Добавки витамина А для профилактики заболеваемости и смертности младенцев в возрасте от 1 до 6 месяцев
Referencias
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomised, placebo‐controlled, double‐blind, 2 x 2 factorial trial Data collection: July 1999 to November 2001 | |
| Participants | n = 564 Inclusion criteria: recently delivered women with live singleton neonates Exclusion criteria: none 51% boys | |
| Interventions | 2 x 2 factorial design Aa group: maternal vitamin A, infant vitamin A (mother received 400,000 IU vitamin A within 24 hours of delivery; infant received 100,000 IU vitamin A at 14 weeks of age with DPT and OPV vaccines; n = 142) Pa group: maternal placebo, infant vitamin A (mother received placebo within 24 hours of delivery; infant received 100,000 IU vitamin A at 14 weeks of age with DPT and OPV vaccines; n = 143) Ap group: maternal vitamin A, infant placebo (mother received 400,000 IU vitamin A within 24 hours of delivery; infant received placebo at 14 weeks of age with DPT and OPV vaccines; n = 140) Pp group: maternal placebo, infant placebo (mother received placebo within 24 hours of delivery; infant received placebo at 14 weeks of age with DPT and OPV vaccines; n = 139) All pregnant women received presumptive malarial treatment in their second and third trimesters In Ayah 2007, we included data for Aa vs. Ap, and in Ayah 2007 (2) we included data for Pa vs. Pp groups | |
| Outcomes | Infant supplementation: all‐cause mortality, bulging fontanelle | |
| Notes | Location: Bondo District, rural western Kenya (Africa) HIV status: earlier HIV prevalence reported as 28% among antenatal clinic attendees; however, the trial was conducted before to the availability of HIV testing and antiretroviral prophylaxis for antenatal women in public sector facilities in western Kenya Mortality outcome data were taken from figure 1 (trial profile). We included raw numbers after mother‐infant pair was randomised. We treated the trial as 2 studies and included data in a way that mother received the same supplementation between the groups and only difference was infant vitamin A supplementation i.e. Aa vs. Ap and Pa vs. Pp Adverse effect of bulging fontanelle was recorded as a comparison of infants receiving vitamin A or placebo irrespective of maternal vitamin A supplementation. We included the data as 1 group for this comparison | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Two random sequences of X and Y were prepared, one for the mothers and one for the infants. Identification numbers from 1 to 700 were assigned consecutively to each of the two lists and mother‐infant pairs of capsules were packaged in zip‐lock bags numbered from 1 to 700 and kept in batches of ten" |
| Allocation concealment (selection bias) | Low risk | "The randomization codes were concealed for the entire trial duration and only revealed after completion of data analysis" |
| Blinding (performance bias and detection bias) | Low risk | "...prepared and supplied the vitamin A and identical‐looking placebo supplements as oily capsules in brown bottles coded as X or Y" |
| Incomplete outcome data (attrition bias) | Low risk | Attrition of 8% at 14 weeks' follow‐up and that of 22% at 26 weeks. The reasons for attrition were described for each group and comparable among groups. All analyses were by intention to treat |
| Selective reporting (reporting bias) | Unclear risk | No protocol was not available to make an assessment |
| Other bias | Low risk | The study seemed to be free of other bias |
| Methods | See Ayah 2007 above | |
| Participants | See Ayah 2007 above | |
| Interventions | 2 x 2 factorial design Aa group: maternal vitamin A, infant vitamin A (mother received 400,000 IU vitamin A within 24 hours of delivery; infant received 100,000 IU vitamin A at 14 weeks of age with DPT and OPV vaccines; n = 142) Pa group: maternal placebo, infant vitamin A (mother received placebo within 24 hours of delivery; infant received 100,000 IU vitamin A at 14 weeks of age with DPT and OPV vaccines; n = 143) Ap group: maternal vitamin A, infant placebo (mother received 400,000 IU vitamin A within 24 hours of delivery; infant received placebo at 14 weeks of age with DPT and OPV vaccines; n = 140) Pp group: maternal placebo, infant placebo (mother received placebo within 24 hours of delivery; infant received placebo at 14 weeks of age with DPT and OPV vaccines; n = 139) All pregnant women received presumptive malarial treatment in their second and third trimesters In Ayah 2007, we included data for Aa vs. Ap, and in Ayah 2007 (2), we included data for Pa vs. Pp groups | |
| Outcomes | See Ayah 2007 above | |
| Notes | See Ayah 2007 above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Ayah 2007 above |
| Allocation concealment (selection bias) | Low risk | See Ayah 2007 above |
| Blinding (performance bias and detection bias) | Low risk | See Ayah 2007 above |
| Incomplete outcome data (attrition bias) | Low risk | See Ayah 2007 above |
| Selective reporting (reporting bias) | Unclear risk | See Ayah 2007 above |
| Other bias | Low risk | See Ayah 2007 above |
| Methods | Randomised, double‐blind, placebo‐controlled trial Data collection: 1993 | |
| Participants | n = 167 Inclusion criteria: infants registered in local demographic surveillance system aged 6 to 7 weeks Exclusion criteria: severe malnutrition (defined as weight/age < 60% of the National Center for Health Statistics reference median); clinical vitamin A deficiency (any signs or symptoms) 41% boys | |
| Interventions | Intervention: vitamin A 25,000 IU palmitate in peanut oil and transport media, given at 6, 10 and 14 weeks of age (n = 86) Control: soybean oil and the same transport media given at 6, 10 and 14 weeks of age (n = 81) | |
