.
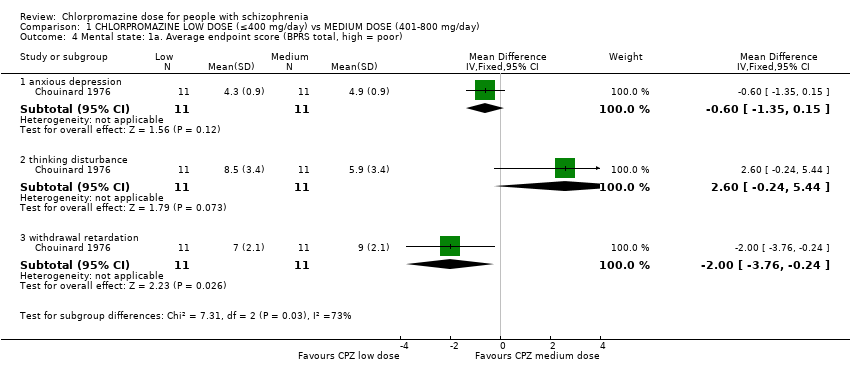
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 4 Mental state: 1a. Average endpoint score (BPRS total, high = poor).
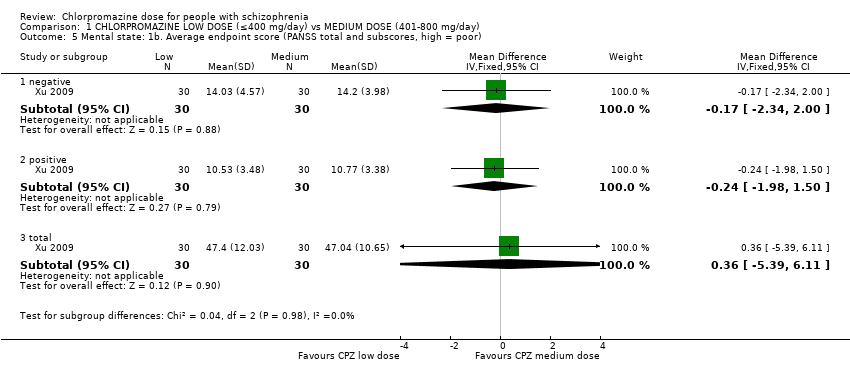
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 5 Mental state: 1b. Average endpoint score (PANSS total and subscores, high = poor).
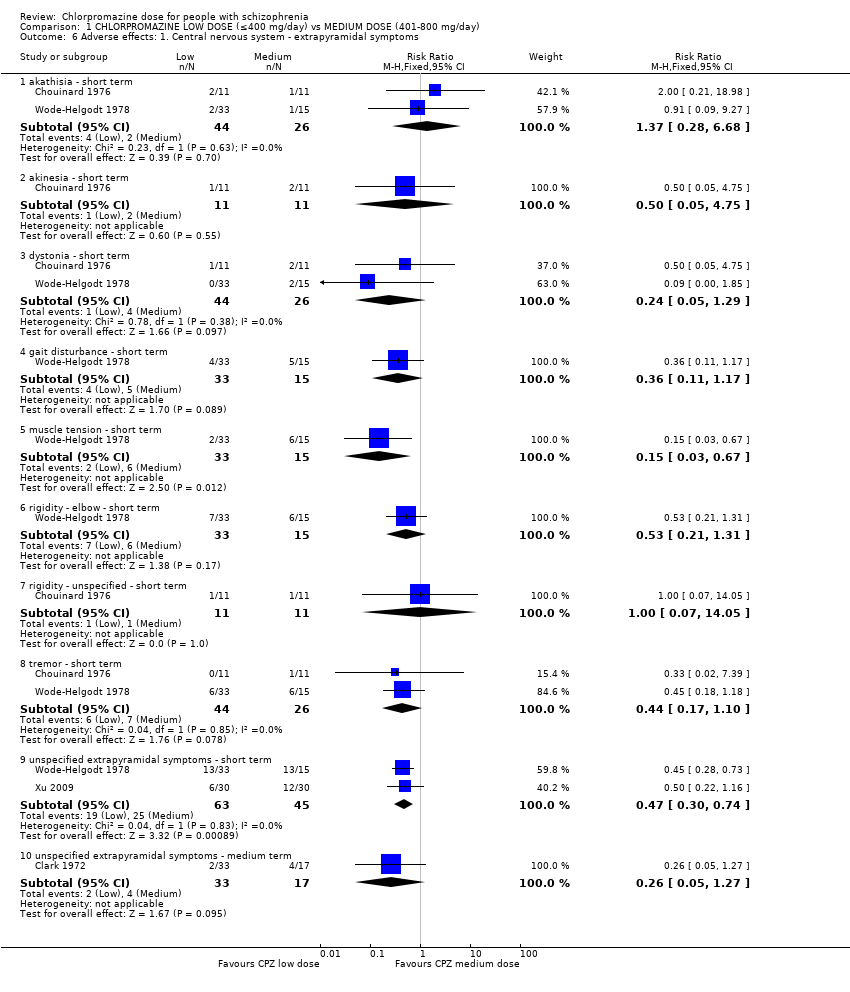
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 6 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms.
