AINE tópicos para el dolor musculoesquelético agudo en adultos
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007402.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 junio 2015see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
For the earlier review, Tom Massey and SD identified studies, and carried out data extraction, analysis and drafting. RAM and HJM were involved in planning, acted as adjudicators, and were involved with writing.
For this update, SD and RAM carried out searches, data extraction, and analysis. All authors were involved with writing the review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
No sources of support supplied
Declarations of interest
SD has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
PW has no conflicts relating to this review or any similar product.
HG has no conflicts relating to this review or any similar product.
MM has no conflicts relating to this review or any similar product.
Acknowledgements
The Oxford Pain Relief Trust, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme provided support for the earlier review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jun 15 | Topical NSAIDs for acute musculoskeletal pain in adults | Review | Sheena Derry, R Andrew Moore, Helen Gaskell, Mairead McIntyre, Philip J Wiffen | |
| 2010 Jun 16 | Topical NSAIDs for acute pain in adults | Review | Thomas Massey, Sheena Derry, R Andrew Moore, Henry J McQuay | |
| 2008 Oct 08 | Topical NSAIDs for acute pain in adults | Protocol | Thomas Massey, Sheena Derry, R Andrew Moore, Philip J Wiffen, Henry J McQuay | |
Differences between protocol and review
For this update in 2015, we have changed the title to specify musculoskeletal pain because topical NSAIDs are not normally used to treat visceral pain or headache. We felt that the new title better reflected the content of the review. We have also changed the focus of the review from pooled analysis of all topical NSAIDs and all studies of a particular NSAID to an examination of individual drug and its formulation. This makes the review much more relevant. We have expanded the 'Risk of bias' assessment, and added a 'Summary of findings' table and PRISMA flow chart. We have removed a number of sensitivity analyses because they were not appropriate given the current information on the impact of formulation on efficacy. The sensitivity analyses have been superseded by the 'Risk of bias' assessment and taken into account in the 'Summary of findings' tables.
An earlier review in 2004 chose to exclude studies using benzydamine, on the grounds that it was no longer considered to be an NSAID (Mason 2004a). Although the protocol for this review stated that we would not include benzydamine, after further consultation we now believe that it should be classified as an NSAID, albeit with a different mode of action, which is not fully understood (Quane 1998). Thus, we have reinstated studies using topical benzydamine.
Notes
2019
In March 2019, this review was stabilised following discussion with the authors and editors. Restricted searches in March 2019 identified another two new studies (Bussin 2017; Lai 2017), but we judged that including them would not affect the conclusions of the review. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Bussin ER, Cairns B, Bovard J, Scott A. Randomised controlled trial evaluating the short‐term analgesic effect of topical diclofenac on chronic Achilles tendon pain: a pilot study. BMJ Open. 2017 May 4;7(4):e015126. doi:10.1136/bmjopen‐2016‐015126.
Lai PM, Collaku A, Reed K. Efficacy and safety of topical diclofenac/menthol gel for ankle sprain: A randomized, double‐blind, placebo‐ and active‐controlled trial. J Int Med Res. 2017 Apr;45(2):647‐661. doi: 10.1177/0300060517700322.
2017
In February 2017, this review was stabilised following discussion with the authors and editors. Restricted searches in February 2017 identified two new studies (Cheechareoan 2016; Predel 2016), but we judged that including them would not affect the conclusions of the review. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Cheechareoan S, Pathanawiriyasirikul T, Manmee C, Janpol K. Efficacy of Plai Cream in Adult Patients with Muscle Strain: A Randomized, Double‐Blind, Placebo‐Controlled Trial. Journal of the Medical Association of Thailand 2016;99(Suppl 2):S147‐52.
No significant difference between groups in mean pain intensity at two weeks (N = 140).
Predel HG, Pabst H, Schäfer A, Voss D, Giordan N. Diclofenac patch for the treatment of acute pain caused by soft tissue injuries of limbs: a randomized, placebo‐controlled clinical trial. The Journal of Sports Medicine and Physical Fitness. 2016:56(1‐2):92‐9.
