Contenido relacionado
Revisiones y protocolos relacionados
Andrea Siebenhofer, Sebastian Winterholer, Klaus Jeitler, Karl Horvath, Andrea Berghold, Cornelia Krenn, Thomas Semlitsch | 17 enero 2021
Thomas Semlitsch, Cornelia Krenn, Klaus Jeitler, Andrea Berghold, Karl Horvath, Andrea Siebenhofer | 8 febrero 2021
Raj S Padwal, Diana Rucker, Stephanie K Li, Cintia Curioni, David CW Lau | 20 octubre 2003
Marco I Perez, Vijaya M Musini, James M Wright | 7 octubre 2009
Xiao-Yin Zhang, Sam Soufi, Colin Dormuth, Vijaya M Musini | 5 septiembre 2020
Emily Reeve, Vanessa Jordan, Wade Thompson, Mouna Sawan, Adam Todd, Todd M Gammie, Ingrid Hopper, Sarah N Hilmer, Danijela Gnjidic | 10 junio 2020
Heather O Dickinson, Donald Nicolson, Fiona Campbell, Julia V Cook, Fiona R Beyer, Gary A Ford, James Mason | 19 julio 2006
Meghan J Ho, Edmond CK Li, James M Wright | 3 marzo 2016
Cintia Curioni, Charles André | 18 octubre 2006
Sarah N Stabler, Aaron M Tejani, Fong Huynh, Claire Fowkes | 15 agosto 2012
Respuestas clínicas Cochrane
Meghan Ho | 19 septiembre 2019

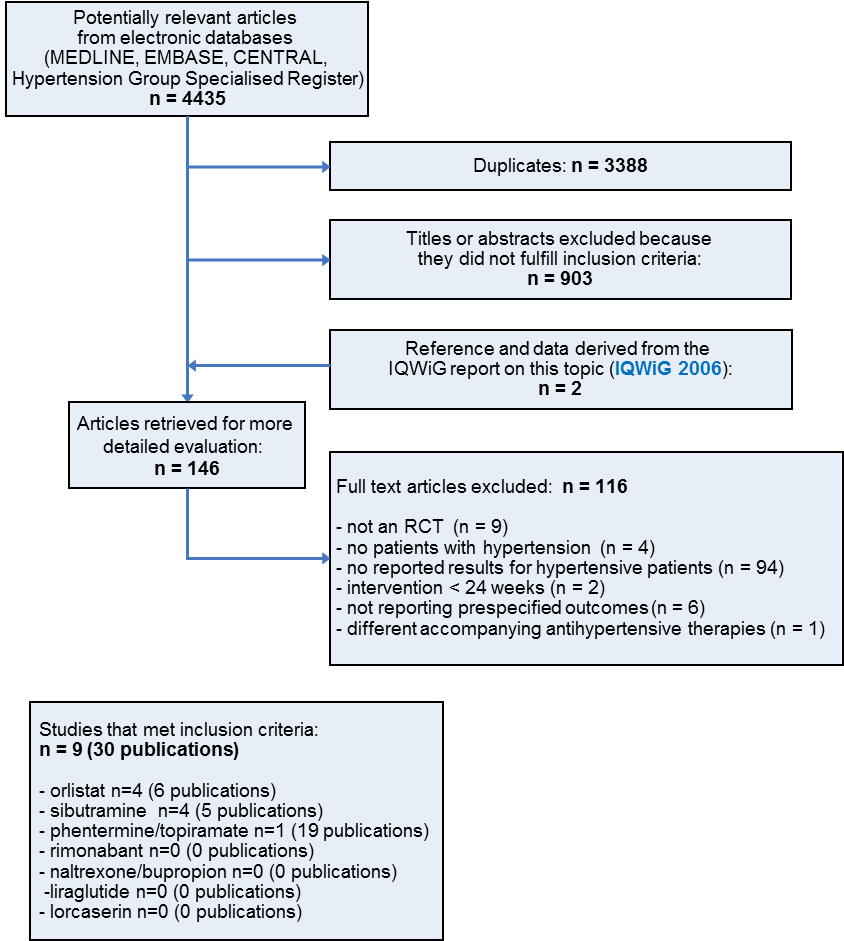

![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.1 Change in systolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG04.png)
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.2 Change in diastolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG05.png)
![Forest plot of comparison: 1 Orlistat versus placebo, outcome: 1.3 Change in body weight from baseline to endpoint [kg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG06.png)
![Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.1 Change in diastolic blood pressure from baseline to endpoint [mm Hg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG07.png)
![Forest plot of comparison: 2 Sibutramine versus placebo, outcome: 2.2 Change in body weight from baseline to endpoint [kg].](/es/cdsr/doi/10.1002/14651858.CD007654.pub4/media/CDSR/CD007654/rel0004/CD007654/image_n/nCD007654-AFig-FIG08.png)





