Heparina no fraccionada versus heparina de bajo peso molecular para evitar la trombocitopenia inducida por heparina en pacientes posoperatorios
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators did not state the method of randomisation neither in the protocol of the study (ClinicalTrials.gov ID NCT00196417) nor in the trial report |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed envelopes to conceal allocation of treatment groups |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel to the assigned treatment group was assured by a special coding of the medications and by the use of placebo injections when necessary |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of investigators who assessed the primary outcome (HIT) was probably not done since participants were assessed by the investigators using the 4T's score and after they were known to be positive in at least one of the HIT tests used |
| Blinding of outcome assessment (detection bias) | Low risk | The study report states that abnormal findings were adjudicated by an investigator blinded to treatment assignment to the participant during the evaluation of venous thrombosis |
| Incomplete outcome data (attrition bias) | High risk | Analyses were conducted 'per protocol'. Attrition accounted for 12% of the randomised participants. Numbers of exclusions and reasons for exclusions were described, but not detailed according to treatment arm. The high ratio of participants with missing data to participants' events might have affected the results |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest for this review have been reported in the pre‐specified way |
| Adequacy of HIT monitoring | Low risk | Assessment of HIT antibodies occurred independently of clinical suspicion of HIT |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes | Funding was provided by the Canadian Institutes of Health Research, the Australian and New Zealand College of Anesthetists Research Foundation, and the Heart and Stroke Foundation of Canada. Study drugs were provided by Pfizer and by Eisai. Neither the funders nor the drug manufacturers played any role in the design or conduct of the trial or in the analysis or interpretation of the data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pharmacist used a centralised electronic system to randomise participants to either intervention group |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was detailed in the study protocol report: "Allocation concealment is ensured by access via a password‐protected Web site or voice‐activated telephone system. Research pharmacists prepare identical syringes for twice‐daily subcutaneous injection of UFH 5000 IU or dalteparin 5000 IU once daily plus once‐daily placebo injection, which are administered by bedside nurses for the duration of ICU stay." |
| Blinding of participants and personnel (performance bias) | High risk | According to correspondence with study authors, the study drugs were given in an open label fashion |
| Blinding of outcome assessment (detection bias) | Unclear risk | The study report did not describe any procedure to blind adjudicators for HIT diagnosis |
| Blinding of outcome assessment (detection bias) | Low risk | Diagnosis of venous thromboembolism was done by two adjudicators who were unaware of study‐group assignments and of one another’s assessments |
| Incomplete outcome data (attrition bias) | Low risk | Losses of follow‐up of the original trial were minor and adequately reported |
| Selective reporting (reporting bias) | Low risk | The trial reports appeared to include all expected outcomes. Therefore, it was probably free of attrition bias. In addition, complementary data were sought from the study authors and used in the analysis. |
| Adequacy of HIT monitoring | Low risk | HIT was diagnosis based on clinical suspicion and appropriate confirmation through laboratory tests |
| Methods |
| |
| Participants |
| |
| Interventions |
| |
| Outcomes |
| |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial report stated the accomplishment of a randomisation process but no information regarding the method used was available |
| Allocation concealment (selection bias) | Unclear risk | Study authors did not report any allocation concealment approach |
| Blinding of participants and personnel (performance bias) | High risk | Study authors did not report the method assuring blinding of participants. Indeed, they describe the study merely as a randomised trial |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of HIT assessment was probably not done since this event was analysed only in secondary analysis years after the original trial |
| Blinding of outcome assessment (detection bias) | Low risk | Clinical events and radiologic studies for the diagnosis of DVT, PE and haemorrhage were interpreted by a committee that was unaware of the assigned treatments |
| Incomplete outcome data (attrition bias) | High risk | Losses of follow‐up of the original trial were minor and adequately reported. However, the selection process for the subgroup of participants used in the secondary analysis to determine the incidence of HIT was conducted according to researchers' convenience. Therefore, one cannot assure that comparativeness allowed by the randomisation process was not missed |
| Selective reporting (reporting bias) | Low risk | Trial report appears to include all expected outcomes and it is probably free of any suggestion of selective reporting |
| Adequacy of HIT monitoring | Low risk | Assessment of HIT antibodies occurred independently of clinical suspicion of HIT |
DVT: deep vein thrombosis
ELISA: enzyme linked immunoassay
HIPA: heparin‐induced platelet activation
HIT: heparin‐induced thrombocytopenia
ICU: intensive care unit
LMWH: low molecular weight heparin
RCT: randomised controlled trial
SD: standard deviation
SRA: serotonin release assay
UFH: unfractionated heparin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This trial includes non operative participants, therefore not meeting our participant inclusion criteria. Moreover, the definition of thrombocytopenia is obscure | |
| This study was excluded because it recruited non operative participants | |
| Participants recruited for this trial were randomised to receive UFH or enoxaparin at the surgical procedure. However, all participants received enoxaparin plus aspirin during 3 days after surgery. The combined intervention (enoxaparin plus aspirin) and the time of prophylaxis for thrombotic events are not in accordance with our predefined inclusion criteria | |
| The study was a comparison of desidurin (a direct thrombin inhibitor) with enoxaparin, thus not meeting our intervention criteria | |
| This trial includes participants treated with heparin for DVT, PE, peripheral arterial embolism and cerebrovascular thromboembolism. It does not meet our entry criteria for type of participants. Additionally, the study did not confirm the diagnosis of HIT through the demonstration of HIT antibodies by functional or enzyme immunoassays | |
| This study assessed thrombocytopenia but it did not confirm the diagnosis of HIT through the demonstration of HIT antibodies by functional or enzyme immunoassays | |
| The study investigated thrombocytopenia, but not HIT | |
| This trial includes non operative participants, not meeting our participant inclusion criteria | |
| This is not a RCT | |
| Eligible participants in this trial were those requiring treatment for acute PE thus it did not evaluate postoperative patients | |
| This study compares clinical outcomes of two treatments for HIT: danaparoid versus dextran 70 | |
| The CORTES Study investigated the incidence and clinical relevance of platelet factor 4/heparin antibodies in people who had acute DVTof the leg and excluded all people submitted to surgical procedures | |
| This trial includes participants not submitted to a surgical procedure, not meeting our participant inclusion criteria. Additionally, UFH in this trial was administered followed by acenocoumarol | |
| This study is a cohort study and enrolled non operated patients | |
| This is a phase II trial so it studied only healthy volunteers | |
| This study was excluded because it focused on the effect of heparin exposure during coronary surgery and only 18.8% of the participants enrolled used heparin after surgery | |
| This is cohort study | |
| This trial includes non operated participants, therefore did not meet our participant inclusion criteria | |
| This study is a CCT, not a RCT | |
| The trial reports on thrombocytopenia, but does not mention HIT in any section | |
| The study was a comparison of rivaroxaban (a direct factor Xa inhibitor) with enoxaparin, thus not meeting our intervention criteria | |
| The main primary outcome in this trial was antibody formation to heparin/platelet factor 4 complexes studied using the SRA and a heparin/platelet factor 4 ELISA. Platelet counts were monitored only into the postoperative day 5. Thus, the study could not assess clinical HIT and is not in accordance with the defined inclusion criteria for this systematic review | |
| This trial excluded any patient who could be submitted to any invasive procedure, therefore it excluded surgical patients | |
| The trial did not measure the outcomes thrombocytopenia nor HIT | |
| This trial defined thrombocytopenia as a platelet count drop of more than 40% and an absolute count decrease below 100 x 109/L on two consecutive measurements with laboratory confirmation by in vitro aggregation tests. It was excluded because the definition of HIT is not in accordance with our previously defined criteria. Moreover, using such a definition for thrombocytopenia in HIT may underestimate cases of the outcome | |
| This study is a CCT, not a RCT | |
| This trial studied participants receiving treatment for venous thromboembolism during pregnancy and puerperium therefore not meeting our type of participant inclusion criteria | |
| This trial studied efficacy and safety of early initiation of warfarin during heparin therapy in acute thromboembolism. The intervention was heparin (UFH or LMHW) followed by warfarin starting within 48 hours or 96 hours. It does not meet our entry criteria for type of intervention | |
| This was a cohort, not a RCT | |
| The article describing the study could not be located. Review authors have exhausted all means of locating further information about this study, but not even the contact information of the authors could be identified | |
| This study was excluded because it assessed thrombocytopenia, not HIT | |
| Eligible participants in this trial were people requiring haemodialysis for acute renal failure or as adjunctive therapy in systematic inflammatory response syndrome therefore not meeting our type of participant inclusion criteria | |
| This is a study protocol for a RCT comparing two different doses of enoxaparin, a LMWH upon commencement of continuous renal replacement therapy | |
| The objective of this trial was to investigate two types of heparins as bridging therapy in the perioperative outpatient management of people receiving chronic VKA therapy. Participants received heparins 2‐4 days before the surgical procedure and restarted on VKA on day 1 after surgery | |
| This trial compares treatment with UFH and warfarin, therefore it is not in accordance with our inclusion criteria | |
| This trial is not a RCT. It studied the cross‐reactivity of HIT sera with fondaparinux in 39 HIT‐confirmed participants. It does not meet our inclusion criteria | |
| The study did not measure the outcome thrombocytopenia nor HIT | |
| This study is a CCT, not a RCT | |
| This trial includes non operated participants, thus not meeting our participant inclusion criteria | |
| This trial tested patient sera from two randomised, double‐blind clinical trials that compared the LMWH (enoxaparin) with another anticoagulant drug, fondaparinux | |
| This trial enrolled non operative participants with unstable angina or non–ST‐segment‐elevation myocardial infarction |
CCT: controlled clinical trial
DVT: deep venous thrombosis
ELISA: enzyme linked immunosorbent assay
HIT: heparin‐induced thrombocytopenia
PE: pulmonary embolism
RCT: randomised controlled trial
SRA: serotonin release assay
UFH: unfractionated heparin
VKA: Vitamin K antagonist
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double‐blind RCT |
| Participants | People requiring thromboprophylaxis |
| Interventions | UFH compared to LMWH (enoxaparin) |
| Outcomes | ‐ DVT and PE ‐ Major bleeding defined as requirement of urgent blood transfusion or fatal ‐ HIT defined as platelet count dropping to half or less than half of admission value after starting thromboprophylaxis |
| Notes | We contacted study authors aiming to clarify the definition of HIT and the availability of data on the subgroup of postoperative participants. No response received as yet |
DVT: deep vein thrombosis
HIT: heparin‐induced thrombocytopenia
LMWH: low molecular weight heparin
PE: pulmonary embolism
RCT: randomised controlled trial
UFH: unfractionated heparin
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heparin‐induced thrombocytopenia (HIT) Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.73] |
| Analysis 1.1  Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 Heparin‐induced thrombocytopenia (HIT). | ||||
| 2 HIT in people undergoing major surgical procedures Show forest plot | 2 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.75] |
| Analysis 1.2  Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 HIT in people undergoing major surgical procedures. | ||||
| 3 HIT complicated by venous thromboembolism Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.84] |
| Analysis 1.3 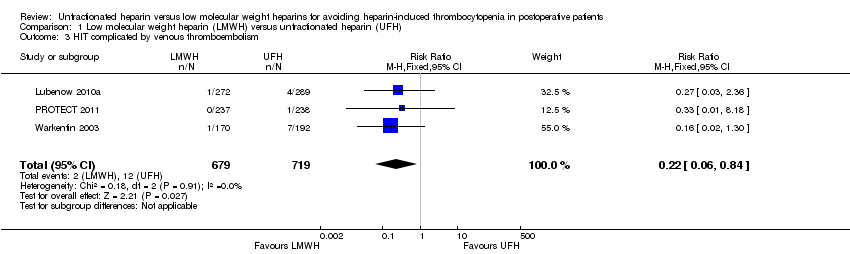 Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 HIT complicated by venous thromboembolism. | ||||

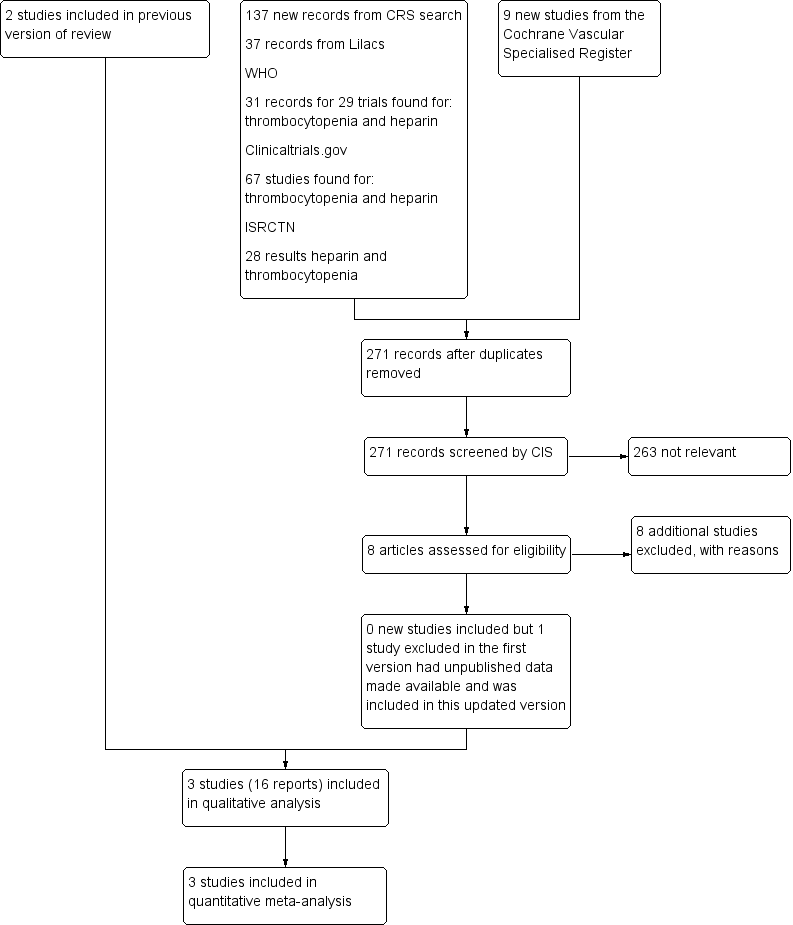
Flowchart illustrating the recruitment of the participants included in and extracted from the study reported by Levine 1991 and Warkentin 2003

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
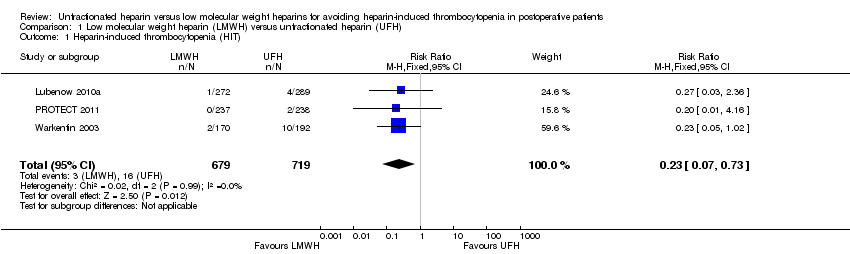
Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 Heparin‐induced thrombocytopenia (HIT).

Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 HIT in people undergoing major surgical procedures.
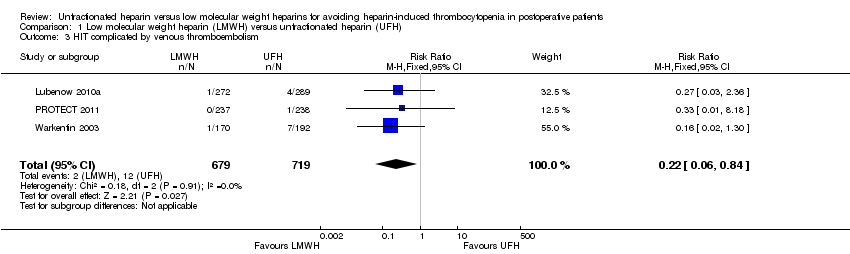
Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 HIT complicated by venous thromboembolism.
| Is unfractionated heparin (UFH) use better than low molecular weight heparin (LMWH) use to avoid heparin‐induced thrombocytopenia? | ||||||
| Patient or population: people undergoing surgical procedures and treated with UFH or LMWH for prophylaxis of thrombotic events lasting at least 5 days | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Heparin‐induced thrombocytopenia (HIT) Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.23 | 1398 | ⊕⊕⊝⊝ | ||
| 22 per 1000 | 5 per 1000 | |||||
| HIT in people undergoing major surgical procedures Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 586 | ⊕⊕⊝⊝ | ||
| 48 per 1000 | 11 per 1000 | |||||
| HIT complicated by venous thromboembolism Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 1398 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 4 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to high risk and unclear risk of bias in the domains: selection bias, performance bias, detection and attrition bias. | ||||||
| Study ID | UFH | Number of participants | Dose | LMWH | Number of participants | Dose | Treatment duration | Time point when plasma samples were obtained for HIT‐IgG antibodies test | Laboratory test for HIT |
| Standard calcium heparin | 192 | 7500 U sc twice daily | Enoxaparin | 170 | 30 mg sc twice daily | Started 12 h‐24 h after surgery and continued for 14 days or until discharge if it occurred sooner | At least 1 plasma sample obtained on postoperative day 7 or later | SRA, with confirmatory investigation for the presence of functional antibodies of IgG class | |
| Standard UFH | 289 | 5000 U SC 3 times daily | Certoparin | 272 | 3000 anti‐factor Xa U sc once daily | Started immediately after admission and continued until day 10 or until discharge. After day 10 all participants received LMWH | Obtained on admission, at discharge (if before | Anti‐platelet factor 4/heparin for immunoglobulin IgG class and platelet‐activating | |
| Standard UFH | 238 | 5000 U sc twice daily | Dalteparin | 237 | 5000 U once daily | At least 5 days of | Data were collected daily in the ICU | Commercially available platelet factor 4 ELISA and SRA | |
| ELISA: enzyme‐linked immunosorbent assay | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heparin‐induced thrombocytopenia (HIT) Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.73] |
| 2 HIT in people undergoing major surgical procedures Show forest plot | 2 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.75] |
| 3 HIT complicated by venous thromboembolism Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.84] |