Fármacos que liberan óxido nítrico para la maduración cervical en el aborto quirúrgico del primer trimestre
Resumen
Antecedentes
En ciertos grupos de mujeres, se recomienda la preparación cervical antes del aborto quirúrgico del primer trimestre. Los fármacos que liberan óxido nítrico (ON) inducen la maduración cervical sin contracciones uterinas, pero existe preocupación sobre la eficacia y los efectos secundarios.
Objetivos
Evaluar los fármacos que liberan ON para la maduración cervical antes del aborto quirúrgico del primer trimestre en cuanto a eficacia, efectos secundarios y reducción de las complicaciones.
Métodos de búsqueda
Se realizaron búsquedas en el Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL), MEDLINE, EMBASE y POPLINE. También se hicieron búsquedas en las listas de referencias de los artículos recuperados. Se estableció contacto con expertos en el área para obtener información sobre ensayos publicados y no publicados.
Criterios de selección
Ensayos controlados aleatorizados que compararon fármacos que liberan ON solos o en combinación con otros métodos para la maduración cervical en el aborto quirúrgico del primer trimestre.
Obtención y análisis de los datos
Dos autores de la revisión de forma independiente seleccionaron y extrajeron los datos en un formulario de extracción de datos. Los datos se procesaron mediante el programa informático Review Manager (RevMan 5).
Resultados principales
Se incluyeron nueve estudios con 766 participantes. En los ensayos incluidos no hubo complicaciones graves (infección que requirió tratamiento con antibióticos, transfusión de sangre, complicaciones que requirieron una operación no programada, lesión cervical, perforación uterina, muerte o morbilidad grave).
Los fármacos que liberan ON fueron más efectivos para la maduración cervical en comparación con placebo o ningún tratamiento. La dilatación cervical inicial antes del procedimiento fue mayor en el grupo de donantes de ON (diferencia de medias [DM] 0,30; intervalo de confianza [IC] del 95%: 0,01 a 0,58). La fuerza acumulativa necesaria para dilatar el cuello del útero hasta 8 mm (DM ‐4,29; IC del 95%: ‐9,92 a 1,35), la cefalea (riesgo relativo [RR] 1,73; IC del 95%: 0,86 a 3,46), el dolor abdominal (RR 0,87; IC del 95%: 0,50 a 1,50), o la satisfacción de la paciente (RR 0,95; IC del 95%: 0,84 a 1,07) no fueron diferentes. Ocurrieron más náuseas y vómitos en las mujeres que recibieron un fármaco que libera ON (RR 2,62; IC del 95%: 1,07 a 6,45).
Los fármacos que liberan ON fueron inferiores a las prostaglandinas en cuanto a la maduración cervical. La fuerza acumulativa necesaria para dilatar el cuello del útero hasta 8 mm a 9 mm fue mayor (DM 13,12; IC del 95%: 9,72 a 16,52), y la dilatación cervical inicial fue menor (DM ‐0,73; IC del 95%: ‐1,01 a ‐0,45) en el grupo de donantes de ON. La probabilidad de dilatación mayor de 8 mm a las tres horas fue significativamente mayor en el grupo de fármacos que liberan ON (RR 6,67; IC del 95%: 2,21 a 20,09). Los efectos secundarios que incluyeron cefalea (RR 5,13; IC del 95%: 3,29 a 8,00), palpitaciones (RR 3,43; IC del 95%: 1,64 a 7,15), mareos (RR 3,29; IC del 95%: 1,46 a 7,41) y pérdida de sangre intraoperatoria (DM 33,59 ml; IC del 95%: 24,50 a 42,67) también fueron mayores. Sin embargo, el dolor abdominal (RR 0,33; IC del 95%: 0,25 a 0,44) y la hemorragia vaginal (RR 0,14; IC del 95%: 0,07 a 0,27) fueron menores en el grupo de donantes de ON. No hay diferencias en cuanto a las náuseas/vómitos en ambos grupos (RR 1,17; IC del 95%: 0,94 a 1,46). La satisfacción de la paciente no fue diferente.
Un ensayo comparó un fármaco que libera ON con un fármaco que libera ON más prostaglandina. La fuerza acumulativa necesaria para dilatar el cuello uterino hasta 8 mm fue mayor (DM 14,50; IC del 95%: 0,50 a 28,50) en el grupo de donantes de ON. No hubo diferencias en la cefalea (RR 0,88; IC del 95%: 0,38 a 2,00), el dolor abdominal (RR 0,14; IC del 95%: 0,02 a 1,07), o la pérdida de sangre intraoperatoria (DM ‐50; IC del 95%: ‐164,19 a 64,19).
Conclusiones de los autores
Los fármacos que liberan ON son superiores a placebo y a ningún tratamiento, pero son inferiores a las prostaglandinas para la maduración cervical en el primer trimestre y se asocian con más efectos secundarios.
PICO
Resumen en términos sencillos
Preparación del cuello del útero con fármacos que liberan óxido nítrico antes del aborto quirúrgico en los tres primeros meses de embarazo
Las posibles complicaciones del aborto quirúrgico en los tres primeros meses del embarazo incluyen lesión del cuello del útero (el cuello de la matriz) y de la propia matriz. La preparación del cuello del útero antes de la cirugía podría hacer el procedimiento más seguro y más fácil. Se utilizan varios métodos para preparar el cuello uterino. Esta revisión comparó los fármacos conocidos como donantes de óxido nítrico (ON) con otros fármacos.
Se realizó una búsqueda electrónica de ensayos aleatorizados de fármacos que liberan ON utilizados antes del aborto quirúrgico. Encontramos que los donantes de ON son mejores que placebo (una píldora de azúcar). Las prostaglandinas son mejores que los donantes de ON para preparar el cuello del útero.
Authors' conclusions
Background
Cervical priming before surgical abortion is beneficial, particularly in women with cervical anomalies or previous surgery, young women, and as gestational age advances. These groups of women have a higher chance of cervical injury and uterine perforation due to forceful dilation or misdirected dilators during surgical abortion (Schulz 1983; Grimes 1984). The incidence of cervical injury ranges from 0.1 to 10 per 1000 (Schulz 1983; Hakim‐Elahi 1990; Zhou 2002).
The Society of Family Planning recommends cervical preparation prior to surgical abortion in the first trimester only for women who may be at increased risk of complications, including those late in the first trimester, adolescents, and women in whom cervical dilation is expected to be difficult due to either patient factors or provider experience (Allen 2007). The Royal College of Obstetricians and Gynaecologists also recommends that cervical preparation prior to surgical abortion should be considered in all cases (RCOG 2011).
The agents commonly used for cervical ripening in the first trimester are prostaglandins (Ekerhovd 2003; Sharma 2005). Prostaglandins prime the cervix but also induce uterine contractions, which may result in bleeding, pain, and incomplete abortion. The ideal cervical priming agent should be easy to use and noninvasive; it should also increase softening, distensibility, and effacement of the cervix within 24 hours without inducing uterine contractions (Hayashi 1993).
The expression of inducible nitric oxide synthase (iNOS) isoforms in the human cervix increases at the end of pregnancy, suggesting that nitric oxide may be involved in the process of cervical ripening (Tschugguel 1999; Ledingham 2000). Vaginal application of a NO donor before first‐trimester abortion has been reported to reduce the cumulative force required to dilate the cervix compared with controls (Thomson 1997). Other reports have suggested that NO donors may be clinically useful for cervical ripening, since softening and effacement may occur without uterine contractions.
This review evaluated the efficacy and side effects of NO donors as a cervical priming agent prior to first‐trimester surgical abortion.
Objectives
To evaluate NO donors for cervical ripening before first‐trimester surgical abortion, in terms of efficacy, side effects, and reduction of complications.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Women undergoing surgical abortion in the first trimester of pregnancy, defined as less than 14 weeks’ gestation.
Types of interventions
NO donors alone or in combination with other methods for cervical ripening in first‐trimester surgical abortion. Four types of NO donors were used: isosorbide mononitrate, isosorbide dinitrate, glyceryl trinitrate, and sodium nitroprusside. Isosorbide mononitrate was the NO donor used most (Thomson 1997; Thomson 1998; Ledingham 2001; Li 2003; Radulovic 2007; Phusaanantakul 2010). All types of NO donors were analysed together. Two types of prostaglandins were used: gemeprost and misoprostol. Both were analysed together.
Types of outcome measures
Primary outcomes
-
Complications (infection requiring antibiotic treatment, need for blood transfusion, any complication requiring an unintended operation, cervical injury, uterine perforation, maternal death, or serious maternal morbidity).
-
Cervical changes: dilatation, length, and cervical resistance.
Secondary outcomes
-
Side effects of drugs
-
Patient satisfaction
Search methods for identification of studies
The search strategy for this review included electronic search using the following key words: (abortion OR “pregnancy termination” OR “termination of pregnancy” OR “induced abortion” OR “therapeutic abortion”) AND “first trimester” AND (“nitric oxide donors” OR “sodiumnitroprusside” OR “isosorbide mononitrate” OR nitroglycerin OR “isosorbide dinitrate”) from
1. The Cochrane Central Register of Controlled Trials (to 16 October 2014)
2. MEDLINE (from 1966 to October 2014)
3. EMBASE (from 1986 to October 2014)
4. POPLINE (from 1964 to October 2014)
We searched reference lists of all retrieved articles. We contacted experts in the field for information on other published or unpublished trials. We imposed no language limitation in the review.
Data collection and analysis
We assessed all potential studies identified as a result of the search strategy for inclusion.
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed trials that met the eligibility criteria for quality using the following criteria:
-
generation of random allocation sequence: adequate, inadequate, unclear;
-
allocation concealment: A = adequate, B = unclear, C = inadequate, D = not used;
-
blinding of participants: yes, no, inadequate, no information;
-
blinding of caregivers: yes, no, inadequate, no information;
-
blinding of outcome assessment: yes, no, inadequate, no information;
-
completeness of follow‐up data (including any differential loss of participants from each group):
-
fewer than 5% of participants excluded;
-
5% to 9.9% of participants excluded;
-
10% to 19.9% of participants excluded;
-
20% or more excluded; and
-
unclear.
-
We excluded trials most susceptible to bias based on the following quality assessment:
those with inadequate allocation concealment (C or D) or high levels of postrandomisation losses or exclusions (d).
Two review authors (Patama Promsonthi and Anyarin Preechapornprasert) independently extracted the data onto a data extraction form. We resolved cases of discrepancy between review authors in either the decision to include or exclude studies or in data extraction by consensus.
We processed data using Review Manager software (RevMan 5).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
We included 9 trials with a total of 766 participants in this review. The included trials compared three different interventions: NO donor versus placebo or no treatment; NO donors versus prostaglandins; and NO donors versus NO donor and prostaglandins. No trial compared NO donor with mifepristone.
Risk of bias in included studies
Seven trials received concealment allocation score A: Thomson 1997; Ledingham 2001; Li 2003; Arteaga‐Troncoso 2005; Chan 2005; Radulovic 2007; Phusaanantakul 2010. Two studies received allocation concealment score B: Thomson 1998; Facchinetti 2000.
Thomson 1998 studied adverse symptoms as a primary outcome, but the investigator who administered the symptom questionnaire was the one who allocated the treatment (not blinded to the allocated treatment). To prevent detection bias, we did not analyse the side effects from this trial. However, the surgeons were blinded to the treatment given. Hence, we included only cervical change from this trial for our analysis.
Facchinetti 2000 calculated the sample size of 36 to reach statistical significance. Thirty‐six women were recruited, but three of them dropped out (one in the placebo group and two in the nitroprusside group). The preparation of placebo and nitroprusside gel were similar in terms of syringe, consistency, and volume, but the active gel was brown and the placebo gel was white. However, the surgeon was unable to see the gel colour.
Ledingham 2001 allocated the treatment and administered the symptom questionnaire. However, the questions were read from a script and the women were asked to respond only "Yes" or "No" to the questions.
Li 2003 did not mention the characteristics of the placebo, and there were significantly more smokers in the misoprostol group.
Chan 2005 performed the operation and supervised the research nurse who administered the drug. However, the surgeons were blinded to the treatment given.
Radulovic 2007 included 76 women for assessment of both cervical ripening and side effects. The calculated sample size to reach statistical significance was 60 for cervical ripening. Sixteen women (21%) did not receive the allocated intervention or were excluded from the analysis. Because of high levels of postrandomisation losses (greater than 20%), we did not include the data for assessment of cervical ripening from this trial in our analysis. The calculated sample size to reach statistical significance was 116 for the assessment of side effects; Radulovic 2007 included a total of 148 women and 28 (18.9%) dropped out. We included this trial for the assessment of side effects only.
Effects of interventions
Three trials compared NO donors with placebo (Facchinetti 2000; Li 2003; Phusaanantakul 2010), and one trial compared NO donor with no treatment (Thomson 1997). A total of 201 participants were included for analysis. Baseline cervical dilatation before the procedure was significantly higher in NO donors group (MD 0.30, 95% CI 0.01 to 0.58) (Analysis 1.1; Figure 1). There was no statistically significant difference in the following outcomes: cumulative force required to dilate cervix to 8 mm (MD ‐4.29, 95% CI ‐9.92 to 1.35) (Analysis 1.2; Figure 2); headache (RR 1.73, 95% CI 0.86 to 3.46) (Analysis 1.3; Figure 3); abdominal pain (RR 0.87, 95% CI 0.50 to 1.50) (Analysis 1.4; Figure 4); or patient satisfaction (RR 0.95, 95% CI 0.84 to 1.07) (Analysis 1.6; Figure 5). There was significantly more nausea and vomiting in the women receiving NO donors compared to placebo or no treatment (RR 2.62, 95% CI 1.07 to 6.45) (Analysis 1.5; Figure 6).

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.1 Baseline cervical dilatation before the procedure.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.2 Cumulative force required to dilate cervix to 8 mm.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.3 Side effect: Headache.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.4 Side effect: Abdominal pain.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.6 Patient satisfaction.
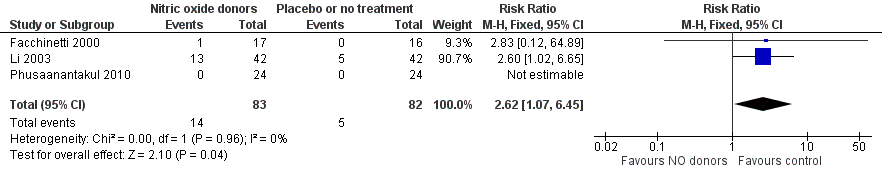
Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.5 Side effect: Nausea/vomiting.
Seven trials compared NO donors with prostaglandins (Thomson 1997; Thomson 1998; Ledingham 2001; Li 2003; Arteaga‐Troncoso 2005; Chan 2005; Radulovic 2007). Most trials evaluated cervical change by the cumulative force required to dilate the cervix to 8 mm to 9 mm diameter or baseline cervical dilatation before the procedure. Only one trial used probability of dilation greater than 8 mm at 3, 6, 9, and 12 hours (Arteaga‐Troncoso 2005). We only analysed cervical change at three hours, which was the time interval used in most studies.