| Outcomes | Mortality: not recorded Morbidity: not recorded Adverse effects: bulging fontanelle Follow‐up on days 1, 2, 3 and 8 | |
| Notes | Location: slum population, Dhaka city, Bangladesh (Asia). The study was carried out in the Urban Surveillance System (USS) area of the Urban Health Extension Project (UHEP) of the International Centre for Diarrhoeal Disease Research, Bangladesh | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "5 different numbers between 1 to 10 were randomly assigned to bottle A and rest 5 were assigned to bottle B. The last digit of the serial number assigned to the infant determined the bottle from which the infant received the supplement, each infant received all doses from bottle with the same code" |
| Allocation concealment (selection bias) | Low risk | "The randomization code was supplied in a sealed envelope to a committee of two paediatricians and a statistician who were not involved in the study. The code was made available after data analysis was completed" |
| Blinding (performance bias and detection bias) | Low risk | "Vitamin A and placebo were supplied by a local pharmaceutical company as 1 ml of fluid in small, dark bottles, which were marked "A" or "B" " |
| Incomplete outcome data (attrition bias) | Unclear risk | 9.7% of infants lost to follow‐up and not accounted for in the analysis. Reason for attrition were not given |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available to make an assessment |
| Other bias | Low risk | Study seemed to be free of other bias |
| Methods | Cluster randomised, non‐placebo controlled trial conducted in Jumla district, Nepal, Asia | |
| Participants | n = 7197 aged 1 to 59 months; 1058 aged 1 to 5 months Inclusion criteria: children 1 to 59 months of age Exclusion criteria: infants < 1 month of age 16 clusters were randomly assigned either to vitamin A or control group. These included 7197 children in which 3786 children were in vitamin A group and 3411 in control group. There were 547 infants in vitamin A group and 511 in placebo group who were aged 1 to 5 months 51% boys | |
| Interventions | Intervention: vitamin A 50,000 IU for infants < 6 months old (n = 547) Control: no intervention (n = 511) Following doses of vitamin A were used for older children: 100,000 IU for infants 6 to 12 months of age and 200,000 IU for children aged 12 to 59 months | |
| Outcomes | Mortality: given Morbidity: not given Adverse effects: not given | |
| Notes | Location: remote mountainous region of north‐western Nepal with a total population of about 80,000, with 12,000 children < 5 years of age. This area was considered as 1 of the poorest and most medically underserved areas of the country. Infant mortality rate was 189 deaths per 1000 live births and child (1 to 4 years of age) mortality rate was 52 per 1000 per year. Malnutrition was prevalent in the study area, and 26% of children aged 1 to 4 years were had substantial malnutrition. A survey of 3651 children in children under 5 years showed active xerophthalmia in 1.3% to 2% of population and 1% to 5% among infants, which is high for this age group. Disaggregated data on mortality were available according to different age groups We included data for infants 1 to 5 months of age only according to the objectives of our review Cluster design; however, the design effect was not given. We adjusted for cluster design by decreasing the effective sample size using methods given in Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used a design effect of 1.92 as calculated previously by Beaton 1993 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly selected by card eight of the 16 sub‐districts for vitamin A supplementation" Probably done |
| Allocation concealment (selection bias) | Low risk | Allocation concealment is usually not a major issue in a cluster randomised trial as sequence generation is done at once for all clusters |
| Blinding (performance bias and detection bias) | High risk | Control group was open so essentially no masking was done |
| Incomplete outcome data (attrition bias) | Low risk | Minimal loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | This study appeared to be free of other bias |
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | n = 191 Inclusion criteria: infants Exclusion criteria: severe malnutrition defined as weight < 60% of National Center for Health Statistics | |
| Interventions | Intervention: vitamin A 50,000 IU (palmitate in peanut oil and transport media) at 1.5, 2.5 and 3.5 months of age (n = 96) Control: soybean oil and the same transport media as above at 1.5, 2.5 and 3.5 months of age (n = 95) Infants were examined on days 1, 2, 3 and 8 after supplementation | |
| Outcomes | Mortality: not recorded Morbidity: not recorded Adverse effects: bulging fontanelle Follow‐up on days 1, 2, 3 and 8 | |
| Notes | Location: rural Bangladesh (Asia). "The trial was conducted in the Matlab Maternal and child health‐family planning (MCH‐FP) programme intervention area. This part of Bangladesh has an agricultural subsistence economy, poor infrastructure and communications, and high poverty and illiteracy rates. Bangladesh is classified as a country where vitamin A deficiency has reached public health significance". Study funded by the USUSAID under grant No. DPE‐5986‐A‐00‐1009‐00 with the International Centre for Diarrhoeal Disease Research, Bangladesh | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerised randomization procedure had been used to assign a bottle code to each infant" |
| Allocation concealment (selection bias) | High risk | "The randomization code was supplied to a committee of two paediatricians and a statistician, who were able to stop the trial" |
