| 1 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) Show forest plot | 3 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.26] |
|
| 2 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) ‐ subgroup by age Show forest plot | 2 | 790 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.16] |
|
| 2.1 Children (< 15 years old) | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults (> or = 15 years old) | 2 | 790 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.16] |
| 3 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) ‐ subgroup by smoking status Show forest plot | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.58, 3.50] |
|
| 3.1 Active smokers | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Ex‐ or never‐smokers | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.58, 3.50] |
| 4 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) ‐ subgroup by time elapsed before treatment initiation Show forest plot | 2 | 680 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.78] |
|
| 4.1 Time elapsed before initiation (< 48h) | 1 | 390 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.51, 1.74] |
| 4.2 Time elapsed before initiation (> or = 48h) | 1 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.58, 3.50] |
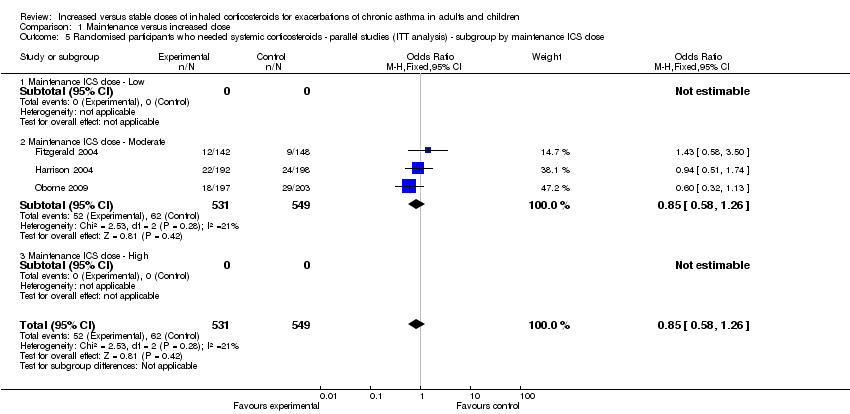
| 5 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) ‐ subgroup by maintenance ICS dose Show forest plot | 3 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.26] |
|
| 5.1 Maintenance ICS dose ‐ Low | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Maintenance ICS dose ‐ Moderate | 3 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.26] |
| 5.3 Maintenance ICS dose ‐ High | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) ‐ subgroup by achieved ICS dose Show forest plot | 3 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.26] |
|
| 6.1 Achieved ICS dose ‐ Low | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Achieved ICS dose ‐ Moderate | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Achieved ICS dose ‐ High | 3 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.26] |
| 7 Randomised participants who needed systemic corticosteroids ‐ parallel studies (ITT analysis) ‐ subgroup by x2 or x4 Show forest plot | 3 | 1080 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.58, 1.26] |
|
| 7.1 Double dose | 2 | 680 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.65, 1.78] |
| 7.2 Quadruple dose | 1 | 400 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.32, 1.13] |
| 8 Need for systemic corticosteroids ‐ cross‐over studies Show forest plot | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.84 [0.47, 130.46] |
|
| 9 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) Show forest plot | 3 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.74] |
|
| 10 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) ‐ subgroup by age Show forest plot | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.18] |
|
| 10.1 Children (< 15 years old) | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults (> or = 15 years old) | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.18] |
| 11 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) ‐ subgroup by smoking status Show forest plot | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.64, 4.47] |
|
| 11.1 Active smokers | 0 | 0 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Ex‐ or never‐smokers | 1 | 98 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.64, 4.47] |
| 12 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) ‐ subgroup by time elapsed before treatment initiation Show forest plot | 2 | 305 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.44, 2.34] |
|
| 12.1 Time elapsed before initiation (< 48 h) | 1 | 207 | Odds Ratio (M‐H, Random, 95% CI) | 0.71 [0.36, 1.41] |
| 12.2 Time elapsed before initiation (> or = 48 h) | 1 | 98 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.64, 4.47] |
| 13 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) ‐ subgroup by maintenance ICS dose Show forest plot | 3 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.74] |
|
| 13.1 Maintenance ICS dose ‐ Low | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Maintenance ICS dose ‐ Moderate | 3 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.74] |
| 13.3 Maintenance ICS dose ‐ High | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) ‐ subgroup by achieved ICS dose Show forest plot | 3 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.74] |
|
| 14.1 Achieved ICS dose ‐ Low | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Achieved ICS dose ‐ Moderate | 0 | 0 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Achieved ICS dose ‐ High | 3 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.74] |
| 15 Participants (with at least one use of study inhaler) who needed systemic corticosteroids ‐ parallel‐group studies (modified ITT analysis) ‐ subgroup by x2 or x4 Show forest plot | 3 | 399 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.74] |
|
| 15.1 Double dose | 2 | 305 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.44, 2.34] |
| 15.2 Quadruple dose | 1 | 94 | Odds Ratio (M‐H, Random, 95% CI) | 0.27 [0.11, 0.67] |
| 16 Sensitivity analysis ‐ effect of funding on need for systemic corticosteroids ‐ parallel‐group studies Show forest plot | 2 | 301 | Odds Ratio (M‐H, Random, 95% CI) | 0.46 [0.18, 1.18] |
|
| 17 Unscheduled physician visits ‐ parallel‐group studies Show forest plot | 2 | | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
|
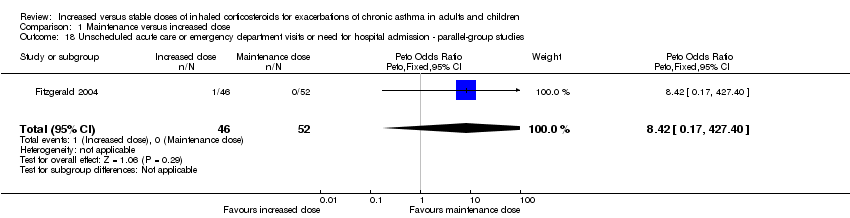
| 18 Unscheduled acute care or emergency department visits or need for hospital admission ‐ parallel‐group studies Show forest plot | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.42 [0.17, 427.40] |
|
| 19 Unscheduled acute care or emergency department visits or need for hospital admission ‐ cross‐over studies Show forest plot | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
|
| 20 Duration of exacerbation as defined by time to recovery of lung function Show forest plot | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
|
| 21 Duration of exacerbation as defined by time to recovery of symptoms Show forest plot | 1 | 207 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
|
| 22 Non‐serious adverse events Show forest plot | 2 | 142 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.68, 6.73] |
|
| 23 Non‐serious adverse event ‐ pharyngitis Show forest plot | 2 | 142 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.18, 11.58] |
|
| 24 Non‐serious adverse event ‐ glossitis Show forest plot | 2 | 142 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.36 [0.54, 163.45] |
|
| 25 Non‐serious adverse event ‐ headaches Show forest plot | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.36 [0.10, 290.73] |
|
| 26 Non‐serious adverse event ‐ psychiatric disturbance (depression, anxiety) Show forest plot | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.36 [0.10, 290.73] |
|
| 27 Non‐serious adverse event ‐ oral candidiasis | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Non‐serious adverse event ‐ GI (nausea, abdominal discomfort) Show forest plot | 1 | 94 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.85 [0.31, 26.51] |
|
| 29 Non‐serious adverse event ‐ upper respiratory tract infections Show forest plot | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.33 [0.03, 3.38] |
|
| 30 Non‐serious adverse event ‐ change in appetite | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31 Non‐serious adverse event ‐ dysphonia | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |