Heparina no fraccionada versus heparina de bajo peso molecular para evitar la trombocitopenia inducida por heparina en pacientes posoperatorios
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007557.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 21 abril 2017see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Vascular
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
DJ: co‐ordinated the review update, led the review team and wrote the review report. She was responsible for identifying potential studies, extracting data and for formatting the review in line with Review Manager 5 requirements. DJ also assessed the quality of trials selected for inclusion and performed analyses.
LZ: provided expert comments on the updated version of this review, assessed the risk of bias the included trials, and revised the review manuscripts.
EP: conceived the review, revised and provided expert comments on the methodology and text of the review. He also supervised the trial selection and data extraction process regarding the methodology of the trials assessed.
Sources of support
Internal sources
-
Center of Drug Studies, The School of Pharmacy, Federal University of Minas Gerais, Brazil.
Workplace and office supplies
-
Coordination for the Improvement of Higher Level Education, CAPES, Brazil.
-
National Council of Technological and Scientific Development, CNPq, Brazil.
-
The Minas Gerais State Research Foundation (Fapemig), Brazil.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
DJ: has declared that she received funds from the Minas Gerais State Research Foundation (Fapemig, Brazil) to prepare the first version of this review (published 2012). DJ received a scholarship from CAPES during her Master degree (2007 to 2008), when the review title was registered, and she was supported by CNPq with a PhD fellowship (2009 to 2012) when the first version of this review was developed and published.
LZ: none known
EP: has declared that he is employed as Professor by the Universidade Federal de Minas Gerais and has no known conflicts. EP has declared that the review authors received funds from the Minas Gerais State Research Foundation (Fapemig, Brazil) for their research. The first version of this review (published 2012) was completed as part of this research.
Acknowledgements
We warmly thank the co‐authors of the previous version of this review, Maria das Graças Carvalho and Raphael Penholati.
This review would have been impossible without the priceless assistance of Dr Heather Maxwell, previous Managing Editor of the Peripheral Vascular Diseases Group, during the title registration and protocol stages.
Dr Marlene Stewart, current Managing Editor of Cochrane Vascular, also offered invaluable assistance and she was certainly essential in the development of the first version and the update version of this review. We would like to deeply thank Marlene for her support and patience.
The Plain language summary of the first version of this review was kindly revised by Dr Andrew Herxheimer, who passed away in February 2016. It was a great honour to receive the support of Dr Andrew Herxheimer.
Version history
| Published | Title | Stage | Authors | Version |
| 2017 Apr 21 | Unfractionated heparin versus low molecular weight heparins for avoiding heparin‐induced thrombocytopenia in postoperative patients | Review | Daniela R Junqueira, Liliane M Zorzela, Edson Perini | |
| 2012 Sep 12 | Unfractionated heparin versus low molecular weight heparin for avoiding heparin‐induced thrombocytopenia in postoperative patients | Review | Daniela RG Junqueira, Edson Perini, Raphael RM Penholati, Maria G Carvalho | |
| 2009 Jan 21 | Unfractionated heparin versus low molecular weight heparin for avoiding heparin‐induced thrombocytopenia in postoperative patients | Protocol | Daniela RG Junqueira, Edson Perini | |
Differences between protocol and review
In the protocol of the review we planned to exclude participants younger than 18 years old of age. However, we realised that there was no need for this exclusion criterion and we did not consider it when evaluating trials. Nevertheless, none of the trials that were assessed would have been excluded on the basis of this criterion.
We also planned to consider trials in which participants were randomly allocated to receive UFH versus LMWH or one type of heparin versus another anticoagulant therapy. However, this approach does not correspond to the title of the review. In order to keep the review faithful to its scope, we accepted only trials comparing UFH to LMWH.
We planned to extract data about 'death', as a secondary outcome, if it was confirmed by autopsy. However, as this is not a rigorous procedure adopted in clinical trials, we accepted extracting data related to this outcome without autopsy confirmation.
In the updated review, we clarified one strategy that was applied in the first version to classify the frequency of HIT as very common, common, uncommon or rare. The edited text reads: "We described the frequency of HIT in terms of absolute risk. We classified the frequency of the adverse drug reaction events according to WHO‐UMC categories: 'very common' when the frequency was more than 10%, 'common' when the frequency was more than 1% but less than 10%, 'uncommon' when the frequency was more than 0.1% but less than 1%, and 'rare' when the frequency was more than 0.01% but less than 0.1% (WHO 2011).".
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Anticoagulants [*administration & dosage, adverse effects];
- Heparin [*administration & dosage, adverse effects];
- Heparin, Low-Molecular-Weight [*administration & dosage, adverse effects];
- Numbers Needed To Treat;
- Postoperative Complications [chemically induced, *prevention & control];
- Randomized Controlled Trials as Topic;
- Surgical Procedures, Operative [statistics & numerical data];
- Thrombocytopenia [chemically induced, complications, *prevention & control];
- Thrombosis [etiology, *prevention & control];
- Venous Thrombosis [etiology, prevention & control];
Medical Subject Headings Check Words
Humans;
PICO

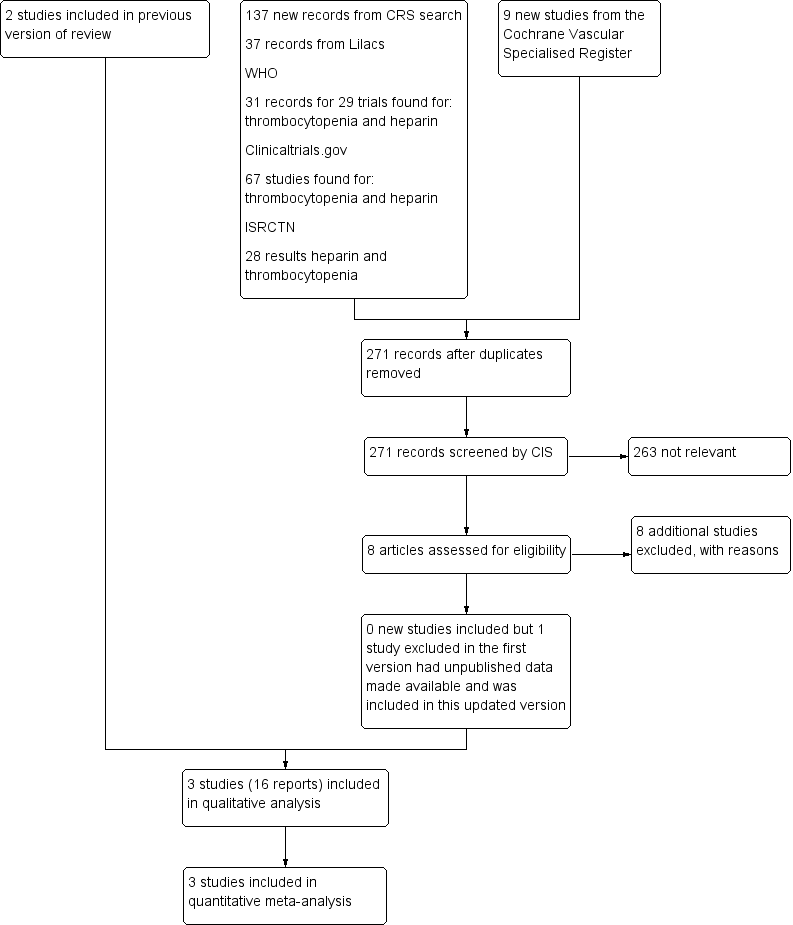
Flowchart illustrating the recruitment of the participants included in and extracted from the study reported by Levine 1991 and Warkentin 2003

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
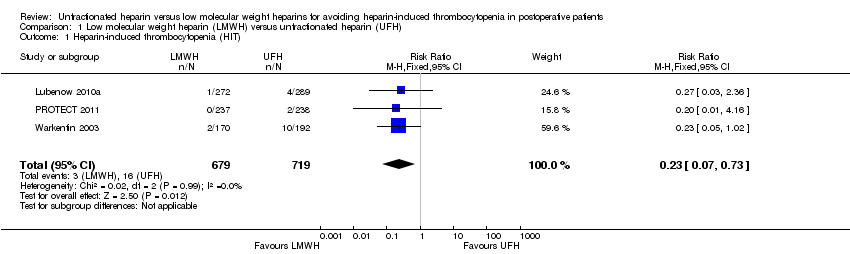
Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 Heparin‐induced thrombocytopenia (HIT).

Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 HIT in people undergoing major surgical procedures.
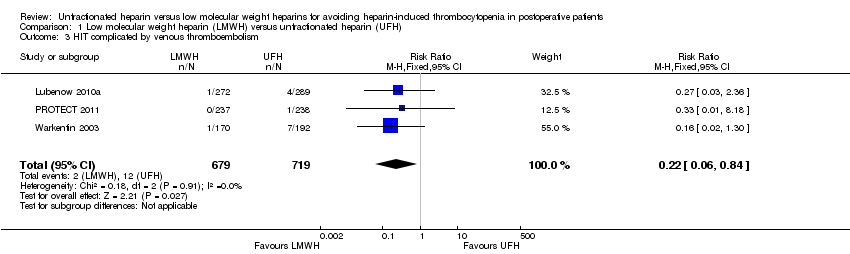
Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 HIT complicated by venous thromboembolism.
| Is unfractionated heparin (UFH) use better than low molecular weight heparin (LMWH) use to avoid heparin‐induced thrombocytopenia? | ||||||
| Patient or population: people undergoing surgical procedures and treated with UFH or LMWH for prophylaxis of thrombotic events lasting at least 5 days | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Heparin‐induced thrombocytopenia (HIT) Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.23 | 1398 | ⊕⊕⊝⊝ | ||
| 22 per 1000 | 5 per 1000 | |||||
| HIT in people undergoing major surgical procedures Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 586 | ⊕⊕⊝⊝ | ||
| 48 per 1000 | 11 per 1000 | |||||
| HIT complicated by venous thromboembolism Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 1398 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 4 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to high risk and unclear risk of bias in the domains: selection bias, performance bias, detection and attrition bias. | ||||||
| Study ID | UFH | Number of participants | Dose | LMWH | Number of participants | Dose | Treatment duration | Time point when plasma samples were obtained for HIT‐IgG antibodies test | Laboratory test for HIT |
| Standard calcium heparin | 192 | 7500 U sc twice daily | Enoxaparin | 170 | 30 mg sc twice daily | Started 12 h‐24 h after surgery and continued for 14 days or until discharge if it occurred sooner | At least 1 plasma sample obtained on postoperative day 7 or later | SRA, with confirmatory investigation for the presence of functional antibodies of IgG class | |
| Standard UFH | 289 | 5000 U SC 3 times daily | Certoparin | 272 | 3000 anti‐factor Xa U sc once daily | Started immediately after admission and continued until day 10 or until discharge. After day 10 all participants received LMWH | Obtained on admission, at discharge (if before | Anti‐platelet factor 4/heparin for immunoglobulin IgG class and platelet‐activating | |
| Standard UFH | 238 | 5000 U sc twice daily | Dalteparin | 237 | 5000 U once daily | At least 5 days of | Data were collected daily in the ICU | Commercially available platelet factor 4 ELISA and SRA | |
| ELISA: enzyme‐linked immunosorbent assay | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heparin‐induced thrombocytopenia (HIT) Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.73] |
| 2 HIT in people undergoing major surgical procedures Show forest plot | 2 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.75] |
| 3 HIT complicated by venous thromboembolism Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.84] |