نقش NSAIDهای موضعی در درمان درد حاد عضلانیاسکلتی در بزرگسالان
Appendices
Appendix 1. CENTRAL search strategy (via CRSO) for 2015 update
-
MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL TREES (13419)
-
(bufexamac OR bufexine OR calmaderm OR ekzemase OR dicoflenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma‐gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR "Trans Act LAT" OR tulip OR ibuprofen OR cuprofen OR "deep relief" OR fenbid OR ibu‐cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR "nurofen gel" OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine): TI,AB,KY (25220)
-
1 OR 2 (32484)
-
MESH DESCRIPTOR Administration, Topical EXPLODE ALL TREES (2169)
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster):TI,AB,KY (67940)
-
4 OR 5 (70486)
-
MESH DESCRIPTOR Athletic Injuries EXPLODE ALL TREES (411)
-
(strain OR sprain* OR contusion OR distortion OR compression OR "sports injur*" OR "soft tissue injur*" OR tend?nitis OR "muscle pain" OR periarthritis OR epicondylitis OR tenosynovitis):TI,AB,KY (9158)
-
7 OR 8 (9448)
-
MESH DESCRIPTOR pain EXPLODE ALL TREES (29943)
-
(pain* OR analgesi*):TI,AB,KY (74815)
-
10 OR 11 (80041)
-
3 AND 6 AND 9 AND 12 (110)
Appendix 2. MEDLINE search strategy via Ovid (for 2015 update)
-
exp Anti‐inflammatory Agents, non‐steroidal/ (162888)
-
(bufexamac OR bufexine OR calmaderm OR ekzemase OR diclofenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma‐gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR "Trans Act LAT" OR tulip OR ibuprofen OR cuprofen OR "deep relief" OR fenbid OR ibu‐cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR "nurofen gel" OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine).mp (558284)
-
1 OR 2 (664691)
-
exp Administration, Topical/ (69697)
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp (1982395)
-
4 OR 5 (1997720)
-
exp Athletic Injuries/ (21464)
-
(strain OR sprain* OR contusion OR distortion OR compression OR "sports injur*" OR "soft tissue injur*" OR tend?nitis OR "muscle pain" OR periarthritis OR epicondylitis OR tenosynovitis).mp (420133)
-
7 OR 8 (439228)
-
Pain/ (113906)
-
(pain* OR analgesi*).mp (585249)
-
10 or 11 (585249)
-
randomized controlled trial.pt (401171)
-
randomized.ab (296222)
-
placebo.ab (155341)
-
drug therapy.fs (1789858)
-
randomly.ab (207517)
-
trial.ab (308477)
-
groups.ab (1318386)
-
OR/13‐19
-
3 AND 6 AND 9 AND 12 AND 20 (139)
-
limit 21 to yr="2008‐Current" (56)
Appendix 3. EMBASE search strategy via Ovid (for 2015 update)
-
exp Anti‐inflammatory Agents, non‐steroidal/ (452266)
-
(bufexamac OR bufexine OR calmaderm OR ekzemase OR dicoflenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma‐gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR "Trans Act LAT" OR tulip OR ibuprofen OR cuprofen OR "deep relief" OR fenbid OR ibu‐cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR "nurofen gel" OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine).mp (790435)
-
1 OR 2 (1108683)
-
exp Administration, Topical/ (68490)
-
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp (3208844)
-
4 OR 5 (3208844)
-
exp Athletic Injuries/ (24675)
-
(strain OR sprain* OR contusion OR distortion OR compression OR "sports injur*" OR "soft tissue injur*" OR tend?nitis OR "muscle pain" OR periarthritis OR epicondylitis OR tenosynovitis).mp (804322)
-
7 OR 8 (825164)
-
Pain/ (216406)
-
(pain* OR analgesi*).mp (971593)
-
10 OR 11 (971593)
-
clinical trials.sh (841252)
-
controlled clinical trials.sh (389335)
-
randomized controlled trial.sh (357169)
-
double‐blind procedure.sh (118945)
-
(clin* adj25 trial*).ab (331002)
-
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab (141726)
-
placebo*.ab (203390)
-
random*.ab (906176)
-
OR/13‐20 (1731380)
-
3 AND 6 AND 9 AND 10 AND 19 (439)
-
limit 22 to yr="2008‐Current" (204)
Appendix 4. Summary of outcomes: efficacy
| Study ID | Treatment | Clinical response | Other response |
| (1) Ketoprofen gel 2 x 5 g (125 mg) daily, n = 29 (2) Placebo gel, n = 27 | PGE "improved" at 7 days (1) 24/29 (2) 14/27 | No additional data | |
| (1) Indomethacin spray 1% (Elmetacin), 3‐5 x 0.5‐1.5 mL daily, n = 23 (2) Indomethacin capsules, 3 x 25 mg daily, n = 23 (3) Placebo spray and capsules, n = 24 | No pain on palpation at 7 days (1) 12/22 (2) 5/22 (3) 6/24 | Patient assessment of improvement at 7 days (scale 0 to 100) (1) 57 (2) 49 (3) 30 | |
| (1) Piroxicam gel 5%, 3 or 4 x 1 g daily, n = 84 (2) Indomethacin gel 1%, 3 or 4 x 1 g daily, n = 84 (3) Placebo gel, n = 84 | PGE (5 point) "better or much better" at 7 days (1) 56/72 (2) 41/64 (3) 33/67 | Pain on movement "reduced" or "disappeared" at 7 days (1) 48/61 (2) 38/60 (3) 35/63 | |
| (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 117 (2) Placebo gel, n = 110 | PGE (5 point) "good or very good" at 7 days (1) 69/117 (2) 54/110 | Pain on palpation "improved" at 7 days (1) 69/117 (2) 53/110 | |
| (1) Ibuprofen microgel 5% 3 x 200 mg daily, n = 80 (2) Placebo gel, n = 80 | Complete remission (1) 25/80 (2) 10/80 | Improvement in pain with movement of 20% at 7 days (1) 65/80 (2) 55/80 | |
| (1) Ibuprofen cream 5% (Proflex) 4 x 4" daily, n = 26 (2) Placebo cream, n = 25 | Improvement in walking ability (4 point) at 7 days (1) 21/26 (2) 19/25 | No additional data | |
| (1) Benzydamine HCl cream 3% 3 x daily, n = 25 (2) Placebo cream, n = 25 | Pain on movement "absent/slight" at 6 days (1) 21/25 (2) 12/25 | Tenderness with pressure "absent/slight" at 6 days (1) 21/25 (2) 12/25 | |
| (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 142 (2) DHEP plaster (Flector) x 1 daily, n = 146 (3) Placebo plaster, n = 142 | No responder analysis for efficacy. Overall treatment efficacy not reported | Mean reduction from baseline for PI on movement at 3 days (from graph) | |