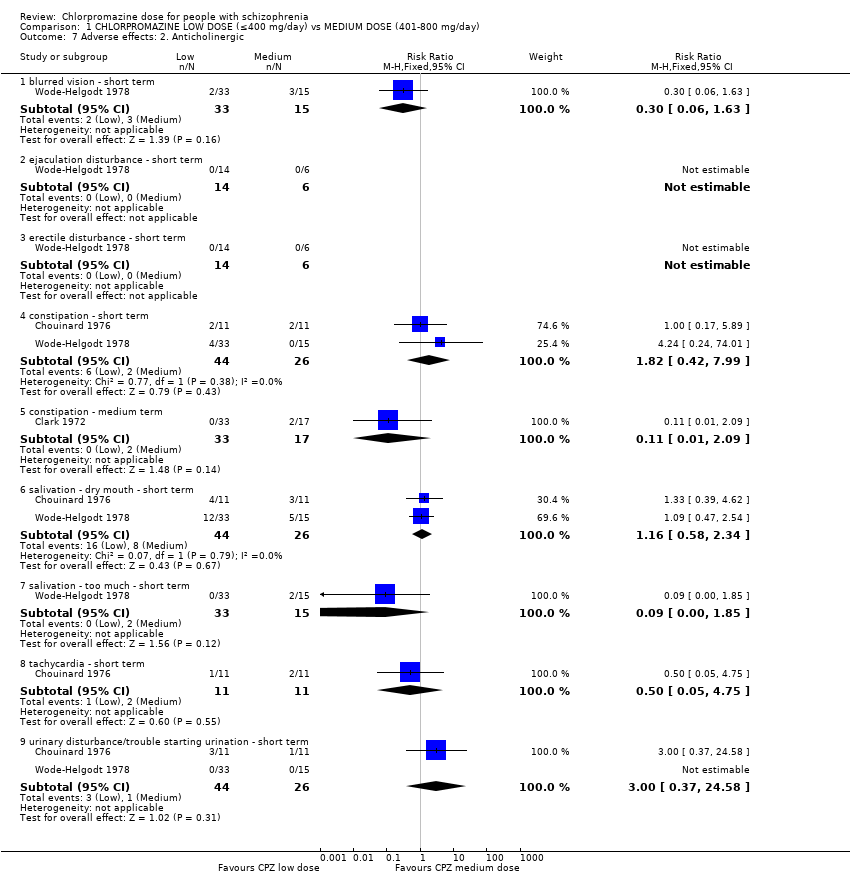
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 7 Adverse effects: 2. Anticholinergic.
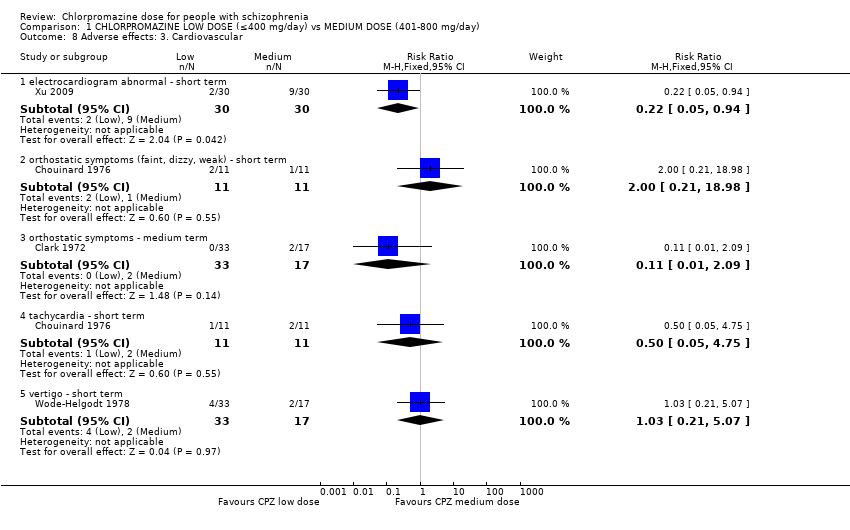
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 8 Adverse effects: 3. Cardiovascular.
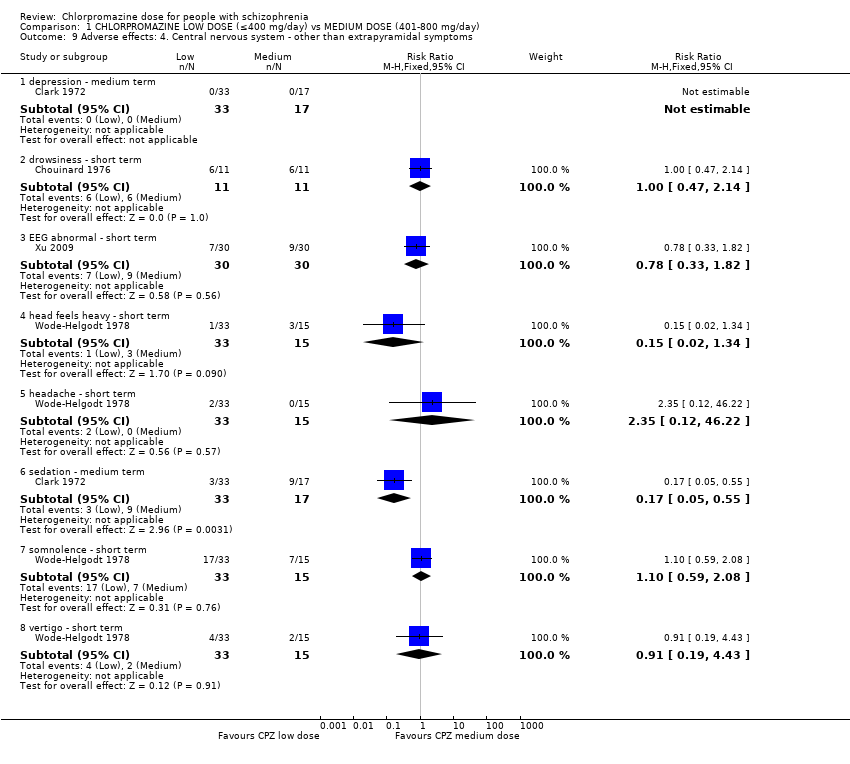
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 9 Adverse effects: 4. Central nervous system ‐ other than extrapyramidal symptoms.
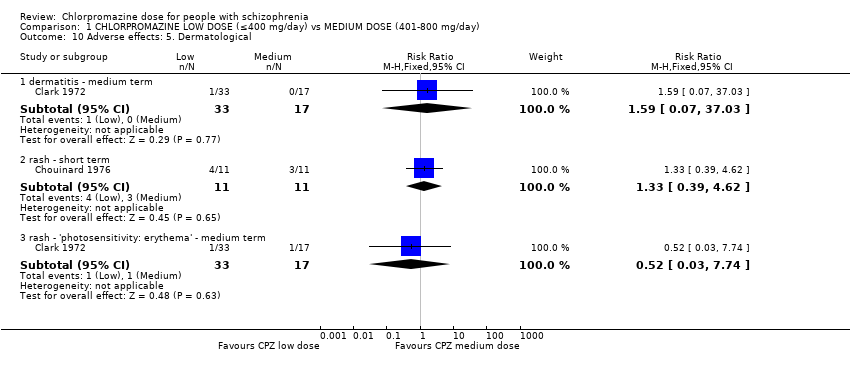
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 10 Adverse effects: 5. Dermatological.
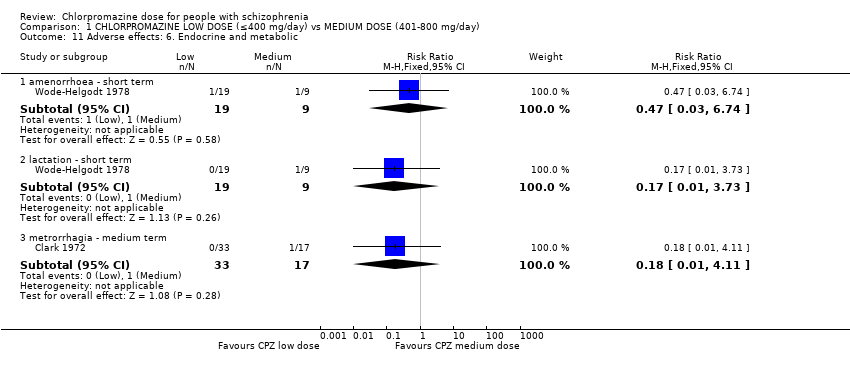
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 11 Adverse effects: 6. Endocrine and metabolic.
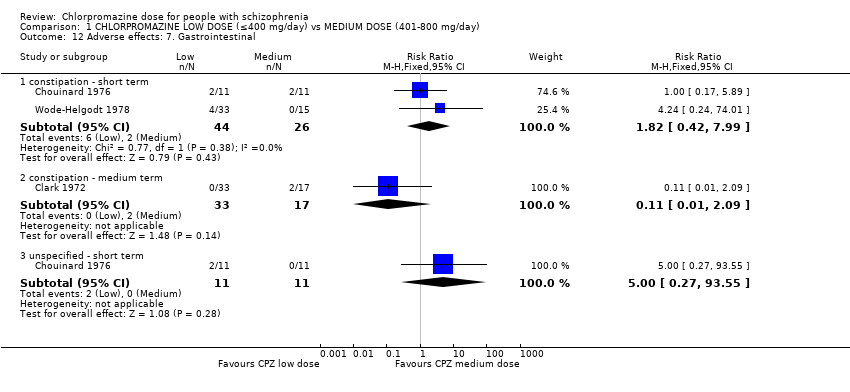
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 12 Adverse effects: 7. Gastrointestinal.
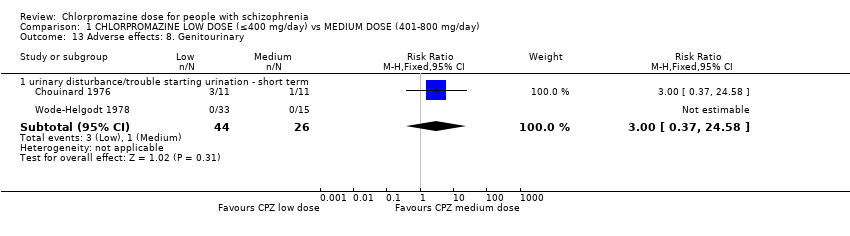
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 13 Adverse effects: 8. Genitourinary.
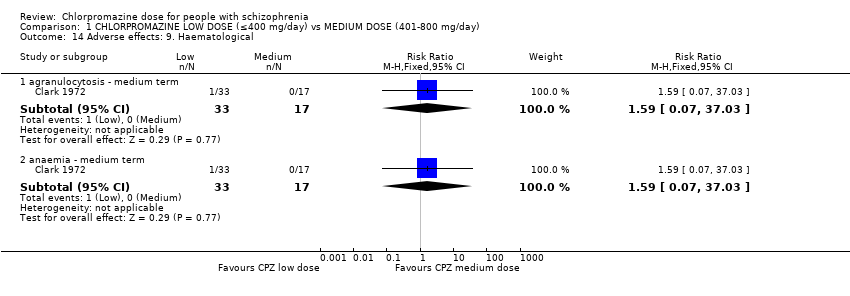
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 14 Adverse effects: 9. Haematological.
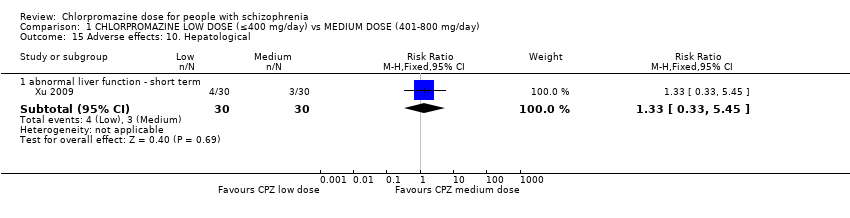
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 15 Adverse effects: 10. Hepatological.
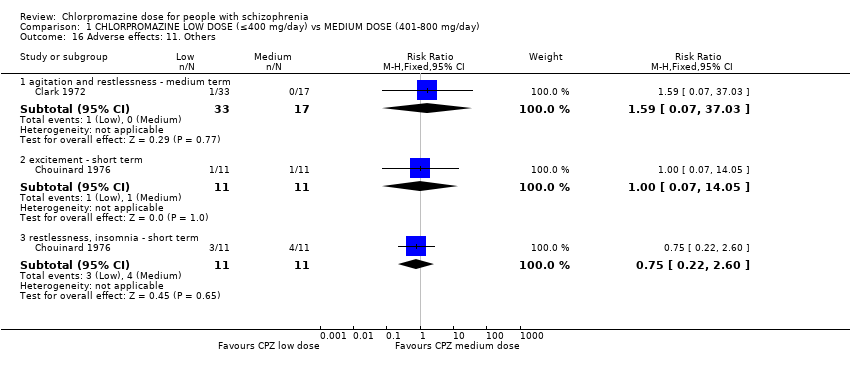
Comparison 1 CHLORPROMAZINE LOW DOSE (≤400 mg/day) vs MEDIUM DOSE (401‐800 mg/day), Outcome 16 Adverse effects: 11. Others.
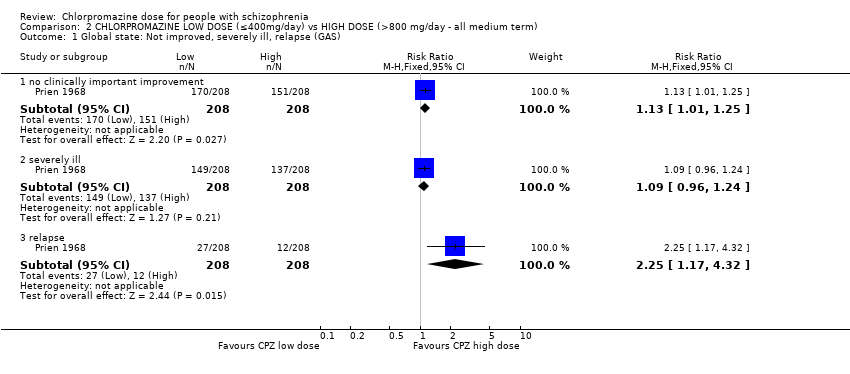
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 1 Global state: Not improved, severely ill, relapse (GAS).
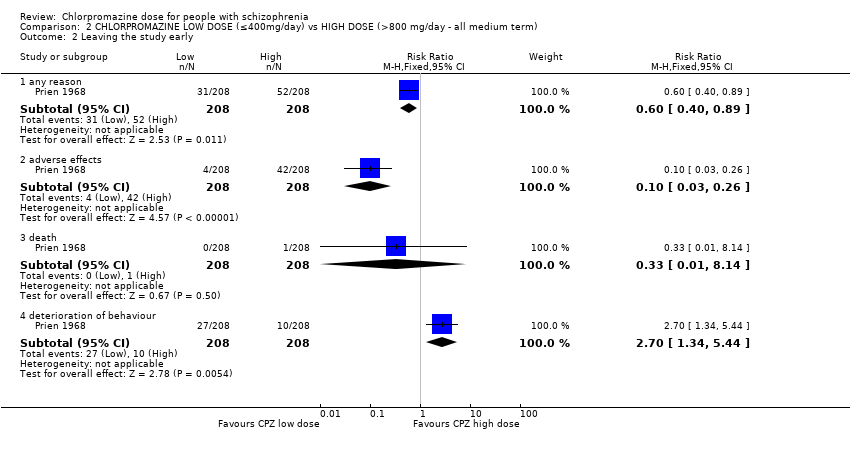
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 2 Leaving the study early.
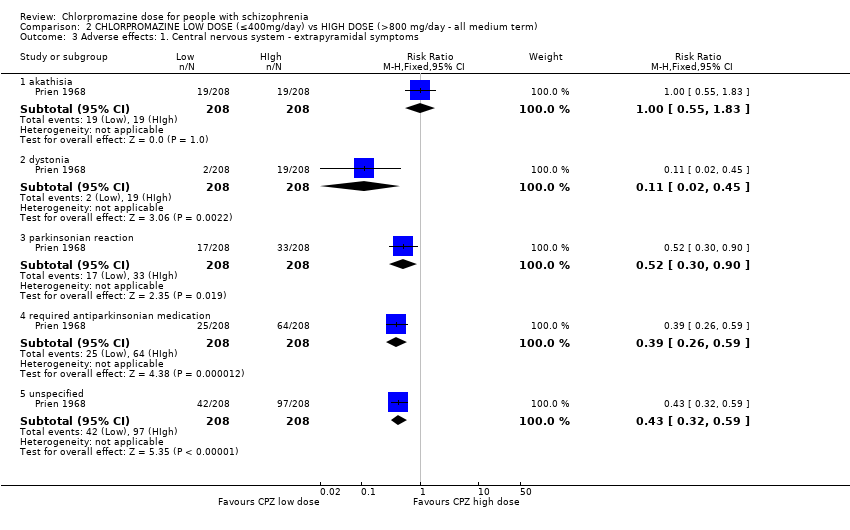
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 3 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms.
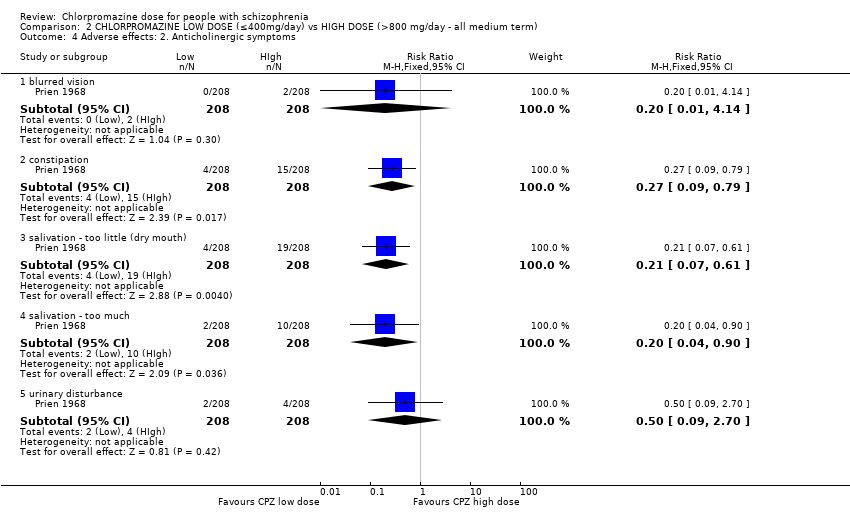
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 4 Adverse effects: 2. Anticholinergic symptoms.
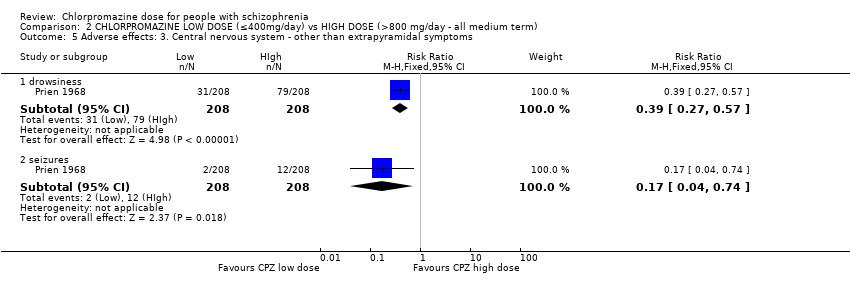
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 5 Adverse effects: 3. Central nervous system ‐ other than extrapyramidal symptoms.
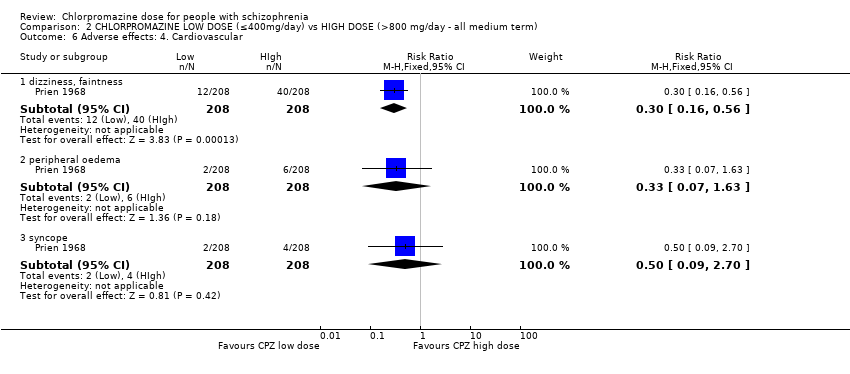
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 6 Adverse effects: 4. Cardiovascular.
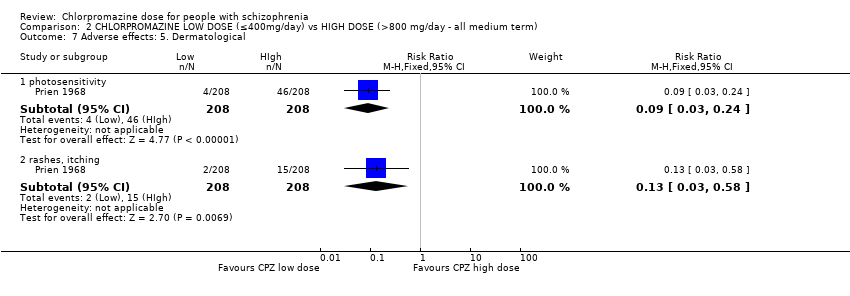
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 7 Adverse effects: 5. Dermatological.
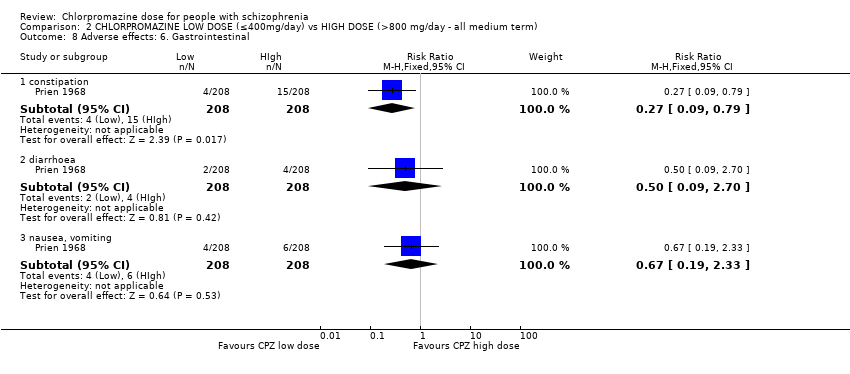
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 8 Adverse effects: 6. Gastrointestinal.
