Rubefacientes que contienen salicilato para el dolor osteomuscular agudo y crónico en adultos
Appendices
Appendix 1. CENTRAL search strategy (2014 update)
-
MESH descriptor Irritants EXPLODE ALL TREES (187)
-
(rubefacient OR "counter‐irritant" OR "ammonium salicylate" OR "radian B" OR "benzyl nicotinate" OR kausalpunkt OR pykaryl OR rubriment OR "bornyl salicylate" OR camphor OR "choline salicylate" OR "diethylamine salicylate" OR algesal OR algoderm OR algoflex OR artogota OR "Lloyd's cream" OR physiogesic OR rheumagel OR "transvasin heat spray" OR "diethyl salicylate" OR "ethyl nicotinate" OR mucotherm OR transvasin "PR heat spray" OR "ethyl salicylate" OR "glycol monosalicylate" OR ralgex OR salonpas OR intralgin OR "glycol salicylate" OR "algipan rub" OR menthol OR "methyl butetisalicylate" OR doloderm OR "methyl gentisate" OR "methyl nicotinate" OR "nella red oil" OR wintergreen OR "sweet birch oil" OR "methyl salicylate" OR aezodent OR argesic OR aspellin OR balmosa OR "bengue's balsam" OR "chymol emollient balm" OR " deep heat" OR dencorub OR dermacreme OR dubam OR eftab OR exocaine OR germolene OR "gone balm" OR gordogesic OR linsal OR salonpas OR intralgin OR mentholatum OR monophytol OR nasciodine OR phlogont rheuma OR "PR heat spray" OR ralgex OR rheumabad OR rheumax OR salonair OR thermo‐rub OR nicoboxil OR finalgon OR ortholan OR nonivamide OR Warme‐Pflaster OR picolamine OR salicylate OR algiospray OR reflex OR "propyl nicotinate" OR elacur OR nicodan OR salicylamide OR isosal OR salicylate OR salycilic OR movelat OR radian OR "thurfyl salicylate" OR "triethanolamine salicylate" OR "analgesia crme" OR antiphlogistine OR aspercreme OR Ben‐Gay OR bexidermil OR dencorub OR exocaine OR metsal OR miosal OR mobisyl OR myoflex OR pro‐gesic OR royflex OR sportscreme OR topicrem): TI,AB,KY (4095)
-
1 OR 2 (4273)
-
MESH descriptor Administration, topical EXPLODE ALL TREES (12155)
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR creme OR lotion OR mousse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster): TI,AB,KY (65584)
-
4 OR 5 (68135)
-
MESH descriptor Athletic injuries EXPLODE ALL TREES (411)
-
(strain OR sprain* OR "sports injury"): TI,AB,KY (3671)
-
MESH descriptor Musculoskeletal diseases EXPLODE ALL TREES (20514)
-
(arthrit* OR rhemat* or osteoarth* OR tend?nitis OR sciatica OR lumbago OR fibrositis): TI,AB,KY (12221)
-
7 OR 8 OR 9 OR 10 (29202)
-
(pain OR painful OR analgesi*): TI,AB,KY (71595)
-
3 AND 6 AND 11 AND 12 (43)
Appendix 2. MEDLINE search strategy (2014 update)
-
exp Irritants/ (12084)
-
(rubefacient OR "counter‐irritant" OR "ammonium salicylate" OR "radian B" OR "benzyl nicotinate" OR kausalpunkt OR pykaryl OR rubriment OR "bornyl salicylate" OR camphor OR "choline salicylate" OR "diethylamine salicylate" OR algesal OR algoderm OR algoflex OR artogota OR "Lloyd's cream" OR physiogesic OR rheumagel OR "transvasin heat spray" OR "diethyl salicylate" OR "ethyl nicotinate" OR mucotherm OR transvasin "PR heat spray" OR "ethyl salicylate" OR "glycol