Suplementação de vitamina A para prevenir morbidade e mortalidade em lactentes de um a seis meses de idade
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007480.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 28 septiembre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Neonatología
- Copyright:
-
- Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
AI and ZA screened the titles, extracted the data and contributed to characteristics of included studies table.
AI conducted the analysis and wrote the manuscript.
ZAB supervised each aspect of this review, helped interpretation of the analyses and contributed to manuscript writing.
Sources of support
Internal sources
-
Sitaram Bhartia Institute of Science and Research, B‐16 Qutab Institutional Area, Delhi 110016, India.
for time support for Prof Sachdev and Dr Gogia to December 2009.
-
Max Hospital, Gurgaon, Haryana, India.
for time support for Dr Gogia from January 2010.
External sources
-
Department of Nutrition for Health and Development, World Health Organization, Switzerland.
for providing funding for the preparation and update of this review.
-
Child and Adolescent Health Division, World Health Organization, Switzerland.
for providing funding for the initial preparation of this review.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
None known.
Acknowledgements
The review authors thank the Cochrane Editorial Unit, especially Toby Lasserson, for their advice and tremendous support in preparing the 'Summary of findings' tables for this review. We thank Nazia Darvesh who helped with screening of titles for this review.
We thank Dr Siddhartha Gogia and Dr Harshpal S Sachdev for their work on the protocol and the last published version of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Sep 28 | Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age | Review | Aamer Imdad, Zunirah Ahmed, Zulfiqar A Bhutta | |
| 2011 Oct 05 | Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less | Review | Siddhartha Gogia, Harshpal S Sachdev | |
| 2008 Oct 08 | Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less | Protocol | Siddhartha Gogia, Harshpal S Sachdev | |
Differences between protocol and review
This update included infants one to six months of age only, compared to the previous review, which also included neonates (Gogia 2011).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- *Developing Countries;
- *Dietary Supplements [adverse effects];
- *Infant Mortality;
- Breast Feeding;
- Cause of Death;
- Diarrhea [epidemiology];
- Lactation;
- Milk, Human [chemistry];
- Postpartum Period;
- Randomized Controlled Trials as Topic;
- Respiratory Tract Infections [epidemiology];
- Vitamin A [*administration & dosage, adverse effects, physiology];
- Vitamin A Deficiency [mortality, *therapy];
- Vitamins [*administration & dosage, adverse effects];
Medical Subject Headings Check Words
Female; Humans; Infant; Infant, Newborn;
PICO
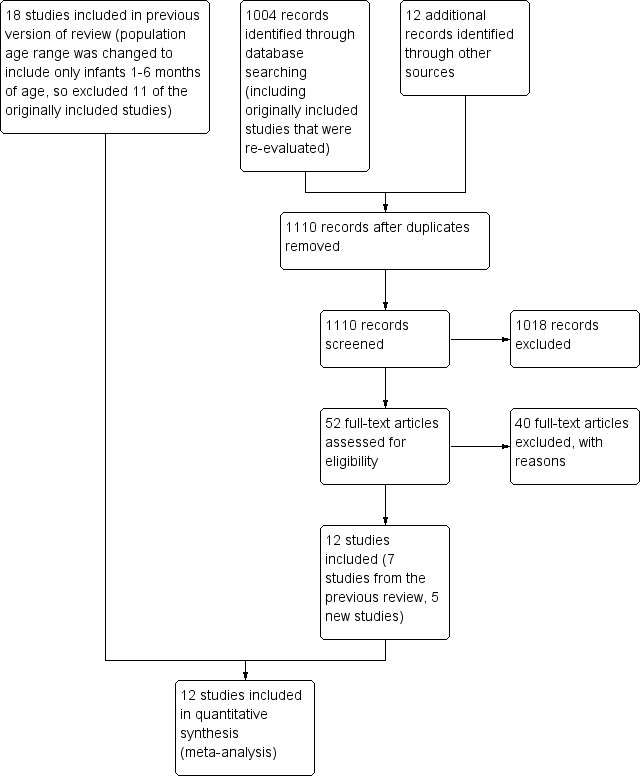
Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
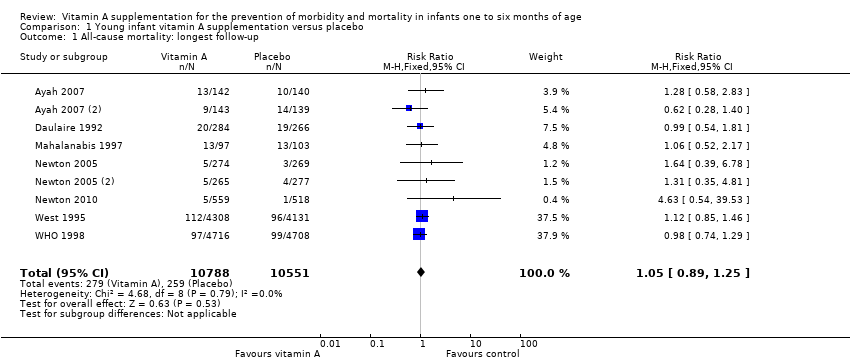
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 1 All‐cause mortality: longest follow‐up.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 2 Diarrhoea‐specific mortality at longest follow‐up.
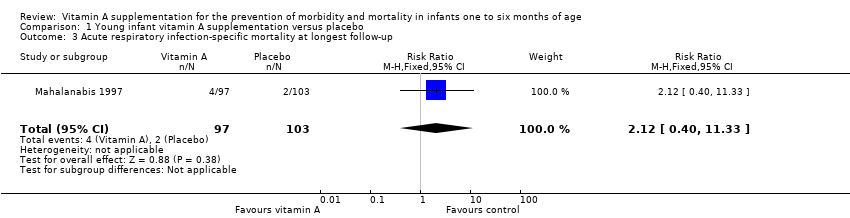
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 3 Acute respiratory infection‐specific mortality at longest follow‐up.
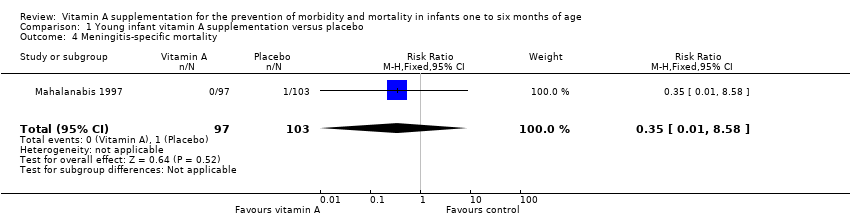
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 4 Meningitis‐specific mortality.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 5 Morbidity: diarrhoea: point prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 6 Morbidity: lower respiratory tract infection: period prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 7 Morbidity: fever: period prevalence.
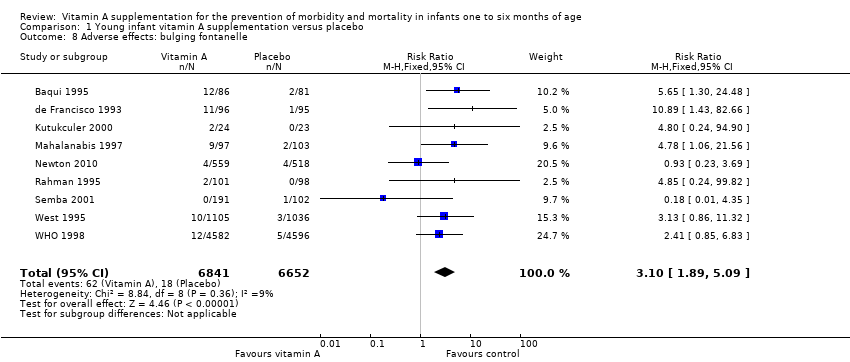
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 8 Adverse effects: bulging fontanelle.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 9 Adverse effects: vomiting.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 10 Adverse effects: irritability.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 11 Adverse effects: diarrhoea.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 12 Adverse effects: fever.