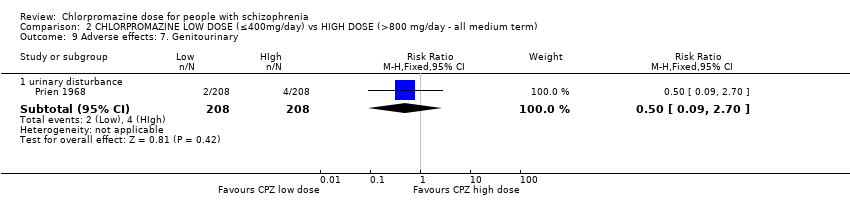
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 9 Adverse effects: 7. Genitourinary.
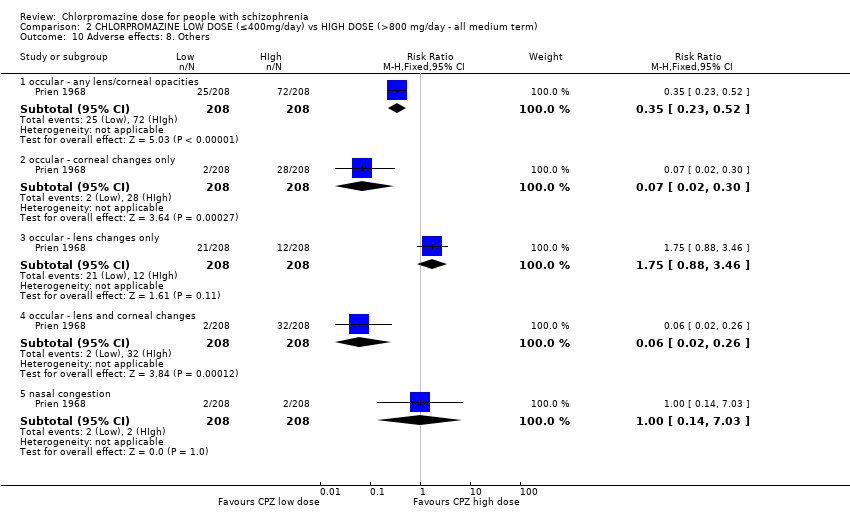
Comparison 2 CHLORPROMAZINE LOW DOSE (≤400mg/day) vs HIGH DOSE (>800 mg/day ‐ all medium term), Outcome 10 Adverse effects: 8. Others.
| Medication | Comparison | For people with | Relevant excluded study | Relevant existing Cochrane review | |
| Antiparkinsonian drug ‐ unspecified | Antiparkinsonian treatment versus placebo | Movement disorders | ‐ | ||
| Antipsychotic drugs ‐ unspecified | Doses | Any antipsychotic at high chlorpromazine equivalent dosages vs lower chlorpromazine equivalent dosages | Schizophrenia | ‐ | |
| Dose reduction of multiple antipsychotics versus control group | ‐ | ||||
| Timing of treatment | Treatment days per week of antipsychotics | ‐ | |||
| Chlorpromazine | Mostly versus other antipsychotic drug | Chlorpromazine brand comparisons | ‐ | ||
| Chlorpromazine versus clopenthixol | |||||
| Chlorpromazine versus clozapine | |||||
| Chlorpromazine versus haloperidol | |||||
| Chlorpromazine versus placebo | Borison 1991; Clark 1967; Clark 1970a; Clark 1970b; Eitan 1992 | ||||
| Chlorpromazine versus rimcazole | ‐ | ||||
| Chlorpromazine versus risperidone | |||||
| Chlorpromazine versus thioridazine | |||||
| Chlorpromazine versus trifluoperazine | |||||
| Doses | Ultra‐low doses of chlorpromazine | ‐ | |||
| Ultra‐low doses of thioridazine | ‐ | ||||
| Timing of treatment | Night doses versus daytime doses of chlorpromazine | ‐ | |||
| Single doses versus divided doses of chlorpromazine | ‐ | ||||
| Q.I.D versus O.D of chlorpromazine | ‐ | ||||
| Clopenthixol | Clopenthixol versus placebo | ||||
| Clozapine | Clozapine versus risperidone | ||||
| Dextroamphetamine | Dextroamphetamine versus imipramine | ||||
| Dextroamphetamine versus isocarboxazid | |||||
| Dextroamphetamine versus placebo | ‐ | ||||
| Dextroamphetamine versus trifluoperazine | ‐ | ||||
| Haloperidol | Haloperidol versus placebo | ||||
| Haloperidol versus trifluoperazine | |||||
| Haloperidol versus thioridazine | |||||
| Imipramine | ‐ | ‐ | |||
| Isocarboxazid | Isocarboxazid versus placebo | ||||
| Isocarboxazid versus trifluoperazine | |||||
| Rimcazole | Rimcazole versus placebo | ‐ | |||
| Doses | Rimcazole dose | ‐ | |||
| Risperidone | ‐ | ‐ | |||
| Thioridazine | Thioridazine versus placebo | ||||
| Timing of treatment | Night doses versus daytime doses of thioridazine | ‐ | |||
| Timing of treatment | Thioridazine ‐ single doses versus divided doses | ‐ | |||
| Trifluoperazine | Trifluoperazine versus placebo | ||||
| Trifluoperazine versus thioridazine | |||||
| Trihexyphenidyl | Trihexyphenidyl versus placebo | Movement disorders | ‐ | ||
| Vitamin preparation | Vitamin preparation versus placebo | Schizophrenia | |||
| Methods | Allocation: clearly randomised, well‐described concealment. Blindness: triple‐blinding clearly described including information on method administration, volume and concentration of chlorpromazine. Duration: 28 weeks (2 weeks of no antipsychotic medication). |
| Participants | Diagnosis: people with schizophrenia ‐ diagnosed by any criteria. Age: any. Sex: both. N = 450.* History: acute or long‐term illness. Excluded: borderline cases, mental deficiency (IQ < 70), bad physical health with complicating organic illness or known brain damage, medical conditions constraining use of high doses, alcoholism or drug use, showing severe suicidal or aggressive impulses. |
| Interventions | 1. Chlorpromazine: dose 300 mg/day. N = 150. |
| Outcomes | Leaving the study early (reasons, timing, gender and treatment group provided). Deaths. Adverse effects (clinically important and specific). Needing additional medication (information about doses, timings and reasons for drug administration provided). Effectiveness:
|
| Notes | * 150 in each group allows for good statistical power. |
| CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) compared to MEDIUM DOSE (401 mg/day to 800 mg/day) for people with schizophrenia | |||||
| Patient or population: people with schizophrenia | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| MEDIUM DOSE (401‐800 mg/day) | CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) | ||||
| Global state: no improvement ‐ short term | Low1 | RR 0.83 | 60 | ⊕⊝⊝⊝ | |
| 100 per 1000 | 83 per 1000 | ||||
| Moderate1 | |||||
| 200 per 1000 | 166 per 1000 | ||||
| High1 | |||||
| 300 per 1000 | 249 per 1000 | ||||
| Global state: needing additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term | Low1 | RR 3.09 | 50 | ⊕⊕⊝⊝ | |
| 20 per 1000 | 62 per 1000 | ||||
| Moderate1 | |||||
| 60 per 1000 | 185 per 1000 | ||||
| High1 | |||||
| 100 per 1000 | 309 per 1000 | ||||
| Mental state: average endpoint score (PANSS total, high=poor) | The mean mental state: average endpoint score (PANSS total, high = poor) in the intervention groups was | 60 | ⊕⊝⊝⊝ | ||
| Leaving the study early ‐ any reason | Low1 | RR 1.06 | 70 | ⊕⊕⊕⊝ | |
| 50 per 1000 | 53 per 1000 | ||||
| Moderate1 | |||||
| 100 per 1000 | 106 per 1000 | ||||
| High1 | |||||
| 150 per 1000 | 159 per 1000 | ||||
| Behaviour: agitation and restlessness ‐ medium term (categorised as adverse event) | Study population | RR 1.59 | 50 | ⊕⊝⊝⊝ | |
| 0 per 1000 | 0 per 1000 | ||||
| Moderate | |||||
| 0 per 1000 | 0 per 1000 | ||||
| Adverse effects: extrapyramidal symptoms (unspecified extrapyramidal symptoms ‐ short term) | Low | RR 0.47 | 108 | ⊕⊕⊕⊝ | |
| 200 per 1000 | 94 per 1000 | ||||
| Moderate | |||||
| 500 per 1000 | 235 per 1000 | ||||
| High | |||||
| 800 per 1000 | 376 per 1000 | ||||
| Adverse event: death | No trial reported this outcome | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 Indirectness: rated serious: downgraded by 1 ‐ unclear if clinically important improvement, no prespecified medium‐term data available. | |||||
| CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) compared to HIGH DOSE (> 800 mg/day‐ all medium term) for people with schizophrenia | ||||||
| Patient or population: patients with people with schizophrenia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comment | |
| Assumed risk | Corresponding risk | |||||
