Liječenje akutne mišićno‐koštane boli u odraslih lokalnim nesteroidnim protuupalnim lijekovima
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | R, DB, PC, parallel groups Gel applied to the painful area twice daily for 7 days Assessment at baseline, 3, 7 days | |
| Participants | Minor soft tissue injuries (< 7 days) N = 56 M 45, F 11 Age not reported Mean baseline pain at rest 25‐26 mm | |
| Interventions | Ketoprofen gel, 2 x 5 g (125 mg) daily, n = 29 Placebo gel, n = 27 Rescue medication paracetamol 500 mg No other treatment allowed | |
| Outcomes | PGE: 5‐point scale but reported as "improved" or "same or worse" (responder = "improved") Improvement in pain with movement: 100 mm VAS, reported as group mean Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of early withdrawals or method of imputation |
| Size | High risk | < 50 participants per treatment group |
| Methods | R, DB, PC, AC, parallel groups Gel applied to affected area 3 or 4 times daily, with no occlusion for 7 days Assessment at baseline, 3, 7 days | |
| Participants | Acute orthopaedic trauma (contusion, distortion, fracture, < 7 days) N = 252 (203 analysed for efficacy) M 98, F 105 Age range 8 to 86 years, 13% younger than 20 years Baseline pain mild in 35% Exclusions: 23 protocol violations, 26 reasons "not related" to drug. Equally distributed between groups | |
| Interventions | Piroxicam gel 0.5%, 1 g 3 to 4 x daily, n = 84 Indomethacin gel 1%, 1 g 3 to 4 x daily, n = 84 Placebo gel, n = 84 No other medication or initiation of physical therapy allowed | |
| Outcomes | PGE: 5‐point scale (responder = "better" and "much better") Adverse events Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "key code sealed until end of study" |
| Blinding (performance bias and detection bias) | Low risk | Gels in "identical tubes" |
| Incomplete outcome data (attrition bias) | Unclear risk | > 10% withdrawals "unrelated to treatment" and for "protocol violations". No further details, but no significant differences between groups |
| Size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | R, DB, PC, parallel groups Gel massaged into skin over affected heel 3 times daily after cleaning with soap and water for up to 21 days Assessment at baseline, 7, 21 days | |
| Participants | Acute Achilles heel tendinitis (not associated with continuous pain at rest or > 1 month history) N = 243 (227 analysed for efficacy) M/F not reported Mean age 29 years Baseline pain: ˜ 10% had < 26 mm on palpation of tendon, ˜ 30% had mild or no pain on dorsiflexion of foot Exclusions: failure to meet inclusion criteria, major protocol violations, failure to take study medication for full duration | |
| Interventions | Niflumic acid gel 2.5%, 3 x 5 g daily, n = 117 Placebo gel, n = 110 No other analgesics and anti‐inflammatories, physiotherapy or supportive measures allowed | |
| Outcomes | PGE: 5‐point scale (responder = "good" or "very good") Pain improved or disappeared on dorsiflexion Adverse events Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | 10% excluded for "failing to meet entry criteria and protocol violations". No further details |
| Size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | R, DB, PC, parallel groups Gel applied 3 times daily with rubbing Assessed at baseline, 3, 5, 7 days | |
| Participants | Distortion of ankle joint N = 160 M and F Age 18+ years Baseline pain not reported | |
| Interventions | Ibuprofen microgel 5%, 3 x 10 cm (= 200 mg) daily, n = 80 Placebo gel, n = 80 | |
| Outcomes | Pain with movement: VAS (responder = decreased by 20%) Complete remission Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient data to assess |
| Size | Unclear risk | 50 to 200 participants per treatment group |
| Methods | R, DB, PC, parallel groups Cream applied 4 times daily for 7 days (up to 14 days optional) Self assessed using daily diary for 7 days, and up to 14 days | |
| Participants | Acute ankle sprain (< 24 hours, no fracture) N = 100 (51 analysed) M 33, F 18 Mean age 29 years Baseline pain at rest > 35 mm, on walking 80 mm Exclusions: did not return diaries, protocol exclusions (25 ibuprofen, 24 placebo) | |
| Interventions | Ibuprofen cream 5% (Proflex), 4 x 4" (10 cm) daily, n = 26 Placebo cream, n = 25 Advised to use rest and regular icing for 48 hours, then walking and exercise Rescue medication: paracetamol | |
| Outcomes | Improvement in walking ability: 4‐point scale (responder = "improvement") Pain on walking: 100 mm VAS (mean data) Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out by sponsor. Tubes dispensed by hospital pharmacy who held the codes. |
| Blinding (performance bias and detection bias) | Low risk | "identical cream" |
| Incomplete outcome data (attrition bias) | High risk | > 40% lost to follow‐up. Approximately equal between groups |
| Size | High risk | < 50 participants per treatment arm as analysed |
| Methods | R, DB, PC, parallel groups Cream applied to site of injury 3 times daily for 6 days Assessment at baseline, 2, 6 days | |
| Participants | Soft tissue injuries (recent) N = 51 M/F not reported Age not reported Baseline pain on passive movement moderate or severe in all but 3 participants | |
| Interventions | Benzydamine HCl cream 3%, 3 x daily, n = 25 Placebo cream, n = 25 (5 active, 6 placebo participants also received ultrasound) No other topical agent allowed | |
| Outcomes | Pain on passive movement: 4‐point scale (responder = "absent" or "slight") Tenderness with pressure: 4‐point scale (responder = "absent" or "slight") Adverse events Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predetermined randomised schedule" |
| Allocation concealment (selection bias) | Low risk | Sealed copy of schedule held by investigator and duplicate copy kept by clinical trial co‐ordinator. Looked at only in event of adverse reaction (not necessary) |
| Blinding (performance bias and detection bias) | Low risk | "indistinguishable .... in appearance and consistency" |
| Incomplete outcome data (attrition bias) | Low risk | 2% lost to follow‐up, no withdrawals due to adverse events. Responder analysis |
| Size | Unclear risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, multicentre, parallel group Plaster applied daily for 7 days | |
| Participants | Grade I or II ankle sprain (< 48 hours) with lateral external ligament involvement N = 430 M 249, F 175 (for analysis) Mean age 35 years Baseline PI on movement 72/100 (SD 12) | |
| Interventions | DHEP/hep, n = 142 Rescue medication: paracetamol to maximum 3 g daily | |
| Outcomes | Mean change in PI on movement from baseline to 3 days Mean reduction in oedema at 3 days Tolerability at 3 days Rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelopes" |
| Blinding (performance bias and detection bias) | Low risk | Shape, colour, size, and application method identical for all plasters |
| Incomplete outcome data (attrition bias) | Unclear risk | Very few withdrawals, but used LOCF |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, multicentre, parallel group Plaster applied daily for 7 days PI assessed twice daily over 3 days, then day 7. Overall treatment efficacy and tolerability assessed on days 3, 7 | |
| Participants | Ankle sprain (< 48 hours) with lateral external ligament involvement N = 240 (233 for analysis) M 148, F 86, 6 unknown | |
| Interventions | DHEP/hep, n = 120 Rescue medication: paracetamol to maximum 4 g daily | |
| Outcomes | Global efficacy at 7 days: 4‐point scale (responder = "excellent" or "good") Mean change in PI on movement at 6 hours and 7 days Mean change in oedema at 3 and 7 days Tolerability Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelopes" |
| Blinding (performance bias and detection bias) | Low risk | Appearance and odour identical for all plasters |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis, < 5% excluded for missing data, equal between groups |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, AC, parallel groups Gel rubbed into affected area until absorbed, twice daily for 10 days Assessed at baseline, and daily to 10 days | |
| Participants | Acute soft tissue injuries N = 60 M 33, F 27 Median age 33 years Baseline pain not given | |
| Interventions | Ibuproxam gel 10%, n = 20 Ketoprofen gel, n = 20 Etofenamate gel, n = 20 | |
| Outcomes | PGE: 4‐point scale ("good" or "excellent") Resolution of symptoms Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Medication supplied in identical tubes |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, AC, parallel groups Gel applied 3 times daily, without occlusion, for 14 days Assessment at baseline, 2, 3, 4, 8, 15 days | |
| Participants | Ankle sprain (< 24 hours) N = 37 M 24, F 13 Mean age 28 years Baseline pain slight to moderate | |
| Interventions | Ketorolac gel 2%, 3 x 3 g daily, n = 13 Etofenamate gel 5%, 3 x 3 g daily, n = 12 Placebo gel, n = 12 Rescue medication: paracetamol No other analgesic or anti‐inflammatory medication, ice packs, or physiotherapy allowed | |
| Outcomes | Reduction in PI: 100 mm VAS and 4‐point scale (responder = "improved") Adverse events Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Medication assignment ... supplied in a sealed envelope." Opened only if serious participant event necessitation treatment disclosure occurred (not necessary) |
| Blinding (performance bias and detection bias) | Low risk | "identical appearance" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Cream applied 3 times daily Assessment at baseline, 7 days | |
| Participants | Acute tendinitis (< 1 month) N = 64 M 35, F 25 Mean age 36 years Baseline spontaneous pain ≥ 60 mm | |
| Interventions | Ibuprofen cream 5%, 3 x 4 cm daily, n = 32 (3 x 10 cm for large joints) Placebo cream, n = 32 No other topical, systemic, or physical treatment allowed | |
| Outcomes | PGE: scale not reported (responder = "improvement" or "complete relief") Improvement in pain: VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 10/16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Missing participants added back using BOCF |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied twice daily to affected area with light massage, then covered with standard compress Assessed at baseline, 3, 7 days | |
| Participants | Uncomplicated, recent ankle sprain N = 60 M 36, F 24 Mean age 33 years Mean baseline pain 54 mm | |
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm daily, n = 30 Placebo gel, n = 30 No concomitant therapy other than simple oral analgesia allowed | |
| Outcomes | PGE: 3‐point scale (responder = "better") Improvement in pain: VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Treatments "identical in every way except that placebo did not contain active principle" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participant per treatment arm |
| Methods | R, DB, PC, parallel groups Gel lightly massaged into skin over affected area 3 times daily, then covered with standard compress Assessed at baseline, 3, 7 days | |
| Participants | Uncomplicated, ankle sprain (< 4 days) N = 60 (59 analysed) M 29, F 29 (not stated for 1 participant) Mean age 33 years Baseline pain ≥ moderately severe Exclusions: 1 participant had only moderate pain at baseline | |
| Interventions | Niflumic acid gel 2.5%, 3 x 5 g daily, n = 30 Placebo gel, n = 30 Concomitant treatment with systemic NSAIDs, local therapies, or physiotherapy were not allowed | |
| Outcomes | PGE: 4‐point scale (responder = "cured" or "improved") Improvement in pain: VAS (mean data) Adverse events Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Patch applied twice daily Assessed at baseline, 3, 7 days | |
| Participants | Traumatic ankle sprain (< 2 days) N = 131 M 84, F 47 Mean age 34 years Baseline pain ≥ 50 mm | |
| Interventions | Flurbiprofen patch, 2 x 40 mg daily, n = 65 Placebo patch, n = 66 Rescue medication: paracetamol. Ice or light restraint allowed Exclusions: 1 from flurbiprofen group for protocol violation | |
| Outcomes | PGE: 4‐point scale (responder = "good" or "very good") Improvement in pain: VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Placebo patch was "non‐medicated (but otherwise identical)" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel lightly massaged into skin 3 times daily, and kept dry for 6 to 8 hours Assessed at baseline, 3, 10 days | |
| Participants | Peri and extra‐articular inflammatory diseases N = 100 M 32, F 68 Mean age 49 years Baseline spontaneous pain ≥ 40 mm | |
| Interventions | DHEP lecithin gel, 3 x 5 g (= 65 mg) daily, n = 50 DHEP gel, 3 x 5 g (= 65 mg) daily, n = 50 | |
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Pain on movement: mean Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 participants per treatment arm |
| Methods | R, DB, PC, AC, parallel groups Gel applied to affected area 3 or 4 times daily with no occlusion for up to 14 days Assessed at baseline, 7, 14 days | |
| Participants | Muscle pain or inflammation in neck, shoulder, back, chest and upper and lower extremities, or a combination N = 271 (247 analysed) M 97, F 149 Age < 20 to 89 years Baseline pain mostly mild to moderate Exclusions: 24 due to protocol violations, loss to follow‐up | |
| Interventions | Piroxicam gel 0.5% 1 g, 3 to 4 x daily, n = 92 Indomethacin gel 1% 1 g, 3 to 4 x daily, n = 90 Placebo gel, n = 89 No concomitant oral or topical analgesic or anti‐inflammatory medication allowed. No physical therapy initiated after start of study | |
| Outcomes | PGE: 5‐point scale (responder = "better" or "much better") Physician rated improvement: 5‐point scale (responder = "marked improvement") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total =4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Cartons numbered randomly and numbers held in a key code until study completion |
| Blinding (performance bias and detection bias) | Low risk | "identical tubes" packed in numbered carton. Gel bases slightly different in appearance, so dispensing physician did not have access to them |
| Incomplete outcome data (attrition bias) | Unclear risk | ˜ 8% excluded from analysis for unknown reasons or lost to or inadequate follow‐up. Approximately equal between groups |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel applied to affected area 4 times daily, with light massage, for 14 days Assessment at baseline, 7, 14 days | |
| Participants | Painful inflammatory conditions N = 50 M 20, F 30 Mean age 50 years Baseline pain ≥ moderate severity | |
| Interventions | Diclofenac gel 1%, 2 g 4 x daily, n = 25 (Flector) Diclofenac sodium 1%, 2 g 4 x daily, n = 25 (Voltaren Emugel) No other medication that could interfere with test drugs allowed | |
| Outcomes | PGE: 5‐point scale (responder = "good" or "excellent") Improvement in pain on pressure: 4‐point scale (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB (for gels), SB (for ointment), AC, multicentre, parallel groups 2 g (˜ 6 cm of gel) applied over injured area x 3 daily for 14 days Assessments at 0, 4, 7, 14 days of treatment, and day 42 follow‐up | |
| Participants | Grade I or II ankle sprain (< 24 hours) with lateral external ligament involvement, PI on weight bearing ≥ 30/100 N = 449 M = 308, F = 112 (for analysis) Mean age 28 years | |
| Interventions | Traumeel gel, 3 x 2 g daily, n = 140 Traumeel ointment, n = 143 (not analysed in this review) Rescue medication: paracetamol to maximum 2 g daily | |
| Outcomes | Pain‐free on day 7 Global efficacy: 5‐point scale (responder = "good" and "very good") on day 14 Normal function on day 14: yes or no (responder = "yes") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2 (gels), W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation, kits assigned in order received, and used envelopes (no further details) |
| Blinding (performance bias and detection bias) | Low risk | For gel comparison: "identical containers" |
| Incomplete outcome data (attrition bias) | Unclear risk | Used LOCF. Most withdrawals due to early recovery (within 14 days), approximately equal between groups |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel or cream applied 3 times daily for up to 14 days Assessed at baseline, 7, 14 days | |
| Participants | Soft tissue injuries + 2 fractures N = 30 M = 21, F = 9 Median age 38 years Mean baseline pain on movement moderate to severe (2.8, scale 0 to 4) | |
| Interventions | Ketoprofen gel 5%, 3 x 2 to 3 g daily, n = 15 Ketoprofen cream 1%, 3 x 2 to 3 g daily, n = 15 | |
| Outcomes | PGE: 5‐point scale (responder = "good" and "excellent") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total =3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Treatments were given in identical tubes and measurements made by blinded observers, but one was a cream and the other a gel |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel applied twice daily for 10 days Assessed at baseline, 4, 7, 10 days | |
| Participants | Soft tissue injuries N = 60 M = 37, F = 23 Mean age 32 years (range 13 to 78) Mean baseline pain on movement moderate to severe (2.2, scale 0 to 3) | |
| Interventions | Flunoxaprofen gel, 2 x 3 to 5 cm daily, n = 30 Ketoprofen gel, 2 x 3 to 5 cm daily, n = 30 | |
| Outcomes | Improvement in pain on pressure (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient data |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Cream applied lightly to affected area 6 times daily for 6 days Assessed at baseline, 2, 4, 6 days | |
| Participants | Soft tissue injuries (< 24 hours) N = 43 M/F not reported Age not reported Baseline pain not reported | |
| Interventions | Benzydamine cream 3%, 6 x daily, n = 21 Placebo cream, n = 22 | |
| Outcomes | Pain on movement: 4‐point scale (responder = "improved") Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) | Low risk | All participants apparently included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, multicentre, parallel group Plaster applied x 1 daily (minimum of 20 consecutive hours of application per day) for 14 days PI assessed daily, overall efficacy assessed at 7 and 14 days | |