| Blinding (performance bias and detection bias) | Low risk | "Vitamin A (50,000 IU palmitate in peanut oil and transport media) and a placebo (soybean oil and the same transport media) were supplied as 1 mL liquid in dark small, bottles, which were marked A or B" Outcome assessors were unaware of the bottle code |
| Incomplete outcome data (attrition bias) | Low risk | "Losses of follow‐up were minimal and equally distributed in the vitamin A and placebo groups" |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available to make an assessment |
| Other bias | Low risk | This study seemed to be free of other bias |
| Methods | Randomised control trial | |
| Participants | n = 89 Inclusion criteria: healthy infants aged 2 months Exclusion criteria: infants who have prematurity, low birthweight (< 10%), congenital anomalies, systemic diseases, intrauterine infections. In addition, infants born to once or twice‐immunised mothers against tetanus during pregnancy not included | |
| Interventions | Group A: vitamin A 30,000 IU orally (retinol palmitate 30,000 IU) for 3 days just after all 3 doses of primary vaccination (n = 24) Group E: vitamin E 100 IU oral for only 1 day after the injections for primary immunisation (n = 21) Group AE: vitamin A 30,000 IU + vitamin E 100 IU as a single dose (n = 21) Group C: control with no vitamin supplementation after DPT immunisation (n = 23) | |
| Outcomes | The geometric mean titers of serum tetanus antitoxin were measured in response to vitamin A supplementation at 2, 5, and 6 to 18 months. Adverse effects were also reported at 24 and 48 hours after vitamin supplementation | |
| Notes | Conducted in Turkey All the infants received vitamin D 400 IU daily for 1 year. All infants breastfed until 4 to 6 months of age. Then, fed with a standard feeding schedule in order to minimise the effects of feeding on the development of the immune system This study contributed data for only one outcome, i.e. bulging fontanelle and data were given and included only for vitamin A alone (group A) and control (group C) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient data make an assessment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data make an assessment |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient data make an assessment |
| Incomplete outcome data (attrition bias) | High risk | All participants enrolled were not accounted for in the analysis. During follow‐up, some of the infants who had pulmonary infections or diarrhoea lasting > 1 week and infants who did not receive vitamins regularly and who were not vaccinated on time were excluded from the study. As a result, 70 infants were evaluated at 5 months and 40 at 16 to 18 months. It was not further described if there was a differential attrition among groups |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make an assessment |
| Other bias | Low risk | This study seemed to be free of other bias |
| Methods | Randomised, double blind, clinical trial | |
| Participants | n = 210 Infants aged 6 to 17 weeks attending immunisation clinics for first dose of DPT and OPV | |
| Interventions | Intervention: vitamin A 50,000 IU in peanut oil given with immunisation contact (n = 97) Control: soybean oil given at each immunisation contact (n = 103) 3 doses were given at 1, 4 and 8 weeks along with immunisation. Follow‐up at 1, 3 and 6 months after the third dose | |
| Outcomes | Mortality: given Adverse effects: given | |
| Notes | Location: urban area in Dhaka, Bangladesh Participants were recruited from diarrhoea treatment centre of International Centre for Diarrhoeal Disease Research, Bangladesh and it was not clear if participants actually had diarrhoea at enrolment. We included this study as children were given vitamin A beyond hospitalisation and we assumed that at least 2nd and 3rd dose was given when child was free of diarrhoea Morbidity outcomes were given in a separate publication and numbers were reported per child year. The exact denominator was not given so data were not pooled | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomization table was prepared by a person not directly involved with the conduct of the study using permuted blocks of random numbers" |
| Allocation concealment (selection bias) | Low risk | "Sets of three bottles containing either vitamin A or placebo for each patient were serially numbered according to the randomization chart and |
| Blinding (performance bias and detection bias) | Low risk | "Vitamin A palmitate 50,000 IU in peanut oil made up into a liquid formulation and a placebo made from soybean oil in a liquid formulation were provided as individual doses in screw‐capped dark bottles by a pharmaceutical company" Probably done |
| Incomplete outcome data (attrition bias) | Low risk | Reasons for attrition was given in table 1 of the study |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to permit judgement |
| Other bias | High risk | Study participants were recruited from diarrhoea treatment centre of International Centre for Diarrhoeal Disease Research, Bangladesh and it was not clear if participants actually had diarrhoea at the of enrolment. Therefore, it is not clear if the study participants were representative of otherwise healthy children from the community |
| Methods | Randomised, placebo‐controlled, 2 x 2 factorial design trial Data collection: November 1996 to January 1999 | |
| Participants | n = 1085, mother‐infant pair Inclusion criteria: newly delivered mother and her infant recruited 3 to 4 weeks' postpartum Exclusion criteria: families intending to move out of the study area | |