monosalicylate" OR ralgex OR salonpas OR intralgin OR "glycol salicylate" OR "algipan rub" OR menthol OR "methyl butetisalicylate" OR doloderm OR "methyl gentisate" OR "methyl nicotinate" OR "nella red oil" OR wintergreen OR "sweet birch oil" OR "methyl salicylate" OR aezodent OR argesic OR aspellin OR balmosa OR "bengue's balsam" OR "chymol emollient balm" OR " deep heat" OR dencorub OR dermacreme OR dubam OR eftab OR exocaine OR germolene OR "gone balm" OR gordogesic OR linsal OR salonpas OR intralgin OR mentholatum OR monophytol OR nasciodine OR phlogont rheuma OR "PR heat spray" OR ralgex OR rheumabad OR rheumax OR salonair OR thermo‐rub OR nicoboxil OR finalgon OR ortholan OR nonivamide OR Warme‐Pflaster OR picolamine OR salicylate OR algiospray OR reflex OR "propyl nicotinate" OR elacur OR nicodan OR salicylamide OR isosal OR salicylate OR salycilic OR movelat OR radian OR "thurfyl salicylate" OR "triethanolamine salicylate" OR "analgesia crme" OR antiphlogistine OR aspercreme OR Ben‐Gay OR bexidermil OR dencorub OR exocaine OR metsal OR miosal OR mobisyl OR myoflex OR pro‐gesic OR royflex OR sportscreme OR topicrem).mp. (99561)
-
1 OR 2 (111385)
-
exp Administration, topical/ (67911)
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR creme OR lotion OR mousse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp. (1380924)
-
4 OR 5 (1396722)
-
exp Athletic injuries/ (29773)
-
(strain OR sprain* OR "sports injury").mp. (296402)
-
exp Musculoskeletal diseases/ (842839)
-
(arthrit* OR rhemat$* or osteoarth* OR tend?nitis OR sciatica OR lumbago OR fibrositis).mp. (215793)
-
7 OR 8 OR 9 OR 10 (1179700)
-
(pain OR painful OR analgesi*).mp. (537105)
-
randomized controlled trial.pt. (385551)
-
controlled clinical trial.pt. (89638)
-
randomized.ab. (282279)
-
placebo.ab. (149897)
-
drug therapy.fs. (1733690)
-
randomly.ab. (199106)
-
trial.ab. (292620)
-
groups.ab. (1274063)
-
13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 (3262413)
-
3 AND 6 AND 11 AND 12 AND 21 (106)
-
Limit 22 to yr="2008 ‐ Current" (35)
Appendix 3. EMBASE search strategy (2014 update)
-
exp Irritants/ (2671)
-
(rubefacient OR "counter‐irritant" OR "ammonium salicylate" OR "radian B" OR "benzyl nicotinate" OR kausalpunkt OR pykaryl OR rubriment OR "bornyl salicylate" OR camphor OR "choline salicylate" OR "diethylamine salicylate" OR algesal OR algoderm OR algoflex OR artogota OR "Lloyd's cream" OR physiogesic OR rheumagel OR "transvasin heat spray" OR "diethyl salicylate" OR "ethyl nicotinate" OR mucotherm OR transvasin "PR heat spray" OR "ethyl salicylate" OR "glycol monosalicylate" OR ralgex OR salonpas OR intralgin OR "glycol salicylate" OR "algipan rub" OR menthol OR "methyl butetisalicylate" OR doloderm OR "methyl gentisate" OR "methyl nicotinate" OR "nella red oil" OR wintergreen OR "sweet birch oil" OR "methyl salicylate" OR aezodent OR argesic OR aspellin OR balmosa OR "bengue's balsam" OR "chymol emollient balm" OR " deep heat" OR dencorub OR dermacreme OR dubam OR eftab OR exocaine OR germolene OR "gone balm" OR gordogesic OR linsal OR salonpas OR intralgin OR mentholatum OR monophytol OR nasciodine OR phlogont rheuma OR "PR heat spray" OR ralgex OR rheumabad OR rheumax OR salonair OR thermo‐rub OR nicoboxil OR finalgon OR ortholan OR nonivamide OR Warme‐Pflaster OR picolamine OR salicylate OR algiospray OR reflex OR "propyl nicotinate" OR elacur OR nicodan OR salicylamide OR isosal OR salicylate OR salycilic OR movelat OR radian OR "thurfyl salicylate" OR "triethanolamine salicylate" OR "analgesia crme" OR antiphlogistine OR aspercreme OR Ben‐Gay OR bexidermil OR dencorub OR exocaine OR metsal OR miosal OR mobisyl OR myoflex OR pro‐gesic OR royflex OR sportscreme OR topicrem).mp. (89247)
-
1 OR 2 (91804)
-
exp Administration, topical/ (14446)
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR creme OR lotion OR mousse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp. (1214203)
-
4 OR 5 (1214204)
-
exp Athletic injuries/ (15192)
-
(strain OR sprain* OR "sports injury").mp. (461094)
-
exp Musculoskeletal diseases/ (1041957)
-
(arthrit* OR rhemat* or osteoarth* OR tend?nitis OR sciatica OR lumbago OR fibrositis).mp. (222168)
-
7 OR 8 OR 9 OR 10 (1505520)
-
(pain OR painful OR analgesi*).mp. (706918)
-
clinical trial.sh. (686097)
-
controlled clinical trial.sh. (340752)
-
randomized controlled trial.sh. (302635)
-
double‐blind procedure.sh. (90931)
-
(clin* adj25 trial*).ab. (285240)
-
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab. (98024)
-
placebo*.ab. (151862)
-
random*.ab. (749790)
-
13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20
-
3 AND 6 AND 11 AND 12 AND 21
-
Limit 22 to yr="2008 ‐ Current" (74)
Appendix 4. Summary of outcomes in individual studies: efficacy and use of rescue medication
| Analgesia | ||||
| Study ID | Treatment | Outcome measure | Success | Rescue Medication |
| Acute | ||||
| (1) Salicylate, adrenal extract, and mucopolysaccharide ointment (Mobilat) (2) Placebo ointment | Movement pain on 100 mm VAS at: (a) 8 days (b) 15 days | No dichotomous data (a) Significant difference in favour of (1) (b) Significant difference in favour of (1) | No data | |
| (1) Salicylate and mucopolysaccharide cream (Movelat) (2) Placebo cream | Movement pain on 100 mm VAS at: (a) 9 days (b) 11 days | No dichotomous data (a) Significant difference in favour of (1) (b) No significant difference | No data | |
| (1) Salicylate and capsicum oleoresin ointment (Rado‐Salil) (2) Placebo ointment | Patient global assessment ('excellent' or 'good') at: (a) 3 days (b) 14 days | (a) (1) 5/20 (2) 0/20 (b) (1) 10/20 (2) 2/20 | Total number of rescue tablets (250 mg paracetamol) used: (1) 24 (2) 36 | |
| (1) Salicylate spray (2) Fepradinol spray active control | "Cure" at 12 days | (1) 23/35 (2) 85/102 | No data | |
| (1) Salicylate, adrenal extract, and mucopolysaccharide gel (Movelat) (2) Placebo gel | Relief of pain by 7 days | (1) 18/20 (2) 13/22 | No data | |
| (1) Salicylate gel (Reparil‐Gel) (2) Placebo gel | Patient global assessment ('very good' or 'good') at 9 days | (1) 37/39 (2) 3/42 | No data | |
| (1) Salicylate, nicotinate, capsicum oleoresin, and histamine gel (Cremor Capsici Compositus FNA) (2) Herbal gel (Spiroflor SRL) active control | 80% reduction in pain on 100 mm VAS at 7 days | (1) 41/78 (2) 40/83 | Number using rescue medication (paracetamol): (1) 65/82 (2) 56/75 | |
| Chronic | ||||
| (1) Salicylate cream (Myoflex) (2) Placebo cream | Pain relief score at 7 days favours (1) or (2) | No first period data. Combined periods: (1) 10/25 (2) 8/25 | No data | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | Improvement in rest pain score at 10 days | (1) 8/10 (2) 3/10 | No data | |
| (1) Salicylate and heparin gel (Dolo‐Menthoneurin) (2) Etofenamate gel active control | Patient global score ('very good' or 'good') after phase 1 at 7 days | First period data (1) 24/25 (2) 8/25 | No data | |
| (1) Salicylate cream (Aspercreme) + placebo tablets (2) Aspirin tablets + placebo cream active control | Patient global assessment of pain relief ('excellent' or 'good') at 7 days | (1) 13/20 (2) 10/20 | No data | |
| (1) Salicylate cream (Theraflex‐TMJ) | Spontaneous pain VAS (10 cm) at: (a) 15 days (b) 10 days | No dichotomous data (a) Significant difference in favour of (1) (b) No significant difference | No data | |
| (1) Salicylate gel (Phardol‐Mono) (2) Placebo gel | Dropout 'pain free' by day 14 | (1) 21/54 (2) 18/59 | No data | |
| (1) Salicylate gel (2) Placebo gel | Patient global assessment ('very good' or 'good') at 28 days | (1) 22/58 (2) 21/56 | Number using rescue medication (paracetamol): (1) 43/56 (2) 39/55 (1) 555 (2) 600 | |
| (1) Salicylate and nonivamide in heparin and salicylate ointment (Enelbin‐Rheuma) (2) Salicylate in heparin and salicylate ointment active control | Global assessment ('very good' or 'good') at 14 days | (1) 27/50 (2) 10/50 | No data | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | Rest pain score at 15 days | (1) 15/32 (2) 4/24 | No data | |
| (1) Salicylate ointment (2) Herbal (cinnamon, ginger, mastic, sesame oil) ointment | Reduction in pain intensity (group mean) | 14 days (1) 13/100 (2) 13/100 28 days (1) 19/100 (2) 21/100 42 days (1) 22/100 (2) 25/100 | No data | |
| VAS: visual analogue scale | ||||
Appendix 5. Summary of outcomes in individual studies: adverse events and withdrawals
| Withdrawals and exclusions | Adverse events | |||||
| Study ID | Treatment | All withdrawals and exclusions | Lack of efficacy | Adverse events | All adverse events | Local adverse events |
| (1) Salicylate cream (Myoflex) (2) Placebo cream | 1/26 unrelated to study | (1) 0/25 (2) 0/25 | (1) 0/25 (2) 0/25 | (1) 0/25 (2) 0/25 | (1) 0/25 (1) 0/25 | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | No data | No data | No data | No data | No data | |
| (1) Salicylate, adrenal extract, and mucopolysaccharide ointment (Mobilat) (2) Placebo ointment | No data | No data | (1) 0/40 (2) 0/40 | (1) 0/40 (2) 0/40 | (1) 0/40 (2) 0/40 | |
| (1) Salicylate and mucopolysaccharide cream (Movelat) (2) Placebo cream | 7/16 violation of protocol | (1) 0/78 (2) 0/78 | (1) 0/78 (2) 0/78 | (1) 0/78 (2) 1/78 | (1) 0/78 (2) 1/78 | |
| (1) Salicylate and heparin gel (Dolo‐Menthoneurin) (2) Etofenamate gel active control | Phase 1: (1) 0/25 (2) 0/25 | Phase 1: (1) 0/25 (2) 0/25 | Phase 1: (1) 0/25 (2) 0/25 Phase 2: (1) 0/25 (2) 0/25 | Phases 1 and 2 combined: (1) 2/50 (2) 2/50 | Phases 1 and 2 combined: (1) 2/50 (2) 2/50 | |
| (1) Salicylate and capsicum oleoresin ointment (Rado‐Salil) (2) Placebo ointment | No data | No data | No data | (1) 4/20 (2) 1/20 | (1) 4/20 (2) 1/20 | |
| (1) Salicylate cream (Aspercreme) + placebo tablets (2) Aspirin tablets + placebo cream active control | (1) 1/20 (2) 8/20 | (1) 1/20 (2) 2/20 | (1) 0/20 (2) 6/20 | (1) 3/20 (2) 12/20 | (1) 0/20 (2) 0/20 | |
| (1) Salicylate spray (2) Fepradinol spray active control | No data | No data | (1) 0/35 (2) 0/102 | (1) 0/35 (2) 0/102 | (1) 0/35 (2) 0/102 | |
| (1) Salicylate, adrenal extract, and mucopolysaccharide gel (Movelat) (2) Placebo gel | 8/50 4 excluded due to fractures, 4 lost to follow‐up | No data | No data | (1) 0/20 (2) 2/22 | (1) 0/20 (2) 2/22 | |
| (1) Salicylate cream (Theraflex‐TMJ) | No data | No data | No data | (1) 2/26 (2) 2/26 | (1) 2/26 (2) 2/26 | |
| (1) Salicylate gel (Reparil‐Gel) (2) Placebo gel | (1) 13/50 11 with no data, rest lack of efficacy (2) 24/50 8 with no data, rest lack of efficacy | (1) 2/39 (2) 16/42 | (1) 0/39 (2) 0/42 | (1) 0/39 (2) 0/42 | (1) 0/39 (2) 0/42 | |
| (1) Salicylate gel (Phardol‐Mono) (2) Placebo gel | 7/136 lost to follow‐up | No data | No data | (1) 1/54 unrelated disc prolapse (2) 0/59 | (1) 0/54 (2) 0/59 | |
| (1) Salicylate gel (2) Placebo gel | (1) 15/58 14 withdrew during trial, 1 lost to follow‐up (2) 10/58 2 withdrew before treatment, 7 withdrew during trial, 1 lost to follow‐up | (1) 3/58 | (1) 10/58 | (1) 48/58 | Total number of adverse events: (1) 80 (2) 27 | |
| (1) Salicylate, nicotinate, capsicum oleoresin, and histamine gel (Cremor Capsici Compositus FNA) (2) Herbal gel (Spiroflor SRL) active control | (1) 4/78 lost to follow‐up (2) 2/83 1 death, 1 lost to follow‐up | No data | (1) 8/74 (2) 1/82 unrelated death | (1) 19/74 (2) 10/82 | (1) 18/74 (2) 3/81 | |
| (1) Salicylate and nonivamide in heparin and salicylate ointment (Enelbin‐Rheuma) (2) Salicylate in heparin and salicylate ointment active control | (1) 0/50 (2) 2/50 | (1) 1/50 (2) 0/50 | (1) 0/50 (2) 2/50 | (1) 0/50 (2) 2/50 | (1) 0/50 (2) 2/50 | |
| (1) Salicylate and myrtecaine cream (Algesal Suractive) (2) Placebo cream | No data | No data | No data | No data | No data | |
| (1) Salicylate ointment (2) Herbal (cinnamon, ginger, mastic, sesame oil) ointment | (1) 3/46 (2) 4/46 All lost to follow‐up | No data | No data | No data | No data | |

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
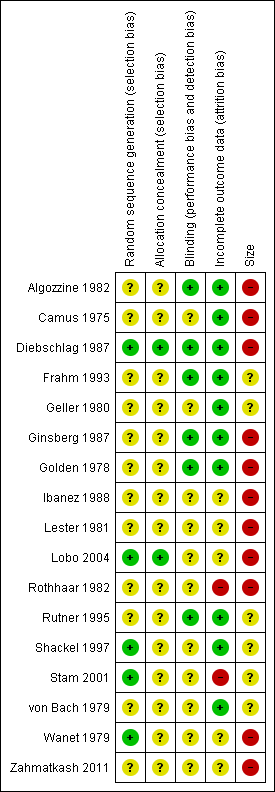
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Rubefacient versus placebo, outcome: 1.1 Clinical success (eg 50% reduction in pain).