Statistically significant difference between groups in mean pain intensity at two days, and comparable adverse events between groups (N = 164).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Acute Pain [*drug therapy, etiology];
- Administration, Topical;
- Anti‐Inflammatory Agents, Non‐Steroidal [*administration & dosage, adverse effects];
- Athletic Injuries [complications];
- Musculoskeletal Pain [*drug therapy, etiology];
- Randomized Controlled Trials as Topic;
- Sprains and Strains [complications];
Medical Subject Headings Check Words
Adult; Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
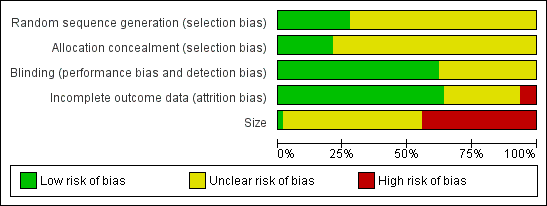
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
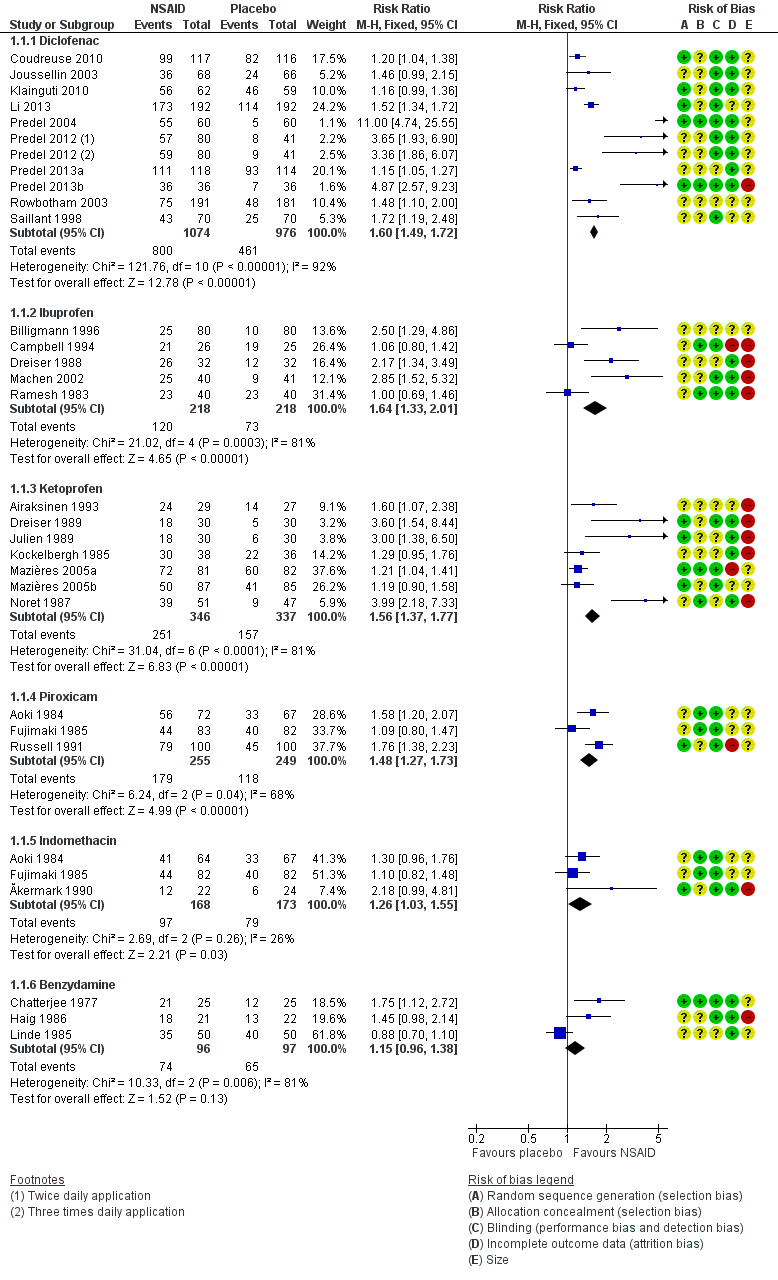
Forest plot of comparison: 2 Individual NSAID versus placebo, outcome: 2.1 Clinical success.

L'Abbé plot of clinical success in studies of topical diclofenac versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Dark blue: Emulgel; light blue: spray/gel; red: Flector; pink: other patch or plaster.

L'Abbé plot of clinical success in studies of topical ketoprofen versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Light blue: ketoprofen gel; pink: ketoprofen plaster.

Comparison 1 Individual NSAID versus placebo, Outcome 1 Clinical success.

Comparison 1 Individual NSAID versus placebo, Outcome 2 Local adverse events.

Comparison 2 Diclofenac versus placebo (effect of formulation), Outcome 1 Clinical success.

Comparison 3 Ibuprofen versus placebo (effect of formulation), Outcome 1 Clinical success.
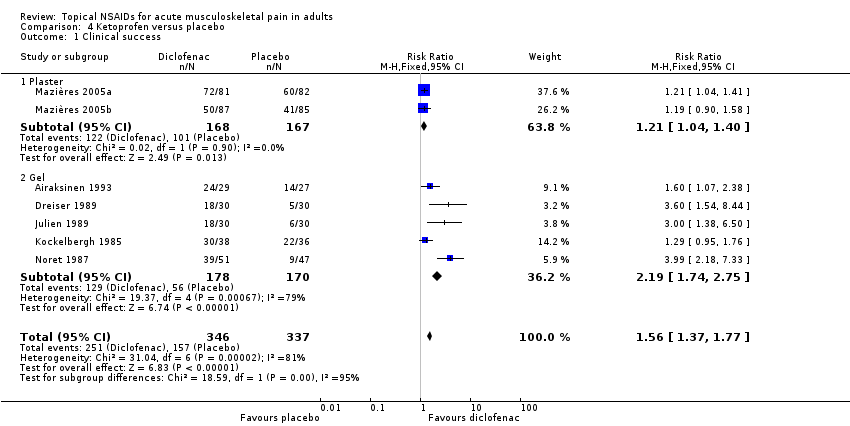
Comparison 4 Ketoprofen versus placebo, Outcome 1 Clinical success.
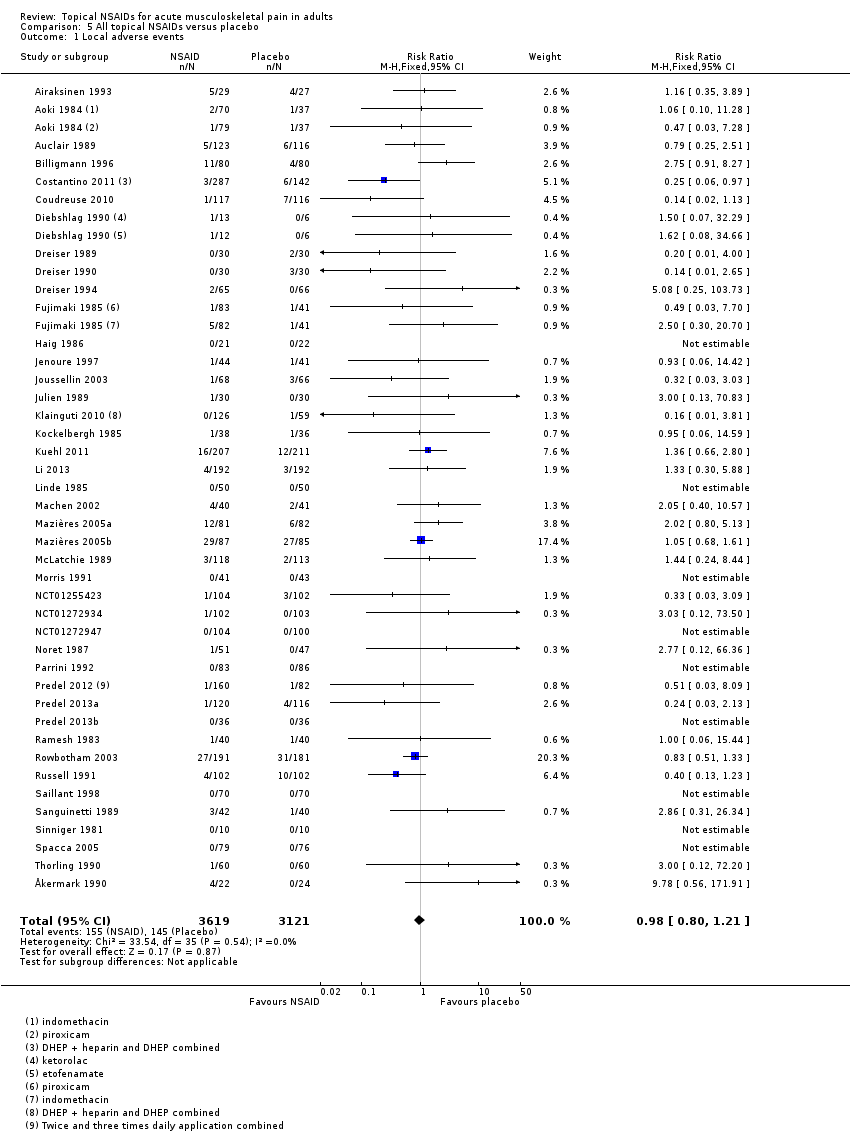
Comparison 5 All topical NSAIDs versus placebo, Outcome 1 Local adverse events.
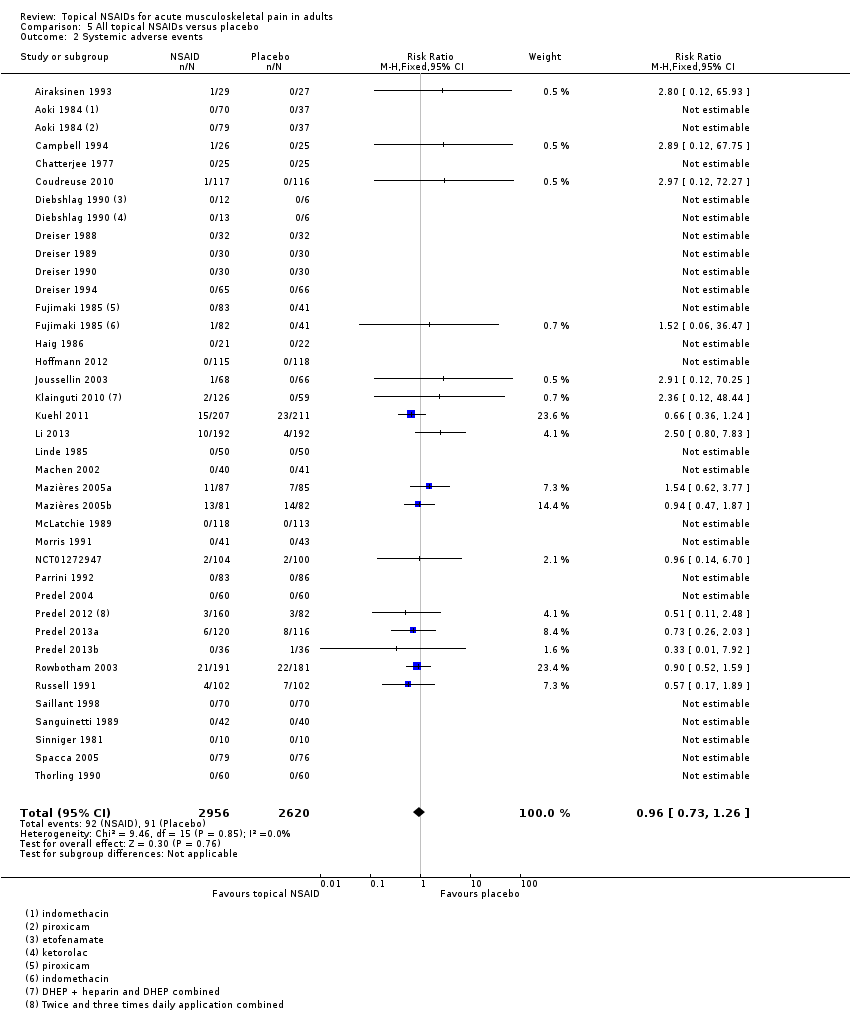
Comparison 5 All topical NSAIDs versus placebo, Outcome 2 Systemic adverse events.
