Fármacos que liberan óxido nítrico para la maduración cervical en el aborto quirúrgico del primer trimestre
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ir a:
| Methods | Randomisation: computer‐generated randomisation | |
| Participants | 60 women, 30 in each group Inclusion: first trimester of pregnancy, no evidence of cervical change, no history of hypotension, and ultrasound evidence of a gestational sac and a nonviable embryo Exclusion: no information provided | |
| Interventions | Endocervical Group 1: isosorbide dinitrate 80 mg/1.5 ml gel solution Group 2: misoprostol 400 μg/1.5 ml gel solution Every 3 hours until reaching cervical dilation > 8 mm (maximum 4 doses) | |
| Outcomes | Probability of reaching cervical dilation > 8 mm at 3, 6, 9, 12 hours Side effects | |
| Notes | 3 cases in misoprostol group required hospital admission due to pain. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: randomisation table, nulliparous and multiparous women were randomised separately | |
| Participants | 200 women (100 nulliparous and 100 multiparous), 100 in each group Inclusion: primipara or multipara admitted for abortion at 8 to 12 weeks by suction evacuation Exclusion: previous uterine operation, allergy to sodium nitroprusside or misoprostol, chronic disease requiring medication, unable to understand the consent form, and age < 18 years | |
| Interventions | Group 1: vaginal misoprostol 400 μg and intracervical placebo gel Group 2: intracervical sodium nitroprusside gel 10 mg and vaginal vitamin B6 tablets 3 hours before the procedure | |
| Outcomes | Cumulative force required to dilate cervix from 4 mm to 9 mm Baseline cervical dilation Changes in blood pressure Duration of operation Operative blood loss Side effects | |
| Notes | The investigator who performed the operation supervised the nurse administering the drug. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: computer‐generated randomisation | |
| Participants | 36 women, 18 in each group Inclusion: first pregnancy, pregnancy dating between 9 and 12.5 weeks determined by last menstrual period and clinical examination, filling requirement of Italian Law number 194/78, and undergoing uterine evacuation under local anesthesia Exclusion: inability to understand the consent form, multiple pregnancy, previous uterine surgery, known allergy to drugs, and any chronic disease requiring medication | |
| Interventions | First series (18 women): intracervical Group 1: placebo Group 2: 1% nitroprusside gel (5 mg) Followed by uterine evacuation 6 hours after treatment Second series (18 women): intracervical Group 1: placebo Group 2: 2% nitroprusside gel (10 mg) Followed by uterine evacuation 3 hours after treatment | |
| Outcomes | Cumulative force required to dilate cervix from 4 mm to 8 mm Blood pressure change | |
| Notes | The calculated sample size to reach statistical significance was 36. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ unclear |
| Methods | Randomisation: random number table | |
| Participants | 66 women, 22 in each group Inclusion: primigravid women undergoing surgical termination by vacuum extraction before 12 weeks of gestation Exclusion: previous cervical surgery, threatened miscarriage | |
| Interventions | Intravaginal Group 1: isosorbide mononitrate 40 mg Group 2: misoprostol 400 μg Group 3: isosorbide mononitrate 40 mg and misoprostol 400 μg 3 hours before surgery | |
| Outcomes | Cumulative force required to dilate cervix to 8 mm Intraoperative blood loss Side effects | |
| Notes | The investigator who allocated the treatment administered the symptom questionnaires (the questions were read from a script and the women were asked to respond "Yes" or "No" to the questions). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: computer‐generated randomisation; nulliparous and multiparous women were randomised separately | |
| Participants | 126 women (63 nulliparous and 63 multiparous), 42 in each group Inclusion: healthy women requesting termination of pregnancy between 9 and 12 weeks gestation Exclusion: no information provided | |
| Interventions | Intravaginal moistened drugs 4 to 6 hours before suction evacuation Group 1: placebo Group 2: isosorbide mononitrate 40 mg Group 3: misoprostol 400 μg | |
| Outcomes | Baseline cervical dilatation Cumulative force for cervical dilation up to 8 mm Side effects Patient satisfaction | |
| Notes | Significantly more smokers in the misoprostol group The author did not state the characteristics of placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: computer‐generated randomisation | |
| Participants | 48 women, 24 in each group Inclusion criteria: nonviable fetus of less than 12 weeks of gestation, undergoing suction evacuation under general anesthesia, and no history of hypotension Exclusion criteria: previous cervical surgery, inevitable abortion, incomplete abortion, underlying medical diseases, allergy to isosorbide mononitrate, and unwilling to participate | |
| Interventions | Intravaginal Group I: isosorbide mononitrate 20 mg Group II: placebo 4 hours before suction evacuation | |
| Outcomes | Cervical width prior to suction evacuation Complications of the procedure Patient satisfaction Adverse effects of the drugs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: method of generation of the random allocation sequence: unclear | |