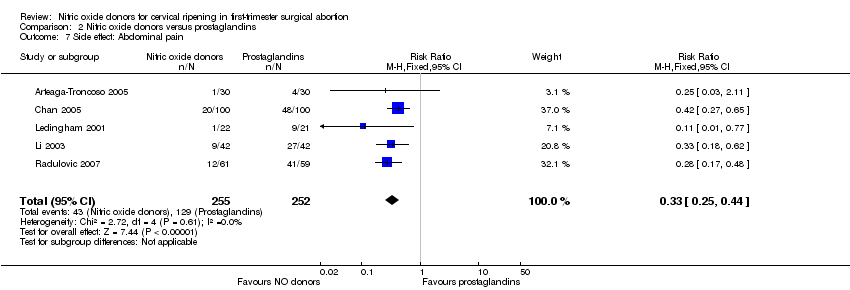
NO donors were inferior to prostaglandins for cervical ripening. The cumulative force required to dilate the cervix to 8 mm to 9 mm was significantly higher (MD 13.12, 95% CI 9.72 to 16.52) (Analysis 2.1; Figure 7), and baseline cervical dilatation was significantly less (MD ‐0.73, 95% CI ‐1.01 to ‐0.45) (Analysis 2.2; Figure 8) in the NO donor group. The probability of dilation greater than 8 mm at three hours was significantly higher in the NO donor group (RR 6.67, 95% CI 2.21 to 20.09) (Analysis 2.3; Figure 9). NO donors caused significantly more side effects, including headache (RR 5.13, 95% CI 3.29 to 8.00) (Analysis 2.4; Figure 10), palpitation (RR 3.43, 95% CI 1.64 to 7.15) (Analysis 2.5; Figure 11), and dizziness (RR 3.29, 95% CI 1.46 to 7.41) (Analysis 2.6; Figure 12). However, NO donors caused less abdominal pain (RR 0.33, 95% CI 0.25 to 0.44) (Analysis 2.7; Figure 13) and vaginal bleeding (RR 0.14, 95% CI 0.07 to 0.27) (Analysis 2.8; Figure 14). There was no statistically significant difference for nausea/vomiting (RR 1.17, 95% CI 0.94 to 1.46) (Analysis 2.9; Figure 15) or patient satisfaction (RR 1.09, 95% CI 0.92 to 1.28) (Analysis 2.10; Figure 16). NO donors were associated with significantly more intraoperative bleeding than prostaglandins (MD 33.59, 95% CI 24.50 to 42.67) (Analysis 2.11; Figure 17).

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.1 Cumulative force required to dilate cervix to 8‐9 mm.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.2 Baseline cervical dilatation before the procedure.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.11 Probability of reaching cervical ripening > 8 mm at 3 hours.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.3 Side effect: Headache.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.6 Side effect: Palpitation.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.7 Side effect: Dizziness.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.4 Side effect: Abdominal pain.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.5 Side effect: Vaginal bleeding.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.8 Side effect: Nausea/Vomiting.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.10 Patient satisfaction.
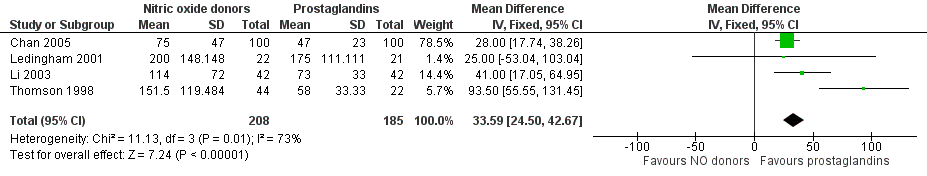
Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.9 Intraoperative blood loss.
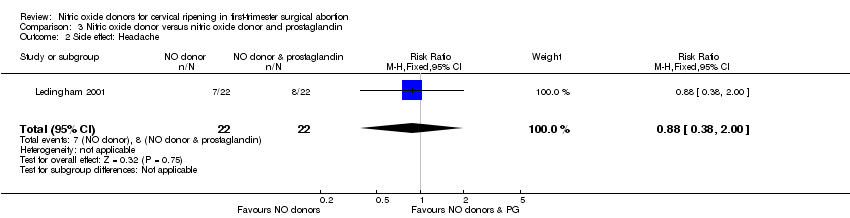
One small trial compared a NO donor versus a NO donor plus prostaglandin (Ledingham 2001). The cumulative force required to dilate the cervix to 8 mm diameter was higher (MD 14.50, 95% CI 0.50 to 28.50) (Analysis 3.1; Figure 18) in those who received the NO donor alone. No important differences were seen in headache (RR 0.88, 95% CI 0.38 to 2.00) (Analysis 3.2; Figure 19), abdominal pain (RR 0.14, 95% CI 0.02 to 1.07) (Analysis 3.3; Figure 20), or intraoperative blood loss (MD ‐50, 95% CI ‐164.19 to 64.19) (Analysis 3.4; Figure 21).