| HIGH DOSE (>800 mg/day‐ all medium term) | CHLORPROMAZINE LOW DOSE (≤ 400 mg/day) | |||||
| Global state: no clinically important improvement ‐ medium term | Low1 | RR 1.13 | 416 | ⊕⊕⊕⊝ | ||
| 300 per 1000 | 339 per 1000 | |||||
| Moderate1 | ||||||
| 700 per 1000 | 791 per 1000 | |||||
| High1 | ||||||
| 900 per 1000 | 1000 per 1000 | |||||
| Global state: requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) | See comment | See comment | Not estimable | 0 | See comment | No data available |
| Mental state: no clinically important change in mental state | See comment | See comment | Not estimable | 0 | See comment | No data available |
| Leaving the study early ‐ any reason | Low1 | RR 0.60 | 416 | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 60 per 1000 | |||||
| Moderate1 | ||||||
| 250 per 1000 | 150 per 1000 | |||||
| High1 | ||||||
| 500 per 1000 | 300 per 1000 | |||||
| Behaviour: deterioration of behaviour (categorised as reason to leave early) | Low | RR 2.70 | 416 | ⊕⊕⊝⊝ | ||
| 20 per 1000 | 54 per 1000 | |||||
| Moderate | ||||||
| 50 per 1000 | 135 per 1000 | |||||
| High | ||||||
| 100 per 1000 | 270 per 1000 | |||||
| Adverse effects: unspecified extrapyramidal symptoms | Low | RR 0.43 | 416 | ⊕⊕⊕⊝ | ||
| 200 per 1000 | 86 per 1000 | |||||
| Moderate | ||||||
| 500 per 1000 | 215 per 1000 | |||||
| High | ||||||
| 700 per 1000 | 301 per 1000 | |||||
| Adverse event: death | Low | RR 0.33 | 416 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Moderate | ||||||
| 5 per 1000 | 2 per 1000 | |||||
| High | ||||||
| 50 per 1000 | 17 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: rated serious: downgraded by 1 ‐ randomisation not described well, no mention of allocation concealment, concern regarding selective reporting. 2Indirectness: rated serious: downgraded by 1 ‐ unclear if clinically important change in behaviour, rated within trial as leaving the study early outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: 1. Not improved ‐ short term (CGI‐SI) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.28, 2.44] |
| 2 Global state: 2. Requiring additional medication (sedation with chloral hydrate or a barbiturate > 5 times) ‐ medium term Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.40, 23.64] |
| 3 Leaving the study early Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 any reason | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.32, 3.55] |
| 3.2 adverse effects | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.02, 2.32] |
| 3.3 inefficacy of treatment | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.24 [0.24, 74.01] |
| 3.4 other reason | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.09, 9.27] |
| 4 Mental state: 1a. Average endpoint score (BPRS total, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 anxious depression | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.35, 0.15] |
| 4.2 thinking disturbance | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐0.24, 5.44] |
| 4.3 withdrawal retardation | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐3.76, ‐0.24] |
| 5 Mental state: 1b. Average endpoint score (PANSS total and subscores, high = poor) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 negative | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐2.34, 2.00] |
| 5.2 positive | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐1.98, 1.50] |
| 5.3 total | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐5.39, 6.11] |
| 6 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 akathisia ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.28, 6.68] |
| 6.2 akinesia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 6.3 dystonia ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.29] |
| 6.4 gait disturbance ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.11, 1.17] |
| 6.5 muscle tension ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.67] |
| 6.6 rigidity ‐ elbow ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.21, 1.31] |
| 6.7 rigidity ‐ unspecified ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 6.8 tremor ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.10] |
| 6.9 unspecified extrapyramidal symptoms ‐ short term | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.30, 0.74] |
| 6.10 unspecified extrapyramidal symptoms ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.27] |
| 7 Adverse effects: 2. Anticholinergic Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 blurred vision ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.63] |
| 7.2 ejaculation disturbance ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 erectile disturbance ‐ short term | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.4 constipation ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.42, 7.99] |
| 7.5 constipation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 7.6 salivation ‐ dry mouth ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.58, 2.34] |
| 7.7 salivation ‐ too much ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.85] |
| 7.8 tachycardia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 7.9 urinary disturbance/trouble starting urination ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.37, 24.58] |
| 8 Adverse effects: 3. Cardiovascular Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 electrocardiogram abnormal ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.94] |
| 8.2 orthostatic symptoms (faint, dizzy, weak) ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.21, 18.98] |
| 8.3 orthostatic symptoms ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 8.4 tachycardia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 4.75] |
| 8.5 vertigo ‐ short term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.21, 5.07] |
| 9 Adverse effects: 4. Central nervous system ‐ other than extrapyramidal symptoms Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 depression ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 drowsiness ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 9.3 EEG abnormal ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.82] |
| 9.4 head feels heavy ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.34] |
| 9.5 headache ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [0.12, 46.22] |
