Intervenciones para el tratamiento de la disfunción sexual en la nefropatía crónica
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Unclear risk | Blinding of investigators |
| Incomplete outcome data addressed? | High risk | Patients excluded at various stage (one patient died and three others dropped out because of intercurrent illness) |
| Free of selective reporting? | Low risk | Primary outcomes for this review (plasma gonadotropins concentration) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Unclear risk | Data only available from conference proceedings abstract |
| Incomplete outcome data addressed? | Unclear risk | Patients excluded at various stages (one transplanted, one died, seven lost to follow‐up) |
| Free of selective reporting? | Unclear risk | Primary outcomes for this review (IIEF score) have been reported, however data only available from conference proceedings abstract |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Unclear risk | Blinding of participants |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (plasma gonadotropins concentration) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Unclear risk | Blinding of participants |
| Incomplete outcome data addressed? | Unclear risk | Patients excluded at various stage (only 7/15 patients were able to complete the study; bromocriptine caused serious hypotension in the remaining 8 men) |
| Free of selective reporting? | High risk | Primary outcomes for this review not reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (plasma gonadotropins concentration) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Unclear risk | NS |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (IIEF score) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Low risk | Consecutively numbered sealed envelopes |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (plasma gonadotropins concentration) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria Myocardial infarction within the last 6 months; cerebrovascular event within the last 6 months; severe hepatic impairment; penile anatomic deformities; severe cardiac disease; concomitant nitrate therapy | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | High risk | Continuous results are reported as mean and no SD |
| Free of selective reporting? | Unclear risk | Primary outcomes for this review (IIEF score) have not been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Low risk | Tablets were dispensed in code by the hospital pharmacy |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Unclear risk | Patients excluded at various stage (two received kidney transplants and nine stopped owing to side effects) |
| Free of selective reporting? | Low risk | Primary outcomes for this review (testosterone concentration) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | "24 patients in each group received a sealed box containing the capsules" |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Unclear risk | Patients excluded at various stages (one patient asked to be excluded, two died, one withdrew the consent and three had initial IIEF scores higher than 26) |
| Free of selective reporting? | Low risk | Primary outcomes for this review (IIEF score) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "randomisation table generated by the method of random permuted blocks" |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (IIEF score) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria
| |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer‐generated randomisation sequence |
| Allocation concealment? | Low risk | Patients were randomised into either sildenafil or vardenafil groups by opening pre‐numbered sealed opaque envelopes |
| Blinding? | High risk | Open‐label study |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (IIEF‐5 score) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Low risk | Blinding of participants, investigators and outcomes assessors |
| Incomplete outcome data addressed? | Unclear risk | Data only available for 7/8 patients |
| Free of selective reporting? | High risk | Primary outcomes for this review have not been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (IIEF score) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
| Methods |
| |
| Participants | Inclusion criteria
Exclusion criteria: NS | |
| Interventions | Treatment group
Control group
| |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Stated "randomised" no further information provided |
| Allocation concealment? | Unclear risk | NS |
| Blinding? | Low risk | Blinding of participants and investigators |
| Incomplete outcome data addressed? | Low risk | All patients followed up or accounted for |
| Free of selective reporting? | Low risk | Primary outcomes for this review (plasma gonadotropins concentration) have been reported |
| Free of other bias? | Unclear risk | Funding source: NS |