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.1 Cumulative force required to dilate cervix to 8 mm.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.2 Side effect: Headache.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.3 Side effect: Abdominal pain.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.4 Intraoperative blood loss.
No serious complications occurred in any trial. No incomplete abortion was reported in any included trial.
Discussion
The primary objective of cervical ripening in this setting is to reduce surgical complications. Serious complications in surgical termination of pregnancy such as maternal mortality, uterine perforation, postabortion infection, or cervical laceration are rare and unlikely to be assessed with randomised controlled trials. Hence, these trials focused on surrogate markers, such as amount of dilation, rather than on true clinical outcomes of interest, such as cervical injury. The results from this review should be interpreted cautiously, since all included trials had small sample sizes, and some outcomes were based on one trial only.
Four types of NO donors were used in the trials: sodium nitroprusside, glyceryl trinitrate, isosorbide mononitrate and isosorbide dinitrate. Two types of prostaglandins were used: gemeprost and misoprostol. Different doses and routes of administration complicated the meta‐analysis. However, different types of drugs may have unequal potency and different side effects.
Four trials with a total of 201 participants compared NO donor with placebo or no treatment (Thomson 1997; Facchinetti 2000; Li 2003; Phusaanantakul 2010). NO donors were more effective than placebo or no treatment for cervical ripening (more baseline cervical dilatation before the procedure and less force required to dilate the cervix). However, the frequency of gastrointestinal side effects were also higher in No donors group.
Prostaglandins were more effective than NO donors for cervical ripening. All but one trial (Arteaga‐Troncoso 2005) concurred that prostaglandins were superior to NO donors. We cannot combine the outcome from this one study with others due to different methods of measurement. NO donors were associated with more headache, palpitation, and dizziness. As expected, abdominal pain and vaginal bleeding were more common in women given prostaglandins, due to uterine contractions. However, no woman aborted from the prostaglandin used as a priming agent.
Only one trial compared NO donor with combination of NO donor and prostaglandin (Ledingham 2001). Although the combination regimen seemed more effective than the NO donor alone for cervical ripening, the 95% confidence interval was wide, reflecting imprecision.
This review suggested that prostaglandins are preferred to NO donors for cervical ripening before first‐trimester surgical abortion. However, NO donors may be an option for patients who are contraindicated to prostaglandins. The recommended agents prostaglandins are oral or vaginal misoprostol, osmotic dilators, or oral mifepristone (RCOG 2011; WHO 2012). Women should be informed of the potential side effects of vaginal bleeding and abdominal cramping, as well as the possibility of aborting after the priming dose.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.1 Baseline cervical dilatation before the procedure.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.2 Cumulative force required to dilate cervix to 8 mm.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.3 Side effect: Headache.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.4 Side effect: Abdominal pain.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.6 Patient satisfaction.

Forest plot of comparison: 1 Nitric oxide donors versus placebo or no treatment, outcome: 1.5 Side effect: Nausea/vomiting.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.1 Cumulative force required to dilate cervix to 8‐9 mm.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.2 Baseline cervical dilatation before the procedure.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.11 Probability of reaching cervical ripening > 8 mm at 3 hours.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.3 Side effect: Headache.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.6 Side effect: Palpitation.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.7 Side effect: Dizziness.
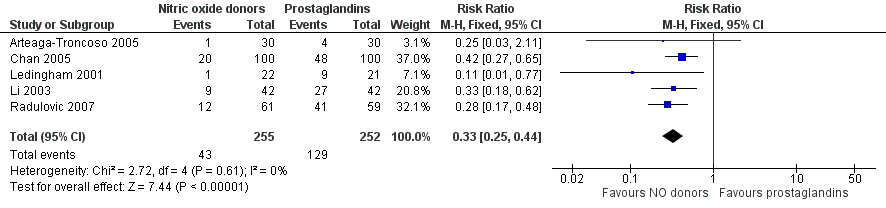
Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.4 Side effect: Abdominal pain.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.5 Side effect: Vaginal bleeding.
