The use of propofol for procedural sedation in emergency departments
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized single‐blind control trial | |
| Participants | "Adult patients who presented to the ED for either brief or intensely painful procedures, having marked anxiety or requiring some degree of immobilization" | |
| Interventions | Group A: IV fentanyl 1 μg/kg as a titration dose and propofol 1 mg/kg followed by propofol 0.5 mg/kg if needed Group B: IV fentanyl 1 μg/kg as a bolus dose and a titration dose of midazolam 0.1 mg/kg followed by midazolam 0.1 mg/kg if needed | |
| Outcomes | Level of sedation, vital signs, cardiorespiratory adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "all 40 patients were selected by convenience sampling, and further randomization into two groups was carried out by using the computer‐generated random permuted blocks of four patients" |
| Allocation concealment (selection bias) | Unclear risk | No information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) | High risk | There were 70 study participants in a published abstract of the study, but only 40 study participants in the final published study |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "the drugs were single blinded. They were supplied by the pharmacy department, wrapped individually and placed in an envelope. Each envelope was sealed and labelled accordingly as drug A or B. The operators, emergency physicians and residents in Emergency Medicine, including the main researcher, were unaware of the exact drug to be given until the envelope was opened" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information to permit judgement of 'low risk' or 'high risk' |
| Methods | Prospective randomized trial | |
| Participants | Adults > 18 years undergoing ED cardioversion for a supraventricular arrhythmia | |
| Interventions | Group 1: etomidate 0.2 mg/kg Group 2: propofol 1.5 mg/kg Group 3: midazolam 0.2 mg/kg Group 4: midazolam followed by flumazenil (0.5 mg in bolus followed by 0.5 mg in IV infusion) | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "we prepared the allocation sequence by using a random number table and sequentially numbered envelopes containing each assignment" |
| Allocation concealment (selection bias) | Low risk | Quote: "we prepared the allocation sequence by using a random number table and sequentially numbered envelopes containing each assignment. At each sedation, the investigator then selected the next envelope in sequence to determine the group allocation" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants completed the study |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | Unclear risk | Quote; "the physician responsible for recording the time intervals and the recovery durations was not blinded to the agent used; therefore, we performed 4 objective tests to minimize the potential for observer bias" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: the published study did not provide any information about outcome assessment |
| Methods | Prospective non‐blinded randomized trial | |
| Participants | Adults aged 16‐65 years, with shoulder dislocation | |
| Interventions | Propofol 0.5 mg/kg, with subsequent dose of 0.25 mg/kg and remifentanil 0.5 μg/kg, with subsequent dose 0.5 μg/kg compared with morphine 2.5 mg at 3‐minute increments with midazolam in 1‐mg increments every 3 minute | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "treatment was allocated in equal proportions (20 per group), to either morphine and midazolam or remifentanil and propofol by sequentially selecting sealed envelopes that had been shuffled and numbered" |
| Allocation concealment (selection bias) | Low risk | Quote: "treatment was allocated in equal proportions (20 per group), to either morphine and midazolam or remifentanil and propofol by sequentially selecting sealed envelopes that had been shuffled and numbered" |
| Incomplete outcome data (attrition bias) | Low risk | Details included for all 40 participants completing study |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported |
| Methods | Prospective, partially blinded, controlled, comparative trial | |
| Participants | Convenience sample of children aged 3‐18 years requiring PSA for emergency orthopaedic procedures | |
| Interventions | Comparison of IV P/F with K/M for brief procedural sedation. In the P/F group, IV fentanyl 1‐2 μg/kg was given slowly over 1‐2 minutes and titrated to provide adequate analgesia. After 5 minutes, an initial slow bolus of IV propofol 1 mg/kg was followed by subsequent administration of smaller aliquots based on participant response. In the K/M group, midazolam 0.05 mg/kg IV to a maximum of 2 mg was given slowly over 1‐2 minutes. After 3 minutes, this was followed by ketamine 1‐2 mg/kg IV given slowly over 1‐2 minutes | |
| Outcomes | Comparison of effectiveness of P/F with K/M for brief procedural sedation. Effectiveness was measured using 6 parameters
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "patients were assigned to P/F on odd days and K/M on even enrolment days" |
