Retirada versus continuación del uso de medicamentos antipsicóticos a largo plazo para los síntomas conductuales y psicológicos en las personas mayores con demencia
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007726.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 01 abril 2018see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Demencia y trastornos cognitivos
- Copyright:
-
- Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
2018 update
EVL: lead author for the review; searched for and selected trials, obtained copies of trial reports and correspondence, 'risk of bias' table, entry of data into RevMan and into GRADEpro, did grading, interpretation of data analyses and drafting review.
MP: selection of included studies and interpretation of data analyses, contributed to the text.
MVD: selection of included studies, interpretation of data analyses, grading and 'risk of bias' table, contributed to the text and text editing.
ADS: interpretation data analyses and grading, contributed to the text.
RVDS: interpretation of data analyses, contributed to the text.
TD: performed previous work that was used in the current review: obtaining copies of trial reports, extraction of data and interpretation of data analyses. Contributed to the text.
TC: arbiter in the selection of trials, interpretation of data analyses and grading, contributed to the text.
Sources of support
Internal sources
-
No internal financial support received for this review, Other
External sources
-
NIHR, UK
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
-
NIHR, UK
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Ellen Van Leeuwen: none known
Mirko Petrovic: none known
Mieke L van Driel: none known
An IM De Sutter: none known
Robert Vander Stichele: none known
Tom Declercq: none known
Thierry Christiaens: none known
Acknowledgements
The review authors are grateful to Sue Marcus, Managing Editor, for her support throughout the review. The review authors would also like to thank Dr Jenny McCleery, Co‐ordinating Editor of the Cochrane Dementia and Cognitive Improvement Group, for her comments and contributions in the process of the review.
Version history
| Published | Title | Stage | Authors | Version |
| 2018 Apr 01 | Withdrawal versus continuation of long‐term antipsychotic drug use for behavioural and psychological symptoms in older people with dementia | Review | Ellen Van Leeuwen, Mirko Petrovic, Mieke L Driel, An IM De Sutter, Robert Vander Stichele, Tom Declercq, Thierry Christiaens | |
| 2013 Mar 28 | Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and psychological symptoms in older people with dementia | Review | Tom Declercq, Mirko Petrovic, Majda Azermai, Robert Vander Stichele, An IM De Sutter, Mieke L van Driel, Thierry Christiaens | |
| 2009 Apr 15 | Withdrawal versus continuation of chronic antipsychotic drugs for behavioural and neuropsychiatric symptoms in elderly patients with dementia | Protocol | Tom Declercq, Mirko Petrovic, Robert Vander Stichele, An IM De Sutter, Mieke L van Driel, Thierry Christiaens | |
Differences between protocol and review
Changes between the 2009 protocol and the 2013 review
Of the nine included studies, only Ruths 2008 established the dementia diagnosis according to DSM‐IV or ICD‐10. Findlay 1989 used ICD‐9 criteria. Devanand 2011 and Devanand 2012 used the clinical diagnoses of dementia by DSM‐IV criteria and probable Alzheimer's disease by NINCDS/ADRDA criteria. All other studies included older participants with dementia diagnosed in another way: Ballard 2008 and Ballard 2004 used new NINCDS/ADRDA criteria for possible or probable Alzheimer's disease, van Reekum 2002 and Bridges‐Parlet 1997 included participants with dementia without any specification (diagnostic criteria unclear). The author of Cohen‐Mansfield 1999 stated in her email (Declerck 2009a [pers comm]) that "she was quite sure all participants had dementia". All studies included, since they all studied participants with dementia. Devanand 2011 and Devanand 2012 included participants aged 50 to 95 years. The Ballard 2008 and Devanand 2012 trials did not report schizophrenia in the exclusion criteria, therefore it may be possible that some participants with dementia and schizophrenia are included in these trials.
Changes between the 2009 protocol and this update
Ellen Van Leeuwen joined the review author team in 2017.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Aged; Humans;
PICO

Forest plot of comparison: 1 Discontinuation versus continuation of long‐term antipsychotic drug use: continuous data, analysis method: mean difference, outcome: 1.1 Behavioural assessment by using Neuropsychiatric Inventory (NPI) measuring neuropsychiatric symptoms (NPS) at 3 months (Ballard 2004 and Ballard DART‐AD) (Analysis 1.1).
