Penggunaan pesanan ringkas telefon bimbit sebagai peringatan untuk menghadiri temujanji kesihatan
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
-
cellular phone/
-
((cell* or mobile or wireless) adj (phone* or telephon*)).tw.
-
(cellphone* or mobiles or mhealth or m‐health).tw.
-
((mobile or handheld or hand‐held) adj2 (device* or technolog* or app* or health*)).tw.
-
(smart phone* or smartphone* or blackberry or iphone* or android phone* or google android or ipod touch or personal digital assistant* or pda or pdas).tw.
-
or/1‐5
-
(text* or messag* or multimedia or multi‐media or imag* or mms or data or input* or application* or app?).tw.
-
6 and 7
-
text messaging/
-
((text or short or multimedia or multi‐media) adj1 messag*).tw.
-
sms.tw.
-
(texting* or texted or texter*).tw.
-
(mms and (multimedia or multi‐media or messag*)).mp.
-
or/8‐13
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
drug therapy.fs.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
or/15‐22
-
exp animals/ not humans.sh.
-
23 not 24
-
14 and 25
-
limit 26 to yr="1993 ‐ 2012"
Appendix 2. EMBASE (Ovid) search strategy
-
mobile phone/
-
((cell* or mobile or wireless) adj (phone* or telephon*)).ti,ab,kw
-
(cellphone* or mobiles or mhealth or m‐health).ti,ab,kw
-
((mobile or handheld or hand‐held) adj2 (device* or technolog* or app* or health*)).ti,ab,kw
-
personal digital assistant/
-
(smart phone* or smartphone* or blackberry or iphone* or android phone* or google android or ipod touch or personal digital assistant* or pda or pdas).ti,ab,kw.
-
or/1‐6
-
(text* or messag* or multimedia or multi‐media or imag* or mms or data or input* or application* or app?).ti,ab,kw.
-
7 and 8
-
text messaging/
-
((text or short or multimedia or multi‐media) adj1 messag*).ti,ab,kw.
-
sms.ti,ab,kw.
-
(texting* or texted or texter*).ti,ab,kw.
-
(mms and (multimedia or multi‐media or messag*)).mp.
-
or/9‐14
-
randomized controlled trial/
-
controlled clinical trial/
-
single blind procedure/ or double blind procedure/
-
crossover procedure/
-
random*.tw.
-
placebo*.tw.
-
((singl* or doubl*) adj (blind* or mask*)).tw.
-
(crossover or cross over or factorial* or latin square).tw.
-
(assign* or allocat* or volunteer*).tw.
-
or/16‐24
-
15 and 25
-
limit 26 to yr="1993 ‐ 2012"
Appendix 3. PsycINFO (Ovid) search strategy
-
cellular phones/
-
((cell* or mobile or wireless) adj (phone* or telephon*)).ti,ab,id.
-
(cellphone* or mobiles or mhealth or m‐health).ti,ab,hw,id.
-
((mobile or handheld or hand‐held) adj2 (device* or technolog* or app* or health*)).ti,ab,hw,id.
-
mobile devices/
-
(smart phone* or smartphone* or blackberry or iphone* or android phone* or google android or ipod touch or personal digital assistant* or pda or pdas).ti,ab,hw,id.
-
or/1‐6
-
(text* or messag* or multimedia or multi‐media or imag* or mms or data or input* or application* or app?).ti,ab,hw,id.
-
7 and 8
-
((text or short or multimedia or multi‐media) adj1 messag*).ti,ab,id.
-
sms.ti,ab,id.
-
(texting* or texted or texter*).ti,ab,id.
-
(mms and (multimedia or multi‐media or messag*)).ti,ab,hw,id.
-
or/9‐13
-
random*.ti,ab,hw,id.
-
trial*.ti,ab,hw,id.
-
controlled stud*.ti,ab,hw,id.
-
placebo*.ti,ab,hw,id.
-
((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
-
(cross over or crossover or factorial* or latin square).ti,ab,hw,id.
-
(assign* or allocat* or volunteer*).ti,ab,hw,id.
-
treatment effectiveness evaluation/
-
mental health program evaluation/
-
exp experimental design/
-
"2000".md.