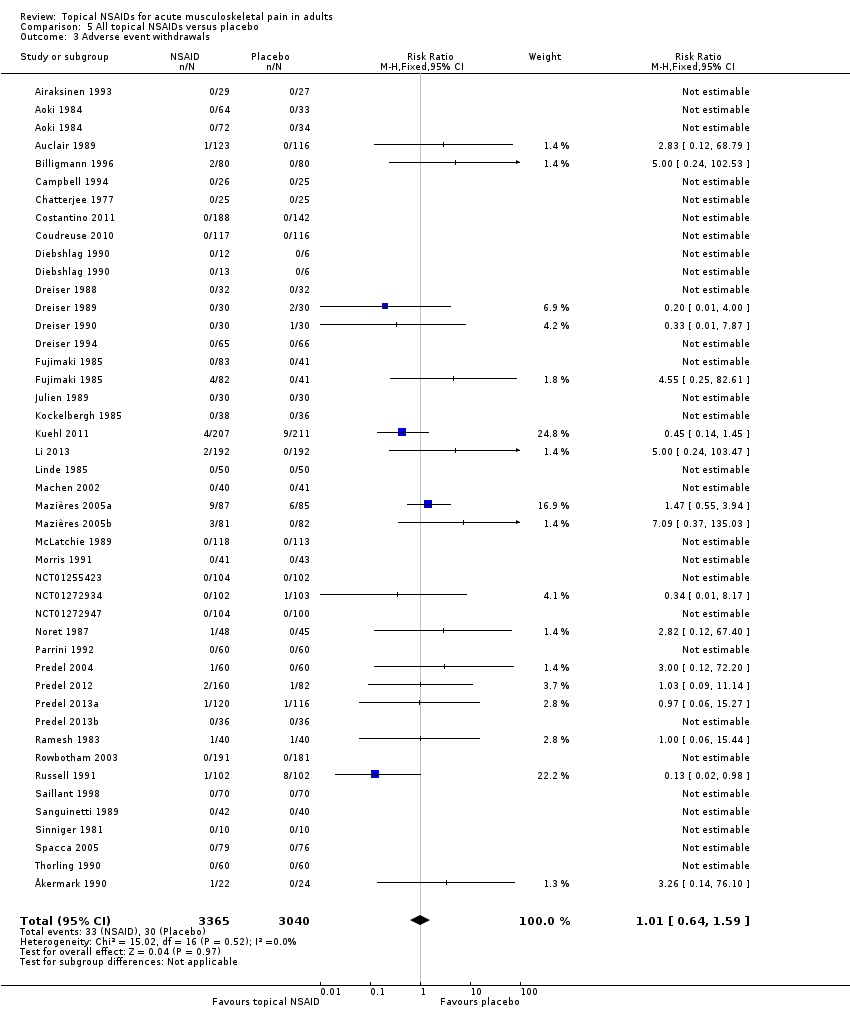
Comparison 5 All topical NSAIDs versus placebo, Outcome 3 Adverse event withdrawals.