| Participants | 148 women scheduled for surgical termination of pregnancy 76 for assessment of cervical ripening and side effects, 72 for assessment of side effects only Inclusion: healthy women with a viable singleton pregnancy and gestational age less than 12 weeks, assessed by transvaginal ultrasonography Exclusion: previous cervical surgery, ongoing vaginal bleeding, uterine‐related pain, and known allergy to either isosorbide mononitrate or misoprostol | |
| Interventions | The women self‐administered intravaginally Group 1: isosorbide mononitrate 40 mg Group 2: misoprostol 200 μg 9 to 13 hours before surgery | |
| Outcomes | Baseline cervical dilatation Cumulative force to dilate the cervix to 9 mm Side effects | |
| Notes | The calculated sample size to reach statistical significance was 30 in each group for cervical ripening and 58 in each group for side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: random‐number table | |
| Participants | 48 women, 12 in each group Inclusion: primigravid women referred for surgical termination of pregnancy by vacuum aspiration before 12 weeks of gestation Exclusion: no information provided | |
| Interventions | Intravaginal Group 1: isosorbide mononitrate 40 mg Group 2: glyceryl trinitrate 500 μg Group 3: gemeprost 1 mg Group 4: no treatment (vaginal examination only) 3 hours before surgery | |
| Outcomes | Cumulative force to dilate the cervix to 8 mm Cervical diameter before surgical dilatation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ adequate |
| Methods | Randomisation: random‐number table | |
| Participants | 66 women, 22 in each group Inclusion: primigravid women scheduled for vacuum aspiration in the first trimester Exclusion: any previous pregnancy, previous cervical surgery, signs of threatened miscarriage, or concurrent maternal disease | |
| Interventions | Intravaginal Group 1: gemeprost 1 mg Group 2: isosorbide mononitrate 40 mg Group 3: isosorbide mononitrate 80 mg 3 hours before surgery | |
| Outcomes | Onset of new symptoms before abortion Cervical diameter before surgical dilatation Cumulative force to dilate the cervix to 8 mm | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ unclear |
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| No information about randomisation, allocation concealment, or blinding provided. Number of participants described in the article's results section was different from number cited in the table. We could not contact the author. | |
| Brief communication. No information about randomisation, allocation concealment, or blinding provided. The results were published without details of the outcomes. We could not contact the author. | |
| No information about randomisation, allocation concealment, or blinding provided. Incomplete data in the published results. We could not contact the author. | |
| No information about randomisation, allocation concealment, or blinding provided. We could not contact the author. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Baseline cervical dilatation before the procedure Show forest plot | 3 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.01, 0.58] |
| Analysis 1.1  Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 1 Baseline cervical dilatation before the procedure. | ||||
| 2 Cumulative force required to dilate cervix to 8 mm Show forest plot | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐4.29 [‐9.92, 1.35] |
| Analysis 1.2  Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 2 Cumulative force required to dilate cervix to 8 mm. | ||||
| 3 Side effects: Headache Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.86, 3.46] |
| Analysis 1.3  Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 3 Side effects: Headache. | ||||
| 4 Side effect: Abdominal pain Show forest plot | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.50] |
| Analysis 1.4  Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 4 Side effect: Abdominal pain. | ||||
| 5 Side effect: Nausea/vomiting Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.07, 6.45] |
| Analysis 1.5  Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 5 Side effect: Nausea/vomiting. | ||||
| 6 Patient satisfaction Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| Analysis 1.6  Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 6 Patient satisfaction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative force required to dilate cervix to 8‐9 mm Show forest plot | 5 | 429 | Mean Difference (IV, Fixed, 95% CI) | 13.12 [9.72, 16.52] |
| Analysis 2.1  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 1 Cumulative force required to dilate cervix to 8‐9 mm. | ||||
| 2 Baseline cervical dilatation before the procedure Show forest plot | 4 | 386 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.01, ‐0.45] |
| Analysis 2.2  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 2 Baseline cervical dilatation before the procedure. | ||||
| 3 Probability of reaching cervical ripening > 8 mm at 3 hours Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.21, 20.09] |
| Analysis 2.3  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 3 Probability of reaching cervical ripening > 8 mm at 3 hours. | ||||
| 4 Side effect: Headache Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [3.29, 8.00] |
| Analysis 2.4  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 4 Side effect: Headache. | ||||
| 5 Side effect: Palpitation Show forest plot | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.64, 7.15] |
| Analysis 2.5  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 5 Side effect: Palpitation. | ||||
| 6 Side effect: Dizziness Show forest plot | 3 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.46, 7.41] |
| Analysis 2.6  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 6 Side effect: Dizziness. | ||||
| 7 Side effect: Abdominal pain Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.25, 0.44] |
| Analysis 2.7  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 7 Side effect: Abdominal pain. | ||||
| 8 Side effect: Vaginal bleeding Show forest plot | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.07, 0.27] |
| Analysis 2.8  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 8 Side effect: Vaginal bleeding. | ||||
| 9 Side effect: Nausea/Vomiting Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.46] |
| Analysis 2.9  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 9 Side effect: Nausea/Vomiting. | ||||
| 10 Patient satisfaction Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.28] |
| Analysis 2.10  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 10 Patient satisfaction. | ||||
| 11 Intraoperative blood loss Show forest plot | 4 | 393 | Mean Difference (IV, Fixed, 95% CI) | 33.59 [24.50, 42.67] |
| Analysis 2.11  Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 11 Intraoperative blood loss. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative force required to dilate cervix to 8 mm Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 14.5 [0.50, 28.50] |
| Analysis 3.1  Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 1 Cumulative force required to dilate cervix to 8 mm. | ||||
| 2 Side effect: Headache Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.38, 2.00] |
| Analysis 3.2  Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 2 Side effect: Headache. | ||||
| 3 Side effect: Abdominal pain Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.07] |
| Analysis 3.3  Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 3 Side effect: Abdominal pain. | ||||
| 4 Intraoperative blood loss Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐50.0 [‐164.19, 64.19] |
| Analysis 3.4  Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 4 Intraoperative blood loss. | ||||

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.1 Baseline cervical dilatation before the procedure.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.2 Cumulative force required to dilate cervix to 8 mm.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.3 Side effect: Headache.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.4 Side effect: Abdominal pain.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.6 Patient satisfaction.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.5 Side effect: Nausea/vomiting.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.1 Cumulative force required to dilate cervix to 8‐9 mm.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.2 Baseline cervical dilatation before the procedure.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.11 Probability of reaching cervical ripening > 8 mm at 3 hours.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.3 Side effect: Headache.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.6 Side effect: Palpitation.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.7 Side effect: Dizziness.
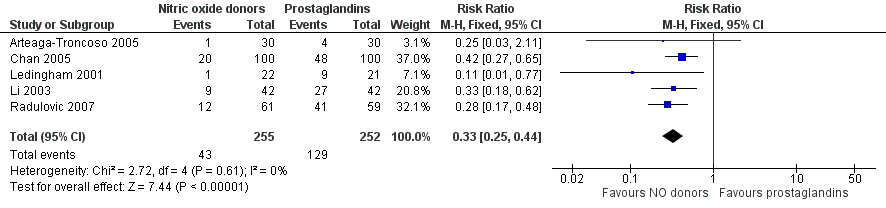
Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.4 Side effect: Abdominal pain.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.5 Side effect: Vaginal bleeding.
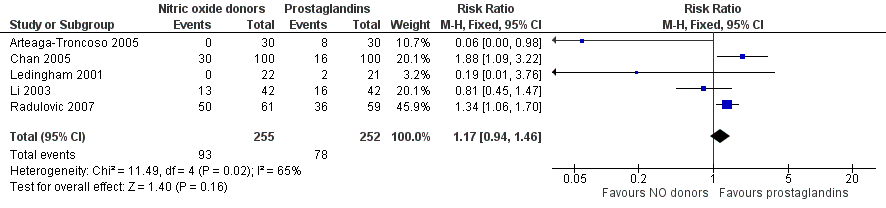
Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.8 Side effect: Nausea/Vomiting.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.10 Patient satisfaction.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.9 Intraoperative blood loss.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.1 Cumulative force required to dilate cervix to 8 mm.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.2 Side effect: Headache.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.3 Side effect: Abdominal pain.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.4 Intraoperative blood loss.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 1 Baseline cervical dilatation before the procedure.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 2 Cumulative force required to dilate cervix to 8 mm.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 3 Side effects: Headache.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 4 Side effect: Abdominal pain.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 5 Side effect: Nausea/vomiting.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 6 Patient satisfaction.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 1 Cumulative force required to dilate cervix to 8‐9 mm.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 2 Baseline cervical dilatation before the procedure.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 3 Probability of reaching cervical ripening > 8 mm at 3 hours.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 4 Side effect: Headache.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 5 Side effect: Palpitation.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 6 Side effect: Dizziness.