-
or/15‐25
-
14 and 26
-
limit 27 to yr="1993 ‐ 2012"
Appendix 4. CENTRAL search strategy
#1 ((cell* or mobile or wireless) next (phone* or telephon* or communication)):ti,ab,kw
#2 ((mobile or handheld or hand‐held) near/2 (device or technology or app or apps or health*)):ti,ab,kw
#3 (cellphone or mhealth or m‐health or smart‐phone or smartphone or blackberry or iphone or android‐phone or google‐android or ipod‐touch or personal‐digital‐assistant or pda or pdas):ti,ab,kw
#4 ((text or short or multimedia or multi‐media) next messag*):ti,ab,kw
#5 (texting* or texted or texter or sms or mms):ti,ab,kw
#6 (#1 OR #2 OR #3 OR #4 OR #5)
Appendix 5. CINAHL (EBSCO) search strategy
| S19 | S17 and S18 |
| S18 | EM 199301‐ |
| S17 | S6 and S16 |
| S16 | S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 |
| S15 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) |
| S14 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) |
| S13 | AB (random* or trial or placebo*) or TI (random* or trial or placebo*) |
| S12 | MH Quantitative Studies |
| S11 | MH Placebos |
| S10 | MH Random Assignment |
| S9 | MH Clinical Trials+ |
| S8 | PT Clinical Trial |
| S7 | PT randomized controlled trial |
| S6 | S1 or S2 or S3 or S4 or S5 |
| S5 | ((text or short or multimedia or "multi‐media") N1 messag*) or texting* or texted or texter* or sms or mms |
| S4 | cellphone* or mobiles or mhealth or "m‐health" or "smart phone*" or smartphone* or blackberry or iphone* or "android phone*" or "google android" or "ipod touch" or "personal digital assistant*" or pda or pdas |
| S3 | (mobile or handheld or "hand‐held") N1 (device* or technolog* or app or apps or health*) |
| S2 | (cell* or mobile or wireless) N1 (phone* or telephon*) |
| S1 | MH Wireless Communications |
Appendix 6. Search Strategy for Trial portals
“cellular phone” OR “mobile phone” OR cellular telephone* OR mobile telephone* OR text messag* OR texting OR texted OR short messag* OR multimedia messag* OR sms OR mms
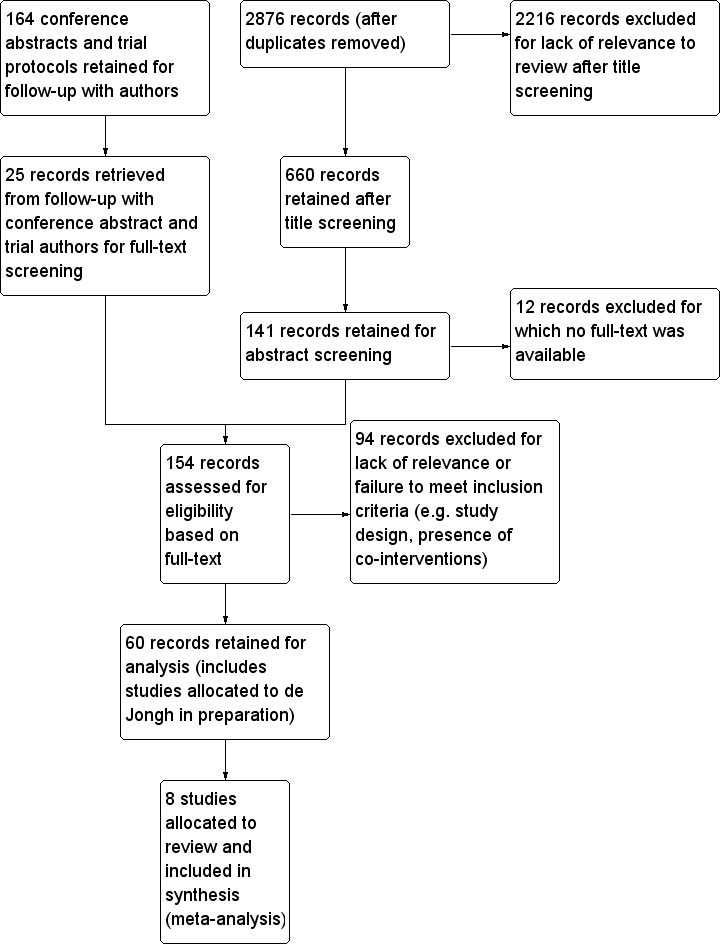
Study flow diagram. (Note: search strategy and screening selection is common for this review and for de Jongh in preparation until the final allocation stage).