| Participants | Unilateral, mild to moderate muscle contusion of upper or lower limb (< 72 hours), PI ≥ 50/100, superficial haematoma ≤ 10 x 14 cm at injured site N = 354 M 126, F 228 Mean age 39 years | |
| Interventions | DHEP/hep plaster, x 1 daily, n = 121 Rescue medication: paracetamol 500 mg (no limit reported) | |
| Outcomes | PGE: 5‐point scale (responder = excellent or good) at 14 days Mean reduction in PI at 3 and 8 days Rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported: "Medication packed and labelled in order to render all participants and personnel fully blinded to treatment administered" |
| Blinding (performance bias and detection bias) | Low risk | "Indistinguishable regarding appearance, shape, colour, size and odour" |
| Incomplete outcome data (attrition bias) | Unclear risk | No responder analysis reported, and imputation method not mentioned |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel applied to affected region 4 times daily, with gentle massage Assessed at baseline, 8 days in clinic and daily diary | |
| Participants | Soft tissue articular pain (≤ 15 days) N = 142 M 19, F 123 Mean age 57 years Mean baseline PI moderate to severe | |
| Interventions | Diclofenac sodium gel 1%, 4 x 2 cm daily, n = 69 Lysine clonixinate gel 5%, 4 x 2 cm daily, n = 73 (2 cm = 22.5 mg) No other analgesic, local treatment (including immobilisation, bandaging), or acupuncture Rescue mediation allowed after 2 applications, if needed | |
| Outcomes | PGE: 3‐point scale ("good") PI: participant diary (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Diclofenac gel repackaged to maintain DB with lysine clonixinate gel. Minor differences between gels only apparent when directly compared |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Foam (approximately the size of a golf ball) applied, and 1 tablet taken, 3 times daily for 7 days and up to 14 days Assessed at baseline, 7, 14 (if necessary) days | |
| Participants | Acute lower back injury (< 1 month) N = 287 (261 analysed for efficacy) M 151, F 136 Mean age 37 years (range 18 to 63) Most participants had moderate to severe pain on movement, one had none Exclusions: 25 lost to follow up, one assessed at 14 days, but not 7 days | |
| Interventions | Felbinac foam 3%, 3 x 2 g daily + placebo tablets, 3 x 1 daily, n = 140 (127 analysed for efficacy) Ibuprofen tablets, 3 x 400 mg daily + placebo foam, 3 x 2 g daily, n = 147 (134 analysed for efficacy, but one had no pain at baseline) No other oral, injectable, or topical analgesic or anti‐inflammatory medication. Ongoing physiotherapy to continue without change | |
| Outcomes | Pain on movement: 5‐point scale (responder = "none" or "mild") Spontaneous pain: 5‐point scale (responder = "none" or "mild") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "double dummy" |
| Incomplete outcome data (attrition bias) | Unclear risk | Imputation method not mentioned |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Plaster applied to skin over affected area twice daily, and kept in place with an elastic bandage Assessed at baseline, 7, 14 days, and after further 14 days without treatment | |
| Participants | Humero‐radial epicondyl pain (tendinopathic) ‐ nearly all tennis elbow N = 85 M 54, F 31 Mean age 45 years Baseline pain: "mild" in ˜ 10% of placebo group and 29% of active group | |
| Interventions | DHEP plaster (Tissugel), x 2 daily, n = 44 Placebo plaster x 2 daily, n = 41 | |
| Outcomes | Pain on pressure: 5‐point scale (responder = "none" or "mild") Spontaneous pain: 5‐point scale (responder = "no pain") Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | "identical characteristics" |
| Incomplete outcome data (attrition bias) | Unclear risk | No useable data |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Plaster applied to skin over affected area once daily Assessed at baseline, 1, 2, 3, 7 days | |
| Participants | Ankle sprain (< 48 hours) N = 134 M 72, F 62 Age range 18 to 65 years Baseline spontaneous pain ≥ 50 mm | |
| Interventions | DHEP plaster (Flector Tissugel 1%), x 1 daily, n = 68 Placebo plaster, x 1 daily, n = 66 Rescue medication: paracetamol | |
| Outcomes | PGE: 4‐point scale (responder = "excellent") Pain on movement: VAS (mean) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Identical in size, appearance and used same formula as active patch, without active ingredient" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in responder analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied to affected area twice daily, with light massage Assessed at baseline, 3, 7 days in clinic and daily diary | |
| Participants | Tendinitis N = 60 M 29, F 31 Mean age 41 years Baseline pain > 50 mm | |
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm (= 50 mg) daily, n = 30 Placebo gel, 2 x 5 cm daily, n = 30 No concomitant therapy other than simple analgesia | |
| Outcomes | PGE: 4‐point scale (responder = "improved" or "recovered") Pain on movement: 4‐point scale (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Randomisation code supplied by Menarini laboratories, remote from allocation |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in responder analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, multicentre, parallel group Plaster applied x 1 daily (minimum 20 consecutive hours of application daily) for 10 days PI assessed daily, Overall treatment efficacy and tolerability assessed at days 3 and 7 | |
| Participants | Unilateral, mild to moderate muscle contusion or strain of upper or lower limb (< 72 hours), superficial haematoma ≤ 140 cm2, PI ≥ 40/100 N = 185 Mean age 39 years | |
| Interventions | DHEP/hep plaster, x 1 daily, n = 65 | |
| Outcomes | Overall treatment efficacy at 3 days: 5‐point scale (responder = "good" or "excellent") Resolution of haematoma at 10 days: yes or no Rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "Identical in size, appearance and odour" |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals < 5%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied twice daily Assessed at baseline, 3, 7 days | |
| Participants | Acute soft tissue trauma (< 24 hours) N = 74 M 60, F 14 Mean age 27 years Baseline pain > 65 mm | |
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm (= 15 mg) daily, n = 38 Placebo gel, 2 x 5 cm daily, n = 36 No concomitant treatment Rescue medication: glafenine | |
| Outcomes | PGE: 3‐point scale (responder = "good") Spontaneous pain: 100 mm VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in responder analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, multicentre, parallel groups Plaster applied x 2 daily for 14 days or until resolution PI assessed daily. Overall treatment efficacy assessed at 14 days | |
| Participants | Mild or moderate sprain, strain or contusion (< 7 days), PI ≥ 5/10 N = 418 M 206, F 212 | |
| Interventions | DHEP patch, x 2 daily, n = 207 Rescue medication: no analgesic allowed (or ice/wrapping): use = discontinuation | |
| Outcomes | Resolution of injury (VAS ≤ 2/10) Overall tolerability: 5‐point scale (responder = "good" or "excellent") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "identical in appearance with same content except diclofenac" |
| Incomplete outcome data (attrition bias) | High risk | Used LOCF, high withdrawal rate (43%). Did not report all efficacy outcomes measured |
| Size | Low risk | > 200 participants per treatment arm |
| Methods | R, DB, PC, multicentre, parallel groups Plaster applied x 2 daily for 7 days PI assessed daily, overall treatment efficacy and tolerability assessed at 7 days | |
| Participants | Mild or moderate ankle or knee sprain, muscle strain or contusion (< 72 hours), PI ≥ 50/100 M 144, F 240 | |
| Interventions | DHEP plaster, x 2 daily, n = 192 Rescue medication: paracetamol to maximum 2 g daily | |
| Outcomes | ≥ 50% reduction in PI at 7 days Overall treatment efficacy: 5‐point scale (responder = "good" or "excellent") Overall tolerability: 5‐point scale (responder = "good" or "excellent") Rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not fully described: "sealed envelopes", "concealed from investigators and participants" |
| Blinding (performance bias and detection bias) | Low risk | "identical in texture, size, color and odor" |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawals 2%. Methods states LOCF, but appears to describe BOCF for withdrawals |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Cream applied x 3 daily for 5 days, with elastic support for the first 3 days Assessed at baseline 4, 8 days | |
| Participants | Sprained ankle (< 24 hours) N = 100 M 58, F 42 Mean age 28 years Baseline pain: all participants had "walking pain" | |
| Interventions | Benzydamine 3% cream, x 3 daily, n = 50 Placebo gel, x 3 daily, n = 50 | |
| Outcomes | Pain on movement: responder = "free of walking pain" Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1 Total = 3/5 *Paper describes a benzydamine cream and a placebo gel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described. Paper describes a benzydamine cream and a placebo gel |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in responder analysis |
| Size | Unclear risk | 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel gently ("minimal rub", not vigorously) massaged into skin over affected site until absorbed 3 times daily until symptoms disappeared or for maximum of 7 days Assessment at baseline and once daily using diary cards to 7 days | |
| Participants | Soft tissue injury (< 2 weeks and untreated) N = 85 (81 analysed) M 42, F 39 Mean age 41 years Baseline pain > 50 mm 4 placebo participants lost to follow‐up | |
| Interventions | Ibuprofen gel 5%, x 3 daily, n = 40 Placebo gel, x 3 daily, n = 41 Initiation of other medication or physiotherapy not allowed during study | |
| Outcomes | PGE: 5‐point scale (responder = "marked improvement" or "complete clearance") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Gels had similar physical characteristics and were supplied in identical tubes |
| Incomplete outcome data (attrition bias) | Low risk | < 5% missing participants. Remaining participants included in responder analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel applied with gentle massage to affected area 3 times daily, without occlusion, for 10 days Assessed at baseline, 3, 10 days in clinic and daily patient diary | |
| Participants | First‐degree ankle or knee sprains, first‐degree muscle strains and mild‐to‐moderate contusions n = 100 M 69, F 31 Mean age 32 years Mean baseline pain with activity ≥ 65 mm | |
| Interventions | DHEP lethicin gel, 3 x 5 g (= 65 mg) daily, n = 52 DHEP gel, 3 x 5 g (= 65 mg) daily, n = 48 All participants treated with ice at site of inflammation for first 48 hours, but no immobilisation allowed Rescue medication: paracetamol 500 mg if strictly necessary | |
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Pain on movement: 100‐mm VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Pharmaceutically inert colouring agents added to reference formulation so that gels were indistinguishable |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in responder analysis |
| Size | High risk | < 50 participants in each treatment arm |
| Methods | R, DB, PC, parallel groups New patch applied directly to skin over painful area each morning Assessed at baseline, 3, 7, 14 days | |
| Participants | Symptomatic tendonitis in upper or lower limbs, not requiring surgery (≤ 15 days) N = 172 M 72, F 100 Mean age 46 years Baseline pain with activity ≥ 40 mm | |
| Interventions | Ketoprofen patch 100 mg, x 1 daily, n = 87 Placebo patch, x 1 daily, n = 85 No analgesic or steroid by any route or other topical medication or physical therapy allowed Rescue medication permitted, but not within 12 hours of assessment | |
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated global randomization code" |
| Allocation concealment (selection bias) | Low risk | "The randomization list and code envelopes were prepared by the company appointed for clinical supplies packaging. The random code was disclosed only after study completion and database closure" |
| Blinding (performance bias and detection bias) | Low risk | "the same indistinguishable patch with no ingredient" |
| Incomplete outcome data (attrition bias) | High risk | LOCF, withdrawals 12%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups New patch applied directly to skin over painful area each morning Assessed at baseline, 3, 7, 14 days | |
| Participants | Painful, benign ankle sprain (≤ 48 hours) N = 163 M 83, F 80 Mean age 37 years Baseline spontaneous pain ≥ 50 mm | |
| Interventions | Ketoprofen patch 100 mg, x 1 daily, n = 81 Placebo patch, x 1 daily, n = 82 No analgesic or steroid by any route or other topical medication or physical therapy allowed Rescue medication permitted, but not within 12 hours of assessment | |
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated global randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | The same transdermal patch with no active ingredient |
| Incomplete outcome data (attrition bias) | Unclear risk | LOCF, withdrawals 5% to 6%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied to injured site 3 times daily for 7 days Assessment at baseline 4, 7 days at clinic, daily patient diary | |
| Participants | Acute soft tissue injury (< 48 hours) N = 231 M 143, F 88 Mean age 33 years Baseline pain moderate to severe | |
| Interventions | Felbinac gel 3%, 3 x 3 cm daily, n = 118 Placebo gel, 3 x 3 cm daily, n = 113 Rescue medication: paracetamol | |
| Outcomes | Patient diary: mean change Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "tubes identical in all aspects" |
| Incomplete outcome data (attrition bias) | Unclear risk | No usable efficacy data |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied to site of injury 3 times daily for 7 days Assessed at baseline, 7 days at clinic, and daily patient diary | |
| Participants | Acute soft tissue injury (< 3 days) N = 100 (84 analysed for efficacy) M 70, F 14 Mean age 25 years Baseline pain moderate to severe Exclusions: 1 participant in placebo group lost to follow‐up, 15 protocol violations | |
| Interventions | Felbinac gel 3%, 3 x 1 cm daily, n = 41 Placebo gel, n = 43 Ice, joint immobilisation, bandaging and compression allowed No concomitant oral NSAID, occlusive dressing, physiotherapy, or liniments allowed Rescue medication: paracetamol | |
| Outcomes | PGE: 5‐point scale (responder = "good" and "very good") Change in PI: patient diary 10 cm VAS (mean data) Adverse events Withdrawals and exclusions | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Randomisation was undertaken at the production facility and a sealed copy of the list supplied to the investigator for reference, only in defined circumstances" |
| Blinding (performance bias and detection bias) | Low risk | "identical tubes and outer boxes", "placebo was a similarly constituted gel" |
| Incomplete outcome data (attrition bias) | Low risk | All relevant participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel group study Gel applied 4 x daily | |
| Participants | Acute lateral ankle sprain, Grade I to II, ≤ 12 hours Mean (± SD) age 31 years (± 13) | |
| Interventions | Diclofenac sodium gel 1% x 4 daily, n = 104 | |
| Outcomes | Mean PI on movement Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Method of blinding not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel group study Gel applied 4 x daily | |
| Participants | Acute lateral ankle sprain, Grade I to II, ≤ 12 hours M 101, F 104 | |
| Interventions | Diclofenac sodium gel 1%, x 4 daily, n = 102 | |
| Outcomes | Mean PI on movement Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Method of blinding not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC parallel groups Gel applied 4 x daily | |
| Participants | Acute blunt soft tissue injuries/contusions of the limbs, < 3 hours M 101, F 103 | |
| Interventions | Diclofenac sodium gel 1%, x 4 daily, n = 104 | |
| Outcomes | Mean PI on movement Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Method of blinding not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants accounted for |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC parallel groups Gel applied twice daily for 7 days Assessment at baseline, 3, 8 days | |
| Participants | Minor sports injuries (< 24 hours) N = 98 (93 analysed) M 71, F 27 Mean age 29 years Baseline pain > 60 mm | |
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm daily (= 15 mg), n = 48 Placebo gel, n = 45 No other treatment given | |
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Spontaneous pain: 100 mm VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "allocated according to a randomization list and a corresponding code in a sealed envelope" |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Foam (the size of a walnut, or a 1‐second spray) applied with massage 3 x daily for 7 days | |
| Participants | Articular trauma, strains, distortions N = 169 M 94, F 75 Mean age 37 years Mean baseline pain on movement 3.1 (scale 1 to 4) | |
| Interventions | Ketoprofen foam 15%, 3 x 2 g (= 600 mg) daily, n = 83 Placebo foam, 3 x 2 g, n = 86 | |
| Outcomes | Pain on movement: 4‐point scale (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised according to the method of random numbers" [translated] |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No usable efficacy data |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Cream applied with slight massage until completely absorbed, 3 times daily for up to 16 days Assessed at baseline, 4, 8, 12, 16 days | |
| Participants | Acute sports injuries N = 40 M 24, F 16 Mean age 22 years (range 12 to 46) Most participants had mild to moderate baseline pain (12 and 9 with slight pain on movement) | |
| Interventions | Ibuprofen gel 10%, 3 x daily, n = 20 Ketoprofen gel 1%, 3 x daily, n = 20 | |
| Outcomes | Pain on movement (responder = "none") Adverse events | |
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | "tubes were identical in appearance" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups New patch applied to injured area twice daily for 7 days. Contact of patch with humidity or water to be avoided Assessment at baseline 3, 7 days | |
| Participants | Traumatic blunt soft tissue injuries (< 3 hours, no treatment) N = 120 M 73, F 47 Mean age 32 years Baseline pain > 60 mm | |
| Interventions | Diclofenac sodium patch, 2 x daily (140 mg per patch), n = 60 Placebo patch, 2 x daily, n = 60 NSAIDs, analgesics, psychotropic agents, other topical preparations, and bandages not allowed | |
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Pain on movement: 10 cm VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated block randomisation list |
| Allocation concealment (selection bias) | Low risk | An independent statistician produced randomisation list, and an independent contract research organisation packaged medication according to list. Nobody else had access to the randomisation list until the database was closed |
| Blinding (performance bias and detection bias) | Low risk | "The placebo patch was visually indistinguishable from the active patch." To avoid unblinding due to different smell, any study nurse involved with medication was not involved in outcome assessment |
| Incomplete outcome data (attrition bias) | Low risk | Withdrawals < 1%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups | |
| Participants | Grade I or II ankle sprain with lateral external ligament involvement, < 12 hours M 152, F 90 | |
| Interventions | Diclofenac gel (Voltaren Emulgel 2.32%), 2 x 5 cm + 1 x 5 cm placebo gel daily, n = 80 Rescue medication: paracetamol (to maximum 2 g daily) | |
| Outcomes | ≥ 50% reduction in PI on movement at 5 days PGE: 5‐point scale (responder = "good" or 'very good") Patient satisfaction: 5‐point scale (responder = "good" or 'very good" or "excellent") Rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "identical in composition, appearance, texture and smell" |
| Incomplete outcome data (attrition bias) | Low risk | LOCF, but withdrawals < 3% in all treatment arms |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups | |
| Participants | Uncomplicated, one‐sided ankle sprain with swelling ≥ 12 mm (2 to 18 hours) M 126, F 106 | |
| Interventions | Diclofenac 4% spray gel, 3 x 4 to 5 sprays daily (96 to 120 mg diclofenac sodium), n = 118 Rescue medication: paracetamol 500 mg (maximum 10 tablets per week) | |
| Outcomes | None or slight PI on movement at 3 and 7 days PGE: 4‐point scale (responder = "very good" at 14 days) > 50% reduction in swelling at 10 days Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | "placebo (vehicle only, no active ingredient)" |
| Incomplete outcome data (attrition bias) | Low risk | LOCF for full or early cure. Withdrawals < 2% |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups | |
| Participants | Uncomplicated neck pain originating from cervical joints and accompanying soft tissues (≥ 12 hours but < 3 months), PI ≥ 50/100 M 33, F 39 | |
| Interventions | Diclofenac gel (Voltaren Emulgel), 4 x 2 g daily, n = 36 Rescue medication: paracetamol up to 2 g daily | |
| Outcomes | ≥ 50% decrease in PI on movement after 48 hours Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sponsor "produced randomisation list" |
| Allocation concealment (selection bias) | Low risk | Automated remote system |
| Blinding (performance bias and detection bias) | Low risk | "identical in packaging, labelling, schedule of administration, appearance and odour" |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Cream applied to painful area and rubbed into skin over a large area for up to 10 days Assessment at baseline, 3, 7, 10 days | |
| Participants | Strains, sprains, contusions, compressions N = 80 M 42, F 38 Age 11 to 81 years Baseline pain: 5 ibuprofen, 2 placebo participants had no or slight pain | |
| Interventions | Ibuprofen cream 5%, 3 to 4 x 5 to 10 cm daily, n = 40 Placebo cream, 3 to 4 x 5 to 10 cm daily, n = 40 Adjuvant therapy was not administered | |
| Outcomes | Pain on movement: 4‐point scale (responder = "none" or "slight") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation key in sealed envelope, available for emergencies, but opened only after completion |
| Blinding (performance bias and detection bias) | Low risk | "identical appearance and odour" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups New patches applied to the affected painful area for 12 consecutive hours twice daily, for up to 14 days Assessed at baseline, 14 days in clinic and daily patient diary | |
| Participants | Minor sports injuries (sprains, sprains, contusions, < 72 hours) N = 372 M 253, F 119 Mean age 33 years Baseline pain at rest ≥ 5/10 | |
| Interventions | DHEP patch (Flector Tissuegel), 2 x daily (equivalent to diclofenac sodium 140 mg per patch), n = 191 Placebo patch, 2 x daily, n = 181 | |
| Outcomes | PGE: 5‐point scale (responder = "good" and "excellent") Pain resolved: < moderate for 2 days Spontaneous pain: 10 cm VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | "Système identique" without diclofenac |
| Incomplete outcome data (attrition bias) | Unclear risk | No information about imputation. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Affected area washed with soap and water and dried, then gel applied and carefully rubbed into skin, 4 times daily for at least 7 days Assessed at baseline, 4, 8, 15 (if necessary) days at clinic, and daily patient diary | |
| Participants | Acute soft tissue injuries (recent, not recurrent) N = 214 (200 analysed) M 95, F 105 Mean age 40 years Baseline pain > 65 mm | |
| Interventions | Piroxicam gel 0.5%, 4 x 5 mg daily, n = 100 Placebo gel, n = 100 No other NSAIDs or analgesic drugs, including liniments containing salicylates, allowed. Ancillary therapy at the discretion of the investigator | |
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Spontaneous pain: mean reduction Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "identical base formulation" |
| Incomplete outcome data (attrition bias) | High risk | High withdrawal rate (8% with piroxicam, 50% with placebo). No information about imputation |
| Size | Unclear risk | 50 to 100 participants per treatment group |
| Methods | R, DB, PC, parallel groups | |
| Participants | Ankle sprain (< 48 hours) | |
| Interventions | (1) DHEP plaster (Flector Tissugel 1%), 1 x daily, n = 70 Rescue medication: paracetamol (to maximum 3 g daily) | |
| Outcomes | ≥ 30% decrease in PI at 7 days PGE: 4‐point scale (responder = "excellent") No or low pain on passive stretch Single foot leaning OK without pain Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Identical in size, appearance, and used same formula as active patch, without active ingredient |
| Incomplete outcome data (attrition bias) | Unclear risk | Used LOCF for primary outcome. Withdrawals < 7% and equal between groups. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied 3 times daily for 7 consecutive days Assessment at baseline, 7 days | |
| Participants | Soft tissue trauma (< 48 hours) N = 82 M = 47, F = 35 Mean age 34 years Baseline pain moderate to severe | |
| Interventions | Felbinac* gel 3%, 3 x daily, n = 42 Placebo gel, 3 x daily, n = 40 No other NSAID, steroid, other topical application allowed Rescue medication: paracetamol * felbinac is an active metabolite of the NSAID fenbufen | |
| Outcomes | PGE: scale not reported (responder = "good" and "very good") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "indistinguishable in appearance, colour or odour" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Cream applied 2 to 3 times daily, with gentle massage, or if massage not possible (too painful) with protective dressing Assessment at baseline, 5, 10 days | |
| Participants | Minor soft tissue injuries N = 20 M 11, F 9 Mean age 40 years Baseline pain not reported | |
| Interventions | Fentiazac cream 5%, 2 to 3 x daily, n = 10 Placebo cream, 2 to 3 x daily, n = 10 All participants told to rest No other local and systemic treatments allowed Rescue medication: analgesic if actually needed | |
| Outcomes | Pain relief: scale not reported (responder = total pain relief) % improvement in pain on movement: pain scale not reported (mean data) Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied 3 times daily, with gentle massage until complete absorption, for up to 10 days Assessment at baseline, 10 days in clinic, and daily patient diary | |
| Participants | Shoulder periarthritis or lateral epicondylitis (< 5 days) N = 155 M 74, F 81 Mean age 51 years Baseline pain with activity > 70 mm | |
| Interventions | DHEP lecithin gel (Effigel), 3 x 5 g daily, n = 79 Placebo gel, 3 x 5 g daily, n = 76 Rescue medication (paracetamol) allowed if pain unbearable No other analgesic or anti‐inflammatory drug allowed | |
| Outcomes | Improvement in pain: 100 mm VAS (mean data) Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | No usable efficacy data |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel applied to affected area 3 to 4 times daily, without occlusion, for 14 days Assessed at baseline, 7, 14 days | |
| Participants | Non‐traumatic diseases of muscle or tendon N = 366 (340 analysed for efficacy) M 115, F 202 (completers) Age range 12 to 84 years (most 30 to 70) Baseline pain on movement "none" or "mild" in about 1/3 of participants Exclusions for protocol violations: 8 piroxicam, 18 indomethacin | |
| Interventions | Piroxicam gel 0.5%, 3 to 4 x 1 g daily, n = 183 Indomethacin gel 1%, 3 to 4 x 1 g daily, n = 183 No concomitant anti‐inflammatory or analgesic drug, including steroids, or initiation of physical therapy allowed | |
| Outcomes | PGE: 5‐point scale (responder = "better" or "much better") Pain on movement: 4‐point scale (responder = "reduced" or "disappeared") | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Key code sealed and retained until end of study |
| Blinding (performance bias and detection bias) | Low risk | "both packages were of the same appearance and indistinguishable", and investigators did not see contents |
| Incomplete outcome data (attrition bias) | Low risk | All relevant participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Participants given specific instructions on how to apply gel (not reported) to affected area 2 to 6 times daily as required Assessment at baseline, 3, 7 days in clinic | |
| Participants | Soft tissue injuries (< 48 hours) N = 120 M 85, F 35 Mean age 27 years Baseline pain moderate to severe | |
| Interventions | Naproxen gel 10%, 2 to 6 x daily, n = 60 Placebo gel, 2 to 6 x daily, n = 60 Rescue medication: paracetamol 500 mg | |
| Outcomes | PGE: 5‐point scale (responder = "good" and "very good") Pain on passive movement: 4‐point scale (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "supplied in unmarked tubes" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
| Methods | R, DB, AC, parallel groups Gel applied 3 times daily for 2 to 3 weeks Assessed at baseline, and intervals of 7 days | |
| Participants | Muscle or joint trauma N = 30 M 20, F 10 Mean age 34 years 1 participant had injury of mild severity. Mean baseline pain on active movement 2.8 (scale 0 to 4) | |
| Interventions | Ketoprofen gel 5%, 3 x 2 to 3 g daily, n = 15 Etofenamate gel 5%, 3 x 2 to 3 g, n = 15 No concomitant treatment with NSAID, aspirin, steroid or physical therapy | |
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Pain on movement: 5‐point scale (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | "the two drugs were packed in indistinguishable tubes" |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB, PC, parallel groups Gel applied to the skin on and around painful area and gently rubbed in until absorbed, twice daily for up to 10 days Assessed at baseline, 5, 10 days | |
| Participants | Soft tissue trauma (minor sports injuries) N = 60 M 60 Mean age 25 years Mean baseline pain on active movement: moderate | |
| Interventions | Meclofenamic acid gel 5%, 2 x 10 cm daily (= 4 g), n = 30 Placebo gel, 2 x 10 cm daily, n = 30 Both groups treated with ice, rest, and bandage for first 48 hours before starting test treatment Rescue medication: paracetamol | |
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Pain on movement: 4‐point scale (mean data) Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
| Methods | R, DB (double dummy), AC, parallel groups Gel applied to affected site, with gentle massage, and 1 tablet taken 3 times daily for at least 7 days Assessed at baseline, 7, 14 (if necessary) days in clinic, and daily patient diary | |
| Participants | Soft tissue injuries (< 24 hours) N = 100 M 95, F 5 Mean age 26 years (range 18 to 50) Mean baseline pain on movement 2.2 cm | |
| Interventions | Ibuprofen gel 5% + placebo tablet 3 x daily, n = 50 Ibuprofen 400 mg tablet + placebo gel 3 x daily, n = 50 No other medication or physical therapy was prescribed and no other analgesics were allowed | |
| Outcomes | PGE: 3‐point scale (responder = "excellent") Change in condition of injury site: 5‐point scale (responder = "completely better") Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | Placebo tablets Identical in appearance to active tablets. Active and placebo gels had similar physical characteristics and were supplied in identical tubes |
| Incomplete outcome data (attrition bias) | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 participants per treatment arm |
| Methods | R, DB (double dummy), PC, AC, parallel groups Spray applied to affected area, and capsules taken 3 times daily for 2 weeks Assessment at baseline, 3 or 4, 7, and 14 days | |
| Participants | Superficial overuse sports injuries (symptom onset 7.4 weeks) N = 70 M 44, F 18 (completers) Mean age 30 years Baseline pain on palpation mostly slight to moderate | |
| Interventions | Elmetacin spray (indomethacin 1%), 3 to 5 x 0.5 to 1.5 ml daily + placebo capsules, n = 23 Indomethacin capsules, 3 x 25 mg daily + placebo spray, n = 23 Placebo spray and capsules, n = 24 Rescue medication: paracetamol | |
| Outcomes | No pain on palpation (= responder) Participant improvement: 100 mm VAS (mean data) Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | "identical in appearance" |
| Incomplete outcome data (attrition bias) | Low risk | Responder analysis, < 5% withdrawals |
| Size | High risk | < 50 participants per treatment group |
AC: active control; BOCF: baseline observation carried forward; DB: double‐blind; DHEP: diclofenac hydroxyethylpyrrolidine, or diclofenac epolamine; F: female; HCl: hydrochloride; hep: heparin; LOCF: last observation carried forward; M: male; N: number of participants in study; n: number of participants in treatment arm; PC: placebo control; PGE: Participant Global Evaluation; PI: pain intensity; R: randomised; VAS: visual analogue scale; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No usable dichotomous data | |
| Not double‐blind | |
| No usable dichotomous data | |
| Not an RCT | |
| No usable data | |
| <10 participants/treatment arm in first period of crossover study | |
| Active control invalid | |
| Not an RCT | |
| Not double‐blind | |
| No usable dichotomous data | |
| Inappropriate randomisation | |
| No usable dichotomous data | |
| No usable dichotomous data | |
| No usable data | |
| Not double‐blind | |
| Not double‐blind | |
| Inappropriate randomisation ‐ quasi‐randomised | |
| Treatment not applied daily | |
| Used NSAID‐lidocaine combination (conference abstract) | |
| Not an RCT | |
| No usable dichotomous data | |
| No usable dichotomous data | |
| Inappropriate comparator | |
| Not an RCT | |
| Not an RCT | |
| Dose and duration of treatment unclear | |
| Not double‐blind | |
| Not an RCT | |
| Chronic and acute outcomes combined |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | R, DB, PC, parallel groups |
| Participants | Ankle sprain or strain, Grade I or II. Age ≥ 18 years N = 220 |
| Interventions | Ketoprofen patch 20% x 1 daily |
| Outcomes | Assessments days 3, 7, 21 |
| Notes | Sponsor: Endo Pharmaceuticals Estimated completion February 2007. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Shoulder, elbow, or knee tendonitis or bursitis. Age ≥ 18 years N = 330 |
| Interventions | Ketoprofen patch 20% x 1 daily |
| Outcomes | Assessments days 3, 7, 21 |
| Notes | Sponsor: Endo Pharmaceuticals Estimated completion February 2007. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Shoulder, elbow, or knee tendonitis or bursitis. Age ≥ 18 years |
| Interventions | Ketoprofen patch 20% x 1 daily |
| Outcomes | PI during activity Safety |
| Notes | Study terminated May 2008, but "sufficient number of subjects accrued to conduct analysis". No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Ankle sprain or strain, Grade I or II, ≤ 48 hours, PI 5/10 to 9/10. Age 18 to 75 years N = 170 |
| Interventions | Diclofenac sodium patch (15 mg) x 1 daily |
| Outcomes | Efficacy Safety |
| Notes | Study terminated July 2008 (Sponsor decision ‐ no further details). No results posted |
| Methods | R, DB, PC, parallel groups |
| Participants | Shoulder, elbow, or knee tendonitis or bursitis. Age 18 to 75 years N = 308 |
| Interventions | Diclofenac sodium patch 1% (15 mg) x 1 daily Duration of treatment not reported, probably 14 days |
| Outcomes | Efficacy Safety |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion April 2008. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute unilateral shoulder pain, requiring treatment for ≥ 2 weeks. Age ≥ 18 years N = 368 |
| Interventions | Ketoprofen patch (HKT‐500) x 1 daily |
| Outcomes | Pain Safety |
| Notes | Sponsor: Hisamitsu Pharmaceutical Co., Inc. Estimated completion October 2008. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute benign ankle sprain, Grade I‐II, ≤ 48 hours. Age ≥ 18 years N = 260 |
| Interventions | Ketoprofen patch (HKT‐500) x 1 daily |
| Outcomes | Pain Safety |
| Notes | Sponsor: Hisamitsu Pharmaceutical Co., Inc. Estimated completion November 2008. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute sprain or strain of upper and lower extremities, mild to moderate injuries, ≤ 72 hours. Age 18 to 70 years N = 364 |
| Interventions | Ketoprofen cream 10% 1 g x 3 daily |
| Outcomes | Pain Safety |
| Notes | Sponsor: Imprimis Pharmaceuticals, Inc. Estimated completion September 2009. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Wrist sprain, strain, or contusion (mild to moderate), "recent". Age 17 to 75 years N = 214 |
| Interventions | Diclofenac sodium patch 1% x 1 daily |
| Outcomes | Change in PI during activity at 3 and 7 days Safety |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion September 2009. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Unilateral, ankle sprain (mild or moderate), "recent". Age 17 to 75 years N = 219 |
| Interventions | Diclofenac sodium patch x 1 daily |
| Outcomes | Change in PI during activity at 3 and 7 days |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion August 2009. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Unilateral soft tissue injury between mid‐bicep to wrist or mid‐thigh to ankle (mild to moderate), "recent". Age 18 to 75 years N = 407 |
| Interventions | Diclofenac sodium patch x 1 daily |
| Outcomes | Change in PI during activity at 7 and 14 days Safety |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion December 2009. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute ankle sprain, Grade I or II ≤ 24 hours. Age ≥ 16 years. Baseline PI ≥ 5/10 N = 305 |
| Interventions | Ibuprofen cream 200 mg in 2.7 g cream x 4 daily |
| Outcomes | PI on movement daily to 7 days Systemic and local adverse events |
| Notes | Estimated completion January 2014. No results |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute lateral ankle sprain, Grade I‐II, ≤ 24 hours, PI ≥ 5/10. Age 18 to 65 years N = 270 |
| Interventions | Indomethacin patch x 2 daily Duration of treatment not reported, probably 7 days |
| Outcomes | PI on movement daily |
| Notes | Estimated completion September 2014. No results |
| Methods | R, DB, PC and AC, parallel groups |
| Participants | Uncomplicated acute ankle sprain, Grade I or II, < 48 hours, PI > 50/100. Age 18 to 65 years N = 658 |
| Interventions | Diclofenac epolamine (Flector) 1.3% patch Duration of treatment not reported |
| Outcomes | Bioequivalence study Application site reactions |
| Notes | Estimated completion December 2014. No results |
| Methods | R, DB, AC, parallel group, non‐inferiority study Duration 7 days |
| Participants | Acute musculoskeletal injury (mainly muscular, joint, tendon) N = 697 M 271, F 426 Mean age 52 years |
| Interventions | Ketoprofen (SKP‐021) patch, ketoprofen 30 mg Diclofenac (Voltadola), patch diclofenac sodium 140 mg Patches applied twice daily for 7 days |
| Outcomes | ≥ 50% reduction in PI from baseline to end of study (100 mm VAS) Patient overall rating Clinical symptoms (4‐point scale) Time to response Adverse events, skin reactions Serious adverse events |
| Notes | "The analysis of the data of this trial showed that the two formulations were equally effective and well tolerated in the treatment of acute musculoskeletal injuries." |
AC: active‐controlled; DB: double‐blind; F: female; M: male; N: number of participants in study; PC: placebo‐controlled; PGE: Patient Global Evaluation of treatment; PI: pain intensity; R: randomised; VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Placebo‐controlled, double‐blind evaluation of the efficacy and safety of ibuprofen 5% topical gel for the treatment of ankle sprain |
| Methods | R, DB, PC, parallel groups |
| Participants | Ankle sprain, Grade I or II, age ≥ 12 years Estimated enrolment 280 |
| Interventions | Ibuprofen gel 5% x 2 daily Ibuprofen gel 5% x 3 daily |
| Outcomes | PI on weight bearing and rest at 7 days Safety |
| Starting date | November 2013 |
| Contact information | Pfizer |
| Notes | Estimated completion February 2015 Includes participants aged ≥ 12 years |
| Trial name or title | A clinical study to assess the efficacy and onset of pain relief of topical MFC51123 diclofenac‐menthol gel versus controls in ankle sprain |
| Methods | R, DB, PC, and AC, parallel groups |
| Participants | Acute lateral ankle sprain, Grade I or II, ≤ 24 hours, PI ≥ 5/10. Age 16 to 65 years Estimated enrolment 400 |
| Interventions | Diclofenac sodium 1% + methanol 3% gel |
| Outcomes | PR Adverse events |
| Starting date | November 2013 |
| Contact information | |
| Notes | Estimated completion November 2014 |
| Trial name or title | A randomised, double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy and safety of diclofenac sodium topical gel (DSG) 1% applied four times daily in subjects with acute blunt soft tissue injuries/contusions of the limbs |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute blunt soft tissue injuries or contusions of the limbs, ≤ 6 hours ("fresh"). Age ≥ 16 years Estimated enrolment 200 |
| Interventions | Diclofenac sodium gel 1% x 4 daily Duration of treatment not reported |
| Outcomes | Pain Safety |
| Starting date | December 2014 |
| Contact information | Novartis |
| Notes | Estimated completion August 2015 |
AC: active control; DB: double‐blind; PC: placebo‐controlled; PGE: Patient Global Evaluation of treatment; PI: pain intensity; PID: pain intensity difference; PR: pain relief; R: randomised
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Individual NSAID versus placebo, Outcome 1 Clinical success. | ||||
| 1.1 Diclofenac | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events Show forest plot | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Individual NSAID versus placebo, Outcome 2 Local adverse events. | ||||
| 2.1 Diclofenac | 15 | 3271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.10] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| Analysis 2.1  Comparison 2 Diclofenac versus placebo (effect of formulation), Outcome 1 Clinical success. | ||||
| 1.1 Plaster ‐ Flector | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.36, 1.71] |
| 1.2 Plaster ‐ other | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.37, 1.75] |
| 1.3 Gel ‐ Emulgel | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [2.68, 5.50] |
| 1.4 Gel ‐ other | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| Analysis 3.1 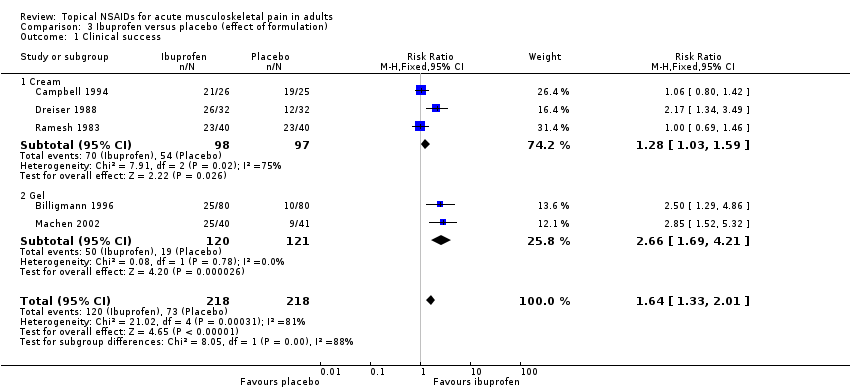 Comparison 3 Ibuprofen versus placebo (effect of formulation), Outcome 1 Clinical success. | ||||
| 1.1 Cream | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 1.2 Gel | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.69, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| Analysis 4.1  Comparison 4 Ketoprofen versus placebo, Outcome 1 Clinical success. | ||||
| 1.1 Plaster | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.2 Gel | 5 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local adverse events Show forest plot | 42 | 6740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| Analysis 5.1 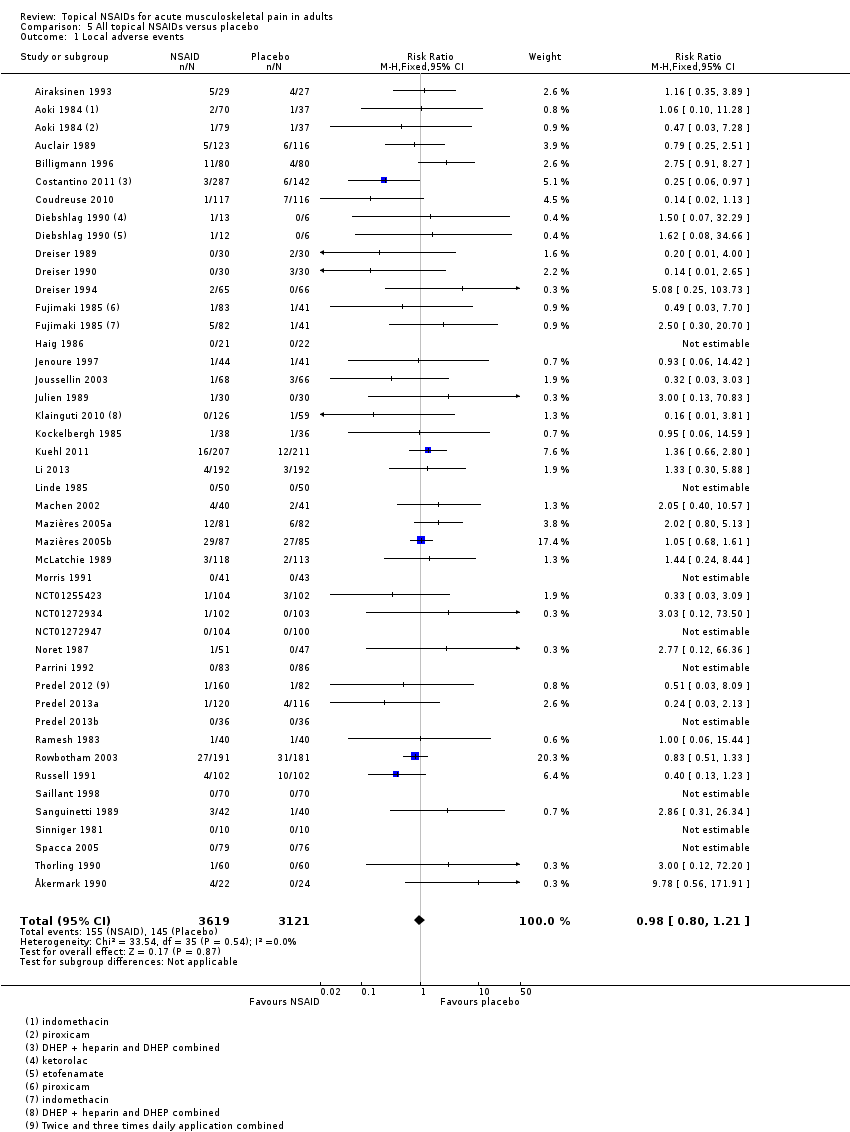 Comparison 5 All topical NSAIDs versus placebo, Outcome 1 Local adverse events. | ||||
| 2 Systemic adverse events Show forest plot | 36 | 5576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| Analysis 5.2 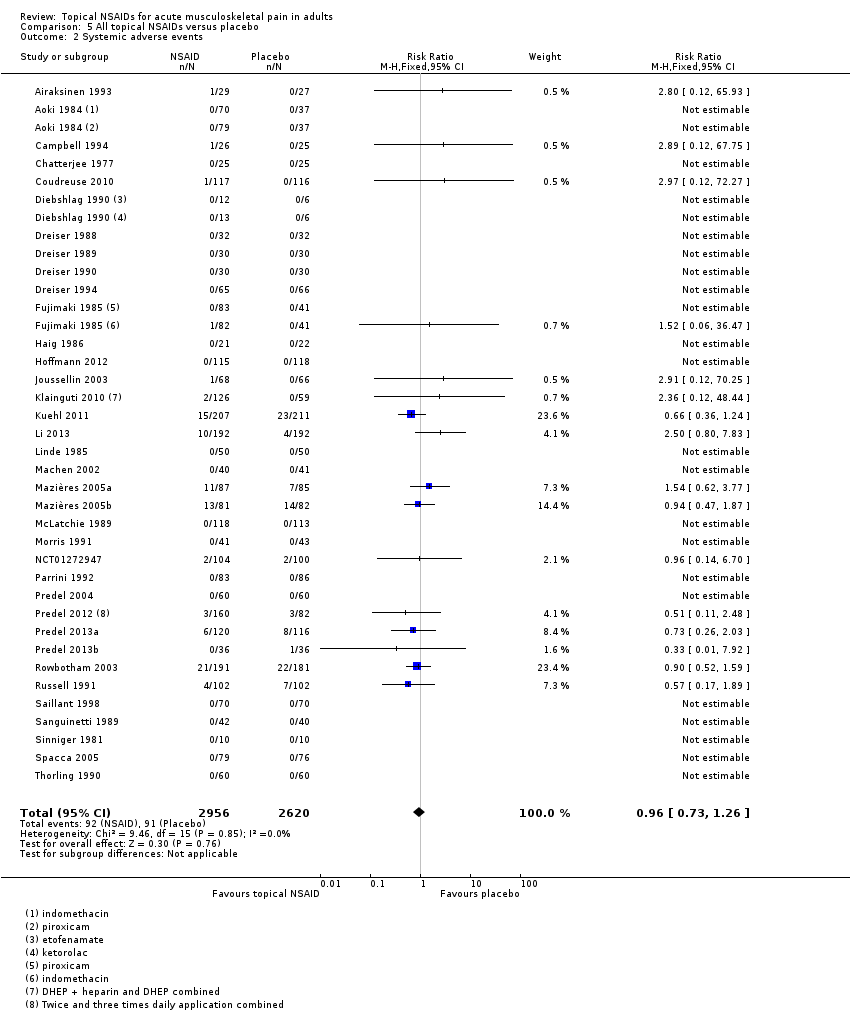 Comparison 5 All topical NSAIDs versus placebo, Outcome 2 Systemic adverse events. | ||||
| 3 Adverse event withdrawals Show forest plot | 42 | 6405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.59] |
| Analysis 5.3  Comparison 5 All topical NSAIDs versus placebo, Outcome 3 Adverse event withdrawals. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| Analysis 6.1  Comparison 6 Topical NSAID versus active comparator, Outcome 1 Clinical success ‐ topical piroxicam vs topical indomethacin. | ||||
| 2 Local adverse events ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |
| Analysis 6.2  Comparison 6 Topical NSAID versus active comparator, Outcome 2 Local adverse events ‐ topical piroxicam vs topical indomethacin. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
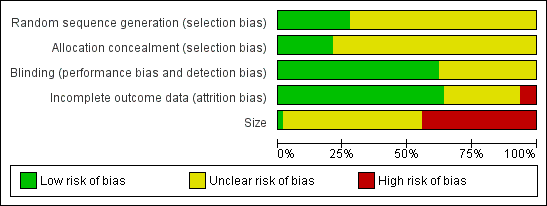
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
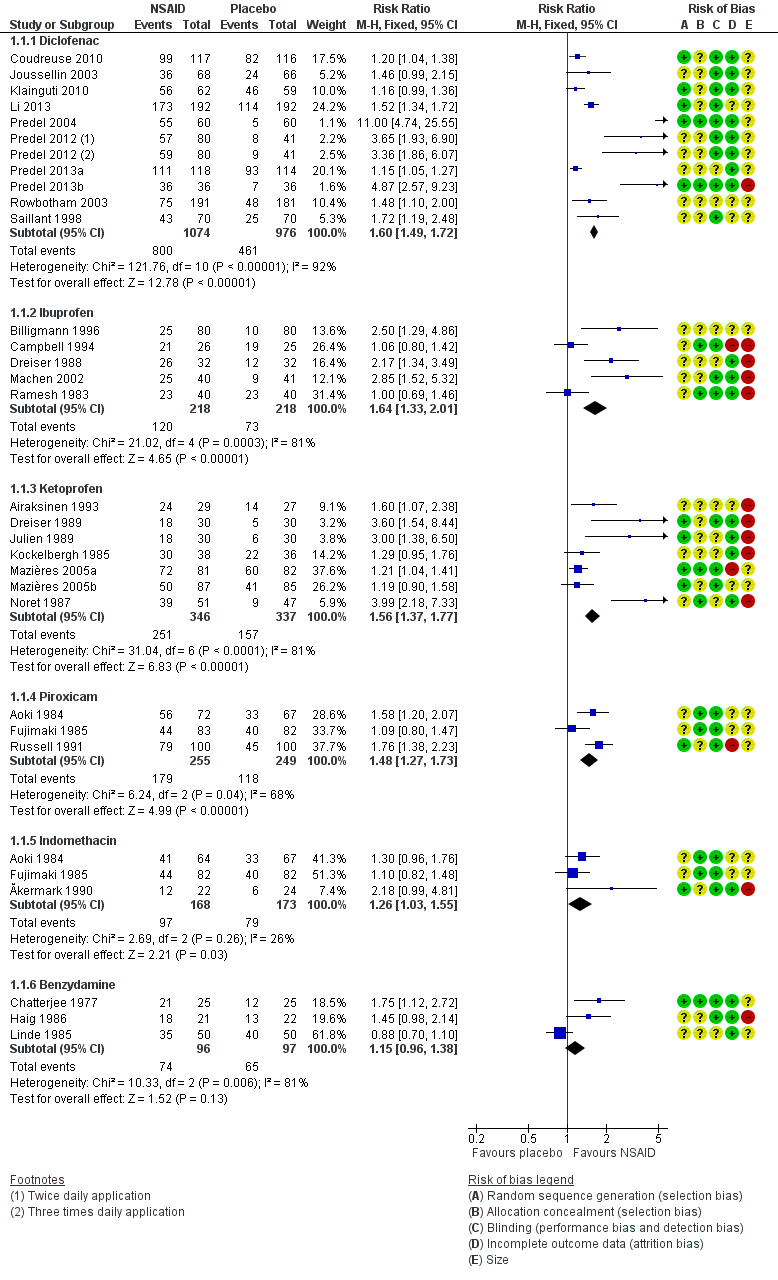
Forest plot of comparison: 2 Individual NSAID versus placebo, outcome: 2.1 Clinical success.

L'Abbé plot of clinical success in studies of topical diclofenac versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Dark blue: Emulgel; light blue: spray/gel; red: Flector; pink: other patch or plaster.

L'Abbé plot of clinical success in studies of topical ketoprofen versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Light blue: ketoprofen gel; pink: ketoprofen plaster.

Comparison 1 Individual NSAID versus placebo, Outcome 1 Clinical success.

Comparison 1 Individual NSAID versus placebo, Outcome 2 Local adverse events.

Comparison 2 Diclofenac versus placebo (effect of formulation), Outcome 1 Clinical success.

Comparison 3 Ibuprofen versus placebo (effect of formulation), Outcome 1 Clinical success.
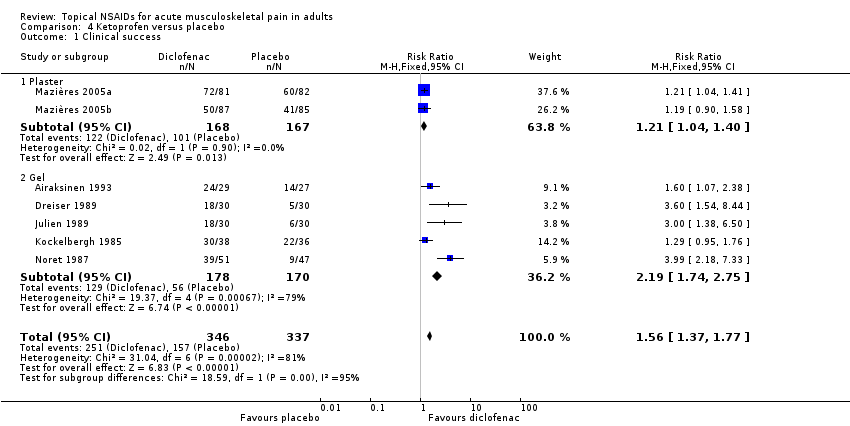
Comparison 4 Ketoprofen versus placebo, Outcome 1 Clinical success.
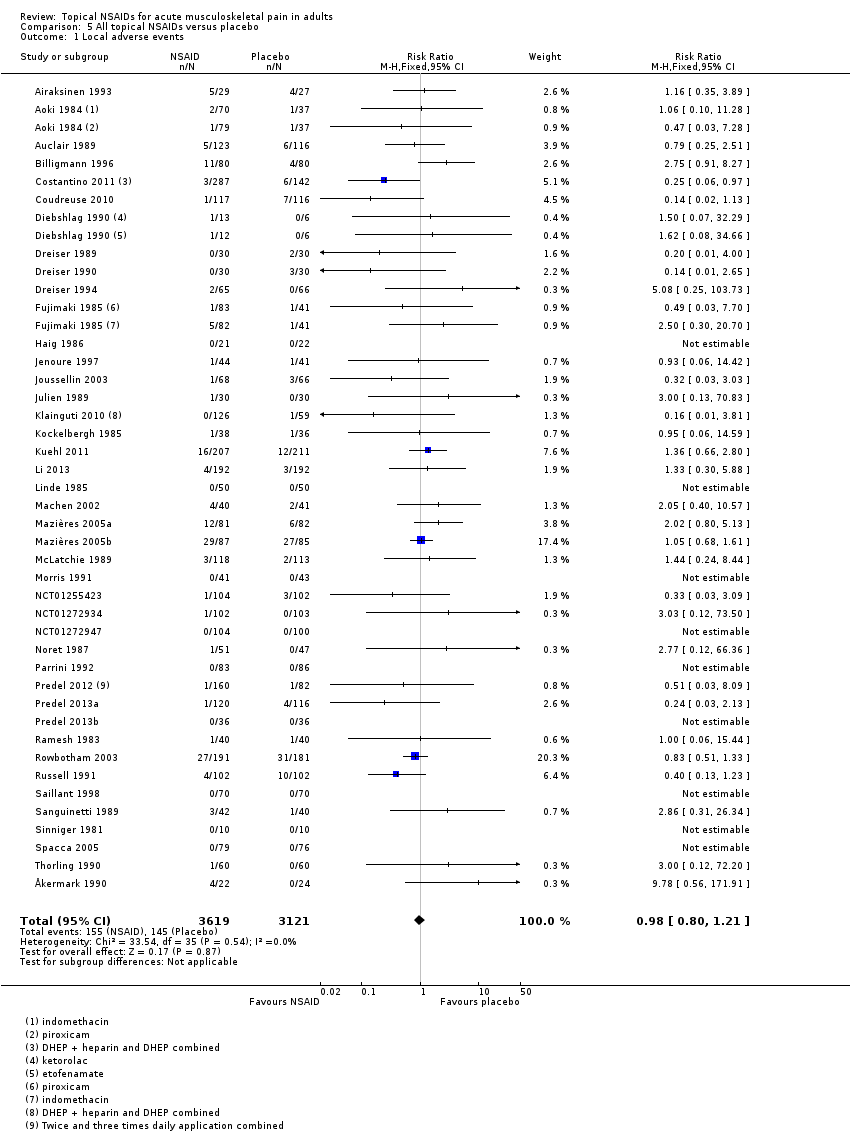
Comparison 5 All topical NSAIDs versus placebo, Outcome 1 Local adverse events.
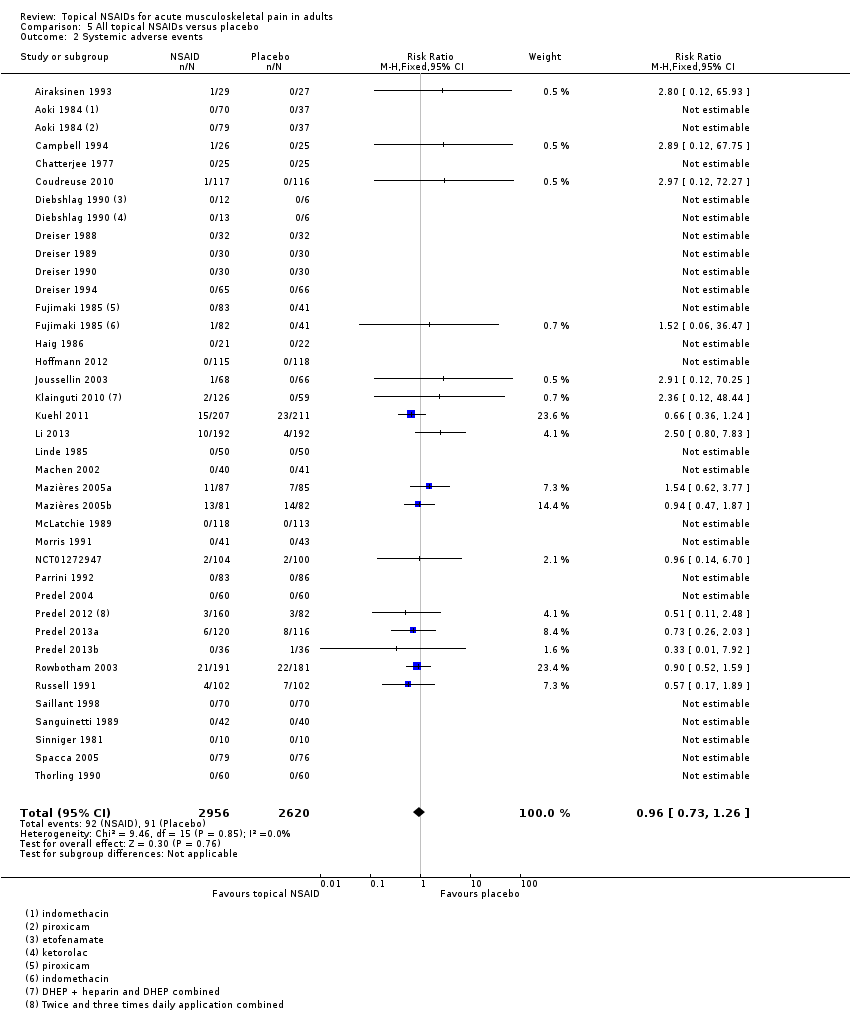
Comparison 5 All topical NSAIDs versus placebo, Outcome 2 Systemic adverse events.
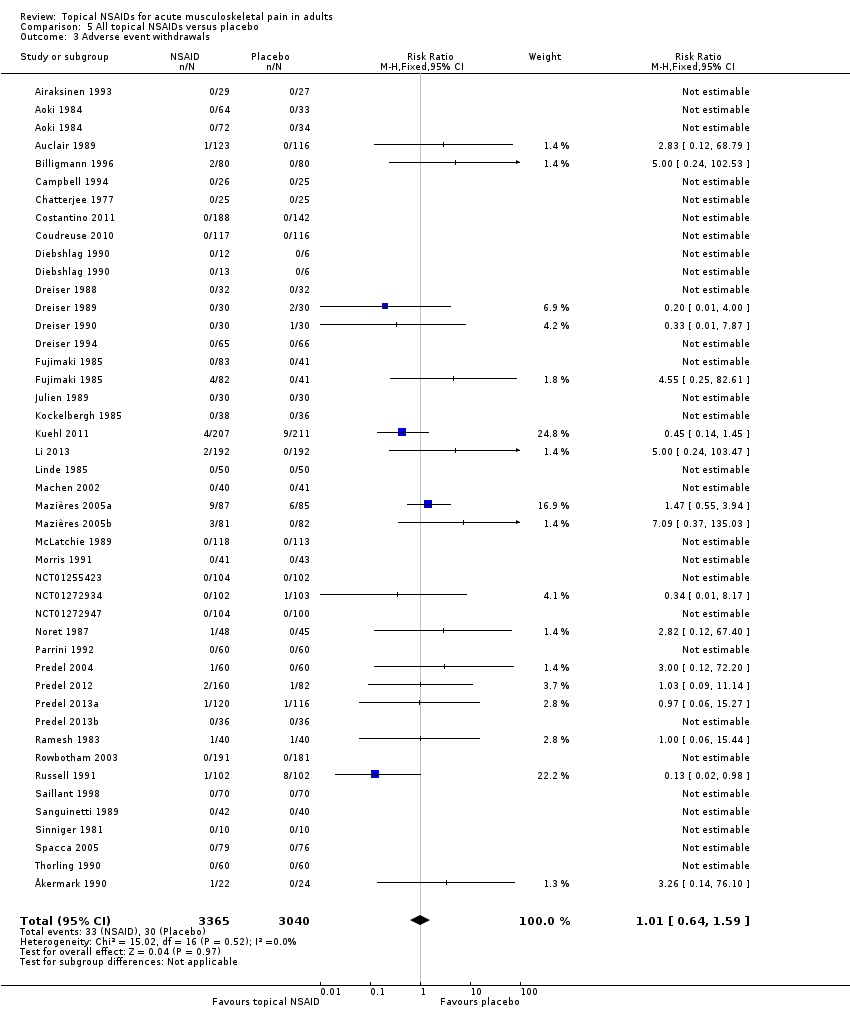
Comparison 5 All topical NSAIDs versus placebo, Outcome 3 Adverse event withdrawals.
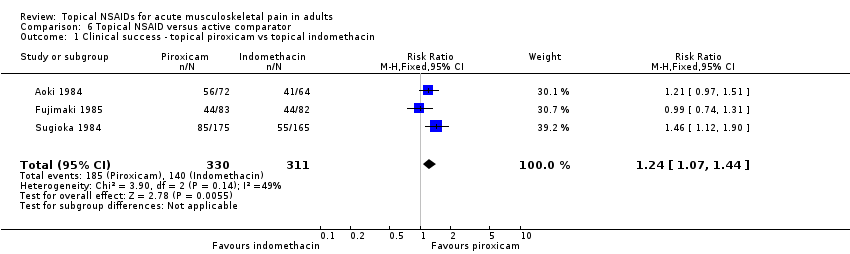
Comparison 6 Topical NSAID versus active comparator, Outcome 1 Clinical success ‐ topical piroxicam vs topical indomethacin.

Comparison 6 Topical NSAID versus active comparator, Outcome 2 Local adverse events ‐ topical piroxicam vs topical indomethacin.
| Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults | ||||||
| Patient or population: adults with strains, sprains, or muscle pull Settings: community Intervention: topical NSAID (topical diclofenac, ibuprofen, and ketoprofen gels only shown here for efficacy) Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | RR, NNT, NNTp, or NNH | No of studies, participants | Quality of the evidence | Comments |
| Topical diclofenac gel (as Emulgel) Clinical success (eg 50% reduction in pain) | 780 in 1000 | 200 in 1000 | RR 3.4 (2.7 to 55) NNT 1.8 (1.5 to 2.1) | 2 studies 314 participants | High | Consistent results in 2 moderately sized recent studies of high quality |
| Topical ibuprofen gel Clinical success (eg 50% reduction in pain) | 420 in 1000 | 160 in 1000 | RR 2.7 (1.7 to 4.2) NNT 3.9 (2.7 to 6.7) | 2 studies 241 participants | Moderate | Modest effect size and numbers of participants |
| Topical ketoprofen gel Clinical success (eg 50% reduction in pain) | 720 in 1000 | 330 in 1000 | RR 2.2 (1.7 to 2.8) NNT 2.5 (2.0 to 3.4) | 5 studies 348 participants | Moderate | Modest effect size and numbers of participants, but studies small, with none recent |
| All topical NSAIDs Local adverse events | 46 in 1000 | 50 in 1000 | RR 1.0 (0.80 to 1.2) NNH not calculated | 42 studies 6125 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Systemic adverse events | 32 in 1000 | 35 in 1000 | RR 1.0 (0.7 to 1.3) NNH not calculated | 38 studies 5372 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Withdrawals ‐ adverse events | 11 in 1000 | 11 in 1000 | RR 1.0 (0.7 to 1.7) NNH not calculated | 42 studies 5790 participants | High | Large number of studies and participants with consistent results |
| Serious adverse events | 1 in total | 0 in total | Not calculated | All data | Low | Small numbers of events |
| GRADE Working Group grades of evidence | ||||||
| CI: confidence interval; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event happening; NNH: number needed to treat for an additional harmful outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Diclofenac | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events Show forest plot | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac | 15 | 3271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.10] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.1 Plaster ‐ Flector | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.36, 1.71] |
| 1.2 Plaster ‐ other | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.37, 1.75] |
| 1.3 Gel ‐ Emulgel | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [2.68, 5.50] |
| 1.4 Gel ‐ other | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.1 Cream | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 1.2 Gel | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.69, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.1 Plaster | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.2 Gel | 5 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local adverse events Show forest plot | 42 | 6740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Systemic adverse events Show forest plot | 36 | 5576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 3 Adverse event withdrawals Show forest plot | 42 | 6405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| 2 Local adverse events ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |