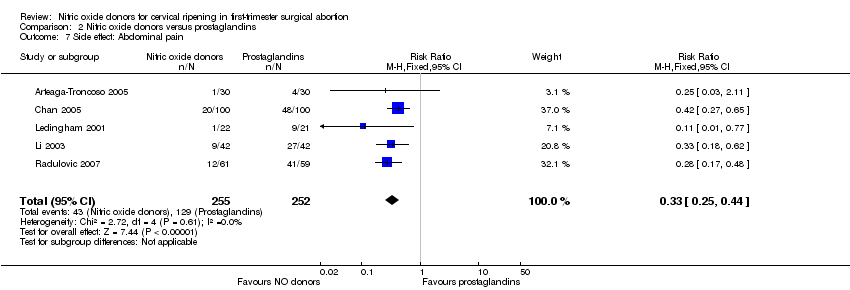
Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 7 Side effect: Abdominal pain.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 8 Side effect: Vaginal bleeding.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 9 Side effect: Nausea/Vomiting.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 10 Patient satisfaction.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 11 Intraoperative blood loss.

Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 1 Cumulative force required to dilate cervix to 8 mm.
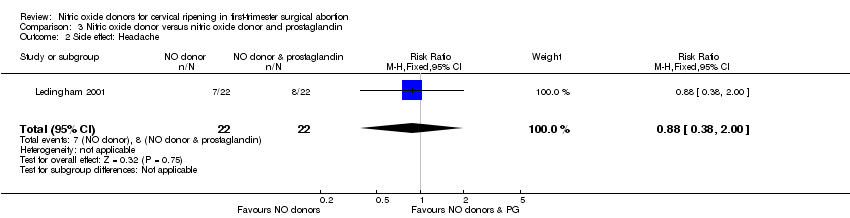
Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 2 Side effect: Headache.

Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 3 Side effect: Abdominal pain.

Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 4 Intraoperative blood loss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Baseline cervical dilatation before the procedure Show forest plot | 3 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.01, 0.58] |
| 2 Cumulative force required to dilate cervix to 8 mm Show forest plot | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐4.29 [‐9.92, 1.35] |
| 3 Side effects: Headache Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.86, 3.46] |
| 4 Side effect: Abdominal pain Show forest plot | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.50] |
| 5 Side effect: Nausea/vomiting Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.07, 6.45] |
| 6 Patient satisfaction Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative force required to dilate cervix to 8‐9 mm Show forest plot | 5 | 429 | Mean Difference (IV, Fixed, 95% CI) | 13.12 [9.72, 16.52] |
| 2 Baseline cervical dilatation before the procedure Show forest plot | 4 | 386 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.01, ‐0.45] |
| 3 Probability of reaching cervical ripening > 8 mm at 3 hours Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.21, 20.09] |
| 4 Side effect: Headache Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [3.29, 8.00] |
| 5 Side effect: Palpitation Show forest plot | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.64, 7.15] |
| 6 Side effect: Dizziness Show forest plot | 3 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.46, 7.41] |
| 7 Side effect: Abdominal pain Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.25, 0.44] |
| 8 Side effect: Vaginal bleeding Show forest plot | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.07, 0.27] |
| 9 Side effect: Nausea/Vomiting Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.46] |
| 10 Patient satisfaction Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.28] |
| 11 Intraoperative blood loss Show forest plot | 4 | 393 | Mean Difference (IV, Fixed, 95% CI) | 33.59 [24.50, 42.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative force required to dilate cervix to 8 mm Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 14.5 [0.50, 28.50] |
| 2 Side effect: Headache Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.38, 2.00] |
| 3 Side effect: Abdominal pain Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.07] |
| 4 Intraoperative blood loss Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐50.0 [‐164.19, 64.19] |