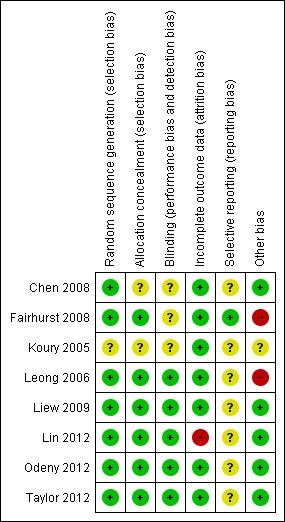
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
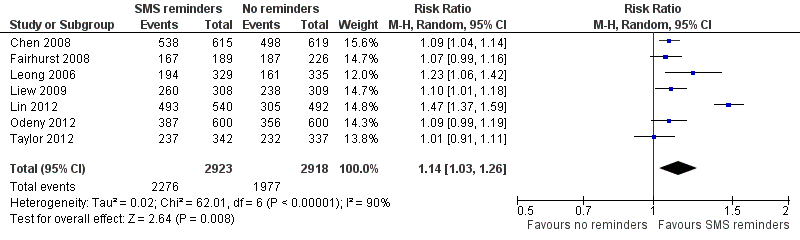
Forest plot of comparison: 1 Mobile phone text message reminders vs no reminders, outcome: 1.1 Attendance rate at healthcare appointments.

Forest plot of comparison: 2 Mobile phone message text reminders plus postal reminders vs postal reminders, outcome: 2.1 attendance rate of scheduled healthcare appointments.

Forest plot of comparison: 3 Mobile phone message reminders vs phone call reminders, outcome: 3.1 Attendance rate at healthcare appointments.

Comparison 1 Mobile phone text message reminders vs no reminders, Outcome 1 Attendance rate at healthcare appointments.

Comparison 1 Mobile phone text message reminders vs no reminders, Outcome 2 Attendance rate at healthcare appointments (sensitivity analysis).
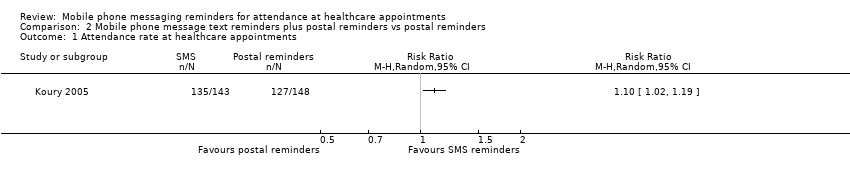
Comparison 2 Mobile phone message text reminders plus postal reminders vs postal reminders, Outcome 1 Attendance rate at healthcare appointments.
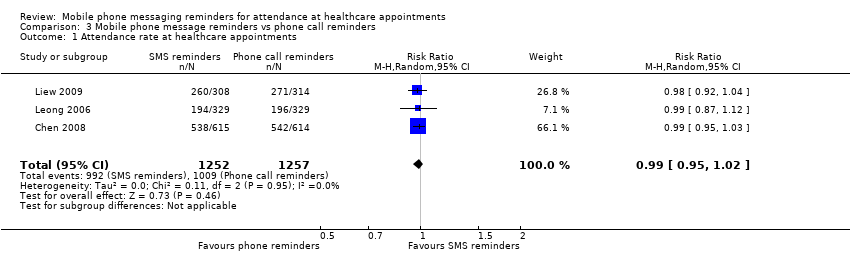
Comparison 3 Mobile phone message reminders vs phone call reminders, Outcome 1 Attendance rate at healthcare appointments.
| Patient or population: Patients with healthcare appointments | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| No reminders | Mobile phone text message reminders | ||||
| Attendance rate at healthcare appointments | 678 per 1000 | 773 per 1000 | RR 1.14 (1.03 to 1.26) | 5841 | ⊕⊕⊕⊝ |
| Other outcomes | None of the included studies reported on health outcomes, costs, user evaluation of the intervention, user perception of safety, potential harms or adverse effects of the intervention. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Unclear risk of bias for several categories in the included studies. | |||||
| Patient or population: Patients with healthcare appointments | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Postal reminders | Mobile phone message text plus postal reminders | ||||
| Attendance rate at healthcare appointments | 858 per 1000 | 944 per 1000 | RR 1.10 | 291 | ⊕⊕⊝⊝ |
| Other outcomes | The included study did not report on health outcomes, costs, user evaluation of the intervention, user perception of safety, potential harms or adverse effects of the intervention. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| aOnly one study included, with small number or participants. No information provided about the method of randomisation, allocation concealment, blinding and selective outcome reporting (unclear risk of bias). Low risk only for attrition bias. | |||||
| Mobile phone message reminders compared to phone call reminders for patients with healthcare appointments | |||||
| Patient or population: patients with healthcare appointments | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Phone call reminders | Mobile phone message reminders | ||||
| Attendance rate at healthcare appointments | 803 per 1000 | 795 per 1000 | RR 0.99 | 2509 | ⊕⊕⊕⊝ |
| Costs | While the attendance rates after text messages versus phone reminders were similar, the costs per text message per attendance were 55% and 65% lower than costs per phone call reminder in two included studies. | ||||
| Adverse outcomes | One study reported that there were no adverse events during the study period. Two studies did not report on adverse events. | ||||
| Other outcomes | None of the included studies reported on health outcomes, user evaluation of the intervention or user perception of safety. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| a Unclear risk of bias for several categories in the included studies. | |||||
| Face‐to‐face | Postal Letter | Call to Landline | Call to Mobile | Web Based (Electronic Health Record) | | SMS / MMS | |
| Immediacy | Slow: Requires a visit to the provider | Slow: around 2 days | Immediate, if person is at home. Return call may be necessary. | Immediate, if person answers (more likely than landline). | Immediate | Immediate | Immediate |
| Privacy and Confidentiality | High: | High: | Low: Confidentiality prevents message being left as others may answer or retrieve it. | High: | Moderate: | Moderate: | High, if |
| Likelihood of misinterpretation | Low | Moderate | Low, as patient can request immediate clarification | Low, as patient can request immediate clarification | Moderate | Moderate | Moderate |
| Delivery confirmation possible | Not applicable | Yes, but only at significant expense | Unnecessary if call is answered. No, if message was left. | Unnecessary if call is answered. No, if message was left. | Not applicable | Yes | Yes |
| Cost | High | Moderate | Low | Moderate | Low | Low | Low |
| Study | Costs and cost effectiveness (monetary unit as specified in the study) | Participant evaluation of the intervention (as reported in the study) | Potential harms or adverse effects of the intervention (as reported in the study) |
| Cost per attendance: SMS group: 0.31 Yuan (4.7 GBP) Telephone group: 0.48 Yuan (7.3 GBP) Ratio of total cost per attendance: SMS group: 0.65 (relative to telephone group) | Not reported | Not reported | |
| Not reported | 98% willing to receive routine reminders of their appointments. Usefulness of the intervention:
| Not reported | |
| Cost per attendance: SMS group: 0.45 RM (0.67 GBP) Mobile phone group: 0.82 RM (0.123 GBP) Ratio of total cost per attendance: SMS group: 0.55 (relative to mobile phone group) | Not reported | No adverse events reported during the study period. | |
| Not reported | 132 out of 135 (97.8%) reported they would like the intervention to continue | Not reported |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance rate at healthcare appointments Show forest plot | 7 | 5841 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.03, 1.26] |
| 2 Attendance rate at healthcare appointments (sensitivity analysis) Show forest plot | 6 | 4809 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.05, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance rate at healthcare appointments Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Attendance rate at healthcare appointments Show forest plot | 3 | 2509 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.95, 1.02] |