1,25(OH)2D3 ‐ 1,25 dihydroxycholecalciferol; ED ‐ erectile dysfunction; ESKD ‐ end‐stage kidney disease; FSH ‐ follicle‐stimulating hormone; HD ‐ haemodialysis; IIEF ‐ International Index of Erectile Function; LH ‐ luteinizing hormone; NS ‐ not stated
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not an RCT | |
| One off pharmacokinetic study, not an intervention for sexual dysfunction | |
| Wrong population | |
| Intervention not for sexual dysfunction | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT | |
| Not an RCT |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efficacy and safety of tadalafil 20 mg for the treatment of erectile dysfunction in chronic renal patients in haemodialysis |
| Methods |
|
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | NS |
| Contact information | Bruno SP Carvalho: 558199757974 [email protected] |
| Notes |
NS ‐ not stated
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sexual function using IIEF‐5 Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 PDE inhibitors versus placebo, Outcome 1 Sexual function using IIEF‐5. | ||||
| 1.1 Total score | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 10.65 [5.34, 15.96] |
| 1.2 Erection frequency | 3 | 149 | Mean Difference (IV, Random, 95% CI) | 1.54 [1.14, 1.93] |
| 1.3 Erection quality (Q2) | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 1.78 [1.04, 2.53] |
| 1.4 Penetration ability (Q3) | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 1.70 [1.16, 2.24] |
| 1.5 Maintenance frequency of penetration (Q4) | 4 | 193 | Mean Difference (IV, Random, 95% CI) | 1.60 [1.02, 2.18] |
| 1.6 Maintenance of erection after penetration (Q5) | 4 | 193 | Mean Difference (IV, Random, 95% CI) | 1.83 [1.17, 2.50] |
| 1.7 Erection confidence (Q15) | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 1.39 [0.84, 1.95] |
| 2 Sexual function using IIEF‐15 Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 PDE inhibitors versus placebo, Outcome 2 Sexual function using IIEF‐15. | ||||
| 2.1 Overall satisfaction | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 2.64 [1.32, 3.96] |
| 2.2 Erectile function | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 10.64 [5.32, 15.96] |
| 2.3 Orgasmic function | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.70 [0.35, 3.05] |
| 2.4 Intercourse satisfaction | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.71 [0.11, 3.31] |
| 2.5 Sexual desire | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.67, 1.65] |
| 3 Headache Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 PDE inhibitors versus placebo, Outcome 3 Headache. | ||||
| 4 Improvement in erectile function Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 PDE inhibitors versus placebo, Outcome 4 Improvement in erectile function. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum testosterone Show forest plot | 3 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.05, 2.45] |
| Analysis 2.1  Comparison 2 Zinc versus placebo, Outcome 1 Serum testosterone. | ||||
| 1.1 Zinc in dialysate | 2 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐2.14, 2.55] |
| 1.2 Oral zinc | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 1.62 [0.58, 2.66] |
| 2 Improvement of libido Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Zinc versus placebo, Outcome 2 Improvement of libido. | ||||
| 3 Plasma FSH Show forest plot | 2 | 28 | Mean Difference (IV, Random, 95% CI) | ‐9.69 [‐23.72, 4.34] |
| Analysis 2.3  Comparison 2 Zinc versus placebo, Outcome 3 Plasma FSH. | ||||
| 4 Plasma LH Show forest plot | 2 | 28 | Mean Difference (IV, Random, 95% CI) | 18.80 [‐26.16, 63.76] |
| Analysis 2.4  Comparison 2 Zinc versus placebo, Outcome 4 Plasma LH. | ||||
| 5 Frequency of intercourse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Zinc versus placebo, Outcome 5 Frequency of intercourse. | ||||
| 6 Total/partial impotence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Zinc versus placebo, Outcome 6 Total/partial impotence. | ||||
| 7 Variations in libido Show forest plot | 2 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.83] |
| Analysis 2.7  Comparison 2 Zinc versus placebo, Outcome 7 Variations in libido. | ||||
| 8 Nocturnal penile tumescence (NPT) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 2.8  Comparison 2 Zinc versus placebo, Outcome 8 Nocturnal penile tumescence (NPT). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prolactin Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Vitamin E versus placebo, Outcome 1 Prolactin. | ||||
| 2 Plasma LH Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Vitamin E versus placebo, Outcome 2 Plasma LH. | ||||
| 3 Plasma FSH Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.3  Comparison 3 Vitamin E versus placebo, Outcome 3 Plasma FSH. | ||||
| 4 Serum testosterone Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 3.4  Comparison 3 Vitamin E versus placebo, Outcome 4 Serum testosterone. | ||||

Flow chart showing the number of citations retrieved by individual searches and number of studies included
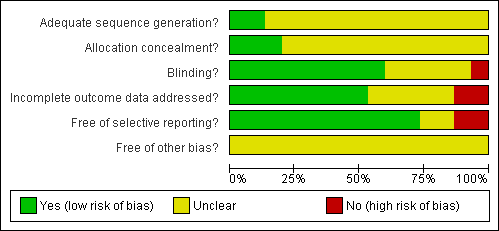
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
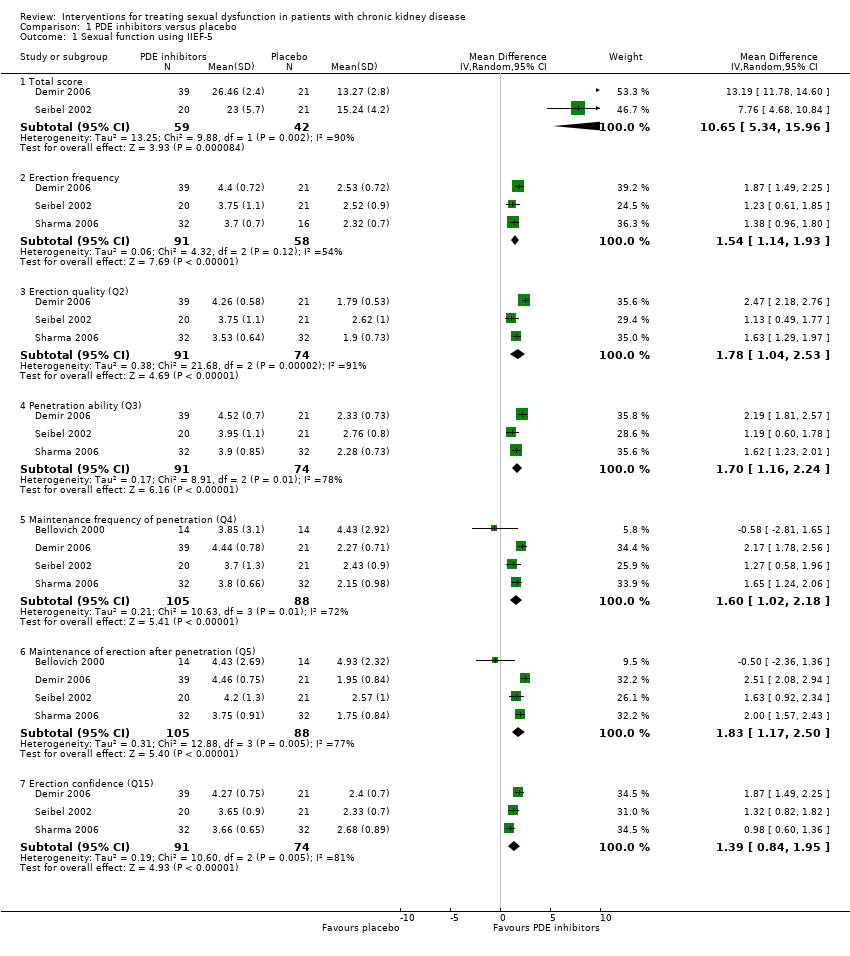
Comparison 1 PDE inhibitors versus placebo, Outcome 1 Sexual function using IIEF‐5.

Comparison 1 PDE inhibitors versus placebo, Outcome 2 Sexual function using IIEF‐15.

Comparison 1 PDE inhibitors versus placebo, Outcome 3 Headache.
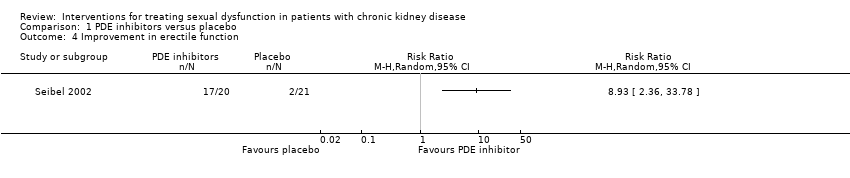
Comparison 1 PDE inhibitors versus placebo, Outcome 4 Improvement in erectile function.

Comparison 2 Zinc versus placebo, Outcome 1 Serum testosterone.

Comparison 2 Zinc versus placebo, Outcome 2 Improvement of libido.

Comparison 2 Zinc versus placebo, Outcome 3 Plasma FSH.

Comparison 2 Zinc versus placebo, Outcome 4 Plasma LH.
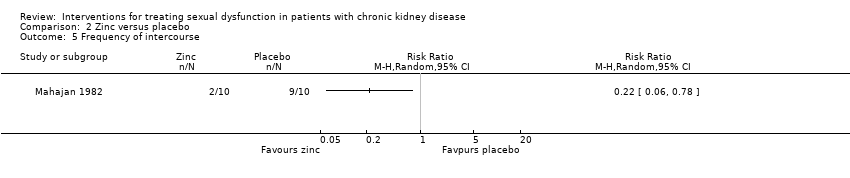
Comparison 2 Zinc versus placebo, Outcome 5 Frequency of intercourse.
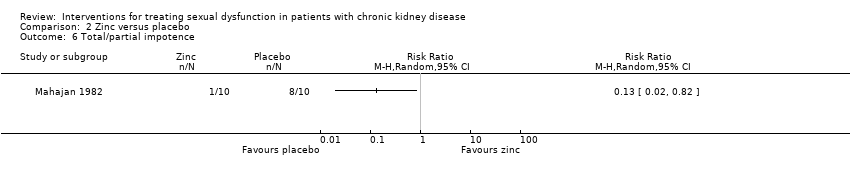
Comparison 2 Zinc versus placebo, Outcome 6 Total/partial impotence.
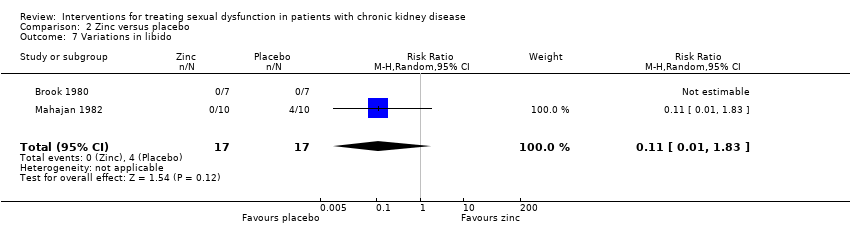
Comparison 2 Zinc versus placebo, Outcome 7 Variations in libido.

Comparison 2 Zinc versus placebo, Outcome 8 Nocturnal penile tumescence (NPT).

Comparison 3 Vitamin E versus placebo, Outcome 1 Prolactin.
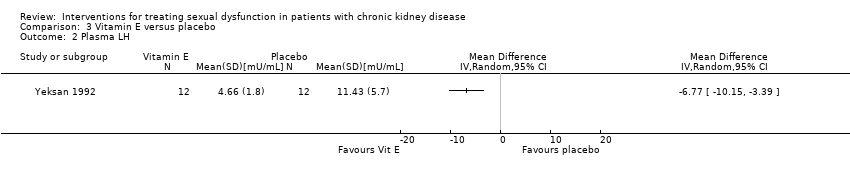
Comparison 3 Vitamin E versus placebo, Outcome 2 Plasma LH.

Comparison 3 Vitamin E versus placebo, Outcome 3 Plasma FSH.
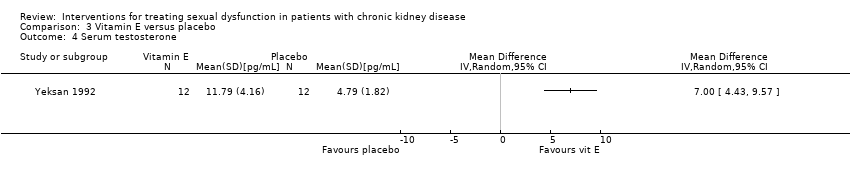
Comparison 3 Vitamin E versus placebo, Outcome 4 Serum testosterone.
| Study ID | N
| Intervention | Outcome | Mean ± SD (treatment group or baseline value) or RR (95% CI) | Mean ± SD (control group or after treatment) |
| 8
| Oral zinc versus placebo | Testosterone concentration (ng/mL)¹ | 8.00 ± 3.50 | 3.20 ± 2.00 | |
| LH (mU/mL)¹ | 85.30 ± 81.00 | 47.30 ± 26.90 | |||
| FSH (mU/mL)¹ | 24.00 ± 24.30 | 33.00 ± 8.70 | |||
| 14
| Sildenafil citrate | IIEF ‐ Frequency of penetration | 3.85 ± 3.10 | 4.43 ± 2.92 | |
| IIEF ‐ Maintenance of erection penetration | 4.43 ± 2.69 | 4.93 ± 2.32 | |||
| 14
| Zinc chloride versus placebo to dialysate | Improvement of libido | 1.00 (0.08 to 13.02) | NA | |
| Plasma testosterone (nmol/L)² | 9.00 ± 4.23 | 12.00 ± 1.32 | |||
| 20 | Oral zinc acetate versus placebo | Total/partial impotence | 0.13 (0.02 to 0.82) | NA | |
| Decreased libido | 0.11 (0.01 to 1.83) | NA | |||
| Decreased frequency of intercourse | 0.22 (0.06 to 0.78) | NA | |||
| Increased plasma testosterone³ | 5.20 ± 1.58 | 3.00 ± 0.95 | |||
| Decreased plasma FSH³ | 25.00 ± 22.14 | 35.00 ± 15.81 | |||
| Decreased plasma LH³ | 49.00 ± 82.22 | 38.00 ± 25.3 | |||
| 13 | Sildenafil citrate versus placebo | Global efficacy question | 2.50 (1.05 to 5.96) | NA | |
| 14 | Bromocriptine versus placebo | Testosterone (nmol/L)¹ | 16.80 ± 4.49 | 17.00 ± 4.11 | |
| 32 | Sildenafil citrate versus placebo | Global efficacy question | 4.33 (2.07 to 9.08) |
| |
| Blood urea nitrogen (mg/dL)² | 18.3 ± 7.6 | 17.9 ± 51.0 | |||
| Creatinine (mg/dL)² | 1.48 ± 0.4 | 1.4 ± 0.4 | |||
| Haemoglobin (g/dL)² | 12.3 ± 1.5 | 13.2 ± 1.4 | |||
| 8 | Oral zinc versus placebo | Tumescence episodes | 0.75 (0.07 to 7.73) | NA | |
| 24 | Vitamin E versus placebo* | Prolactin (ng/mL) | 15.00 ± 4.28 | 56.23 ± 15.66 | |
| LH (mU/mL) | 4.66 ± 1.80 | 11.43 ± 5.70 | |||
| FSH (mU/mL) | 4.23 ± 1.83 | 4.88 ± 2.94 | |||
| Testosterone (pg/mL) | 11.79 ± 4.16 | 4.79 ± 1.82 | |||
| ¹ significance not reported; ² P value not significant; ³ P value < 0.05; * Data pre and post vitamin E treatment only is reported; IIEF ‐ International Index of Erectile Function; LH ‐ luteinizing hormone; FSH ‐ follicular stimulating hormone; SD ‐ standard deviation; RR ‐ relative risk; NA ‐ not applicable or not available. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sexual function using IIEF‐5 Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Total score | 2 | 101 | Mean Difference (IV, Random, 95% CI) | 10.65 [5.34, 15.96] |
| 1.2 Erection frequency | 3 | 149 | Mean Difference (IV, Random, 95% CI) | 1.54 [1.14, 1.93] |
| 1.3 Erection quality (Q2) | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 1.78 [1.04, 2.53] |
| 1.4 Penetration ability (Q3) | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 1.70 [1.16, 2.24] |
| 1.5 Maintenance frequency of penetration (Q4) | 4 | 193 | Mean Difference (IV, Random, 95% CI) | 1.60 [1.02, 2.18] |
| 1.6 Maintenance of erection after penetration (Q5) | 4 | 193 | Mean Difference (IV, Random, 95% CI) | 1.83 [1.17, 2.50] |
| 1.7 Erection confidence (Q15) | 3 | 165 | Mean Difference (IV, Random, 95% CI) | 1.39 [0.84, 1.95] |
| 2 Sexual function using IIEF‐15 Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Overall satisfaction | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 2.64 [1.32, 3.96] |
| 2.2 Erectile function | 2 | 80 | Mean Difference (IV, Random, 95% CI) | 10.64 [5.32, 15.96] |
| 2.3 Orgasmic function | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.70 [0.35, 3.05] |
| 2.4 Intercourse satisfaction | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 1.71 [0.11, 3.31] |
| 2.5 Sexual desire | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.49 [‐0.67, 1.65] |
| 3 Headache Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Improvement in erectile function Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Serum testosterone Show forest plot | 3 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.05, 2.45] |
| 1.1 Zinc in dialysate | 2 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐2.14, 2.55] |
| 1.2 Oral zinc | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 1.62 [0.58, 2.66] |
| 2 Improvement of libido Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Plasma FSH Show forest plot | 2 | 28 | Mean Difference (IV, Random, 95% CI) | ‐9.69 [‐23.72, 4.34] |
| 4 Plasma LH Show forest plot | 2 | 28 | Mean Difference (IV, Random, 95% CI) | 18.80 [‐26.16, 63.76] |
| 5 Frequency of intercourse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Total/partial impotence Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Variations in libido Show forest plot | 2 | 34 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.83] |
| 8 Nocturnal penile tumescence (NPT) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Prolactin Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Plasma LH Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Plasma FSH Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Serum testosterone Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |

