Heparin tidak terpecah berbanding heparin berat molekul rendah untuk mengelakkan trombositopenia yang disebabkan oleh heparin pada pesakit selepas pembedahan
Abstract
Background
Heparin‐induced thrombocytopenia (HIT) is an adverse drug reaction presenting as a prothrombotic disorder related to antibody‐mediated platelet activation. It is a paradoxical immune reaction resulting in thrombin generation in vivo, which leads to a hypercoagulable state and the potential to initiate venous or arterial thrombosis. A number of factors are thought to influence the incidence of HIT including the type and preparation of heparin (unfractionated heparin (UFH) or low molecular weight heparin (LMWH)) and the heparin‐exposed patient population, with the postoperative patient population at higher risk.
Although LMWH has largely replaced UFH as a front‐line therapy, there is evidence supporting a lack of superiority of LMWH compared with UFH regarding prevention of deep vein thrombosis and pulmonary embolism following surgery, and similar frequencies of bleeding have been described with LMWH and UFH. The decision as to which of these two preparations of heparin to use may thus be influenced by harmful effects such as HIT. We therefore sought to determine the relative impact of UFH and LMWH on HIT in postoperative patients receiving thromboembolism prophylaxis. This is an update of a review first published in 2012.
Objectives
The objective of this review was to compare the incidence of heparin‐induced thrombocytopenia (HIT) and HIT complicated by venous thromboembolism in postoperative patients exposed to unfractionated heparin (UFH) versus low molecular weight heparin (LMWH).
Search methods
For this update, the Cochrane Vascular Information Specialist searched the Specialised Register (May 2016), CENTRAL (2016, Issue 4) and trials registries. The authors searched Lilacs (June 2016) and additional trials were sought from reference lists of relevant publications.
Selection criteria
We included randomised controlled trials (RCTs) in which participants were postoperative patients allocated to receive prophylaxis with UFH or LMWH, in a blinded or unblinded fashion. Studies were excluded if they did not use the accepted definition of HIT. This was defined as a relative reduction in the platelet count of 50% or greater from the postoperative peak (even if the platelet count at its lowest remained greater than 150 x 109/L) occurring within five to 14 days after the surgery, with or without a thrombotic event occurring in this timeframe. Additionally, we required circulating antibodies associated with the syndrome to have been investigated through laboratory assays.
Data collection and analysis
Two review authors independently extracted data and assessed the risk of bias. Disagreements were resolved by consensus with participation of a third author.
Main results
In this update, we included three trials involving 1398 postoperative participants. Participants were submitted to general surgical procedures, minor and major, and the minimum mean age was 49 years. Pooled analysis showed a significant reduction in the risk of HIT with LMWH compared with UFH (risk ratio (RR) 0.23, 95% confidence interval (CI) 0.07 to 0.73); low‐quality evidence. The number needed to treat for an additional beneficial outcome (NNTB) was 59. The risk of HIT was consistently reduced comparing participants undergoing major surgical procedures exposed to LMWH or UFH (RR 0.22, 95% CI 0.06 to 0.75); low‐quality evidence. The occurrence of HIT complicated by venous thromboembolism was significantly lower in participants receiving LMWH compared with UFH (RR 0.22, 95% CI 0.06 to 0.84); low‐quality evidence. The NNTB was 75. Arterial thrombosis occurred in only one participant who received UFH. There were no amputations or deaths documented. Although limited evidence is available, it appears that HIT induced by both types of heparins is common in people undergoing major surgical procedures (incidence greater than 1% and less than 10%).
Authors' conclusions
This updated review demonstrated low‐quality evidence of a lower incidence of HIT, and HIT complicated by venous thromboembolism, in postoperative patients undergoing thromboprophylaxis with LMWH compared with UFH. Similarily, the risk of HIT in people undergoing major surgical procedures was lower when treated with LMWH compared to UFH (low‐quality evidence). The quality of the evidence was downgraded due to concerns about the risk of bias in the included studies and imprecision of the study results. These findings may support current clinical use of LMWH over UFH as front‐line heparin therapy. However, our conclusions are limited and there was an unexpected paucity of RCTs including HIT as an outcome. To address the scarcity of clinically‐relevant information on HIT, HIT must be included as a core harmful outcome in future RCTs of heparin.
PICO
Ringkasan bahasa mudah
Heparin tidak terpecah berbanding heparin berat molekul rendah untuk mengelakkan trombositopenia yang disebabkan oleh heparinpada pesakit selepas pembedahan
Soalan ulasan
Adakah insiden trombositopenia yang disebabkan oleh heparin lebih rendah pada pesakit pasca operasi yang menerima tromboprofilaksis dengan heparin berat molekul rendah berbanding dengan pesakit yang menerima heparin tanpa pecahan?
Latar belakang
Heparin adalah agen semulajadi yang digunakan untuk mencegah pembentukan bekuan di dalam saluran. Dua jenis heparin digunakan secara meluas, heparin tidak terpecah (UFH) dan heparin berat molekul rendah (LMWH). Trombositopenia yang disebabkan oleh heparin (HIT) adalah kesan sampingan yang boleh berlaku semasa dirawat dengan heparin. Ia adalah perkara biasa dalam amalan dan akibat yang paling penting ialah peningkatan paradoks dalam risiko komplikasi pembekuan (tromboembolik). Beberapa faktor dianggap mempengaruhi kekerapannya, termasuk jenis heparin dan jenis pesakit, dengan pesakit yang telah menjalani pembedahan berisiko lebih tinggi. Kami membandingkan risiko HIT pada orang yang telah menjalani pembedahan dan pernah terdedah kepada UFH atau LMWH. Pemahaman yang lebih baik mengenai masalah ini membolehkan pengurusan lebih selamat pada pesakit selepas pembedahan yang memerlukan tromboprofilaksis dengan heparin.
Ciri‐ciri kajian dan keputusan utama
Bukti berkualiti tinggi tentang HIT daripada percubaan rawak terkawal (RCT) adalah jarang. Hanya tiga RCT dengan jumlah 1398 peserta yang sesuai untuk dimasukkan dalam ulasan ini (semasa sehingga Mei 2016). Pesakit selepas pembedahan yang diberi LMWH mempunyai risiko HIT yang lebih rendah daripada mereka yang diberi UFH (nisbah risiko (RR) 0.23, 95% selang keyakinan (CI) 0.07 hingga 0.73); bukti berkualiti rendah. Kejadian HIT yang dirumitkan dengan pembekuan darah adalah jauh lebih rendah pada peserta yang menerima LMWH berbanding dengan UFH (RR 0.22, 95% CI 0.06 hingga 0.84); bukti berkualiti rendah. Dua kes HIT dapat dielakkan bagi setiap 100 orang dirawat dengan LMWH dan bukannya UFH. Kes komplikasi pembekuan HIT dapat dielakkan bagi setiap 75 orang yang dirawat dengan LMWH. Risiko HIT dikurangkan secara konsisten pada orang yang menjalani prosedur pembedahan utama yang terdedah kepada LMWH atau UFH (RR 0.22, 95% CI 0.06 hingga 0.75); bukti berkualiti rendah. Walaupun bukti terhad tersedia, nampaknya HIT yang disebabkan oleh kedua‐dua jenis heparin adalah perkara biasa pada orang yang menjalani prosedur pembedahan utama (insiden lebih besar daripada 1% dan kurang daripada 10%).
Keputusan sistematik ini menyokong cadangan klinikal mengenai pemantauan kiraan platelet untuk HIT.
Kualiti bukti
Bukti yang terkumpul dalam ulasan ini dianggap berkualiti rendah. Kami menurunkan kualiti bukti kerana kami mempunyai kebimbangan tentang risiko berat sebelah dalam kajian yang disertakan dan ketidaktepatan keputusan. Ada kemungkinan bahawa prognosis pesakit atau keutamaan doktor mempengaruhi peruntukan peserta untuk menerima satu atau ubat yang lain. Proses ini harus dilaksanakan secara kebetulan untuk membolehkan perbandingan yang adil antara terapi. Kami tidak yakin bahawa kakitangan yang melaksanakan kajian tidak mengetahui rawatan mana yang diterima oleh peserta, dan berkemungkinan bahawa pelaporan data yang tidak lengkap boleh menjejaskan anggaran. Pengesanan HIT sepanjang percubaan kajian juga mempunyai masalah, dan kami tidak yakin bahawa ia telah dilakukan dengan secukupnya. Keputusan mungkin betul, tetapi ia mungkin diubah dengan penyelidikan masa depan.
Authors' conclusions
Summary of findings
| Is unfractionated heparin (UFH) use better than low molecular weight heparin (LMWH) use to avoid heparin‐induced thrombocytopenia? | ||||||
| Patient or population: people undergoing surgical procedures and treated with UFH or LMWH for prophylaxis of thrombotic events lasting at least 5 days | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Heparin‐induced thrombocytopenia (HIT) Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.23 | 1398 | ⊕⊕⊝⊝ | ||
| 22 per 1000 | 5 per 1000 | |||||
| HIT in people undergoing major surgical procedures Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 586 | ⊕⊕⊝⊝ | ||
| 48 per 1000 | 11 per 1000 | |||||
| HIT complicated by venous thromboembolism Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 1398 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 4 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to high risk and unclear risk of bias in the domains: selection bias, performance bias, detection and attrition bias. | ||||||
Background
Description of the condition
Heparin is a commonly used medication worldwide since it is essential in the treatment and prophylaxis of thromboembolic disorders. There are two types of heparin drugs comprising unfractionated heparin (UFH), also known as standard heparin, and low molecular weight heparin (LMWH). LMWH is constituted by a group of several drugs (for example, enoxaparin, dalteparin, nadroparin, tinzaparin, certoparin) (Hirsh 2004). LMWH has been largely replacing UFH as front‐line therapy as it is judged to be at least as efficacious in preventing thromboembolic complications and in inducing fewer bleeding adverse outcomes (Alikhan 2014; Barrera 2013; Falck‐Ytter 2012; Gould 2012; Kahn 2012; Schulman 2008). However, similar efficacy and risks have been described (Akl 2014; Handoll 2002; Wille‐Jørgensen 2003), as well as insufficient and low‐quality evidence (Bain 2014; Barrera 2013). Inadequate assessment of adverse effects is also demonstrated even in the most recent evidence (Di Nisio 2016).
Although haemorrhagic events are the main recognised risk of heparin use, heparin‐induced thrombocytopenia (HIT) is a potentially severe, morbid complication of heparin therapy. HIT is a prothrombotic disorder defined as a relative reduction in platelet count of about 50% (even if the platelet count at its lowest remains greater than 150 x 109/L) occurring within five to 14 days after the start of heparin therapy (Warkentin 2003; Warkentin 2003a; Warkentin 2006). People re‐exposed after a recent treatment may develop a rapid onset of HIT within 24 hours of heparin administration (Warkentin 2009). Also, a less frequent delayed onset of HIT, when it occurs after discontinuation of heparin, has been described (Smythe 2005; Warkentin 2001). Formerly designated as white clot syndrome or HIT type II, it is considered an acquired hypercoagulability syndrome caused by an immune‐mediated reaction which is commonly followed by venous or arterial thrombosis (Greinacher 1995; Hong 2003; Walenga 2000; Warkentin 1995).
Description of the intervention
The mechanism of HIT is an immune response to heparin exposure which appears to be a misdirected host defence mechanism (Greinacher 2015; Prechel 2013). Platelet factor 4 (PF4), which is released by activated platelets, undergoes a conformational change binding to heparin and forming an immunogenic complex PF4/heparin (Brandt 2014). IgG antibodies recognise this immunogenic complex, thus determining an immunocomplex (PF4/heparin/IgG) (Amiral 1992; Joglekar 2015; Kelton 1994). The immunocomplex formed by PF4/heparin/IgG (HIT antibodies) promotes platelet activation and aggregation (Januzzi 2000; Warkentin 1994a), which leads to intravascular platelet consumption. HIT antibodies also activate monocytes and endothelium, which increase thrombin generation (Greinacher 2015; Joglekar 2015; Rauova 2010; Tutwiler 2016). In a few HIT cases (less than 10%) only IgA or IgM antibodies against PF4/heparin complexes are detectable (Amiral 1996), but their clinical importance remains uncertain (Bircher 2006; Warkentin 2009). Considering that people requiring antithrombotic therapy with heparin may be bedridden, at least to some extent, the procoagulant state together with vascular injury and stasis may be a central mechanism of the venous and arterial thrombosis associated with HIT. Therefore, HIT may be a devastating adverse effect of heparin use and lead to loss of life or limb.
The diagnosis of HIT requires the combination of clinical likelihood and laboratory tests to detect platelet activation induced by the HIT antibodies (Keeling 2006). The functional assays,14C‐serotonin release assay (SRA) and heparin‐induced platelet activation assay (HIPA), present the most favourable sensitivity and specificity trade‐off (Warkentin 2008), as they demonstrate the presence of clinically relevant antibodies (Otis 2010). The platelet aggregation assay is not generally recommended (Leo 2003). Also, a number of commercial enzyme‐linked immunoassays (ELISA) are available to diagnose HIT. These immunoassays represent an ideal test to rule out HIT but they may be combined with functional assays to confirm a diagnosis, since they detect both pathogenic and non‐pathogenic antibodies (Otis 2010). Better approaches to interpreted optical density in enzyme immunoassays have been investigated (Greinacher 2010), and scoring systems may also be helpful to help the diagnosis of HIT in clinical practice (Crowther 2014; Cuker 2010; Joseph 2015; Junqueira 2011a; Lo 2006).
How the intervention might work
HIT can occur following any mode of heparin administration (Januzzi 2000; Warkentin 2008), including parenteral infusions (Smythe 2005), subcutaneous therapy (Girolami 2003), and even with low‐grade exposures such as heparin line flushes or following the insertion of heparin‐bonded pulmonary artery catheters (Mureebe 2004). All sources of heparin must be suspended when the reaction occurs and the rationale for the treatment is the use of direct thrombin inhibitors and anti‐factor Xa agents (Warkentin 2008).
The precise incidence of HIT varies due to different definitions of thrombocytopenia in HIT and due to distinctive methods to demonstrate the HIT antibodies (Warkentin 2008). Moreover, the development of HIT is associated with the type of heparin used (UFH or LMWH) and the type of heparin‐exposed patient population (Warkentin 2008). The incidence of HIT appears to be higher with the use of bovine heparin when compared with porcine heparin (Ahmad 2007; Francis 2003).
Overall, an absolute risk of HIT induced by UFH or LMWH has been estimated to be approximately 2% to 3% and 0.2% to 0.6%, respectively (Martel 2005; Warkentin 2003). This association of HIT with the type of heparin may be justified by a different immunogenicity attributed to UFH as it has a higher molecular weight and degree of sulphation. The high‐risk subgroup is constituted of postoperative patients receiving UFH (incidence estimated between 1% to 5%) (Warkentin 2008). Postoperative patients receiving LMWH show a lower risk of HIT (incidence estimated between 0.1% to 1%) as do medical and obstetrical patients exposed to subcutaneous UFH (Girolami 2003). Specific characteristics of the patients and of certain surgery types have been shown to influence the risk profile of HIT (Lubenow 2010; Warkentin 2000), but most of the studies comprise patients after orthopaedic surgery.
Timing is also essential for the adequate diagnosis of HIT since this is an immune response mediated by B lymphocytes. Although there are exceptions, typically the induction of the immune response causing HIT takes at least five days to manifest. In surgery settings, platelet counts drop due to destruction or transfusions, reaching a nadir in days 2 to 4 and subsequently a peak (Linkins 2012; Greinacher 2015). This can mislead the clinical diagnosis of HIT. The relative reduction in the platelet count of 50% or greater from the postoperative peak (even if the platelet count at its lowest remains greater than 150 x 109/L) allows an adequate assessment of HIT.
Why it is important to do this review
Heparin‐induced thrombocytopenia is an important adverse drug reaction and delayed recognition contributes to patient morbidity and mortality (Rice 2005). However, there is a lack of robust evidence supporting knowledge on the frequency of HIT, which weakens the decision‐making therapeutical process. Postoperative patients are a group of people at high risk of developing HIT, and also at high risk of thrombotic disorders like deep vein thrombosis and pulmonary embolism. Therefore, we aimed to compare the risk of HIT in postoperative patients exposed to UFH or LMWH.
Objectives
The objective of this review was to compare the incidence of heparin‐induced thrombocytopenia (HIT) and HIT complicated by venous thromboembolism in postoperative patients exposed to unfractionated heparin (UFH) versus low molecular weight heparin (LMWH).
Methods
Criteria for considering studies for this review
Types of studies
This review included randomised controlled trials (RCTs) in which participants were postoperative patients allocated to receive UFH or LMWH, in a blinded or unblinded fashion. Studies were excluded if they did not use the accepted definition of HIT. This was defined as a relative reduction in the platelet count of 50% or greater from the postoperative peak (even if the platelet count at its lowest remained greater than 150 x 109/L) occurring within five to 14 days after the surgery, with or without a thrombotic event occurring in this timeframe. Additionally, we required circulating antibodies associated with the syndrome to have been investigated through functional or immunological laboratory assays.
Types of participants
We included people undergoing surgical procedures and treated with UFH or LMWH for prophylaxis of thrombotic events lasting at least five days.
Types of interventions
We were interested in the incidence of HIT occurring during prophylaxis with either UFH or LMWH after any surgical intervention. In order to achieve this objective, we studied RCTs in which participants were postoperative patients allocated to receive UFH or LMWH in a blinded or unblinded fashion.
Types of outcome measures
Primary outcomes
The main outcome of interest was the occurrence of HIT. The accepted definition of HIT was relative reduction in the platelet count of 50% or greater from the postoperative peak (even if the platelet count at its lowest remained greater than 150 x 109/L) occurring within five to 14 days after the surgery and confirmed through laboratory assays. The onset of a thromboembolic event in the time frame defined above could also prompt suspicion of HIT, though all clinically suspected cases needed to have the diagnosis of HIT confirmed through the demonstration of HIT antibodies by functional or immunological laboratory tests.
We also considered cases of early‐onset or delayed‐onset HIT as long as they had properly performed laboratory tests for HIT. These outcomes are defined as follows:
-
early‐onset is when HIT develops within 24 hours of heparin administration;
-
delayed‐onset is when HIT is diagnosed after the discontinuation of heparin.
Secondary outcomes
The secondary outcomes investigated were HIT complicated by the following events:
-
venous thromboembolism (presenting clinically as deep vein thrombosis, pulmonary embolism, or both);
-
arterial thrombosis (presenting clinically as myocardial infarction, stroke, or other artery thrombosis);
-
amputation;
-
death.
All secondary outcomes had to be confirmed by an objective method (Büller 2003; EMEA 1998; EMEA 2008; Prandoni 2005), depending on the specific situation, as follows:
-
Arterial thrombosis:
-
arteriography for an arterial thrombosis investigation;
-
electrocardiography with enzymatic support in the case of suspected myocardial infarction;
-
cerebral computed tomography (CT) scan or magnetic resonance imaging (MRI) in the case of suspected stroke.
-
-
Venous thromboembolism had to be confirmed by at least one objective test:
-
ascending contrast venography;
-
duplex venous ultrasonography, MRI or venography in the case of suspected deep vein thrombosis (DVT);
-
ventilation/perfusion lung scan, pulmonary angiogram or spiral CT lung scan for clinical diagnosis of pulmonary embolism.
-
Recurrent venous thrombosis would also be considered as a secondary outcome and the criteria for its diagnosis included abnormal venous ultrasonography where compression had been normal or there was a substantial increase (4 mm or more) in the diameter of the thrombus during full compression.
Search methods for identification of studies
We performed electronic and manual searches with no restriction on language.
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials:
-
The Cochrane Vascular Specialised Register (May 2016);
-
The Cochrane Central Register of Controlled Trials (CENTRAL, 2016, Issue 4) via the Cochrane Register of Studies Online.
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings that have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com).
The CIS also searched the following trials registries for details of ongoing and unpublished studies in May 2016 using the terms thrombocytopenia and heparin:
-
ClinicalTrials.gov (www.clinicaltrials.gov)
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch)
-
ISRCTN Register (www.isrctn.com/)
In addition, the review authors updated their search of Lilacs (iAHx interface) using the search strategy shown in Appendix 2.
Searching other resources
We searched the reference lists of relevant articles.
Data collection and analysis
Selection of studies
For this 2016 update, two review authors, Daniela RG Junqueira (DJ) and Edson Perini (EP) screened study titles and abstracts. We obtained full‐text articles of studies considered potentially relevant and assessed them for eligibility. We resolved disagreements by consensus. We contacted trialists to request further information when study reports lacked data to allow a decision on its inclusion or exclusion and also in order to clarify the definition of HIT used or to obtain detailed data needed to perform analyses.
Data extraction and management
To collect data on each trial, we used a standard form that addressed characteristics of the trials and participants:
-
details of trial design;
-
setting where trial was conducted;
-
eligibility criteria and trial exclusion criteria;
-
number of participants randomised for each intervention group;
-
mean age of participants;
-
losses to follow‐up;
-
randomisation and concealment allocation method;
-
type of heparin used (dose, commencement of therapy relative to surgery, duration of therapy);
-
definition of HIT;
-
time points when clinical and laboratory measurements were made to diagnose HIT during the study;
-
type of laboratory assay performed to confirm HIT;
-
number of primary and secondary outcomes (as mentioned in the section Criteria for considering studies for this review).
For this update, two review authors (DJ and EP, or DJ and Liliane Zorzela (LZ)) independently carried out data extraction. We applied the same standardised data extraction form used in the first version of the review and sought additional information from the trialists when needed.
Assessment of risk of bias in included studies
Two review authors, DJ and LZ assessed the internal validity of the included studies following the current recommended approach for assessing risk of bias in studies included in Cochrane Reviews (Higgins 2011a). We assessed the following domains in the 'Risk of bias' tool:
-
selection bias (random sequence generation);
-
selection bias (allocation concealment);
-
performance bias (blinding of participants and personnel);
-
detection bias (blinding of outcome assessment): HIT;
-
detection bias (blinding of outcome assessment): HIT complicated by venous thromboembolism;
-
attrition bias (incomplete outcome data);
-
reporting bias (selective reporting);
-
adequacy of HIT monitoring.
Measures of treatment effect
We extracted dichotomous data and calculated the absolute risk (incidence) of HIT with corresponding 95% confidence interval (CI).
We described the frequency of HIT in terms of absolute risk. We classified the frequency of the adverse drug reaction events according to WHO‐UMC categories: 'very common' when the frequency was more than 10%, 'common' when the frequency was more than 1% but less than 10%, 'uncommon' when the frequency was more than 0.1% but less than 1%, and 'rare' when the frequency was more than 0.01% but less than 0.1% (WHO 2011).
We used outcome frequencies to estimate the risk ratio (RR) with 95% CI in the meta‐analysis.
Unit of analysis issues
Throughout the systematic review and in all statistical analyses, we defined participants, not limbs, as the unit of analysis.
Dealing with missing data
We contacted the authors of trials when there was a lack of data presented. We also sought clarifications whenever facing unclear data.
Assessment of heterogeneity
We tested statistical heterogeneity using the Chi2 test (Deeks 2011) and I2 statistic (Higgins 2003). We used the Chi2 test to assess whether observed differences in results were compatible with chance alone and the I2 statistic to quantify inconsistency across studies.
Assessment of reporting biases
We obtained data from full papers and sought unpublished data. However, there were insufficient studies to provide appropriate visual inspection for asymmetry on the scatter plot and therefore we did not use funnel plots to look for publication bias.
Data synthesis
The outcomes of interest in this review were dichotomous and we recorded the number of participants who developed the outcomes according to the allocated group of heparin type. We calculated the risk of HIT according to the type of heparin used. We used outcome frequencies to estimate the risk ratio (RR) with 95% CI in the meta‐analysis. We used a fixed‐effect model for pooling data. In the event of a statistical difference, we calculated the relative risk reduction (RRR) and the number needed to treat for an additional beneficial outcome (NNTB).
Subgroup analysis and investigation of heterogeneity
Because of a lack of trials, we could not carry out a subgroup analysis exploring the effect of different laboratory methods for the diagnosis of HIT or the different types of heparins. One subgroup analysis, not planned in advance, was possible. Thus, we performed an analysis exploring the risk of HIT with LMWH versus UFH in people undergoing major surgical procedures (as opposed to people undergoing any, that is major or minor, surgical procedure). Major surgical procedures include procedures involving inner body cavities, usually requiring general anaesthesia and posing a high risk to the patient (Earl 1917).
Sensitivity analysis
We had planned to perform a sensitivity analysis exploring heterogeneity according to factors previously stated (methodological quality, blinding method performed, unpublished studies, study sample size, age of participants, gender of participants, drug posology) if sufficient numbers of trials were identified.
Summary of findings
For this update, we presented the main findings of the review results concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data for the most relevant outcomes of this review in summary of findings Table for the main comparison, created according to Schünemann 2011 and Atkins 2004. We used the GRADE profiler Guideline Development Tool (GRADEpro GDT) software (www.guidelinedevelopment.org) to assist in the preparation of the 'Summary of findings' table. We used the system developed by the GRADE working group for grading the quality of evidence as high, moderate, low and very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of population bias (Atkins 2004).
We included the following outcomes according to their priority:
-
HIT;
-
HIT complicated by venous thromboembolism;
-
HIT in participants undergoing major surgical procedure
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
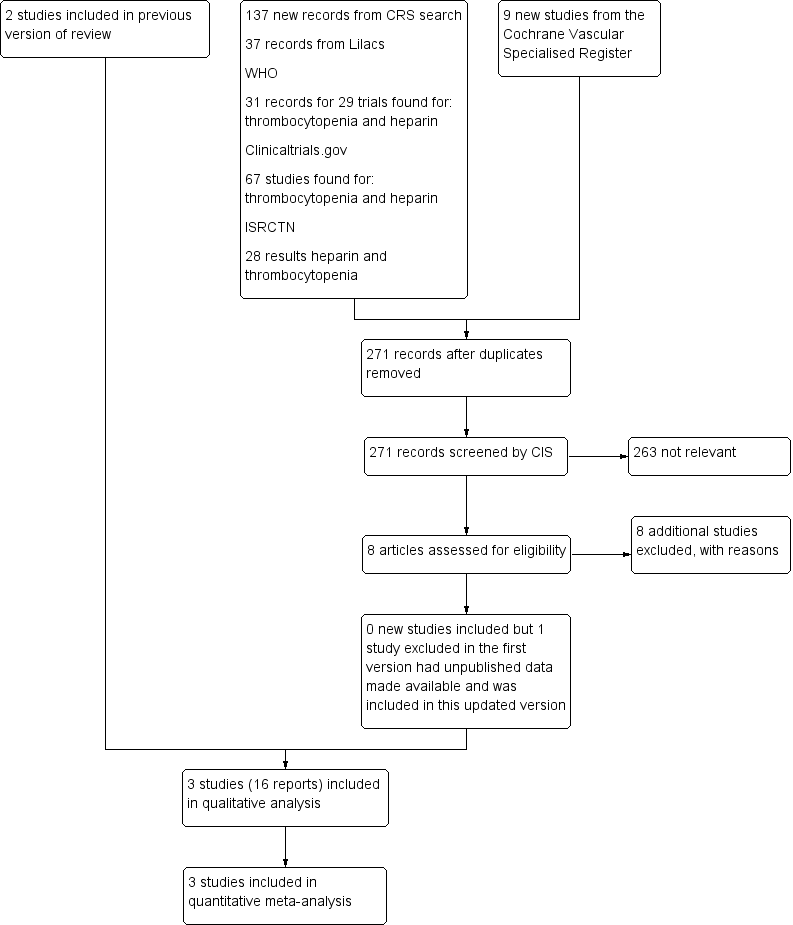
See Figure 1.

Flow diagram
Included studies
One new study was included for this 2016 update (PROTECT 2011), as we were able to acquire a subset of data related to postoperative patients who received, at least, five days of heparin (UFH or LMWH). PROTECT 2011 was previously excluded as we did not have these data. Two studies were included in the previous version (Lubenow 2010a; Warkentin 2003).
In total, in our updated analysis, we have included data from 1398 postoperative participants recruited in hospitals located in Germany (Lubenow 2010a), Canada and Germany (Warkentin 2003), and in Canada, Australia, Brazil, Saudi Arabia, the USA, and the UK (PROTECT 2011).
One included study (Warkentin 2003), compared the incidence of HIT in orthopaedic postoperative participants allocated to one of two groups treated with standard heparin or enoxaparin, a type of LMWH. This study represents a secondary analysis using participants enrolled in a major clinical trial (Levine 1991). The participants were originally recruited to study the efficacy and safety of enoxaparin compared with standard calcium heparin for the prevention of postoperative DVT in people undergoing elective hip replacement (total hip arthroplasty). A total of 665 participants were randomised in the original trial and 362 underwent systematic monitoring for HIT and had the presence of HIT antibodies determined (n = 192 in the UFH group, n = 170 in the LMWH group). A flowchart illustrating the sequence of participants enrolled in these two studies is provided (Figure 2).

Flowchart illustrating the recruitment of the participants included in and extracted from the study reported by Levine 1991 and Warkentin 2003
Participants enrolled in this trial received treatment until postoperative day 14 or until discharge. Preoperative and daily postoperative platelet counts were measured for all participants and the peak postoperative platelet count represented the platelet count baseline. Plasma samples used to investigate HIT were collected around postoperative day 7 or later (Warkentin 2003). The study confirmed the presence of functional antibodies of HIT through the SRA. Warkentin 2003 reported "serotonin release assay was considered positive if the sample caused greater than 20% serotonin release at 0.1 U/mL heparin, less than 20% serotonin release at 100 U/mL heparin, and less than 20% serotonin release at 0.1 U/mL heparin in the presence of Fc receptor blocking monoclonal antibody". Samples tested positive in the SRA were screened through enzyme immunoassay to confirm the presence of antibodies of the IgG class in the sample.
The trial reported by Lubenow 2010a compared the incidence of HIT in people admitted to a trauma surgery department (n = 614). Although 8.6% of the participants enrolled were not submitted to a surgical procedure, we could extract data separately for participants who underwent major or minor surgical procedures (n = 561). Participants in this study received UFH (n = 289) or certoparin (n = 272), a type of LMWH, until median postoperative day 10. Daily platelet counts were measured and HIT was clinically defined when a participant scored four or more points on the 4T's score system. The 4T's score system is a risk assessment tool that classifies patients according to their probability for having HIT based on the sum of points attributed to four clinical features of HIT (magnitude of thrombocytopenia, timing of thrombocytopenia regarding heparin exposure, occurrence of thrombosis or other sequelae, and the absence of other explanations for the thrombocytopenia) (Lo 2006; Warkentin 2003a). Considering the controlled design of the trial, any participant with a relative reduction in the platelet count of 50% or greater from the postoperative peak, occurring within days five to 14 after the surgery, or presenting with a thromboembolic event in this time frame would score four points in the score system and therefore fall within the definition of HIT specified for the inclusion in this systematic review. The study confirmed the presence of functional antibodies of HIT through the HIPA test and an in‐house enzyme immunoassay for IgG, IgM and IgA (cut off 0.5 optical density units).
The PROTECT 2011 study was a multicentre trial planned to test the superiority of the LMWH, dalteparin, over the UFH in people admitted to the intensive care unit (ICU). Intention‐to‐treat analysis included 3746 participants; 1873 assigned to receive UFH and 1873 assigned to receive LMWH. Both interventions were administered for a median duration of seven days (interquartile range, 4 to 12). HIT was reported if the platelet count was less than 50 x 109/L or decreased more than 50% of the baseline value, or if otherwise suspected. An anti‐PF4–polyanion enzyme immunoassay was performed and a platelet 14C‐serotonin–release assay was performed to confirm HIT. Correspondence with the study authors made data available on 475 participants who were admitted to the trial with a surgical diagnosis or had to undergo a surgical procedure after being enrolled in the study (n = 238 in the UFH group, n = 237 in the LMWH group). These participants received heparin for at least five days in an open‐label fashion (information stated in the study author's email).
Details of the study design are shown in the table Characteristics of included studies and in Table 1.
| Study ID | UFH | Number of participants | Dose | LMWH | Number of participants | Dose | Treatment duration | Time point when plasma samples were obtained for HIT‐IgG antibodies test | Laboratory test for HIT |
| Standard calcium heparin | 192 | 7500 U sc twice daily | Enoxaparin | 170 | 30 mg sc twice daily | Started 12 h‐24 h after surgery and continued for 14 days or until discharge if it occurred sooner | At least 1 plasma sample obtained on postoperative day 7 or later | SRA, with confirmatory investigation for the presence of functional antibodies of IgG class | |
| Standard UFH | 289 | 5000 U SC 3 times daily | Certoparin | 272 | 3000 anti‐factor Xa U sc once daily | Started immediately after admission and continued until day 10 or until discharge. After day 10 all participants received LMWH | Obtained on admission, at discharge (if before | Anti‐platelet factor 4/heparin for immunoglobulin IgG class and platelet‐activating | |
| Standard UFH | 238 | 5000 U sc twice daily | Dalteparin | 237 | 5000 U once daily | At least 5 days of | Data were collected daily in the ICU | Commercially available platelet factor 4 ELISA and SRA |
ELISA: enzyme‐linked immunosorbent assay
HIPA: heparin‐induced platelet activation
h: hours
HIT: heparin‐induced thrombocytopenia
ICU: intensive care unit
LMWH: low molecular weight heparin
mg: milligrams
sc: subcutaneously
SRA: serotonin release assay
U: units
UFH: unfractionated heparin
Excluded studies
For this update, we excluded an additional eight studies (Avidan 2011; Brambila 1998; Kakkar 2014; Lastória 2006; Polanco 1997; Robinson 2014; Santamaria 2013; Schwartsmann 1996).
We excluded a total of 41 trials due to one or more of the following reasons: nonoperative participants (Ahmad 2003; Ansell 1980; Bailey 1986; Berkowitz 2001; Chen 2005; CORTES Study; Daskalopoulos 2005; Fier 2011; Harenberg 1996; Lage 2007; Mitic 2010; Reeves 1999; Wang 2006; Yeh 2007); no randomisation (Brambila 1998; Eika 1980; Funk 2000; Huhle 2000; Mahlfeld 2002; Oliveira 2008; Savi 2005; Stenske 1998); irrelevant intervention (studies of drugs used to treat HIT), combined intervention (heparin plus another drug or heparin compared with a different anticoagulant drug) or comparison not in accordance with the inclusion criteria (Assadian 2008; Avidan 2011; Chong 2001; Daskalopoulos 2005; Francis 2003; Kanan 2008; Mohiuddin 1992; Robinson 2014; Santamaria 2013; Sarduy 2004; Savi 2005; Warkentin 2005); length of treatment with heparin (Francis 2003; Mohiuddin 1992); definition or monitoring of HIT not in accordance with the inclusion criteria of this review (Bell 1980; Bergqvist 1997; Kakkar 2014; Konkle 2001; Lastória 2006; Leyvraz 1991; Schwartsmann 1996; Powers 1984). Despite our efforts, we could not locate one study (Polanco 1997).
As stated above, some excluded studies reported the assessment of HIT but applied a definition not in accordance with the current accepted definition, which is detailed in the Criteria for considering studies for this review section. We describe below the diverse definitions for thrombocytopenia and HIT used in these studies. More information about our reasons for the exclusion of these studies are detailed in Characteristics of excluded studies.
No reporting on thrombocytopenia nor HIT
Two studies did not report any assessment or results regarding thrombocytopenia nor HIT: Schwartsmann 1996, Lastória 2006.
Thrombocytopenia
Two studies (Bell 1980; Powers 1984), compared the use of UFH from different sources (bovine lung versus porcine intestinal mucosa). Thrombocytopenia was defined as platelet count less than 100 x 109/L (Bell 1980), and as platelet count less than 150 x 109/L on at least two determinations taken 24 hours apart (Powers 1984). Per‐protocol analysis of the 149 participants randomised to receive bovine or porcine heparin in the study by Bell 1980 estimated that 13 out 50 participants (26%, 95% CI 15.87 to 39.55) developed thrombocytopenia in the bovine heparin arm, and 8 out of 90 participants (8.08%, 95% CI 3.81 to 15.76) developed thrombocytopenia in the porcine heparin group. According to these results, bovine heparin would be associated with an increased risk of thrombocytopenia (RR 3.22, 95% CI 1.43 to 7.25). Analysis of all randomised participants included in the study by Powers 1984 observed that five out 65 participants (7.69%, 95 CI 3.31 to 16.78) exposed to bovine heparin developed thrombocytopenia while none of the participants exposed to porcine heparin was diagnosed with thrombocytopenia.
One study (Bergqvist 1997) defined thrombocytopenia as a platelet count below 70 x 109/L and accessed in a per‐protocol analysis of participants randomised to receive UFH or LMWH (enoxaparin). The incidence of thrombocytopenia according to this definition was 1.27% (95% CI 0.49 to 3.21) in the UFH group of participants (4/319) and equal to 0.32% (95% CI 0.06 to 1.79) in the LMWH arm.
The study by Kakkar 2014 compared the effects of semuloparin (an ultra LMWH) started postoperatively versus enoxaparin started preoperatively in major abdominal surgery. It reports thrombocytopenia related to treatment‐emergent events without any clear definition of the meaning of the terminology applied. Thrombocytopenia was therefore reported to occur in 52 out of 2177 participants in the LMWH group (2.39%, 95% CI 1.81 to 3.12), and in 36 out of 2175 (1.66%, 95% CI 1.20 to 2.28) of the semuloparin group.
HIT during the first five days of therapy
HIT assessed through platelet counts on the postoperative days one to five, serotonin‐released assay (SRA) and enzyme‐linked immunoassays (ELISA), was reported by Konkle 2001. During this limited observation period, no cases of thrombocytopenia were associated with antibody formation, and thus no diagnosis of HIT was made among the 98 participants randomised to receive bovine heparin (n = 49) or porcine heparin (n = 49) for cardiopulmonary bypass.
HIT defined as platelet count of less than 40% and an absolute count below 100 x 109/L on two consecutive measurements, followed by in vitro aggregation test
One open‐label randomised trial investigated HIT defined as a drop in platelet count of more than 40% and absolute count less than 100 x 109/L on two consecutive measurements, confirmed by in vitro aggregation tests (Leyvraz 1991). Two participants receiving UFH developed HIT according to this definition (2/175; 1.15%, 95% CI 0.29 to 4,53), while no participant was reported with a diagnosis of HIT among participants receiving the LMWH fraxiparine (0/175).
Studies awaiting classification
We were not able to acquire data from postoperative participants in one newly identified study (ISHI 2013). We contacted the study authors, but have not yet received any data; or information on data available for sharing up to submitting this review, thus the study is considered 'awaiting classification'. See Characteristics of studies awaiting classification for further details.
Risk of bias in included studies
Details are described in Characteristics of included studies. Visual information on the risk of bias is provided (Figure 3, Figure 4). The risk of bias of the included trials varied from low to high, and it was unclear for some domains in some studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We classified selection bias due to random sequence generation as unclear risk in Warkentin 2003 and Lubenow 2010a, since they provided no information regarding the method of randomisation. One study used an adequate method of generation of the randomisation and we assessed it as low risk of bias for this domain (PROTECT 2011).
We considered one study (Warkentin 2003) at unclear risk of selection bias due to allocation concealment due to no reporting of any concealment allocation approach. Two studies reported adequate methods of concealment allocation and we judged them at low risk of bias (Lubenow 2010a; PROTECT 2011).
Blinding
We classified two studies (PROTECT 2011; Warkentin 2003) as having high risk of bias for blinding of participants and personnel (performance bias). In PROTECT 2011, correspondence with the study authors confirmed that the study drugs were given in an open label fashion. The study of Warkentin 2003 was reported as merely a randomised trial, and no method of blinding was detailed. We judged blinding of participants and personnel adequate in one trial (Lubenow 2010a), that is, at low risk of bias.
We classified one trial (Warkentin 2003) as having high risk of bias for blinding of outcome assessment (detection bias) regarding HIT. Our judgement considered that blinding of HIT assessment was probably not done since this event was analysed only in secondary analysis years after the original trial. We also judged blinding of outcome assessment (detection bias) of high risk in Lubenow 2010a since participants were assessed by the investigators using the 4T's score and after they were known to be positive in at least one of the HIT tests used. This could possibly have introduced systematic differences between groups and how outcomes were determined.
We judged that PROTECT 2011 was at unclear risk of bias since it did not describe any procedure to blind adjudicators for HIT diagnosis.
We classified all trials (Lubenow 2010a; PROTECT 2011; Warkentin 2003) as having low risk of bias for blinding of outcome assessment (detection bias) regarding venous thromboembolism in , since they describe adequate methods on this domain.
Incomplete outcome data
We considered two trials (Lubenow 2010a; Warkentin 2003) at high risk of bias for incomplete outcome assessment (attrition bias). In particular, one study (Warkentin 2003) represented a secondary analysis using participants enrolled in a major clinical trial. A total of 665 participants were randomised in the original trial (Levine 1991) and Warkentin 2003 determined the presence of HIT antibodies with the ability to activate platelets in a subgroup consisting of 362 participants in whom serial plasma samples were available. Losses to follow‐up in the original trial were minor and adequately reported. However, the selection process for the subgroup of participants used in the secondary analysis regarding HIT was not described and may have been conducted according to the researcher's convenience. Therefore, one cannot be confident that the randomisation process was respected.
Lubenow 2010a conducted analysis 'per protocol' and did not detail numbers of exclusions and reasons for exclusions according to treatment arm.
We deemed the PROTECT 2011 trial at low risk of bias (PROTECT 2011), since losses to follow‐up of the original trial were minor and adequately reported. In addition, we sought complementary data from the study authors and used them in the analysis.
Selective reporting
We considered all the included studies to be at low risk of reporting bias since the trials' reports appeared to include all expected outcomes.
Other potential sources of bias
Adequacy of HIT monitoring
We paid special attention to the appropriateness of HIT monitoring and of the diagnosis process according to the clinical and serological profile of the syndrome (see Table 1 and Characteristics of included studies). Addressing this issue is essential to avoid under‐recognition of the condition. All of the included studies performed systematic assays to determine the presence of functional antibodies related to HIT and we judged them to be at low risk of bias.
Effects of interventions
Primary outcome: HIT
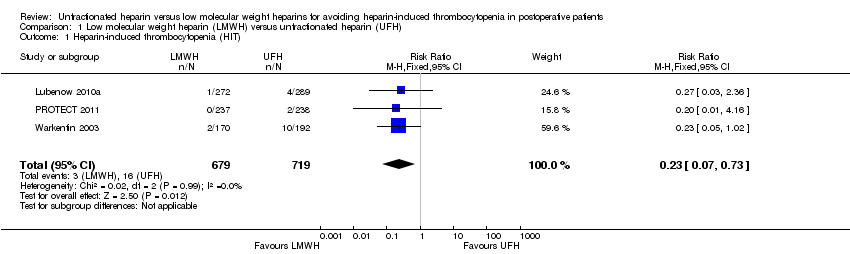
Three studies involving 1398 participants provided data to a pooled analysis evaluating the risk of HIT comparing LMWH versus UFH (Lubenow 2010a; PROTECT 2011; Warkentin 2003). The analysis indicated a significant reduction in the risk of HIT with LMWH compared with UFH (RR 0.23, 95% CI 0.07 to 0.73; low‐quality evidence; Analysis 1.1). There was no evidence of heterogeneity (P = 0.99; I2 = 0%). This result suggests that people treated with LMWH would have a RRR of 77% in the probability of developing HIT compared with people receiving UFH, and 1000 people would need to receive LMWH rather than UFH for 16 people to avoid HIT (NNTB = 59). The absolute incidence of HIT was estimated as equal to 0.44% (95% CI 0.15 to 1.29) in participants exposed to LMWH (uncommon reaction), and equal to 2.23% (95% CI 1.37 to 3.58) in participants receiving UFH (common reaction).
Combining the subgroup of participants undergoing major surgical procedures was possible since one study included only this type of participants (Warkentin 2003), and another presented extractable data according to type of surgical procedure (Lubenow 2010a). In these trials, major surgical procedures consisted of people undergoing hip arthroplasty (Warkentin 2003); fracture of humerus, hip/pelvis, femur, head of tibia, tibia, or knee endoprosthesis (Lubenow 2010a). The absolute incidence of HIT in participants submitted to major surgical procedures was estimated as equal to 1.02% (95% CI 0.35 to 2.96) in participants exposed to LMWH, and equal to 4.79% (95% CI 2.88 to 7.89) in participants receiving UFH. In both cases, HIT would be classified as a common adverse drug reaction. The meta‐analysis (the scores of 586 participants undergoing major surgical procedures) demonstrated a significant reduction in the risk of HIT with LMWH compared with UFH (RR 0.22, 95% CI 0.06 to 0.75; low‐quality evidence; Analysis 1.2). There was no evidence of heterogeneity (P = 0.93; I2 = 0%). The RRR of HIT with LMWH versus UFH is equal to 78%, and 26 people would need to be treated with LMWH rather than UFH for one additional person avoid HIT (NNTB = 26).
Secondary outcomes
HIT complicated by venous thromboembolism
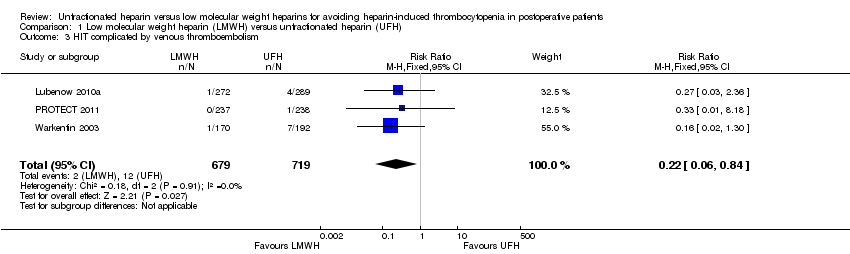
The absolute incidence of HIT complicated by venous thromboembolism was estimated as equal to 0.29% (95% CI 0.08 to 1.07) in participants exposed to LMWH, and equal to 1.67% (95% CI 0.91 to 2.89) in participants receiving UFH. In both cases, HIT would be classified as an uncommon adverse drug reaction. Pooled analysis showed a significant reduction in HIT complicated by venous thromboembolism with LMWH compared with UFH (RR 0.22, 95% CI 0.06 to 0.84; low‐quality evidence, Analysis 1.3), with no evidence of heterogeneity (P = 0.91; I2 = 0%). This result indicates that people using LMWH would have a RRR of 78% for developing HIT complicated by venous thromboembolism, and 75 people would need to be treated with LMWH rather than UFH to avoid one additional case of HIT (NNTB = 75). Three studies involving 1398 participants provided data to compare the risk of HIT complicated by venous thromboembolism in people receiving LMWH versus UFH (Lubenow 2010a; PROTECT 2011; Warkentin 2003).
HIT complicated by arterial thrombosis, amputation, or death
Arterial thrombosis occurred in one participant who received UFH (Warkentin 2003). There were no cases of HIT complicated by amputations, deaths or recurrent venous thromboses documented in any of the included studies.
Discussion
Summary of main results
An adverse drug reaction is defined as "a response to a drug which is noxious and unintended, and which occurs at doses normally used in humans for the prophylaxis, diagnosis, or therapy of a disease, or for the modification of a physiological function" (ASHP 1995; EMEA 2006; WHO 1972). Adverse drug reactions are the most clinically significant medication‐related problems, and evidence continues to mount that adverse reactions to medicines are a common cause of illness, disability and even death (Ernst 2001; Forster 2004; Handler 2007; Lazarou 1998; WHO 1994).
Heparin‐induced thrombocytopenia (HIT) is an adverse drug reaction induced by exposure to heparin. It is a potentially morbid syndrome since its main consequence is a hypercoagulate state associated with an increased risk of thrombotic events. However, despite its clinical importance and life‐threatening presentation, HIT continues to be unrecognised, and most physicians think that they have never seen a case (Levine 2005). Heparins are one of the most widely used drugs in hospitals worldwide, and heparin is an essential drug in many clinical settings, from cardiovascular and surgical interventions to haemodialysis and pregnancy management. Postoperative patients in particular are a subgroup with an important risk of developing thromboembolic complications. The surgical procedure itself and the associated mobility restrictions contribute to this exacerbated occurrence of thromboembolic events. Moreover, it has been shown that this subgroup of patients is at a higher risk of developing HIT (Warkentin 2008). Therefore, increasing the knowledge of the incidence of HIT in postoperative patients should improve the clinician's decision‐making processes.
We carried out a comprehensive search of the medical literature. Although we identified up to 11 potentially eligible trials to be included in our review, only three studies fulfilled the inclusion criteria for this review. We included two randomised controlled trials in the first version this systematic review, and an additional trial in this updated version, thanks to additional data provided by the trialists. See summary of findings Table for the main comparison. We judged the totality of the evidence analysed to be of low quality, which means that new data could provide evidence that the effect estimate is substantially different. We downgraded the evidence because of serious concerns over study risk of bias and imprecision.
The incidence of HIT was shown to be reduced by the use of LMWH in comparison with UFH. If confirmed, the results may be of substantial clinical relevance and will corroborate data from non‐randomised studies (Girolami 2003; Warkentin 2008). The rates of HIT complicated by venous thromboembolism between heparin types were also significantly different when comparing LMWH and UFH. Clinical significance was also seen since one HIT case may be avoided for 75 treated people. The determination of HIT and HIT complicated by venous thromboembolism as common events in postoperative patients receiving UFH, and as uncommon events following LMWH exposure is relevant for pharmacovigilance activities. In particular, people from developing countries without access to laboratory tests to confirm or rule out HIT because of financial limitations, could benefit from the use of a medication proved to reduce the occurrence of a dangerous adverse drug reaction such as HIT.
Interestingly, subgroup analysis exploring the risk of HIT in people who had undergone major surgical procedures showed that this subgroup presented a similar risk of HIT in comparison with the general sample of patients. Only two trials provided data to this analysis. Of note, the observed incidence of HIT in people undergoing major surgical procedures and treated with LMWH was higher than previously described in the scientific literature. This finding could indicate that, at least in people submitted to undergo major surgery, HIT is a frequent occurrence following exposure to UFH and LMWH.
Overall completeness and applicability of evidence
There are some limitations in these results. The number of clinical trials that we identified that were able to provide data to answer the question addressed by this systematic review was small. Moreover, the sample sizes of the trials included in the analysis were underpowered to detect HIT. From the frequencies demonstrated by the studies individually, the power to detect HIT was equal to 56.0% (Warkentin 2003) and to 24.6% (Lubenow 2010a) (results from OpenEpi, Version 2 (www.openepi.com), open source calculator‐power RCT, two‐sided test at a significant level of 95%). The remaining trial (PROTECT 2011) contributed only a subgroup of the total study population, which was also a limitation. The subgroup analysis also used a small sample size. Although there was no evidence of statistical heterogeneity, the studies presented some level of clinical and methodological diversity. For instance, doses of heparin and duration of treatment were different between studies. Moreover, from a methodological view point, the follow‐up strategy for HIT assessment differed between the studies. It is difficult to determine exactly if and how these characteristics could affect the results. Another issue which should be considered is the existence of the different types of standard heparin which are commercially available in different countries. Whereas in the USA and in Europe, bovine UFH is no longer produced, because of the bovine spongiform encephalopathy epidemic (Blossom 2008; Brown 2001), in developing countries, the delivery of heparins extracted from both bovine and porcine animal sources remains a reality (Junqueira 2011).
Quality of the evidence
Three RCTs provided the evidence included in this review, involving 1398 postoperative patients, of which 679 received LMWH (enoxaparin or certoparin) and 719 received UFH. We judged the quality of the evidence for all the outcomes assessed: HIT, HIT complicated by venous thromboembolism and HIT in people undergoing major surgical procedures, to be of low quality. We downgraded the evidence due to serious concerns over individual study risk of bias (high or unclear risk of bias in the domains selection bias, performance bias and attrition bias, and high or unclear risk of bias related to the detection of HIT) and imprecision (due to the small number of events and large confidence interval). See summary of findings Table for the main comparison.
Due to these limitations, we judged that the evidence showing that the incidence of HIT could be significantly reduced with the administration of LMWH instead of UFH was of low quality. In the first version of this systematic review, we considered that the evidence available was not strong enough to support a definitive conclusion regarding HIT as a more preventable adverse drug reaction by using LMWH compared with the use of UFH. We still believe that we need more reliable evidence to consider HIT as a preventable adverse drug reaction by the use of LMWH.
Potential biases in the review process
Randomised controlled trials are largely used to study therapeutic interventions. However, the emphasis on treatment benefit, together with omission of information on harmful effects, could misinform anyone trying to make balanced decisions. Therefore, this review has drawn on the knowledge that harmful effects of any intervention may be reviewed with similar rigour as treatment benefits (Loke 2007). Although non‐randomised trials may be an optimised approach to evaluate data on rare harmful effects, we opted to limit this review to RCTs only, due to HIT being a widely acknowledged adverse drug reaction occurring from short‐term heparin use. Any trial assessing heparin use could also assess HIT incidence and report accordingly. We followed a well‐recognised methodology, which assures the quality of the Cochrane Reviews for interventions (Higgins 2011). We avoided publication bias by searching numerous databases and performing manual citation tracking. However, it was not evaluated by objective methods because of the small numbers of studies included. Despite our efforts, we identified a limited number of clinical trials which fitted the inclusion criteria.
The resulting low number of studies identified to answer the clinical question of this review may illustrate a limitation of RCTs to accurately evaluate adverse effects induced by drug use, since this design is typically applied to study positive outcomes, particularly those with a higher frequency. However, considering the available body of knowledge related to HIT, and the fact that HIT is not a rare event, any clinical trial studying heparins must be designed to consider monitoring of HIT. It is an important limitation, and even unethical, when clinical trials are conducted focusing on just the efficacy or when they do not perform screening for a well‐known and potentially dangerous adverse reaction to the drug under study.
We are aware that the systematic evaluation of adverse effects of drugs may require other study designs, mainly non‐randomised ones. The methodology for inclusion of observational studies in Cochrane systematic reviews to improve data on harmful effects such as HIT are evolving. We believe that the continuous search for high‐quality studies focusing on HIT and the ongoing development of the methodology for systematic reviews of adverse effects according to Cochrane quality standards (Cochrane 2010; Loke 2007), may improve the evidence on the risk of HIT highlighted in this systematic review. Of relevance, pharmaceutical industries and researchers involved in clinical trials regarding heparins have the responsibility to use high quality methods to assess HIT.
Agreements and disagreements with other studies or reviews
The incidence of HIT among people exposed to heparin is highly variable and is influenced by the type of heparin used (UFH or LMWH) and the type of heparin‐exposed patient population. One important evidence‐based clinical practice guideline summarises the various risk factors for HIT and classifies them into three categories: high risk (incidence greater than 1.0%), intermediate risk (incidence ranging from 0.1% to 1.0%), and low risk (incidence less than 0.1%) (Warkentin 2008). Patient groups with a risk estimated to be higher than 1.0% are postoperative patients receiving prophylactic or therapeutic doses of UFH. Medical and obstetric patients receiving a prophylactic or therapeutic dose of UFH, postsurgery patients receiving LMWH, postsurgery patients receiving UFH 'flushes', and medical and obstetric patients receiving LMWH after first receiving UFH, are groups with a risk for HIT that is estimated to be intermediate. Medical and obstetric patients receiving LMWH and medical and obstetric patients receiving only heparin 'flushes' are groups at lower risk. The methodological quality supporting this guideline is based on evidence from observational studies, case series, RCTs with serious flaws, or indirect evidence. This means that higher‐quality research is likely to have an important impact on the confidence in the estimates of the effect and may well change them (Guyatt 2008).
Our systematic review corroborates the evidence that LMWH induces HIT to a lower degree than UFH (Martel 2005; Prandoni 2005; Warkentin 2000; Warkentin 2003). Also, the analyses demonstrated a high risk of HIT complicated by venous thromboembolism events when participants were exposed to UFH. The number of venous thromboembolism events linked to HIT was striking (Greinacher 1995; Hong 2003; Walenga 2000; Warkentin 1995). In disagreement with other studies, our results demonstrated a higher than expected incidence of HIT (absolute risk) with LMWH exposure in those participants submitted to major surgery.
It has been demonstrated that people who have had major surgical procedures have a much greater risk of developing an immune response to platelet factor 4/heparin than people undergoing minor surgical procedures, irrespective of the type of heparin received (Lubenow 2010a). In line with this previous evidence, we also showed a higher risk of HIT associated with major surgery. Together, these findings may support the existence of a non‐drug factor acting as a marker for the immune response which leads to this adverse drug reaction, as discussed in the paper of Lubenow 2010a.
An interesting systematic review with meta‐analysis (Morris 2007), evaluated the incidence of thrombocytopenia and heparin‐induced thrombocytopenia in people treated with either LMWH or UFH for venous thromboembolism. The results showed no statistically significant difference between UFH and LMWH with regards to the incidence of thrombocytopenia alone. The incidence of HIT highlighted by Morris 2007 was too low to permit any firm conclusion. However, the definition of HIT used by Morris 2007 was significantly less rigorous than the definition considered in the present review, thus weakening any comparison.
Another systematic review with meta‐analysis screened 368 articles with the aim of evaluating HIT incidence in people receiving thromboprophylaxis with both types of heparin (Martel 2005) and considered two RCTs addressing HIT following orthopaedic surgery (Leyvraz 1991; Warkentin 1995) and three non‐RCT prospective studies (two addressing HIT following orthopaedic surgery and one addressing HIT following cardiac surgery) eligible for the review. Martel 2005 also analysed other studies addressing only thrombocytopenia (without confirmatory tests for HIT). Our electronic searches retrieved both RCTs included in Martel 2005. We did not consider the trial performed by Leyvraz 1991 in the quantitative analysis of our review because it used an obsolete laboratory test to diagnose HIT, which would underestimate cases of the outcome. We considered the study performed by Warkentin 1995 to be a previous report of the study reported by Warkentin 2003. We included the latter in the present systematic review.
Warkentin and colleagues (Warkentin 1995) published a report investigating HIT in a total of 665 participants who had been randomised in a larger trial (Levine 1991) to receive UFH or LMWH. HIT was defined as a decrease in platelet count below 150 x 109/L beginning five or more days after initiation of heparin therapy together with a positive test for PF4/heparin IgG antibodies. Plasma samples were not available from all the 665 participants and the article analysed data describing the study design and results for the 387 participants tested. In 2003, the same group of researchers published a secondary report (Warkentin 2003), discussing a more accurate definition for thrombocytopenia in HIT (the currently accepted definition) and re‐analysing the same data from the previous article of 1995. A detailed analysis of the report shows that Warkentin 2003 determined the presence of HIT antibodies in a subgroup consisting of 362 participants who underwent systematic monitoring for HIT and had serial plasma samples available. Therefore, the study actually investigated 362 participants according to our definition of HIT, resulting in a description of 12 HIT cases: 2/170 in the LMWH group and 10/192 in the UFH group. There were another three participants in the UFH group with serologically‐proven HIT (positive SRA in all three, positive immunoassay IgG specific in 2/2 tested) and with a large magnitude drop in platelet count (more than 50% fall and timing consistent with HIT) who were not in the 362‐participant subgroup but who underwent serological testing for HIT antibodies as ordered by the treating physicians. The study author included these three participants in his analysis and also extended the analysis approaching the problem based on the total sample initially randomised (665 participants).The resulting analysis was 13 cases of proven HIT among 332 participants exposed to UFH versus two HIT cases among 333 participants exposed to LMWH. These detailed data were available thanks to information provided by the trialists through electronic correspondence.
When extracting data from Warkentin 2003 for this review, we considered the cohort of participants who actually followed the same methodological approach, which is the 362 participants who had platelets monitored and serological tests performed for HIT according to standard protocols predefined in the trial. The meta‐analysis performed by Martel 2005 considered eight cases of HIT in the UFH arm (8/332) and no cases of HIT in the LMWH arm (0/333). The resulting Mantzel‐Haensel odds ratio, using a random‐effects model, was 0.06 (95% CI 0.00 to 1.00). The total of HIT cases extracted in the meta‐analysis by Martel 2005 is therefore not in accordance with ours.

Flowchart illustrating the recruitment of the participants included in and extracted from the study reported by Levine 1991 and Warkentin 2003

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1 Heparin‐induced thrombocytopenia (HIT).

Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2 HIT in people undergoing major surgical procedures.
Comparison 1 Low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3 HIT complicated by venous thromboembolism.
| Is unfractionated heparin (UFH) use better than low molecular weight heparin (LMWH) use to avoid heparin‐induced thrombocytopenia? | ||||||
| Patient or population: people undergoing surgical procedures and treated with UFH or LMWH for prophylaxis of thrombotic events lasting at least 5 days | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with UFH | Risk with LMWH | |||||
| Heparin‐induced thrombocytopenia (HIT) Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.23 | 1398 | ⊕⊕⊝⊝ | ||
| 22 per 1000 | 5 per 1000 | |||||
| HIT in people undergoing major surgical procedures Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 586 | ⊕⊕⊝⊝ | ||
| 48 per 1000 | 11 per 1000 | |||||
| HIT complicated by venous thromboembolism Follow‐up: range 10 days to 14 days, or until discharge | Study population | RR 0.22 | 1398 | ⊕⊕⊝⊝ | ||
| 17 per 1000 | 4 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded by one level due to high risk and unclear risk of bias in the domains: selection bias, performance bias, detection and attrition bias. | ||||||
| Study ID | UFH | Number of participants | Dose | LMWH | Number of participants | Dose | Treatment duration | Time point when plasma samples were obtained for HIT‐IgG antibodies test | Laboratory test for HIT |
| Standard calcium heparin | 192 | 7500 U sc twice daily | Enoxaparin | 170 | 30 mg sc twice daily | Started 12 h‐24 h after surgery and continued for 14 days or until discharge if it occurred sooner | At least 1 plasma sample obtained on postoperative day 7 or later | SRA, with confirmatory investigation for the presence of functional antibodies of IgG class | |
| Standard UFH | 289 | 5000 U SC 3 times daily | Certoparin | 272 | 3000 anti‐factor Xa U sc once daily | Started immediately after admission and continued until day 10 or until discharge. After day 10 all participants received LMWH | Obtained on admission, at discharge (if before | Anti‐platelet factor 4/heparin for immunoglobulin IgG class and platelet‐activating | |
| Standard UFH | 238 | 5000 U sc twice daily | Dalteparin | 237 | 5000 U once daily | At least 5 days of | Data were collected daily in the ICU | Commercially available platelet factor 4 ELISA and SRA | |
| ELISA: enzyme‐linked immunosorbent assay | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Heparin‐induced thrombocytopenia (HIT) Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.73] |
| 2 HIT in people undergoing major surgical procedures Show forest plot | 2 | 586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.75] |
| 3 HIT complicated by venous thromboembolism Show forest plot | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.06, 0.84] |