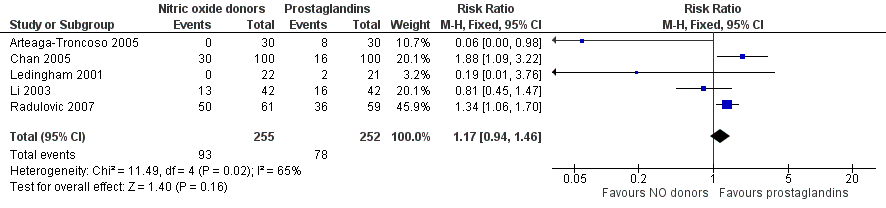
Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.8 Side effect: Nausea/Vomiting.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.10 Patient satisfaction.

Forest plot of comparison: 2 Nitric oxide donors versus prostaglandins, outcome: 2.9 Intraoperative blood loss.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.1 Cumulative force required to dilate cervix to 8 mm.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.2 Side effect: Headache.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.3 Side effect: Abdominal pain.

Forest plot of comparison: 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, outcome: 3.4 Intraoperative blood loss.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 1 Baseline cervical dilatation before the procedure.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 2 Cumulative force required to dilate cervix to 8 mm.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 3 Side effects: Headache.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 4 Side effect: Abdominal pain.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 5 Side effect: Nausea/vomiting.

Comparison 1 Nitric oxide donors versus placebo or no treatment, Outcome 6 Patient satisfaction.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 1 Cumulative force required to dilate cervix to 8‐9 mm.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 2 Baseline cervical dilatation before the procedure.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 3 Probability of reaching cervical ripening > 8 mm at 3 hours.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 4 Side effect: Headache.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 5 Side effect: Palpitation.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 6 Side effect: Dizziness.
Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 7 Side effect: Abdominal pain.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 8 Side effect: Vaginal bleeding.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 9 Side effect: Nausea/Vomiting.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 10 Patient satisfaction.

Comparison 2 Nitric oxide donors versus prostaglandins, Outcome 11 Intraoperative blood loss.

Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 1 Cumulative force required to dilate cervix to 8 mm.
Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 2 Side effect: Headache.

Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 3 Side effect: Abdominal pain.

Comparison 3 Nitric oxide donor versus nitric oxide donor and prostaglandin, Outcome 4 Intraoperative blood loss.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Baseline cervical dilatation before the procedure Show forest plot | 3 | 168 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.01, 0.58] |
| 2 Cumulative force required to dilate cervix to 8 mm Show forest plot | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | ‐4.29 [‐9.92, 1.35] |
| 3 Side effects: Headache Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.86, 3.46] |
| 4 Side effect: Abdominal pain Show forest plot | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.50] |
| 5 Side effect: Nausea/vomiting Show forest plot | 3 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.07, 6.45] |
| 6 Patient satisfaction Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative force required to dilate cervix to 8‐9 mm Show forest plot | 5 | 429 | Mean Difference (IV, Fixed, 95% CI) | 13.12 [9.72, 16.52] |
| 2 Baseline cervical dilatation before the procedure Show forest plot | 4 | 386 | Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.01, ‐0.45] |
| 3 Probability of reaching cervical ripening > 8 mm at 3 hours Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [2.21, 20.09] |
| 4 Side effect: Headache Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [3.29, 8.00] |
| 5 Side effect: Palpitation Show forest plot | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.43 [1.64, 7.15] |
| 6 Side effect: Dizziness Show forest plot | 3 | 327 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.29 [1.46, 7.41] |
| 7 Side effect: Abdominal pain Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.25, 0.44] |
| 8 Side effect: Vaginal bleeding Show forest plot | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.07, 0.27] |
| 9 Side effect: Nausea/Vomiting Show forest plot | 5 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.46] |
| 10 Patient satisfaction Show forest plot | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.28] |
| 11 Intraoperative blood loss Show forest plot | 4 | 393 | Mean Difference (IV, Fixed, 95% CI) | 33.59 [24.50, 42.67] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Cumulative force required to dilate cervix to 8 mm Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 14.5 [0.50, 28.50] |
| 2 Side effect: Headache Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.38, 2.00] |
| 3 Side effect: Abdominal pain Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.07] |
| 4 Intraoperative blood loss Show forest plot | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐50.0 [‐164.19, 64.19] |