| Allocation concealment (selection bias) | High risk | Quote: "patients were assigned to P/F on odd days and K/M on even enrolment days" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants completed the study |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the procedure was videotaped for independent review of outcomes. One or both investigators were present for all sedations. Ketamine is known to produce a characteristic dissociative state with nystagmus and a vacant gaze. To mask these "tell tale" facies from the reviewer, patients wore dark goggles (sunglasses) for the duration of the video recording. The patients, their parents, and the reviewers of the tapes were blinded to the type of medication administered. All medications and tubing were covered from view of the video camera and the parents (or legal guardians). Both investigators reviewed all the tapes continuously to ensure that the protocol was followed. In anticipation of a greater frequency of airway repositioning manoeuvres during propofol use, random mock jaw thrusts were performed to reduce bias on the part of the reviewers. Equal numbers of jaw thrusts, both actual and mock, were recorded for both medication groups" Quote: "the independent blinded reviewers assessed the tapes in random order for patient's behavioral distress as measured by the previously validated pain scale known as the OSBD‐r" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: the published study does not provide any information about outcome assessment |
| Methods | Prospective, blinded, randomized clinical trial | |
| Participants | Aged 2‐18 years with isolated extremity injury necessitating closed reduction | |
| Interventions | Propofol (1 mg/kg bolus) followed by infusion (67‐100 μg/kg/minute) until cast completion Midazolam (0.1 mg/kg IV) with additional doses (0.05‐0.1 mg/kg) as required | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized in blocks of 10 using the Moses‐Oakford algorithm" |
| Allocation concealment (selection bias) | Unclear risk | Comment: individual responsible for allocations not described in the paper |
| Incomplete outcome data (attrition bias) | High risk | Comment: 37.1% of participants were uncontactable at follow‐up, missing additional possible complication data |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: sedation nurse performed scoring, recorded time to recovery and complications was blinded. Participants were blinded, submitted follow‐up questionnaire |
| Blinding of outcome assessment (detection bias) | Low risk | Comment: the blinded sedation nurse recorded most measurements. In addition, unblinded sedation scores were done and used for comparison Quote: "there was good strength of agreement between blinded and unblinded scores" |
| Methods | Prospective randomized trial | |
| Participants | Convenience sample of adults 18‐65 years, requiring sedation for painful procedures | |
| Interventions | Propofol (0.5 mg/kg IV) with boluses (0.25 mg/kg IV) every 30‐45 seconds until minimum sedation score reached. Additional boluses as required to maintain sedation Midazolam (1 mg IV) followed by 1 mg every 2 minutes until minimum sedation score reached. Additional boluses as required to maintain sedation level | |
| Outcomes | Primary outcome measure was nurse monitoring time Other outcome measures were:
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details provided regarding method of randomization Quote: "patients were randomized to either the propofol or the midazolam group" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided regarding allocation of group assignment |
| Incomplete outcome data (attrition bias) | High risk | Quote: "eight patients failed to reach an RSS of 3 and were excluded. seven of these 8 were assigned to the midazolam group, 1 to the propofol group" Comment: this represents 20% of the total number of participants excluded from analysis due to inadequate sedation (7/8 from 1 group), thus biasing outcome data. While it is acknowledged in the results section ‐ quote: "the majority of physicians stated frustration with waiting for an RSS of 3 to occur as reason to drop the patient from the study", it represents a significant loss of data and source of bias |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | High risk | Comment: only participants were blinded |
| Blinding of outcome assessment (detection bias) | High risk | Quote: "neither the RN nor the physician was blinded to the randomized drug" Comment: this is significant as subjective assessment scales were used as measure of outcome ‐ RSS and VAS |
| Methods | Randomized, non‐blinded prospective trial | |
| Participants | Adults > 18 years old requiring procedural sedation | |
| Interventions | Propofol 1 mg/kg bolus, followed by 0.5 mg/kg every 3 minutes as needed Etomidate 0.1 mg/kg, followed by 0.05 mg/kg every 3‐5 minutes as needed | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using computer generated random numbers by the investigators" |
| Allocation concealment (selection bias) | Low risk | Quote: "selecting a sequentially numbered sealed envelope containing the group assignment" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all participants who received study drugs were analysed. 109/110 allocated to propofol received the drug. 105/110 allocated to etomidate received the drug |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "we were unable to blind patients, physicians, or data collectors to the agent used in each procedural sedation" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is unclear whether assessors were blinded to outcome measurements. Some measurements were recorded by research assistants who may not have been aware of outcome measures but some were documented by the physicians who ‐ quote: "were likely to have preconceived notions about the 2 agents". Some outcome measures came from the participants themselves, who may have been unaware of the outcomes |
| Methods | Prospective, randomized, non‐blinded trial | |
| Participants | Adults > 18 years, requiring moderate procedural sedation | |
| Interventions | Propofol 1 mg/kg IV bolus followed by 0.5 mg/kg every 3 minutes as needed Ketamine 1 mg/kg IV followed by 0.5 mg/kg every 3 minutes as needed | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "determined using a computer generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was achieved by selecting a sequentially numbered sealed envelopes containing the group assignment" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: of 100 randomized participants, 97 completed the analysis |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | High risk | Comment: no blinding Quote: "all of the physicians who enrolled patients in this study are familiar with both propofol and ketamine and likely had preconceived notions about the two agents" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: there is no comment in the paper regarding blinding of those involved in measurements to the outcome measures. As part of OAAS, and subjective physicians assessment of complications, bias is likely |
| Methods | Stratified, randomized, blinded trial | |
| Participants | 2 adult groups: 18‐65 years and ≥ 65 years old, requiring sedation for cardioversion | |
| Interventions | Group 1: < 65 years: fentanyl 1 μg/kg IV, followed by midazolam 2 mg, then midazolam 1 mg every 2 minutes Group 2: < 65 years: fentanyl 1 μg/kg IV, followed by propofol 20 mg IV, then 20 mg every 2 minutes Group 3: ≥ 65 years: fentanyl 0.5 μg/kg IV, followed by midazolam 2 mg, then 1 mg every 2 minutes Group 4: ≥ 65 years: fentanyl 0.5 μg/kg, followed by propofol 20 mg IV, then 20 mg every 2 minutes | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization was achieved by first doing a stratification on age and then using computer software to generate random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "a study nurse obtained the randomization scheme from a computer and prepared the medications" |
| Incomplete outcome data (attrition bias) | Unclear risk | Comment: data for 4 participants in propofol groups not collected (4/33) |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "the researcher who collected the data was blinded to patient treatment allocation. Blinding was achieved by obscuring the patient's arm from the person collecting information" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Comment: it is unclear whether the researcher who recorded the data was blinded to the outcome measures |
| Methods | Multicentre, randomized clinical trial | |
| Participants | Adults aged ≥ 18 years with anterior shoulder dislocation suitable for ED reduction | |
| Interventions | Propofol (bolus dose by slow IV, titrated to clinical sedation endpoint of spontaneous eye closure) Fentanyl (1.25 μg/kg IV) followed after 2 minutes by a bolus of midazolam (slow IV over 30 seconds titrated to the same sedation endpoint) | |
| Outcomes |
| |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "pharmacist used a random number table to block randomize subjects at each of the three participating hospitals (randomization blocks)" Quote: "despite block randomization at each of the study sites, there were more patients randomized to the propofol group" Quote: "there were a greater proportion of men in the propofol group, and this group had a significantly bigger body build" |
| Allocation concealment (selection bias) | Low risk | Quote: "no other person knew the nature of the drug to be used for any patient until the study packs were opened" |
| Incomplete outcome data (attrition bias) | Low risk | Comment: all randomized participants were included in analysis |
| Other bias | Low risk | Comment: no obvious "other bias" identified |
| Blinding of participants and personnel (performance bias) | Low risk | Procedure operator blinded to study drug ‐ responsible for measurement of muscle tone, ease of reduction and attempts required. ED nurse recorded time to wakening and was unaware of study endpoints |
| Blinding of outcome assessment (detection bias) | Unclear risk | Quote: "ED nurses were not aware of the study end points" Comment: this nurse was responsible for recording times to first and full wakening Quote: "staff members at each participating ED were fully briefed on study procedure" Comment: unclear whether this means they were aware of outcome measures but seems likely |
ED: emergency department; EtCO2: end‐tidal carbon dioxide; GCS: Glasgow Coma Scale; IV: intravenous; K/M: ketamine/midazolam; O2: oxygen; OAAS: Observer's Assessment of Alertness Score; OSBD‐r: Observational Score of Behavioural Distress ‐ revised; P/F: propofol/fentanyl; PSA: procedural sedation and analgesia; RN: registered nurse; RSS: Ramsay Sedation Scale; SpO2: peripheral capillary oxygen saturation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Unsatisfactorily explained discrepancy between the number of participants in 2 publications based on the same study | |
| This was a prospective RCT of ED procedural sedation with propofol alone. There was no comparator intravenous sedative or hypnotic. Participants were randomized to have the treating physician either blinded or not blinded to information from the BIS monitor | |
| This was a non‐blinded prospective RCT of ED deep procedural sedation with propofol with or without an intravenous opioid analgesic (alfentanil). There was no comparator intravenous sedative or hypnotic. Participants were randomized to either propofol alone or propofol with alfentanil for ED procedural sedation of people undergoing painful procedures |
BIS: bispectral index; ED: emergency department; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized single‐blinded control trial |
| Participants | "Adult patients with isolated anterior shoulder dislocations who gave written approval for application of the procedure and who accepted to take part in the study were enrolled" |
| Interventions | Group A: "patients received first intravenous fentanyl 1.5 mcg/kg with 50 ml normal saline in 2 minutes and additional doses if needed for pain control. Then midazolam was given 0.1 mg/kg intravenous bolus, and titrated with additional 0.05 mg/kg doses in two minutes up to the desired sedation level" Group B: "patients received first intravenous fentanyl 1.5 mcg/kg with 50 ml normal saline in 2 minutes and additional doses if needed for pain control. Then propofol 1 mg/kg intravenous bolus was given and additional doses of 0.5 mg/kg in 3 minutes was administered if needed for predetermined sedation level" |
| Outcomes |
|
| Notes |
VAS: visual analogue scale.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Desaturation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Adverse effects, Outcome 1 Desaturation. | ||||
| 1.1 Propofol vs. midazolam (aged < 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Propofol vs. midazolam (aged ≥ 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recovery agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Adverse effects, Outcome 2 Recovery agitation. | ||||
| 3 Pain with injection Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Adverse effects, Outcome 3 Pain with injection. | ||||
| 3.1 Propofol vs. midazolam | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Propofol vs. midazolam/fentanyl | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Propofol vs. etomidate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Oversedation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Adverse effects, Outcome 4 Oversedation. | ||||
| 5 Agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Adverse effects, Outcome 5 Agitation. | ||||
| 6 Post‐discharge nausea/vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Adverse effects, Outcome 6 Post‐discharge nausea/vomiting. | ||||
| 7 Post‐discharge persistent sedation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Adverse effects, Outcome 7 Post‐discharge persistent sedation. | ||||
| 8 Post‐discharge fever Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Adverse effects, Outcome 8 Post‐discharge fever. | ||||
| 9 Post‐discharge recall Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.9  Comparison 1 Adverse effects, Outcome 9 Post‐discharge recall. | ||||
| 10 Agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Adverse effects, Outcome 10 Agitation. | ||||
| 11 Laryngospasm Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.11  Comparison 1 Adverse effects, Outcome 11 Laryngospasm. | ||||
| 12 Moaning Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.12  Comparison 1 Adverse effects, Outcome 12 Moaning. | ||||
| 13 Partial airway obstruction Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 Adverse effects, Outcome 13 Partial airway obstruction. | ||||
| 14 Vomiting Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14  Comparison 1 Adverse effects, Outcome 14 Vomiting. | ||||
| 14.1 Propofol/fentanyl vs. ketamine/midazolam | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Propofol vs. midazolam/fentanyl | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Apnoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.15  Comparison 1 Adverse effects, Outcome 15 Apnoea. | ||||
| 15.1 Propofol vs. midazolam (aged < 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Propofol vs. midazolam (aged ≥ 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||
| 1 Participant satisfaction using a visual analogue scale Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 2.1
Comparison 2 Participant satisfaction, Outcome 1 Participant satisfaction using a visual analogue scale. | ||||||||||||||||||||||||
| 1.1 Propofol vs. etomidate | Other data | No numeric data | ||||||||||||||||||||||
| 2 Participant satisfaction by asking if satisfied with treatment received Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 2.2
Comparison 2 Participant satisfaction, Outcome 2 Participant satisfaction by asking if satisfied with treatment received. | ||||||||||||||||||||||||
| 2.1 Propofol vs. ketamine | Other data | No numeric data | ||||||||||||||||||||||
| 3 Participant satisfaction by using a Likert‐type questionnaire Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 2.3
Comparison 2 Participant satisfaction, Outcome 3 Participant satisfaction by using a Likert‐type questionnaire. | ||||||||||||||||||||||||
| 3.1 Propofol vs. midazolam (aged < 65 years) | Other data | No numeric data | ||||||||||||||||||||||
| 3.2 Propofol vs. midazolam (aged ≥ 65 years) | Other data | No numeric data | ||||||||||||||||||||||
| 4 Participant satisfaction using an ordinal scale Show forest plot | Other data | No numeric data | ||||||||||||||||||||||
| Analysis 2.4
Comparison 2 Participant satisfaction, Outcome 4 Participant satisfaction using an ordinal scale. | ||||||||||||||||||||||||
| 4.1 Propofol vs. etomidate vs. midazolam (with or without flumazenil) | Other data | No numeric data | ||||||||||||||||||||||

Study flow diagram.