| (1) DHEP‐heparin plaster (Flectorparin), n = 120 | PGE "good" or "excellent" at 7 days (1) 99/117 (2) 82/116 These are results for physician‐reported judgement ‐ not participant | Mean reduction from baseline for PI on movement over 6 hours | |
| (1) Ibuproxam, n = 20 (2) Ketoprofen, n = 20 (3) Etofenamate, n = 20 | Resolution of symptoms by 7 days (1) 15/20 (2) 13/20 (3) 13/20 | PGE "good" or "excellent" at 10 days (1) 19/20 (2) not reported (3) 16/20 | |
| (1) Ketorolac gel 2% 3 x 3 g daily, n = 13 (2) Etofenamate gel 5% 3 x 3 g daily, n = 12 (3) Placebo gel, n = 12 | Improvement in pain at 7 days (1) 12/13 (2) 10/12 (3) 9/12 | No additional data | |
| (1) Ibuprofen cream 5%, 3 x 4 cm daily, n = 32 (3 x 10 cm for large joints) (2) Placebo cream, n = 32 | PGE "improvement" or "complete relief" at 7 days (1) 26/32 (2) 12/32 | (1) significantly better than (2) for mean improvement in spontaneous pain, movement pain, rest pain, tenderness to pressure (VAS) | |
| (1) Ketoprofen gel 2.5%, 2 x 5 cm daily, n = 30 (2) Placebo gel, n = 30 | PGE (3 point) "better" at 7 days (1) 18/30 (2) 5/30 | (1) significantly better than (2) for mean improvement in pain (rest and movement) (VAS) | |
| (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 30 (2) Placebo gel, n = 30 | PGE (4 point) "cured" or "improved" at 7 days (1) 23/30 (2) 10/30 | (1) significantly better than (2) for mean improvement in pain (VAS) | |
| (1) Flurbiprofen plaster 2 x 40 mg daily, n = 65 (2) Placebo plaster, n = 66 | PGE (4 point) "good" or "very good" at 7 days (1) 48/65 (2) 41/66 | (1) significantly better than (2) for mean improvement in spontaneous pain, but not pain on movement or palpation (VAS) | |
| (1) DHEP lecithin gel 3 x 5 g (= 65 mg) daily, n = 50 (2) DHEP gel 3 x 5 g (= 65 mg) daily, n = 50 | PGE (4 point) "good" or "excellent" at 10 days (1) 35/50 (2) 35/50 | (1) significantly better than (2) for mean improvement in spontaneous pain at 7 days, but not for pain on movement at 10 days (VAS) | |
| (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 92 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 90 (3) Placebo gel, n = 89 | PGE (5 point) "better" or "much better" at end of treatment at 14 days (1) 44/83 (2) 44/82 (3) 40/82 | No additional data | |
| (1) Diclofenac hydroxyethylpyrrolidine gel 1%, 4 x 2 g daily, n = 25 (Flector gel) (2) Diclofenac sodium 1% 4 x 2 g daily, n = 25 (Voltaren Emugel) | PGE (5 point) "good" or "excellent" at 14 days (1) 19/25 (2) 19/25 | No significant difference between groups for pain on applied pressure at 7 and 14 days | |
| (1) Traumeel gel 3 x 2 g daily, n = 140 (2) Traumeel ointment, n = 143 (3) Diclofenac gel 1% 3 x 2 g daily, n = 137 | Pain‐free at 7 days PGE (5 point) "good" or "very good" (1) 128/140 (3) 127/137 | Normal function (5 point) score of 0 or 1 at 14 days (1) 133/140 (3) 131/137 | |
| (1) Ketoprofen gel 5% 3 x 2‐3 g daily, n = 15 (2) Ketoprofen cream 1%, 3 x 2‐3 g daily, n = 15 | PGE (5 point) "good" or "excellent" at 7 days (1) 14/15 (2) 4/15 | No additional data | |
| (1) Flunaxaprofen gel 2 x 3‐5 cm daily, n = 30 (2) Ketoprofen gel 2 x 3‐5 cm daily, n = 30 | No dichotomous data | No significant difference between groups for pain on movement at 7 days | |
| (1) Benzydamine cream 3%, 6 x daily, n = 21 (2) Placebo cream, n = 22 | Pain on movement "improved" by 6 days (1) 18/21 (2) 13/22 | No additional data | |
| (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 121 | PGE (5 point) "good" or "excellent": over 80% in all groups | Mean reduction from baseline in PI (100 mm VAS) | |
| (1) Diclofenac sodium gel 1%, 4 x 2 cm daily, n = 69 (2) Lysine clonixinate gel 5%, 4 x 2 cm (22.5 mg) daily, n = 73 | PGE (3 point) at 8 days: "good" (1) 38/69 (2) 36/73 | No significant difference between treatments for any pain outcomes | |
| (1) Felbinac foam 3% 3 x 2 g daily + placebo tablets, 3 x 1 daily, n = 140 (127 analysed for efficacy) (2) Ibuprofen tablets 3 x 400 mg daily + placebo foam 3 x 2 g daily, n = 147 (134 analysed for efficacy) | Pain on movement "none" or "mild" at 7 days (1) 81/127 (2) 96/133 | Spontaneous pain "none" or "mild" at 7 days (1) 99/127 (2) 108/134 | |
| (1) DHEP plaster (Tissugel), 2 x daily, n = 44 (2) Placebo plaster 2 x daily, n = 41 | Baseline pain in two groups not balanced, and data in table and figure do not agree, so efficacy outcomes not used | No additional data | |
| (1) DHEP plaster (Flector Tissugel 1%), 1 x daily, n = 68 (2) Placebo plaster 1 x daily, n = 66 | PGE (4 point) "excellent" at 7 days (1) 36/68 (2) 24/66 ≥ 30% reduction in PI at 7 days (1) 25/68 (2) 11/66 | (1) significantly better than (2) for mean pain on movement at 6 days | |
| (1) Ketoprofen gel 2.5% 2 x 5 cm (= 50 mg) daily, n = 30 (2) Placebo gel, n = 30 | PGE (4 point) "recovered" at 7 days (1) 18/30 (2) 6/30 | PGE (4 point) "recovered" or "improved" at 7 days (1) 25/30 (2) 13/30 | |
| (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 62 (2) DHEP plaster (Flector) x 1 daily, n = 61 (3) Placebo, n = 59 | Overall treatment efficacy (participant and investigator) (5 point) "good" or "excellent" at 3 days (1) 56/62 (2) not reported (3) 46/59 | Mean reduction in PI at day 3 (from graph) (2) Not reported | |
| (1) Ketoprofen gel 2.5% 2 x 5 cm (= 15 mg) daily, n = 38 (2) Placebo gel, n = 36 | PGE (3 point) "good" at 7 days (1) 30/38 (2) 22/36 | (1) and (2) slightly better than (3) for mean spontaneous pain at 7 days | |
| (1) DETP 1.3% plaster 2 x daily, n = 207 | No dichotomous data | Percentage reduction from baseline at last application (1) 73% (2) 62% | |
| (1) DHEP plaster (Flector) 2 x daily, n = 192 | ≥ 50% pain reduction at day 7 (posthoc analysis) (1) 173/192 (2) 114/192 | Overall treatment efficacy (5 point) "good" or "excellent" (1) 161/192 (2) 81/192 Participant and investigator rating Mean ± SD reduction in PI on movement at 7 days | |
| (1) Benzydamine 3% cream 3 x daily, n = 50 (2) Placebo gel, n = 50 | No pain on movement (walking) at 8 days (1) 35/50 (2) 40/50 | No additional data | |
| (1) Ibuprofen gel 5% 3 x daily, n = 40 (2) Placebo gel, n = 41 | PGE: (5 point) "marked improvement" or "complete clearance" at 7 days (1) 25/40 (2) 9/41 | Clinically meaningful (≥ 30 mm) pain relief at day 7 (1) 30/40 (2) 16/41 | |
| (1) DHEP + lethicin gel 3 x 5 g daily, n = 52 (2) DHEP gel 3 x 5 g daily, n = 48 | PGE (4 point) "good" or "excellent" at 10 days (1) 49/52 (2) 39/48 | Mean reduction in pain on movement at 3 and 10 days significantly greater with (1) than (2) | |
| (1) Ketoprofen plaster 100 mg, x 1 daily, n = 81 (2) Placebo plaster, n = 82 | PGE (4 point) "good" or "excellent" at 14 days (1) 72/81 (2) 60/82 | All mean efficacy measures improved more for (1) than (2), most were statistically significant | |
| (1) Ketoprofen plaster 100 mg, x 1 daily, n = 87 (2) Placebo plaster, n = 85 | PGE (4 point) "good" or "excellent" at 14 days (1) 50/87 (2) 41/85 | All mean efficacy measures improved more for (1) than (2), most were statistically significant | |
| (1) Felbinac gel 3% 3 x 3 cm daily, n = 118 (2) Placebo gel, n = 113 | No dichotomous data | Patient daily self‐assessment for mean pain on rest, movement, at night, interference with normal and leisure activities show better efficacy for (1) than (2) from day 2 (VAS) | |
| (1) Felbinac gel 3% 3 x 1 cm daily, n = 41 (2) Placebo gel, n = 43 | PGE (5 point) "good" or "very good" at 7 days (1) 23/41 (2) 27/43 | (1) better than (2) for mean improvement in symptoms and sporting function at 7 days | |
| (1) Diclofenac sodium gel 1% x 4 daily, n = 104 | No dichotomous data | VAS (mean ± SD) at 72 hours (baseline PI not reported) (1) 37.4 ± 25.2 (2) 38.8 ± 24.1 | |
| (1) Diclofenac sodium gel 1% x 4 daily, n = 104 | No dichotomous data | VAS (mean ± SD) at 72 hours (baseline PI not reported) (1) 25.6 ± 15.9 (2) 61.2 ± 16.6 | |
| (1) Diclofenac sodium gel 1% x 4 daily, n = 104 | No dichotomous data | VAS (mean ± SD) at 24 hours (baseline PI not reported) (1) 33.1 ± 21.4 (2) 65.4 ± 16.9 | |
| (1) Ketoprofen gel 2.5% 2 x 5 cm (7.5 mg) daily, n = 48 (2) Placebo gel, n = 45 | PGE (4 point) "good" or "excellent" at 8 days (1) 39/48 (2) 9/45 | Decrease in mean spontaneous pain significantly greater in (1) than (2) by 3 days | |
| (1) Ketoprofen foam 15% 3 x 2 g (200 mg) daily, n = 83 (2) Placebo foam, n = 86 | No dichotomous data | Mean pain on movement and pressure significantly decreased by 7 days in (1) compared with (2) | |
| (1) Ibuprofen gel 10% 3 x daily , n = 20 (2) Ketoprofen gel 1% 3 x daily, n = 20 | No pain on movement at 8 days (1) 3/20 (2) 0/20 | Spontaneous pain "none" at 8 days (1) 6/20 (2) 0/20 | |
| (1) Diclofenac sodium plaster, 2 x daily (140 mg/plaster), n = 60 (2) Placebo plaster, n = 60 | PGE (4 point) "good" "excellent" at 7 days (1) 55/60 (2) 5/60 | (1) better than (2) for reduction in tenderness, pain, and speed of pain reduction | |
| (1) Diclofenac gel (Voltaren Emulgel 2.32%) 2 x 5 cm daily, n = 80 | ≥ 50% red in PI on movement at 5 days (1) 57/80 | PGE efficacy (5 point) "good" or "very good" at 8 days (1) 68/80 | |
| (1) Diclofenac 4% spray gel 4 or 5 sprays 3 x daily (96‐120 mg diclofenac sodium), n = 118 | None or slight PI on movement at 7‐8 days (1) 111/118 (2) 93/114 | None or slight PI on movement at 3‐4 days | |
| (1) Diclofenac gel (Voltaren Emulgel) 4 x 2 g daily, n = 36 | PGE (5 point) "good" or "excellent" (1) 36/36 (2) 7/36 | ≥ 50% reduction in PI on movement after 48 hours (1) 34/36 (2) 3/36 | |
| (1) Ibuprofen cream 5% 3 or 4 x 5‐10 cm daily, n = 40 (2) Placebo cream, n = 40 | Pain on movement (4 point) "none" or "slight" at 7 days (1) 23/40 (2) 23/40 | Physician global assessment at 10 days: "good" | |
| (1) Diclofenac epolamine plaster (Flector Tissuegel) 2 x daily, n = 191 (2) Placebo plaster, n = 181 | Pain intensity ≤ 2/10 for 2 days or 4 consecutive evaluations, by 7 days (1) 75/191 (2) 48/181 | Mean pain on rest significantly better with (1) than (2) after 7 days | |
| (1) Piroxicam gel 0.5% 4 x 5 mg daily, n = 100 (2) Placebo gel, n = 100 | PGE (4 point) "good" or "excellent" at 8 days (1) 79/100 (2) 45/100 | Statistically greater reduction in mean pain on movement at 8 days with (1) than (2) | |
| (1) DHEP plaster (Flector Tissugel 1%) 1 x daily, n = 70 | PGE (4 point) "excellent" at 7 days (1) 43/70 (2) 25/70 | ≥ 30% decrease in PI at 7 days (1) 64/70 (2) 50/70 | |
| (1) Felbinac gel 3% 3 x daily, n = 42 (2) Placebo gel, n = 40 | PGE "good" or "very good" at 7 days (1) 34/42 (2) 11/40 | (1) better than (2) by 2 days | |
| (1) Fentiazac cream 5% 2 or 3 x daily, n = 10 (2) Placebo cream, n = 10 | Complete pain relief within 10 days (1) 7/10 (2) 1/10 | Improvement in active pain on movement at 5 days (1) 67% (2) 32% | |
| (1) DHEP lecithin gel (Effigel), 3 x 5 g, daily, n = 79 (2) Placebo gel, n = 76 | No dichotomous data | Mean pain scores improved more rapidly in (1) than (2) ‐ statistically significant at 3 and 6 days | |
| (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 183 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 183 | PGE (5 point) "better" or "much better" at 14 days (1) 85/175 (2) 55/165 | Pain on movement "reduced" or "disappeared" at 7 days (1) 77/175 (2) 63/165 | |
| (1) Naproxen gel 10% 2‐6 x daily, n = 60 (2) Placebo gel, n = 60 | PGE (5 point) "good" or "very good" at 7 days (1) 38/60 (2) 27/60 | Participants using naproxen improved more rapidly and had significantly lower severity scores by day 3 | |
| (1) Ketoprofen gel 5%, 3 x 2‐3 g daily, n = 15 (2) Etofenamate gel 5%, 3 x 2‐3 g, n = 15 | PGE (4 point) "good" or "excellent" at 7 days (1) 10/15 (2) 11/15 | Significant reductions in pain on movement by 7 days in both groups | |
