Estiramiento para el tratamiento y la prevención de contracturas
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Children with spastic cerebral palsy Sample size: Experimental group: 13, Control group: 12, Other group: 14 Setting, Country: Outpatient clinics, USA Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (range): Experimental group: 6 years (3‐8), Control group: 6 years (3‐9), Other group: 6 years (3‐9) Gender: Experimental group: 54% female, Control group: 50% female, Other group: 57% female | |
| Interventions | Groups included in this review: Experimental group: Botulinum toxin plus cast Participants received botulinum toxin injections into gastrocnemius muscle followed by cast for 3 weeks at baseline, 3 months and 6 months. Ankle‐foot orthosis (AFO) worn in between casting periods for 20‐22 h/d Total stretch time: 24 h x 7 d x 9 weeks = 1512 hours over a 6‐month period Control group: Botulinum toxin Participants received botulinum toxin injections into gastrocnemius muscle at baseline, 3 months and 6 months. AFO worn for 20‐22 h/d Other group: Placebo plus cast Participants received placebo injections into gastrocnemius muscle followed by cast for 3 weeks at baseline, 3 months and 6 months. AFO worn in between casting periods for 20‐22 h/d Total stretch time: 24 h x 7 d x 3 weeks = 504 hours over a 3‐week period | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive ankle dorsiflexion (knee flexed), active ankle dorsiflexion (knee flexed), ankle dorsiflexion at initial contact during gait, peak ankle dorsiflexion during stance, peak ankle dorsiflexion during swing, triceps surae spasticity (Tardieu), walking velocity, stride length, ankle plantarflexion strength, ankle dorsiflexion strength, ankle power generation Time points included in this review: Outcomes measured at 12 months Other time points: Outcomes also measured at baseline, 3 months, 6 months and 7.5 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...block design randomisation sequence", p 621 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported. If concealment was used, every third allocation could be determined due to the use of a fixed blocked sequence |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...the children and parents were instructed not to discuss their treatment with the evaluating clinician to ensure that the clinician maintained blinding to the treatment group", p 6 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 5/39 (13%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | High risk | Quote: "...leading to an early termination of the study before obtaining the projected number of children", p 622 Comment: possible cause of bias introduced by early termination of study |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 18, Control group: 18 Setting, Country: Inpatient rehabilitation units of 4 metropolitan hospitals, Australia Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants were at risk of developing contracture Mean age (SD): Experimental group: 70 years (7), Control group: 64 years (9) Gender: Experimental group: 60% female, Control group: 56% female | |
| Interventions | Groups included in this review: Experimental group: Shoulder positioning and routine care Participants received 2 x 30‐min sessions of shoulder positioning: Participants also received up to 10 min shoulder exercises and routine upper‐limb care Total stretch time: 30 min x 5 days x 4 weeks = 10 h for each position over a 4‐week period Control group: Routine care Participants received up to 10 min shoulder exercises and routine upper‐limb care Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Maximum passive shoulder flexion (affected limb), shoulder contracture in external rotation (as compared to intact limb), shoulder contracture in flexion (as compared to intact limb), pain experienced during maximal flexion Time points included in this review: Outcomes measured at discharge (or 4 weeks) ‐ whichever was the sooner Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: Insufficient detail reported in paper. Author correspondence revealed that the randomisation sequence was computer generated |
| Allocation concealment (selection bias) | Low risk | Quote: "...centrally randomized into either the experimental or the control group", p 231 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...an assessor blinded to group allocation carried out measurements", p 231 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 5/36 (14%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with knee osteoarthritis Sample size: Experimental group: 17 (33 knees), Control group: 19 (33 knees) Setting, Country: Outpatient clinic of a large metropolitan hospital, Japan Joint of interest: Knee Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 72 years (5), Control group: 74 years (6) Gender: Experimental group: 100% female, Control group: 100% female | |
| Interventions | Groups included in this review: Experimental group : Home‐based stretch Participants self‐administered two knee flexion stretches (sitting on the floor and prone) Total stretch time1: 5 min x 7 d x 11.6 weeks = 6.7 h over a 3‐month period Control group : Maintain usual physical activity Instructed to maintain their current level of physical activity Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Knee ROM during gait Time points included in this review: Outcomes measured at time of admission (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | 1The mean duration of treatment (81 days) was used to estimate the total stretch time for the Experimental group. Also assumed that participants performed 10 repetitions each day, not 10 repetitions of each exercise (20 repetitions). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “…they were randomly allocated to stretching … and control groups”, p 114 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…they were randomly allocated to stretching … and control groups”, p 114 Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "S‐ROM was measured by a physiotherapist blinded to the participants", p 115 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Insufficient detail reported |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes stated were reported |
| Other bias | High risk | Comment: More than one joint per participant but authors have not adequately accounted for this in the analysis |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 13, Control group: 13, Other group: 13 Setting, Country: Rehabilitation department in a university hospital, Turkey Joint of interest: Wrist Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 55 years (12) Control group: 60 years (10), Other group: 52 years (11) Gender: Experimental group: 46% female, Control group: 42% female, Other group: 38% female | |
| Interventions | Groups included in this review: Experimental group : Volar splint and home‐based exercise programme Participants wore each night a custom‐made static volar splint (thermoplastic resin with plastazote on the inner surface) with the hand positioned beyond the angle of 'catch' Participants also did home‐based exercise programme (details below) Total stretch time: 10 hx 7 d x 5 weeks = 350 h over a 1.25‐month period Control group : Home‐based exercise programme only Participants stretched the wrist and finger flexors plus practiced reaching and grasping an object, 10 repetitions of each 3 x d. In addition they were instructed to use their hands as much as possible during daily activities Other group : Dorsal splint and home‐based exercise programme Custom‐made static dorsal splint (thermoplastic resin with plastazote on the inner surface) with the hand positioned beyond the angle of 'catch' worn overnight Total stretch time: 8 h x 7 d x 5 weeks = 280 h over a 1.25‐month period | |
| Outcomes | Outcomes included in this review:
Other outcomes: H latency of flexor carpi radialis, Hmax:Mmax ratio of flexor carpi radialis Time points included in this review: Outcomes measured at 5 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | Assumed participants wore the splint for 8 h per night when calculating total stretch time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “…subjects were randomly allocated to control and experimental groups by using a simple randomization process (computer‐generated random numbers) after baseline measurements”, p 330 |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent person was responsible for randomization and group assignment”, p 330 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | High risk | Quote: "Measurements associated with electroneuromyography (ENMG) were blinded ...but the others were not", p 331‐2 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1/39 (3%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes stated were reported |
| Other bias | Low risk | Comment: Appears to be free of other bias |
| Methods | Design: Randomised within‐subjects study | |
| Participants | Health condition: Adults with spinal cord injury Sample size: Experimental group: 20 legs, Control group: 20 legs Setting, Country: Inpatient rehabilitation unit, Australia Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 34 years (15), Control group: 34 years (15) Gender: Experimental group: 20% female, Control group: 20% female | |
| Interventions | Groups included in this review: Experimental group: Weight‐bearing and stretch Participants were stood on a tilt table with a 15° wedge on a high block placed under the experimental foot Total stretch time: 30 min x 3 d x 12 weeks = 18 h over a 12‐week period Control group: Non weight‐bearing and non stretch Participants were stood on a tilt table but with nothing placed underneath the control foot Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Total proximal femur bone mineral density, total proximal femur bone mineral density (% initial), total proximal femur bone mineral density (% loss of control) Time points included in this review: Outcomes measured at 12 weeks (≥ 24 h after last intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated random allocation schedule", p 253 |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocations were placed in sealed, opaque, sequentially numbered envelopes. The envelopes were not opened until after the initial tests had been performed", p 253 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...measurements were taken...by an independent ..", p 253 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults post‐radiation therapy for the jaw Sample size: Experimental group: 9, Control group: 5, Other group: 7 Setting, Country: Oral and maxillofacial surgery clinic, USA Joint of interest: Mandibular Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 51 years (14), Control group: 62 years (9), Other group: 59 years (8) Gender: Experimental group: 33% female, Control group: 40% female, Other group: 0% female | |
| Interventions | Groups included in this review: Experimental group: Therabite System plus unassisted exercise (Group 3) Participants used the Therabite System to sustain a maximum comfortable stretch of the jaw. Also performed 10 cycles/d of unassisted exercise ‐ opening to maximal inter‐incisal distance, closing, then moving maximally to the left and right and protrusively. Total stretch time: 5 x 30 s x (6‐10 sessions) x 7 d x 10 weeks = 17.5 h‐29.2 h over a 10‐week period Control group: Unassisted exercise (Group 1) Participants performed 10 cycles/d of unassisted exercise ‐ opening to maximal inter‐incisal distance, closing, then moving maximally to the left and right and protrusively Other group: Stacked tongue depressors plus unassisted exercise (Group 2) Participants used stacked tongue depressors to maximally open the mouth. Also performed 10 cycles/d of unassisted exercise ‐ opening to maximal inter‐incisal distance, closing, then moving maximally to the left and right and protrusively Total stretch time: 5 x 30 s x (6‐10 sessions) x 7 d x 10 weeks = 17.5 h‐29.2 h over a 10‐week period | |
| Outcomes | Outcomes included in this review:
Other outcomes: Subjective rating of ROM, lateral jaw movements, protrusive jaw movements Time points included in this review: Outcomes measured at 10 weeks (end of intervention) Other time points: Outcomes also measured at baseline, 2 weeks, 4 weeks, 6 weeks and 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...patients were randomly assigned to one of three groups", p 864 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Comment: Insufficient detail reported |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No dropouts reported |
| Selective reporting (reporting bias) | High risk | Comment: Lateral and protrusive jaw movements, pain, subjective ROM, and subjective well‐being all listed as outcomes in the methods but no data reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with ankylosing spondylitis Sample size: Experimental group: 27, Control group: 12 Setting, Country: Inpatient hospital, UK Joint of interest: Hip Inclusion criteria:
Exclusion criteria: Nil reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: not reported, Control group: not reported Gender: Not reported | |
| Interventions | Groups included in this review: Experimental group: Stretch plus conventional care Participants received cycles of 3 contract relax stretches to the hip muscles Total stretch time: not reported Control group: Conventional care Participants received active exercises in gymnasium and hydrotherapy pool to increase strength and joint mobility Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Hip flexion, hip extension with knee in flexion, single leg abduction, bimalleolar abduction, medial hip rotation, lateral hip rotation Time points included in this review: Outcomes measured at 15 days (end of intervention), 6 months following end of intervention Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were allocated at random ... in blocks of nine to give two in the treatment group for every one control", p 40 |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...measurements were recorded by an independent assessor who did not know to which group the patients had been allocated", p 40‐1 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: No dropouts for 3‐week data, 7/39 (18%) dropouts at 6 months No data reported for 6 months |
| Selective reporting (reporting bias) | High risk | Comment: 6‐month joint mobility data were not reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 31, Control group: 16 Setting, Country: Inpatient rehabilitation unit, Switzerland Joint of interest: Wrist Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 68 years (12), Control group: 64 years (14) Gender: Experimental group: 60% female, Control group: 67% female | |
| Interventions | Groups included in this review: Experimental group: Orthosis plus conventional care Participants were issued a thermoplastic customised wrist splint made following biomechanical principles. The wrist was maintained in a neutral position. Total stretch time: not reported Control group: Conventional care 2 sessions of physical therapy/d, 1 session of occupational therapy/d, and, if indicated, neuropsychologic and speech therapy Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: FMA sub‐scale for ROM of forearm, FMA sub‐scale for ROM of fingers, hand oedema, participant satisfaction with splint Time points included in this review: Outcomes measured at 13 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...allocation schedule was computer generated", p 1858 |
| Allocation concealment (selection bias) | Low risk | Quote: "concealed in opaque, consecutively numbered sealed envelopes by a person not otherwise involved in the study", p 1858 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "independent blinded assessor however, complete blinding of the assessor to the group assignment proved to be difficult in practice because some patients would spontaneously comment on their splint type", p 1858 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4/31 (13%) dropouts at 13 weeks |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported to include in meta‐analysis |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults following surgical release for Dupuytren’s contracture Sample size: Experimental group: 26, Control group: 30 Setting, Country: Hand therapy clinic, New Zealand Joint of interest: Hand Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 68 years (8), Control group: 67 years (9) Gender: Experimental group: 15% female, Control group: 23% female | |
| Interventions | Groups included in this review: Experimental group : Night extension orthosis plus hand therapy Participants wore each night a thermoplastic orthosis that was custom‐fabricated (moulded on the dorsum of the hand holding the operated fingers in maximal comfortable extension without placing undue tension on the wound). The orthosis was adjusted to apply greater extension force to the operated fingers if the therapist deemed this necessary. Participants also received hand therapy (details below) Total stretch time: 8 h x 7 d x 12 weeks = 672 h over a 3‐month period Control group : Hand therapy alone Participants received a standard hand therapy programme delivered by an occupational therapist, physiotherapist or hand therapist, which could include active tendon gliding ROM exercises, education, wound care, oedema management, scar management, graded return to usual daily activities, passive stretch with or without heat to increase finger extension and/or flexion, intermittent use of daytime finger‐based dynamic proximal interphalangeal joint extension orthoses, and grip strengthening Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Active extension of each operated finger, active flexion of each operated finger, distal palmar crease of each operated finger, grip strength of left and right hand Time points included in this review: Outcomes measured at 3 months (end of intervention) Other time points: Outcomes also measured at before surgery, at the first postoperative hand therapy visit and 6 weeks | |
| Notes | Assumed participants wore the splint for 8 h per night when calculating total stretch time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly allocated to 1 of 2 treatment groups ... This occurred at the first postoperative hand therapy appointment by the participant selecting a tag from an envelope with group allocation concealed", p 1286 |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomly allocated to 1 of 2 treatment groups ... This occurred at the first postoperative hand therapy appointment by the participant selecting a tag from an envelope [LH1] with group allocation concealed", p 1286 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Quote: "1 therapist took nearly all of the measurements. When she was unavailable, 2 other therapists, trained by the first to measure uniformly, filled in", p 1287 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 3/56 (5%) dropouts at 6 weeks and 2/56 (4%) dropouts at 3 months |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | High risk | Comment: Protocol allowed "rescue". Also a unit of analysis issue. Analysed joints |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with acquired brain injury Sample size: Experimental group: 6, Control group: 4 Setting, Country: Brain injury and geriatric assessment/rehabilitation units of a major metropolitan hospital, Australia Joint of interest: Wrist and fingers Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants were at risk of developing contracture Mean age (SD): Experimental group: 40 years (16), Control group: 54 years (6) Gender: Experimental group: 33% female, Control group: 50% female | |
| Interventions | Groups included in this review: Experimental group : Splint and standard practice occupational therapy programme Participants wore an individualised, thermoplastic resting mitt splint designed to approximate the standard resting position (20° wrist extension) but tailored to place each participant’s hypertonic muscle groups on low load, prolonged stretch. The splint was worn for 2‐4 h during the day and overnight. Participants also received an occupational therapy programme (details below) Total stretch time: 10 h x 90 d (3 months) = 900 h over a 3‐month period Control group : Standard practice occupational therapy programme only Participants received a standard practice occupational therapy programme as typically provided to people with upper limb hypertonicity (various combinations of movement training, stretches and functional splinting). Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Wrist extension with the fingers flexed, wrist flexor spasticity, wrist flexor muscle stiffness, finger flexor muscle stiffness. Time points included in this review: Outcomes measured at 3 months (end of intervention period) and 4 months Other time points: Outcomes also measured at baseline, 1 month and 2 months | |
| Notes | Assumed participants wore the splint for 10 h per day when calculating total stretch time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A random number table was generated by an independent researcher and used to allocate participants to control (no‐splint) and experimental (splint) groups", p 888 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "A random number table was generated by an independent researcher and used to allocate participants to control (no‐splint) and experimental (splint) groups", p 888 Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "Measures were completed by a blinded assessor", p 888 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 3/10 (30%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes stated were reported |
| Other bias | High risk | Comment: 3 people were included in ITT analysis but not clear how this was done |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with oral submucous fibrosis Sample size: Experimental group: 54, Control group: 23 Setting, Country: Hospital, Nepal Joint of interest: Jaw/mouth Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 36 years (15), Control group: 35 years (13), Other group: 44 years (19) Gender: Experimental group: 30% female, Control group: 30% female, Other group: 10% female | |
| Interventions | Groups included in this review: Experimental group: Physiotherapy (stacked tongue depressors) plus conventional care Participants undertook jaw exercises 5 x d in which tongue spatulas were positioned passively between anterior teeth, spatula number determined by comfortable maximal oral opening. The jaws were opened 5 times in each session, and held in position with the teeth resting on the spatulas for 1 min on each occasion. An additional spatula was added every fifth day unless this caused pain in which case the additional spatula was added on the tenth day. Participants also received conventional care. Total stretch time: 5 min x 5 sessions x 7 d x 17 weeks = 2975 min = 49.6 h over a 17‐week period Control group: Conventional care Participants were recommended to cease areca nut use, given dietary advice and received conventional care Other groups: Hyaluronidase and steroid injections plus conventional care Participants received bi‐weekly submucosal injections over 4 weeks of hyaluronidase (1500 units) and hydrocortisone (100 mg) | |
| Outcomes | Outcomes included in this review:
Other outcomes: Reported areca nut use, progressive involvement of oral mucosa Time points included in this review: Outcomes measured at 4 months (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random numbers were used for assignation", p 221 |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Comment: Insufficient detail reported |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 26/54 (48%) dropouts at 4 months |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported to include in meta‐analysis |
| Other bias | High risk | Quote: "patients unable to attend bi‐weekly injection were assigned for physiotherapy with the next subject assigned for injection"; "control and injection enrolment ceased for ethical reasons when sufficient control patients returned, and injection was recognized as having poor outcomes", p 221 |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with spinal cord injury Sample size: Experimental group: 18, Control group: 21 Setting, Country: Acute hospital, Canada Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants were at risk of developing contracture Mean age (SD): Experimental group: 34 years (15), Control group: 44 years (19) Gender: Experimental group: 11% female, Control group: 10% female | |
| Interventions | Groups included in this review: Experimental group: Positioning plus conventional care (Group 2) Participants received 2 sessions of shoulder positioning: Total stretch time: 45 min x 5 d x (2‐16 weeks) = 7.5 h‐60 h over a 2‐16‐week period Control group: Conventional care (Group 1) Participants received full passive movements on their upper limbs (either passive, active assisted, active or resisted), scapula stretches, modalities and medications as required for shoulder pain. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive shoulder abduction (left arm), passive shoulder flexion (right arm), passive shoulder flexion (left arm), passive shoulder medial rotation (right arm), passive shoulder medial rotation (left arm), passive shoulder lateral rotation (right arm), passive shoulder lateral rotation (left arm), pain during preceding 24 h (left shoulder), hours sitting in chair. Time points included in this review: Outcomes measured at 2 weeks1 Other time points: Outcomes also measured at baseline, week 1, week 3, week 4, week 5, week 6, week 7, week 8, week 9, week 10, week 11 and week 12 | |
| Notes | 1The intervention was ceased early with some participants (from after week 2) while others were treated up until week 12. We included outcomes from week 2 as all participants received at least 2 weeks of stretch | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...patients were randomly assigned (using a random number generator...)", p 268 |
| Allocation concealment (selection bias) | Low risk | Quote: "…and a system of sealed envelopes", p 268 Comment: Insufficient detail reported in paper. Author correspondence revealed that a system of sequentially‐numbered, sealed, opaque envelopes was used to conceal allocation |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...data were collected by a single therapist at each site who was blinded to the treatment allocation of the patient", p 269 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Length of intervention was different for participants, determined by when they were transferred to another facility. Insufficient detail reported to accurately determine dropouts |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported to include in meta‐analysis |
| Other bias | High risk | Quote: "...the trial was terminated with 39 subjects after 3 years of data collection", p 272 Comment 1: possible cause of bias introduced by early termination of study Comment 2: No standard treatment protocol for participants as they were given varying amounts of treatment dependent on length of stay |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 10, Control group: 9 Setting, Country: Rehabilitation unit, Netherlands Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 53 years (10.2)1, Control group: 52 years (8.8)1 Gender: Experimental group: 33% female1, Control group: 63% female1 | |
| Interventions | Groups included in this review: Experimental group: Positioning plus conventional care Participant was positioned in supine with arm in maximal shoulder abduction, shoulder external rotation, elbow extension and supination of the forearm that could be tolerated without any pain. The arm was always supported by a pillow and, if necessary, held in position with a sandbag. Participants also received conventional rehabilitation Total stretch time: 30 min x 2 sessions x 5 d x (5‐10 weeks) = 25 h‐50 h over a 5‐10‐week period Control group: Conventional care Participants received conventional rehabilitation Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive shoulder flexion, passive shoulder external rotation, passive elbow extension, passive forearm supination, Barthel Index Time points included in this review: Outcomes measured at 10 weeks (end of intervention). Other time points: Outcomes also measured at baseline and 5 weeks | |
| Notes | 1Data obtained via correspondence with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"...an independent person carried out the randomization procedure. The envelopes were shuffled and drawn blindfolded", p 658 |
| Allocation concealment (selection bias) | Low risk | Quote: "...subjects were randomly assigned to one of the two groups using opaque, sealed envelopes...The envelopes were shuffled and drawn blindfolded", p 658 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...the same two raters, unaware of group allocation and not involved in the treatment of subjects, carried out all the measurements", p 658 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 2/19 (11%) dropouts at 5‐week outcome assessment, 9/19 (47%) dropouts at 10‐week outcome assessment |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported on pain to include in meta‐analysis |
| Other bias | High risk | Quote: "...after nearly two years the trial had to be terminated because of set time limits, leaving only 19 subjects who met all inclusion criteria" p 663 Comment 1: Possible cause of bias introduced by early termination of study Comment 2: Unclear whether the protocol was for a 10‐week or 5‐week study |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 14, Control group: 14 Setting, Country: Inpatient rehabilitation unit, Australia Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 58 years (13), Control group: 58 years (11) Gender: Experimental group: 50% female, Control group: 15% female | |
| Interventions | Groups included in this review: Experimental group: Shoulder positioning plus conventional care Participants received 3 x 20 min sessions of shoulder positioning: Participants also received active training of reaching and manipulation tasks Total stretch time: 3 sessions x 20 min x 5 d x 6 weeks = 30 h over a 6‐week period Control group: Conventional care Participants received active training of reaching and manipulation tasks. No formal stretches were applied to the shoulder joint complex Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Active shoulder abduction, pain on dressing Time points included in this review: Outcomes measured at 6 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"...random number tables to determine the subject’s group allocation", p 36 |
| Allocation concealment (selection bias) | Low risk | Quote: "...group allocation was completed by a person independent of the recruitment process...the recruiter telephoned another person", p 36 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists. |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...measurements were made by an assessor who was blinded to the subject s group allocation", p 37 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 5/28 (18%) dropouts, with four from experimental group |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with spinal cord injury Sample size: Experimental group: 7, Control group: 6 Setting, Country: Rehabilitation unit, USA Joint of interest: Hand Inclusion criteria:
Exclusion criteria: Nil reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (range): Experimental group: not reported, Control group: not reported, both groups: 26 years (18‐42) Gender: Experimental group: not reported, Control group: not reported, both groups: 8% female | |
| Interventions | Groups included in this review: Experimental group: Positional orthosis plus conventional rehabilitation Participants were issued a short opponens or long opponens orthosis, depending on the strength of their wrist extensors. Both orthoses maintained the distal transverse arch and the thumb web space in 35° of CMC abduction, the metacarpophalangeal joint in full extension, and the interphalangeal joint in slight flexion. Participants also received conventional rehabilitation Total stretch time: 8 h x 7 d x 12 weeks = 672 h over a 12‐week period Control group: Conventional rehabilitation Participants received conventional rehabilitation Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive MCP flexion, passive proximal interphalangeal (PIP) extension, passive PIP flexion, passive distal interphalangeal (DIP) extension, passive DIP flexion, size of opening the hand when releasing, size of closing the hand with tenodesis, Jebsen hand test ‐ 6 other sub‐items, thumb/finger opposition, palmar abduction, passive lateral prehension grasp, wrist extensor strength Time points included in this review: Outcomes measured at 12 weeks (end of intervention) Other time points: Outcomes also measured at baseline, 4 weeks and 8 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...subjects were randomly assigned", p 140 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Comment: Insufficient detail reported |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 4/13 (31%) dropouts |
| Selective reporting (reporting bias) | High risk | Comment: Not all pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised cross‐over study | |
| Participants | Health condition: Elderly nursing‐home residents Sample size: Experimental group: 9, Control group: 9 Setting, Country: Chronic care hospital, Canada Joint of interest: Knee Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (range): Experimental group: not reported, Control group: not reported, both groups: 82 years (71‐93) Gender: Experimental group: not reported, Control group: not reported, both groups: 63% female | |
| Interventions | Groups included in this review: Experimental group: Bed positioning programme (low‐load prolonged knee stretch) 1 Participants were positioned in supine with their knee extended as much as possible. The position was maintained using bed sheets secured under the mattress Total stretch time: 40 min x 4 d x 8 weeks = 21.3 h over an 8‐week period Control group: No intervention 1 Participants received no intervention Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Nil Time points included in this review: Outcomes measuring combined effect after 8 weeks of stretch (both cross‐over periods combined) Other time points: Outcomes also measured at baseline, 1 week , 2 weeks, 3 weeks, 4 weeks, 5 weeks, 6 weeks, 7 weeks, 8 weeks (end of first cross‐over period), 9 weeks, 10 weeks, 11 weeks, 12 weeks, 13 weeks, 14 weeks, 15 weeks and 16 weeks (end of second cross‐over period) | |
| Notes | 1Only includes details of the first period of the cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomly assigned to 2 groups by a random numbers table", p 365 |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...a single rater blinded to the intervention assessed the participants", p 366 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 6/18 (33%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | High risk | Comment: One participant's group allocation was changed to create even group numbers |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 17, Control group: 17 Setting, Country: Inpatient rehabilitation hospital, Australia Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 66 years (16), Control group: 67 years (14) Gender: Experimental group: 41% female1, Control group: 40% female1 | |
| Interventions | Groups included in this review: Experimental group: Shoulder positioning plus conventional care Participants received 2 x 20 min sessions of shoulder positioning: Participants also received an additional shoulder positioning programme for remainder of days during the intervention period: Participants also received 30 min upper limb therapy Total stretch time: 24 h x 30 d2 = 720 h over a 30‐d period Control group: Conventional care Participants received 30 min upper limb therapy. Participants also used locally fabricated cushion supports for their stroke‐affected upper limb when seated and in bed Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Hemiplegic shoulder pain during assessment (Ritchie Articular Index), hemiplegic shoulder pain with movement (VAS), MAS for stroke Time points included in this review: Outcomes measured at discharge (end of intervention) and 6 months following discharge Other time points: Outcomes also measured at baseline | |
| Notes | 1Data obtained via correspondence with study author 2Length of intervention was calculated as an average of 30 days for the intervention and control groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...referred to a random number table to identify the predetermined, random allocation", p 279 |
| Allocation concealment (selection bias) | Low risk | Quote: "...once consent was obtained, the primary investigator referred to a random number table to identify the predetermined, random allocation of that participant to either the treatment or comparison group", p 279 Comment: Author correspondence revealed that central allocation was used. The person recruiting participants did not have access to the random number table |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | High risk | Quote: "...a blinded assessor completed the measurement of the dependent variables at admission and discharge from rehabilitation", p 279 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/17 (12%) dropouts in control group, no dropouts in experimental group |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Unclear risk | Comment: It was identified that 38 people would be needed in the power analysis but only 34 were recruited. Author correspondence revealed that the study was stopped due to participant recruitment difficulties |
| Methods | Design: Randomised within‐subjects study | |
| Participants | Health condition: Adults with spinal cord injury Sample size: Experimental group: 14 legs, Control group: 14 legs Setting, Country: 2 spinal injury rehabilitation units, Australia Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 36 years (16), Control group: 36 years (16) Gender: Experimental group: 0% female, Control group: 0% female | |
| Interventions | Groups included in this review: Experimental group: Stretch Participants received a constant stretch on the experimental ankle into dorsiflexion with the knee extended using a purpose‐built device. Total stretch time: 30 minutes x (5 ‐ 7 days) x 4 weeks = 10 hours to 14 hours over a 4‐week period. Control group: Non‐stretch Participants did not receive any type of manual therapy to either ankle nor did they stand or walk. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Ankle angle at 10 Nm torque with the knee flexed, ankle mobility with knee extended (slope of torque/angle curve), ankle mobility with knee flexed (slope of torque/angle curve), baseline ankle angle with knee extended, baseline ankle angle with knee flexed Time points included in this review: Outcomes measured at 4 weeks (end of intervention) Other time points: Outcomes also measured at baseline, 2 weeks and 5 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a computer generated random allocation schedule was determined before the study by an investigator who was not involved in patient recruitment or group allocation", p 1342 |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocations were placed in sealed, opaque, sequentially numbered envelopes by an investigator who was not involved in determining eligibility for the trial. The envelopes were not opened until after the initial tests had been performed", p 1342 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...a blinded therapist was responsible for all measurements", p 1344 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised within‐subjects study | |
| Participants | Health condition: Adults with spinal cord injury Sample size: Experimental group: 16 legs , Control group: 16 legs Setting, Country: 2 spinal injury rehabilitation units, Australia Joint of interest: Hip Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 33 years (15), Control group: 33 years (15) Gender: Not reported | |
| Interventions | Groups included in this review: Experimental group: Stretch Participants received a stretch to the hamstring muscles with a 30 Nm torque using a purpose‐built device. Participants also performed normal activities of daily living Total stretch time: 30 min x 5 d x 4 weeks = 10 h over a 4‐week period Control group: Non‐stretch Participants did not receive any stretches to the hamstring muscles Participants performed normal activities of daily living Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Nil Time points included in this review: Outcomes measured at 4 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a computer‐generated random allocation schedule was produced prior to the study by one of the authors who was not otherwise involved in subject recruitment or allocation", p 178 |
| Allocation concealment (selection bias) | Low risk | Quote: "...to ensure concealment, the same person placed allocations in sealed, opaque, sequentially‐numbered envelopes. The envelopes were not opened until after the initial tests had been performed", p 178 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...measurements were taken...by an independent therapist who was blinded to allocation", p179 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised within‐subjects and parallel‐group study | |
| Participants | Health condition: Adults with spinal cord injury, stroke or traumatic brain injury Sample size: Total: Experimental group: 30 thumbs, Control group: 30 thumbs Spinal cord injury1: Experimental group: 19 thumbs, Control group: 20 thumbs Stroke2: Experimental group: 7 thumbs, Control group: 7 thumbs Traumatic brain injury3: Experimental group: 4 thumbs, Control group: 3 thumbs Setting, Country: Community participants, Australia Joint of interest: Thumb carpometacarpal Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (range):Unilateral participants: Experimental group: 58 years (49‐67), Control group: 64 years (50‐71) Gender: Experimental group: 13% female, Control group: 30% female | |
| Interventions | Groups included in this review: Experimental group: Thumb splint Participants' thumbs were stretched by splinting them into abduction. One of two splints was used: Participants were also instructed to refrain from self‐administering any other stretch Total stretch time: 8 h x 7 d x 12 weeks = 672 h over a 12‐week period Control group: No splint Participants received no intervention. Participants were instructed to refrain from self‐administering any stretch. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Questionnaire on participants’ attitudes towards the effectiveness and convenience of the splinting regime Time points included in this review: Outcomes measured at 12 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | 1Spinal cord injury subgroup of Harvey 2006 study; 2Stroke subgroup of Harvey 2006 study; 3Traumatic brain injury subgroup of Harvey 2006 study; data obtained via correspondence with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...an independent person used a computer to generate the random allocation schedules", p 252 |
| Allocation concealment (selection bias) | Low risk | Quote: "...these were placed in opaque, sequentially numbered envelopes which were sealed and kept off site", p 252 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...the assessors were blinded to participant allocation and participants were asked not to discuss any aspect of the trial with the assessors in order to maintain blinding", p 252 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1/60 (2%) dropouts |
| Selective reporting (reporting bias) | Unclear risk | Comment: Canadian Outcome Performance Measure was discontinued |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised cross‐over study | |
| Participants | Health condition: Adults with brain injury Sample size: Experimental group: 81, Control group: 71 Setting, Country: Inpatient rehabilitation hospital, USA Joint of interest: Elbow and wrist Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (range): Experimental group: 25 years (9‐44), Control group: 32 years (19‐48) Gender: Not reported | |
| Interventions | Groups included in this review: Experimental group: Serial casting followed by therapy (Group 1) Participants wore rigid circular elbow or wrist casts. Casts were re‐applied each 5‐7 d, with 4‐6 casts applied in total. Limbs were positioned 5°‐10° off maximal ROM Total stretch time: 24 h x 7 d x 4.33 weeks = 728 h over a 4‐week period Control group: Therapy followed by serial casting (Group 2) Participants received traditional treatments included passive and active movements, prolonged stretch, splinting, neurophysiological treatment techniques and relaxation techniques Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Observation of rapid alternating movements Time points included in this review: Outcomes measured at 1 month (cross‐over point) Other time points: Outcomes also measured at baseline, 2 months (end of intervention) | |
| Notes | 1Number of participants who were analysed by the study authors (i.e. these numbers do not include dropouts). Study authors did not report the size of the group allocations at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Subjects were alternately assigned", p 220 |
| Allocation concealment (selection bias) | High risk | Quote: "Subjects were alternately assigned", p 220 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "The evaluations were performed by an experienced occupational therapist who was blind to the treatment each patient was receiving", p 220 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 5/20 (25%) dropouts |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported to include in meta‐analysis |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke or stroke‐like brain injury Sample size: Experimental group: 20, Control group: 20 Setting, Country: Inpatient rehabilitation hospital, Australia Joint of interest: Wrist Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 61 (21), Control group: 62 (17) Gender: Experimental group: 70% female, Control group: 35% female | |
| Interventions | Groups included in this review: Experimental group: Stretch plus usual care Participants received a weight‐bearing stretch of the arm in sitting, with the shoulder positioned in external rotation, slight abduction and extension, elbow in extension, forearm in supination and wrist and fingers in maximum extension. If unable to do stretch using this method, stretch performed manually or with a stretch board. Participants also received usual upper limb care. No wrist or finger stretches were administered. Total stretch time: 30 min x 5 d x 4 weeks = 10 h over a 4‐week period Control group: Usual care Participants received usual upper limb care. No wrist or finger stretches were administered Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Nil Time points included in this review: Outcomes measured at 4 weeks (end of intervention) and 9 weeks (5 weeks after last intervention) Other time points: Outcomes also measured at baseline and 5 weeks (1 week after last intervention) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated randomisation table", p 240 |
| Allocation concealment (selection bias) | Low risk | Quote: "...kept by a person who was remote from the study site and independent of recruitment, and group allocation was revealed by phone call", p 240 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...outcome measures were collected by therapists...who were blind to group allocation", p 240 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/40 (5%) dropouts at 5 weeks, 3/40 (8%) dropouts at 9 weeks |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults following total knee replacement Sample size: Experimental group: 27, Control group: 28 Setting, Country: Acute hospital, UK Joint of interest: Knee Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 66 years (14), Control group: 69 years (10) Gender: Experimental group: 59% female, Control group: 46% female | |
| Interventions | Groups included in this review: Experimental group: Splint Participants received a semi‐rigid knee extension splint for the first 48 hours after total knee replacement surgery. Participants also received usual care Total stretch time: 24 h x 2 d = 48 h over 2 d Control group: No splint Participants received no splint after total knee replacement surgery Participants received usual care. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Knee extension lag, active knee flexion and length of hospital stay Time points included in this review: Outcomes measured at 2 d (end of intervention) and 3 months (˜ 3 months after last intervention) Other time points: Outcomes also measured at baseline and 1 week (5 days after last intervention) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"...patients were randomly assigned to two groups", p 229 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...randomisation was achieved by the closed envelope technique at the time of wound closure, blinding the surgeon to the intended study group until this time", p 229 Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Quote: "...to ensure she would remain blinded to the splint allocation, a second person was trained to take the 48‐h measurements when the splints were still in use", p230 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/55 (4%) dropouts at 3‐month follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: No data reported for 3‐month follow‐up |
| Other bias | Unclear risk | Comment: More participants were recruited than original power calculations indicated were necessary. No reason given |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with shoulder adhesive capsulitis Sample size: Experimental group: 30, Control group: 30 Setting, Country: Outpatient facility, USA Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 52 years (not reported), Control group: 51 years (not reported) Gender: Not reported | |
| Interventions | Experimental group : Static progressive stretch device plus traditional therapy Particiapnts used a static progressive stretch device once daily for 30 min/session in week 1, twice daily for 30 min/session in weeks 2‐3 and thrice daily for 30 min/session in week 4 (readjusting the position of the stretch to tolerance every 5 min). Participants also received traditional therapy (details below) Total stretch time: (30 min x 7 d x 1 week) + (60 min x 7 d x 2 weeks) + (90 min x 7 d x 1 week) = 28 h over a 1‐month period Control group : Traditional therapy Participants received 3 physical therapy sessions per week for 4 weeks (hot pack followed by manual therapy) with a home exercise programme (pulley, wand and pendulum exercises performed 3 times daily with 10 repetitions each) Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive shoulder abduction, passive shoulder external rotation, active shoulder external rotation. Time points included in this review: Outcomes measured at 4 weeks (end intervention) and 12 weeks Other | |
| Notes | Nil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Subjects were randomly assigned by a computerized random number generator created by an independent biostatistician at an independent treatment center”, p 140 |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Subjects were randomly assigned by a computerized random number generator created by an independent biostatistician at an independent treatment center”, p 140 Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "All clinical outcome measures were assessed by an independent physical therapist who was blinded to subjects' group allocation", p 140 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 0/63 (0%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes stated were reported |
| Other bias | High risk | Comment: The 100% follow‐up rates at 2 years and the extremely large treatment effects were together highly improbable and raised suspicions about the conduct of the trial. In addition, the stretch devices used in this study were extremely costly yet the authors stated that they received no funding. It is not clear whether the company provided the devices. |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Children with Duchenne muscular dystrophy Sample size: Experimental group: 15, Control group: 12 Setting; Country: 3 institutions; Norway, Sweden and Denmark Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 7 years (2), Control group: 6 years (2) Gender: Experimental group: 0% female, Control group: 0% female | |
| Interventions | Groups included in this review: Experimental group: Night splint plus passive stretch Participants received below‐knee splints to be worn during the night. Participants also received passive stretches to the tendo‐achilles, hip flexors, knee flexors and iliotibial band. These stretches were performed 10 times per day Total stretch time: not reported Control group: Passive stretch Participants received passive stretches to the tendo‐achilles, hip flexors, knee flexors and iliotibial band. These stretches were performed 10 times per day Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Hip flexor contracture, time taken to run 10 m, Gowers manoeuvre (time taken to move from supine to standing), voluntary muscle strength Time points included in this review: Outcomes measured at 32 months (assessment 12) Other time points: Outcomes also measured at baseline (assessment 1), 1 month, 4 months (randomisation), 7 months, 10 months, 13 months, 17 months, 20 months, 23 months, 26 months and 29 months (assessment 11) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomization numbers from standard statistical tables for random numbers", p 258 |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...the evaluators...were blinded to the randomized treatment group allocation and to the previous assessment", p 258 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 16/27 (59%) dropouts over length of study |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported to include in meta‐analysis |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with recent (< 30 days) burns around the shoulder joint Sample size: Experimental group: 11, Control group: 13 Setting, Country: Inpatient rehabilitation centre in a general hospital, South Korea Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 43.5 years (10.4), Control group: 48.3 years (6.9) Gender: Experimental group: 18% female, Control group: 23% female | |
| Interventions | Groups included in this review: Experimental group : Shoulder splint and usual care Participants wore a multi‐axis shoulder abduction splint to keep the shoulder abducted at 90° abduction after shoulder burn. Participants also received usual care (details below) Total stretch time: 24 h x 7 d x 4 weeks = 672 h over a 1‐month period Control group: Usual care Participants were prescribed an exercise programme which consisted of sessions of passive and active mobilisation and stretching for 30 min twice a day Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Active shoulder flexion, active shoulder external rotation Time points included in this review: Outcomes were measured at 4 weeks (end of intervention) Other time points: Outcomes also measured at baselines, week 1, week 2 and week 3 | |
| Notes | Participants exercised for 30 min twice daily, so the total splint wear time was 23 h/day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...randomization procedure involving a computer‐generated random number sequence…", p 440 |
| Allocation concealment (selection bias) | Low risk | Quote: "...sealed envelopes with random numbers were used to allocate the patients", p 440 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...by assessors who were blinded to whether the patient was being splinted", p 441 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Figure 1: 24/26 (8%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Unclear risk | Comment: Insufficient detail provided |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults following surgical release for Dupuytren’s contracture Sample size: Experimental group: 77, Control group: 77 Setting, Country: 5 National Health Service Hospital Trusts, UK Joint of interest: Hand Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 67 years (10), Control group: 68 years (9) Gender: Experimental group: 21% female, Control group: 23% female | |
| Interventions | Groups included in this review: Experimental group : Static night splint plus hand therapy Particicpants wore a custom‐made thermoplastic splint which accommodated the operated rays of the hand with the metacarpophalangeal joints and/or proximal interphalangeal joints held in maximum extension without causing any tension to the wound. The splint was remoulded intermittently to achieve a greater extension force. Participants were instructed to wear the splint at night only. Participants also received hand therapy (details below). Total stretch time: 8 h x 182 d (6 months) = 1456 h over a 6‐month period Control group: Hand therapy Participants received hand therapy aimed at reducing oedema, promoting wound healing, maximising finger range of movement and facilitating full return to functional use of the hand, including oedema control, exercises and advice. If a participant had a net loss of 15 degrees or more at the proximal interphalangeal joint and/or a net loss of 20 degrees or more at the metacarpal phalangeal joint of the operated fingers, they were then given a splint. Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes Active flexion of the metacarpophalangeal, proximal interphalangeal and distal interphalangeal joints of the operated fingers, patient satisfaction with the outcome, recurrence at 1 year Time points included in this review: Outcomes measured at 6 months (end intervention) and 12 months after surgery Other time points: Outcomes also measured prior to surgery, and at 3 months after surgery Patient satisfaction was assessed only at 6 and 12 months | |
| Notes | Assumed participants wore the splint for 8 h per night when calculating total stretch time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomisation was stratified by centre (five centres) and by surgical procedure (fasciectomy or dermofasciectomy) in block lengths of 4. The allocation sequence was generated and administered independently through a central telephone randomisation service”, p 4 Comment: Not clear how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Low risk | Quote: “The allocation sequence was generated and administered independently through a central telephone randomisation service”, p 4 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | High risk | Quote: "The primary outcome measure was patient‐reported and participants could not be blinded. Secondary outcomes were collected by the research associates who were also not blinded, although they were independent of the clinical staff delivering the interventions", p 5 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 6/154 (3%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes stated were reported. Abandoned the recurrence at 1‐year outcome |
| Other bias | High risk | Quote: “13 patients allocated to the no‐splint group (17%) went on to develop a contracture of the PIPJ which exceeded the agreed threshold and were subsequently given a splint as per protocol”, p 4 Comment: Crossover from control to experimental group, but analysis was by intention‐to‐treat |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with hallux limitus in the first metatarsophalangeal joint following surgery Sample size: Experimental group: 25, Control group: 25 Setting, Country: Outpatient clinics, USA Joint of interest: Metatarsophalangeal joint of great toe Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Not reported (Range: 29‐69 years) Gender: Experimental group: 44% female, Control group: 60% female | |
| Interventions | Groups included in this review: Experimental group :Dynamic splint and usual care Participant wore a dynamic splint for first metatarsophalangeal joint of the great toe. They also received usual care (details below) Total stretch time: 3 h x 7 d x 8 weeks = 168 h over a 2‐month period Control group : Usual care Participants were prescribed nonsteroidal anti‐inflammatory drugs and orthotics. They were also given instructions for home exercises Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Nil Time points included in this review: Outcomes measured at 8 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | Nil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Comment: Insufficient detail reported |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Quote: “Two control patients withdrew from the study because of excessive pain that required additional treatment”, p 287 |
| Selective reporting (reporting bias) | Unclear risk | Comment: Does not clearly state outcomes and only reports on one outcome |
| Other bias | High risk | Comment: Inadequate reporting to gauge other possible sources of bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with contracture following distal radial fracture Sample size: Experimental group: 19, Control group: 21 Setting, Country: Outpatient clinics, Australia Joint of interest: Wrist Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (IQR): Experimental group: 66 years (56‐72), Control group: 58 years (52‐65) Gender: Experimental group: 79% female, Control group: 62% female | |
| Interventions | Groups included in this review: Experimental group : Splint and routine care Participants wore a dynamic splint during the day which stretched the wrist into extension but allowed intermittent movement. They also received routine care (details below) Total stretch time: 6 h x 7 d x 8 weeks = 336 h over a 2‐month period Control group : Routine care Participants received exercises and advice for 8 weeks Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Active wrist extension, active wrist flexion, active radial deviation, active ulnar deviation, Canadian Occupational Performance Measure for Satisfaction Time points included in this review: Outcomes measured at 8 weeks (end of intervention) and 12 weeks Other time points: Outcomes also measured at baseline | |
| Notes | Nil | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "..a computerised blocked randomisation sequence was generated prior to the commencement of the trial by an independent offsite person", p 174 |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants’ allocations were placed in opaque sealed and sequentially numbered envelopes that were held off‐site", p 174 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "A blinded assessor performed assessments at 8 weeks, ....an assessor not blinded to group allocation performed assessments at 12 weeks", p 174 Comment: Only data from the 8‐week assessments were used in the meta‐analyses. |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4/40 (10%) dropouts at 8 weeks and 8/40 (20%) dropouts at 12 weeks |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with Dupuytren’s disease Sample size: Experimental group: 28, Control group: 26 Setting, Country: Outpatient clinics, Netherlands Joint of interest: Proximal interphalangeal Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants were at risk of developing contracture Mean age (SD): Experimental group: 63 years (9), Control group: 64 years (11) Gender: Experimental group: 18% female (n = 5), Control group: 12% female (n = 3) | |
| Interventions | Groups included in this review: Experimental group : Hand splint and usual therapy Participants wore a dorsal static extension splint postoperative. They also received usual therapy (details below) Total stretch time: (24 h x 28 d) + (8 h x 7 weeks x 7 d) = 672 h + 392 h = 1,064 h over a 3‐month period1 Control group : Usual therapy Participants received a standardised programme of graded exercises designed to improve the strength, mobility and function of the affected hand (30 min twice weekly; total duration 3 months, starting 10 d after surgery) Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Global perceived effect, comfort of wearing splint Time points included in this review: Outcomes measured at 6 weeks and 1 year Other time points: Outcomes also measured at 3 months (but only at 1 site) | |
| Notes | 1Total stretch time calculations based on: participants were instructed to apply the splint day and night during the first 4 weeks, but removed for exercises at least 5 times/d for 15 min. Then: participants gradually began to use their hands normally in the daytime and the night splintage was continued | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Table of random numbers was used to make the treatment assignments", p 734 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The assignments were made by a research assistant", p 734 Comment: Not clear if the research assistant had access to the allocation schedule or was involved in making decisions about inclusion |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "..concealed from the outcome assessor", p 734 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "After 1 year, all patients were available for follow‐up", p 735 |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Unclear risk | Comment: The 6‐week and 3‐month data were only collected at one site (n = 36) |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with an axillary burn (anterior chest involving the axillary fold, anterior, lateral or posterior shoulder and the axillary region) Sample size: Experimental group: 27, Control group: 25 Setting, Country: Burns unit of an acute hospital, Australia Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants were at risk of developing contracture Mean age (SD): Experimental group: 49 years (19), Control group: 44 years (18) Gender: Experimental group: 30% female, Control group: 40% female | |
| Interventions | Groups included in this review: Experimental group : Shoulder splint and usual care Participants wore an Otto Bock Omo Immobil shoulder splint, holding the shoulder in 90° abduction for 12 weeks. They also received usual care (details below) Total stretch time: (24 h x 7 d x 6 weeks) + (8 h x 7 d x 6 weeks) = 1344 h over a 3‐month period1 Control group : Usual care Participants received a daily exercise programme which included stretching, strengthening and functional retraining of the affected upper limb Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Shoulder range of flexion, the Grocery Shelving Task, length of stay Time points included in this review: Outcomes measured at 12 weeks (end of intervention) Other time points: Outcomes also measured at baseline and 6 weeks | |
| Notes | 1 “… adherence with splint use was generally poor…” p 640 (no detailed adherence data provided) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was completed via a computer generated program", p 639 |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocation was concealed using opaque envelopes", p 639 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "Outcomes measured by an independent data collector who was blinded to group allocation", p 639 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: Figure 1, Week 12: 40/52 = 77% |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Unclear risk | Comment: Table 2 contains data on length of stay that were not described as an outcome in the text |
| Methods | Design: Randomised cross‐over study | |
| Participants | Health condition: Children with cerebral palsy (12 children had unilateral and 14 bilateral cerebral palsy) Sample size: 37 children (cross‐over) Setting, Country: Hand clinic, Sweden Joint of interest: Wrist and thumb Inclusion criteria:
Exclusion criteria: Nil reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Both groups: 10 years (range 1–16) Gender: Not reported | |
| Interventions | Groups included in this review: Experimental group : Hand splint and usual care Participants received a hand splint for 6 months. Total stretch time: 8 h x 7 d x 26 weeks = 1456 h1 Control group : Usual care Participants did not receive a hand splint Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive thumb abduction Time points included in this review: Outcomes measured at 6 months (end of intervention) Other time points: Outcomes also measured at 3 months, 9 months and 12 months | |
| Notes | 1This assumes participants wore the splint each night for 8 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported. |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...blinded to group allocation", p 26 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Quote: “During the 12 month trial period 11 [of 37] dropped out leaving 26 children”, p 27 |
| Selective reporting (reporting bias) | Unclear risk | Comment: Insufficient detail reported |
| Other bias | Unclear risk | Comment: Only an abstract so difficult to assess susceptibility to bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 151, Control group: 151 Setting, Country: Not reported, USA Joint of interest: Elbow Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 49 years (4), Control group: 56 years (5) Gender: Experimental group: 53% female, Control group: 33% female | |
| Interventions | Groups included in this review: Experimental group: Extension splint plus botulinum toxin and therapy Participants wore an elbow extension dynasplint in addition to botulinum toxin and therapy. Tension was increased 1 increment every 2 weeks, based on participant's tolerance. The initial tension setting was #2 (16 kg/cm of torque), and the mean final tension setting was #6 (58 kg/cm of torque). Participants also received botulinum toxin and therapy (details below) Total stretch time: (6‐8 h) x 7 d x 14 weeks = 588 h to 784 h over a 14‐week period Control group: Botulinum toxin and therapy All participants received botulinum toxin injections and occupational and manual therapies. The botulinum toxin injections were injected into the biceps brachialis, and brachioradialis muscles, and the occupational and manual therapies occurred weekly for 16 weeks. The occupational and manual therapy protocols included moist heat, education, joint mobilisation, passive ROM, active ROM, proprio‐neural facilitation and therapeutic exercise Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Nil Time points included in this review: Outcomes measured at 14 weeks (end of intervention) Other time points: Outcomes also measured at baseline, 1 week | |
| Notes | 1Number of participants analysed by the study authors (i.e. these numbers do not include dropouts). Authors did not report the size of the group allocations at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...selected with a randomized list", p 244 |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...selected with a randomized list", p 244 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Quote: "Upon enrolment, all patients ... measured by the same therapist before and after the BTX injections", p 243 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 6/36 (17%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Unclear risk | Quote: "This study was funded by Dynasplint Systems Inc.", p 246 |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke or brain injury Sample size: Experimental group: 17, Control group: 11 Setting, Country: Inpatient rehabilitation unit, Australia Joint of interest: Wrist (long finger flexors) Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 65 years (16), Control group: 68 years (7) Gender: Experimental group: 53% female, Control group: 55% female | |
| Interventions | Groups included in this review: Experimental group: Splint plus routine therapy Participants wore a static, palmar resting mitt splint on a daily basis for a maximum of 12 h each night. Participants also received routine therapy (details below) Total stretch time: 12 h x 7 d x 4 weeks = 336 h over a 4‐week period Control group: Routine therapy Participants received routine therapy for individual motor training and upper‐limb stretches 5 d/week. Upper limb stretches involved a seated weight‐bearing stretch and a seated upper limb stretch using an inflatable long‐arm air splint Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: MAS ‐ item 6, MAS ‐ item 7, MAS ‐ item 8 Time points included in this review: Outcomes measured at 4 weeks (end of intervention) Other time points: Outcomes also measured at baseline and 5 weeks (1 week after end of intervention) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...a random number table was used to generate the random number sequence", p 298 |
| Allocation concealment (selection bias) | Low risk | Quote: "...the investigator contacted an independent person to obtain group allocation for each subject. This ensured concealed randomization", p 298 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...both assessors were blinded to allocation", p 298 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 3/28 (10%) dropouts for 4‐week outcomes, 1/28 (4%) dropouts for 5‐week outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 21, Control group: 21, Other group: 21 Setting, Country: 9 inpatient rehabilitation units, Australia Joint of interest: Wrist (long finger flexors) Inclusion criteria:
Exclusion criteria: Nil reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 69 years (12), Control group: 75 years (11), Other group: 70 years (13) Gender: Experimental group: 43% female, Control group: 57% female, Other group: 52% female | |
| Interventions | Groups included in this review: Experimental group: Wrist extension splint and usual rehabilitation Participants wore a custom‐made, static, palmar mitt splint for up to 12 h overnight. The wrist was positioned in a comfortable end‐of‐range extended position with the metacarpophalangeal and interphalangeal joints extended. Participants also received usual rehabilitation, except that stretches of the wrist or long finger flexor muscles were not performed during the study period. A maximum of 10 min of isolated wrist and finger extension practice was permitted per day Total stretch time: 12 h x 7 d x 4 weeks = 336 h over a 4‐week period Control group: No splint and usual rehabilitation Participants did not wear a hand splint for the study period Participants received usual rehabilitation, except that stretches of the wrist or long finger flexor muscles were not performed during the study period. A maximum of 10 min of isolated wrist and finger extension practice was permitted per day Other group: Neutral wrist splint Participants wore a custom‐made, static, palmar mitt splint for up to 12 h overnight. The wrist was positioned in 0‐10° extension Participants also received usual rehabilitation, except that stretches of the wrist or long finger flexor muscles were not performed during the study period. A maximum of 10 min of isolated wrist and finger extension practice was permitted per day Total stretch time: 12 h x 7 d x 4 weeks = 336 h over a 4‐week period | |
| Outcomes | Outcomes included in this review:
Other outcomes: Upper limb activity (composite of 3 items of MAS), Spasticity rating (Tardieu) Time points included in this review: Outcomes measured at 4 weeks (end of intervention) Other time points: Outcomes also measured at baseline and 6 weeks (2 weeks after end of intervention) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the allocation schedule was computer generated", p 112 |
| Allocation concealment (selection bias) | Low risk | Quote: "...concealed in opaque, consecutively numbered envelopes by a person not otherwise involved in the study", p 112 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...measures were assessed...by an independent assessor who was unaware of which treatment the patient had received", p 112 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1/63 (2%) dropouts at 4‐week assessment, 4/63 (6%) dropouts for primary outcome at 6‐week assessment |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Children with spastic cerebral palsy Sample size: Experimental group: 191, Control group: 181, Other group A: 171, Other group B: 181, Entire sample: 792 Setting, Country: 3 treatment centres for disabled children, Canada Joint of interest: Wrist (wrist flexors) Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Not reported Gender: Experimental group: 68% female, Control group: 56% female, Other group A: 59% female, Other group B: 61% female | |
| Interventions | Groups included in this review: Experimental group: Cast plus intensive neurodevelopmental therapy (NDT) Participants wore an upper extremity inhibitory short arm cast for a minimum of 4 h per day. The cast immobilised the wrist in neutral to 10° extension. Participants also received 45 min of NDT therapy twice weekly plus a home programme for 30 min/d Total stretch time: 4 h x 7 d x 26 weeks = 728 h over a 26‐week period Control group: Intensive neurodevelopmental therapy (NDT) Participants received 45 min of NDT therapy twice weekly plus a home programme for 30 min/d Other group A: Regular neurodevelopmental therapy (NDT) plus cast Participants wore an upper extremity inhibitory short arm cast for a minimum of 4 h/d. The cast immobilised the wrist in neutral to 10 degrees extension Participants also received NDT therapy for a minimum of once per month up to a maximum of once per week. Participants performed a home programme for 15 min, 3 times per week Total stretch time: 4 h x 7 days x 26 weeks = 728 h over a 26‐week period Other group B: Regular neurodevelopmental therapy (NDT) Participants received NDT therapy for a minimum of once per month up to a maximum of once per week. Participants performed a home programme for 15 min, 3 times per week | |
| Outcomes | Outcomes included in this review:
Other outcomes: Quality of Upper Extremity Skills Test (QUEST) Time points included in this review: Outcomes measured at 6 months (end of intervention) and 9 months (3 months after end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | 1Number of participants who were analysed by the study authors (i.e. these numbers do not include dropouts). Authors did not report the size of the group allocations at baseline. 2 Number of participants who were randomised. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...outcomes were assessed by an evaluator, blind to the children's status at commencement", p 381 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 7/79 (9%) dropouts |
| Selective reporting (reporting bias) | High risk | Comment: Insufficient detail reported to include in meta‐analysis |
| Other bias | High risk | Quote: "...one nine month assessment was omitted because of consistently missed appointments", p 382 Comment: Not analysed by intention‐to‐treat |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adult women following radiotherapy for breast cancer Sample size: Experimental group: 31, Control group: 30 Setting, Country: Outpatients department, Australia Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 55 years (13), Control group: 53 years (12) Gender: Experimental group: 100% female, Control group: 100% female | |
| Interventions | Groups included in this review: Experimental group: Stretch plus usual care Participants received an individualised pectoral muscle stretching programme consisting of low‐load, prolonged, passive stretches of pectoralis major and minor while in supine‐lying. Participants also followed an independent exercise programme outlined in a pamphlet given to them after breast cancer surgery, which consisted of gentle shoulder ROM exercises. Participants were seen by the physiotherapist on a weekly basis during their radiotherapy for skin care, lymphoedema information and reviewing the above stretches. Total stretch time: 10 min x 2 muscles x 2 sessions x 7 d x 30.33 weeks = 141.5 h over a 30‐week period Control group: Usual care Participants followed an independent exercise programme outlined in a pamphlet given to them after breast cancer surgery. The exercise programme consisted of gentle shoulder ROM exercises. Participants were also seen by the physiotherapist on a weekly basis during their radiotherapy for skin care and lymphoedema information only Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive shoulder horizontal extension ROM ‐ unaffected, passive shoulder forward flexion ROM ‐ affected, passive shoulder forward flexion ROM ‐ unaffected, passive shoulder external rotation ROM ‐ affected, passive shoulder external rotation ROM ‐ unaffected, active shoulder abduction ROM ‐ affected, active shoulder abduction ROM ‐ unaffected, pain after arm ROM measurement ‐ unaffected, European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ‐C30), shoulder horizontal flexion strength ‐ affected, shoulder horizontal flexion strength ‐ unaffected, shoulder forward flexion strength ‐ affected, shoulder forward flexion strength ‐ unaffected, shoulder horizontal extension ‐ affected, shoulder horizontal extension strength ‐ unaffected, shoulder abduction strength ‐ affected, shoulder abduction strength ‐ unaffected, Shoulder external rotation strength ‐ affected, shoulder external rotation strength ‐ unaffected, arm swelling. Time points included in this review: Outcomes measured at 7 months (end of intervention). Other time points: Outcomes also measured at baseline and 6 weeks (end of radiotherapy). | |
| Notes | Pain and quality of life data were not reported in the publications and were therefore obtained directly from the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...participants were randomised to either a control or stretch group using computer‐generated randomisation schedule", p 314 |
| Allocation concealment (selection bias) | Low risk | Quote: "...allocation was concealed by the use of numbered opaque envelopes", p 314 Comment: Insufficient detail reported in paper whether envelopes were sealed. Correspondence with study author revealed that envelopes were sealed prior to randomisation |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...participants were measured by a physiotherapist blinded to group allocation at each of the three measurements.." |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 5/61 (8%) dropouts (Note: Review authors treated self‐reported outcomes as dropouts) |
| Selective reporting (reporting bias) | High risk | Comment: No results reported for pain and quality of life |
| Other bias | High risk | Comment: Changed protocol midway through trial |
| Methods | Design: Randomised cross‐over study | |
| Participants | Health condition: Children with cerebral palsy Sample size: Experimental group: 5, Control group: 4 Setting, Country: Outpatient clinic, UK Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 7 years (not reported), Control group: 7 years (not reported) Gender: Experimental group: 40% female, Control group: 75% female | |
| Interventions | Groups included in this review: Experimental group: Cast 1 Participants had a short leg cast applied in prone with the knee flexed. Casts were re‐applied each week. Casts were not re‐applied if there had been no improvement in ROM or if a target ROM (10° dorsiflexion) had been achieved. Total stretch time: 24 h x 7 d x (3‐4 weeks) = 504‐672 h over a 3‐4‐week period Control group: No cast 1 Participants did not receive a cast Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Maximum passive ankle dorsiflexion with the knee flexed, maximum ankle dorsiflexion in single‐support, maximum ankle dorsiflexion in swing, minimum knee flexion in stance, minimum hip flexion in stance, Gillette functional assessment questionnaire, walking speed, cadence, stride length, time in single‐support. Time points included in this review: Outcomes measured at 12 weeks (8‐9 weeks after end of intervention). Other time points: Outcomes also measured at baseline and 5 weeks. | |
| Notes | 1Only includes details of the first period of the cross‐over. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...participants in the study were allocated to one of two groups", p 465 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Comment: Insufficient detail reported |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: Insufficient detail reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: Not clear how many kinematic variables were measured |
| Other bias | High risk | Comment: Some participants had treatments applied bilaterally. Not clear how bilateral data were dealt with. |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with primary anterior cruciate ligament reconstruction Sample size: Experimental group: 18, Control group: 18 Setting, Country: Not reported, Italy Joint of interest: Knee Inclusion criteria:
Exclusion criteria: Nil reported Existing contracture, at risk of contracture, or combination of both: Participants were at risk of developing contracture Mean age (SD): Experimental group: 28 years (3), Control group: 30 years (7) Gender: Experimental group: 0% female, Control group: 0% female | |
| Interventions | Groups included in this review: Experimental group: Knee extension brace Participants wore a rehabilitation brace, locked in full extension, applied during the first postoperative week. The brace was only unlocked during ROM exercises. Full extension was maintained during gait and rest, including night‐time. Total stretch time: 23 h x 7 d = 161 h over a 1‐week period Control group: ROM brace (0 °‐90 °) Participants wore a rehabilitation brace locked from 0°‐90°, applied from the day of surgery to the seventh postoperative day. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: KT1000 measurement of ACL laxity Time points included in this review: Outcomes measured at 8 weeks post surgery (7 weeks after end of intervention) Other time points: Outcomes also measured at baseline, 2 weeks, 4 weeks, 4 months post surgery (KT1000 measurement only at this time point) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "...who were alternately distributed into the groups after the operation", p 323 |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information on allocation concealment reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...the physician didn't know to which group the patients belonged", |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: No dropouts reported |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised cross‐over study | |
| Participants | Health condition: Adults with traumatic brain injury Sample size: Experimental group: 5, Control group: 5 Setting, Country: Inpatient rehabilitation unit, Australia Joint of interest: Ankle (plantarflexors) Inclusion criteria:
Exclusion criteria: Nil reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: not reported, Control group: not reported, both groups: 29 years (11) Gender: Experimental group: not reported, Control group: not reported, both groups: 11% female | |
| Interventions | Groups included in this review: Experimental group: Cast 1 Participants had a short leg cast applied in prone with the knee flexed. Gastrocnemius was stretched by placing knee in extension for prolonged periods of time. Participants also received motor training aimed at improving the performance of everyday tasks. Total stretch time: 24 h x 7 d = 168 h over a 1‐week period Control group: No cast 1 Participants did not receive a cast and did not stretch. Participants received motor training aimed at improving the performance of everyday tasks. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Nil Time points included in this review: Outcomes measured at 7 d (end of intervention). Other time points: Outcomes also measured at baseline | |
| Notes | 1Only includes details of the first period of the cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...the experimental and control conditions occurred in random order", p 243 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | High risk | Quote: "...one potential threat to the validity of the study was the use of a |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 1/10 (10%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with ankle fracture Sample size: Experimental group: 51, Control group: 50, Other group: 49 Setting, Country: Outpatient clinics, Australia Joint of interest: Ankle Inclusion criteria:
Exclusion criteria: Not reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 47 years (15), Control group: 49 years (15), Other group: 43 yeas (15) Gender: Experimental group: 53% female, Control group: 52% female, Other group: 53% female | |
| Interventions | Groups included in this review: Experimental group: Long‐duration stretch plus exercise Participants performed long‐duration stretches by standing with the affected foot on a wedge with the back against a wall or, if weight bearing was not tolerated, in a sitting position. The slope of the wedge and the amount of weight borne through the leg were adjusted so that the participant felt a comfortable stretch in the ankle or calf muscles. Both the slope and the weight were progressed throughout the course of treatment. Participants also received ankle mobility and strengthening exercises, stepping exercises, and exercises involving weight bearing and balancing on the affected leg. Participants completed 30 repetitions of each exercise every day. Participants received gait training and advice. Total stretch time: 30 min x 7 d x 4 weeks = 14 h over a 4‐week period Control group: Exercise Participants received ankle mobility and strengthening exercises, stepping exercises, and exercises involving weight bearing and balancing on the affected leg. Participants completed 30 repetitions of each exercise every day. Participants received gait training and advice. Other group: Short‐duration stretch plus exercise Short duration stretches could be applied in a non–weight bearing position initially, with progression to standing as tolerated. Participants also received ankle mobility and strengthening exercises, stepping exercises, and exercises involving weight bearing and balancing on the affected leg. Participants completed 30 repetitions of each exercise every day. Participants received gait training and advice. Total stretch time: 6 min x 7 d x 4 weeks = 2.8 h over a 4‐week period | |
| Outcomes | Outcomes included in this review:
Other outcomes: Dorsiflexion angle at peak baseline torque with knee bent, peak ankle dorsiflexion ROM with knee straight, peak ankle dorsiflexion ROM with knee bent, measures of ankle stiffness with knee straight, measures of ankle stiffness with knee bent, preload co‐efficient with knee straight, preload co‐efficient with knee bent, ankle torque at the peak baseline dorsiflexion angle with knee straight, ankle torque at the peak baseline dorsiflexion angle with knee bent, pain during stair descent, perceived adverse effects of treatment, return to usual sport and leisure activities, speed when walking, step length asymmetry, stepping rate when stair climbing, global perception of effect of treatment, satisfaction with treatment, duration of PT treatment Time points included in this review: Outcomes measured at 4 weeks (end of intervention) and 3 months (2 months after end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...subjects were randomly allocated into 1 of 3 groups… using a procedure that was stratified and blocked by site", p 1119 Comment: Insufficient detail reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "...the randomization sequence was concealed by using consecutively numbered, sealed, opaque envelopes", p 1119 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...all measurements were made by assessors who were blind to group allocation", p 1112 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 11/150 (7%) dropouts at 4 week assessment, 16/150 (11%) dropouts at 3 month assessment |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with adhesive capsulitis (frozen shoulder) Sample size: Experimental group: 50, Control group: 50 Setting, Country: Outpatient clinic, India Joint of interest: Shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 49 years (6), Control group: 53 years (7) Gender: Experimental group: 36% female, Control group: 34% female | |
| Interventions | Groups included in this review: Experimental group : Stretch with countertraction device and usual care Participants received a shoulder stretch using an overhead device that provided a weighted shoulder countertraction (3 kg distracted load). This was administered during shoulder mobilisation. Participants also received usual care (details below) Total stretch time: 10 min x 5 d x 2 weeks = 1.7 h over a 2‐week period Control group : Usual care Participants received physiotherapy which consisted of heat prior to shoulder mobilisation, mobilisation to improve flexion & abduction range, and electrotherapy (ultrasound or shortwave diathermy) Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: shoulder abduction, shoulder function (Oxford Shoulder Score) Time points included in this review: Outcomes measured at 2 weeks (end of intervention) Other time points: Outcomes also measured at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer generated", p 2263 |
| Allocation concealment (selection bias) | Low risk | Quote: "...based on a sealed‐envelope system", p 2263 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "The outcomes were recorded by an independent outcome assessment trained physiotherapist (DJ), who was not involved in the intervention procedures and also was unaware of participants |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "The outcomes were measured and calculated after the intervention period of 2 weeks and no participants dropped out of the study", p 2265 |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Unclear risk | Comment: Insufficient detail provided |
| Methods | Design: Randomised within‐subjects cross‐over study | |
| Participants | Health condition: Children and young adults with Charcot‐Marie‐Tooth disease Sample size: Experimental group: 14 legs, Control group: 14 legs Setting, Country: Outpatient clinic, Australia Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 15 years (8), Control group: 15 years (8) Gender: Experimental group: 57% female, Control group: 57% female | |
| Interventions | Groups included in this review: Experimental group: Night splint 1 Participants wore a pre‐formed splint which was adjusted into dorsiflexion by the treating physiotherapist until participants felt a stretch in their calf muscles which could be tolerated during sleeping. The amount of dorsiflexion was increased if the stretch was felt to be insufficient. Participants were instructed to wear the splint for the whole night. Participants were also requested to avoid performing additional stretches or exercises that deviated from their normal routine. Total stretch time: (4‐9 h) x 7 d x 6 weeks = 168 h‐78 h over a 6‐week period Control group: No splint 1 Participants were requested to avoid performing additional stretches or exercises that deviated from their normal routine. Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive ankle eversion, isometric ankle dorsiflexion strength, isometric ankle eversion strength, isometric ankle inversion strength Time points included in this review: Outcomes measured at 6 weeks (end of 1st period) Other time points: Outcomes also measured at baseline, 12 weeks (end of 2nd period) and 26 weeks | |
| Notes | 1Only includes details of the first period of the cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...at the initial assessment, the treating physiotherapist randomly selected the leg to be splinted first by tossing a coin after baseline measurements were completed", p 194 |
| Allocation concealment (selection bias) | High risk | Quote: "...at the initial assessment, the treating physiotherapist randomly selected the leg to be splinted first by tossing a coin after baseline measurements were completed", p 194 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...the same assessor, who was blinded to group allocation, made all measurements for each participant", p 194 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 4/56 (7%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Randomised parallel‐group study | |
| Participants | Health condition: Children and young adults with Charcot‐Marie‐Tooth disease and restricted ankle dorsiflexion range Sample size: Experimental group: 15, Control group: 15 Setting, Country: Outpatient clinic, Australia Joint of interest: Ankle Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 10 years (4), Control group: 11 years (3) Gender: Experimental group: 60% female, Control group: 47% female | |
| Interventions | Groups included in this review: Experimental group : Night cast for 4 weeks followed by stretches in standing for 4 weeks Participants wore a fibreglass cast with the ankle positioned in dorsiflexion (knee not included). The casts were bivalved and applied only at night for the first 4 weeks. The casts were remade after 2 weeks. At 4 weeks, the stretches were administered in standing using 2 types of stretches. Each stretch was held for 1 min and performed 3 times a day. Total stretch time: (6‐10 h x 7 d x 4 weeks) + (1 min x 6 times per day x 7 d x 4 weeks) = 170.8‐282.2 h over an 8‐week period Control group : No intervention Participants received no intervention. Other groups : Nil | |
| Outcomes | Outcomes included in this review: 1. Ankle dorsiflexion during a lunge test (degrees) 2. Speed of preferred walking (m/sec) Other outcomes: Foot Posture Index (points), Patient Specific Functional Scale (points), standing up speed (stands/sec), speed of fast walking (m/sec), speed of ascending stairs (stairs/sec), balance with feet together (sec), balance with feet toe to heel (sec), balance in tandem stance (sec), number of falls (no.) Time points included in this review: Outcomes measured at 8 weeks (end of intervention). Other time points: Outcomes also measured at baseline and 4 weeks. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation sequence was computer‐generated...", p 114 |
| Allocation concealment (selection bias) | Low risk | Quote: "…telephoned the administrative assistant to obtain the participant's random allocation..." p 114 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...assessor blinding...", p 113 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: Table 2 ‐ all outcomes at all end‐points |
| Selective reporting (reporting bias) | Low risk | Comment: Table 2 ‐ all outcomes at all end‐points |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised within‐subjects study | |
| Participants | Health condition: Adults with systemic sclerosis (scleroderma) Sample size: Experimental group: 19 hands, Control group: 19 hands Setting, Country: Outpatient clinic, USA Joint of interest: Proximal interphalangeal Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (range): Experimental group: 48 years (31‐61)1, Control group: 48 years (31‐61)1 Gender: Experimental group: 100% female1, Control group: 100% female1, both groups including dropouts: 89% female | |
| Interventions | Groups included in this review: Experimental group: Splint Participants wore a dynamic splint on the experimental hand which provided a sustained stretch into extension on the interphalangeal and metacarpophalangeal joints. Total stretch time: 8 h x 7 d x 8 weeks = 448 h over an 8‐week period Control group: No splint Participants did not wear a splint on the control hand Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Combined proximal interphalangeal active extension, index finger proximal interphalangeal passive extension, index finger proximal interphalangeal active extension, middle finger proximal interphalangeal passive extension, middle finger proximal interphalangeal active extension, ring finger proximal interphalangeal passive extension, ring finger proximal interphalangeal active extension, little finger proximal interphalangeal passive extension, little finger proximal interphalangeal active extension Time points included in this review: Outcomes measured at 2 months (end of intervention) Other time points: Outcomes also measured at baseline and 1 month | |
| Notes | 1 Excludes dropouts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random number table", p 119 |
| Allocation concealment (selection bias) | High risk | Comment: Insufficient detail reported in paper. Correspondence with study author revealed that allocation was not concealed |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...measurements were done by the same evaluator who was blind to the study", p 119 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Quote: "...2 were dropped for non‐compliance", p 120 Comment: 12/19 (63%) dropouts for PROM outcome |
| Selective reporting (reporting bias) | High risk | Comment: At least 8 ROM outcomes were measured but only 2 were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 6, Control group: 8 Setting, Country: Inpatient and outpatient rehabilitation centres, Australia Joint of interest: Wrist (finger flexors) Inclusion criteria:
Exclusion criteria: Not reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 74 years (8.7)1, Control group: 70 years (7.5)1 Gender: Experimental group: 0% female, Control group: 17% female1 | |
| Interventions | Groups included in this review: Experimental group: Splint on 2nd week (Group 2) Participants wore a thermoplastic resting splint during the 2nd week (i.e. participants wore the splint from the 2nd week to the 7th week). Participants received no other upper limb treatment interventions. Total stretch time: 8 h1 x 7 d x 1 week = 56 h over a 1‐week period Control group: No splint on 2nd week (Group 1) Participants did not wear a thermoplastic resting splint during the 2nd week (i.e. participants wore the splint from the 3rd week to the 7th week). Participants received no other upper limb treatment interventions Other groups : Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Resistance at 10° wrist extension, resistance at 0° wrist extension, resistance at 10° wrist flexion, resistance at 20° wrist flexion, rate of change of resistance. Time points included in this review: Outcomes measured at 2 weeks (end of intervention) Other time points: Outcomes also measured at baseline and 7 weeks | |
| Notes | 1Data obtained from correspondence with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...random numbers table", p 1033 |
| Allocation concealment (selection bias) | Low risk | Quote: "...the slips of paper containing the random numbers were replaced in a black bag that was kept in a locked drawer in the independent clinician’s desk...with vision occluded, the independent clinician drew a number from the bag and the participant was allocated to the group", p 1033 |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "The researcher was not involved in the random allocation of subjects and was thus blinded to group allocation" (correspondence with study author) |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/14 (14%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | Low risk | Comment: Appears free of other bias |
| Methods | Design: Randomised within‐subjects study | |
| Participants | Health condition: Elderly people with bilateral knee contractures Sample size: Experimental group: 14, Control group: 14 Setting, Country: Nursing homes, USA Joint of interest: Knee Inclusion criteria:
Exclusion criteria: Not reported Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture Mean age (SD): Experimental group: 86 years (7), Control group: 86 years (7) Gender: Experimental group: 79% female, Control group: 79% female | |
| Interventions | Groups included in this review: Experimental group: Knee splint (prolonged stretch) plus passive ROM exercises and manually administered stretches Participants wore a knee extension splint from the second month of the study through to the seventh month (total = 6 months). The tension setting on the splint was initially 0 and progressed to 6 (62.2 kg‐cm) between weeks 2 and 5 of the study. Participants also received passive ROM and manually administered stretches (details below). Total stretch time: 3 h x 5 d x 26 weeks = 390 h over a 26‐week period Control group: Passive ROM exercises and manually administered stretches Each participant received passive ROM exercises and manually administered stretches to both lower extremities twice a week by on‐site physiotherapists trained in the standardised protocol Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive hip extension, passive ankle dorsiflexion, torque required to maintain maximum passive knee extension. Time points included in this review: Outcomes measured at 7 months (end of intervention). Other time points: Outcomes also measured at baseline, 2 weeks, 4 weeks, 2 months, 3 months, 4 months, 5 months and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Use of the prolonged stretch was alternately assigned to the right or left knee", p 889 |
| Allocation concealment (selection bias) | High risk | Comment: Alternate assignment means allocation was not concealed |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "The physiotherapists performing the measurements were not aware of the side of the experimental treatments", p 888 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 10/28 (36%) dropouts reported |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported |
| Other bias | High risk | Quote: "All the subjects in two of the nursing homes were checked for fit by the designer of the splint, who also owns the company that makes the splint", p 889 |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults with stroke Sample size: Experimental group: 14, Control group: 15 Setting, Country: Hospital inpatients, UK Joint of interest: Wrist and shoulder Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 70 years (10)1, Control group: 66 years (14)1 Gender: Experimental group: 31% female2, Control group: 33% female2 | |
| Interventions | Groups included in this review: Experimental group: Stretch plus usual care Participants received two 30‐min sessions of positioning in each of these positions: Participants also received usual care (details below) Total stretch time (maximum) = 2 wrist stretches x 30 min x 7 d x 12 weeks = 84 h3 Control group: Usual care All participants received the standard arm care which did not include sustained stretches. The affected arm was supported on a Bexhill arm support or pillow in sitting Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Passive wrist extension contracture (unaffected minus affected), passive shoulder external rotation ‐ affected, passive shoulder external rotation contracture (unaffected minus affected), active wrist extension ROM ‐ affected, active shoulder external rotation ROM ‐ affected. Time points included in this review: Outcomes measured at 12 weeks post‐stroke4 Other time points: Outcomes also measured at 4 weeks post‐stroke5 and 8 weeks4 | |
| Notes | 1Mean age (SD) of participants at 4 weeks post stroke. Study authors did not measure participants at point of randomisation 2Gender of participants at 4 weeks post stroke. Study authors did not measure participants at point of randomisation 3Total stretch time varied between participants because of varying recruitment timing and discharge timing. The intervention was also stopped if participants reached a certain criteria for arm function or if they reached 12 weeks post‐stroke 4Considerable variation in time since last stretch intervention ranging from less than 24 h to greater than 1 week 5At least 4 participants were already randomised prior to this first measure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer‐generated sequence for group allocation", p 601 |
| Allocation concealment (selection bias) | Low risk | Quote: "...group allocation was kept by a person who was independent of the recruitment process", p 601 Comment: Off‐site allocation |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Low risk | Quote: "...readings were taken by the assistant who was (when possible) kept blind to the subject s allocation", p 604 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | High risk | Comment: 6/29 (21%) dropouts for 12 week assessment |
| Selective reporting (reporting bias) | High risk | Comment: No outcomes reported for active ROM measures |
| Other bias | High risk | Comment: No standard treatment protocol. Experimental participants given varying amounts of treatment dependent on length of stay or arm function |
| Methods | Design: Randomised parallel‐group study | |
| Participants | Health condition: Adults following total knee replacement Sample size: Experimental group: 42, Control group: 39 Setting, Country: Acute hospital, UK Joint of interest: Knee Inclusion criteria:
Exclusion criteria:
Existing contracture, at risk of contracture, or combination of both: Participants had existing contracture and were at risk of developing contracture Mean age (SD): Experimental group: 71 years (7), Control group: 71 years (8) Gender: Experimental group: 69% female, Control group: 67% female | |
| Interventions | Groups included in this review: Experimental group: Splint Participants' knees were splinted into extension using a cricket pad splint in the early postoperative period. The splint was removed for the participants to do physiotherapy exercises twice a day. Splints were removed when the participant could straight leg raise. Total stretch time: 23 h x 3 d = 69 h over a 3‐day period Control group: No splint Participants had a wool and crepe bandage applied around their knee and were allowed to fully mobilise from the first day. The bandage was removed at 48 hours post‐op. Participants in this group, in addition to the twice a day physiotherapy regime were encouraged to actively flex the knee from the first postoperative day Other groups: Nil | |
| Outcomes | Outcomes included in this review:
Other outcomes: Knee flexion ROM, time to straight leg raise, wound drainage, amount of analgesia required Time points included in this review: Outcomes measured at 6 weeks (end of intervention) Other time points: Outcomes also measured at baseline and 5 days post‐op1 | |
| Notes | 1 Unclear whether the intervention was still continuing in some participants at the 5‐day outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised into two groups", p 225 Comment: Insufficient detail reported |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...using a sealed envelope technique", p 225 Comment: Insufficient detail reported |
| Blinding (performance bias and detection bias) | High risk | Comment: Not possible to blind participants or therapists |
| Blinding of outcome assessors (detection bias) ‐ objective outcomes | Unclear risk | Quote: "...measurements were recorded by an independent observer", p 226 |
| Blinding of outcome assessors (detection bias) ‐ self‐reported outcomes | High risk | Comment: Not possible to blind participants |
| Incomplete outcome data (attrition bias) | Low risk | Comment: 2/81 (2%) dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: All pre‐stated outcomes were reported. Details of secondary outcomes are unclear |
| Other bias | Low risk | Comment: Appears free of other bias |
FMA: Fugl‐Meyer Assessment; ITT: intention‐to‐treat; MAS: Motor Assessment Scale; ROM: range of movement; VAS: Visual Analogue Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Did not measure joint mobility | |
| Stretch compared to serial casting. Unable to isolate the effects of stretch | |
| Not a RCT. | |
| Correspondence with the study author revealed that participants received a confounding intervention. Compared stretch to home exercises. Different home exercises were given to each group | |
| Participants were not at risk of contracture | |
| Stretch compared to two other stretch interventions. Unable to isolate the effects of stretch | |
| Did not measure joint mobility | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Did not measure joint mobility | |
| Not a RCT | |
| Stretch compared to two other stretch interventions. Unable to isolate the effects of stretch | |
| Not a stretch intervention | |
| Splint not applied for the purpose of maintaining or increasing joint mobility | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to two other stretch interventions. Unable to isolate the effects of stretch | |
| Stretch compared to two other stretch interventions. Unable to isolate the effects of stretch | |
| Stretch compared to two other stretch interventions. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Not a stretch intervention | |
| Compared casting and botulinum toxin. No botulinum toxin given to casting group. Unable to isolate the effects of stretch | |
| Stretch compared to two other stretch interventions. Unable to isolate the effects of stretch | |
| Stretch applied in combination with electrical stimulation. Unable to isolate the effects of stretch. | |
| Compared different timings of same stretch before and after botulinum toxin. Unable to isolate effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| This study was registered and noted as a study in progress in the 2009 version of this Cochrane review. However, according to the clinical trials registry, it never started and has since been withdrawn | |
| Did not measure joint mobility | |
| Did not measure joint mobility | |
| Participants were not at risk of contracture | |
| Compared casting to botulinum toxin. No botulinum toxin given to casting group. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Participants were not at risk of contracture | |
| Participants were not at risk of contracture | |
| Not a RCT | |
| Participants were not at risk of contracture | |
| Participants were not at risk of contracture | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to massage. Unable to isolate the effects of stretch | |
| Participants were not at risk of contracture | |
| Splint applied on one occasion only | |
| Not a RCT | |
| Not a stretch intervention | |
| Not a RCT | |
| Did not measure joint mobility | |
| Not a stretch intervention | |
| Orthosis applied on one occasion only | |
| Compared stretch to muscle strengthening. No muscle strengthening performed by stretch group. Unable to isolate the effects of stretch | |
| Did not measure joint mobility | |
| Not a RCT | |
| Not a RCT | |
| Not a RCT | |
| Compared resistance and stretching exercises to no exercises. Unable to isolate the effects of stretch | |
| Not a stretch intervention, involved primarily active exercises | |
| Did not measure joint mobility | |
| Did not measure joint mobility | |
| Not a stretch intervention | |
| Compared intensive neurodevelopmental therapy plus casting to regular occupational therapy. Unable to isolate the effects of casting | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Did not measure joint mobility | |
| Participants were not at risk of contracture | |
| Participants were not at risk of contracture | |
| Stretch compared to passive movements. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch compared to another stretch intervention. Participants were not at risk of contracture | |
| Stretch applied on one occasion only | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Compared different timings of the same stretch intervention. Unable to isolate the effects of stretch | |
| Participants were not at risk of contracture | |
| Not a RCT | |
| Not a stretch intervention | |
| Participants were not at risk of contracture | |
| Compared botulinum toxin to botulinum toxin plus taping. Different botulinum toxin dosages and injection sites were used between groups. Unable to isolate the effects of taping | |
| Not a stretch intervention | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Splint applied on one occasion only | |
| Not a RCT | |
| Not a stretch intervention | |
| Stretch compared to another stretch intervention. Unable to isolate the effects of stretch | |
| Stretch applied on one occasion only | |
| Not a RCT | |
| Participants were not at risk of contracture | |
| Not a RCT | |
| Participants were not at risk of contracture |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Published in Arabic ‐ awaiting translation. Unable to determine if eligible |
| Participants | Unable to determine |
| Interventions | Unable to determine |
| Outcomes | Unable to determine |
| Notes | Unable to determine |
| Methods | Published in Arabic ‐ awaiting translation. Unable to determine if eligible |
| Participants | Unable to determine |
| Interventions | Unable to determine |
| Outcomes | Unable to determine |
| Notes | Unable to determine |
| Methods | Unable to attain full text. Unable to determine if eligible |
| Participants | Unable to determine |
| Interventions | Unable to determine |
| Outcomes | Unable to determine |
| Notes | Unable to determine |
| Methods | Published in Arabic ‐ awaiting translation. Unable to determine if eligible |
| Participants | Unable to determine |
| Interventions | Unable to determine |
| Outcomes | Unable to determine |
| Notes | Unable to determine |
| Methods | Published in Italian ‐ awaiting translation. Unable to determine if eligible |
| Participants | Unable to determine |
| Interventions | Unable to determine |
| Outcomes | Unable to determine |
| Notes | Unable to determine |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
| Trial name or title | Splint: the efficacy of orthotic management in rest to prevent equinus in children with cerebral palsy, a randomised controlled trial |
| Methods | RCT |
| Participants | Children with cerebral palsy |
| Interventions | Orthoses worn for 1 year to prevent a decrease in ROM in the ankle |
| Outcomes | Ankle dorsiflexion |
| Starting date | January 2010 |
| Contact information | Josina C Maas. Department of Rehabilitation Medicine and the EGMO + Institute for Health and Care Research and Research Institute MOVE, VU University Medical Center, De Boelelaan 1117, 1081 HV Amsterdam, The Netherlands, [email protected] |
| Notes | The published trial protocol indicates that the trial will be completed by December 2012. We contacted the study authors in May 2016 to clarify status of the trial but have not had a response. |
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
ROM: range of motion
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 18 | 549 | Mean Difference (IV, Random, 95% CI) | 1.81 [0.45, 3.17] |
| Analysis 1.1  Comparison 1 Joint mobility ‐ short‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 11 | 295 | Mean Difference (IV, Random, 95% CI) | 0.56 [‐1.56, 2.68] |
| 1.2 Charcot‐Marie‐Tooth disease | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 2.27 [0.16, 4.38] |
| 1.3 Acquired brain injury | 3 | 35 | Mean Difference (IV, Random, 95% CI) | 8.48 [0.60, 16.36] |
| 1.4 Spinal cord injury | 4 | 137 | Mean Difference (IV, Random, 95% CI) | 1.42 [‐0.54, 3.37] |
| 2 Non‐neurological conditions Show forest plot | 18 | 865 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.00, 0.33] |
| Analysis 1.2  Comparison 1 Joint mobility ‐ short‐term effects following stretch, Outcome 2 Non‐neurological conditions. | ||||
| 2.1 Frail elderly | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.28, 0.74] |
| 2.2 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.46, 0.35] |
| 2.3 Anklylosing spondylitis | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.07, 1.32] |
| 2.4 Oral submucous fibrosis | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.05, 1.72] |
| 2.5 Post‐radiation therapy to breast | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.47, 0.58] |
| 2.6 Post‐radiation therapy to jaw | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 1.54 [0.25, 2.82] |
| 2.7 Progressive systemic sclerosis | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.32, 1.88] |
| 2.8 Total knee replacement | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.72, 0.34] |
| 2.9 Arthritis | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.25, 1.07] |
| 2.10 Dupuytren's contractures | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.27, 0.45] |
| 2.11 Shoulder adhesive capsulitis/frozen shoulder | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.67, 0.11] |
| 2.12 Hallux limitus | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.14, 1.01] |
| 2.13 Wrist fracture | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.41, 0.90] |
| 2.14 Burns | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.35, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 8 | 211 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐1.37, 2.82] |
| Analysis 2.1  Comparison 2 Joint mobility ‐ long‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 4 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐4.09, 3.44] |
| 1.2 Cerebral palsy | 2 | 39 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐2.05, 4.79] |
| 1.3 Spinal cord injury | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.05, 3.05] |
| 1.4 Acquired brain injury | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 10.42 [0.62, 20.22] |
| 2 Non‐neurological conditions Show forest plot | 6 | 438 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| Analysis 2.2  Comparison 2 Joint mobility ‐ long‐term effects following stretch, Outcome 2 Non‐neurological conditions. | ||||
| 2.1 ACL reconstruction | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.54, 0.77] |
| 2.2 Ankle fracture | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.62, 0.21] |
| 2.3 Total knee replacement | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.80, 0.09] |
| 2.4 Dupuytren's contracture | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 2.5 Wrist fracture | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.80 [0.07, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐neurological conditions Show forest plot | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.09, 0.71] |
| Analysis 3.1  Comparison 3 Quality of life ‐ short‐term effects following stretch, Outcome 1 Non‐neurological conditions. | ||||
| 1.1 Post‐radiation therapy to breast | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.37, 0.67] |
| 1.2 Burns | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.08, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 5 | 174 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.10, 0.50] |
| Analysis 4.1  Comparison 4 Pain ‐ short‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 4 | 135 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.03, 0.66] |
| 1.2 Spinal cord injury | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.81, 0.45] |
| 2 Non‐neurological conditions Show forest plot | 7 | 422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.43, 0.10] |
| Analysis 4.2  Comparison 4 Pain ‐ short‐term effects following stretch, Outcome 2 Non‐neurological conditions. | ||||
| 2.1 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.41, 0.41] |
| 2.2 Frail elderly | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.10, 0.51] |
| 2.3 Post‐radiotherapy to breast | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.63, 0.43] |
| 2.4 Arthritis | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.96, 0.35] |
| 2.5 Shoulder adhesive capsulitis/frozen shoulder | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.17, 0.78] |
| 2.6 Dupuytren's contracture | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.62, 0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 4 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.41, 0.47] |
| Analysis 5.1  Comparison 5 Pain ‐ long‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 4 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.41, 0.47] |
| 2 Non‐neurological conditions Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Pain ‐ long‐term effects following stretch, Outcome 2 Non‐neurological conditions. | ||||
| 2.1 Ankle fracture | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Shoulder adhesive capsulitis | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 7 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.13, 0.52] |
| Analysis 6.1  Comparison 6 Activity limitations ‐ short‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 5 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.09, 0.63] |
| 1.2 Cerebral palsy | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.21, 1.09] |
| 1.3 Charcot‐Marie‐Tooth disease | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.21, 0.24] |
| 2 Non‐neurological conditions Show forest plot | 5 | 356 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.17, 0.34] |
| Analysis 6.2  Comparison 6 Activity limitations ‐ short‐term effects following stretch, Outcome 2 Non‐neurological conditions. | ||||
| 2.1 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.30, 0.51] |
| 2.2 Arthritis | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.20, 1.13] |
| 2.3 Dupuytren's contracture | 1 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.39, 0.25] |
| 2.4 Wrist fracture | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.97, 0.35] |
| 2.5 Burns | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.12, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 6 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.11, 0.56] |
| Analysis 7.1  Comparison 7 Activity limitations ‐ long‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 4 | 136 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.29, 0.58] |
| 1.2 Cerebral palsy | 2 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.17, 1.00] |
| 2 Non‐neurological conditions Show forest plot | 3 | 268 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.32, 0.15] |
| Analysis 7.2  Comparison 7 Activity limitations ‐ long‐term effects following stretch, Outcome 2 Non‐neurological conditions. | ||||
| 2.1 Ankle fracture | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.48, 0.35] |
| 2.2 Dupuytren's contracture | 1 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 2.3 Wrist fracture | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.86, 0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐neurological conditions Show forest plot | 2 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.57, 0.12] |
| Analysis 8.1  Comparison 8 Participation restrictions ‐ short‐term effects following stretch, Outcome 1 Non‐neurological conditions. | ||||
| 1.1 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.72, 0.10] |
| 1.2 Wrist fracture | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.65, 0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐neurological conditions Show forest plot | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.60, 0.29] |
| Analysis 9.1  Comparison 9 Participation restrictions ‐ long‐term effects following stretch, Outcome 1 Non‐neurological conditions. | ||||
| 1.1 Ankle fracture | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.41, 0.41] |
| 1.2 Wrist fracture | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.20, 0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 6 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.36] |
| Analysis 10.1  Comparison 10 Spasticity ‐ short‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 5 | 134 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.29, 0.39] |
| 1.2 Acquired brain injury | 1 | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.55, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 3 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.81, 0.13] |
| Analysis 11.1  Comparison 11 Spasticity ‐ long‐term effects following stretch, Outcome 1 Neurological conditions. | ||||
| 1.1 Stroke | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.12, 0.11] |
| 1.2 Cerebral palsy | 1 | 21 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.73, 1.00] |
| 1.3 Traumatic brain injury | 1 | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.03, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Types of stretch intervention Show forest plot | 36 | 1470 | Mean Difference (IV, Random, 95% CI) | 1.07 [0.03, 2.10] |
| Analysis 12.1  Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 1 Types of stretch intervention. | ||||
| 1.1 Cast | 3 | 57 | Mean Difference (IV, Random, 95% CI) | 4.59 [‐2.60, 11.78] |
| 1.2 Splint | 17 | 787 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐1.02, 1.55] |
| 1.3 Self‐administered | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 3.07 [0.19, 5.94] |
| 1.4 Positioning | 7 | 165 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐2.73, 8.33] |
| 1.5 Other sustained passive stretch | 7 | 386 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐1.07, 2.61] |
| 2 Large versus small joints Show forest plot | 36 | 1467 | Mean Difference (IV, Random, 95% CI) | 1.03 [‐0.02, 2.09] |
| Analysis 12.2  Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 2 Large versus small joints. | ||||
| 2.1 Large joints | 16 | 645 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.89, 2.03] |
| 2.2 Small joints | 20 | 822 | Mean Difference (IV, Random, 95% CI) | 1.44 [‐0.11, 3.00] |
| 3 Influence of discomfort Show forest plot | 36 | 1470 | Mean Difference (IV, Random, 95% CI) | 1.07 [0.01, 2.13] |
| Analysis 12.3  Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 3 Influence of discomfort. | ||||
| 3.1 Measurements influenced by discomfort | 25 | 1009 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐0.41, 2.78] |
| 3.2 Measurements not influenced by discomfort | 11 | 461 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.42, 2.52] |
| 4 Joint mobility measured less than one day versus more than one day Show forest plot | 34 | 1400 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.50, 1.85] |
| Analysis 12.4  Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 4 Joint mobility measured less than one day versus more than one day. | ||||
| 4.1 Less than one day | 28 | 1155 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.20, 2.00] |
| 4.2 More than one day | 7 | 245 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [0.24, 2.28] |

Study flow diagram
1. These numbers are approximate only

Methodological quality summary: review authors' judgements about each methodological quality item for each included study

Forest plot of comparison: Joint mobility ‐ short‐term effects following stretch ‐ neurological conditions (degrees)
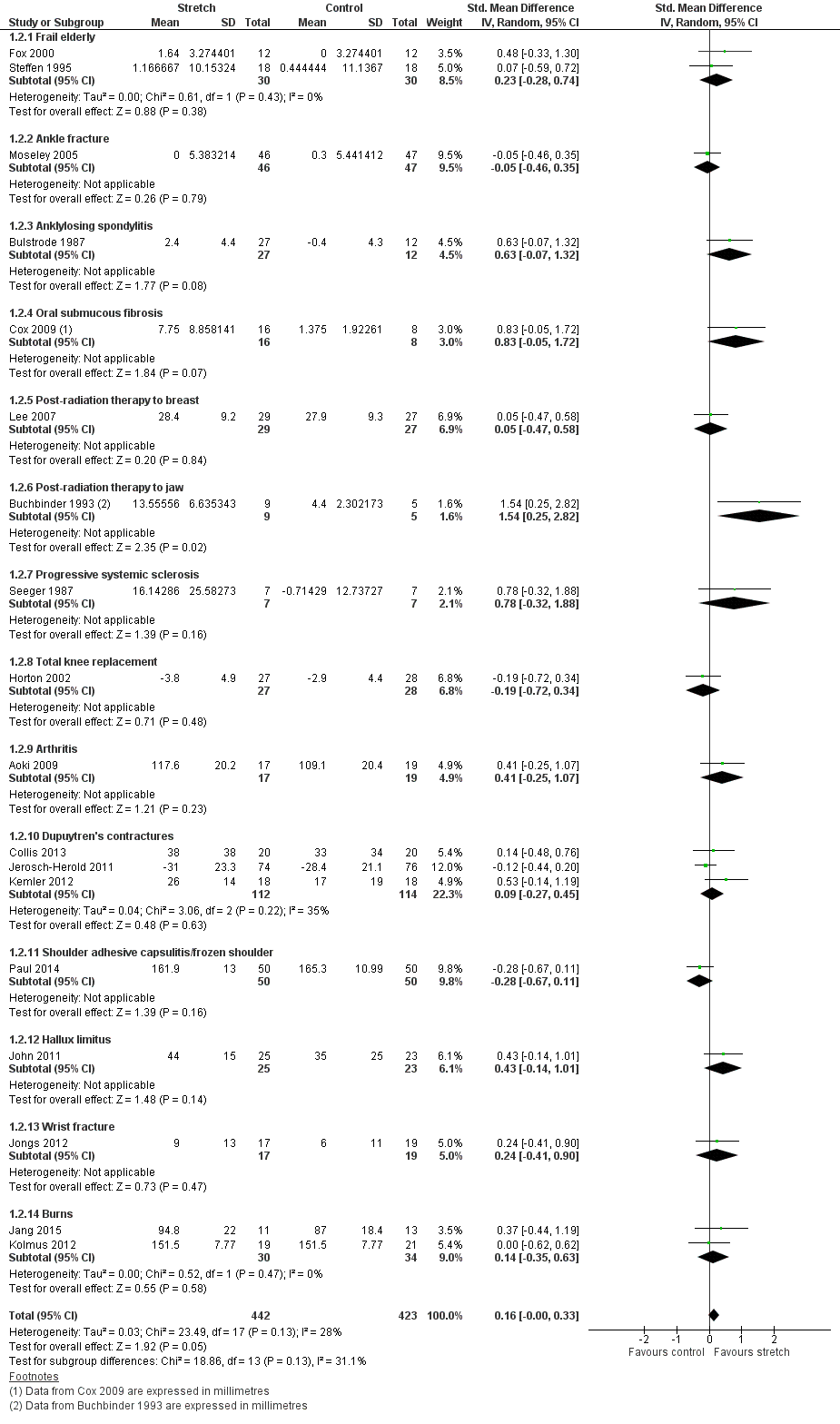
Forest plot of comparison: Joint mobility ‐ short‐term effects following stretch ‐ non‐neurological conditions (SMD)

Forest plot of comparison: Joint mobility ‐ long‐term effects following stretch ‐ neurological conditions (degrees)

Bubble plot of meta‐regression analysis: Joint mobility ‐ effects of total stretch time on joint mobility ‐ all conditions (degrees)

Forest plot of comparison: Joint mobility ‐ subgroup analyses by type of stretch intervention ‐ neurological conditions (degrees)

Funnel plot of comparison: 1 Joint mobility ‐ short‐term effects following stretch, outcome: 1.1 Neurological conditions (degrees)

Funnel plot of comparison: 1 Joint mobility ‐ short‐term effects following stretch, outcome: 1.2 Non‐neurological conditions

Comparison 1 Joint mobility ‐ short‐term effects following stretch, Outcome 1 Neurological conditions.

Comparison 1 Joint mobility ‐ short‐term effects following stretch, Outcome 2 Non‐neurological conditions.

Comparison 2 Joint mobility ‐ long‐term effects following stretch, Outcome 1 Neurological conditions.
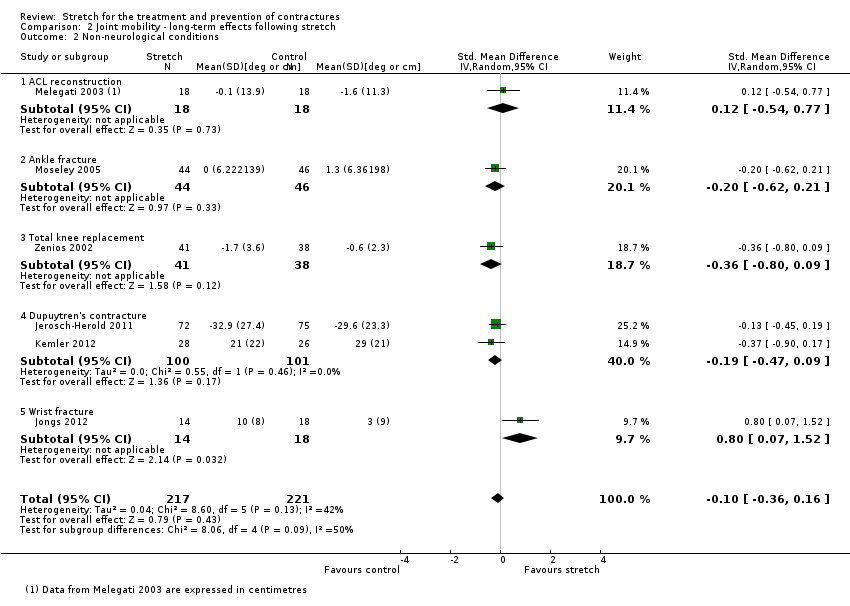
Comparison 2 Joint mobility ‐ long‐term effects following stretch, Outcome 2 Non‐neurological conditions.

Comparison 3 Quality of life ‐ short‐term effects following stretch, Outcome 1 Non‐neurological conditions.
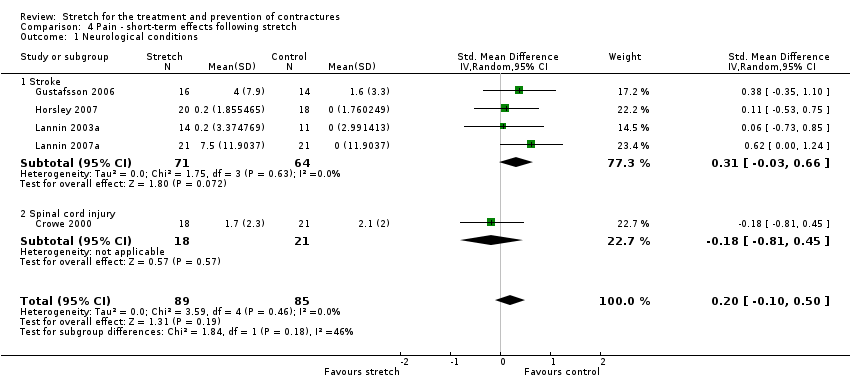
Comparison 4 Pain ‐ short‐term effects following stretch, Outcome 1 Neurological conditions.

Comparison 4 Pain ‐ short‐term effects following stretch, Outcome 2 Non‐neurological conditions.

Comparison 5 Pain ‐ long‐term effects following stretch, Outcome 1 Neurological conditions.

Comparison 5 Pain ‐ long‐term effects following stretch, Outcome 2 Non‐neurological conditions.
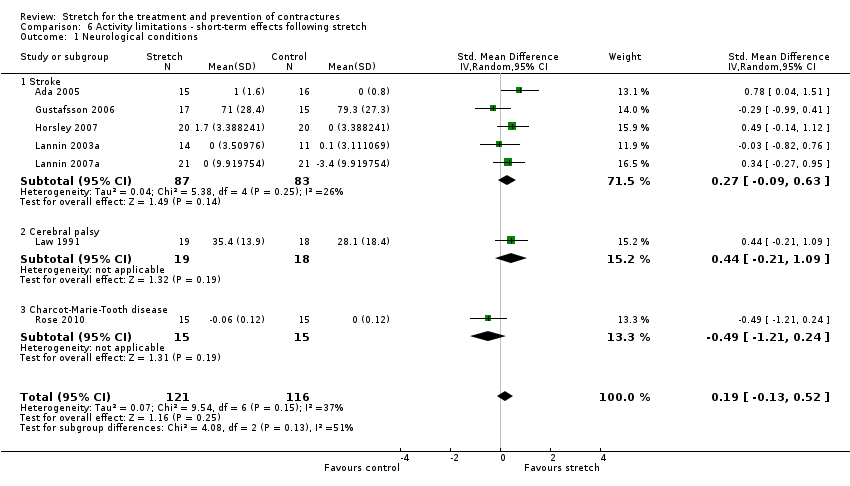
Comparison 6 Activity limitations ‐ short‐term effects following stretch, Outcome 1 Neurological conditions.

Comparison 6 Activity limitations ‐ short‐term effects following stretch, Outcome 2 Non‐neurological conditions.
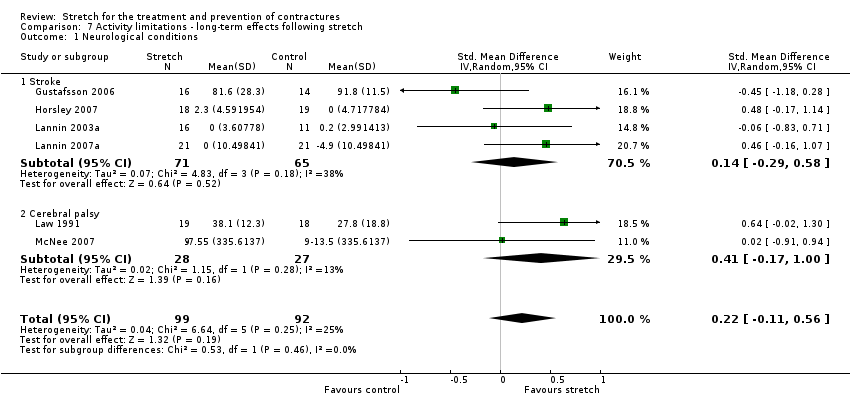
Comparison 7 Activity limitations ‐ long‐term effects following stretch, Outcome 1 Neurological conditions.

Comparison 7 Activity limitations ‐ long‐term effects following stretch, Outcome 2 Non‐neurological conditions.
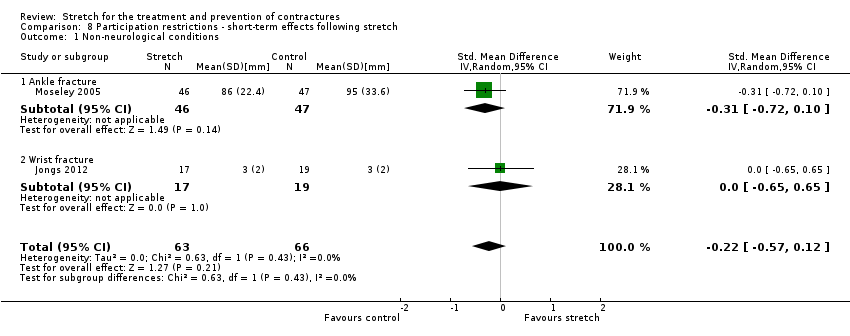
Comparison 8 Participation restrictions ‐ short‐term effects following stretch, Outcome 1 Non‐neurological conditions.
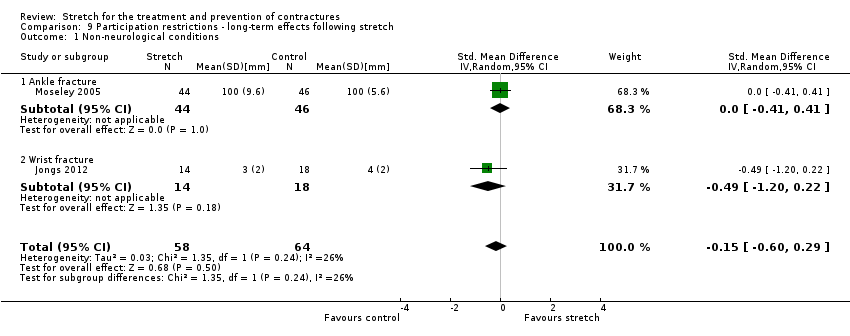
Comparison 9 Participation restrictions ‐ long‐term effects following stretch, Outcome 1 Non‐neurological conditions.

Comparison 10 Spasticity ‐ short‐term effects following stretch, Outcome 1 Neurological conditions.

Comparison 11 Spasticity ‐ long‐term effects following stretch, Outcome 1 Neurological conditions.
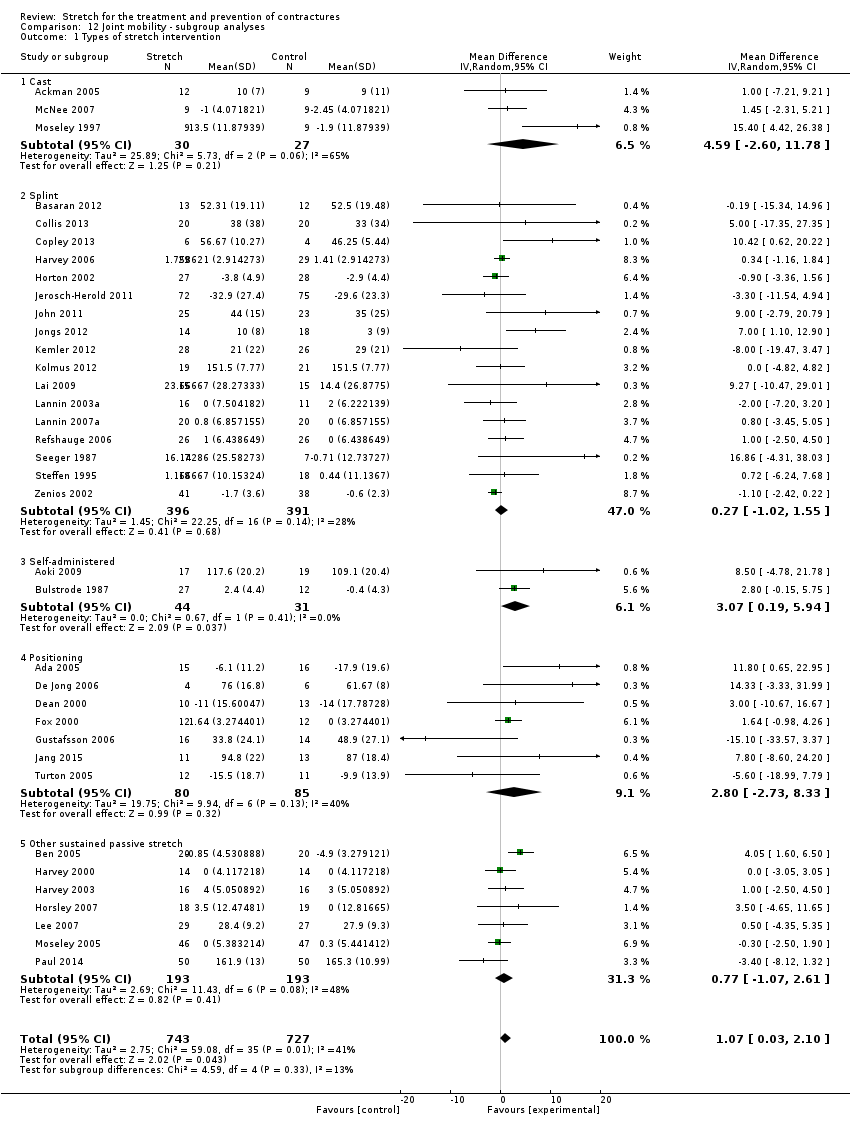
Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 1 Types of stretch intervention.

Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 2 Large versus small joints.
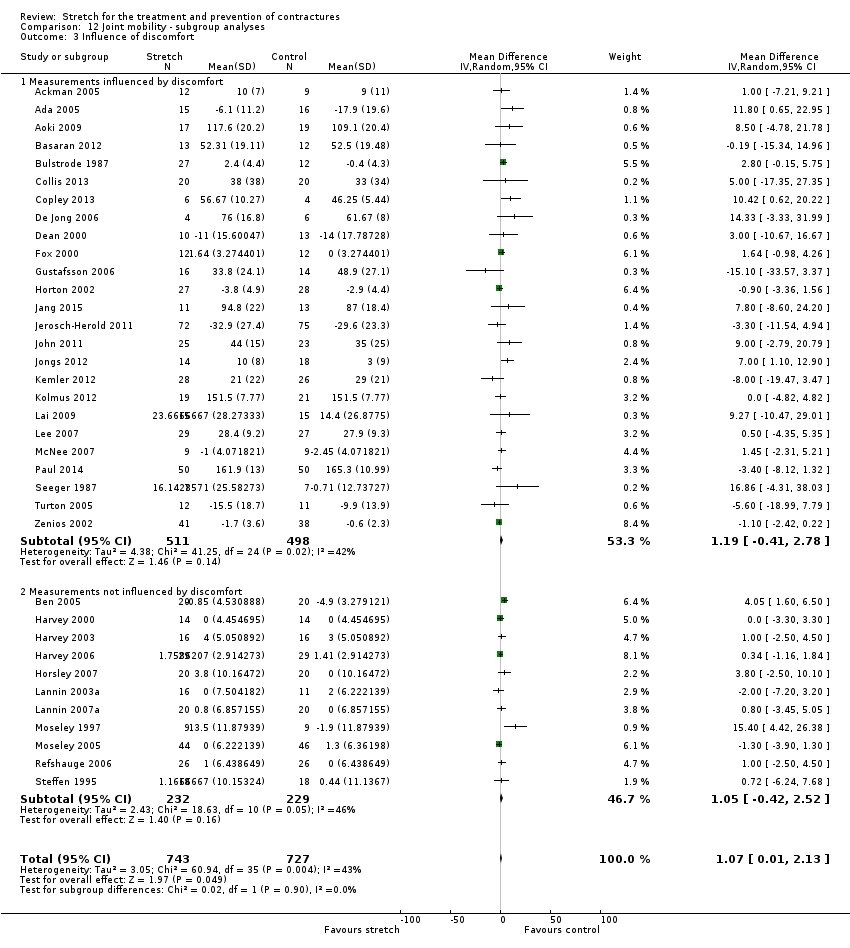
Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 3 Influence of discomfort.

Comparison 12 Joint mobility ‐ subgroup analyses, Outcome 4 Joint mobility measured less than one day versus more than one day.
| Short‐term effects of stretch for the treatment and prevention of contractures | ||||||
| Patient or population: people with neurological conditions1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments, summary statistics, NNTB and absolute risk difference (ARD) | |
| Assumed risk | Corresponding risk | |||||
| Control | Short‐term effects of stretch | |||||
| Joint mobility | Mean joint mobility in the control groups was 10°2 | The mean joint mobility in the intervention groups was 2° higher (0° to 3° higher) | 549 | ⊕⊕⊕⊕ | Absolute change = 1% better (0% to 2% better) Relative change = 2% better (0% to 3% better) | |
| Quality of life | No studies measured quality of life | Not estimable | Not estimable | Not estimable | Not measured | |
| Pain 10‐point VAS | The mean pain in the control group was 0.6 points on a 10‐point VAS4 | This translates to an absolute mean increase of 0.2 higher (‐0.1 to 0.6) points compared with control group on a 10‐point scale.5 | 174 | ⊕⊕⊝⊝ | SMD = 0.2 higher (0.1 lower to 0.5 higher) Absolute change = 2% worse (1% better to 6% worse) Relative change = 55% worse (28% better to 138% worse) | |
| Activity limitations 18‐point upper limb scale | The mean activity limitation in the control group was 0.9 points on an 18‐point upper limb scale7 | This translates to an absolute mean increase of 0.1 (‐0.1 to 0.3) points compared with control group on an 18‐point scale8 | 237 | ⊕⊕⊝⊝ | SMD = 0.2 higher (0.1 lower to 0.5 higher) Absolute change = 1% better (0% to 2% better) Relative change = 38% better (26% worse to 104% better) | |
| Participation restrictions | 1 study measured participation restrictions but it did not provide useable data | Not estimable | Not estimable | Not estimable | Not estimable | |
| Adverse events | Five studies involving 145 participants reported 8 adverse events that may have been related to the intervention. These included skin breakdown, bruising or blisters from plaster casts, and shoulder and wrist pain from stretches applied through positioning | Not estimable | Not estimable | Not estimable | Not estimable | |
| *The assumed risk (e.g. the mean control group risk across studies) is based on one representative study chosen on the basis of its size and susceptibility to bias. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All the studies included in this review and included in the 'Summary of findings' outcomes included people with the following neurological conditions: stroke, Charcot‐Marie‐Tooth disease, acquired brain injury, spinal cord injury and cerebral palsy. The treatment effects were consistent across all types of neurological conditions except acquired brain injury (see Discussion). 2 Post data of the control group in Refshauge 2006 (the corresponding data in Analysis 1.1 is not raw data). 3 The quality of evidence was not downgraded due to risk of bias even though at least some of the included trials had selection, performance, detection, attrition and reporting bias. These types of bias would tend to exaggerate treatment effectiveness. Given this review did not demonstrate treatment effectiveness these forms of bias are probably not important. 4 Post data of the control group in Horsley 2007 (the corresponding data in Analysis 4.1 is not post data). 5 Calculations based on the control group baseline mean (SD) pain: 0.4 (1.1) points on a 0‐10 scale (from Horsley 2007). 6 The quality of the evidence was downgraded due to indirectness and imprecision. The downgrading for indirectness was because the results are only based on studies involving people with stroke and spinal cord injury thereby limiting their generalisability. The downgrading for imprecision was because the 95% CI is wide, particularly when the results are expressed as a relative % change (the 95% CI is narrow when the results are expressed as an absolute risk difference). 7 Post data of the control group in Horsley 2007 (the corresponding data in Analysis 6.1 is not post data). 8 Calculations based on the control group baseline mean (standard deviation) activity limitation: 0.3 (0.6) points on an 18‐point Upper Limb Activity scale (from Horsley 2007). 9 The quality of the evidence was downgraded due to indirectness and imprecision. The downgrading for indirectness was because the results are only based on studies involving people with stroke, cerebral palsy and Charcot‐Marie‐Tooth disease thereby limiting their generalisability. The downgrading for imprecision was because the 95% CI was wide particularly when the results are expressed as a relative % change (the 95% CI is narrow when the results are expressed as an absolute risk difference). | ||||||
| Short‐term effects of stretch for the treatment and prevention of contractures | ||||||
| Patient or population: people with non‐neurological conditions1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative % change | No of Participants | Quality of the evidence | Comments, summary statistics and absolute risk difference | |
| Assumed risk | Corresponding risk | |||||
| Control | Short‐term effects of stretch | |||||
| Joint mobility Range of motion | The mean joint mobility in the control groups was 104°2 | This translates to an absolute mean increase of 1° higher (0° to 2° higher) compared with control group on a 90° scale3 | 865 | ⊕⊕⊕⊕ | SMD = 0.2 higher (0.0 to 0.3 higher) Absolute change = 1% better (0% to 2% better) Relative change = 1% better (0% to 2% better) | |
| Quality of life 160‐point Burn Specific Health Scale‐Brief questionnaire | The mean quality of life in the control group was 128 points on a 160‐point scale6 | This translates to an absolute mean increase of 3 (‐1 to 6) points compared with control group on a 160‐point scale7 | 97 | ⊕⊕⊕⊝ | SMD = 0.3 higher (0.1 lower to 0.7 higher) Absolute change = 2% better (1% worse to 4% better) Relative change = 2% better (1% worse to 5% better) | |
| Pain 10‐point VAS | The mean pain in the control group was 4 points on a 10‐point VAS10 | This translates to an absolute mean decrease of 0.2 (‐0.4 to 0.1) points compared with control group on an 10‐point scale11 | 422 | ⊕⊕⊕⊕ | SMD 0.2 lower (0.4 lower to 0.1 higher) Absolute change = 1% better (3% better to 1% worse) Relative change = 2% better (4% better to 1% worse) | |
| Activity limitations 100‐point Disabilities of the Arm, Shoulder and Hand questionnaire (lower score reflects better outcome) | The mean activity limitation in the control group was 7 points on a 100‐point upper limb scale12 | This translates to an absolute mean increase of 1.2 (‐2.2 to 4.5) points compared with control group on a 100‐point scale13 | 356 | ⊕⊕⊕⊕ | SMD = 0.1 higher (0.2 lower to 0.3 higher) Absolute change = 1% better (2% worse to 4% better) Relative change= 8% better (15% worse to 29% better) | |
| Participation restrictions 100 mm return to usual work activities VAS | The mean participant restriction in the control group was 39 points on a 100‐point VAS for return to work activities14 | This translates to an absolute mean decrease of 11 points (‐30 to 6) points compared with control group on a 100‐point scale15 | 129 | ⊕⊕⊝⊝ | SMD = 0.2 lower (0.6 lower to 0.1 higher) Absolute change = 12% worse (31% worse to 6% better) Relative change = 31% worse (79% worse to 17% better) | |
| Adverse events | Nine studies involving 635 participants reported 41 adverse events that may have been related to the intervention. These included transient numbness (n = 10), pain (n = 1), Raynauds’ phenomenon (n = 4), venous thrombosis (n = 1), need for manipulation under anaesthesia (n = 1), wound infections (n = 10), haematoma (n = 5), flexion deficits (n= 8) and swelling (n = 1). These were predominantly from splints | Not estimable | Not estimable | Not estimable | Not estimable | |
| *The assumed risk (e.g. the mean control group risk across studies) is based on one representative study chosen on the basis of its size and susceptibility to bias. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 All the studies included in this review and included in the 'Summary of Findings' outcomes included people with the following non‐neurological conditions: frail elderly and people with ankle fracture, anklylosing spondylitis, oral submucous fibrosis, post‐radiation therapy to the breast, post‐radiation therapy to jaw, progressive systemic sclerosis, total knee replacement, arthritis, Dupuytren's contractures, shoulder adhesive capsulitis/frozen shoulder, hallux limitus, wrist fracture and burns. An additional study included in this review but not included in the 'Summary of Findings' outcomes included people following anterior cruciate ligament reconstruction. The treatment effects were consistent across all types of non‐neurological conditions. 2 Post data of the control group in Moseley 2005 (the corresponding data in Analysis 1.2 is not post data). 3 Calculations based on the control group baseline mean (SD) range of motion: 98.4 (5.5) points on a 90‐degree range of motion measure (from Moseley 2005). 4 The quality of evidence was not downgraded due to risk of bias even though at least some of the included trials had selection, performance, detection, attrition and reporting bias. These types of bias would tend to exaggerate treatment effectiveness. Given this review did not demonstrate treatment effectiveness these forms of bias are probably not important. 5 The quality of the evidence was not downgraded due to indirectness because the results are based on studies involving people with many different types of underlying conditions (e.g. arthritis, frail elderly,ankle fractures). 6 Post data of the control group in Kolmus 2012 (see Analysis 3.1). 7 Calculations based on the control group post mean (SD) quality of life: 123 (9) on the 160‐point Burn Specific Health Scale Brief (no study provided baseline mean (SD) data for quality of life) (from Kolmus 2012). 8 The quality of the evidence was not downgraded due to imprecision because the point estimate is reasonably precise if expressed as relative % change and absolute risk difference. 9 The quality of the evidence was downgraded due to indirectness because the results are based on only two studies involving people with burns and post radiation therapy to the breast thereby limiting their generalisability. 10 Post data of the control group in Paul 2014 (see Analysis 4.1). 11 Calculations based on the control group baseline mean (SD) pain: 8.0 (0.8) on a 10‐point pain scale (from Paul 2014). 12 Post data of the control group in Jerosch‐Herold 2011 (see Analysis 6.2). 13 Calculations based on the control group baseline mean (SD) activity limitation: 15.4 (13.2) on a 100‐point scale (from Jerosch‐Herold 2011). 14 Post data of the control group in Moseley 2005 (see Analysis 8.1). 15 Calculations based on the control group baseline mean (SD) participation restriction: 39.0 (54.1) on a 100‐point scale (from Moseley 2005). 16 The quality of the evidence was downgraded due to indirectness because the results are based on only two studies involving people with ankle and wrist fracture thereby limiting their generalisability. | ||||||
| Joint mobility ‐ neurological conditions | Pooled results | Randomisation (studies with adequate sequence generation) | Allocation (studies with concealed allocation) | Assessors (studies with blinded assessors) | Dropout rate (studies with ≤ 15% dropouts) |
| Short‐term effects following stretch | 2 ° (0 to 3) n = 18 | 2 ° (0 to 3) n = 16 | 1 ° (0 to 3) n = 15 | 2 ° (0 to 3) | 2 ° (0 to 3) n = 13 |
| Long‐term effects following stretch | 1 ° (‐1 to 3) n = 8 | 1 ° (‐3 to 4) n = 6 | 0 ° (‐2 to 2) n = 5 | 1 ° (‐2 to 3) n = 6 | 0 ° (‐2 to 2) n = 6 |
| Results are presented in degrees; mean (95% CI). n = number of studies included in analysis | |||||
| Joint mobility ‐ non‐neurological conditions | Pooled results | Randomisation (studies with adequate sequence generation) | Allocation (studies with concealed allocation) | Assessors (studies with blinded assessors) | Dropout rate (studies with ≤ 15% dropouts) |
| Short‐term effects following stretch | 1° (‐1 to 2) n = 16 | 1° (‐1 to 3) n = 9 | ‐1° (‐2 to 1) n = 8 | 1° (‐1 to 3) n = 12 | 0° (‐2 to 1) n = 10 |
| Long‐term effects following stretch | ‐1° (‐3 to 2) n = 5 | 0° (‐6 to 7) n = 3 | 1° (‐5 to 7) n = 3 | 0° (‐7 to 7) n = 3 | ‐1° (‐3 to 2) n = 5 |
| Results are presented in degrees; mean (95%CI). Studies in which data were no expressed in degrees were excluded from all analyses (Buchbinder 1993, Cox 2009 and Melegati 2003). n = number of studies included in analysis. | |||||
| Neurological conditions | Non‐neurological conditions | |||
| Short‐term | Long‐term | Short‐term | Long‐term | |
| Joint ROM | Ineffective1 – HIGH | Ineffective1 | Ineffective1 – HIGH | Ineffective1 |
| QOL | Not measured | Not measured | Ineffective2 – MOD | Not measured |
| Pain* | Uncertain ‐ LOW | Uncertain | Ineffective3 – HIGH | Uncertain |
| Spasticity* | Uncertain | Uncertain | Not relevant for people with non‐neurological conditions | Not relevant or people with non‐neurological conditions |
| Activity limitations | Uncertain – LOW | Uncertain | Uncertain ‐ HIGH | Uncertain |
| Participation restrictions | Not measured | Not measured | Uncertain ‐ LOW | Uncertain |
| * Negative value favours stretch Ineffective = the results rule out a clinically important treatment effect. The quality of the evidence for the short‐term effects was rated using GRADE and is indicated by high, moderate (mod) or low. GRADE was not used to rate the quality of evidence for the long‐term effects. 1 The results rule out a clinically important treatment effect of 5°. Results expressed as SMD were back converted to degrees (see summary of findings Table for the main comparison). 2 The results rule out a clinically important treatment effect equivalent to 10 points on a 160‐point scale, and an absolute change and relative change of 5% (see summary of findings Table 2). 3 The results rule out a clinically important treatment effect equivalent to 2 points on a 10‐point pain scale, and an absolute change and relative change of 5% (see summary of findings Table 2). 4 A meta‐analysis was not performed on the two studies because of clinical heterogeneity between studies (see Results). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 18 | 549 | Mean Difference (IV, Random, 95% CI) | 1.81 [0.45, 3.17] |
| 1.1 Stroke | 11 | 295 | Mean Difference (IV, Random, 95% CI) | 0.56 [‐1.56, 2.68] |
| 1.2 Charcot‐Marie‐Tooth disease | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 2.27 [0.16, 4.38] |
| 1.3 Acquired brain injury | 3 | 35 | Mean Difference (IV, Random, 95% CI) | 8.48 [0.60, 16.36] |
| 1.4 Spinal cord injury | 4 | 137 | Mean Difference (IV, Random, 95% CI) | 1.42 [‐0.54, 3.37] |
| 2 Non‐neurological conditions Show forest plot | 18 | 865 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.00, 0.33] |
| 2.1 Frail elderly | 2 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.28, 0.74] |
| 2.2 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.46, 0.35] |
| 2.3 Anklylosing spondylitis | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.07, 1.32] |
| 2.4 Oral submucous fibrosis | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | 0.83 [‐0.05, 1.72] |
| 2.5 Post‐radiation therapy to breast | 1 | 56 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.47, 0.58] |
| 2.6 Post‐radiation therapy to jaw | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 1.54 [0.25, 2.82] |
| 2.7 Progressive systemic sclerosis | 1 | 14 | Std. Mean Difference (IV, Random, 95% CI) | 0.78 [‐0.32, 1.88] |
| 2.8 Total knee replacement | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.72, 0.34] |
| 2.9 Arthritis | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.25, 1.07] |
| 2.10 Dupuytren's contractures | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.27, 0.45] |
| 2.11 Shoulder adhesive capsulitis/frozen shoulder | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.67, 0.11] |
| 2.12 Hallux limitus | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.14, 1.01] |
| 2.13 Wrist fracture | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.41, 0.90] |
| 2.14 Burns | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.35, 0.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 8 | 211 | Mean Difference (IV, Random, 95% CI) | 0.73 [‐1.37, 2.82] |
| 1.1 Stroke | 4 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐4.09, 3.44] |
| 1.2 Cerebral palsy | 2 | 39 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐2.05, 4.79] |
| 1.3 Spinal cord injury | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐3.05, 3.05] |
| 1.4 Acquired brain injury | 1 | 10 | Mean Difference (IV, Random, 95% CI) | 10.42 [0.62, 20.22] |
| 2 Non‐neurological conditions Show forest plot | 6 | 438 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 2.1 ACL reconstruction | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.54, 0.77] |
| 2.2 Ankle fracture | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.62, 0.21] |
| 2.3 Total knee replacement | 1 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.80, 0.09] |
| 2.4 Dupuytren's contracture | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.47, 0.09] |
| 2.5 Wrist fracture | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | 0.80 [0.07, 1.52] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐neurological conditions Show forest plot | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.09, 0.71] |
| 1.1 Post‐radiation therapy to breast | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.37, 0.67] |
| 1.2 Burns | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.55 [‐0.08, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 5 | 174 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.10, 0.50] |
| 1.1 Stroke | 4 | 135 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.03, 0.66] |
| 1.2 Spinal cord injury | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.81, 0.45] |
| 2 Non‐neurological conditions Show forest plot | 7 | 422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.43, 0.10] |
| 2.1 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.41, 0.41] |
| 2.2 Frail elderly | 1 | 24 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.10, 0.51] |
| 2.3 Post‐radiotherapy to breast | 1 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.63, 0.43] |
| 2.4 Arthritis | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.96, 0.35] |
| 2.5 Shoulder adhesive capsulitis/frozen shoulder | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.17, 0.78] |
| 2.6 Dupuytren's contracture | 1 | 54 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.62, 0.44] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 4 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.41, 0.47] |
| 1.1 Stroke | 4 | 132 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.41, 0.47] |
| 2 Non‐neurological conditions Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Ankle fracture | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Shoulder adhesive capsulitis | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 7 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.13, 0.52] |
| 1.1 Stroke | 5 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.09, 0.63] |
| 1.2 Cerebral palsy | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.21, 1.09] |
| 1.3 Charcot‐Marie‐Tooth disease | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.21, 0.24] |
| 2 Non‐neurological conditions Show forest plot | 5 | 356 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.17, 0.34] |
| 2.1 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.30, 0.51] |
| 2.2 Arthritis | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.20, 1.13] |
| 2.3 Dupuytren's contracture | 1 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.39, 0.25] |
| 2.4 Wrist fracture | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.97, 0.35] |
| 2.5 Burns | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.12, 1.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 6 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.11, 0.56] |
| 1.1 Stroke | 4 | 136 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.29, 0.58] |
| 1.2 Cerebral palsy | 2 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.17, 1.00] |
| 2 Non‐neurological conditions Show forest plot | 3 | 268 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.32, 0.15] |
| 2.1 Ankle fracture | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.48, 0.35] |
| 2.2 Dupuytren's contracture | 1 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 2.3 Wrist fracture | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.86, 0.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐neurological conditions Show forest plot | 2 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.57, 0.12] |
| 1.1 Ankle fracture | 1 | 93 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.72, 0.10] |
| 1.2 Wrist fracture | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.65, 0.65] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Non‐neurological conditions Show forest plot | 2 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.60, 0.29] |
| 1.1 Ankle fracture | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.41, 0.41] |
| 1.2 Wrist fracture | 1 | 32 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.20, 0.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 6 | 144 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.30, 0.36] |
| 1.1 Stroke | 5 | 134 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.29, 0.39] |
| 1.2 Acquired brain injury | 1 | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.55, 1.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Neurological conditions Show forest plot | 3 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.81, 0.13] |
| 1.1 Stroke | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.12, 0.11] |
| 1.2 Cerebral palsy | 1 | 21 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.73, 1.00] |
| 1.3 Traumatic brain injury | 1 | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐2.03, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Types of stretch intervention Show forest plot | 36 | 1470 | Mean Difference (IV, Random, 95% CI) | 1.07 [0.03, 2.10] |
| 1.1 Cast | 3 | 57 | Mean Difference (IV, Random, 95% CI) | 4.59 [‐2.60, 11.78] |
| 1.2 Splint | 17 | 787 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐1.02, 1.55] |
| 1.3 Self‐administered | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 3.07 [0.19, 5.94] |
| 1.4 Positioning | 7 | 165 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐2.73, 8.33] |
| 1.5 Other sustained passive stretch | 7 | 386 | Mean Difference (IV, Random, 95% CI) | 0.77 [‐1.07, 2.61] |
| 2 Large versus small joints Show forest plot | 36 | 1467 | Mean Difference (IV, Random, 95% CI) | 1.03 [‐0.02, 2.09] |
| 2.1 Large joints | 16 | 645 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐0.89, 2.03] |
| 2.2 Small joints | 20 | 822 | Mean Difference (IV, Random, 95% CI) | 1.44 [‐0.11, 3.00] |
| 3 Influence of discomfort Show forest plot | 36 | 1470 | Mean Difference (IV, Random, 95% CI) | 1.07 [0.01, 2.13] |
| 3.1 Measurements influenced by discomfort | 25 | 1009 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐0.41, 2.78] |
| 3.2 Measurements not influenced by discomfort | 11 | 461 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐0.42, 2.52] |
| 4 Joint mobility measured less than one day versus more than one day Show forest plot | 34 | 1400 | Mean Difference (IV, Fixed, 95% CI) | 1.17 [0.50, 1.85] |
| 4.1 Less than one day | 28 | 1155 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.20, 2.00] |
| 4.2 More than one day | 7 | 245 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [0.24, 2.28] |