Forest plot of comparison: 1 Rubefacient versus placebo, outcome: 1.4 Adverse events.

Comparison 1 Rubefacient versus placebo, Outcome 1 Clinical success (eg 50% reduction in pain).
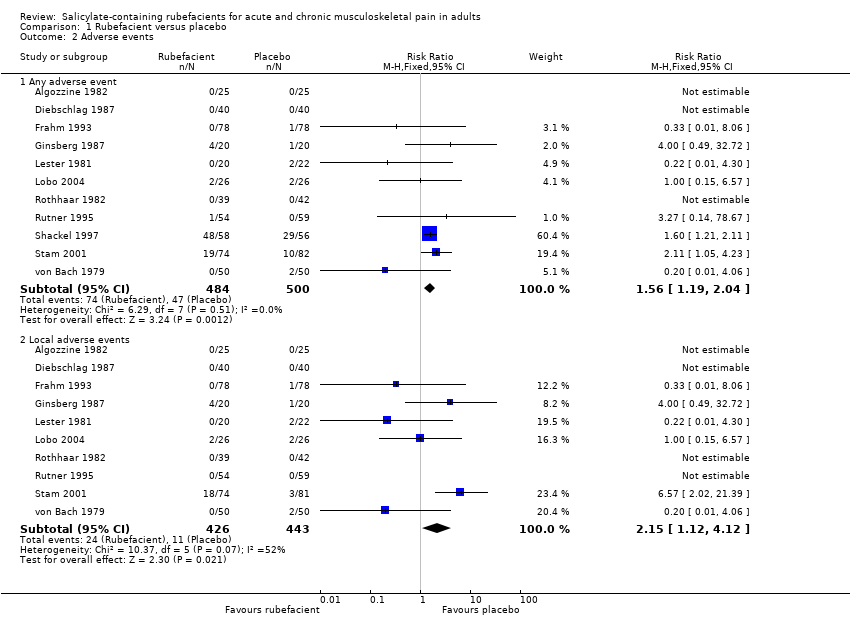
Comparison 1 Rubefacient versus placebo, Outcome 2 Adverse events.
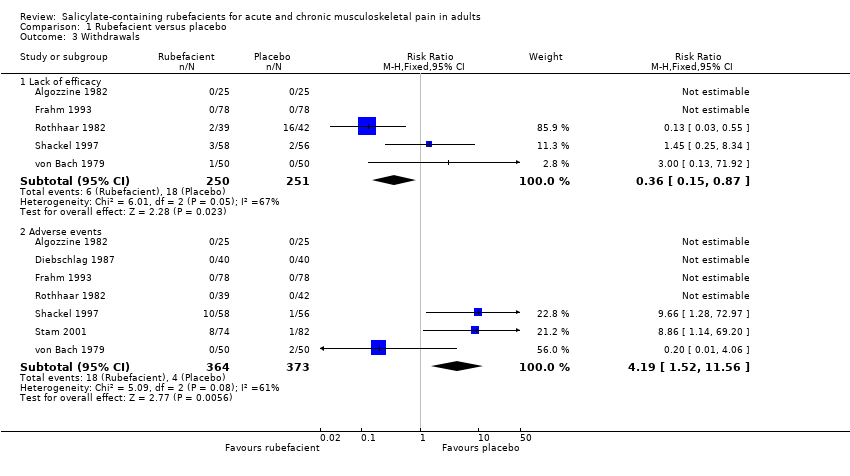
Comparison 1 Rubefacient versus placebo, Outcome 3 Withdrawals.
