补充维生素A用于降低1‐6月龄幼儿的发病率和死亡率。
Appendices
Appendix 1. Standard search methodology
Search Strategy 2010:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Updated Search Date: March 5, 2016
Search Terms: (vitamin A OR retinol OR retinoid OR retinoic OR vitamin A[MeSH])
Plus the following database‐specific terms:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
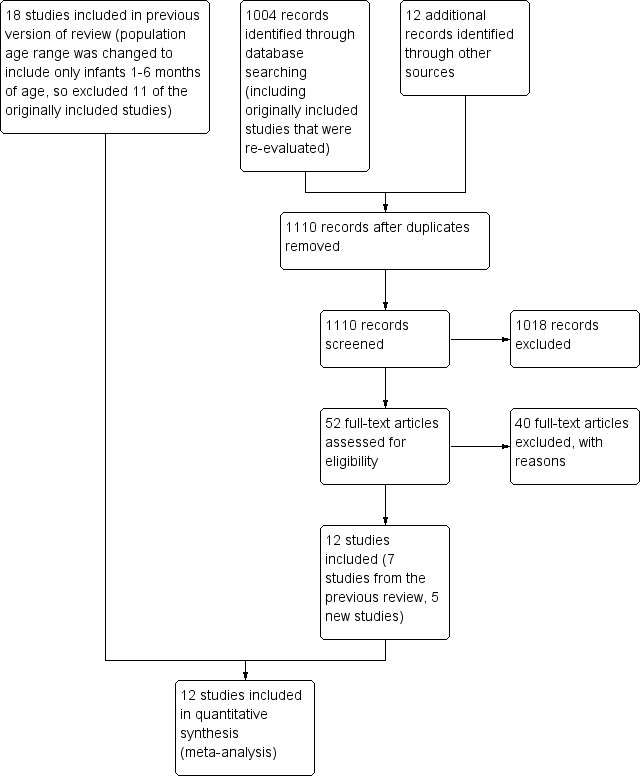
Study flow diagram: review update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
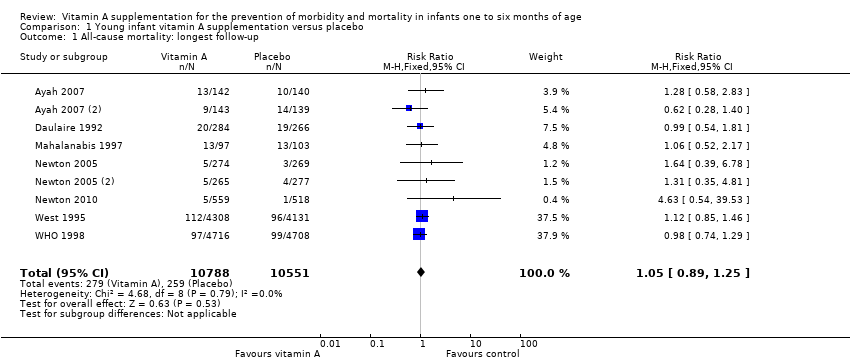
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 1 All‐cause mortality: longest follow‐up.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 2 Diarrhoea‐specific mortality at longest follow‐up.
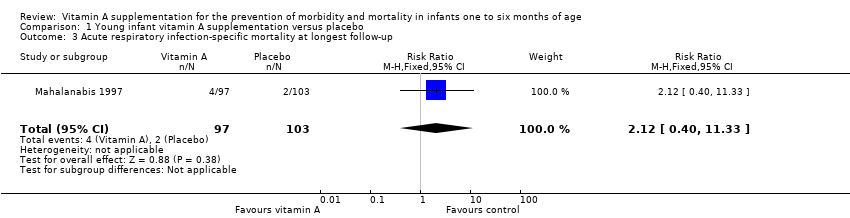
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 3 Acute respiratory infection‐specific mortality at longest follow‐up.
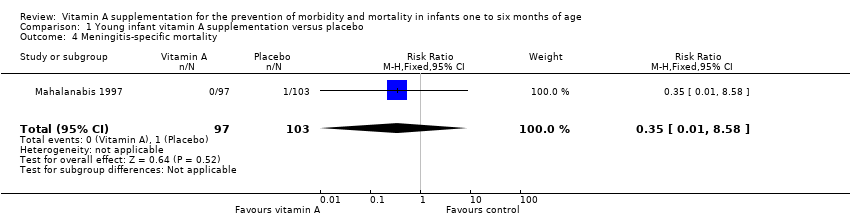
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 4 Meningitis‐specific mortality.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 5 Morbidity: diarrhoea: point prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 6 Morbidity: lower respiratory tract infection: period prevalence.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 7 Morbidity: fever: period prevalence.
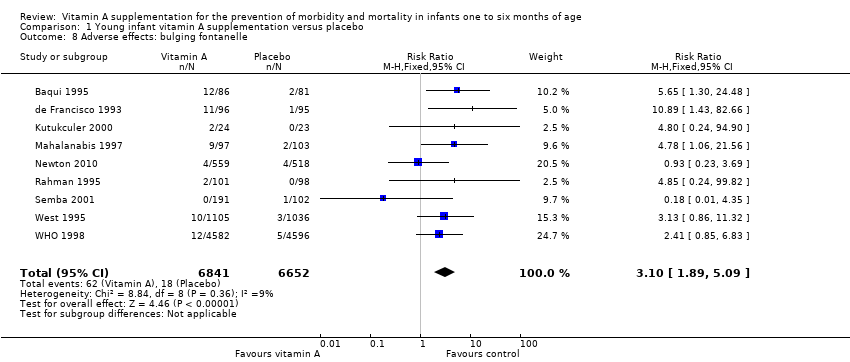
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 8 Adverse effects: bulging fontanelle.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 9 Adverse effects: vomiting.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 10 Adverse effects: irritability.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 11 Adverse effects: diarrhoea.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 12 Adverse effects: fever.
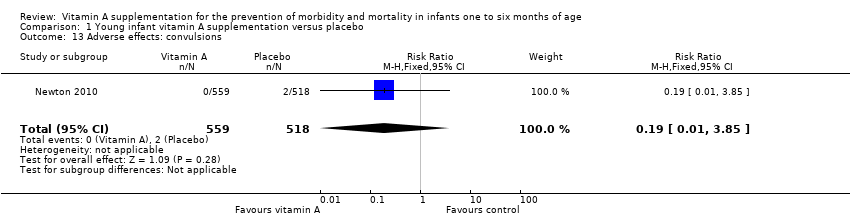
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 13 Adverse effects: convulsions.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 14 Vitamin A deficiency: retinol < 0.7 μmol/L.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination.

Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation.
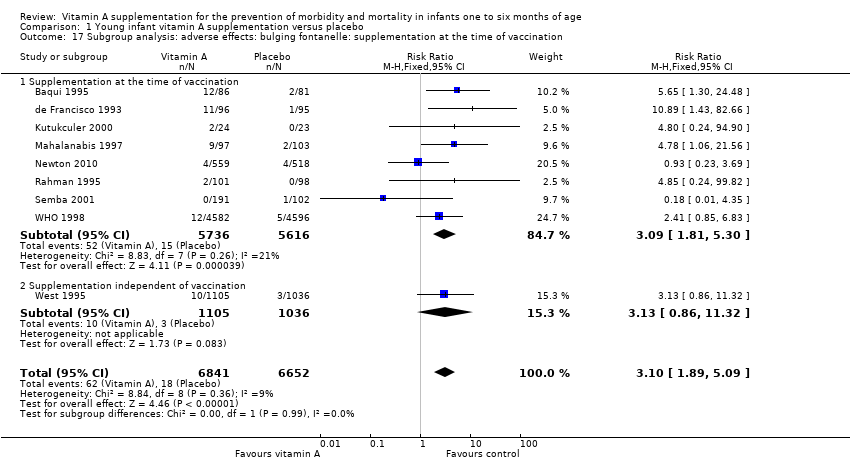
Comparison 1 Young infant vitamin A supplementation versus placebo, Outcome 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination.
| Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age | ||||||
| Patient or population: infants 1 to 6 months of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with young infant vitamin A supplementation | |||||
| All‐cause mortality: longest follow‐up, i.e. until 1 year of age | Study population | RR 1.05 | 21,339 (9 RCTs) | ⊕⊕⊕⊝ | 2 studies contributed about 76% to the overall estimate (West 1995; WHO 1998). There was no substantial heterogeneity in the pooled data. Two studies were 2 x 2 factorial design trials and data were added as two data sets for each study. | |
| 25 per 1000 | 26 per 1000 | |||||
| Morbidity: diarrhoea: point prevalence | Study population | RR 0.99 | 9891 (2 RCTs) | ⊕⊕⊕⊝ | Even though the final quality assignment was moderate, the effect was from only 2 studies. In addition, prevalence was not as good an indicator as incidence to establish a causal association | |
| 0 per 1000 | 0 per 1000 | |||||
| Adverse effects: bulging fontanelle within 48 to 72 hours | Study population | RR 3.10 | 13,493 | ⊕⊕⊕⊕ | Consistent effect across the studies | |
| 3 per 1000 | 8 per 1000 | |||||
| Adverse effects: vomiting 48 to 72 hours | Study population | RR 0.95 | 2187 | ⊕⊕⊝⊝ | ‐ | |
| 49 per 1000 | 47 per 1000 | |||||
| Adverse effects: diarrhoea 48 to 72 hours | Study population | RR 1.07 | 2176 | ⊕⊕⊝⊝ | ‐ | |
| 89 per 1000 | 95 per 1000 | |||||
| Adverse effects: fever 48 to 72 hours | Study population | RR 0.94 | 3187 | ⊕⊕⊝⊝ | ‐ | |
| 194 per 1000 | 183 per 1000 | |||||
| Vitamin A deficiency: retinol < 0.7 μmol/L | Study population | RR 0.86 (0.70 to 1.06) | 1204 | ⊕⊕⊕⊝ | ‐ | |
| 221 per 1000 | 190 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious imprecision (confidence interval for summary estimate included unity). 2 Downgraded one level due to serious risk of bias. 3 Downgraded one level due to serious inconsistency (statistical heterogeneity was 94%). | ||||||
| Study ID | Intervention | Type of vaccination | Response to vaccine |
| Vitamin A dose and frequency: vitamin A 30,000 IU for 3 days just after each 3 doses of DPT Control: no vitamin A supplementation | DPT | Vitamin A administered orally for 3 consecutive days after each 3 doses of DPT for primary immunisation did not affect the specific antibody response against tetanus toxoid | |
| Vitamin A dose and frequency: vitamin A 25,000 IU RE at 6, 10 and 14 weeks Control: placebo | DPT/OPV | Vitamin A supplementation does not affect infants' antibody responses to tetanus toxoid or OPV delivered at EPI contacts | |
| Vitamin A dose and frequency: vitamin A 50,000 IU at 6, 10 and 14 weeks Control: no vitamin A supplementation | Hib + Hep | No significant difference (P = 0.93) in the geometric mean concentration of Haemophilus influenzae type b antibodies in the intervention (2.45) and in the control group (2.51); ratio of geometric mean concentration 0.98 (95% CI 0.59 to 1.62). Similarly, no significant difference (P = 0.29) in the geometric mean concentration of hepatitis B antibodies in the intervention (1.28) and in the control group (1.71); ratio of geometric mean concentration 0.74 (95% CI 0.43 to 1.28) | |
| Vitamin A dose and frequency: vitamin A 25,000 RE; vitamin A 50,000 IU at 6, 10 and 14 week of age; vitamin A 100 000 IU at 9 months of age Placebo | DPT/OPV and measles | There was no differential effect of vitamin A supplementation in favour or against measles vaccination | |
| Vitamin A dose and frequency: vitamin A 25,000 IU with the first, second and third doses of DPT/OPV at 6, 10 and 14 weeks in India and Ghana and at 2, 3 and 4 months in Peru; vitamin A 25,000 IU at 9 months Placebo: soybean oil. Received vitamin A 100,000 IU at 9 months | DPT/OPV and measles | "Vitamin A given to the mothers in the postpartum period and their infants with OPV did not interfere with the antibody response to any of the three polioviruses and enhanced the response to poliovirus type 1" (Data from Indian site only) | |
| DPT: diphtheria, pertussis (whooping cough) and tetanus; EPI: extended programme of immunisation; Hep: hepatitis; Hib: Haemophilus influenzae type b; IU: international unit; OPV: oral polio vaccine; RE: retinol equivalent. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality: longest follow‐up Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 2 Diarrhoea‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.20, 1.70] |
| 3 Acute respiratory infection‐specific mortality at longest follow‐up Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [0.40, 11.33] |
| 4 Meningitis‐specific mortality Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.58] |
| 5 Morbidity: diarrhoea: point prevalence Show forest plot | 2 | Risk Ratio (Fixed, 95% CI) | 0.99 [0.93, 1.05] | |
| 6 Morbidity: lower respiratory tract infection: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.98 [0.81, 1.19] | |
| 7 Morbidity: fever: period prevalence Show forest plot | 1 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.56, 0.85] | |
| 8 Adverse effects: bulging fontanelle Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 9 Adverse effects: vomiting Show forest plot | 2 | 2187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.67, 1.35] |
| 10 Adverse effects: irritability Show forest plot | 4 | 3416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.65, 1.20] |
| 11 Adverse effects: diarrhoea Show forest plot | 3 | 2176 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.82, 1.40] |
| 12 Adverse effects: fever Show forest plot | 3 | 3187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.07] |
| 13 Adverse effects: convulsions Show forest plot | 1 | 1077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.85] |
| 14 Vitamin A deficiency: retinol < 0.7 μmol/L Show forest plot | 4 | 1204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.70, 1.06] |
| 15 Subgroup analysis: all‐cause mortality: co‐supplementation with vaccination Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 15.1 Supplementation at the time of vaccination | 7 | 12350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.28] |
| 15.2 Supplementation independent of vaccination | 2 | 8989 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.86, 1.40] |
| 16 Subgroup analysis: all‐cause mortality: maternal vitamin A supplementation Show forest plot | 9 | 21339 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.25] |
| 16.1 Maternal vitamin A supplementation | 3 | 10249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.79, 1.32] |
| 16.2 No maternal vitamin A supplementation | 6 | 11090 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 17 Subgroup analysis: adverse effects: bulging fontanelle: supplementation at the time of vaccination Show forest plot | 9 | 13493 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [1.89, 5.09] |
| 17.1 Supplementation at the time of vaccination | 8 | 11352 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [1.81, 5.30] |
| 17.2 Supplementation independent of vaccination | 1 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.13 [0.86, 11.32] |