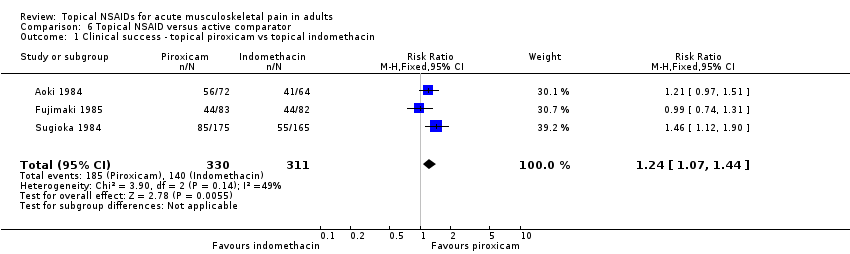
Comparison 6 Topical NSAID versus active comparator, Outcome 1 Clinical success ‐ topical piroxicam vs topical indomethacin.

Comparison 6 Topical NSAID versus active comparator, Outcome 2 Local adverse events ‐ topical piroxicam vs topical indomethacin.
| Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults | ||||||
| Patient or population: adults with strains, sprains, or muscle pull Settings: community Intervention: topical NSAID (topical diclofenac, ibuprofen, and ketoprofen gels only shown here for efficacy) Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | RR, NNT, NNTp, or NNH | No of studies, participants | Quality of the evidence | Comments |
| Topical diclofenac gel (as Emulgel) Clinical success (eg 50% reduction in pain) | 780 in 1000 | 200 in 1000 | RR 3.4 (2.7 to 55) NNT 1.8 (1.5 to 2.1) | 2 studies 314 participants | High | Consistent results in 2 moderately sized recent studies of high quality |
| Topical ibuprofen gel Clinical success (eg 50% reduction in pain) | 420 in 1000 | 160 in 1000 | RR 2.7 (1.7 to 4.2) NNT 3.9 (2.7 to 6.7) | 2 studies 241 participants | Moderate | Modest effect size and numbers of participants |
| Topical ketoprofen gel Clinical success (eg 50% reduction in pain) | 720 in 1000 | 330 in 1000 | RR 2.2 (1.7 to 2.8) NNT 2.5 (2.0 to 3.4) | 5 studies 348 participants | Moderate | Modest effect size and numbers of participants, but studies small, with none recent |
| All topical NSAIDs Local adverse events | 46 in 1000 | 50 in 1000 | RR 1.0 (0.80 to 1.2) NNH not calculated | 42 studies 6125 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Systemic adverse events | 32 in 1000 | 35 in 1000 | RR 1.0 (0.7 to 1.3) NNH not calculated | 38 studies 5372 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Withdrawals ‐ adverse events | 11 in 1000 | 11 in 1000 | RR 1.0 (0.7 to 1.7) NNH not calculated | 42 studies 5790 participants | High | Large number of studies and participants with consistent results |
| Serious adverse events | 1 in total | 0 in total | Not calculated | All data | Low | Small numbers of events |
| GRADE Working Group grades of evidence | ||||||
| CI: confidence interval; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event happening; NNH: number needed to treat for an additional harmful outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Diclofenac | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events Show forest plot | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac | 15 | 3271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.10] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.1 Plaster ‐ Flector | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.36, 1.71] |
| 1.2 Plaster ‐ other | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.37, 1.75] |
| 1.3 Gel ‐ Emulgel | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [2.68, 5.50] |
| 1.4 Gel ‐ other | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.1 Cream | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 1.2 Gel | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.69, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.1 Plaster | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.2 Gel | 5 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local adverse events Show forest plot | 42 | 6740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Systemic adverse events Show forest plot | 36 | 5576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 3 Adverse event withdrawals Show forest plot | 42 | 6405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| 2 Local adverse events ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |

