Prostaciclinas en aerosol para el síndrome de dificultad respiratoria aguda (SDRA)
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007733.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 14 agosto 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Atención crítica y de emergencia
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Updated review (2017)
AA, ABB and MA: involved in literature search, quality assessment and data abstraction of trials.
AA and MA: involved in writing the review.
Original published review
All four authors (Arash Afshari, Jesper Brok, Jørn Wetterslev and Ann Merete Møller) were involved in protocol development, literature searching, quality assessment and data abstraction of trials, and writing the review.
Sources of support
Internal sources
-
Cochrane Anaesthesia Critical and Emergency Care Group (CARG), Denmark.
Support from former trial search co‐ordinator (Karen Hovhannisyan) in designing search strategy
External sources
-
No sources of support supplied
Declarations of interest
AA: none known.
ABB: none known.
MA: none known.
Acknowledgements
We would like to thank Dr Peter Dahlem and Dr Shahla Siddiqui for providing further information about their trials.
From the Cochrane Anaesthesia, Critical and Emergency Care Group we would like to thank:
Prof Harald Herkner (content editor), Jing Xie (statistical editor), Neill Adhikari (peer reviewer), Janet Wale (consumer editor), Janne Vendt (trial search co‐ordinator) for their help and editorial advice during the preparation of this updated systematic review.
We would also like to thank Karen Hovhannisyan (former trials search co‐ordinator) for his assistance in providing our different search strategies and Jane Cracknell (Managing Editor) for her valuable assistance during the entire process.
Finally, special thanks to Prof Harald Herkner and Prof Nathan L Pace for their great editorial criticism and assistance, enabling us to improve the overall quality of the first edition of this paper (Afshari 2010).
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Aug 14 | Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS) | Review | Arash Afshari, Anders Bastholm Bille, Mikkel Allingstrup | |
| 2010 Aug 04 | Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) | Review | Arash Afshari, Jesper Brok, Ann Merete Møller, Jørn Wetterslev | |
| 2009 Apr 15 | Aerosolized prostacyclin for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) | Protocol | Arash Afshari, Jesper Brok, Ann Merete Møller, Jørn Wetterslev | |
Differences between protocol and review
May 2017
The title of this review has been changed. Acute lung injury is no longer mentioned in the title of the review. As described in Description of the condition section, the term 'acute lung injury' no longer exists and has instead been replaced by a new ARDS definition based on the severity of hypoxaemia; the title of this updated review reflects this change.
Furthermore, in this updated review, we decided to expand our 'Risk of bias' table. Therefore, we added the following domains: blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. We also added two 'Risk of bias' figures (Figure 2; Figure 3).
We also applied the principles of the GRADE approach to provide an overall assessment of the evidence relating to our outcomes. We constructed a summary of findings Table for the main comparison table using GRADEpro software.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Child; Humans;
PICO

Study flow diagram.
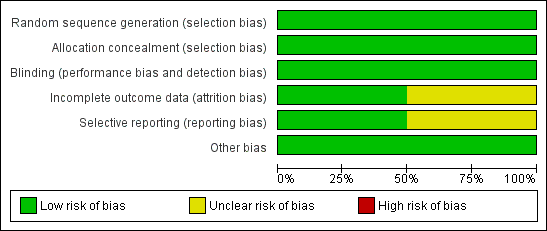
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
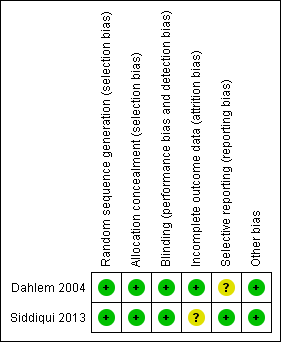
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
| Aerosolized prostacyclin compared to placebo for acute respiratory distress syndrome (ARDS) | ||||||
| Patient or population: people with ARDS Setting: intensive care unit in the Netherlands and Pakistan Intervention: aerosolized prostacyclin Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with aerosolized prostacyclin | |||||
| Mortality | Study population | RR 1.50 | 14 | ⊕⊝⊝⊝ | Only 1 small paediatric trial with cross‐over design provided mortality data (Dahlem 2004). Thus, no meta‐analysis carried out. | |
| 167 per 1000 | 250 per 1000 | |||||
| PaO2/FiO2 ratio5 | ‐ | MD 25.35 lower | ‐ | 67 | ⊕⊝⊝⊝ | Only 1 trial provided data (Siddiqui 2013). Thus, no meta‐analysis was carried out. |
| Improvement in mean pulmonary arterial pressure | ‐ | ‐ | ‐ | ‐ | ‐ | No data is available for meta‐analysis (Characteristics of included studies, Siddiqui 2013) |
| Adverse events7 | ‐ | ‐ | ‐ | 81 (2 studies) | ⊕⊝⊝⊝ | Only descriptive assessment of safety with no available data to carry out meaningful analyses (Dahlem 2004; Siddiqui 2013). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FiO2: fraction of inspired oxygen; MD: mean difference; PaO2: partial pressure of oxygen in arterial blood; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Mortality at 28 to 30 days. 2Required information size for paediatric population depending on the level of heterogeneity adjustment was between 2897 (I2 = 0) and 3862 (I2 = 25%). 4This outcome was downgraded from high to low quality of evidence due to limitations in design (small sample size, few events, cross‐over design) suggesting high likelihood of bias, indirectness of evidence and high probability of publication bias. (Dahlem 2004). 5Despite the fact that biochemical markers of clinical outcomes are often not included in SoF tables, we have chosen to include this outcomes since it is widely used in clinical practice to guide treatment. 6The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size, few events and wide 95% CI suggesting high likelihood of bias and indirectness of evidence. (Siddiqui 2013). 7Adverse events such as bleeding or organ dysfunction 8The outcome was downgraded two levels (from high to very low quality of evidence) for very serious imprecision due to small sample size and few events and since only descriptive assessment of safety and adverse events were provided in the included trials with no data being available for meta‐analyses. | ||||||

