Dosagem de procalcitonina para decidir quando iniciar ou interromper o uso de antibióticos em infecções respiratórias agudas
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised, multicentre, single‐blind clinical trial in 8 French ICUs | |
| Participants | Inclusion criteria: Adults admitted to a participating ICU were eligible if they, < 48 h, had SIRS, acute dysfunction of at least 1 organ, absence of indisputable clinical infection, and negative microbial cultures | |
| Interventions | Guiding antibiotic decisions in ICU patients with non‐microbiologically proven apparent severe sepsis | |
| Outcomes |
| |
| Notes | Funding: Research grant partly by Thermo Fisher B·R·A·H·M·S France. The sponsor had no input in study design, conduct, or reporting. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1 ratio according to a computer‐generated list. Randomisation was centralised through a secured web site and performed by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation using permutation blocks |
| Blinding of participants and personnel (performance bias) | Unclear risk | The control arm remained blinded to PCT levels. Masking of antibiotic therapy was not feasible in this study. |
| Blinding of outcome assessment (detection bias) | High risk | Investigators remained blinded to PCT levels in the control arm, but no blinding of overall outcome assessment was mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for participants on antibiotic on day 5 was: 58/62. 4 participants withdrew their consent. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01025180). |
| Other bias | Unclear risk | Moderate adherence in the PCT arm: physicians were non‐compliant with the PCT‐based algorithm in 19% of participants at 6 h, 17% on day 3, and 37% on day 5. |
| Methods | Randomised, investigator‐initiated, multicentre, partially blinded clinical trial, in 33 multidisciplinary ICUs across Germany | |
| Participants | Inclusion criteria: Adults with severe sepsis or septic shock (severe sepsis was defined as SIRS caused by infection combined with acute organ dysfunction. Septic shock was defined as sepsis in combination with arterial hypotension or need for vasopressor therapy despite adequate fluid resuscitation) | |
| Interventions | Guiding antibiotic decisions and effect of sodium selenite administration in people with severe sepsis or septic shock Algorithm used in this study: Using a 2 × 2 factorial design, participants were randomly assigned to receive intravenous sodium selenite or placebo as well as antimicrobial therapy guided by a PCT algorithm or conventional antimicrobial therapy. | |
| Outcomes |
| |
| Notes | Funding: The study infrastructure was partially funded by grant 01 KI 0106 from the German Federal Ministry of Education and Research. Biosyn (Germany) and Thermo Fisher (Germany) provided study medication and financial support via unrestricted grants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web‐based, centralised randomisation |
| Allocation concealment (selection bias) | Low risk | quote "Using a 2 × 2 factorial design, we randomly assigned patients to receive intravenous sodium selenite or placebo as well as antimicrobial therapy guided by a PCT algorithm or conventional antimicrobial therapy without PCT guidance with an allocation ratio of 1:1:1:1 by use of a central randomisation web server. Randomisation was stratified by study centre, sex, and sepsis severity" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Due to the study design, blinding of physicians was not feasible. Procalcitonin values were only available in participants with study intervention |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment was mentioned in study |
| Incomplete outcome data (attrition bias) | Low risk | Postrandomisation exclusions were about even among intervention arms with a very low dropout rate for mortality |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to trial registration (NCT00832039) |
| Other bias | Unclear risk | Overruling of the PCT algorithm was very high (adherence was lower than 50%) |
| Methods | Randomised, multicentre clinical trial in 9 French ICUs | |
| Participants | Inclusion criteria: Patients with suspected bacterial infections during ICU stay without prior AB (> 24 h) Exclusion criteria: Aged under 18 years; known pregnancy; expected stay in the ICU of less than 3 days; bone marrow transplant or chemotherapy‐induced neutropenia (< 500 neutrophils per mL); infections for which long‐term antibiotic treatment is strongly recommended (i.e. infective endocarditis, osteoarticular infections, anterior mediastinitis after cardiac surgery, hepatic or cerebral abscesses, chronic prostatitis, or infection with Mycobacterium tuberculosis, Pneumocystis jirovecii, or Toxoplasma gondii); poor chance of survival, defined as a simplified acute physiology score (SAPS II) of more than 65 points at screening; and do‐not‐resuscitate orders. Included in this analysis: 394 participants with CAP and VAP out of 630 randomised participants; 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice), and 227 not considered for this analysis due to diagnosis other than ARI | |
| Interventions | Guiding antibiotic decisions in ICU patients with repeated PCT measurements Algorithm used in this study: Investigators were encouraged to discontinue ABs when PCT concentration was less than 80% of the peak concentration or an absolute concentration of less than 0.5 μg/L was reached. | |
| Outcomes |
| |
| Notes | Funding: Research grant from the Départment à la Recherche Clinique et au Développement, Assistance Publique‐Hopitaux de Paris (PHRC AOR06019), France. B·R·A·H·M·S, Germany (manufacturer of PCT assay) provided all assay‐related materials free of charge for the study (Kryptor machines if not already available on‐site and kits and maintenance required for study‐related measurements). Follow‐up: Fixed period of 60 days for mortality Registration: NCT00472667 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent, centralised, computer‐generated randomisation sequence (CleanWEB, Telemedicine Technologies, Boulogne, France) |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Although treatment assignments were not masked, all investigators were unaware of aggregate outcomes during the study and primary endpoints were strictly defined and not patient‐reported." Quote: "An adjudication committee comprised of 4 specialists in infectious diseases and critical care medicine who were masked to the randomisation assignment reviewed and validated all infectious episode classifications by consensus." |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 393/394 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT00472667). |
| Other bias | Unclear risk | Low adherence to PCT algorithm in PCT group (47%) |
| Methods | Single‐centre, randomised, open‐label clinical trial in Rochester, NY, USA | |
| Participants | Inclusion criteria: Adults ≥ 21 years of age with symptoms compatible with LRTI (i.e. admission diagnosis of pneumonia, acute exacerbations of COPD, bronchitis, asthma, influenza, viral syndrome, respiratory failure, and congestive heart failure CHF) were identified by reviewing the daily admission census. | |
| Interventions | Guiding antibiotic decisions in hospitalised patients with respiratory infections Algorithm used in this study: In the intervention group serum PCT and viral/atypical pathogen PCR testing were performed on admission (in addition to all standard‐of‐care diagnostic tests). Antibiotic decisions were made based on a PCT algorithm (for PCT values of ≤ 0.1 ng/mL, initiation of antibiotic treatment is strongly discouraged; for values of 0.11 to 0.24 ng/mL, initiation is discouraged; for values of 0.25 to 0.49 ng/mL, initiation is encouraged; and for values of ≥ 0.5 ng/ mL, initiation is strongly encouraged). In the standard care group standard‐of‐care testing (bacterial and viral cultures of respiratory samples, hospital influenza/RSV duplex PCR, and urine Legionella antigen analysis) were obtained on admission and antibiotic decisions were made by the attending physician. Participants in the standard care group had PCT and viral testing samples frozen and tested at study termination. | |
| Outcomes |
| |
| Notes | Funding: This work was supported by Rochester General Hospital (KIDD Fund); the National Institutes of Health, National Institute of Allergy and Infectious Diseases (contract HHSN27220120005C); and BioFire (FilmArray respiratory panel instrument). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by the presence of COPD and were randomly assigned at a ratio of 1:1, using blocks of 4, to receive standard care or the intervention. |
| Allocation concealment (selection bias) | Low risk | Small block size (4) for randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not feasible due to the study design. Although the control arm remained blinded, there was a risk for spillover care resulting from providers caring for participants in both arms. |
| Blinding of outcome assessment (detection bias) | Low risk | Participants in the standard care group had blood samples frozen and tested at study termination, making the data only available at the end of the study. Phone follow‐up by personnel blinded to randomisation |
| Incomplete outcome data (attrition bias) | Low risk | Over 3 months of follow‐up 36 participants were lost to follow‐up, 13 died, and 4 withdrew their consent (237/300 completed 3‐month follow‐up). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01907659). |
| Other bias | High risk | Low overall adherence to the PCT algorithm (64%) |
| Methods | Randomised clinical trial, multicentre in 53 primary care practices in northwest Switzerland | |
| Participants | Inclusion criteria: People with upper or lower ARIs in primary care and the physician's intention to prescribe antibiotics on the basis of evidence‐based guidelines Exclusion criteria: Antibiotic use within the previous 28 days, psychiatric disorders or inability to give written informed consent, not being available for follow‐up, not fluent in German, severe immunosuppression, cystic fibrosis, active tuberculosis, and the need for immediate hospitalisation Included in this analysis: 458 out of 458 randomised participants | |
| Interventions | Guiding antibiotic decisions in primary care with repeated measurements Algorithm used in this study: In participants with PCT levels lower than 0.1 μg/L, a bacterial infection was considered highly unlikely and the use of ABs was discouraged. In participants with a PCT level higher than 0.25 μg/L, a bacterial infection was considered likely and the use of ABs was recommended. For PCT concentrations of 0.1 to 0.25 μg/L, a bacterial infection was considered unlikely and the use of ABs was not recommended. When ABs were withheld from participants, a second measurement of the PCT level was mandatory within 6 to 24 hours for safety reasons. The use of ABs was recommended if this second measurement was higher than 0.25 μg/L or if the PCT level had increased from the first measurement by more than 50% and the participant showed no clinical improvement. All participants given ABs based on PCT level were reassessed after 3 days. Discontinuation of AB treatment was then recommended in participants with a PCT level of 0.25 μg/L or lower. | |
| Outcomes |
| |
| Notes | Funding: Swiss National Science Foundation (Grant 3300C0‐107772) and Association for the Promotion of Science and Postgraduate Training of the University Hospital Basel, Switzerland. B·R·A·H·M·S, Germany provided assay and kit material related to the study. Follow‐up: Fixed period of 28 days Registration: ISRCTN73182671 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician generated the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation communicated by phone to physician. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded medical students performed interviews with participants at 14 and 28 days. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 454/458 (99%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 85% adherence to PCT algorithm in PCT group |
| Methods | Randomised clinical trial, multicentre in 15 primary care practices in the Hanover, Germany area | |
| Participants | Inclusion criteria: Adults with upper or lower ARIs in primary care Exclusion criteria: Treatment with antibiotics during the previous 2 weeks, chronic liver disease, major surgery that had required hospitalisation during the last 4 weeks, autoimmune or systemic disorders, dialysis, medullary C‐cell carcinoma and other inflammatory diseases Included in this analysis: 550 out of 571 randomised participants; 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) | |
| Interventions | Guiding antibiotic decisions in primary care with initial measurement only Algorithm used in this study: PCT value < 0.25 µg/L indicated that a relevant bacterial infection of the respiratory tract is unlikely. | |
| Outcomes |
| |
| Notes | Funding: B·R·A·H·M·S AG, Germany Follow‐up: Fixed period of 28 days Registration: NCT00827060 and NCT00688610 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated Quote: "Baseline adaptive randomisation was realised through a web‐based randomisation data bank (IOMTech GmbH, Berlin, Germany), which had been programmed specifically for that purpose." |
| Allocation concealment (selection bias) | Low risk | Central randomisation Quote: "In the central laboratory, the web‐based randomisation of the patient into the PCT group or the control group took place." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | Low risk | Structured interviews by blinded personnel |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 546/550 (99%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 87% adherence to PCT algorithm in PCT group |
| Methods | Randomised clinical trial, single‐centre, emergency department at the University Hospital Basel, Switzerland | |
| Participants | Inclusion criteria: People with lower ARIs presenting at a medical emergency department Exclusion criteria: Severely immunocompromised people, i.e. with HIV infection and a CD4 count less than 200 cells per mL, neutropenic patients, and stem cell transplant recipients; those with cystic fibrosis or active tuberculosis; and individuals with nosocomial pneumonia Included in this analysis: 243 participants out of 243 randomised participants | |
| Interventions | Guiding antibiotic decisions in emergency department patients with different ARIs with initial PCT values only Algorithm used in this study: A PCT value of 0.1 to 0.25 µg/L was regarded as an indication that bacterial infection was unlikely and use of ABs was discouraged. A serum PCT between 0.25 and 0.5 g/L was deemed indicative of a possible bacterial infection, and the treating doctor was advised to initiate antimicrobial treatment. A PCT value of 0.5 µg/L or greater was judged suggestive of the presence of bacterial infection and AB treatment was strongly recommended. For participants on antimicrobial therapy at the time of admission, discontinuation of ABs was recommended if PCT concentrations were less than 0.25 µg/L. | |
| Outcomes |
| |
| Notes | Funding: Freiwillige Akademische Gesellschaft Basel, Switzerland; Department of Internal Medicine and the Divisions of Endocrinology and Pneumology, University Hospital Basel; B·R·A·H·M·S AG, Germany and Orgenium Laboratories, Finland, provided assay material and partial support of the investigator‐initiated study. Follow‐up: Fixed period of 10 to 14 days; in participants with acute exacerbations of COPD the follow‐up period comprised 4 to 6 months Registration: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly assigned eligible patients either standard antimicrobial therapy (standard group) or PCT‐guided antimicrobial treatment (PCT group) according to a computer‐generated week wise‐randomisation scheme." |
| Allocation concealment (selection bias) | High risk | Recruiting physicians were aware of group allocation based on week‐wise randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded investigators |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 230/243 (95%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 83% adherence to PCT algorithm in PCT group |
| Methods | Randomised clinical trial, single‐centre, emergency department at the University Hospital Basel, Switzerland | |
| Participants | Inclusion criteria: CAP with X‐ray confirmation in the emergency department Exclusion criteria: People with cystic fibrosis or active pulmonary tuberculosis, people with hospital‐acquired pneumonia, and severely immunocompromised individuals Included in this analysis: 302 out of 302 randomised participants | |
| Interventions | Guiding antibiotic decisions in emergency department patients with CAP with repeated PCT measurements Algorithm used in this study: a PCT level of less than 0.1 µg/L suggested the absence of bacterial infection, and the initiation or continuation of ABs was strongly discouraged. A PCT level between 0.1 and 0.25 µg/L indicated that bacterial infection was unlikely, and the initiation or continuation of ABs was discouraged. A PCT level from 0.25 to 0.5 µg/L was considered indicative of a possible bacterial infection, and the initiation or continuation of AB therapy was encouraged. A PCT level greater than 0.5 µg/L strongly suggested the presence of bacterial infection, and AB treatment and continuation was strongly encouraged. Re‐evaluation of the clinical status and measurement of serum PCT levels were recommended after 6 to 24 h in all participants from whom ABs were withheld. PCT levels were reassessed after 4, 6, and 8 d. Antibiotics were discontinued on the basis of the PCT cut‐offs defined above. In participants with very high PCT values on admission (e.g. greater than 10 µg/L), discontinuation of ABs was encouraged if levels decreased to less than 10% of the initial value (e.g. 1 µg/L, instead of less than 0.25 µg/L). | |
| Outcomes |
| |
| Notes | Funding: Funding obtained from B·R·A·H·M·S (Hennigsdorf, Germany), Pfizer (Schweiz AG), and Mepha (Schweiz AG) was used for assay material and salaries of technical personnel involved in laboratory work and for shipping and handling of data and specimens and presentation of data at scientific meetings. Additional support, which provided more than two‐thirds of the total study costs, was granted by funds from the Departments of Internal Medicine and Emergency Medicine, the Stiftung Forschung Infektionskrankheiten (SFI), and mainly from the Departments of Endocrinology and Pulmonary Medicine, University Hospital Basel, Switzerland. Follow‐up: Fixed period of 6 weeks Registration: ISRCTN04176397 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "On admission, patients were randomly assigned to one of the two groups by sealed, opaque envelopes." Envelopes were not numbered. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 300/302 (99%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 87% adherence to PCT algorithm in PCT group |
| Methods | Randomised, single‐centre clinical trial in an ED of a university hospital in Denmark | |
| Participants | Inclusion criteria: 1) 18 years old and 2) admitted with an AECOPD (clinician’s diagnosis at admission), defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) | |
| Interventions | The aim was to assess whether PCT‐guided antibiotic treatment could reduce the overall use of antibiotics among people hospitalised for AECOPD. | |
| Outcomes |
| |
| Notes | Funding: Thermo Fisher Scientific, MA, USA, and bioMérieux Denmark ApS supported the study non‐financially. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated according to the random part of the civil registration number in Denmark |
| Allocation concealment (selection bias) | Low risk | The randomisation algorithm was concealed to treating clinicians and participants |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not feasible, but PCT was only measured in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | No mentioning of blinding |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 120/120 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01950936) |
| Other bias | High risk | Moderate adherence to the PCT algorithm in PCT group (61.1%) |
| Methods | Randomised, multicentre, controlled, open‐label intervention trial in 15 hospitals in the Netherlands | |
| Participants | Inclusion criteria: Eligible patients had to be at least 18 years of age, be admitted to the ICU, and have received their first dose of antibiotics no longer than 24 h before inclusion to the trial for an assumed or proven infection. | |
| Interventions | Guiding antibiotic decisions in critically ill ICU patients Algorithm used in this study: Antibiotics in the standard‐of care group were stopped according to local or national guidelines and according to the discretion of attending physicians. Procalcitonin concentration was not measured in the standard‐of‐care group. | |
| Outcomes |
| |
| Notes | Funding: Thermo Fisher Scientific | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done centrally by use of a computer‐generated list produced by an independent research organisation (the Julius Centre for Human Research, Utrecht, Netherlands). |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation by an independent organisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants and investigators were aware of treatment assignment. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | 1546 of 1575 participants included in the intention‐to‐treat population analysis |
| Selective reporting (reporting bias) | Low risk | The outcomes correspond to the trial registration (NCT01139489). |
| Other bias | High risk | Physicians did not adhere to the stopping advice in more than half of the participants. |
| Methods | Randomised, single‐centre, controlled clinical trial at an ICU of a tertiary care, private hospital in São Paulo, Brazil | |
| Participants | Inclusion criteria: People with microbiologically confirmed infections (blood, urine, tracheal aspirate, or bronchoalveolar lavage fluid cultures) with sepsis, severe sepsis, and septic shock | |
| Interventions | Guiding antibiotic decisions in ICU patients with proven bacterial infection Algorithm used in this study: All participants received antibiotic therapy. For the control group, stopping antibiotic therapy was at the discretion of the attending physician. For the intervention group, physicians were guided by the PCT protocol to stop antibiotic treatment. The protocol stated 2 conditions: 1) PCT dropped more than 90% from the peak level, or 2) an absolute value < 0.5 ng/mL was reached. | |
| Outcomes |
| |
| Notes | Funding: No funding declared in the main article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A blind randomisation scheme was used where a black box contained 100 folders, and 2 authors randomly drew 1 folder as soon as an informed consent was present. |
| Allocation concealment (selection bias) | Low risk | Folders were randomly and blindly assigned as “PCT group” or “standard group”. 2 of the authors would randomly draw 1 folder from a black box containing 100 folders (50 “PCT group” and 50 “control group”). |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of the treating physicians was not feasible in this study. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of the outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | In the intention‐to‐treat analysis the follow‐up for mortality was 81/81 (100%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT01494675). |
| Other bias | High risk | Low adherence to the PCT algorithm (47.6%) |
| Methods | Randomised, single‐centre, open‐label, controlled clinical trial in HeNan Hospital, China | |
| Participants | Inclusion criteria: All patients with suspected AE‐IPF admitted to the respiratory department were assessed for eligibility from January 2009 to December 2011. Acute exacerbation of idiopathic pulmonary fibrosis was defined according to the criteria established by the Idiopathic Pulmonary Fibrosis Clinical Research Network: (1) previous or concurrent diagnosis of idiopathic pulmonary fibrosis; (2) unexplained worsening or development of dyspnoea within 30 days; (3) high‐resolution computed tomography with new bilateral ground‐glass; (4) abnormality and/or consolidation superimposed on a background reticular or honeycomb pattern consistent with usual interstitial pneumonia pattern; (5) no evidence of pulmonary infection by endotracheal aspirate or bronchoalveolar | |
| Interventions | Guiding antibiotic decisions in people with acute exacerbation of idiopathic pulmonary fibrosis Algorithm used in this study: Serum PCT level was measured every 3 days. The first PCT measurement was available before the clinical decision to start antibiotics treatment. Participants whose serum PCT value exceeded the threshold of 0.25 ng/mL were administered antibiotics and were treated until PCT value fell to ≤ 0.25 ng/mL. In the routine treatment group, the decision to administer antibiotics was guided by the clinical experience of the clinician, typically by conventional laboratory tests such as sputum bacteriology and white blood cell count. | |
| Outcomes |
| |
| Notes | Funding: No funding declared in the main paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to either PCT‐guided antibiotic treatment or a control group receiving routine antibiotic therapy by the statistician using computer‐generated random numbers. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding was performed. "In the routine treatment group, patients were treated by antibiotics according to the clinical experience of clinicians typically guided by conventional laboratory tests, such as sputum bacteriology and white blood cell count." |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment was mentioned in this study. |
| Incomplete outcome data (attrition bias) | Unclear risk | Postrandomisation exclusion rate was relatively high but similar in both study arms (6 in the intervention arm versus 4 in the control group). |
| Selective reporting (reporting bias) | Unclear risk | No trial registration was found for this trial. |
| Other bias | Low risk | 100% adherence to PCT protocol in the per‐protocol analysed participants (all protocol violations were defined as "withdrawn"). |
| Methods | Randomised clinical trial, single‐centre, ICU in Germany | |
| Participants | Inclusion criteria: Patients in the surgical ICU with suspected bacterial infections and > 1 SIRS criteria Exclusion criteria: Patients who refused study consent, whose antibiotic treatment had been initiated before intensive care admission, or who had therapy limitations Included in this analysis: 43 (110); 67 not considered for this analysis due to diagnosis other than ARI | |
| Interventions | Guiding antibiotic decisions in postoperative patients in a surgical ICU Algorithm used in this study: Antibiotic therapy in the PCT‐guided group was discontinued if clinical signs and symptoms of infection improved and PCT decreased to less than 1 µg/L, or if the PCT value was more than 1 µg/L, but had dropped to 25% to 35% of the initial value over 3 days. | |
| Outcomes |
| |
| Notes | Funding: B·R·A·H·M·S AG Follow‐up: Until hospital discharge Registration: ISRCTN10288268 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unconcealed drawing of lots |
| Allocation concealment (selection bias) | High risk | Unconcealed drawing of lots |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 393/394 (100%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed |
| Methods | Randomised clinical trial, multicentre, 3 hospitals in Denmark | |
| Participants | Inclusion criteria: Hospitalised patients with suspected pneumonia (no X‐ray confirmation); quote: "The assessment of eligibility (i.e. the clinical diagnosis) was made by the admitting physician and was based on medical history and physical examination." Exclusion criteria: Not meeting the diagnostic criteria Included in this analysis: 210 out of 223 randomised participants; 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) | |
| Interventions | Guiding antibiotic decisions in CAP patients with initial values only Algorithm used in this study: Physicians were not asked to wait for PCT results before initiating antimicrobial therapy, therefore PCT values were, in most cases, used to motivate either cessation or continuation of already initiated treatments. Discontinuation of AB treatment was recommended if PCT at admission was below 0.25 µg/L, despite delays in test results. | |
| Outcomes |
| |
| Notes | Funding: The Danish Medical Research Council and the Danish Lung Association provided financial support. Follow‐up: Until hospital discharge Registration: NCT00415753 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 210/210 (100% until discharge) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | High risk | 59% adherence to PCT algorithm in PCT group |
| Methods | Randomised, single‐centre, prospective, controlled clinical study in 5 ICUs in Belgium | |
| Participants | Inclusion criteria: Patients older than 18 yrs of age and hospitalised for > 2 days in 1 of the 5 ICUs | |
| Interventions | Guiding antibiotic decisions in ICU patients Algorithm used in this study: According to the proposal by Mueller and colleagues, for participants in the PCT group, the use of antibiotics was more or less strongly discouraged if PCT level was < 0.25 μg/L or 0.50 μg/L, respectively, and more or less recommended if PCT level was above 1 μg/L or 0.50 μg/L, respectively. This strategy was applied to all infectious episodes encountered during participants' ICU stay. | |
| Outcomes |
| |
| Notes | Funding: No information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on method of randomisation provided. |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Procalcitonin levels in the control arm were blinded for treating physicians, but the study arm to which participants had been assigned was not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | At the end of ICU stay, participants’ charts were reviewed by infectious disease specialists blinded to PCT results, who classified them as confirmed, probable, possible, or no infection using all the clinical data and biological results including microbiological cultures and results from investigational procedures. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality 509/509 (100%) |
| Selective reporting (reporting bias) | Unclear risk | No trial registration found for this research article. |
| Other bias | High risk | Low adherence to PCT algorithm (46.3%) |
| Methods | Randomised, single‐centre, prospective, controlled clinical trial in Minas Gerais, Brazil | |
| Participants | Inclusion criteria: Presence of febrile neutropenia (axillary temperature ˜37.8°C, neutrophils count < 500 cells/mm3) in people with diagnosis of haematological disease (except people undergoing allogeneic stem cell transplantation); with expected duration of neutropenia of more than 3 days; ongoing broad‐spectrum antibiotic therapy according to institutional guideline, based on Infectious Diseases Society of America; and no current use of therapeutic antibiotics or antifungals with the last day of use was > 14 days before inclusion in the study | |
| Interventions | Guiding antibiotic decisions in people with febrile neutropenia Algorithm used in this study: Attending physicians were encouraged to discontinue antibiotics in participants when both of the following criteria were met: (i) no febrile episodes for 2 consecutive days (if no Ionger neutropenia) or 3 consecutive days (if still neutropenia), and (ii) PCT concentration at least 90% lower than highest baseline Ievels or lower than 0.5 ng/mL for 2 consecutive days, regardless of the initial Ievels. The final decision to discontinue antibiotics was left at the attending physician's discretion. Procalcitonin Ievels were measured for 2 additional days following antibiotics interruption to monitor a possible relapse of infection. For safety reasons, at least 5 days of antibiotic therapy were ensured for all included participants. Participants with bacteraemia were treated for at least 7 days. In the control group, duration of antibiotic therapy was based on institutional protocol, according to Infectious Diseases Society of America recommendations. | |
| Outcomes |
| |
| Notes | Funding: This work was supported by Funda of the de Amparo a Pesquisa do Eatado dc MiDaa Ocrais (.APQ‐01956‐10). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised on the third day of follow‐up, in a 1:1 ratio for the PCT group and the control group. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using a table of random, computer‐generated numbers, and sealed, opaque envelopes were used. |
| Blinding of participants and personnel (performance bias) | Unclear risk | For participants randomised into the control group, results of PCT serum Ievels were kept concealed during the study and were only revealed for the final analysis. However, physicians were aware of the study group. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment was mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | Most randomised participants finished follow‐up. Only 1 post randomisation exclusion |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to trial registration. |
| Other bias | Low risk | High adherence to the PCT protocol (73.3%) |
| Methods | Randomised clinical trial, single‐centre, emergency department outpatients in China | |
| Participants | Inclusion criteria: CAP with X‐ray confirmation Exclusion criteria: Use of antibiotic therapy in 2 weeks before enrolment, systemic immune deficiency, organ dysfunction, tumour, mental illness, CAP onset ≥ 5 days, coexisting extrapulmonary infection requiring antibiotic therapy Included in this analysis: 127 out of 127 randomised participants | |
| Interventions | Guiding antibiotic decisions in CAP patients with repeated levels Algorithm used in this study: A PCT level of less than 0.1 µg/L suggested the absence of bacterial infection, and the initiation or continuation of ABs was strongly discouraged. A PCT level between 0.1 and 0.25 µg/L indicated that bacterial infection was unlikely, and the initiation or continuation of ABs was discouraged. A PCT level of 0.25 µg/L or greater was considered indicative of a possible bacterial infection, and the initiation or continuation of AB therapy was encouraged. Re‐evaluation of the clinical status and measurement of PCT levels was recommended after 6 to 12 h in all participants from whom ABs were withheld. | |
| Outcomes |
| |
| Notes | Funding: Training fund of Shanghai No. 5 Hospital Follow‐up: 28 days Registration: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd and even patient ID numbers |
| Allocation concealment (selection bias) | High risk | Odd and even patient ID numbers |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | 210/210 (100% until discharge) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
| Methods | Randomised clinical trial, single‐centre, emergency department outpatients in China | |
| Participants | Inclusion criteria: CAP with X‐ray confirmation in an outpatient setting Exclusion criteria: Pregnancy, commencement of antibiotic therapy ≥ 48 h before enrolment, systemic immune deficiency, withholding of life‐support, and active tuberculosis Included in this analysis: 156 out of 172 randomised participants; 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) | |
| Interventions | Guiding antibiotic decisions in CAP patients with repeated levels Algorithm used in this study: A PCT level of less than 0.1 µg/L suggested the absence of bacterial infection, and the initiation or continuation of ABs was strongly discouraged. A PCT level between 0.1 and 0.25 µg/L indicated that bacterial infection was unlikely, and the initiation or continuation of ABs was discouraged. A PCT level of 0.25 µg/L or greater was considered indicative of a possible bacterial infection, and the initiation or continuation of AB therapy was encouraged. Re‐evaluation of the clinical status and measurement of PCT levels was recommended after 6 to 12 h in all participants from whom ABs were withheld. | |
| Outcomes |
| |
| Notes | Funding: The study was sponsored by a grant from the Shanghai Fifth People's Hospital Science Foundation (09YRCPY11). Follow‐up: Fixed period of 4 weeks Registration: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Odd and even patient ID numbers |
| Allocation concealment (selection bias) | High risk | Odd and even patient ID numbers |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 156 (156) (100%) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
| Methods | Randomised, single‐centre, open‐label, controlled clinical trial in Shanghai, China | |
| Participants | Inclusion criteria: People aged 18 to 65 years with severe acute exacerbations of asthma. A severe asthma exacerbation was defined as at least 1 of the following: need for systemic corticosteroids, or an increase from a stable maintenance dose, for at least 3 days and/or hospitalisation or ED visit because of asthma requiring systemic corticosteroids. | |
| Interventions | Guiding antibiotic decisions in people with acute severe exacerbation of asthma Algorithm used in this study: Antibiotic treatment was strongly discouraged when serum PCT level was less than 0.1 μg/L; antibiotic treatment was discouraged when serum PCT level was less than 0.25 μg/L; and antibiotic treatment was encouraged when serum PCT level was higher than 0.25 μg/L. When antibiotics were withheld from participants, a second measurement of the PCT level was mandatory within 6 to 24 hours for safety reasons. The use of antibiotics was recommended if this second measurement was higher than 0.25 μg/L. Physicians were permitted to overrule the algorithm, but they had to indicate the reasons for overruling. The control group received antibiotic according to the discretion of the treating physician, who was unaware of the participant’s PCT levels. | |
| Outcomes |
| |
| Notes | Funding: The study was sponsored by a grant from the Shanghai Fifth People’s Hospital Science Foundation and Minhang District Natural Science Foundation of Shanghai. The funding bodies had no involvement in the design, collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to either intervention was conducted according to computer‐generated random numbers produced by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | After randomisation, an opaque, sealed, sequentially numbered envelope containing the PCT or control protocol was prepared for each participant according to group assignment. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label study with blinding of PCT level in the control group |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | 169/180 participants completed 1‐year follow‐up visit (11 participants lost to follow‐up). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (ChiCTR‐TRC‐12002534). |
| Other bias | Low risk | High adherence to PCT algorithm (93.3%) |
| Methods | Randomised, single‐centre, open‐label, controlled clinical trial at a 200‐bed academic tertiary care hospital in Belgrade, Serbia | |
| Participants | Inclusion criteria: People scheduled to undergo open heart surgery on cardio‐pulmonal bypass. We assessed people who were selected for elective cardiac surgery at the 200‐bed academic tertiary care hospital. The criterion for inclusion in the study was the type of operation: coronary artery bypass grafting (CABG) surgery, valve reconstruction, combined CABG and valve procedures. Entry criteria included stable and unstable angina pectoris, valve insufficiency, left ventricle ejection fraction (LVEF) above 30%, and epidemiological status with saprophyte bacteria. | |
| Interventions | Guiding antibiotic decisions in patients after cardiac surgery Algorithm used in this study: Antibiotic prophylaxis was performed in all participants. The participants were divided at the time of surgery into the standard group and the PCT group. | |
| Outcomes |
| |
| Notes | Funding: No funding is mentioned in the main article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Low risk | The participants were divided at the time of surgery into the standard group and the PCT group by centralised randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding of physicians due to the study design |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 205/205 (100%) |
| Selective reporting (reporting bias) | Unclear risk | No trial registration found. |
| Other bias | Unclear risk | No information about adherence |
| Methods | Randomised, single‐centre, single‐blinded clinical trial in a 30‐bed ICU in Tehran, Iran | |
| Participants | Inclusion criteria: Patients with at least 2 of 4 criteria including body temperature above 38°C or below 36°C; tachycardia > 90/min; tachypnoea > 20/min; and leukocytosis > 12 × 109/L or a leftward shift with more than 10% band cells or leukopenia < 4 × 109/L were defined as patient with SIRS. | |
| Interventions | Guiding antibiotic decisions in critically ill ICU patients Algorithm used in this study: In case group, according to serum level of PCT, participants were divided into 3 groups as: PCT level 0.5 ng/mL or less (group A), PCT value of 0.5 to 2 ng/mL (group B), and PCT level 2 ng/mL or more (group C). Group A indicated a low probability of bacterial infection; use of antibiotics was discouraged, and PCT level was rechecked after 12 hours. In group B, with a medium probability of infection, antibiotic therapy was not administered, and PCT level was rechecked after 8 hours. In group C, with a high probability of bacterial infection, participants underwent antibiotic treatment. If the PCT level was higher than 2 ng/mL after recheck in group A and B, antibiotics therapy was administered; if the PCT level was lower than 2 ng/mL, participants underwent close observation, and PCT was rechecked until culture results were obtained. | |
| Outcomes |
| |
| Notes | Funding: No funding declared in the original research article. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participants were randomly divided into 2 groups by computer‐based random number generation. |
| Allocation concealment (selection bias) | Low risk | All participants were randomly divided into 2 groups by computer‐based random number generation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Procalcitonin was only measured in the intervention arm, but blinding was not feasible due to the study design. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment was mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants finished the study and were assessed for the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No trial registration was found for this study. |
| Other bias | Unclear risk | Adherence to the PCT algorithm was not known. |
| Methods | Randomised clinical trial, single‐centre, medical ICU in Switzerland | |
| Participants | Inclusion criteria: Suspected severe sepsis or septic shock in the ICU Exclusion criteria:
Included in this analysis: 52 out of 79 randomised participants; 27 not considered for this analysis due to a diagnosis other than RTI | |
| Interventions | Guiding antibiotic decisions in ICU patients with repeated measurements Algorithm used in this study: Procalcitonin levels measured at baseline and daily. For participants presenting a favourable clinical course, investigators used predefined “stopping rules” based on circulating PCT levels to encourage physicians to discontinue ABs. Participants with baseline PCT level ≥ 1 µg/L were re‐evaluated at day 5. Investigators encouraged treating physicians to discontinue ABs when:
Participants with PCT level below 1 µg/L at baseline were re‐evaluated at day 3; treating physicians were encouraged to discontinue ABs when PCT level was below 0.1 µg/L and careful clinical evaluation ruled out severe infection. | |
| Outcomes |
| |
| Notes | Funding: B·R·A·H·M·S AG Follow‐up: Fixed follow‐up period of 28 days Registration: NCT00250666 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generation |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 52/52 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Low risk | 81% adherence to PCT algorithm in PCT group |
| Methods | Randomised, single‐centre, prospective, open‐label, non‐inferiority clinical trial in Shizuoka, Japan | |
| Participants | Inclusion criteria: Patients at risk for aspiration, who had been hospitalised after developing pneumonia, were enrolled. Aspiration pneumonia was clinically diagnosed on the basis of the findings on computed tomography (e.g. bronchopneumonia in the dorsal lower lobes), combined with a history of aspiration pneumonia, stroke or dementia, poor systemic condition, or any combination of these (e.g. bedridden patients or patients fed by a nasogastric tube or percutaneous endoscopic gastrostomy). Selection criteria included the following: at least 1 month had elapsed since the last treatment for relapsed pneumonia, and ventilator use was not scheduled for the pneumonia treatment | |
| Interventions | Guiding antibiotic decisions in patients with aspiration pneumonia and assessment of the continuation of oral intake Algorithm used in this study: Procalcitonin levels were measured via outsourcing to SRL (Tokyo, Japan); the results were obtained 2 or 3 days after admission. In the PCT group, if the PCT levels upon admission were < 0.5 ng/mL, 0.5 to 1.0 ng/mL, or > 1.0 ng/mL, the duration of antibiotic therapy was determined to be 3, 5, or 7 days, respectively. If the PCT level upon admission was 45.0 ng/mL, antibiotic treatment was continued until it was less than 10% of the peak PCT level reached. | |
| Outcomes |
| |
| Notes | Funding: No funding declared in the main research paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated in 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No clear mention of allocation concealment is made in the main article. Citation "Following enrolment, the patients were randomly allocated in a 1:1 ratio to groups assigned different durations of antibiotic therapy…" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Procalcitonin was only measured in the intervention arm, but study design was open‐label. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | Most participants were followed for 30 days. Reasons for post randomisation exclusions are clearly reported in the article. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to trial registration. |
| Other bias | Unclear risk | As cited by authors under limitations, time until analysis of PCT concentrations was available was relatively long (3 days). Adherence to PCT algorithm not known |
| Methods | Randomised, multicentre, open‐label, controlled clinical trial in the ICUs of 2 university hospitals in Brazil | |
| Participants | Inclusion criteria: All adult patients 18 years of age or older with suspected severe sepsis or septic shock were assessed for potential inclusion. | |
| Interventions | Guiding antibiotic decisions with CRP versus PCT in septic patients Algorithm used in this study: Antibiotic therapy was discontinued following a protocol based on serum levels of these markers, according to the allocation group. For both groups, at least 7 full days of antibiotic therapy were ensured in participants with SOFA score greater than 10 and/or bacteraemia at inclusion, and participants with evident resolution of the infectious process had antibiotics stopped after 7 days, despite biomarker levels. | |
| Outcomes |
| |
| Notes | Funding: Supported in part by the Minas Gerais Research Foundation (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, FAPEMIG) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes were used for the randomisation. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding due to the study design |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment mentioned in the study. |
| Incomplete outcome data (attrition bias) | Low risk | All participants were included in the ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (NCT00934011). |
| Other bias | Low risk | High adherence to the PCT protocol (87.8%) |
| Methods | Randomised clinical trial, single‐centre, surgical ICU in Germany | |
| Participants | Inclusion criteria: Patients after abdominal surgery with antibiotic treatment because of severe sepsis in the surgical ICU Exclusion criteria: Patients were excluded if they did not meet the respective inclusion criteria, refused informed consent, or had already received antibiotic treatment prior to admission to the ICU. Included in this analysis: 8 out of 27 randomised participants; 19 not considered for this analysis due to diagnosis other than RTI | |
| Interventions | Guiding antibiotic decisions in postoperative patients in a surgical ICU Algorithm used in this study: In the PCT‐guided group, antibiotic therapy was discontinued if clinical signs and symptoms of sepsis improved and PCT values had either decreased to 1 µg/L or less or had dropped to 25% to 35% of the initial PCT concentration over 3 consecutive days. | |
| Outcomes |
| |
| Notes | Funding: NA Follow‐up: Until hospital discharge Registration: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unconcealed drawing of lots |
| Allocation concealment (selection bias) | High risk | Unconcealed drawing of lots |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Non‐blinded study members |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 8/8 (100% until discharge) |
| Selective reporting (reporting bias) | Low risk | No selective reporting (oral verification with first author) |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
| Methods | Randomised clinical trial, multicentre, 6 sites in Switzerland | |
| Participants | Inclusion criteria: Clinical diagnosis of CAP, ECOPD, bronchitis with X‐ray confirmation Exclusion criteria: People with active intravenous drug use, severe immunosuppression other than corticosteroid use, life‐threatening medical comorbidity leading to possible imminent death, hospital‐acquired pneumonia (development of pneumonia 48 hours after hospital admission or if they were hospitalised within 14 days before presentation), and chronic infection necessitating antibiotic treatment Included in this analysis: 1359 out of 1381 randomised participants; 22 post randomisation exclusions due to withdrawal of consent | |
| Interventions | Guiding antibiotic decisions in emergency department patients with different ARIs with repeated measurements Algorithm used in this study: Initiation or continuation of ABs was strongly discouraged if PCT was less than 0.1 µg/L and discouraged if levels were 0.25 µg/L or lower. Initiation or continuation of ABs was strongly encouraged if PCT was higher than 0.5 µg/L and encouraged if levels were higher than 0.25 µg/L. If ABs were withheld, hospitalised patients were clinically re‐evaluated and PCT measurement was repeated after 6 to 24 hours. | |
| Outcomes |
| |
| Notes | Funding: This work was supported in part by grant SNF 3200BO‐116177/1 from the Swiss National Science Foundation and contributions from santésuisse and the Gottfried and Julia Bangerter‐Rhyner‐Foundation, the University Hospital Basel, the Medical University Clinic Liestal, the Medical Clinic Buergerspital Solothurn, the Cantonal Hospitals Muensterlingen, Aarau and Lucerne, respectively, the Swiss Society for Internal Medicine, and the Department of Endocrinology, Diabetology and Clinical Nutrition, University Hospital Basel. B·R·A·H·M·S Inc, the major manufacturer of the PCT assay, provided all assay‐related material, Kryptor machines if not already available onsite, and kits and maintenance required for 10,000 measurements related to the study. Follow‐up: Fixed follow‐up period after 30 days and 180 days Registration: ISRCTN95122877 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Central randomisation using a study web site |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | Low risk | Interviews by blinded medical students, data safety monitoring board |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 1358/1359 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes match previously published protocol. |
| Other bias | Low risk | 91% adherence to PCT algorithm in PCT group |
| Methods | Randomised, multicentre, single‐blind, controlled clinical trial in 11 ICUs in Australia | |
| Participants | Inclusion criteria: Patients older than 18 years of age, admitted to ICU within the previous 72 hours, receiving parenteral and/or enteral antibiotics for a suspected bacterial infection (with 2 or more SIRS criteria) and expected to remain in the ICU for longer than 24 hours were eligible. | |
| Interventions | Guiding antibiotic decisions in critically ill patients in the ICU with undifferentiated infection or suspected sepsis Algorithm used in this study: Cessation of antibiotics was recommended if initial or any subsequent PCT was negative (<0.10 ng/mL) or if initial or any subsequent PCT was between 0.10 to 0.25 ng/mL, and infection was highly unlikely; Subsequent PCT level declined more than 90% from baseline, and 2. Assess antibiotic appropriateness and/or adequacy of source control if PCT level at 48 hours is 70% of baseline value. Daily PCT results were made available to the treating clinician for participants randomised to the PCT group. Antibiotic prescription in both the standard care and PCT groups was according to the Australian Antibiotics Therapeutic Guidelines and the antimicrobial stewardship (implemented by infectious diseases twice‐weekly rounds and on‐need consultations). The algorithm was implemented only in the ICU. | |
| Outcomes |
| |
| Notes | Funding: Funded by a competitive grant from the Intensive Care Foundation of Australia and New Zealand. Material support was provided by Roche Diagnostics, Thermo Fisher Scientific, and bioMérieux. Roche Diagnostics and Thermo Fisher Scientific provided additional unrestricted grant funding. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were variable block randomised 1:1 via a secured central study web site into either a PCT‐guided or clinician‐guided group. |
| Allocation concealment (selection bias) | Low risk | Central randomisation with variable blocks |
| Blinding of participants and personnel (performance bias) | Unclear risk | For the standard care group, clinicians were blinded to the PCT levels, but the physicians were aware of the participants' study group due to the study design. |
| Blinding of outcome assessment (detection bias) | Low risk | Data were collected by professional research personnel at each site and entered into a central secured database at the Clinical Informatics and Data Management Unit, Department of Epidemiology and Preventive Medicine, Monash University and analysed by a blinded biostatistician at Monash University, Melbourne, Australia. The study was monitored by an independent data safety and monitoring committee, with no interim analysis performed. |
| Incomplete outcome data (attrition bias) | Low risk | The follow‐up for mortality was 394/394 (100%). |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. Trial registered (ACTRN12610000809033). |
| Other bias | Low risk | High adherence to the PCT algorithm (97%). "The proportion of study days where the PCT algorithm was not followed was less than 3%, the majority of which was due to missed PCT sampling." |
| Methods | Randomised clinical trial, single‐centre, University Hospital Basel, Switzerland | |
| Participants | Inclusion criteria: Clinical diagnosis of COPD exacerbation Exclusion criteria: People who were considered to be vulnerable study participants (i.e. those with psychiatric comorbidity) were excluded from the study. Other exclusion criteria were immunosuppression, asthma, cystic fibrosis, and the presence of infiltrates on chest radiographs on hospital admission. Included in this analysis: 208 out of 226 randomised participants; 18 post randomisation exclusions due to absence of COPD according to GOLD criteria | |
| Interventions | Guiding antibiotic decisions in COPD patients with repeated measurements Algorithm used in this study: Procalcitonin level of 0.1 µg/L was considered indicative of the absence of bacterial infection, and the use of ABs was discouraged. A level of 0.1 to 0.25 µg/L indicated possible bacterial infection, and the use of ABs was discouraged or encouraged, respectively, based on the stability of the participant’s clinical condition. A PCT level of 0.25 µg/L was considered suggestive of the presence of bacterial infection, and AB treatment was encouraged. | |
| Outcomes |
| |
| Notes | Funding: This study was funded by the Clinic of Pulmonary Medicine; the Clinic of Endocrinology, Diabetes and Clinical Nutrition; and the Emergency Department of the University Hospital Basel. B·R·A·H·M·S provided PCT assays for this investigator‐driven study. Follow‐up: Short‐term follow‐up visit after 14 to 21 days; long‐term follow‐up visit at 6 months Registration: ISRCTN77261143 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation list. |
| Allocation concealment (selection bias) | High risk | Sealed envelopes, not numbered |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | Low risk | Blinded personnel |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 208/208 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
| Methods | Randomised clinical trial, multicentre with 7 European and US intensive care units | |
| Participants | Inclusion criteria: VAP when intubated for > 48 h Exclusion criteria: Patients were excluded it they 1) were pregnant; 2) were enrolled in another trial; 3) had received immunosuppressants or long‐term corticosteroid therapy (> 0.5 mg/kg per day for > 1 month); 4) were severely immunosuppressed, including AIDS; or 5) had a coexisting extrapulmonary infection diagnosed between day 1 and 3 requiring antibiotic therapy for > 3 days. Included in this analysis: 101 (101) (100%) | |
| Interventions | Guiding antibiotic decisions in VAP patients with repeated measurements Algorithm used in this study: A PCT level of < 0.25 µg/L suggested the absence of VAP, and discontinuation of ABs was strongly encouraged. A PCT level between 0.25 µg/L and 0.5 µg/L or a decrease by ≥ 80% as compared to day 0 indicated that bacterial infection was unlikely, and reduction or discontinuation of ABs was encouraged. A PCT level ≥ 0.5 µg/L or decrease by < 80% as compared to day 0 was considered indicative of unresolved bacterial infection, and reduction or discontinuation of AB was discouraged. A PCT level of > 1 µg/L strongly suggested unresolved bacterial infection, and AB discontinuation was strongly discouraged. | |
| Outcomes |
| |
| Notes | Funding: Funding was granted by the Clinic of Pulmonary Medicine, University Hospital Basel. Funding obtained from B·R·A·H·M·S AG (Hennigsdorf, Germany) Follow‐up: Fixed follow‐up period of 28 days Registration: ISRCTN61015974 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent statistician created randomisation list. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was through arbitrary allocation to one of the two treatment assignments based on sealed, opaque envelopes. Block size was 20 envelopes. Treating physicians were not aware of envelope contents before randomisation" |
| Blinding of participants and personnel (performance bias) | Unclear risk | Open‐label trial where physicians knew to which group participants had been assigned and where PCT levels were only communicated in the intervention arm |
| Blinding of outcome assessment (detection bias) | High risk | Unblinded study member |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up for mortality: 101/101 (100%) |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol. |
| Other bias | Unclear risk | Adherence to PCT protocol not reported/assessed. |
| Methods | Randomised, single‐centre, single‐blinded, controlled clinical trial in the emergency department of the Fifth People’s Hospital of Shanghai, China | |
| Participants | Inclusion criteria: 1) ≥ 18 years old; 2) has any, or all, of the following clinical features as defined by the Global Initiative for National Asthma (GINA) Guidelines: dyspnoea, wheeze, acute cough, increased work of breathing, increased requirement for beta2‐agonist from baseline use, O2 saturation < 95%, a peak expiratory flow (PEF) at randomisation ≤ 80% of their known best (within the last 12 months) or, in the absence of this information, of their predicted PEF | |
| Interventions | Guiding antibiotic decisions in patients with acute exacerbation of asthma Algorithm used in this study: Participants in the PCT group were treated with antibiotics based on their PCT serum level according to the following guidelines: antibiotics treatment was strongly discouraged when serum PCT level was < 0.1 μg/L; antibiotics treatment was discouraged when serum PCT level was < 0.25 μg/L; antibiotics treatment was encouraged when serum PCT level was > 0.25 μg/L. | |
| Outcomes |
| |
| Notes | Funding: The study was sponsored by a grant from the Shanghai Fifth People’s Hospital Science Foundation and Minhang District Natural Science Foundation of Shanghai. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to either intervention was conducted according to computer‐generated random numbers produced by an independent statistician. |
| Allocation concealment (selection bias) | Low risk | After randomisation, an opaque, sealed, and sequentially numbered envelope containing the PCT or control protocol was prepared for each participant according to the group. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Attending physicians responsible for participants in the control group remained unaware of the participants’ PCT concentrations throughout the study, but blinding was not feasible due to the study design. |
| Blinding of outcome assessment (detection bias) | Low risk | All participants, laboratory technicians, investigators, and research designers were blinded to participant assignments until the data analysis was completed. There were no protocol violations during the study. |
| Incomplete outcome data (attrition bias) | Low risk | 265/265 participants completed follow‐up for mortality. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to study protocol: ICTRP ChiCTR‐TRC‐12002534. |
| Other bias | Low risk | 100% adherence to the PCT algorithm. "There were no protocol violations during the study." |
| Methods | Randomised, multicentre, open, controlled, parallel‐group, non‐inferiority trial involving 18 university/city hospital pulmonary departments in Italy | |
| Participants | Inclusion criteria: Study participants were male or female, 18 years of age, current or former smokers, and diagnosed with COPD stages I‐IV as defined by GOLD guidelines available at the time the study was designed, with protocol deviation. Participants were hospitalised for severe ECOPD requiring antibiotic treatment, i.e. type 1 exacerbation (increased dyspnoea, sputum volume, and sputum purulence verified by the attending clinician) according to Anthonisen, and/or characterised by respiratory failure. Exacerbation of chronic obstructive pulmonary disease was defined as “an acute event characterised by a worsening of the patient’s respiratory symptoms that is beyond normal day‐to‐day variations and leads to a change in medication”. | |
| Interventions | Guiding antibiotic decisions in people with severe exacerbations of COPD Algorithm used in this study: On admission, all patients received a 3‐day course of antibiotics (either amoxicillin plus clavulanate or quinolones) according to 2005 international guidelines. Procalcitonin levels were measured on hospital admission, on day 1, and on day 2. On day 2 each eligible patient was randomly assigned to 1 of the 2 treatment plans. | |
| Outcomes |
| |
| Notes | Funding: The trial was approved and funded by the Agenzia Italiana del Farmaco (AIFA), the Italian agency for drugs, which is an official body of the Italian Ministry of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was web based, and only statisticians and the web site administrator knew the randomisation sequence |
| Allocation concealment (selection bias) | Low risk | Eligible patients were randomly assigned to receive standard antibiotic therapy (standard group) or PCT‐guided antibiotic treatment (PCT group) according to a 1:1 permuted block computer‐generated scheme, stratified according to hospital |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding was not feasible due to the study design |
| Blinding of outcome assessment (detection bias) | High risk | No blinding of outcome assessment mentioned in the study. The authors state that: "... because we anticipated that the primary outcome (exacerbations of COPD) would be strong and easy to identify, and thus unlikely to be biased by investigator influence, we did not adopt any procedure to reduce bias during the follow‐up part of the trial." |
| Incomplete outcome data (attrition bias) | Low risk | 187 of 192 randomised participants were included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Results correspond to the trial registration. |
| Other bias | Low risk | High adherence to the PCT protocol (95.5%) The study planned to enrol 400 participants to have enough statistical power for the primary endpoint, but randomised only 183. |
| Methods | Randomised, single‐centre, single‐blinded, controlled clinical trial in the Beijing Luhe Hospital, China | |
| Participants | Inclusion criteria: Patients with AECOPD who were 40 years of age, had sound understanding and language abilities, and who had a PCT level < 0.1 ng/mL were included. | |
| Interventions | Guiding antibiotic decisions in patients with acute exacerbation of chronic obstructive pulmonary disease Algorithm used in this study: Patients with a PCT concentration < 0.1 ng/mL were randomised. Antibiotics were withheld from participants in the control group. However, antibiotics could be administered later for participants whose clinical condition was unstable or who had a worsening of symptoms and signs, and for those with positive evidence of bacteria as assessed by the attending physicians. In the antibiotic group, antibiotics were administered routinely. | |
| Outcomes |
| |
| Notes | Funding: The study was sponsored by the National Science Fund for Distinguished Young Scholars (81425001/H0104) for Dr Bin Cao. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer digital table method was used to generate randomisation numbers. |
| Allocation concealment (selection bias) | Low risk | Researchers in this study had 24‐hour access to randomisation numbers, allowing immediate and concealed allocation to the trial. |
| Blinding of participants and personnel (performance bias) | Unclear risk | No blinding feasible due to the study protocol. |
| Blinding of outcome assessment (detection bias) | High risk | Individuals responsible for allocation concealment were not allowed to take part in the measurement of results, but no overall blinding of the outcome assessment was mentioned in this study. |
| Incomplete outcome data (attrition bias) | Low risk | Only 3 participants were excluded during the 30‐day follow‐up, due to a diagnosis of pneumonia according to chest X‐ray. |
| Selective reporting (reporting bias) | Low risk | Outcomes correspond to the trial registration (ChiCTR‐TRC‐14004726). |
| Other bias | Low risk | High adherence to the PCT protocol (82.3%) |
AB: antibiotic
AECOPD: acute exacerbation of chronic obstructive pulmonary disease
AE‐IPF: acute exacerbation of idiopathic pulmonary fibrosis
ARIs: acute respiratory infections
CAP: community‐acquired pneumonia
COPD: chronic obstructive pulmonary disease
CPIS: Clinical Pulmonary Infection Score
CRP: C‐reactive protein
d: day
ED: emergency department
ECOPD: exacerbation of chronic obstructive pulmonary disease
EMR: electronic medical record
FEV1%: forced expiratory volume for 1 second expressed as a percentage of the forced vital capacity
GOLD: Global Initiative for Chronic Obstructive Lung Disease
h: hour
ICU: intensive care unit
ID: identification
ITT: intention‐to‐treat
LRTI: lower respiratory tract infection
NA: not available
ODIN: Organ Dysfunction and/or Infection
PaO2/FiO2: relationship between arterial oxygen tension (PaO2) and inspiratory oxygen fraction (FiO2)
Pa: arterial
PCR: polymerase chain reaction
PCT: procalcitonin
RSV: respiratory Syncytial virus
RTI: respiratory tract infection
SaO2: oxygen saturation
SIRS: systemic inflammatory response syndrome
SOFA: Sequential Organ Failure Assessment
VAP: ventilator‐associated pneumonia
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Poster presentation only | |
| Not adult participants (paediatrics) | |
| Meta‐analysis of previous RCTs | |
| Not using procalcitonin to de‐escalate antibiotic therapy but for improving mortality by escalation of therapy | |
| Meta‐analysis of observational studies | |
| Not an RCT; before‐after study design | |
| Not an RCT | |
| Not an RCT | |
| Not a respiratory infection (pancreatitis) | |
| Not an RCT | |
| Not an RCT; before‐after study design (post study survey) | |
| Meta‐analysis of observational studies | |
| Meta‐analysis of observational studies | |
| Included a paediatric population only | |
| Meta‐analysis of observational studies | |
| Meta‐analysis of RCTs | |
| Meta‐analysis of observational studies |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | PCT Antibiotic Consensus Trial (ProACT) |
| Methods | Randomised, single‐blind, multicentre study |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Procalcitonin versus usual care group in patients with LRTI in the ED. Procalcitonin cut‐offs: < 0.1 ng/L. Antibiotics strongly discouraged 0.1 to 0.25 ng/L. Antibiotics discouraged > 0.25 to 0.5 ng/L. Antibiotics recommended > 0.5 ng/L. Antibiotics strongly recommended |
| Outcomes |
|
| Starting date | November 2014 |
| Contact information | Elizabeth A Gimbel, BS; [email protected] Kourtney A Wofford, BA; [email protected] |
| Notes | Collaborators: University of Pittsburgh National Institute of General Medical Sciences (NIGMS), bioMérieux Registration: NCT02130986 |
| Trial name or title | Pulmonary embolism and PCT. PE‐PCT study |
| Methods | Single‐centre, prospective, randomised trial in France |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Procalcitonin algorithm to guide antibiotic therapy. In the control group, the use of antibiotics will be guided by clinical criteria |
| Outcomes |
|
| Starting date | November 2014 |
| Contact information | Patrick Lacarin; placarin@chu‐clermontferrand.fr |
| Notes | Collaborators: Thermo Fisher Scientific Registration: NCT02261610 |
| Trial name or title | Study to compare the efficacy of pristinamycin (Pyostacine) versus amoxicillin in the treatment of acute community acquired pneumonia |
| Methods | A multicentre, non‐inferiority, randomised, double‐blind, phase IV study in France and Tunisia |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: pristinamycin + placebo versus amoxicillin + placebo. To evaluate the clinical efficacy of pristinamycin at a dose of 2 g x 2/day for 2 days then 1 g x 3/day for 5 to 7 days versus amoxicillin 1 g x 3/day for 7 to 9 days, 5 to 9 days after the end of treatment |
| Outcomes |
|
| Starting date | April 2015 |
| Contact information | Contact‐[email protected] |
| Notes | Collaborators: Clinical Sciences & Operations Registration: NCT02332577 |
| Trial name or title | Addition of tobramycin inhalation in the treatment of ventilator associated pneumonia (VAPORISE) |
| Methods | Randomised trial, parallel assignment, double‐blind (participant, investigator) in the Netherlands and Spain |
| Participants | Inclusion criteria: 1) mechanical ventilation 48 hours or more; and 2) new or progressive radiologic pulmonary infiltrate; together with at least 2 of the following 3 criteria (< 24 h):
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Experimental arm: tobramycin inhalation twice daily; tobramycin inhalation (Bramitob) 300 mg and standard intravenous antibiotics treatment. Intervention: drug: tobramycin inhalation Placebo comparator: placebo twice daily; placebo inhalation and standard intravenous antibiotics treatment. Intervention: drug: placebo |
| Outcomes |
|
| Starting date | March 2015 |
| Contact information | Rogier Hoek, MD; [email protected] Menno Van der Eerden, MD, PhD; [email protected] |
| Notes | Collaborators: Chiesi Farmaceutici S.p.A. Registration: NCT02440828 |
| Trial name or title | PCT in Early Antibiotic Interruption in Patient With Bacterial Pulmonary infeCtion and Acute Heart Failure (EPICAD) |
| Methods | Randomised trial with parallel assignment, open label in Brazil |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Experimental: group A: interruption of antibiotic treatment based on PCT measurement No intervention: group B: antibiotic therapy period determined by the physician without knowledge of PCT levels |
| Outcomes |
|
| Starting date | January 2015 |
| Contact information | Mucio Tavares, PhD, MD; [email protected] Aline Bossa, MSc; [email protected] |
| Notes | Collaborators: University of Sao Paulo General Hospital, bioMérieux Registration: NCT02787603 |
| Trial name or title | PCT Pneumonia/Pneumonitis Associated With ASPIration (PROPASPI) |
| Methods | Randomised trial, parallel assignment, open label in France |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Experimental: PCT group. The PCT concentration is measured at inclusion. No intervention: control group. Concentrations of PCT are not measured. |
| Outcomes |
|
| Starting date | February 2015 |
| Contact information | Gilles Capellier, MDPH; gilles.capellier@univ‐fcomte.fr Sophie Depierre; sdepierre@chu‐besancon.fr |
| Notes | Collaborators: Centre Hospitalier Universitaire de Besancon Registration: NCT02862314 |
| Trial name or title | Intra‐operative PEEP optimisation: effects on postoperative pulmonary complications and inflammatory response |
| Methods | Randomised trial, parallel assignment, single‐blind in Hungary |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Algorithm used in this study: Experimental: Optimal PEEP patients submitted to general anaesthesia and open radical cystectomy and urinary diversion (20 participants) will be submitted an alveolar recruitment maneuver using the sustained airway pressure by the CPAP method, applying 30 cmH2O PEEP for 30 seconds followed by a decremental PEEP titration procedure directed by static pulmonary compliance (Cstat). During PEEP titration procedure, PEEP will be decreased from 14 cmH2O by 2 cmH2O every 4 minutes, until a final PEEP of 6 cmH2O. Optimal PEEP is considered to be a PEEP value resulting the highest possible Cstat measured by ventilator. After PEEP titration procedure, a lung protective mechanical ventilation will be performed using optimal PEEP and low tidal volumes. Active comparator: Standard PEEP. Patients submitted to general anaesthesia and open radical cystectomy and urinary diversion (20 participants) will be submitted an alveolar recruitment manoeuvre using the sustained airway pressure by the CPAP method, applying 30 cmH2O PEEP for 30 seconds followed by a standard lung protective mechanical ventilation using a PEEP value of 6 cmH2O and low tidal volumes (6 mL/kg) |
| Outcomes |
|
| Starting date | October 2016 |
| Contact information | Zoltán Ruszkai, MD; [email protected] |
| Notes | Collaborator: Péterfy Sándor Hospital, Szeged University Registration: NCT02931409 |
AB: antibiotic
ACCP: American College of Chest Physicians
APACHE II: Acute Physiology and Chronic Health Evaluation II
BMI: body mass index
BNP: B‐type natriuretic peptide
COPD: chronic obstructive pulmonary disease
CPAP: continuous positive airway pressure
CRP: C‐reactive protein
CT: computed tomography
d: day
ED: emergency department
ESBL: extended‐spectrum beta‐lactamase
h: hour
GOLD: Global Initiative for Chronic Obstructive Lung Disease
ICU: intensive care unit
LRTI: lower respiratory tract infection
NT‐proBNP: N‐terminal pro‐B‐type natriuretic peptide
NYHA: New York Heart Association
Pa: arterial
PaO2/FiO2: relationship between arterial oxygen tension (PaO2) and inspiratory oxygen fraction (FiO2)
PCT: procalcitonin
PEEP: positive end‐expiratory pressure
PORT: Pneumonia Patient Outcomes Research Team
RCT: randomised controlled trial
SIRS: systemic inflammatory response syndrome
SOFA: Sequential Organ Failure Assessment score
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 30 days Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| Analysis 1.1  Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 1 Mortality at 30 days. | ||||
| 1.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.98] |
| 1.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.36] |
| 1.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.00] |
| 2 Treatment failure at 30 days Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| Analysis 1.2 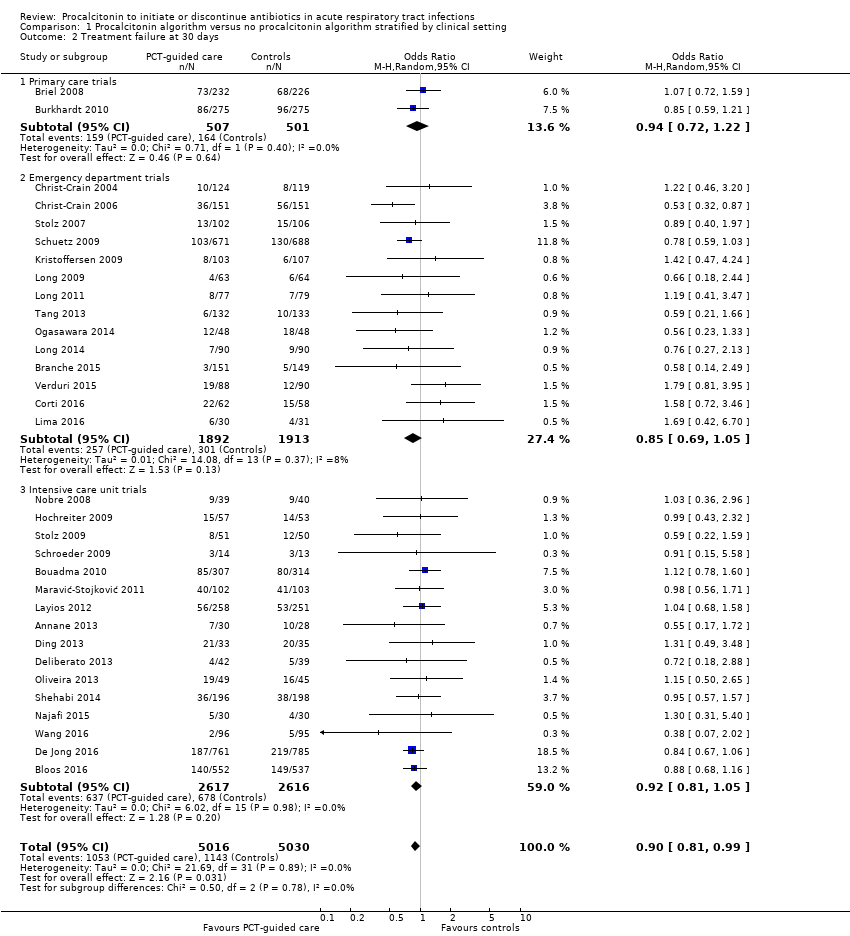 Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 2 Treatment failure at 30 days. | ||||
| 2.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 2.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 2.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 30 days stratified by adherence Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| Analysis 2.1  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 1 Mortality at 30 days stratified by adherence. | ||||
| 1.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.37] |
| 1.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.97] |
| 2 Treatment failure at 30 days stratified by adherence Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| Analysis 2.2  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 2 Treatment failure at 30 days stratified by adherence. | ||||
| 2.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.02] |
| 2.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 3 Mortality at 30 days stratified by allocation concealment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| Analysis 2.3  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 3 Mortality at 30 days stratified by allocation concealment. | ||||
| 3.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.01] |
| 3.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 4 Treatment failure at 30 days stratified by allocation concealment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| Analysis 2.4  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 4 Treatment failure at 30 days stratified by allocation concealment. | ||||
| 4.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 4.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.04] |
| 5 Mortality at 30 days stratified by blinded outcome assessment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| Analysis 2.5  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 5 Mortality at 30 days stratified by blinded outcome assessment. | ||||
| 5.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.32] |
| 5.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.95] |
| 6 Treatment failure at 30 days stratified by blinded outcome assessment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| Analysis 2.6  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 6 Treatment failure at 30 days stratified by blinded outcome assessment. | ||||
| 6.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 6.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 7 Mortality at 30 days stratified by follow up Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| Analysis 2.7  Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 7 Mortality at 30 days stratified by follow up. | ||||
| 7.1 Trials with 1 month follow up for mortality | 18 | 7337 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 7.2 Trials with different follow up for mortality | 14 | 2709 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.30] |

Study flow diagram.
Abbreviations: ARI: acute respiratory infection; IPD: individual participant data; RCT: randomised controlled trial

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.
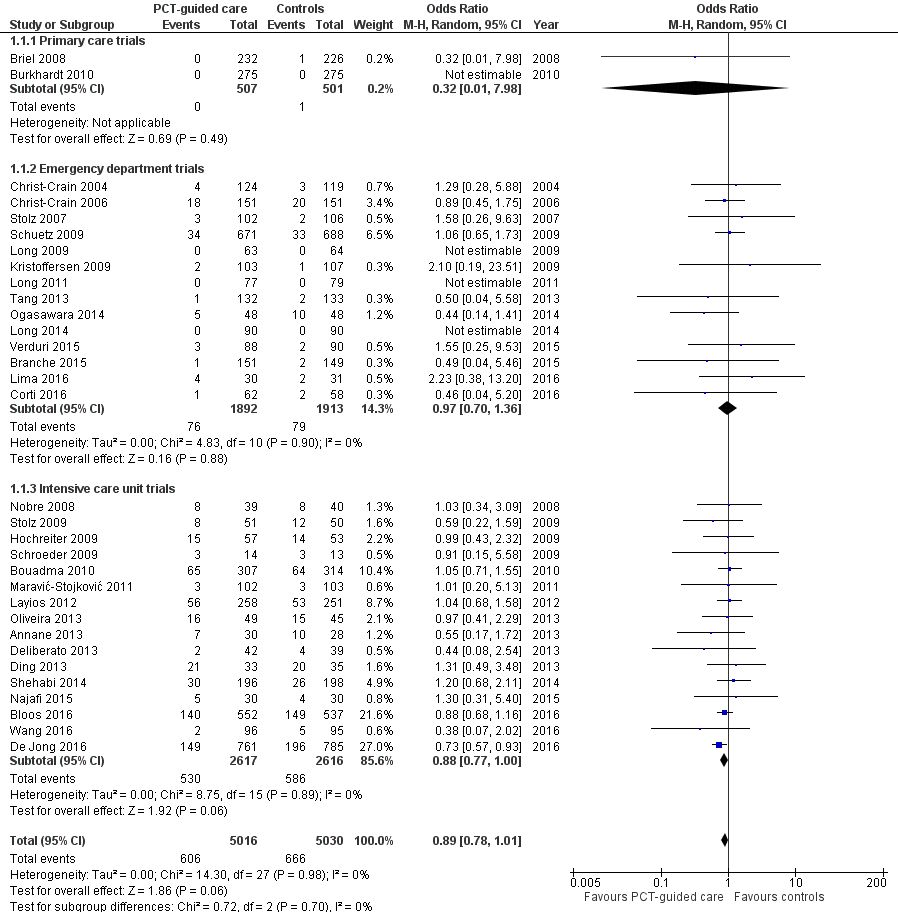
Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.1 Mortality at 30 days.

Forest plot of comparison: 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, outcome: 1.2 Treatment failure at 30 days.

Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 1 Mortality at 30 days.

Comparison 1 Procalcitonin algorithm versus no procalcitonin algorithm stratified by clinical setting, Outcome 2 Treatment failure at 30 days.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 1 Mortality at 30 days stratified by adherence.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 2 Treatment failure at 30 days stratified by adherence.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 3 Mortality at 30 days stratified by allocation concealment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 4 Treatment failure at 30 days stratified by allocation concealment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 5 Mortality at 30 days stratified by blinded outcome assessment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 6 Treatment failure at 30 days stratified by blinded outcome assessment.

Comparison 2 Procalcitonin algorithm versus no procalcitonin algorithm, sensitivity analyses, Outcome 7 Mortality at 30 days stratified by follow up.
| Procalcitonin algorithm compared to standard care for guiding antibiotic therapy in acute respiratory tract infections | ||||||
| Patient or population: people with acute respiratory tract infections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard care | PCTalgorithm | |||||
| Mortality | Study population | OR 0.83 | 6708 | ⊕⊕⊕⊕ | ||
| 100 per 1000 | 86 per 1000 (76 to 95) | |||||
| Treatment failure | Study population | OR 0.90 | 6708 | ⊕⊕⊕⊝ | ||
| 249 per 1000 | 230 per 1000 (216 to 245) | |||||
| Antibiotic‐related side effects Follow‐up: 30 days | Study population 221 per 1000 | 163 per 1000 (145 to 182) | OR 0.68 | 3034 | ⊕⊕⊕⊝ | |
| Antibiotic exposure | The mean antibiotic exposure in the control groups was | The mean antibiotic exposure in the intervention groups was | ‐ | 6708 | ⊕⊕⊕⊕ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1No downgrading for serious concerns. Still, there is some concern about unconcealed allocation in several trials in the emergency department and intensive care settings. There is also some concern about low adherence with the PCT algorithm in the intervention group. We consider unblinded outcome assessment as not relevant for the outcome of death. | ||||||
| Study ID | Country | Setting, type of trial | Clinical diagnosis | Type of PCTalgorithm and PCTcut‐offs used (µg/L) | N: ARI participants (study total) | Primary endpoint | Follow‐up time | Reasons for exclusion of patients |
| France | ICU, multicentre | Severe sepsis without overt source of infection and negative blood culture | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 5.0) | 0 (62) | Participants on AB on day 5 post randomisation | Hospital stay | 62 non‐ARI patients (4 of them with post randomisation consent withdrawal) | |
| Germany | ICU, multicentre | Severe sepsis or septic shock | Discontinuation at day 4, 7, and 10; R against AB: < 1.0 or > 50% drop to previous value | 219 (1180) | 28‐day mortality | 3 months | 91 post randomisation exclusions (informed consent not obtainable), 870 not ARI patients | |
| France | ICU, multicentre | Suspected bacterial infections during ICU stay without prior AB (> 24 h) | Initiation and duration; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 394 (630) | All‐cause mortality | 2 months | 9 post randomisation exclusions (8 withdrew consent, 1 randomised twice); 227 non‐ARI patients | |
| USA | ED, medical ward, single centre | Lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 265 (300) | Antibiotic exposure and safety | 3 months | 35 non‐ARI patients | |
| Switzerland | Primary care, multicentre | Upper and lower ARI | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 458 (458) | Days with restricted activities | 1 month | No exclusions | |
| Germany | Primary care, multicentre | Upper and lower ARI | Initiation; R against AB: < 0.25; R for AB: > 0.25 | 550 (571) | Days with restricted activities | 1 month | 21 post randomisation exclusions (2 withdrew consent, 1 due to loss of sample, 15 with autoimmune, inflammatory, or systemic disease, 2 with advanced liver disease, 1 with prior use of antibiotics) | |
| Switzerland | ED, single centre | Lower ARI with X‐ray confirmation | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 219 (243) | AB use | 2 weeks | 24 non‐ARI patients | |
| Switzerland | ED, medical ward, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 286 (302) | AB use | 6 weeks | 16 non‐ARI patients | |
| Denmark | ED, single centre | AECOPD | Initiation and duration; R against AB < 0.25 (0.15)/80% decrease; R for AB > 0.25 | 120 (120) | AB use | 28 days | No exclusions | |
| Netherlands | ICU, multicentre | Critically ill patients with presumed infection | Duration; R against AB: < 0.5 or > 80% drop | 994 (1575) | AB use | 1 year | 29 post randomisation exclusions (25 protocol violations, 4 withdrew informed consent), 552 non‐ARI patients | |
| Brazil | ICU, single centre | Septic patients with proven bacterial infection | Duration; R against AB: < 0.5 or > 90% drop | 66 (81) | AB use | ICU discharge or 14 days' post randomisation | 15 non‐ARI patients | |
| China | ICU, single centre | Acute exacerbation of pulmonary fibrosis | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 0 (78) | AB use | 1 month | 10 post randomisation exclusions (7 lost to follow‐up, 3 withdrew informed consent), 68 data not shared | |
| Germany | Surgical ICU, single centre | Suspected bacterial infections and > 1 SIRS criteria | Duration; R against AB: < 1 or > 65% drop over 3 d | 43 (110) | AB use | Hospital stay | 67 non‐ARI patients | |
| Denmark | ED, medical ward, multicentre | Lower ARI without X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 (> 0.5) | 210 (223) | AB use | Hospital stay | 13 post randomisation exclusions (3 no PCT testing, 6 not meeting inclusion criteria, 4 withdrew informed consent) | |
| Belgium | ICU, single centre | Suspected infection | Initiation; R against AB: < 0.5 (< 0.25); R for AB: > 0.5 (> 1.0) | 160 (509) | AB use | 1 month | 120 no PCT measurements, 10 missing data, 219 non‐ARI patients | |
| Brazil | ED, medical ward, single centre | Febrile neutropenia | Duration; R against AB: < 0.5 for 2 days or > 90% drop than highest measured concentration | 0 (62) | AB use | 28 days | 1 post randomisation exclusion (withdrew informed consent), 62 non‐ARI patients | |
| China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 127 (149) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent | |
| China | ED, outpatients, single centre | CAP with X‐ray confirmation | Initiation and duration; R against AB: < 0.25; R for AB: > 0.25 | 156 (172) | AB use | 1 month | 16 post randomisation exclusions (6 lost to follow‐up, 7 withdrew consent, 3 with final diagnosis other than CAP) | |
| China | ED, single centre | Severe acute exacerbation of asthma | Initiation; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 180 (180) | AB use | 1 year | No exclusions | |
| Serbia | ICU surgical, single centre | Infection after open heart surgery | Initiation; R for AB: > 0.5 | 5 (205) | AB use, AB cost | Hospital stay | 200 non‐ARI patients | |
| Iran | ICU, single centre | SIRS without apparent source of infection | Initiation; R for AB: > 2 | 0 (60) | AB use | Hospital stay | 60 patient data not shared | |
| Switzerland | ICU, single centre | Suspected severe sepsis or septic shock | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 52 (79) | AB use | 1 month | 27 non‐ARI patients | |
| Japan | Medical ward, single centre | Aspiration pneumonia | Predefined duration; AB for 3 d: < 0.5; AB for 5 d: 0.5 to 1.0; AB for 7 d: > 1 | 0 (105) | Relapse and 30‐day mortality | 1 month | 9 post randomisation exclusions (2 withdrew consent, 7 others), 96 data not shared | |
| Brazil | ICU, multicentre | Severe sepsis or septic shock | Discontinuation; initial < 1.0: R against AB: 0.1 at day 4; initial > 1.0: R against: > 90% drop | 58 (97) | AB use | 28 days or hospital discharge | 3 post randomisation exclusions (2 withdrew consent, 1 technical problems), 36 patients with a final diagnosis other than ARI | |
| Germany | Surgical ICU, single centre | Severe sepsis following abdominal surgery | Duration; R against AB: < 1 or > 65% drop over 3 d | 8 (27) | AB use | Hospital stay | 19 non‐ARI patients | |
| Switzerland | ED, medical ward, multicentre | Lower ARI with X‐ray confirmation | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 1304 (1381) | AB use | 1 month | 22 post randomisation exclusions due to withdrawal of consent, 55 non‐ARI patients | |
| Australia | ICU, multicentre | Suspected sepsis, undifferentiated infections | Duration; R against AB: < 0.25 (< 0.1) or > 90% drop | 156 (400) | AB use | 3 months | 6 post randomisation exclusions (6 withdrew consent), 238 non‐ARI patients | |
| Switzerland | ED, medical ward, single centre | Exacerbated COPD | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 (> 0.5) | 208 (226) | AB use | 2 to 3 weeks | 18 post randomisation exclusions (absence of COPD) | |
| Switzerland, USA | ICU, multicentre | VAP when intubated > 48 h | Duration; R against AB: < 0.5 (< 0.25) or > 80% drop; R for AB: > 0.5 (> 1.0) | 101 (101) | AB‐free days alive | 1 month | No exclusions | |
| China | ED, single centre | Exacerbation of asthma | Initiation and duration; R against AB: < 0.25 (< 0.1); R for AB: > 0.25 | 0 (265) | AB use | 6 weeks | 10 post randomisation exclusions (5 lost to follow‐up, 3 died, 2 withdrew consent), 255 data not shared | |
| Italy | ED, medical ward, multicentre | AECOPD | Initiation; R against AB:< 0.1; R for AB: > 0.25 | 178 (183) | Number of exacerbations | 6 months | 5 post randomisation exclusions (5 lost to follow‐up because they did not meet the inclusion criteria) | |
| China | ICU, single centre | AECOPD | All participants had initial PCT < 0.1; AB group treated with AB for at least 3 days, control group no AB in the first 10 days | 191 (194) | Treatment success within 10 days | 30 days | 3 post randomisation exclusions (3 with pneumonia according to CT scan) | |
| AB: antibiotic | ||||||||
| Parameter | Control (n = 3372) | PCT group (n = 3336) |
| Demographics | ||
| Age (year), mean (SD) | 61.2 ± 18.4 | 60.7 ± 18.8 |
| Male gender, n (%) | 1910 (56.6%) | 1898 (56.9%) |
| Clinical setting, no (%) | ||
| Primary care | 501 (14.9%) | 507 (15.2%) |
| Emergency department | 1638 (48.6%) | 1615 (48.4%) |
| Intensive care unit | 1233 (36.6%) | 1214 (36.4%) |
| Primary diagnosis | ||
| Total upper ARI, n (%) | 280 (8.3%) | 292 (8.8%) |
| Common cold | 156 (4.6%) | 149 (4.5%) |
| Rhino‐sinusitis, otitis | 67 (2.0%) | 73 (2.2%) |
| Pharyngitis, tonsillitis | 46 (1.4%) | 61 (1.8%) |
| Total lower ARI, n (%) | 3092 (91.7%) | 3044 (91.2%) |
| Community‐acquired pneumonia | 1468 (43.5%) | 1442 (43.2%) |
| Hospital‐acquired pneumonia | 262 (7.8%) | 243 (7.3%) |
| Ventilator‐associated pneumonia | 186 (5.5%) | 194 (5.8%) |
| Acute bronchitis | 287 (8.5%) | 257 (7.7%) |
| Exacerbation of COPD | 631 (18.7%) | 621 (18.6%) |
| Exacerbation of asthma | 127 (3.8%) | 143 (4.3%) |
| Other lower ARI | 131 (3.9%) | 144 (4.3%) |
| Procalcitonin upon enrolment | ||
| PCT< 0.1 ug/L | 921 (35.6%) | 981 (30.9%) |
| PCT 0.1 to 0.25 ug/L | 521 (20.1%) | 608 (19.2%) |
| PCT > 0.25 to 0.5 ug/L | 308 (11.9%) | 383 (12.1%) |
| PCT > 0.5 to 2.0 ug/L | 358 (13.8%) | 520 (16.4%) |
| PCT > 2.0 ug/L | 482 (18.6%) | 679 (21.4%) |
| ARI: acute respiratory infection | ||
| Study ID | Allocation concealment | Blinded | Follow‐up for mortality | Adherence to PCT algorithm in PCT group | Follow‐up for mortality |
| Yes (central randomisation) | No | 58/58 (100%) | 63% adherence | LOS | |
| Yes (central randomisation) | No | 1045/1089 (96%) | 49.6% adherence | 28 days and 90 days | |
| Yes (central randomisation) | Yes | 393/394 (100%) | 47% adherence | 28 days and 60 days | |
| Yes (central randomisation using blocks of 4) | No | 250/300 (83.3%) | 64% adherence | 1 month and 3 months | |
| Yes (central randomisation) | Yes | 454/458 (99%) | 85% adherence | 28 days | |
| Yes (central randomisation) | Yes | 546/550 (99%) | 87% adherence | 28 days | |
| No (alternating weeks) | No | 230/243 (95%) | 83% adherence | 10 to 14 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 300/302 (99%) | 87% adherence | 56 days | |
| Yes (randomisation algorithm was concealed to treating clinicians and participants) | No | 120/120 (100%) | 61.1% adherence | 28 days | |
| Yes (central randomisation) | No | 1546/1546 (100%) | 44% adherence | 28 days and 1 year | |
| Yes (opaque, sealed envelopes) | No | 81/81 (100%) | 52% adherence | LOS | |
| Yes (central randomisation) | No | 68/78 (87.2%) | Not reported | 30 days | |
| No (unconcealed drawing of lots) | No | 43/43 (100% until discharge) | Not reported | LOS | |
| Yes (central randomisation) | No | 210/210 (100% until discharge) | 59% adherence | LOS | |
| Not reported | No | 509/509 (100%) | Not reported | Intensive care unit LOS | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 61/62 (98.4%) | 73.3% adherence | 28 days and 90 days | |
| No (odd and even patient ID numbers) | No | 127/127 (100%) | Not reported | Not reported | |
| No (odd and even patient ID numbers) | No | 156/156 (100%) | Not reported | 28 days | |
| Yes (central randomisation) | No | 169/180 (93.9%) | 96.6% adherence | LOS and 1 year | |
| Yes (central randomisation) | No | 205/205 (100%) | Not reported | 30 days and LOS | |
| Yes (central randomisation) | No | 30/30 (100%) | Not reported | LOS | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 52/52 (100%) | 81% adherence | 28 days and LOS | |
| Not reported | No | 96/96 (100%) | Not reported | 30 days | |
| Yes (central randomisation) | No | 94/94 (100%) | 86.2% adherence | 28 days | |
| No (unconcealed drawing of lots) | No | 8/8 (100% until discharge) | Not reported | LOS | |
| Yes (central randomisation) | Yes | 1358/1359 (100%) | 91% adherence | 28 days | |
| Yes (central randomisation) | Yes | 394/394 (100%) | Not reported | LOS and 90 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 208/208 (100%) | Not reported | 6 months | |
| Yes (sequentially numbered, opaque, sealed envelopes) | No | 101/101 (100%) | Not reported | 28 days | |
| Yes (sequentially numbered, opaque, sealed envelopes) | Yes | 258/265 (97.4%) | Not reported | 6 weeks | |
| Yes (central randomisation) | No | 178/178 (100%) | Not reported | 6 months | |
| Yes (those responsible for allocation concealment were not involved in the measurement of results) | No | 191/191 (100%) | 82.3% adherence (17 participants in the control group received AB) | 30 days | |
| LOS: length of stay | |||||
| Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction | |
| Overall | 3372 | 3336 | ||
| 30 days mortality, n (%) | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Treatment failure, n (%) | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Length of ICU stay, mean (±SD) | 13.3 ± 16.0 | 13.7 ± 17.2 | 0.39 (‐0.81 to 1.58), P = 0.524 | NA |
| Length of hospital stay, mean (±SD) | 13.7 ± 20.6 | 13.4 ± 18.4 | ‐0.19 (‐0.96 to 0.58), P = 0.626 | NA |
| Antibiotic‐related side effects, n (%) | 336 (22.1%) | 247 (16.3%) | 0.68 (0.57 to 0.82), P < 0.001 | NA |
| According to setting | ||||
| Primary care | 501 | 507 | ||
| 30 days mortality, n (%) | 1 (0.2%) | 0 (0.0%) | NA | NA |
| Treatment failure, n (%) | 164 (32.7%) | 159 (31.4%) | 0.96 (0.73 to 1.25), P = 0.751 | 0.715 |
| Days with restricted activities, mean (±SD) | 8.9 ± 4.2 | 8.9 ± 4.1 | 0.07 (‐0.44 to 0.59), P = 0.777 | NA |
| Antibiotic‐related side effects, n (%) | 128 (25.7%) | 102 (20.2%) | 0.65 (0.46 to 0.91), P = 0.012 | 0.596 |
| Emergency department | 1638 | 1615 | ||
| 30 days mortality, n (%) | 62 (3.8%) | 57 (3.5%) | 0.91 (0.63 to 1.33), P = 0.635 | 0.546 |
| Treatment failure, n (%) | 292 (17.8%) | 259 (16.0%) | 0.87 (0.72 to 1.05), P = 0.141 | 0.807 |
| Length of hospital stay, mean (±SD) | 8.2 ± 10.5 | 8.1 ± 7.5 | ‐0.14 (‐0.73 to 0.44), P = 0.631 | 0.684 |
| Antibiotic‐related side effects, n (%) | 208 (20.3%) | 145 (14.4%) | 0.66 (0.52 to 0.83), P = 0.001 | 0.596 |
| Intensive care unit | 1233 | 1214 | ||
| 30 days mortality, n (%) | 273 (22.3%) | 229 (19.0%) | 0.84 (0.69 to 1.02), P = 0.081 | 0.619 |
| Length of ICU stay, mean (±SD) | 14.8 ± 16.2 | 15.3 ± 17.5 | 0.56 (‐0.82 to 1.93), P = 0.427 | 0.849 |
| Length of hospital stay, mean (±SD) | 26.3 ± 26.9 | 25.8 ± 23.9 | ‐0.33 (‐2.28 to 1.62), P = 0.739 | 0.641 |
| According to diagnosis | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| 30 days mortality, n (%) | 206 (14.1%) | 175 (12.2%) | 0.82 (0.66 to 1.03), P = 0.083 | 0.958 |
| Treatment failure, n (%) | 385 (26.2%) | 317 (22.0%) | 0.78 (0.66 to 0.93), P = 0.005 | 0.052 |
| Length of ICU stay, mean (±SD) | 10.5 ± 10.3 | 11.9 ± 13.3 | 1.45 (0.15 to 2.75), P = 0.029 | 0.119 |
| Length of hospital stay, mean (±SD) | 13.3 ± 15.7 | 13.9 ± 16.1 | 0.74 (‐0.25 to 1.73), P = 0.143 | 0.094 |
| Antibiotic‐related side effects, n (%) | 186 (27.7%) | 127 (19.1%) | 0.62 (0.48 to 0.80), P < 0.001 | 0.227 |
| Exacerbation of COPD | 631 | 621 | ||
| 30 days mortality, n (%) | 24 (3.8%) | 19 (3.1%) | 0.8 (0.43 to 1.48), P = 0.472 | 0.847 |
| Treatment failure, n (%) | 110 (17.4%) | 104 (16.7%) | 0.94 (0.70 to 1.27), P = 0.704 | 0.676 |
| Length of hospital stay, mean (±SD) | 9.3 ± 13.9 | 8.4 ± 7.2 | ‐0.60 (‐1.84 to 0.64), P = 0.342 | 0.658 |
| Antibiotic‐related side effects, n (%) | 30 (10.9%) | 29 (10.5%) | 0.93 (0.53 to 1.63), P = 0.805 | 0.198 |
| Acute bronchitis | 287 | 257 | ||
| 30 days mortality, n (%) | 0 (0.0%) | 2 (0.8%) | NA | NA |
| Treatment failure, n (%) | 55 (19.2%) | 52 (20.2%) | 1.11 (0.72 to 1.70), P = 0.643 | 0.4 |
| Length of hospital stay, mean (±SD) | 2.6 ± 5.7 | 2.2 ± 4.7 | ‐0.21 (‐0.90 to 0.48), P = 0.556 | 0.97 |
| Antibiotic‐related side effects, n (%) | 54 (21.6%) | 39 (17.3%) | 0.77 (0.49 to 1.22), P = 0.263 | 0.657 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| 30 days mortality, n (%) | 29 (15.6%) | 23 (12.0%) | 0.75 (0.41 to 1.39), P = 0.366 | 0.644 |
| Treatment failure, n (%) | 51 (27.4%) | 44 (22.7%) | 0.78 (0.48 to 1.28), P = 0.332 | 0.522 |
| Length of ICU stay, mean (±SD) | 23.5 ± 20.5 | 21.8 ± 19.1 | ‐1.74 (‐5.64 to 2.17), P = 0.383 | 0.441 |
| Length of hospital stay, mean (±SD) | 33.8 ± 27.6 | 32.0 ± 23.1 | ‐2.14 (‐7.04 to 2.75), P = 0.391 | 0.448 |
| Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals ARI: acute respiratory infection | ||||
| Mortality | ||||
| Main analysis | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 336 (10.0%) | 286 (8.6%) | 0.83 (0.70 to 0.99), P = 0.037 | NA |
| Adherence | ||||
| High adherence | 82 (4.5%) | 75 (4.1%) | 0.88 (0.63 to 1.22), P = 0.434 | 0.617 |
| Low adherence (< 70% or not reporting) | 254 (16.4%) | 211 (14.0%) | 0.83 (0.67 to 1.02), P = 0.073 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 305 (10.6%) | 250 (8.8%) | 0.80 (0.67 to 0.97), P = 0.021 | 0.229 |
| High risk for allocation concealment bias (or not reporting) | 31 (6.5%) | 36 (7.3%) | 1.12 (0.66 to 1.91), P = 0.672 | |
| Blinding | ||||
| Blinded outcome assessment | 113 (6.5%) | 102 (5.9%) | 0.85 (0.64 to 1.13), P = 0.259 | 0.537 |
| No blinded outcome assessment | 223 (13.8%) | 184 (11.5%) | 0.81 (0.65 to 1.01), P = 0.062 | |
| Follow‐up | ||||
| Follow up for mortality at 1 month | 275 (10.7%) | 224 (8.9%) | 0.81 (0.67 to 0.99), P = 0.039 | 0.325 |
| Follow up for mortality < 1 month | 61 (7.6%) | 62 (7.6%) | 0.94 (0.64 to 1.38), P = 0.756 | |
| Treatment failure | Control group | PCT group | Adjusted OR (95% CI), P value | P for interaction |
| All participants | 841 (24.9%) | 768 (23.0%) | 0.90 (0.80 to 1.01), P = 0.068 | NA |
| Adherence | ||||
| High adherence | 419 (23.1%) | 381 (21.0%) | 0.89 (0.76 to 1.04), P = 0.148 | 0.752 |
| Low adherence (< 70% or not reporting) | 422 (27.1%) | 387 (25.5%) | 0.92 (0.77 to 1.08), P = 0.301 | |
| Allocation | ||||
| Low risk for allocation concealment bias | 776 (26.8%) | 699 (24.6%) | 0.89 (0.79 to 1.01), P = 0.069 | 0.486 |
| High risk for allocation concealment bias (or not reporting) | 65 (13.6%) | 69 (13.8%) | 0.99 (0.68 to 1.44), P = 0.939 | |
| Blinding | ||||
| Blinded outcome assessment | 412 (23.5%) | 367 (21.1%) | 0.88 (0.75 to 1.03), P = 0.117 | 0.574 |
| No blinded outcome assessment | 429 (26.5%) | 401 (25.1%) | 0.94 (0.79 to 1.11), P = 0.438 | |
| CI: confidence interval | ||||
| Parameter | Control group | PCT group | Measures of effect: adjusted OR or difference (95% CI), P value | P for interaction |
| Overall | 3372 | 3336 | ||
| Initiation of antibiotics, n (%) | 2894 (86.3%) | 2351 (71.5%) | 0.27 (0.24 to 0.32), P < 0.001 | |
| Duration of antibiotics (days), mean (±SD) | 9.4 ± 6.2 | 8.0 ± 6.5 | ‐1.83 (‐2.15 to ‐1.50), P < 0.001 | |
| Total exposure of antibiotics (days), mean (±SD) | 8.1 ± 6.6 | 5.7 ± 6.6 | ‐2.43 (‐2.71 to ‐2.15), P < 0.001 | |
| Setting‐specific outcomes | ||||
| Primary care | 501 | 507 | ||
| Initiation of antibiotics, n (%) | 316 (63.1%) | 116 (22.9%) | 0.13 (0.09 to 0.18), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.3 ± 2.5 | 7.0 ± 2.8 | ‐0.52 (‐1.07 to 0.04), P = 0.068 | 0.064 |
| Total exposure of antibiotics (days), mean (±SD) | 4.6 ± 4.1 | 1.6 ± 3.2 | ‐3.02 (‐3.45 to ‐2.58), P < 0.001 | 0.101 |
| Emergency department | 1638 | 1615 | ||
| Initiation of antibiotics, n (%) | 1354 (83.2%) | 1119 (71.3%) | 0.49 (0.41 to 0.58), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.8 ± 5.4 | 7.3 ± 5.1 | ‐2.45 (‐2.86 to ‐2.05), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 8.2 ± 6.2 | 5.2 ± 5.4 | ‐3.02 (‐3.41 to ‐2.62), P < 0.001 | < 0.001 |
| Intensive care unit | 1233 | 1214 | ||
| Initiation of antibiotics, n (%) | 1224 (99.8%) | 1116 (91.9%) | 0.02 (0.01 to 0.05), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.8 ± 7.8 | ‐1.23 (‐1.82 to ‐0.65), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 9.5 ± 7.4 | 8.1 ± 7.9 | ‐1.44 (‐1.99 to ‐0.88), P < 0.001 | < 0.001 |
| Disease‐specific outcomes | ||||
| Community‐acquired pneumonia | 1468 | 1442 | ||
| Initiation of antibiotics, n (%) | 1455 (99.4%) | 1340 (92.9%) | 0.08 (0.04 to 0.15), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 10.5 ± 6.2 | 8.0 ± 5.7 | ‐2.45 (‐2.87 to ‐2.02), P < 0.001 | < 0.001 |
| Total exposure of antibiotics (days), mean (±SD) | 10.4 ± 6.2 | 7.5 ± 5.9 | ‐2.94 (‐3.38 to ‐2.50), P < 0.001 | 0.004 |
| Exacerbation of COPD | 631 | 621 | ||
| Initiation of antibiotics, n (%) | 453 (71.8%) | 266 (42.8%) | 0.29 (0.23 to 0.36), P < 0.001 | 0.017 |
| Duration of antibiotics (days), mean (±SD) | 7.4 ± 5.3 | 7.2 ± 6.7 | ‐1.15 (‐2.00 to ‐0.31), P = 0.007 | 0.003 |
| Total exposure of antibiotics (days), mean (±SD) | 5.3 ± 5.6 | 3.1 ± 5.6 | ‐2.22 (‐2.83 to ‐1.60), P < 0.001 | 0.506 |
| Acute bronchitis | 287 | 257 | ||
| Initiation of antibiotics, n (%) | 189 (65.9%) | 68 (26.5%) | 0.18 (0.12 to 0.26), P < 0.001 | < 0.001 |
| Duration of antibiotics (days), mean (±SD) | 7.1 ± 3.0 | 6.4 ± 3.5 | ‐0.35 (‐1.15 to 0.45), P = 0.393 | 0.359 |
| Total exposure of antibiotics (days), mean (±SD) | 4.7 ± 4.2 | 1.7 ± 3.3 | ‐2.95 (‐3.59 to ‐2.31), P < 0.001 | 0.33 |
| Ventilator‐associated pneumonia | 186 | 194 | ||
| Initiation of antibiotics, n (%) | 186 (100.0%) | 193 (99.5%) | NA | NA |
| Duration of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.22 (‐3.80 to ‐0.65), P = 0.006 | 0.253 |
| Total exposure of antibiotics (days), mean (±SD) | 13.1 ± 7.9 | 10.8 ± 8.7 | ‐2.45 (‐4.09 to ‐0.82), P = 0.003 | 0.786 |
| Note: Duration refers to the total days of antibiotic therapy in participants in whom antibiotics were initiated. Total exposure refers to the total days of antibiotic therapy in all randomised participants. Measures of effect: dichotomous outcomes are reported as adjusted OR (95% CI) and continuous outcomes are adjusted mean differences and confidence intervals ARI: acute respiratory infection | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 30 days Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.98] |
| 1.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.36] |
| 1.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.77, 1.00] |
| 2 Treatment failure at 30 days Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2.1 Primary care trials | 2 | 1008 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.72, 1.22] |
| 2.2 Emergency department trials | 14 | 3805 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 2.3 Intensive care unit trials | 16 | 5233 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality at 30 days stratified by adherence Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 1.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.37] |
| 1.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.73, 0.97] |
| 2 Treatment failure at 30 days stratified by adherence Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 2.1 Adherence to procalcitonin algorithm > 70% | 14 | 4422 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.02] |
| 2.2 Adherence to procalcitonin algorithm < 70% or not available | 18 | 5624 | Odds Ratio (M‐H, Random, 95% CI) | 0.92 [0.81, 1.04] |
| 3 Mortality at 30 days stratified by allocation concealment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.89 [0.78, 1.01] |
| 3.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.76, 1.01] |
| 3.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.70, 1.28] |
| 4 Treatment failure at 30 days stratified by allocation concealment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.81, 0.99] |
| 4.1 Trials with concealed allocation | 22 | 7968 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.82, 1.02] |
| 4.2 Trials without concealed allocation | 10 | 2078 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.64, 1.04] |
| 5 Mortality at 30 days stratified by blinded outcome assessment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 5.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.32] |
| 5.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.95] |
| 6 Treatment failure at 30 days stratified by blinded outcome assessment Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 6.1 Trials with blinded outcome assessment | 9 | 4664 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.06] |
| 6.2 Trials without blinded outcome assessment | 23 | 5382 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 7 Mortality at 30 days stratified by follow up Show forest plot | 32 | 10046 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.00] |
| 7.1 Trials with 1 month follow up for mortality | 18 | 7337 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 7.2 Trials with different follow up for mortality | 14 | 2709 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.30] |