| Interventions | 2 x 2 factorial design Aa group: maternal vitamin A, infant vitamin A (mother received vitamin A 200,000 IU at 3 to 4 weeks' postpartum; infant received vitamin A 25,000 IU at 6, 10 and 14 weeks of age with DPT and OPV vaccines; n = 274) Pa group: maternal placebo, infant vitamin A (mother received placebo within 24 hours of delivery; infant received vitamin A 25,000 IU at 6, 10 and 14 weeks of age with DPT and OPV vaccines; n = 265) Ap group: maternal vitamin A, infant placebo (mother received vitamin A 200,000 IU within 24 hours of delivery; infant received placebo at 6, 10 and 14 weeks of age with DPT and OPV vaccines; n = 269) Pp group: maternal placebo, infant placebo (n = 277) In Newton 2005, we included data for Aa vs. Ap, and in Newton 2005 (2), we included data for Pa vs. Pp | |
| Outcomes | Mortality Morbidity: not recorded Adverse effects: not recorded Follow‐up at 6 months | |
| Notes | Location: Kintampo, Ghana (Africa) Breastfeeding rate almost 100% and 51% of children aged < 5 years in the area had serum retinol concentrations < 0.70 μmol/L 3 vitamin A supplementation strategies were investigated: supplementation of breastfeeding mothers with RE vitamin A 200,000 IU within 4 weeks of delivery; Expanded Program on Immunization‐linked supplementation of infants with RE vitamin A 25,000 IU at 6, 10 and 14 weeks and combined mother and child supplementations. A fourth group in which mother and child were given placebos served as controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Mothers and infants were allocated to 1 of 4 treatment groups, using a blocked randomization scheme" No further details were available to make an assessment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make an assessment |
| Blinding (performance bias and detection bias) | Low risk | "The test and placebo capsules were identical in size colour and shape" |
| Incomplete outcome data (attrition bias) | High risk | Only infants of mothers for which blood sample was obtained in the end of the study were included in the analysis; attrition was 34.6% |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make an assessment |
| Other bias | Low risk | Enrolment of participants was extended due to higher than planned loss to follow‐up; sample size calculation provided, but unclear whether a protocol was published a priori Supported by a grant from the Wellcome Trust |
| Methods | See Newton 2005 above | |
| Participants | See Newton 2005 above | |
| Interventions | 2 x 2 factorial design Aa group: maternal vitamin A, infant vitamin A (mother received vitamin A 200,000 IU at 3 to 4 weeks' postpartum; infant received vitamin A 25,000 IU at 6, 10 and 14 weeks of age with DPT and OPV vaccines; n = 274) Pa group: maternal placebo, infant vitamin A (mother received placebo within 24 hours of delivery; infant received vitamin A 25,000 IU at 6, 10 and 14 weeks of age with DPT and OPV vaccines; n = 265) Ap group: maternal vitamin A, infant placebo (mother received vitamin A 200,000 IU within 24 hours of delivery; infant received placebo at 6, 10 and 14 weeks of age with DPT and OPV vaccines; n = 269) Pp group: maternal placebo, infant placebo (n = 277) In Newton 2005, we included data for Aa vs. Ap, and in Newton 2005 (2), we included data for Pa vs. Pp | |
| Outcomes | See Newton 2005 above | |
| Notes | See Newton 2005 above | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Newton 2005 above |
| Allocation concealment (selection bias) | Unclear risk | See Newton 2005 above |
| Blinding (performance bias and detection bias) | Low risk | See Newton 2005 above |
| Incomplete outcome data (attrition bias) | High risk | See Newton 2005 above |
| Selective reporting (reporting bias) | Unclear risk | See Newton 2005 above |
| Other bias | Low risk | See Newton 2005 above |
| Methods | Open label, quasi‐randomised, controlled trial | |
| Participants | n = 1095 Infants aged 6 to 14 weeks | |
| Interventions | Intervention: vitamin A 50,000 IU orally with vaccines at each visit at 6, 10 and 14 weeks of age (n = 559) Control: no placebo given (n = 518) At the end of the trial, at 18 weeks of age, all infants were given vitamin A 100,000 IU. Mothers of infants in both groups had received vitamin A 400,000 IU as retinol palmitate, post delivery | |
| Outcomes | Mortality: not reported Morbidity: not reported Adverse effects: reported | |
| Notes | Location: 3 towns in the Ashanti region of Ghana Results reported in 3 different publications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants were assigned to intervention or control group based on date of birth |
| Allocation concealment (selection bias) | High risk | Inadequate randomisation methods and open‐label trial. Probably not done |
| Blinding (performance bias and detection bias) | High risk | Blinding was not done due to cost and logistical constraint |
| Incomplete outcome data (attrition bias) | Low risk | Attrition of about 18% that was balanced between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data to assess |
| Other bias | Low risk | This study seemed to be free of other bias |
| Methods | Individual, randomised controlled trial | |
| Participants | n = 199 Inclusion criteria: infants aged 6 to 17 weeks Exclusion criteria: any infant with serious illness | |
| Interventions | Intervention: vitamin A 25,000 IU given for 3 doses (n = 101) Control: soya bean oil (n = 98) Vitamin A was given with vaccination | |
| Outcomes | Adverse effects | |
| Notes | Location: Bangladesh Participants were recruited from a centre where infants were treated for diarrhoea; however, not all the children had diarrhoea at the time of vitamin A supplementation Data for vomiting unclear so not included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomization list was prepared by senior staff...." Most likely done |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make an assessment |
| Blinding (performance bias and detection bias) | Unclear risk | Insufficient information to make an assessment |
| Incomplete outcome data (attrition bias) | Low risk | Minimal loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make an assessment |
| Other bias | High risk | Participants were recruited from a centre where infants were treated for diarrhoea; however, not all the children had diarrhoea at the time of vitamin A supplementation |
| Methods | Randomised, double‐blind, placebo‐controlled clinical trial | |
| Participants | n = 467 Inclusion criteria: infants < 6 weeks of age Exclusion criteria: none | |
| Interventions | Group 1: vitamin A 25,000 IU at 6, 10 and 14 weeks of life (n = 156) Group 2: vitamin A 50,000 IU at 6, 10 and 14 weeks of life (n = 155) Control: placebo (n = 156) Co‐intervention with OPV and DPT vaccine at each visit | |
| Outcomes | Mortality: not recorded Morbidity Adverse effects Follow‐up within 24 hours of first visit at 6 weeks in 293 infants; follow‐up at 10 and 14 weeks and at 9, 10 and 15 months | |
| Notes | Location: Indonesia (Asia). Supported by grants from the National Institutes of Health (AI35143, HD30042); the Thrasher Research Fund; the WHO Expanded Programme on Immunization and the Office of Nutrition, Bureau for Science and Technology, USAID (Cooperative Agreement DAN‐0045‐A‐5094‐00) Protocol mentioned but no details given For morbidity outcomes of diarrhoea and fever, we included data for only 1 group (25,000 IU) to avoid counting the placebo data twice as the individual level data were not available For adverse effects, we combined both vitamin A groups as the data were given for each group separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by number table in blocks of ten" |
| Allocation concealment (selection bias) | Low risk | "Infants received identification numbers as they were enrolled into the study, and each identification number had an envelope with an identical capsule containing either vitamin A or placebo. At the time of treatment allocation, both paediatrician and study nurse were required to verify the identification number of the infant" |
| Blinding (performance bias and detection bias) | Low risk | "Identical capsules containing either vitamin A or placebo" |
| Incomplete outcome data (attrition bias) | Low risk | Exclusions and attrition was 8.4%; reasons for attrition and exclusions not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available to make an assessment |
| Other bias | Low risk | This study seemed to be free of other bias |
| Methods | Cluster‐randomised, double‐masked, placebo‐controlled trial Data collection: September 1989 to December 1991 | |
| Participants | All ages: n = 11,918; 1 to 5 months of age: n = 10,297 Inclusion criteria: infants aged ≤ 6 months Exclusion criteria: none | |
| Interventions | Intervention: vitamin A 50,000 IU 1 oral dose (3 drops of oil) for neonates, 100,000 IU (6 drops of oil) for infants 1 to 5 months of age; n = 5256 aged 1 to 5 months) Control: 1 oral dose of placebo, 75 RE (250 IU) for neonates or 150 RE (500 IU) for infants 1 to 5 months of age; n = 5041 aged 1 to 5 months) All supplements also contained vitamin E ˜ 3.3 IU/drop, added as an antioxidant | |
| Outcomes | Mortality Morbidity: not recorded Adverse effects Follow‐up 4 monthly until 6 months of age | |
| Notes | Location: Sarlahi, Nepal (Asia) Setting: community trial (261 wards in 29 village development areas (33,000 households)) We included only data for infants 1 to 5 months of age Cluster adjustment: we decreased the effective sample size using methods giving in Cochrane Handbook for Systematic Reviews of Interventions and using a design effect of 1.22 as calculated previously by Beaton 1993 Supported by a grant from Johns Hopkins University and assistance from Hoffmann‐La Roche industry (Basel, Switzerland) A protocol was described but no details were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Two hundred sixty‐one wards in 29 contiguous village development areas (VDAs) in the District of Sanlahi were mapped and 33,000 households were numbered. After a random start, wards were systematically assigned, blocked on VDAs, for infants to receive an oral dose of vitamin A" Most likely done |
| Allocation concealment (selection bias) | Low risk | Allocation concealment is usually not a major concern in cluster trial as sequence is generated all at once |
| Blinding (performance bias and detection bias) | Low risk | "The supplements were given as single‐dose gelatin capsules of identical taste and appearance." "Capsule codes were broken" after the study was over |
| Incomplete outcome data (attrition bias) | Low risk | "All analyses were performed on an intention‐to‐treat basis, that is, by randomized treatment group irrespective of individual compliance to the dosing regimen" |
| Selective reporting (reporting bias) | Unclear risk | In the absence of trial protocol, it is unclear if all prespecified outcomes were reported |
| Other bias | Low risk | This study seemed to be free of other bias |
| Methods | Randomised, double‐blind, multicentre trial | |
| Participants | n = 9424 Inclusion criteria: pregnant women and women with newborn babies Exclusion criteria: families intending to leave study site | |
| Interventions | Intervention: vitamin A (mothers 21 to 42 days' postpartum in Ghana and 18 to 28 days' postpartum in India and Peru received vitamin A 200,000 IU at enrolment; infants received 25,000 IU at 6, 10 and 14 weeks in India and Ghana and at 2, 3 and 4 months in Peru) (n = 4716) Control: placebo to both mothers and infants at the same time as the vitamin A group (n = 4708) At 9 months, with measles immunisation, infants in the vitamin A group were given vitamin A 25,000 IU, whereas those in control group received vitamin A 100,000 IU. Vitamin A was provided as retinol palmitate with minute amounts of vitamin E; placebo was soy bean oil | |
| Outcomes | Mortality Morbidity Adverse effects Follow‐up: 4 weekly until 12 months of age | |
| Notes | Location: "The trial was implemented in three countries that have clinical (Ghana and India) or severe subclinical (Peru) vitamin A deficiency. The sites were the Brong Ahafo region of Ghana (Kintampo), New Delhi, India (Dakshinpuri and Tigri), and Lima, Peru (Canto Grande). Available data from the sites indicated these regions to be areas of severe subclinical vitamin A deficiency, according to WHO's criteria. The proportion of children younger than 5 years with serum retinol equal or below 0·70 mmol/L exceeded 20% in the three sites ‐ values range from 44% in New Delhi to 51% in Kintampo" We included mortality, morbidity and adverse effect data for follow‐up until 9 months of age as children got additional vitamin A at 9 months at the time of measles vaccination. We used the raw numbers where possible as per protocol for this review For outcomes of bulging fontanelle outcomes, we included data for first dose as most of other pooled studies reported data for first dose Supported by Child Health and Development Division, WHO (Geneva), Indian Council of Medical Research and Johns Hopkins Family Health and Child Survival Co‐operative agreement with USAID | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Identification numbers were generated by computer at the data management centre at John Hopkins University in Baltimore, and assigned as random permuted blocks of size eight" |
| Allocation concealment (selection bias) | Low risk | "Three sealed copies of study codes were prepared and kept at WHO in Geneva, with the ethics committee of the All India Institute of Medical Sciences in New Delhi, and at the data management centre in Baltimore. Access was limited to one data manager, who had no direct involvement in the data analysis, and who prepared information requested by the treatment effects monitoring committee" |
| Blinding (performance bias and detection bias) | Low risk | "The supplements and placebo, in identical opaque gelatin capsules, were packaged in individually coded blister packs in Baltimore" |
| Incomplete outcome data (attrition bias) | Low risk | All analyses were intention to treat; reasons and distributions in the 2 groups were provided |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcomes reported |
| Other bias | Low risk | Sample size calculation reported; protocol and study standard operating procedure available in the WHO, Geneva on request |
DPT: diphtheria, pertussis (whooping cough) and tetanus; HIV: human immunodeficiency virus; IU: international units; n: number of participants; OPV: oral polio vaccine; RE: retinol equivalent; USAID: US Agency for International Development; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Vitamin A supplemented during neonatal period | |
| Not placebo controlled | |
| Vitamin A supplemented after 6 months of age | |
| Vitamin A supplemented during neonatal period | |
| Vitamin A supplemented during neonatal period | |
| Vitamin A given during neonatal period | |
| Vitamin A given at 9 months of age | |
| Supplementation given to mothers only | |
| Vitamin A supplemented during neonatal period | |
| Vitamin A given during neonatal period | |
| Vitamin A given during neonatal period | |
| Trial conducted on HIV‐positive women | |
| Both treatment groups received vitamin A and it was not possible to study isolated effect of vitamin A supplementation | |
| Supplementation given during neonatal period | |
| Vitamin A given to mothers only | |
| Even though 1 of the group was supplemented with vitamin A (iron + zinc + vitamin A), all the children in study received therapeutic dose of vitamin A, i.e. 100,000 IU. So it was not possible to determine independent effect of vitamin A. Also supplementation started in infants < 6 months of age and continued for 6 months after start of supplementation | |
| Trial conducted on HIV‐positive women | |
| Vitamin A given to mothers only | |
| Follow‐up study of a neonatal randomised trial | |
| Vitamin A given to neonates only | |
| Vitamin A supplemented during neonatal period only | |
| Reports data only on HIV‐positive women | |
| Vitamin A supplemented to mother only | |
| Vitamin A supplemented during neonatal period | |
| Vitamin A given to women of reproductive age only | |
| Vitamin A supplemented during neonatal period | |
| Trial conducted on HIV‐positive women | |
| Vitamin A supplemented during neonatal period | |
| Vitamin A supplemented to mothers and neonates only | |
| Study protocol. Vitamin A supplemented during neonatal period | |
| Trial predominantly (81.1%) on infants born to HIV‐positive mothers | |
| Half of the children were > 6 months of age | |
| Vitamin A supplemented during neonatal period | |
| Maternal supplementation only | |
| Not placebo controlled | |
| Maternal supplementation only in the antenatal period | |
| Most of the participants > 6 months of age. Study was included in review of vitamin A supplementation in children 6 to 59 months of age | |
| Maternal supplementation only | |
| Supplementation given at 6 months of age. Study was included in review on vitamin A supplementation in children 6 to 59 months of age | |
| Not placebo controlled |
IU: international unit.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality: longest follow‐up Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| Analysis 1.1  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 1 All‐cause mortality: longest follow‐up. | ||||
| 2 Diarrhoea‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.20, 1.70] |
| Analysis 1.2 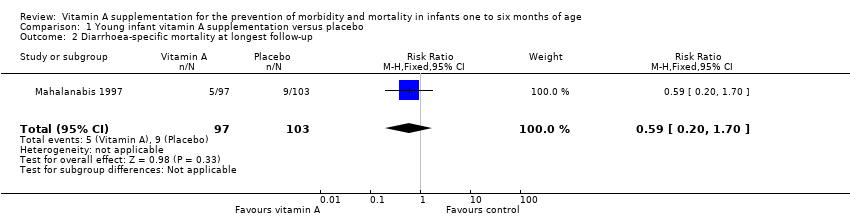 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 2 Diarrhoea‐specific mortality at longest follow‐up. | ||||
| 3 Acute respiratory infection‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.40, 11.33] |
| Analysis 1.3  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 3 Acute respiratory infection‐specific mortality at longest follow‐up. | ||||
| 4 Meningitis‐specific mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.58] |
| Analysis 1.4 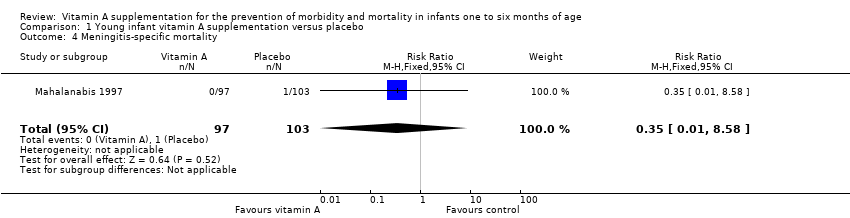 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 4 Meningitis‐specific mortality. | ||||
| 5 Morbidity: diarrhoea: point prevalence Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.93, 1.05] | |
| Analysis 1.5 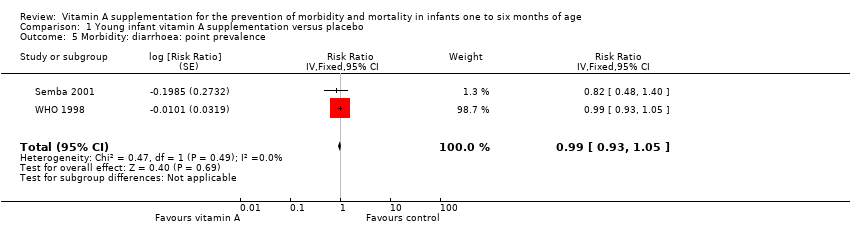 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 5 Morbidity: diarrhoea: point prevalence. | ||||
| 6 Morbidity: lower respiratory tract infection: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.81, 1.19] | |
| Analysis 1.6  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 6 Morbidity: lower respiratory tract infection: period prevalence. | ||||
| 7 Morbidity: fever: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] | |
| Analysis 1.7 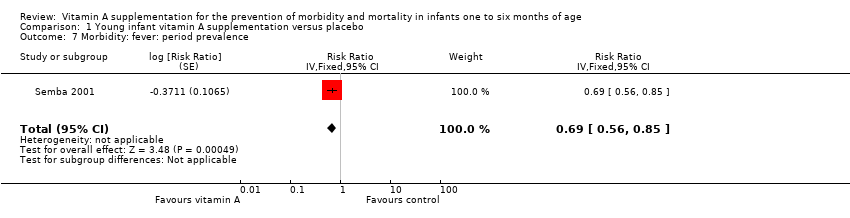 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 7 Morbidity: fever: period prevalence. | ||||
| 8 Adverse effects: bulging fontanelle Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| Analysis 1.8 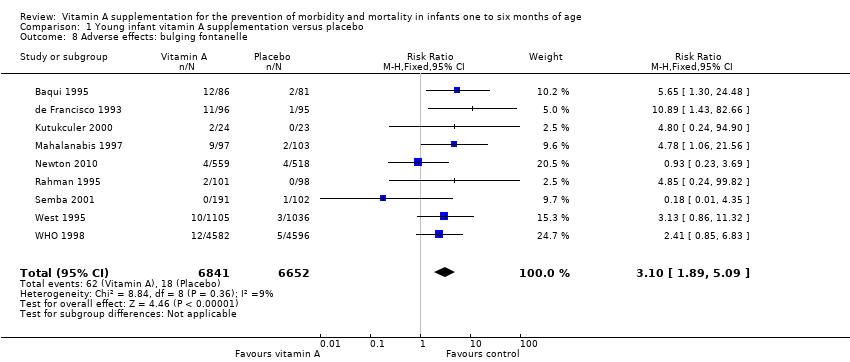 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 8 Adverse effects: bulging fontanelle. | ||||
| 9 Adverse effects: vomiting Show forest plot | 2 | 2187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.35] |
| Analysis 1.9  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 9 Adverse effects: vomiting. | ||||
| 10 Adverse effects: irritability Show forest plot | 4 | 3416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| Analysis 1.10 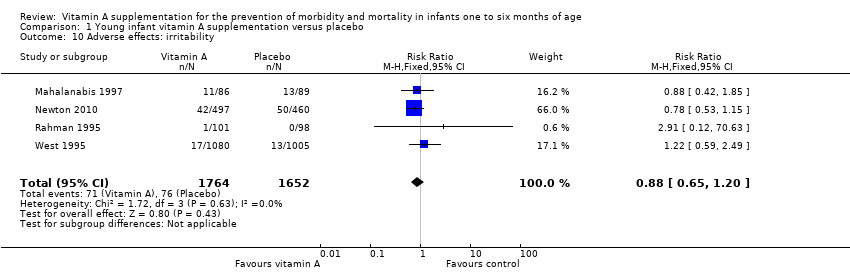 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 10 Adverse effects: irritability. | ||||
| 11 Adverse effects: diarrhoea Show forest plot | 3 | 2176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| Analysis 1.11  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 11 Adverse effects: diarrhoea. | ||||
| 12 Adverse effects: fever Show forest plot | 3 | 3187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| Analysis 1.12  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 12 Adverse effects: fever. | ||||
| 13 Adverse effects: convulsions Show forest plot | 1 | 1077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.85] |
| Analysis 1.13  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 13 Adverse effects: convulsions. | ||||
| 14 Vitamin A deficiency: retinol < 0.7 μmol/L Show forest plot | 4 | 1204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| Analysis 1.14  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 14 Vitamin A deficiency: retinol < 0.7 μmol/L. | ||||
| 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| Analysis 1.15 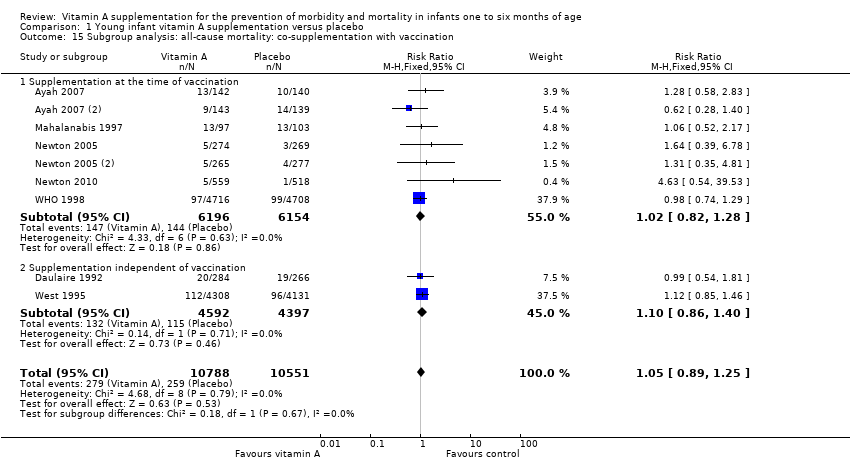 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination. | ||||
| 15.1 Supplementation at the time of vaccination | 7 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 15.2 Supplementation independent of vaccination | 2 | 8989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| Analysis 1.16 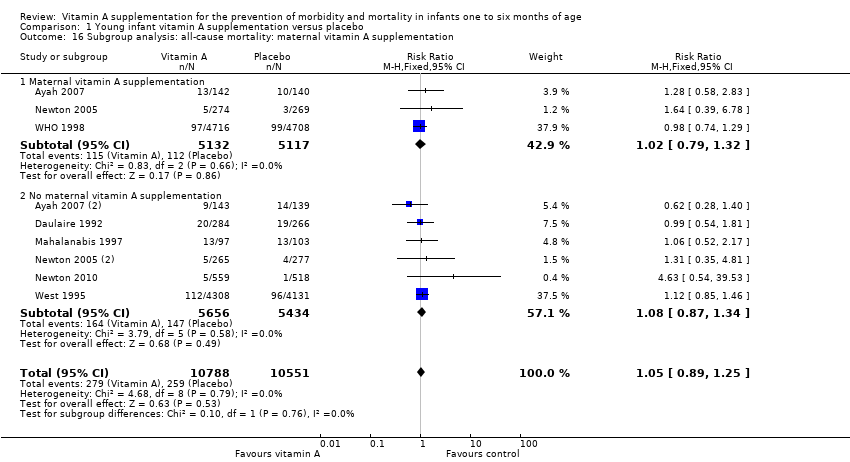 Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation. | ||||
| 16.1 Maternal vitamin A supplementation | 3 | 10249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 16.2 No maternal vitamin A supplementation | 6 | 11090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| Analysis 1.17  Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination. | ||||
| 17.1 Supplementation at the time of vaccination | 8 | 11352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.81, 5.30] |
| 17.2 Supplementation independent of vaccination | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.86, 11.32] |
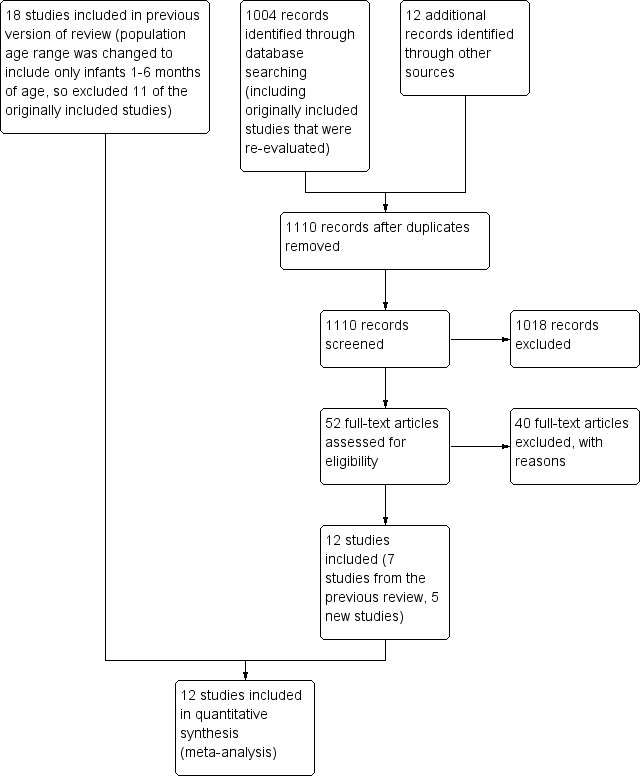
Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
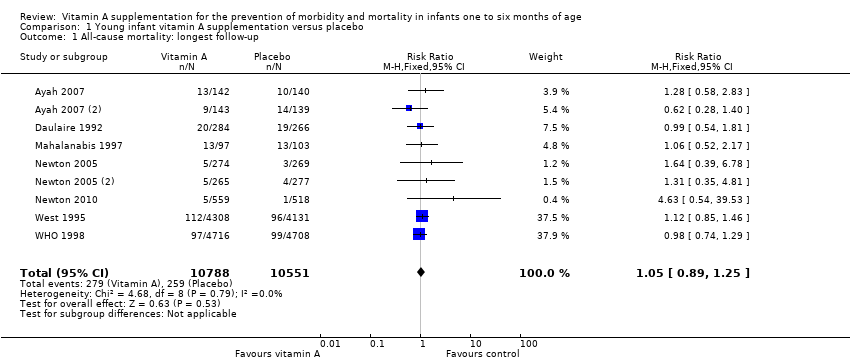
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 1 All‐cause mortality: longest follow‐up.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 2 Diarrhoea‐specific mortality at longest follow‐up.
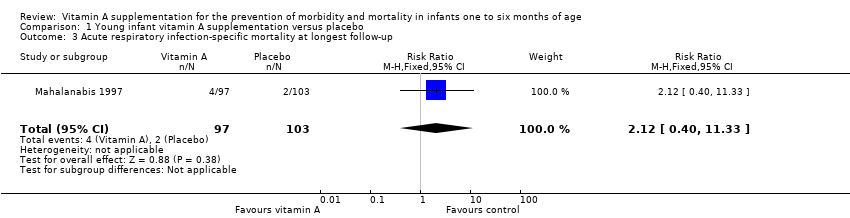
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 3 Acute respiratory infection‐specific mortality at longest follow‐up.
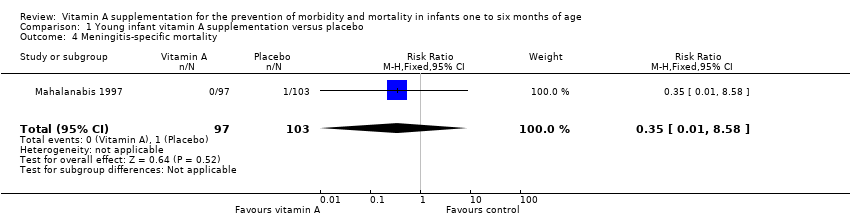
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 4 Meningitis‐specific mortality.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 5 Morbidity: diarrhoea: point prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 6 Morbidity: lower respiratory tract infection: period prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 7 Morbidity: fever: period prevalence.
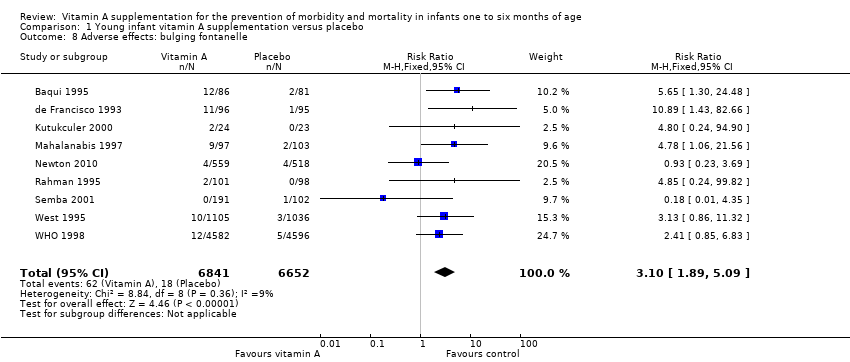
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 8 Adverse effects: bulging fontanelle.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 9 Adverse effects: vomiting.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 10 Adverse effects: irritability.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 11 Adverse effects: diarrhoea.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 12 Adverse effects: fever.
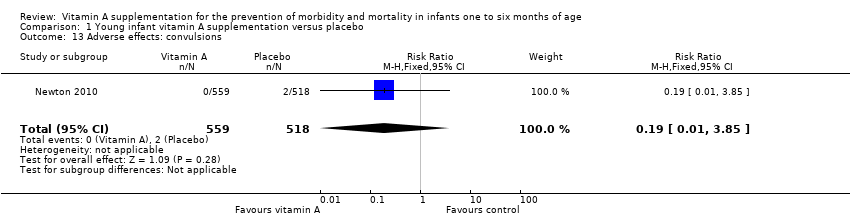
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 13 Adverse effects: convulsions.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 14 Vitamin A deficiency: retinol < 0.7 μmol/L.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation.