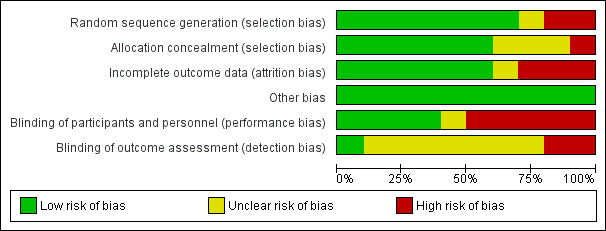
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
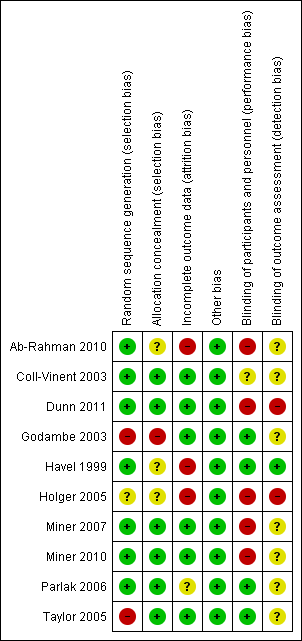
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
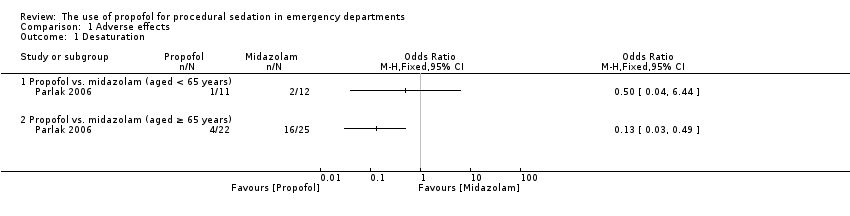
Comparison 1 Adverse effects, Outcome 1 Desaturation.
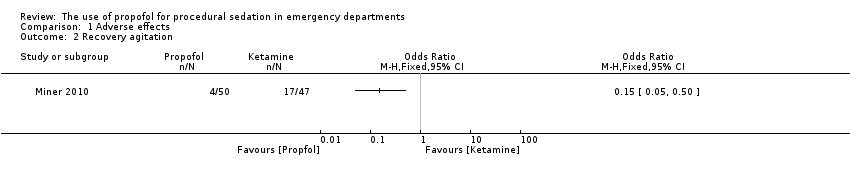
Comparison 1 Adverse effects, Outcome 2 Recovery agitation.
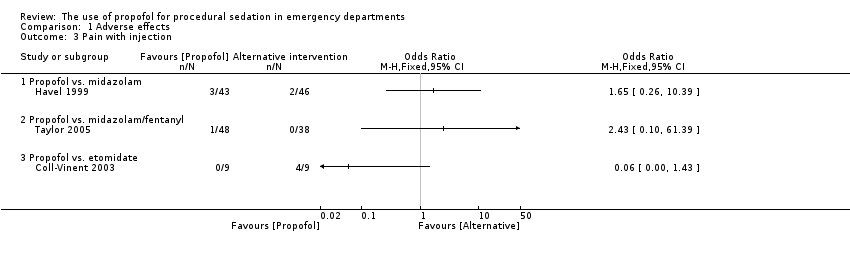
Comparison 1 Adverse effects, Outcome 3 Pain with injection.
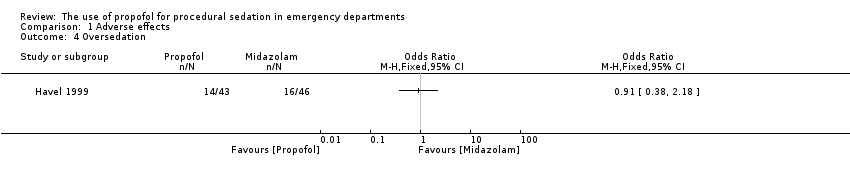
Comparison 1 Adverse effects, Outcome 4 Oversedation.
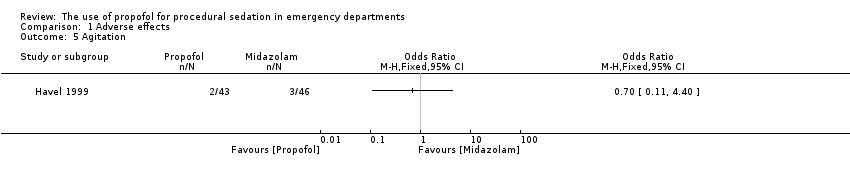
Comparison 1 Adverse effects, Outcome 5 Agitation.
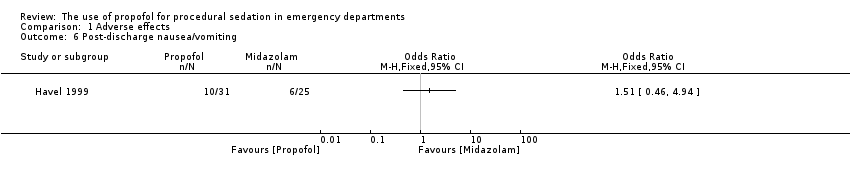
Comparison 1 Adverse effects, Outcome 6 Post‐discharge nausea/vomiting.
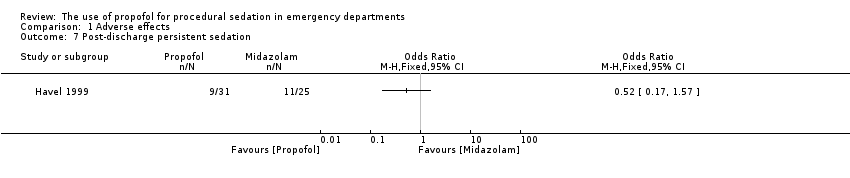
Comparison 1 Adverse effects, Outcome 7 Post‐discharge persistent sedation.

Comparison 1 Adverse effects, Outcome 8 Post‐discharge fever.
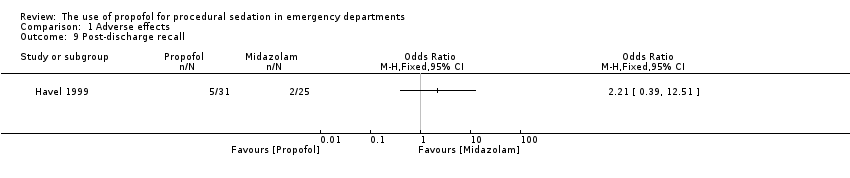
Comparison 1 Adverse effects, Outcome 9 Post‐discharge recall.

Comparison 1 Adverse effects, Outcome 10 Agitation.
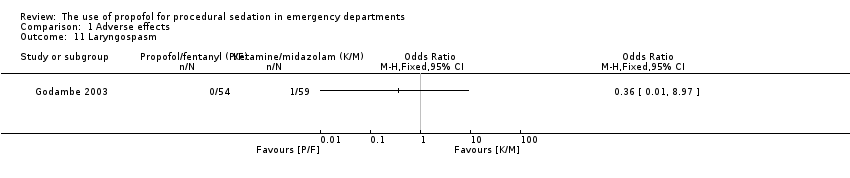
Comparison 1 Adverse effects, Outcome 11 Laryngospasm.

Comparison 1 Adverse effects, Outcome 12 Moaning.

Comparison 1 Adverse effects, Outcome 13 Partial airway obstruction.
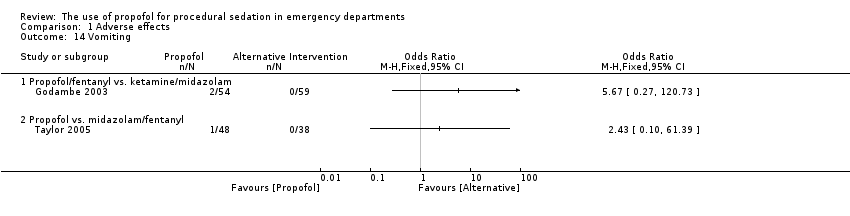
Comparison 1 Adverse effects, Outcome 14 Vomiting.

Comparison 1 Adverse effects, Outcome 15 Apnoea.
| Study | Propofol (n=109) | Etomidate (n=105) |
| Propofol vs. etomidate | ||
| Miner 2007 | 10.3 mm (95% CI 7.0 to 13.6) | 9.8 mm (95% CI 6.1 to 13.6) |
Comparison 2 Participant satisfaction, Outcome 1 Participant satisfaction using a visual analogue scale.
| Study | Propofol (n=50) | Ketamine (n=47) |
| Propofol vs. ketamine | ||
| Miner 2010 | 100% of patients reporting satisfaction with the procedure | 100% of patients reporting satisfaction with the procedure |
Comparison 2 Participant satisfaction, Outcome 2 Participant satisfaction by asking if satisfied with treatment received.
| Study | Propofol (n=11) | Midazolam (n=12) |
| Propofol vs. midazolam (aged < 65 years) | ||
| Parlak 2006 | All 11 patients satisfied with procedure | All 12 patients satisfied with procedure |
| Propofol vs. midazolam (aged ≥ 65 years) | ||
| Parlak 2006 | 20 patients satisfied with procedure; 2 not sure | 20 patients satisfied with procedure; 2 not sure |
Comparison 2 Participant satisfaction, Outcome 3 Participant satisfaction by using a Likert‐type questionnaire.
| Study | Propofol (n=9) | Etomidate (n=9) | Midazolam (n=8) | Midazolam with flumazenil (n=6) |
| Propofol vs. etomidate vs. midazolam (with or without flumazenil) | ||||
| Coll‐Vinent 2003 | 7 patients were "very satisfied"; 2 patients were "satisfied" | 7 patients were "very satisfied"; 2 patients were "satisfied" | 4 patients were "very satisfied"; 4 patients were "satisfied" | 2 patients were "very satisfied"; 4 patients were "satisfied" |
Comparison 2 Participant satisfaction, Outcome 4 Participant satisfaction using an ordinal scale.
| Intravenous propofol compared with alternative intravenous sedative or hypnotic for emergency department procedural sedation | ||||||
| Patient or population: emergency department procedural sedation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Alternative intravenous sedative or hypnotic | Intravenous propofol | |||||
| Adverse effects (as defined by study authors) | Study population | Not estimable | 527 | ⊕⊝⊝⊝ | Clinical heterogeneity prevented a summary statistic | |
| 0 per 1000 | 0 per 1000 | |||||
| Participant satisfaction (as defined by study authors) | Study population | Not estimable | 413 | ⊕⊝⊝⊝ | Clinical heterogeneity prevented a summary statistic | |
| 0 per 1000 | 0 per 1000 | |||||
| Pain with injection | Study population | Not estimable | 193 | ⊕⊝⊝⊝ | Clinical heterogeneity prevented a summary statistic | |
| 0 per 1000 | 0 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The 7 included studies all employed different comparator interventions (Coll‐Vinent 2003; Dunn 2011; Godambe 2003; Havel 1999; Miner 2010; Parlak 2006; Taylor 2005). The quality of evidence was rated down 3 levels for risk of bias because of very serious concerns about inadequate blinding. Coll‐Vinent 2003 reported that the physician responsible for observing time intervals and recovery time was not blinded to the agent used. Dunn 2011 employed no blinding. Godambe 2003 reported blinding of participants, parents and reviewers of the recorded procedure. Havel 1999 reported that blinding during sedation was achieved by shielding medications, infusion tubing and intravenous site from everyone but the study investigator. Miner 2010 reported that neither participants nor staff were blinded to the study drug being administered. Parlak 2006 reported that only the researcher collecting data was blinded to the study drug. Taylor 2005 reported that the doctor performing the procedure was blinded but not the sedation doctor. The quality of evidence was rated down 3 levels for imprecision because the reported CIs around the estimates of treatment effect were very wide. The quality of evidence was rated down 3 levels for inconsistency due to significant clinical heterogeneity in the studies reporting this outcome measure. The quality of evidence was rated down 1 level for indirectness because in 1 study the setting included the coronary care unit (Parlak 2006). The quality of evidence was rated down 3 levels for publication bias because the available evidence comes from small studies (6 of the 7 studies reporting this outcome measure employed fewer than 100 participants). 2The 4 included studies all employed different comparator interventions (Coll‐Vinent 2003; Miner 2007; Miner 2010; Parlak 2006). The quality of evidence was rated down three levels for risk of bias because of very serious concerns about inadequate blinding. Coll‐Vinent 2003 reported that the physician responsible for observing time intervals and recovery time was not blinded to the agent used. Miner 2010 and Miner 2007 reported that neither participants nor staff were blinded to the study drug being administered. Parlak 2006 reported that only the researcher collecting data was blinded to the study drug. The quality of evidence was rated down 3 levels for imprecision because the only reported CI around the estimates of treatment effect was very wide (Miner 2007). The quality of evidence was rated down 3 levels for inconsistency due to significant clinical heterogeneity in the studies reporting this outcome measure. The quality of evidence was rated down 1 level for indirectness because in 1 study the setting included the coronary care unit (Parlak 2006). The quality of evidence was rated down 3 levels for publication bias because the available evidence comes from small studies (3 of the 4 studies reporting this outcome measure employed fewer than 100 participants). 3The 3 included studies all employed different comparator interventions (Coll‐Vinent 2003; Havel 1999; Taylor 2005). The quality of evidence was rated down 3 levels for risk of bias because of very serious concerns about inadequate blinding. Coll‐Vinent 2003 reported that the physician responsible for observing time intervals and recovery time was not blinded to the agent used. Havel 1999 reported that blinding during sedation was achieved by shielding medications, infusion tubing and intravenous site from everyone but the study investigator. Taylor 2005 reported that the doctor performing the procedure was blinded but not the sedation doctor. The quality of evidence was rated down 3 levels for imprecision because the reported CIs around the estimates of treatment effect were very wide (Havel 1999; Taylor 2005). The quality of evidence was rated down 3 levels for inconsistency due to significant clinical heterogeneity in the studies reporting this outcome measure. The quality of evidence was not rated down for indirectness because all the studies reporting this outcome measure, which is important to participants, where applied to the emergency department participant population. The quality of evidence was rated down 3 levels for publication bias because the available evidence comes from small studies (all the studies reporting this outcome measure employed fewer than 100 participants). | ||||||
| Study | Total number of participants randomized | Intervention 1 | Intervention 2 | Intervention 3 |
| 40 | Propofol/fentanyl | Midazolam/fentanyl | None | |
| 32 | Propofol | Etomidate | Midazolam | |
| 40 | Propofol/remifentanyl | Midazolam/fentanyl | None | |
| 113 | Propofol/fentanyl | Ketamine/midazolam | None | |
| 89 | Propofol | Midazolam | None | |
| 32 | Propofol | Midazolam | None | |
| 214 | Propofol | Etomidate | None | |
| 97 | Propofol | Ketamine | None | |
| 70 | Propofol/fentanyl | Midazolam/fentanyl | None | |
| 86 | Propofol | Midazolam/fentanyl | None |
| Study | Adverse effects reported |
| Myoclonus, bronchospasm, pain at injection site, re‐sedation | |
| Oversedation | |
| Agitation, emesis, laryngospasm, apnoea, delayed adverse reactions (nightmares and behavioural change) | |
| Pain with injection, oversedation, post‐discharge complications (nausea/vomiting, persistent sedation, fever and recall) | |
| Recovery agitation | |
| Desaturation, apnoea | |
| Moaning, partial airway obstruction, pain at intravenous site, vomiting |
| Study | Method used to assess participant satisfaction |
| Ordinal scale (not satisfied, moderately satisfied, satisfied, very satisfied) | |
| 100‐mm satisfaction visual analogue scale consisting of the question, How satisfied are you with the treatment you received during this procedure? With the words 'completely satisfied' and 'not satisfied at all' on either side of the 100‐mm line | |
| Quote: "after the patients returned to their baseline mental status, they were asked if they felt any pain during the procedure or were able to recall any of the procedure (yes/no). They were also asked if they were satisfied with the treatment they received during the procedure" | |
| Quote: "patient satisfaction subsequently was evaluated with a questionnaire including Likert‐type questions" |
| Secondary outcome measure | Odds ratio (95% confidence interval) |
| Procedural recall | 0.93 (0.28 to 3.1) |
| Incidence of hypoxia | 1.11 (0.34 to 3.59) |
| Incidence of hypotension | 0.94 (0.13 to 6.94) |
| Secondary outcome measure | Summary statistic (95% confidence interval) |
| Procedural recall | SMD ‐0.24 (‐0.51 to 0.03) |
| BIS nadir | MD 1.6 (‐4.1 to 6.2) |
| Incidence of hypoxia | OR 0.96 (0.38 to 2.41) |
| Need for ventilation | OR 1.21 (0.32 to 4.65) |
| Decrease in SBP from baseline | MD ‐4.1 (‐6.4% to 1.7%) |
| MD: mean difference; OR: odds ratio; SMD: standardized mean difference. | |
| Secondary outcome measure | P value |
| Physician satisfaction score | 0.245 |
| Recall | 1.0 |
| Hypoxia | 0.002 |
| Hypotension | 1.0 |
| Secondary outcome measure | Mean difference (95% confidence interval) | P value |
| Time to first awakening | 4.6 (0.7 to 8.6) | 0.097 |
| Recall | 6.3% (‐6.1% to 18.6%) | 0.309 |
| Hypoxia | 3.1% (‐9.9% to 16%) | 0.69 |
| Hypotension | 2.6% (‐4.8% to 10.1%) | 0.442 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Desaturation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Propofol vs. midazolam (aged < 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Propofol vs. midazolam (aged ≥ 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Recovery agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pain with injection Show forest plot | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Propofol vs. midazolam | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Propofol vs. midazolam/fentanyl | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Propofol vs. etomidate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Oversedation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Post‐discharge nausea/vomiting Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Post‐discharge persistent sedation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Post‐discharge fever Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Post‐discharge recall Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Agitation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Laryngospasm Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Moaning Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Partial airway obstruction Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Vomiting Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 Propofol/fentanyl vs. ketamine/midazolam | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Propofol vs. midazolam/fentanyl | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Apnoea Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Propofol vs. midazolam (aged < 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Propofol vs. midazolam (aged ≥ 65 years) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participant satisfaction using a visual analogue scale Show forest plot | Other data | No numeric data | ||
| 1.1 Propofol vs. etomidate | Other data | No numeric data | ||
| 2 Participant satisfaction by asking if satisfied with treatment received Show forest plot | Other data | No numeric data | ||
| 2.1 Propofol vs. ketamine | Other data | No numeric data | ||
| 3 Participant satisfaction by using a Likert‐type questionnaire Show forest plot | Other data | No numeric data | ||
| 3.1 Propofol vs. midazolam (aged < 65 years) | Other data | No numeric data | ||
| 3.2 Propofol vs. midazolam (aged ≥ 65 years) | Other data | No numeric data | ||
| 4 Participant satisfaction using an ordinal scale Show forest plot | Other data | No numeric data | ||
| 4.1 Propofol vs. etomidate vs. midazolam (with or without flumazenil) | Other data | No numeric data | ||