| (1) Meclofenamic acid gel 5% 2 x 10 cm daily (2 g), n = 30 (2) Placebo, n = 30 | PGE (4 point) "good" or "excellent" at 10 days (1) 30/30 (2) 19/30 | (1) significantly better than (2) for mean improvement in spontaneous pain, movement pain, functional restriction | |
| (1) Ibuprofen gel 5% + placebo tablet 3 x daily, n = 50 (2) Ibuprofen 400 mg tablet + placebo gel 3 x daily, n = 50 | Participant satisfied at 7 days (1) 30/50 (2) 36/50 | "Completely better" at 14 days (1) 24/50 (2) 30/50 | |
| DHEP: diclofenac epolamine; HCl: hydrochloride; n: number; PGE: participant global evaluation; PI: pain intensity; SD: standard deviation; VAS: visual analogue scale. | |||
Appendix 5. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | Local AEs | Systemic AEs | Serious AEs | Withdrawals |
| (1) Ketoprofen gel 2 x 5 g (125 mg) daily, n = 29 (2) Placebo gel, n = 27 | (1) 5/29 (2) 4/27 | (1) 1/29 (nausea after paracetamol) (2) 0/27 | None | AE: none Other: none reported | |
| (1) Indomethacin spray 1% (Elmetacin), 3‐5 x 0.5‐1.5 mL daily, n = 23 (2) Indomethacin capsules, 3 x 25 mg daily, n = 23 (3) Placebo spray and capsules, n = 24 | (1) 4/22 (2) 0/22 (3) 0/24 | No usable data ‐ reported for events not participants | None reported | AE: (1) 1, (2) 1, (3) 0 Lost to follow‐up: (1) 1, (2) 2, (3) 3 | |
| (1) Piroxicam gel 5%, 3 or 4 x 1 g daily, n = 84 (2) Indomethacin gel 1%, 3 or 4 x 1 g daily, n = 84 (3) Placebo gel, n = 84 | (1) 1/79 (2) 2/70 (3) 2/74 | None | None reported | AE: none 23 excluded for protocol violations: (1) 7, (2) 7, (3) 9 26 withdrew for reasons unrelated to treatment: (1) 5, (2) 13, (3) 8 | |
| (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 117 (2) Placebo gel, n = 110 | All AEs (1) 5/123 (2) 6/116 Most commonly cutaneous eruptions | No usable data | None reported | AE: (1) 1/123, (2) 0/116 26 excluded from efficacy analysis for not meeting entry criteria and protocol violations | |
| (1) Ibuprofen microgel 5% 3 x 200 mg daily, n = 80 (2) Placebo gel, n = 80 | (1) 11/80 (2) 4/80 | None reported | None reported | AE: (1) 2/80 (allergic rash, dermatitis) No reason given: (1) 3/80, (2) 5/80 | |
| (1) Ibuprofen cream 5% (Proflex) 4 x 4" daily, n = 26 (2) Placebo cream, n = 25 | No data | (1) 1/26 (headache) (2) 0/25 | No data | AE: none Exclusions 49: 3 presented late, 2 missing forms, 1 appeared twice, 43 did not return diaries | |
| (1) Benzydamine HCl cream 3% 3 x daily, n = 25 (2) Placebo cream, n = 25 | None | None | None | AE: none 1 participant lost to follow‐up (group not reported) | |
| (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 142 (2) DHEP plaster (Flector) x 1 daily, n = 146 (3) Placebo plaster, n = 142 | Possibly or probably drug‐related | Not reported | None | AE: none Other: (1) 0/142 | |
| (1) DHEP‐heparin plaster (Flectorparin), n = 120 | (1) 1/117 | (1) 1/117 | None | AE: (1) 1/117 (increased oedema) (2) 0/116 Other: (1) 6/117 (recovery 3, no reason 3) (2) 3/116 (recovery 1, no reason 2) | |
| (1) Ibuproxam, n = 20 (2) Ketoprofen, n = 20 (3) Etofenamate, n = 20 | None | None | None | AE: none Other: none | |
| (1) Ketorolac gel 2% 3 x 3 g daily, n = 13 (2) Etofenamate gel 5% 3 x 3 g daily, n = 12 (3) Placebo gel, n = 12 | (1) 1/13 (2) 1/12 (3) 0/12 | None | None | AE: none 1 ketorolac participant did not attend 15 day follow‐up due to car accident | |
| (1) Ibuprofen cream 5%, 3 x 4 cm daily, n = 32 (3 x 10 cm for large joints) (2) Placebo cream, n = 32 | No usable data | None | Not reported | AE: none 4 placebo participants lost to follow‐up | |
| (1) Ketoprofen gel 2.5%, 2 x 5 cm daily, n = 30 (2) Placebo gel, n = 30 | (1) 0/30 (2) 2/30 | None | None reported | AE: (2) 2/30 (intolerance) LoE: (1) 1/30, (2) 1/30 | |
| (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 30 (2) Placebo gel, n = 30 | (1) 0/30 (2) 3/30 | None | None | AE: (2) 1/30 (erythema) Exclusion: 1 from (2) from efficacy analysis for inadequate baseline pain | |
| (1) Flurbiprofen plaster 2 x 40 mg daily, n = 65 (2) Placebo plaster, n = 66 | (1) 2/65 (2) 0/66 | None | None | AE: 0 (1) 1/65 excluded from efficacy analysis for protocol violation | |
| (1) DHEP lecithin gel 3 x 5 g (= 65 mg) daily, n = 50 (2) DHEP gel 3 x 5 g (= 65 mg) daily, n = 50 | (1) 0/50 (2) 1/50 | No data | None reported | AE: none Other: none | |
| (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 92 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 90 (3) Placebo gel, n = 89 | (1) 1/83 (2) 5/82 (3) 2/82 | (1) 0/83 (2) 1/82 (nausea and vomiting) (3) 0/82 | None | AE: (1) 0, (2) 4, (3) 0 Unknown reasons: (1) 2, (2) 1 Did not return after 1st visit/irregular visits: (1) 6, (2) 6, (3) 7 | |
| (1) Diclofenac hydroxyethylpyrrolidine gel 1%, 4 x 2 g daily, n = 25 (Flector gel) (2) Diclofenac sodium 1% 4 x 2 g daily, n = 25 (Voltaren Emugel) | No AEs | None | None | AE: none Other: none | |
| (1) Traumeel gel 3 x 2 g daily, n = 140 (2) Traumeel ointment, n = 143 | No usable data | No usable data | None | AE: none Other: (1) 11/140 | |
| (1) Ketoprofen gel 5% 3 x 2‐3 g daily, n = 15 (2) Ketoprofen cream 1%, 3 x 2‐3 g daily, n = 15 | No side effects | None | None | AE: none Other: none | |
| (1) Flunaxaprofen gel 2 x 3‐5 cm daily, n = 30 (2) Ketoprofen gel 2 x 3‐5 cm daily, n = 30 | (1) 1/30 (2) 3/30 | No data | None reported | AE: none Other: none | |
| (1) Benzydamine cream 3%, 6 x daily, n = 21 (2) Placebo cream, n = 22 | No AEs reported | None | None reported | AE: none reported Other: no data | |
| (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 121 | Most AEs were minor local reactions (e.g. pruritus and erythema) in area of plaster, of mild to moderate intensity and resolved without interrupting treatment | No treatment‐related systemic AEs recorded | (2) 1 participant had 3 SAEs, none judged related to study medication | AE: none Exclusions: (1) 5/121 (2) 10/115 (3) 7/118 Excluded from per protocol analysis due to poor compliance or personal decision | |
| (1) Diclofenac sodium gel 1%, 4 x 2 cm daily, n = 69 (2) Lysine clonixinate gel 5%, 4 x 2 cm (22.5 mg) daily, n = 73 | (1) 1/58 (2) 1/61 | None | None | AE: none LoE: (1) 9, (2) 8 | |
| (1) Felbinac foam 3% 3 x 2 g daily + placebo tablets, 3 x 1 daily, n = 140 (127 analysed for efficacy) (2) Ibuprofen tablets 3 x 400 mg daily + placebo foam 3 x 2 g daily, n = 147 (134 analysed for efficacy) | (1) 1/127 (2) 3/134 | GI events: (1) 14/127, (2) 11/134 For (1) more mild, none definitely drug related, for (2) definitely related to study drug | None | AE: none Exclusions: (1) 13, (2) 13 did not return for 7 day follow‐up | |
| (1) DHEP plaster (Tissugel), 2 x daily, n = 44 (2) Placebo plaster 2 x daily, n = 41 | (1) 1/44 (2) 1/41 | No data | None reported | AE: none reported Other: none reported | |
| (1) DHEP plaster (Flector Tissugel 1%), 1 x daily, n = 68 (2) Placebo plaster 1 x daily, n = 66 | (1) 1/68 (pruritus) | (1) 1/68 (allergic reaction) | None reported | AE: (1) 0/66 (2) 1/66 Other: (1) 3/66 (2) 2/66 | |
| (1) Ketoprofen gel 2.5% 2 x 5 cm (= 50 mg) daily, n = 30 (2) Placebo gel, n = 30 | (1) 1/30 (2) 0/30 | Not reported | None | AE: none Other: none | |
| (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 62 | (1) 0/62 (2) 0/61 (3) 1/59 | (1) 1/65 (facial infection) (2) 1/61 (abdominal pain) (3) 0/59 | None | AE: none Other: (1) 3/65 | |
| (1) Ketoprofen gel 2.5% 2 x 5 cm (= 15 mg) daily, n = 38 (2) Placebo gel, n = 36 | (1) 1/38 (2) 1/26 | Not reported | None | AE: none Other: none | |
| (1) DETP 1.3% plaster 2 x daily, n = 207 | (1) 16/207 | (1) 15/207 | None | AE: (1) 4/207 LoE: (1) 21/207 (2) 25/211 Other: (1) 58/207 (2) 62/211 | |
| (1) DHEP plaster (Flector) 2 x daily, n = 192 | (1) 4/192 | (1) 10/192 | None | AE: (1) 2/192 Other: (1) 5/192 | |
| (1) Benzydamine 3% cream 3 x daily, n = 50 (2) Placebo gel, n = 50 | (1) 4/40 (2) 2/41 | None | None | AE: none (1) 6, (2) 6 excluded from 1st assessment | |
| (1) Ibuprofen gel 5% 3 x daily, n = 40 (2) Placebo gel, n = 41 | (1) 4/40 (2) 2/41 | None | None | AE: none (1) 1 LoE, 1 protocol violation | |
| (1) DHEP + lethicin gel 3 x 5 g daily, n = 52 (2) DHEP gel 3 x 5 g daily, n = 48 | (1) 1/52 (2) 0/48 | (1) 1/52 (2) 0/48 | None | AE: none 5 lost to follow‐up | |
| (1) Ketoprofen plaster 100 mg, x 1 daily, n = 81 (2) Placebo plaster, n = 82 | At 21 days: (1) 12/81 (2) 6/82 | (1) 13/81 (2) 14/82 | None | AE: (1) 3/81 (1) 7/81 (1 LoE, 6 cured) (2) 7/82 (5 LoE, 2 cured) | |
| (1) Ketoprofen plaster 100 mg, x 1 daily, n = 87 (2) Placebo plaster, n = 85 | At 21 days: (1) 29/87 (2) 27/85 | (1) 11/87 (2) 7/85 | None | AE: (1) 9/87, (2) 6/85 (1) 6/87 (2 LoE, 4 cured) (2) 5/85 (4 LoE, 1 cured) | |
| (1) Felbinac gel 3% 3 x 3 cm daily, n = 118 (2) Placebo gel, n = 113 | (1) 3/118 (2) 2/113 Mild transient local irritation | None reported | None | AE: none Other: none | |
| (1) Felbinac gel 3% 3 x 1 cm daily, n = 41 (2) Placebo gel, n = 43 | None | None | None | AE: none (1) 4 (protocol violations) (2) 1 (lost to follow‐up) Exclusions: 11 from efficacy analysis because evaluated by 4 different investigators | |
| (1) Diclofenac sodium gel 1% x 4 daily, n = 104 | (1) 1/104 (2) 3/102 | Total AE (1) 11/104 (2) 8/102 | None | None | |
| (1) Diclofenac sodium gel 1% x 4 daily, n = 104 | (1) 1/102 (2) 0/103 | Total AE (excluding SAE) (1) 6/102 (2) 3/103 | (1) 0/102 (2) 1/103 (ruptured ligaments in wrist) | AE: see SAE | |
| (1) Diclofenac sodium gel 1% x 4 daily, n = 104 | None | (1) 2/104 (2) 2/100 | None | None | |
| (1) Ketoprofen gel 2.5% 2 x 5 cm (7.5 mg) daily, n = 48 (2) Placebo gel, n = 45 | (1) 1/51 (2) 0/47 | None reported | Not reported | AE: (1) 1/51 (skin allergy) (1) 1 LoE, 1 unrelated to trial | |
| (1) Ketoprofen foam 15% 3 x 2 g (200 mg) daily, n = 83 (2) Placebo foam, n = 86 | None | None | None | AE: none Other: none | |
| (1) Ibuprofen gel 10% 3 x daily , n = 20 (2) Ketoprofen gel 1% 3 x daily, n = 20 | None | None | None | AE: none Other: not reported | |
| (1) Diclofenac sodium plaster, 2 x daily (140 mg/plaster), n = 60 (2) Placebo plaster, n = 60 | 12 participants experienced 16 mild AEs with no differences between groups | None | None | AE: (1) 1/60 Other: none | |
| (1) Diclofenac gel (Voltaren Emulgel 2.32%) 2 x 5 cm daily, n = 80 | (1) 0/80 (3) 1/82 All mild to moderate | (1) 2/80 | None | AE: (1) 0/80 Other: (1) 1/80 (protocol violations, lost to follow‐up) | |
| (1) Diclofenac 4% spray gel 4 or 5 sprays 3 x daily (96‐120 mg diclofenac sodium), n = 118 | (1) 1/118 | (1) 5/118 All AEs mild, reversible | None | AE: (1) 1/118 Other: (1) 3/118 | |
| (1) Diclofenac gel (Voltaren Emulgel) 4 x 2 g daily, n = 36 | None | (1) 0/36 | None | AE: none Other: none | |
| (1) Ibuprofen cream 5% 3 or 4 x 5‐10 cm daily, n = 40 (2) Placebo cream, n = 40 | (1) 1/40 (2) 1/40 | None reported | Not reported | AE: (1) 1/40, (2) 1/40 Other: none | |
| (1) DHEP plaster (Flector Tissuegel) 2 x daily, n = 191 (2) Placebo plaster, n = 181 | (1) 27/191 (pruritis 14) (2) 31/181 (pruritis 21) | (1) 21/191 (2) 22/181 | None reported ("vast majority mild") | AE: none (1) 3/191, (2) 4/181 (did not finish trial and complete daily diaries) | |
| (1) Piroxicam gel 0.5% 4 x 5 mg daily, n = 100 (2) Placebo gel, n = 100 | (1) 4/102 (2) 10/102 | GI or CNS events: (1) 4, (2) 7 Any AE: (1) 7/102, (2) 15/102 | None reported | AE: (1) 1/102, (2) 8/102 Other: (1) 6 LoE, 1 "other" (2) 42 LoE Exclusions: 7 did not comply with study medication schedule, 6 lost to follow‐up, 1 protocol violation | |
| (1) DHEP plaster (Flector Tissugel 1%) 1 x daily, n = 70 | None | None | None | AE: none Other: (1) 5/70 (2) 5/70 | |
| (1) Felbinac gel 3% 3 x daily, n = 42 (2) Placebo gel, n = 40 | (1) 3/42 | None | None reported | AE: none Other: none reported | |
| (1) Fentiazac cream 5% 2 or 3 x daily, n = 10 (2) Placebo cream, n = 10 | "No untoward side effects" | None | None | AE: none Other: none reported | |
| (1) DHEP lecithin gel (Effigel), 3 x 5 g, daily, n = 79 (2) Placebo gel, n = 76 | "No signs of cutaneous irritation or sensitisation observed" | No AEs observed | None | AE: none Other: none reported | |
| (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 183 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 183 | (1) 5/178 (2) 26/179 | None reported | None reported | AE: none reported Exclusions due to protocol violations: Withdrawals: | |
| (1) Naproxen gel 10% 2‐6 x daily, n = 60 (2) Placebo gel, n = 60 | (1) 1/60 (2) 0/60 | None | None | AE: none (1) 1 LoE, 1 protocol violation | |
| (1) Ketoprofen gel 5%, 3 x 2‐3 g daily, n = 15 (2) Etofenamate gel 5%, 3 x 2‐3 g, n = 15 | None | No AEs attributable to the medication | None | AE: None LoE: (1) 1, (2) 2 | |
| (1) Meclofenamic acid gel 5% 2 x 10 cm daily (2 g), n = 30 (2) Placebo, n = 30 | Tolerability excellent or good in nearly all participants | No data | None | AE: none reported (2) 5 lost to follow‐up | |
| (1) Ibuprofen gel 5% + placebo tablet 3 x daily, n = 50 (2) Ibuprofen 400 mg tablet + placebo gel 3 x daily, n = 50 | No data | 6 AEs reported, none judged related to study medication | None reported | AE: none Recovered: (1) 3, (2) 2 LoE: (2) 1 Lost to follow‐up: (1) 1, (2) 1 | |
| AE: adverse event; CNS: central nervous system; DHEP: diclofenac epolamine; GI: gastrointestinal; HCl: hydrochloride; LoE: lack of efficacy; n: number; SAE: serious adverse event. | |||||
Appendix 6. Concentration, amount, and frequency of dosing
| Study | Drug | Concentration | Quantity | Frequency | Estimated daily dose of topical NSAID |
| Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt | |
| Diclofenac | 1% | Plaster | 2 | 360 mg epolamine salt, 280 mg Na salt | |
| Diclofenac | 1% | Plaster | 2 | 360 mg epolamine salt, 280 mg Na salt | |
| Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt | |
| Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt | |
| Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt | |
| Diclofenac | ‐ | Plaster | 2 | 280 mg Na salt | |
| Diclofenac | 2.32% | 5 cm ribbon/˜ 2 g | 2 or 3 | 92‐138 mg (?Na equiv) as diethylamine salt | |
| Diclofenac | 1.16% | 2 g | 4 | 92 mg (?Na equiv) as diethylamine salt | |
| Diclofenac | 4% | 4‐5 sprays | 3 | 96‐120 mg Na salt | |
| Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt | |
| Diclofenac | ‐ | 5 g | 3 | 195 mg epolamine salt | |
| Diclofenac | 1% | 2 g | 4 | 80 mg | |
| Diclofenac | 1% | 6 cm, 2g | 3 | 60 mg | |
| Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt | |
| Diclofenac | 1% | 2 cm | 4 | probably 30‐40 mg Na salt | |
| Diclofenac | 1% | Plaster | 2 | 360 mg epolamine salt, 280 mg Na salt | |
| Diclofenac | ‐ | 5 g | 3 | 195 mg epolamine salt | |
| Diclofenac | 1% | ‐ | 4 | ‐ | |
| Diclofenac | 1% | ‐ | 4 | ‐ | |
| Diclofenac | 1% | ‐ | 4 | ‐ | |
| Diclofenac | ‐ | 5 g | 3 | 195 mg epolamine salt | |
| Ibuprofen | 5% | 4 inch ribbon | 4 | Assume 800 mg | |
| Ibuprofen | 5% | 4 cm ribbon | 4 | Assume up‐800 mg | |
| Ibuprofen | 5% | 5‐10 cm ribbon | 3‐4 | Assume 300‐800 mg | |
| Ibuprofen | 5% | 10 cm, 4 g gel | 3 | 600 mg | |
| Ibuprofen | 5% | ‐ | 3 | ‐ | |
| Ibuprofen | 10% | ‐ | 3 | ‐ | |
| Ibuprofen | 5% | ‐ | 3 | ‐ | |
| Ketoprofen | ‐ | Plaster | 1 | 100 mg | |
| Ketoprofen | ‐ | Plaster | 1 | 100 mg | |
| Ketoprofen | ‐ | 5 g | 2 | 125 mg | |
| Ketoprofen | 2.5% | 5 cm | 2 | 100 mg | |
| Ketoprofen | 2.5% | 5 cm | 2 | 100 mg | |
| Ketoprofen | 2.5% | 5 cm, 7.5 g | 2 | 375 mg | |
| Ketoprofen | No details | 2 | ‐ | ||
| Ketoprofen | 5% | 2‐3 g | 3 | 300‐450 mg | |
| Ketoprofen | 1% | 2‐3 g | 3 | 60‐90 mg | |
| Ketoprofen | ‐ | 3‐5 cm | 2 | ‐ | |
| Ketoprofen | 2.5% | 5 cm | 2 | 100 mg | |
| Ketoprofen | 15.0% | 2 g | 3 | 600 mg | |
| Ketoprofen | 1% | ‐ | 3 | ‐ | |
| Ketoprofen | 5% | 2‐3 g | 3 | 300‐450 mg | |
| Indomethacin | 1% | 0.5‐1.5 mL | 3‐5 | 12‐60 mg | |
| Indomethacin | 1% | 1 g | 3‐4 | 30‐40 mg | |
| Indomethacin | 1% | 1 g | 3‐4 | 30‐40 mg | |
| Indomethacin | 1% | 1 g | 3‐4 | 30‐40 mg | |
| Piroxicam | 0.5% | 1 g | 3‐4 | 15‐20 mg | |
| Piroxicam | 0.5% | 1 g | 3‐4 | 15‐20 mg | |
| Piroxicam | 0.5% | 5 mg | 4 | 20 mg | |
| Piroxicam | 0.5% | 1 g | 3‐4 | 15‐20 mg | |
| Benzydamine | 3% | ‐ | 3 | ‐ | |
| Benzydamine | 3% | ‐ | 6 | ‐ | |
| Benzydamine | 3% | ‐ | 3 | ‐ | |
| Niflumic acid | 2.5% | 10 cm, 5 g | 3 | 375 mg | |
| Niflumic acid | 2.5% | 10 cm, 5 g | 3 | 375 mg | |
| Ibuproxam gel | 10% | ‐ | 2 | ‐ | |
| Etofenamate | No details | ‐ | 2 | ‐ | |
| Etofenamate | 5% | 3 g | 3 | 450 mg | |
| Etofenamate | 5% | 2‐3 g | 3 | 300‐450 mg | |
| Ketorolac | 2% | 3 g | 3 | 360 mg | |
| Flurbiprofen | Patch | 2 | 80 mg | ||
| Flunoxaprofen | ‐ | 3‐5 cm | 2 | ‐ | |
| Lysine clonixinate | 5% | 2 cm | 4 | 90 mg | |
| Felbinac | 3% | 2 g | 3 | 180 mg | |
| Felbinac | 3% | 3 cm | 3 | ‐ | |
| Felbinac | 3% | 1 cm | 3 | ‐ | |
| Felbinac | 3% | ‐ | 3 | ‐ | |
| Fentiazac | 5% | Varied according to involved areas | 2‐3 | ‐ | |
| Naproxen | 10% | ‐ | 2‐6 | ‐ | |
| Meclofenamic acid | 5% | 10 cm, 4 g | 2 | 400 mg | |
| equiv: equivalent; Na: sodium. | |||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
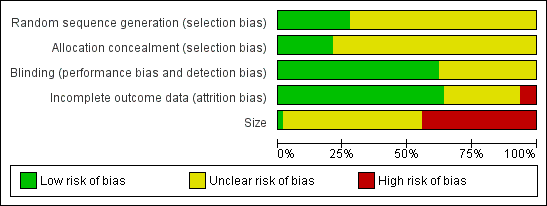
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
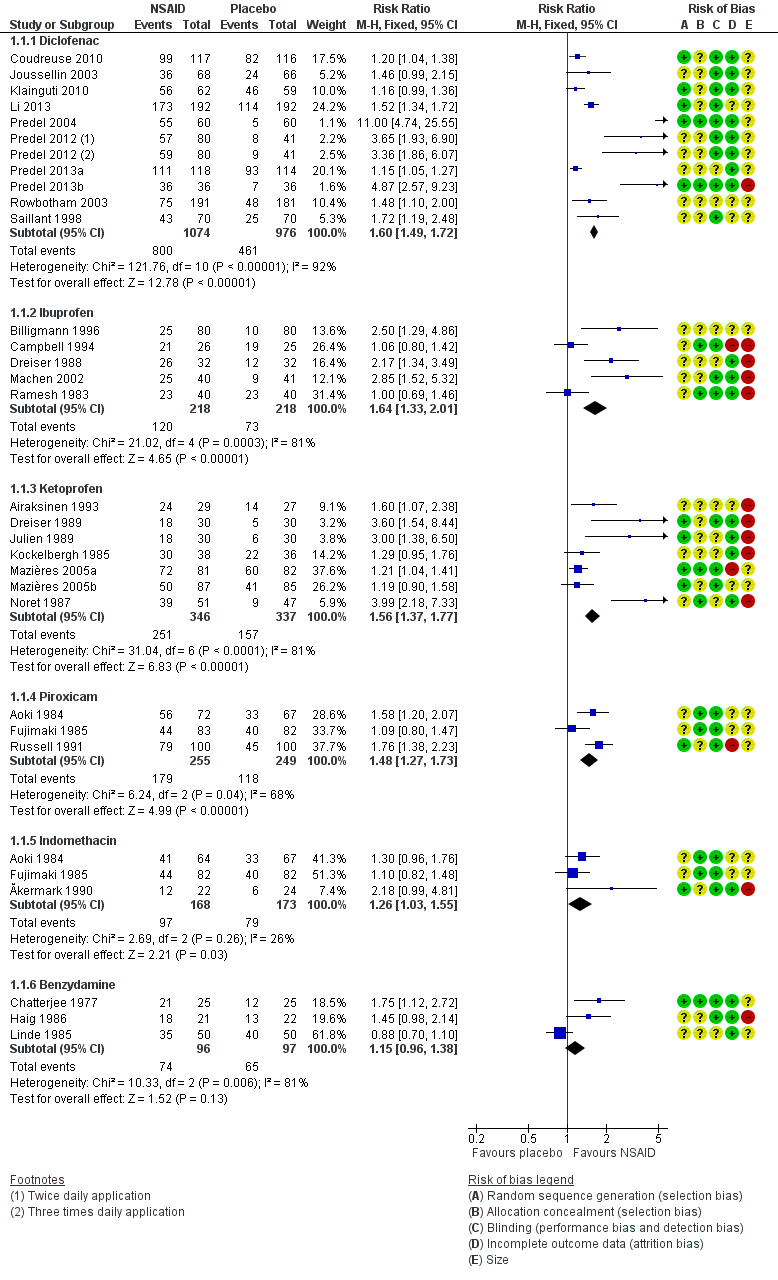
Forest plot of comparison: 2 Individual NSAID versus placebo, outcome: 2.1 Clinical success.

L'Abbé plot of clinical success in studies of topical diclofenac versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Dark blue: Emulgel; light blue: spray/gel; red: Flector; pink: other patch or plaster.

L'Abbé plot of clinical success in studies of topical ketoprofen versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Light blue: ketoprofen gel; pink: ketoprofen plaster.

Comparison 1 Individual NSAID versus placebo, Outcome 1 Clinical success.

Comparison 1 Individual NSAID versus placebo, Outcome 2 Local adverse events.

Comparison 2 Diclofenac versus placebo (effect of formulation), Outcome 1 Clinical success.

Comparison 3 Ibuprofen versus placebo (effect of formulation), Outcome 1 Clinical success.
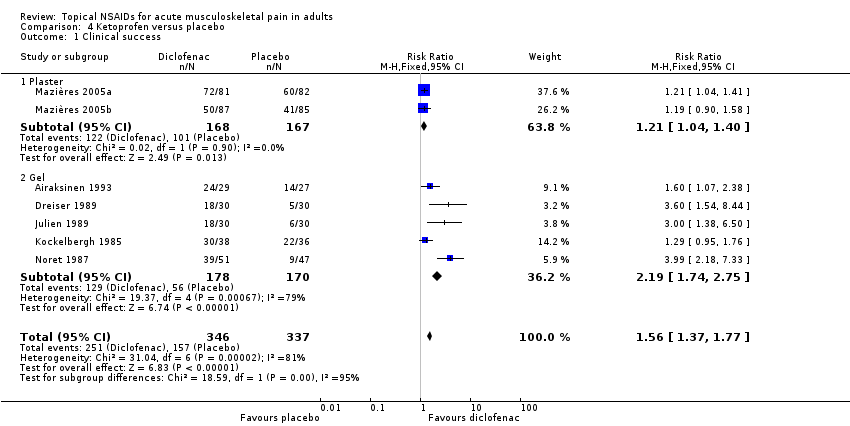
Comparison 4 Ketoprofen versus placebo, Outcome 1 Clinical success.
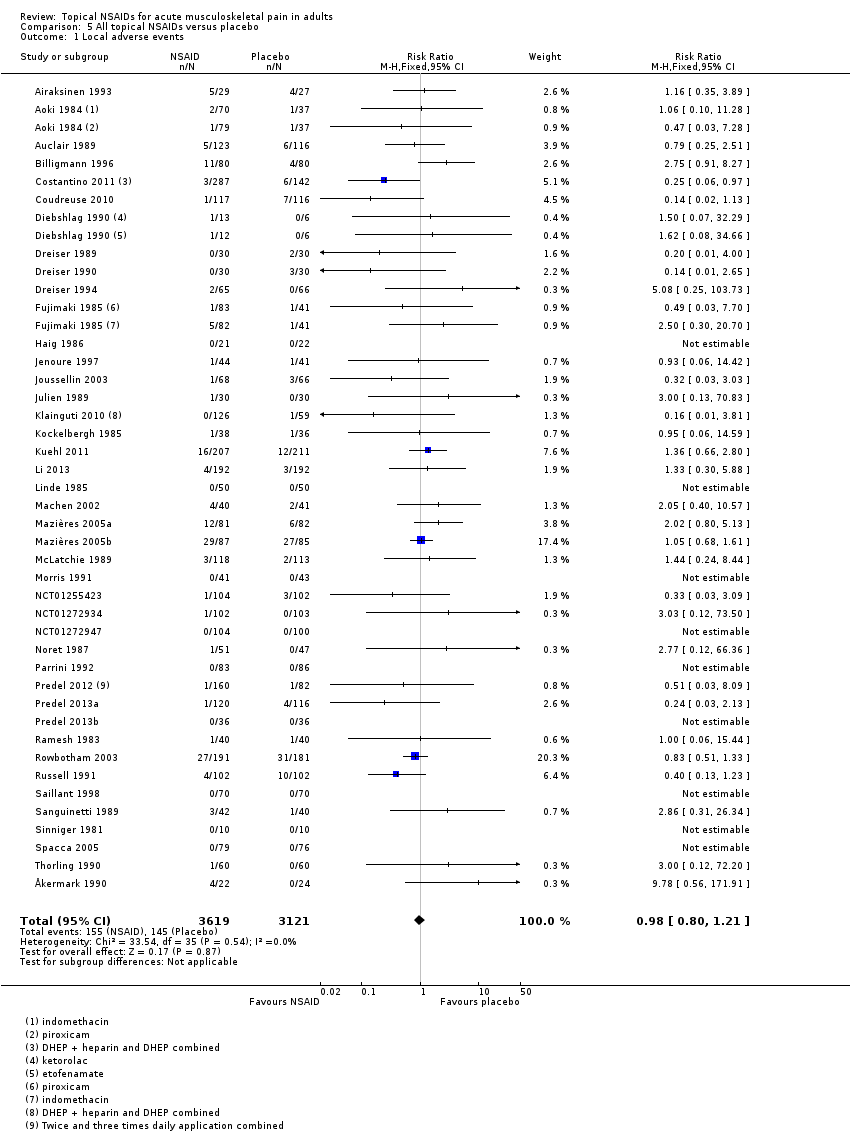
Comparison 5 All topical NSAIDs versus placebo, Outcome 1 Local adverse events.
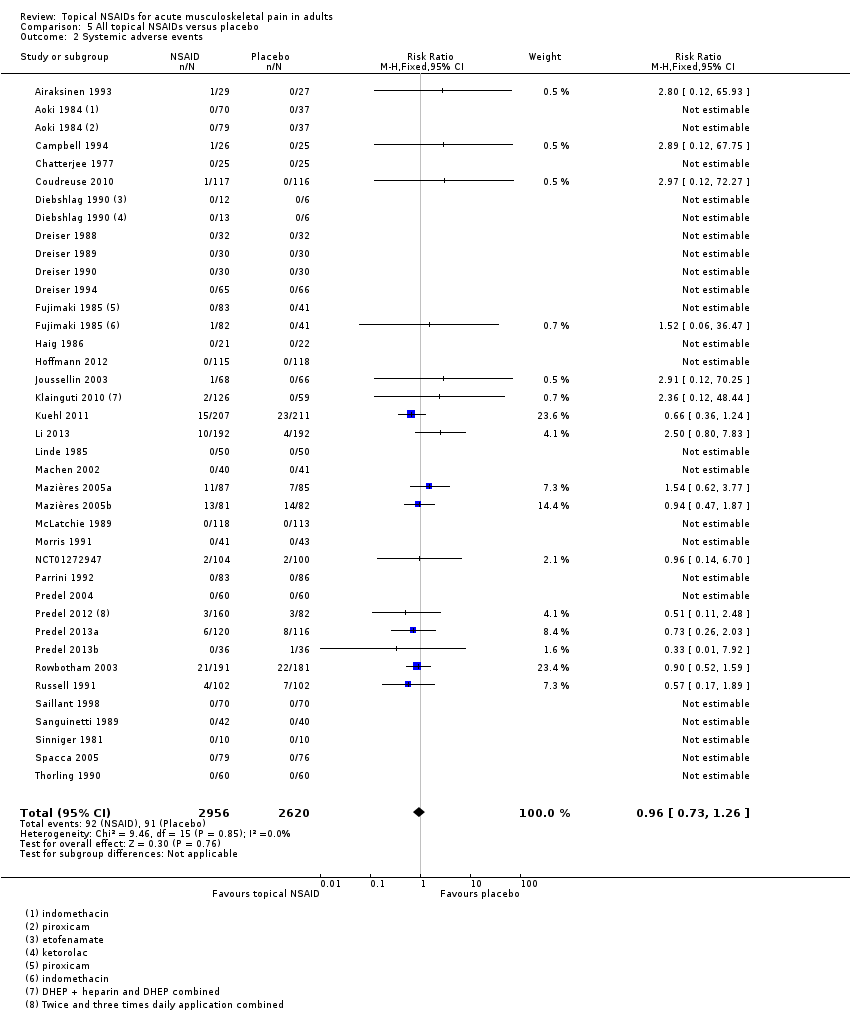
Comparison 5 All topical NSAIDs versus placebo, Outcome 2 Systemic adverse events.