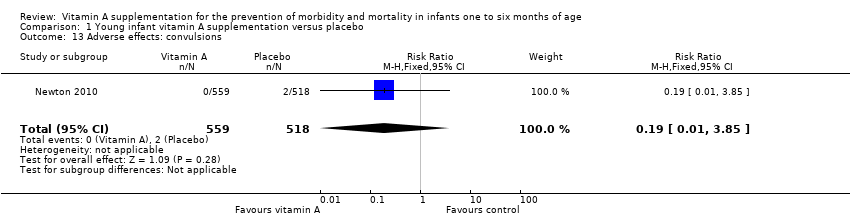
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 13 Adverse effects: convulsions.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 14 Vitamin A deficiency: retinol < 0.7 μmol/L.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation.
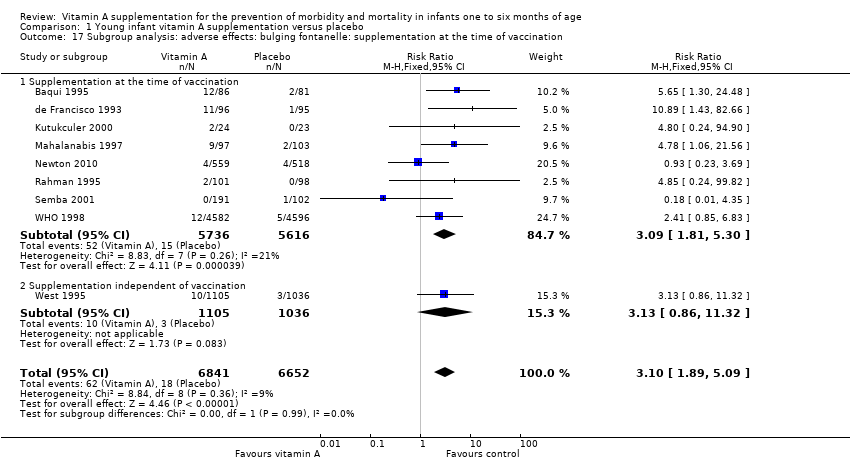
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination.
| Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age | ||||||
| Patient or population: infants 1 to 6 months of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with young infant vitamin A supplementation | |||||
| All‐cause mortality: longest follow‐up, i.e. until 1 year of age | Study population | RR 1.05 | 21,339 (9 RCTs) | ⊕⊕⊕⊝ | 2 studies contributed about 76% to the overall estimate (West 1995; WHO 1998). There was no substantial heterogeneity in the pooled data. Two studies were 2 x 2 factorial design trials and data were added as two data sets for each study. | |
| 25 per 1000 | 26 per 1000 | |||||
| Morbidity: diarrhoea: point prevalence | Study population | RR 0.99 | 9891 (2 RCTs) | ⊕⊕⊕⊝ | Even though the final quality assignment was moderate, the effect was from only 2 studies. In addition, prevalence was not as good an indicator as incidence to establish a causal association | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: bulging fontanelle within 48 to 72 hours | Study population | RR 3.10 | 13,493 | ⊕⊕⊕⊕ | Consistent effect across the studies | |
| 3 per 1000 | 8 per 1000 | |||||
| Adverse effects: vomiting 48 to 72 hours | Study population | RR 0.95 | 2187 | ⊕⊕⊝⊝ | ‐ | |
| 49 per 1000 | 47 per 1000 | |||||
| Adverse effects: diarrhoea 48 to 72 hours | Study population | RR 1.07 | 2176 | ⊕⊕⊝⊝ | ‐ | |
| 89 per 1000 | 95 per 1000 | |||||
| Adverse effects: fever 48 to 72 hours | Study population | RR 0.94 | 3187 | ⊕⊕⊝⊝ | ‐ | |
| 194 per 1000 | 183 per 1000 | |||||
| Vitamin A deficiency: retinol < 0.7 μmol/L | Study population | RR 0.86 (0.70 to 1.06) | 1204 | ⊕⊕⊕⊝ | ‐ | |
| 221 per 1000 | 190 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious imprecision (confidence interval for summary estimate included unity). 2 Downgraded one level due to serious risk of bias. 3 Downgraded one level due to serious inconsistency (statistical heterogeneity was 94%). | ||||||
| Study ID | Intervention | Type of vaccination | Response to vaccine |
| Vitamin A dose and frequency: vitamin A 30,000 IU for 3 days just after each 3 doses of DPT Control: no vitamin A supplementation | DPT | Vitamin A administered orally for 3 consecutive days after each 3 doses of DPT for primary immunisation did not affect the specific antibody response against tetanus toxoid | |
| Vitamin A dose and frequency: vitamin A 25,000 IU RE at 6, 10 and 14 weeks Control: placebo | DPT/OPV | Vitamin A supplementation does not affect infants' antibody responses to tetanus toxoid or OPV delivered at EPI contacts | |
| Vitamin A dose and frequency: vitamin A 50,000 IU at 6, 10 and 14 weeks Control: no vitamin A supplementation | Hib + Hep | No significant difference (P = 0.93) in the geometric mean concentration of Haemophilus influenzae type b antibodies in the intervention (2.45) and in the control group (2.51); ratio of geometric mean concentration 0.98 (95% CI 0.59 to 1.62). Similarly, no significant difference (P = 0.29) in the geometric mean concentration of hepatitis B antibodies in the intervention (1.28) and in the control group (1.71); ratio of geometric mean concentration 0.74 (95% CI 0.43 to 1.28) | |
| Vitamin A dose and frequency: vitamin A 25,000 RE; vitamin A 50,000 IU at 6, 10 and 14 week of age; vitamin A 100 000 IU at 9 months of age Placebo | DPT/OPV and measles | There was no differential effect of vitamin A supplementation in favour or against measles vaccination | |
| Vitamin A dose and frequency: vitamin A 25,000 IU with the first, second and third doses of DPT/OPV at 6, 10 and 14 weeks in India and Ghana and at 2, 3 and 4 months in Peru; vitamin A 25,000 IU at 9 months Placebo: soybean oil. Received vitamin A 100,000 IU at 9 months | DPT/OPV and measles | "Vitamin A given to the mothers in the postpartum period and their infants with OPV did not interfere with the antibody response to any of the three polioviruses and enhanced the response to poliovirus type 1" (Data from Indian site only) | |
| DPT: diphtheria, pertussis (whooping cough) and tetanus; EPI: extended programme of immunisation; Hep: hepatitis; Hib: Haemophilus influenzae type b; IU: international unit; OPV: oral polio vaccine; RE: retinol equivalent. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality: longest follow‐up Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 2 Diarrhoea‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.20, 1.70] |
| 3 Acute respiratory infection‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.40, 11.33] |
| 4 Meningitis‐specific mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.58] |
| 5 Morbidity: diarrhoea: point prevalence Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.93, 1.05] | |
| 6 Morbidity: lower respiratory tract infection: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.81, 1.19] | |
| 7 Morbidity: fever: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] | |
| 8 Adverse effects: bulging fontanelle Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 9 Adverse effects: vomiting Show forest plot | 2 | 2187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.35] |
| 10 Adverse effects: irritability Show forest plot | 4 | 3416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 11 Adverse effects: diarrhoea Show forest plot | 3 | 2176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 12 Adverse effects: fever Show forest plot | 3 | 3187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 13 Adverse effects: convulsions Show forest plot | 1 | 1077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.85] |
| 14 Vitamin A deficiency: retinol < 0.7 μmol/L Show forest plot | 4 | 1204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 15.1 Supplementation at the time of vaccination | 7 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 15.2 Supplementation independent of vaccination | 2 | 8989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 16.1 Maternal vitamin A supplementation | 3 | 10249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 16.2 No maternal vitamin A supplementation | 6 | 11090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 17.1 Supplementation at the time of vaccination | 8 | 11352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.81, 5.30] |
| 17.2 Supplementation independent of vaccination | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.86, 11.32] |