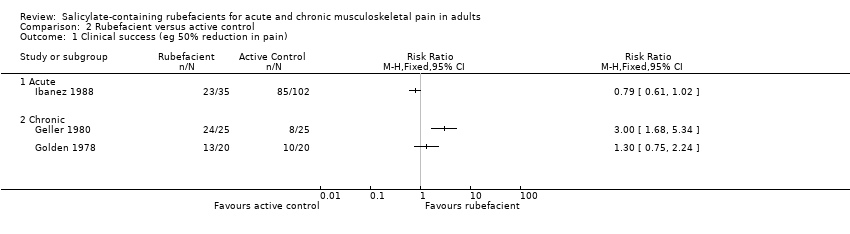
Comparison 2 Rubefacient versus active control, Outcome 1 Clinical success (eg 50% reduction in pain).

Comparison 2 Rubefacient versus active control, Outcome 2 Adverse events.

Comparison 2 Rubefacient versus active control, Outcome 3 Withdrawals.
| Salicylate‐containing topical rubefacients compared with topical placebo for acute and chronic painful conditions | ||||||
| Patient or population: adults with strains or sprains (acute) or osteoarthritis or low back pain (chronic) Settings: community Intervention: salicylate‐containing topical rubefacient Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | RR NNT, NNTp, or NNH | No of studies, participants | Quality of the evidence | Comments |
| Clinical success (eg 50% reduction in pain) Acute conditions | 640 in 1000 | 335 in 1000 | RR 1.9 (1.5 to 2.5) NNT 3.2 (2.4 to 4.9) | 4 studies 324 participants | ⊕⊝⊝⊝ | Most recent, largest study showed no effect Note NNT cannot be trusted because of low numbers and poor quality studies |
| Clinical success (eg 50% reduction in pain) Chronic conditions | 447 in 1000 | 284 in 1000 | RR 1.6 (1.2 to 2.0) NNT 6.2 (4.0 to 13) | 6 studies 455 participants | ⊕⊝⊝⊝ | Most recent, largest studies showed no effect Note NNT cannot be trusted because of low numbers and poor quality studies |
| Adverse events ‐ any adverse events Acute and chronic conditions combined | 152 in 1000 | 94 in 1000 | RR 1.6 (1.2 to 2.0) NNH 17 (9.9 to 58) | 11 studies 984 participants | ⊕⊕⊝⊝ | Inadequate reporting of adverse events is common Acute and chronic conditions combined |
| Adverse events ‐ local adverse events Acute and chronic conditions combined | 56 in 1000 | 24 in 1000 | RR 2.2 (1.1 to 4.1) NNH 31 (16 to 300) | 10 studies 869 participants | ⊕⊝⊝⊝ | Small numbers of events Acute and chronic conditions combined |
| Withdrawals ‐ lack of efficacy Acute and chronic conditions combined | 24 in 1000 | 72 in 1000 | RR 0.4 (0.2 to 0.9) NNTp 21 (12 to 120) | 5 studies 501 participants | ⊕⊝⊝⊝ | Small numbers of events Acute and chronic conditions combined |
| Withdrawals ‐ adverse events Acute and chronic conditions combined | 49 in 1000 | 11 in 1000 | RR 4.2 (1.5 to 12) NNH 26 (15 to 85) | 7 studies 737 participants | ⊕⊝⊝⊝ | Small numbers of events Acute and chronic conditions combined |
| GRADE Working Group grades of evidence | ||||||
| CI: confidence interval; RR: risk ratio; NNT: number needed to treat; NNTp: number needed to prevent an event happening; NNH: number needed to harm | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success (eg 50% reduction in pain) Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Acute conditions | 4 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.51, 2.46] |
| 1.2 Chronic conditions | 6 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.22, 2.04] |
| 2 Adverse events Show forest plot | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any adverse event | 11 | 984 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.19, 2.04] |
| 2.2 Local adverse events | 10 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [1.12, 4.12] |
| 3 Withdrawals Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Lack of efficacy | 5 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.15, 0.87] |
| 3.2 Adverse events | 7 | 737 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.19 [1.52, 11.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success (eg 50% reduction in pain) Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Acute | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Chronic | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Any adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Local adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Withdrawals Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Lack of efficacy | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