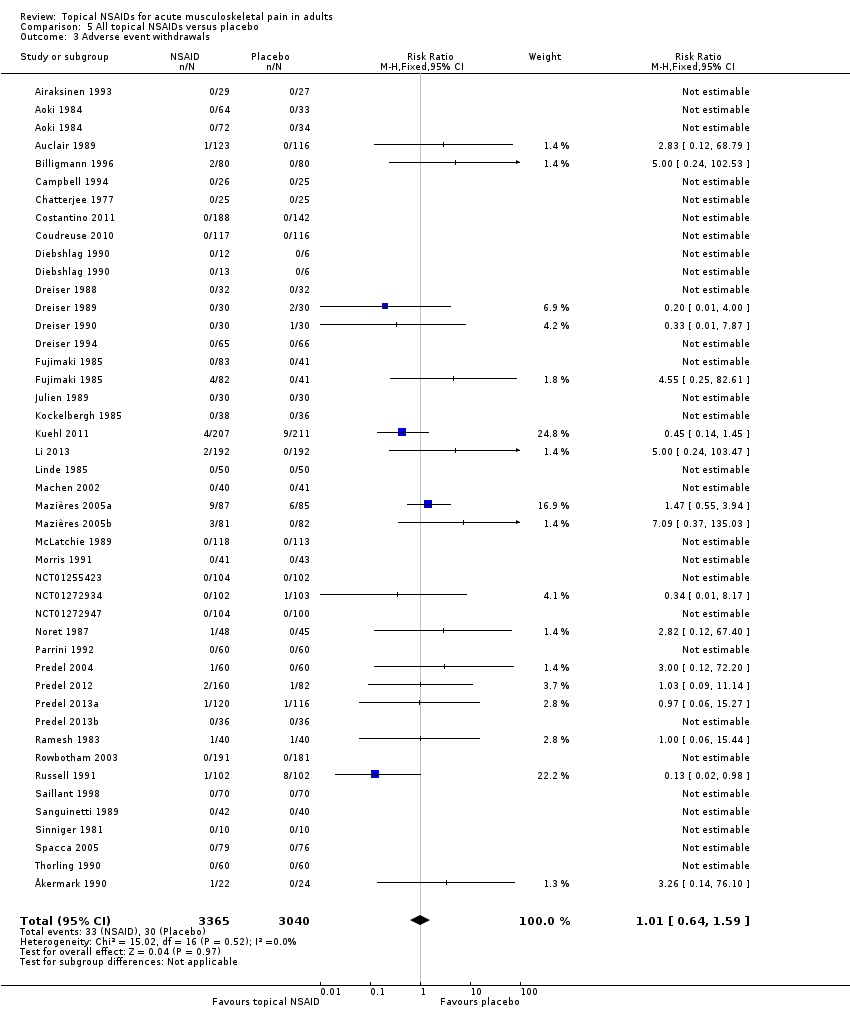
Comparison 5 All topical NSAIDs versus placebo, Outcome 3 Adverse event withdrawals.
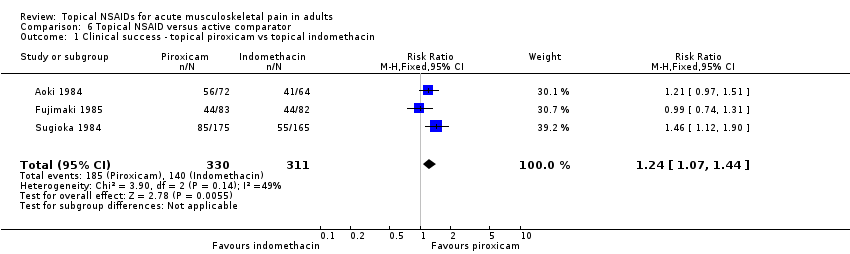
Comparison 6 Topical NSAID versus active comparator, Outcome 1 Clinical success ‐ topical piroxicam vs topical indomethacin.

Comparison 6 Topical NSAID versus active comparator, Outcome 2 Local adverse events ‐ topical piroxicam vs topical indomethacin.
| Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults | ||||||
| Patient or population: adults with strains, sprains, or muscle pull Settings: community Intervention: topical NSAID (topical diclofenac, ibuprofen, and ketoprofen gels only shown here for efficacy) Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with | Probable outcome with | RR, NNT, NNTp, or NNH | No of studies, participants | Quality of the evidence | Comments |
| Topical diclofenac gel (as Emulgel) Clinical success (eg 50% reduction in pain) | 780 in 1000 | 200 in 1000 | RR 3.4 (2.7 to 55) NNT 1.8 (1.5 to 2.1) | 2 studies 314 participants | High | Consistent results in 2 moderately sized recent studies of high quality |
| Topical ibuprofen gel Clinical success (eg 50% reduction in pain) | 420 in 1000 | 160 in 1000 | RR 2.7 (1.7 to 4.2) NNT 3.9 (2.7 to 6.7) | 2 studies 241 participants | Moderate | Modest effect size and numbers of participants |
| Topical ketoprofen gel Clinical success (eg 50% reduction in pain) | 720 in 1000 | 330 in 1000 | RR 2.2 (1.7 to 2.8) NNT 2.5 (2.0 to 3.4) | 5 studies 348 participants | Moderate | Modest effect size and numbers of participants, but studies small, with none recent |
| All topical NSAIDs Local adverse events | 46 in 1000 | 50 in 1000 | RR 1.0 (0.80 to 1.2) NNH not calculated | 42 studies 6125 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Systemic adverse events | 32 in 1000 | 35 in 1000 | RR 1.0 (0.7 to 1.3) NNH not calculated | 38 studies 5372 participants | High | Large number of studies and participants with consistent results |
| All topical NSAIDs Withdrawals ‐ adverse events | 11 in 1000 | 11 in 1000 | RR 1.0 (0.7 to 1.7) NNH not calculated | 42 studies 5790 participants | High | Large number of studies and participants with consistent results |
| Serious adverse events | 1 in total | 0 in total | Not calculated | All data | Low | Small numbers of events |
| GRADE Working Group grades of evidence | ||||||
| CI: confidence interval; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event happening; NNH: number needed to treat for an additional harmful outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Diclofenac | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events Show forest plot | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac | 15 | 3271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.10] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.1 Plaster ‐ Flector | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.36, 1.71] |
| 1.2 Plaster ‐ other | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.37, 1.75] |
| 1.3 Gel ‐ Emulgel | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [2.68, 5.50] |
| 1.4 Gel ‐ other | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.1 Cream | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 1.2 Gel | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.69, 4.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success Show forest plot | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.1 Plaster | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.2 Gel | 5 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local adverse events Show forest plot | 42 | 6740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Systemic adverse events Show forest plot | 36 | 5576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 3 Adverse event withdrawals Show forest plot | 42 | 6405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.59] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical success ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| 2 Local adverse events ‐ topical piroxicam vs topical indomethacin Show forest plot | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |