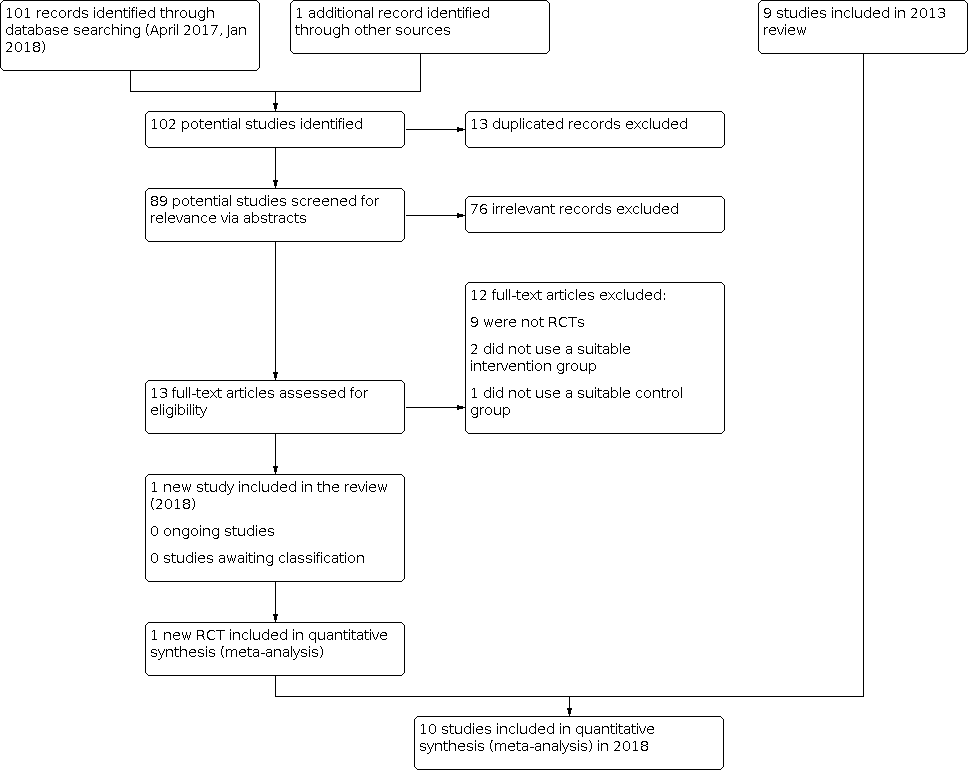
Inclusions of trials of study flow diagram 2018

Risk of bias graph for the 10 included studies in the review.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study in the review.

Comparison 1: Discontinuation versus continuation of long‐term antipsychotic drug use (continuous data, analysis method mean difference), Outcome 1: Behavioural assessment
| Discontinuation compared to continuation of antipsychotic medication for behavioural and psychological symptoms in older participants with dementia | ||||||
| Patient or population: older people with dementia who had been taking an antipsychotic drug for at least 3 months | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk Continuation antipsychotics | Corresponding risk Discontinuation antipsychotics | |||||
| Success of withdrawal from antipsychotics Measured with a variety of outcomes related to failure to complete the study Follow‐up: 1 to 8 months | In 7 studies there was no overall difference in the outcomes reported for success of withdrawal. In two studies of participants with psychosis, aggression or agitation who had responded to antipsychotic treatment, discontinuation accelerated symptomatic relapse without affecting the number of participants experiencing a relapse in one study and was associated with a higher rate of symptomatic relapse in the other study. In one small study a high proportion of the participants in the discontinuation group failed to complete the study. | 575 (9 RCTs) | ⊕⊕⊝⊝ LOWab | Our intended primary outcome, success of withdrawal defined as the ability to complete the study in the allocated study group, i.e. no failure due to worsening of NPS or relapse to antipsychotic drug use, was not reported in any study. We used the difference between groups in the number of non‐completers of the study as a proxy for our primary outcome. However, data could not be pooled due to variability in outcome measures. | ||
| Behavioural and psychological symptoms Assessed with various scales. Follow up: 1 to 8 months | In 2 pooled studies there was no difference in NPI scores between the continuation and discontinuation groups (see Data and analyses and Figure 1). In five non‐pooled studies, there was no difference in the outcomes on scales measuring overall behaviour and psychological symptoms between groups. | 519 (7 RCTs) | ⊕⊕⊝⊝ | Data could only be pooled for 2 studies due to variability in outcome measures. The two pooled studies performed subgroup analyses according to baseline NPI‐score (≤ 14 or > 14). In one study, some participants with milder symptoms at baseline were less agitated at three months in the discontinuation group. In both studies, discontinuation led to worsening of NPS in some participants with more severe baseline NPS. | ||
| Adverse events Assessed with various scales. Follow‐up: 1 to 8 months | In 5 studies, there was no evidence of a difference between groups in adverse events. | 381 (5 RCTs) | ⊕⊕⊝⊝ LOWab | Data could not be pooled due to variability in outcome measures. Adverse events of antipsychotics were not systematically reported. | ||
| Quality of life (QoL) Assessed with DCM or QoL‐AD. Follow‐up: 3 months to 25 weeks | In 2 studies, there was no evidence of an effect on quality of life. | 119 (2 RCTs) | ⊕⊕⊝⊝ | Data could not be pooled due to variability in outcome measures. There was no difference between discontinuation and continuation group in the overall cohort or in subgroups with baseline NPI score above or below the median (14). | ||
| Cognitive function Assessed with various scales. Follow‐up: 1 to 8 months | In 5 studies, there was no evidence of an impact on scales measuring overall cognitive function. In one of these trials, discontinuation improved a measure of verbal fluency. | 365 (5 RCTs) | ⊕⊕⊝⊝ | Data could not be pooled due to variability in outcome measures. | ||
| Use of physical restraint Follow‐up: 1 month | In one study there was no effect on the use of physical restraint. | 36 (1 RCT) | ⊕⊝⊝⊝ | Conclusion made by the authors but not supported by data. | ||
| Mortality Assessed with various scales. Follow‐up: 4 to 12 months | In two studies there was no evidence of an effect on mortality. | 275 (2 RCTs) | ⊕⊝⊝⊝ | Data could not be pooled due to clinical heterogeneity. In a long‐term follow‐up of 36 months after the 12 months randomised discontinuation trial (Devanand 2012), we were uncertain whether discontinuation decreased mortality. | ||
| *The basis for the assumed risk (e.g. the median control group risk across the studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded one level for indirectness. b Downgraded one level for risk of bias. c Downgraded one level for imprecision due to a small number of participants. d Downgraded two level for risk of bias. | ||||||
| Phenothiazines with aliphatic side chain Phenothiazines with piperazine structure Fhenothiazines with piperidine structure Butyrophenone derivatives Indole derivatives Thioxanthene derivatives Diphenylbutylpiperidine derivatives Diazepines, Oxazepines and Thiazepines Benzamides Other antipsychotics |
| Phenothiazines with aliphatic side‐chain N05AA01 Chlorpromazine 0.3 g per os N05AA02 Levomepromazine 0.3 g per os N05AA03 Promazine 0.3 g per os N05AA04 Acepromazine 0.1 g per os N05AA05 Triflupromazine 0.1 g per os N05AA06 Cyamemazine N05AA07 Chlorproethazine |
| Phenothiazines with piperazine structure N05AB01 Dixyrazine 50 mg per os N05AB02 Fluphenazine 10 mg per os N05AB03 Perphenazine 30 mg per os N05AB04 Prochlorperazine 0.1 g per os N05AB05 Thiopropazate 60 mg per os N05AB06 Trifluoperazine 20 mg per os N05AB07 Acetophenazine 50 mg per os N05AB08 Thioproperazine 20 mg per os N05AB09 Butaperazine 10 mg per os N05AB10 Perazine 0.1 g per os N05AB20 Homophenazine |
| Phenothiazines with piperidine structure N05AC01 Periciazine 50 mg per os N05AC02 Thioridazine 0.3 g per os N05AC03 Mesoridazine 0.2 g per os N05AC04 Pipotiazine 10 mg per os |
| Butyrophenone derivatives N05AD01 Haloperidol 8 mg per os N05AD02 Trifluperidol 2 mg per os N05AD03 Melperone* 0.3 g per os N05AD04 Moperon 20 mg per os N05AD05 Pipamperone 0.2 g per os N05AD06 Bromperidol 10 mg per os N05AD07 Benperidol 1.5 mg per os N05AD08 Droperidol N05AD09 Fluanisone |
| N05AE Indole derivatives N05AE01 Oxypertine 0.12 g per os N05AE02 Molindone 50 mg per os N05AE03 Sertindole* 16 mg per os N05AE04 Ziprasidone* 80 mg per os |
| Thioxanthene derivatives N05AF01 Flupentixol 6 mg per os N05AF02 Clopenthixol 0.1 g per os N05AF03 Chlorprothixene 0.3 g per os N05AF04 Tiotixene 30 mg per os N05AF05 Zuclopenthixol 30 mg per os |
| Diphenylbutylpiperidine derivatives N05AG01 Fluspirilene N05AG02 Pimozide 4 mg per os N05AG03 Penfluridol 6 mg per os |
| Diazepines, Oxazepines and Thiazepines N05AH01 Loxapine 0.1 g per os N05AH02 Clozapine* 0.3 g per os N05AH03 Olanzapine* 10 mg per os N05AH04 Quetiapine* 0.4 g per os |
| Benzamides N05AL01 Sulpiride 0.8 g per os N05AL02 Sultopride 1.2 g per os N05AL03 Tiapride 0.4 g per os N05AL04 Remoxipride 0.3 g per os N05AL05 Amisulpride* 0.4 g per os N05AL06 Veralipride N05AL07 Levosulpiride 0.4 g per os |
| Other antipsychotics N05AX07 Prothipendyl 0.24 g per os N05AX08 Risperidone* 5 mg per os N05AX09 Clotiapine 80 mg per os N05AX10 Mosapramine* N05AX11 Zotepine* 0.2 g per os N05AX12 Aripiprazole* 15 mg per os N05AX13 Paliperidone* *atypical antipsychotics |
| * Atypical antipsychotic agents. |
| Study IDI | Setting | Duration | Randomised number | Discontinuation group | Continuation group | Discontinuation schedule | Control | Behavioural inclusion criteria | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Residents in long‐term care facilities | 3 months | 100 | 46 | 54 | Abrupt | Typical APa or risperidone | NPIb not higher than 7 | ||
| Residents in long‐term care facilities | 6 months 12 months | 165 | 82 | 83 | Abrupt | Typical and risperidone | NRc | ||
| Residents in nursing homes | 25 weeks | 19 | 9 | 10 | Tapering over 2 week | Risperidone | NRc | Unpublished study | |
| Residents in long‐term care facilities | 1 month | 36 | 22 | 14 | Abrupt + tapering over 2 weeks | Typical APa | Physically aggressive participants identified by nurse supervisors | ||
| Residents in nursing homes | 7 weeks followed by 7 weeks cross‐over | 58 | 29 | 29 | Tapering over 3 weeks | Typical APa + lorazepam | NRc | Cross‐over study | |
| Residents in the community | 6 months (primary analysis) 12 months | 20 | 10 | 10 | Abrupt + tapering over 2 weeks | Haloperidol | Current symptoms of psychosis, agitation or aggression | Participants had a response to haloperidol open treatment for 20 weeks | |
| Residents in the community and nursing homes | 4 months 8 months | 110 | 70 | 40 | Abrupt + tapering over 2 week | Risperidone | NPIb score higher than 4 on psychosis or agitation/aggression subscale | Participants had a response to risperidone open treatment for 16 weeks | |
| Residents in nursing homes | 1 month | 36 | 18 | 18 | Tapering over 1 week | Thioridazine | NRc | ||
| Residents in nursing homes | 1 month | 55 | 27 | 28 | Abrupt | Haloperidol risperidone, olanzapine | All participants regardless individual symptoms | ||
| Residents in nursing homes | 26 weeks | 34 | 17 | 17 | Tapering over 2 weeks | Typical APa | Stable behaviour | ||
| a AP: antipsychotic drug. b NPI: Neuropsychiatric Inventory. c NR: not reported. | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Behavioural assessment Show forest plot | 2 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐1.49 [‐5.39, 2.40] |