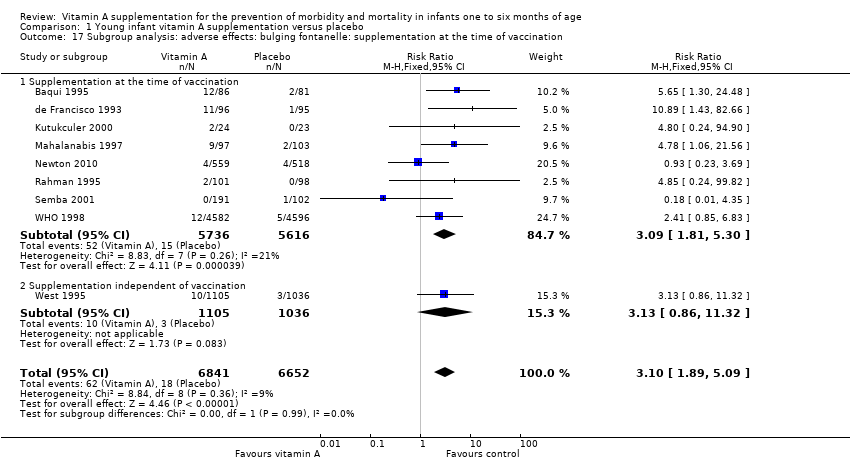
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination.
| Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age | ||||||
| Patient or population: infants 1 to 6 months of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with young infant vitamin A supplementation | |||||
| All‐cause mortality: longest follow‐up, i.e. until 1 year of age | Study population | RR 1.05 | 21,339 (9 RCTs) | ⊕⊕⊕⊝ | 2 studies contributed about 76% to the overall estimate (West 1995; WHO 1998). There was no substantial heterogeneity in the pooled data. Two studies were 2 x 2 factorial design trials and data were added as two data sets for each study. | |
| 25 per 1000 | 26 per 1000 | |||||
| Morbidity: diarrhoea: point prevalence | Study population | RR 0.99 | 9891 (2 RCTs) | ⊕⊕⊕⊝ | Even though the final quality assignment was moderate, the effect was from only 2 studies. In addition, prevalence was not as good an indicator as incidence to establish a causal association | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: bulging fontanelle within 48 to 72 hours | Study population | RR 3.10 | 13,493 | ⊕⊕⊕⊕ | Consistent effect across the studies | |
| 3 per 1000 | 8 per 1000 | |||||
| Adverse effects: vomiting 48 to 72 hours | Study population | RR 0.95 | 2187 | ⊕⊕⊝⊝ | ‐ | |
| 49 per 1000 | 47 per 1000 | |||||
| Adverse effects: diarrhoea 48 to 72 hours | Study population | RR 1.07 | 2176 | ⊕⊕⊝⊝ | ‐ | |
| 89 per 1000 | 95 per 1000 | |||||
| Adverse effects: fever 48 to 72 hours | Study population | RR 0.94 | 3187 | ⊕⊕⊝⊝ | ‐ | |
| 194 per 1000 | 183 per 1000 | |||||
| Vitamin A deficiency: retinol < 0.7 μmol/L | Study population | RR 0.86 (0.70 to 1.06) | 1204 | ⊕⊕⊕⊝ | ‐ | |
| 221 per 1000 | 190 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious imprecision (confidence interval for summary estimate included unity). 2 Downgraded one level due to serious risk of bias. 3 Downgraded one level due to serious inconsistency (statistical heterogeneity was 94%). | ||||||
| Study ID | Intervention | Type of vaccination | Response to vaccine |
| Vitamin A dose and frequency: vitamin A 30,000 IU for 3 days just after each 3 doses of DPT Control: no vitamin A supplementation | DPT | Vitamin A administered orally for 3 consecutive days after each 3 doses of DPT for primary immunisation did not affect the specific antibody response against tetanus toxoid | |
| Vitamin A dose and frequency: vitamin A 25,000 IU RE at 6, 10 and 14 weeks Control: placebo | DPT/OPV | Vitamin A supplementation does not affect infants' antibody responses to tetanus toxoid or OPV delivered at EPI contacts | |
| Vitamin A dose and frequency: vitamin A 50,000 IU at 6, 10 and 14 weeks Control: no vitamin A supplementation | Hib + Hep | No significant difference (P = 0.93) in the geometric mean concentration of Haemophilus influenzae type b antibodies in the intervention (2.45) and in the control group (2.51); ratio of geometric mean concentration 0.98 (95% CI 0.59 to 1.62). Similarly, no significant difference (P = 0.29) in the geometric mean concentration of hepatitis B antibodies in the intervention (1.28) and in the control group (1.71); ratio of geometric mean concentration 0.74 (95% CI 0.43 to 1.28) | |
| Vitamin A dose and frequency: vitamin A 25,000 RE; vitamin A 50,000 IU at 6, 10 and 14 week of age; vitamin A 100 000 IU at 9 months of age Placebo | DPT/OPV and measles | There was no differential effect of vitamin A supplementation in favour or against measles vaccination | |
| Vitamin A dose and frequency: vitamin A 25,000 IU with the first, second and third doses of DPT/OPV at 6, 10 and 14 weeks in India and Ghana and at 2, 3 and 4 months in Peru; vitamin A 25,000 IU at 9 months Placebo: soybean oil. Received vitamin A 100,000 IU at 9 months | DPT/OPV and measles | "Vitamin A given to the mothers in the postpartum period and their infants with OPV did not interfere with the antibody response to any of the three polioviruses and enhanced the response to poliovirus type 1" (Data from Indian site only) | |
| DPT: diphtheria, pertussis (whooping cough) and tetanus; EPI: extended programme of immunisation; Hep: hepatitis; Hib: Haemophilus influenzae type b; IU: international unit; OPV: oral polio vaccine; RE: retinol equivalent. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality: longest follow‐up Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 2 Diarrhoea‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.20, 1.70] |
| 3 Acute respiratory infection‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.40, 11.33] |
| 4 Meningitis‐specific mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.58] |
| 5 Morbidity: diarrhoea: point prevalence Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.93, 1.05] | |
| 6 Morbidity: lower respiratory tract infection: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.81, 1.19] | |
| 7 Morbidity: fever: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] | |
| 8 Adverse effects: bulging fontanelle Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 9 Adverse effects: vomiting Show forest plot | 2 | 2187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.35] |
| 10 Adverse effects: irritability Show forest plot | 4 | 3416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 11 Adverse effects: diarrhoea Show forest plot | 3 | 2176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 12 Adverse effects: fever Show forest plot | 3 | 3187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 13 Adverse effects: convulsions Show forest plot | 1 | 1077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.85] |
| 14 Vitamin A deficiency: retinol < 0.7 μmol/L Show forest plot | 4 | 1204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 15.1 Supplementation at the time of vaccination | 7 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 15.2 Supplementation independent of vaccination | 2 | 8989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 16.1 Maternal vitamin A supplementation | 3 | 10249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 16.2 No maternal vitamin A supplementation | 6 | 11090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 17.1 Supplementation at the time of vaccination | 8 | 11352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.81, 5.30] |
| 17.2 Supplementation independent of vaccination | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.86, 11.32] |