| 9.6 sedation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.05, 0.55] |
| 9.7 somnolence ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.59, 2.08] |
| 9.8 vertigo ‐ short term | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.19, 4.43] |
| 10 Adverse effects: 5. Dermatological Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 dermatitis ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 10.2 rash ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.39, 4.62] |
| 10.3 rash ‐ 'photosensitivity: erythema' ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.03, 7.74] |
| 11 Adverse effects: 6. Endocrine and metabolic Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 amenorrhoea ‐ short term | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.03, 6.74] |
| 11.2 lactation ‐ short term | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.73] |
| 11.3 metrorrhagia ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 4.11] |
| 12 Adverse effects: 7. Gastrointestinal Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 constipation ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.42, 7.99] |
| 12.2 constipation ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.09] |
| 12.3 unspecified ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 93.55] |
| 13 Adverse effects: 8. Genitourinary Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 urinary disturbance/trouble starting urination ‐ short term | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.37, 24.58] |
| 14 Adverse effects: 9. Haematological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 agranulocytosis ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 14.2 anaemia ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 15 Adverse effects: 10. Hepatological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 abnormal liver function ‐ short term | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.45] |
| 16 Adverse effects: 11. Others Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 agitation and restlessness ‐ medium term | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.07, 37.03] |
| 16.2 excitement ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.05] |
| 16.3 restlessness, insomnia ‐ short term | 1 | 22 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.22, 2.60] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Global state: Not improved, severely ill, relapse (GAS) Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 no clinically important improvement | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.01, 1.25] |
| 1.2 severely ill | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 1.3 relapse | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.17, 4.32] |
| 2 Leaving the study early Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 any reason | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.89] |
| 2.2 adverse effects | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.26] |
| 2.3 death | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.14] |
| 2.4 deterioration of behaviour | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.7 [1.34, 5.44] |
| 3 Adverse effects: 1. Central nervous system ‐ extrapyramidal symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 akathisia | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.55, 1.83] |
| 3.2 dystonia | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.02, 0.45] |
| 3.3 parkinsonian reaction | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.90] |
| 3.4 required antiparkinsonian medication | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.26, 0.59] |
| 3.5 unspecified | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.32, 0.59] |
| 4 Adverse effects: 2. Anticholinergic symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 blurred vision | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.14] |
| 4.2 constipation | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.79] |
| 4.3 salivation ‐ too little (dry mouth) | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.07, 0.61] |
| 4.4 salivation ‐ too much | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.04, 0.90] |
| 4.5 urinary disturbance | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 5 Adverse effects: 3. Central nervous system ‐ other than extrapyramidal symptoms Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 drowsiness | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.57] |
| 5.2 seizures | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.04, 0.74] |
| 6 Adverse effects: 4. Cardiovascular Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 dizziness, faintness | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.16, 0.56] |
| 6.2 peripheral oedema | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.63] |
| 6.3 syncope | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 7 Adverse effects: 5. Dermatological Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 photosensitivity | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.24] |
| 7.2 rashes, itching | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.58] |
| 8 Adverse effects: 6. Gastrointestinal Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 constipation | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.09, 0.79] |
| 8.2 diarrhoea | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 8.3 nausea, vomiting | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.33] |
| 9 Adverse effects: 7. Genitourinary Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 urinary disturbance | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.70] |
| 10 Adverse effects: 8. Others Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 occular ‐ any lens/corneal opacities | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.23, 0.52] |
| 10.2 occular ‐ corneal changes only | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.30] |
| 10.3 occular ‐ lens changes only | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.88, 3.46] |
| 10.4 occular ‐ lens and corneal changes | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.02, 0.26] |
| 10.5 nasal congestion | 1 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 7.03] |

