Parenterale Opioide zum Schmerzmanagement bei Wehen
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: (not clear) hospital in Oklahoma, USA 100 women in early active labour (with regular contractions and cervical dilatation 3 cm to 4 cm); at term (at or > 37 weeks' gestation); no medical or obstetric complications or evidence of fetal distress; requesting a "pain shot" rather than an epidural (all women were offered epidural). | |
| Interventions | Both groups had continuous electronic fetal monitoring and intrauterine pressure catheters. Experimental: IV fentanyl 50 µg to 100 µg every 1 to 2 hrs to a max of 5 doses Control: IV butorphanol 1 mg to 2 mg every 1 to 2 hrs to a max 5 doses (Doses of drugs were approximately equivalent in both arms of the trial.) | |
| Outcomes | Maternal uterine activity; adverse effects and side effects (including vomiting and sedation); pain scored using 10‐point VAS (0 = no pain, 10 = excruciating pain) scores were recorded by nurses; Apgar scores at 1 and 5 mins; infant neurological exam 2 to 4 hrs and 24 to 36 hrs after birth. | |
| Notes | Start and end date: December 1992 ‐ June 1993 Power calculation: unclear Baseline imbalances between groups: unclear Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared identical unlabelled, coded syringes |
| Blinding of participants and personnel (performance bias) | Low risk | Identical syringes. Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors reported as blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | It was not clear at what point women were randomised.155 women enrolled; 24 decided to have an epidural and were excluded (it was not clear whether or not this was after randomisation); 19 women delivered within 1 hr of first dose and 12 did not request analgesia and were not included in the analysis. Data available for 100 women; if loss occurred after randomisation this represents a very high level of attrition. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | None apparent |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | Setting: hospital, Germany 45 women, in labour, cephalic presentation | |
| Interventions | Experimental: IM tramadol 50 mg (N = 23) Control: IM pethidine 50 mg (N = 22) | |
| Outcomes | Primary outcome: maternal analgesia. Pain assessed as good, not good relief 5 to 10 mins after injection. Secondary outcomes: maternal side effects and fetal heart changes | |
| Notes | German language paper, translation obtained. Tramadol 100 mg plus antiemetic arm not extracted. If additional analgesia required, repeat doses could be administered within < 1 hr. Tramadol: could have up to 3 repeat doses, 50 mg Pethidine: could have up to 3 repeat doses, 25 mg Start and end date: August 1978 ‐ December 1978 Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Assessor was described as unaware of treatment assignment. |
| Incomplete outcome data (attrition bias) | High risk | Women not having a normal birth were excluded from analyses. No information on pain relief was available for 7/45 women. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | Setting: Belfast hospital, UK 40 women (healthy and well) in labour, ASA I or II Exclusion criteria: women planning to have epidural analgesia, with pre‐eclampsia, multiple pregnancy, premature labour, allergy to study medications | |
| Interventions | Experimental: PCA remifentanil 40 µg with lock‐out of 2 mins Control: PCA pethidine 15 mg with lock‐out of 10 mins Nitrous oxide was available to all women and women were free to choose an epidural at any stage. | |
| Outcomes | Maternal sedation score (1 to 5 fully awake to unrousable); VAS 0 to 10 for pain and satisfaction with pain relief; nausea; anxiety; Apgar scores at 1 min and 5 mins; infant neurological adaptive capacity score (2 hrs and 24 hrs after birth). | |
| Notes | VAS scores were reported as median with inter‐quartile range. We were not able to enter data into RevMan tables but have described findings briefly in the text. Start and end date: not reported. Power calculation: "prospective power calculation showed that a sample size of 20 would give 85% power for detecting a difference of 20 mm on the VAS for overall pain, with SD 21.2 from previous work". Baseline imbalances between groups: "The two groups were similar as to characteristics and duration, stage of labour and use of PCA". Funding source: not specified Conflicts of interest: not declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Not clear when randomisation occurred or how it was carried out |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind study |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was reported that for some outcomes assessment was blinded |
| Incomplete outcome data (attrition bias) | Low risk | 40 women were randomised, 1 women was not included in the analysis because of a "protocol violation". 1 woman that withdrew from the study was included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | No baseline imbalance apparent |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | Hospital setting 199 women: in labour, at term gestation, following normal pregnancy No inclusion or exclusion criteria reported | |
| Interventions | Experimental: IM pentazocine 20 mg to 40 mg (N = 91) Control: IM pethidine 50 mg to 100 mg (N = 89) | |
| Outcomes | Primary: analgesic and sedative effects. Pain assessed at time of birth or when second injection administered, as very good, good, moderate or none. Secondary: maternal and neonatal side effects | |
| Notes | If additional analgesia required opioid repeated once after 3 or > hrs of first injection. Actual dose received by women not reported. Start and end date: unclear Power calculation: unclear Baseline imbalances between groups: unclear Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Ampoules numbered and in random order |
| Blinding of participants and personnel (performance bias) | Unclear risk | Reported as double‐blind, but no description of how achieved. Identical volume but appearance not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All participants analysed, but missing data for some outcome |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | Balanced at baseline for age, parity, blood pressure, pulse, frequency contractions, FHR, augmented labour, intensity of labour, membranes intact or ruptured. |
| Methods | RCT, 3‐arm parallel‐group design | |
| Participants | Setting: hospital in Baltimore, USA 212 women randomised (141 included in the analyses in this review). Inclusion criteria: women admitted to hospital for planned vaginal birth, at term, requesting analgesia (birth under regional anaesthesia) Exclusions: imminent birth, allergy to any study medication or requiring birth under general anaesthesia | |
| Interventions | Interventions at 3 cm to 4 cm dilatation for primiparous, and 4 cm to 5 cm for multiparous women. Group 1: pentobarbital IV (initial dose 200 mg) dosage varied Group 2: pethidine IV (initial dose 100 mg), (69 women) Group 3: morphine IV (initial dose 8 mg), (72 women) All 3 groups also received 0.4 mg of scopolamine. If further analgesia was required, women were given a half of the initial dose and 0.2 mg of scopolamine. If more than 2 additional doses were required analgesia was at the discretion of the attending doctor. In this review we have included groups 2 and 3 only in the analyses; pentobarbital (a barbiturate) is no longer used for pain relief in labour. | |
| Outcomes | Length of labour, amount of analgesia required, obstetric complications and neonatal condition (Apgar score at 1 min). Maternal perceptions were recorded 3 days after birth (satisfaction and amnesia). A focus of this paper was the perception of staff on whether women were "manageable". Unmanageable women were those who were "possibly dangerous to others or themselves, perhaps by leaving her bed". Staff had the option of removing unmanageable women from the study and prescribing whatever medication was deemed suitable. | |
| Notes | All women included delivered under regional anaesthesia. Start and end date: not reported Power calculation: not specified Baseline imbalances between groups: unclear Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "in a random manner." |
| Allocation concealment (selection bias) | Low risk | Coded vials containing study drugs were provided by pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "None of the personnel concerned with the administration of the drugs or the evaluation of the patients' reaction had access to the master list at any time." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "None of the personnel concerned with the administration of the drugs or the evaluation of the patients' reaction had access to the master list at any time." |
| Incomplete outcome data (attrition bias) | Unclear risk | All women appear to be accounted for in the analysis and there were few missing data. The data regarding babies were less clear, denominators were not provided. |
| Selective reporting (reporting bias) | High risk | Results were not provided for babies. There was a statement in the text "there were few infant complications in the neonatal period; none of these appeared related to the drugs". |
| Other bias | Unclear risk | Baseline characteristics described as similar. |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | Setting: hospital, UK 46 women (20 primiparous and 14 multiparous women included in the analyses). Uncomplicated pregnancy. Exclusions: first stage of labour > 12 hr, second stage > 1 hr, body weight < 45 kg, multiple pregnancy, non‐vertex presentation, preterm or postmature labour, previous caesarean section, birthweight outside the 5th and 95th centiles for gestational age, congenital fetal abnormality. | |
| Interventions | Experimental: IM meptazinol 1.5 mg/kg body weight plus 10 mg metoclopramide hydrochloride (N = 17) Control: IM pethidine 1.5 mg/kg body weight plus 10 mg metoclopramide hydrochloride (N = 17) | |
| Outcomes | Neonatal acid‐base balance. Maternal pH pre injection, repeated at head crowning, neonatal pH at 10 and 60 mins PN. | |
| Notes | If additional analgesia required opioid repeated > 3‐hourly. Actual dose received by women not reported. Start and end date: not reported Power calculation: not specified Baseline imbalances between groups: unclear Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but not described. |
| Incomplete outcome data (attrition bias) | High risk | 12 women excluded from analysis, reasons for all exclusions not explained. |
| Selective reporting (reporting bias) | High risk | Reasons why some participant data excluded not explained. 3/12 excluded because problem with pH analyser (meptazinol group). |
| Other bias | Low risk | No baseline imbalances |
| Methods | Randomised clinical trial using individual randomisation. | |
| Participants | Setting: hospital in Iran 90 women randomised: nulli‐parous, aged between 18 and 35 years, singleton pregnancy, spontaneous active labour, cervical dilation between 4 cm and 5 cm, gestational age between 38 and 40 weeks, normal FHR tracings, intact membranes, and vertex presentation. Exclusion criteria: elective labour induction, emergency caesarean delivery, known cephalopelvic disproportion, diagnosed pre eclampsia, chorioamnionitis, pyelonephritis, maternal cardiac, renal disease, intrauterine growth restriction and cervical dilation greater than 5 cm. | |
| Interventions | Experimental group: pethidine 50 mg IM – no further detail given. Not clear if it was given as requested or to all women or whether women could request a subsequent dose. (N = 45). Control group: normal saline IV same volume as pethidine. (N = 45). Amniotomy was performed by a trained midwife when cervical dilation reached 5 cm if the membranes had not ruptured spontaneously. | |
| Outcomes | Mode of birth Duration of active phase | |
| Notes | Start and end date: December 2012 to March 2014 Funding: not stated CoI: reported no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out in the obstetric triage unit using a random‐number chart. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) | High risk | Equal volumes of normal saline and pethidine given but by different routes. Likely that caregiver would realise allocation. Participants likely to be aware of treatment. |
| Blinding of outcome assessment (detection bias) | High risk | Labour outcomes were collected by caregiver. |
| Incomplete outcome data (attrition bias) | Unclear risk | Reported that none of the 90 enrolled women withdrew for any reason. Data reported for all women. However not all data are reported in absolute numbers and denominators and results are not clear for Apgar scores or neonatal admission to intensive care. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Outcomes are not clearly pre‐specified. It was not clear which outcomes the power calculation related to. Important outcomes were not reported. |
| Other bias | Unclear risk | Similar baseline characteristics. Generally poorly reported. |
| Methods | RCT, 3‐arm parallel groups | |
| Participants | Setting: the Netherlands, Department of Obstetrics and Gynaecology 180 enrolled, 159 completed the study. Inclusion criteria: healthy ASA physical status I or II term parturients in an active stage of labour, with singleton cephalic presentation, without prior administration of opioid analgesics. Exclusion criteria: obesity (BMI ≥ 40 kg m‐2), opioid allergy, substance abuse history, and high‐risk patients (pre‐eclampsia, severe asthma, insulin‐dependent diabetes mellitus, hepatic insufficiency, or renal failure). | |
| Interventions |
| |
| Outcomes | Outcomes: pain scores (VAS) every hr; sedation score (1 awake, 2 sleepy, 3 eyes closed, 4 eyes closed but rousable, 5 unrousable; overall satisfaction on 10‐point scale 2 hrs after delivery; side effects – nausea,vomiting, itching; Apgar scores at 1 min, 5 mins; cord blood gas analysis; NACS scores at 15 mins and 2 hrs after delivery; oxytocin use; instrumental delivery; CS; spontaneous delivery. | |
| Notes | Quote: “All women received similar instructions on how to use the PCA device: all parturients were instructed to press the bolus button whenever they needed pain relief." Start and end date: not reported Power calculation: 'For sample size calculation, we hypothesized that average pain scores in the remifentanil or fentanyl group would differ at least 10% from the meperidine group. Assuming an SD of 15 mm based on the previous studies, we calculated a sample size of 60 parturients per group for a power of 0.95 and a two‐sided a level of 0.05 to detect this difference'. Baseline imbalances between groups: 'The characteristics of the parturients did not differ statistically.' Funding source: Bronovo Research Fund Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Established using a computer generated random sequence in numbered envelopes.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Study medication was prepared and blinded by hospital pharmacy.” |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: “Observants and medical personnel attending to the parturient were unaware of the drug assignment.” |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: “with exception of baseline data, all observations and measurements were made by blinded observers.” |
| Incomplete outcome data (attrition bias) | Low risk | 180 enrolled, 159 completed the study: 52 R group; 53 M group; 54 F group; 21 excluded because delivered within 1 hr after randomisation. Quote: “Data analysis was per‐protocol.” |
| Selective reporting (reporting bias) | Unclear risk | All outcomes discussed in methods appear to have been reported upon within results. However, the study protocol was not evaluated. |
| Other bias | Low risk | Baseline characteristics similar |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | Setting: hospital, UK 200 women. 66% primips, 34% multips, > 35 weeks' gestation. Singleton, uncomplicated pregnancy. Exclusions: toxaemia, chronic medical disease, isoimmunisation, obstetric complication | |
| Interventions | Experimental: IM pentazocine 48 mg (N = 100) Control: IM pethidine 120 mg (N = 100) Nalorphine hydrobromide + methylphenidate given if opioid administered within 2/24 of second stage diagnosis and, or fetal distress. | |
| Outcomes | Primary outcome: analgesic effects: pain assessed at time of injection and every 30 mins for 4 hrs. Severe or moderate pain. Pain relief complete, partial or none. Secondary outcomes: maternal: vomiting, blood pressure and pulse. Neonatal: Apgar at 1 min in babies born within 4 hrs of opioid. | |
| Notes | If additional analgesia required opioid repeated after 4 hrs. As inclusion criteria > 35 weeks' gestation, may include preterm infants. Start and end date: not reported Power calculation: not specified Baseline imbalances between groups: the 100 women given each drug was comparable in respect of age, parity, height, last antenatal weight and blood pressure, attendance at preparation classes, and infant weight. Funding source: drug ‐ Pentazocine was supplied by Bayer products Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States "double blind" but does not report how achieved. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States "double blind" but does not report how achieved. |
| Incomplete outcome data (attrition bias) | High risk | 200 women randomised. Exclusion of women from analyses if inadequacy of records, reached second stage before analgesic assessment, operative birth or another intervention. Exclusion of babies from Apgar analysis if additional analgesia given, GA, antidote given to mother pre‐birth or clinical explanation for depressed baby. Denominators for outcomes not clear. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Balanced at baseline for age, parity, height, weight, blood pressure, attendance at antenatal classes and infant weight. |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | Setting: Ain Shams University Maternity Hospital, Egypt 240 women randomised Inclusion criteria: healthy, nulliparous, women aged between 18 and 30 years, at term (37–42 weeks of gestation) with a single fetus in vertex presentation, and diagnosed with prolonged labour due to uterine dystocia during the first stage of labour with a cervical dilatation of 4 cm to 6 cm. (Uterine dystocia was defined as crossing of the alert line on the partogram without abnormal fetal presentation or cephalopelvic disproportion.) Exclusion criteria: meperidine allergy, any contraindication for vaginal delivery, labour induction, use of oxytocin or any type of analgesia prior to randomisation, maternal request of pain relief, fetal death, or evidence of fetal distress. | |
| Interventions | Experimental: Meperidine ‐ single dose of 50 mg meperidine in 10 mL of isotonic saline by slow intravenous administration over 2 mins (50 mg pethidine, 2‐mL solution; Misr Pharmaceutics, Cairo, Egypt) (N = 120). Control: placebo ‐ 10 mL of isotonic saline supplied in identical vials. (N = 120). | |
| Outcomes | Primary outcomes: (i) duration of labour (from the time of the beginning of the intervention to the time of expulsion of the fetal head) and (ii) neonatal acid‐base balance in arterial and venous. Secondary outcomes: severity of labour pain, as assessed by the 10‐cm visual analogue scale (VAS) score (0 defined as no pain) before the intervention and 15, 30, and 60 mins after drug or placebo administration, and during the second stage of labour; maternal adverse effects; requirement for oxytocin augmentation after intervention; mode of delivery; and Apgar score at 1 and 5 mins. | |
| Notes | When labour crossed the alert line on partograph, women were randomised and oxytocin commenced. Start and end date: July 2007 and October 2009 Power calculation: 'The sample size was calculated using a power of 80%, an alpha of 0.05, expected 60‐min reduction in the length of labour, and assumed standard deviation of 158 mins based on a previous report of the length of labor in a population of women similar to our population. A sample size of 220 women was calculated to be necessary on the basis of these assumptions.' Baseline imbalances between groups: 'There were no significant differences between the two groups with regard to maternal age, body mass index, gestational age at delivery, cervical dilatation and length before intervention, and VAS score before drug or placebo administration'. Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were placed in sequentially‐numbered, opaque, sealed envelopes to be opened at time of enrolment by a nurse who prepared the study drug and had no further involvement with the care of the participants. |
| Blinding of participants and personnel (performance bias) | Low risk | The study is described as double‐blind, placebo trial. If the neonatologists needed to know the administered intervention to manage a neonatal side effect, they would call a nurse to obtain such information. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | There was no loss to follow‐up and all women reportedly received their allocated intervention. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in methods appear to be reported. |
| Other bias | Unclear risk | Similar baseline characteristics. Some lack of clarity in results, e.g. unclear if labour durations include women who had a caesarean. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: Cape Town, South Africa 29 women in established labour, not clear how many primips, mean age 24 years, women were expected to have a vaginal birth and have no antenatal medical or obstetric problems. | |
| Interventions | Experimental: pethidine, IV PCA 10‐min lock out, 0.3 mg per kg. Control: pentazocine, IV PCA 10‐min lock out, 0.15 mg per kg. | |
| Outcomes | Pain relief in labour (assessed by midwife); pain relief (measured immediately after labour (10 cm VAS) and 24 hrs postpartum from mother); satisfaction with pain relief; maternal and neonatal serum samples; Apgar score at 1 min and 5 mins; infant weight; neuro‐behavioural examination on 1st and 5th day. | |
| Notes | The study also included a non‐randomised control group; we have not included this group in the analysis. Start and end date: not reported Power calculation: not specified Baseline imbalances between groups: unclear Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | It was reported that women were attended by the same midwife throughout labour who was not informed what medication women received. It is not clear whether this blinding was achieved for all staff. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors of neonatal outcomes were reported to be blind to group allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | Overall attrition not clear, there were some missing data for some outcomes. Denominators were not provided in all of the results tables. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance apparent |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 161 women randomised, data available for 133 women. 52% primips, 48% multips, cx at least 3 cm dilated, 37 or > weeks' gestation in spontaneous or induced labour (induction by amniotomy and IV infusion oxytocin). | |
| Interventions | Experimental: IM diamorphine 7.5 mg (primips), 5 mg (multips) plus 12.5 mg prochlorperazine (N = 65) Control: IM pethidine 150 mg (primips), 100 mg (multips) plus 12.5 mg prochlorperazine (N = 68) | |
| Outcomes | Primary outcome: maternal pain at 1 hr VAS (0‐100), pain intensity (0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain), pain relief (0 = none, 1 = slight, 2 = moderate, 3 = good, 4 = complete). Secondary outcomes: maternal: vomiting, sedation, global analgesia assessment at 24 hr (good or poor). Neonatal: Apgar at 1 min and 5 mins, resuscitation, naloxone administration, SCBU admission, significant morbidity (seizures, respiratory distress, intraventricular haemorrhage, necrotising enterocolitis). | |
| Notes | Second dose at maternal request: her choice of drug or epidural. Stratified by maternal parity. Trial stopped early after recruitment of 150 women. Planned sample size was 200 women. Start and end date: May 1990 ‐ February 1992 Power calculation: not specified Baseline imbalances between groups: unclear Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block sizes of 6 |
| Allocation concealment (selection bias) | Low risk | Coded drug containers, randomisation code not broken until analysis. |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind, drug containers identical in appearance. |
| Blinding of outcome assessment (detection bias) | Low risk | It was stated that the randomisation code was not broken until the analysis stage. |
| Incomplete outcome data (attrition bias) | High risk | 28 (17%) excluded as delivered within 1 hr of administration of study drug. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | Balanced at baseline |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Italy: hospital care setting 40 women. Full‐term pregnancy, cx ≥ 4 cm, in spontaneous active labour and requiring analgesia. | |
| Interventions | Experimental: IM tramadol 100 mg (N = 20) Control: IM pethidine 75 mg (N = 20) | |
| Outcomes | Primary outcome: maternal pain relief and acceptability. Pain assessed hourly up to 5 hrs, VAS 1‐3. Secondary outcomes: maternal: observations (pulse, BP, respiratory rate, arterial oxygen saturation). Neonatal: Apgar at 1 min and 5 mins. Umbilcal cord pH. | |
| Notes | Second dose of study drug allowed after 2 hrs as required. Italian language, translation obtained. Data were presented in a way in which we were not able to incorporate them into data tables in RevMan. Start and end date: unclear Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many women analysed as only percentages reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | No baseline characteristics table ‐ unclear re maternal parity. Likely response bias as no information on whom women reported to about their pain post injection. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: London hospital, UK 60 healthy women at term (38‐42 weeks) requiring pain relief in labour. Women requesting epidural, that had already received opioid analgesia, were receiving treatment for depression or where the fetus was at risk were excluded | |
| Interventions | Experimental: (30 women) nalbuphine, 3 mg with 3 mg increments to a max of 18 mg per hr; lockout time 10 mins (total max dose = 42 mg). Control: (30 women) pethidine, 15 mg, 15 mg increments to a max of 90 mg per hr; lockout time 10 mins (total max dose = 210 mg). Entonox ® was available to women in both groups but was withheld for 30 mins for analgesia assessment. Analgesia was stopped in the 2nd stage if there were side effects or if the woman requested an alternative method. | |
| Outcomes | Pain (measured on 5‐point scale from 1‐ no pain to 5 ‐ very severe); pain relief (assessed 1 day after birth; pain relief rated as good or excellent and women saying they would use the same method again); sedation (1 awake, 3 asleep); neuro‐behavioural assessment 6 to 10 hrs after birth; FHR. | |
| Notes | Start and end date: not reported Power calculation: not specified Baseline imbalances between groups: unclear Funding source: Dupont (UK) Ltd Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated." |
| Allocation concealment (selection bias) | Unclear risk | Described as double‐blind but allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Very little information. Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | There were some outcome data for all but one of the women randomised, but there were high levels of missing data for some neonatal outcomes (e.g. neurological infant assessments 40/60 babies available for analysis). |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | There was some baseline imbalance; 6/30 in the nalbuphine group were multiparous compared with 12/30 in the pethidine group. The authors report that they took this into account in the analysis. In this review data have not been adjusted for baseline imbalance. |
| Methods | RCT, 2‐arm parallel groups | |
| Participants | New Jersey USA, hospital setting, 1994 28 women in labour (36 randomised) with uncomplicated pregnancies, singleton, vertex presentation, at term (37 to 41 weeks), 4 cm or less cervical dilatation, at least 3 contractions in 10 mins, no known maternal or fetal conditions that would affect FHR tracings, fetal reactive, no medications that would affect FHR in the previous 2 weeks. Exclusions criteria: meconium staining, pregnancy‐induced hypertension, fetal tachy‐ or brady‐cardia, arrhythmias or decelerations, chorioamnionitis, FGR, abnormal placenta, maternal fever, fetal chromosomal disorder of structural abnormality. | |
| Interventions | Experimental: IV nalbuphine10 mg Control: IV pethidine 50 mg Both groups had continuous fetal monitoring for 1 hr following medication. | |
| Outcomes | FHR (accelerations, high and low variation); Apgar scores < 8 at 1 min and 5 mins; mode of birth; cord pH < 7.15. | |
| Notes | Start and end date: March 1994 ‐ August 1994. Power calculation: 'Using the normal reference ranges for long‐term variation and acceleration of ten beats per minute for a 15‐second duration, the study would require 28 women to achieve a power of 90% to detect a change from values at the 50th percentiles to values below the fifth percentile at an alpha error of 0.05.' Baseline imbalances between groups: there was statistical difference between the groups. Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) | Unclear risk | 36 women were enrolled. 8 women did not have sufficient FHR tracings and were not included in the analysis (22% attrition). |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | No apparent baseline imbalance |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital in USA 500 women admitted to hospital in labour. Little information provided. | |
| Interventions | Experimental: (185 women) alphaprodine (Nisentil), initial dose 40 mg IV, subsequent doses IM Control: (210 women) pethidine, initial dose 100 mg IV, subsequent doses IM Both groups received scopolamine. Analgesia was for the first stage of labour, birth was carried out "with rare exception" under "saddle block or pudendal block terminal anesthesia". | |
| Outcomes | Pain relief (rated just before leaving the room for childbirth); side effects and length of labour. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | Coded drug containers |
| Blinding of participants and personnel (performance bias) | Low risk | Drugs were prepared by pharmacy in coded containers and the codes were not revealed until after birth. |
| Blinding of outcome assessment (detection bias) | Low risk | Drugs were prepared by pharmacy in coded containers and the codes were not revealed until after birth. |
| Incomplete outcome data (attrition bias) | High risk | 500 women were randomised, 55 women received no analgesia and were excluded, 22 women received more than 1 dose of opioid (not necessarily the same drug) and were excluded, 21 women who were in preterm labour or had a CS were excluded and 1 woman was excluded because she was sensitive to study medication. Data available for 395 women (21% attrition). |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Study medication was for pain relief in the first stage of labour, most women received a pudendal block for birth so outcomes relating to birth may not be attributable to study medication alone. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 212 women in spontaneous or induced labour with cephalic presentation at > 36 weeks' gestation. Recruited to the trial at 36 week antenatal clinic visit. | |
| Interventions | Experimental: IM phenazocine 3 mg (N = 107) Control: IM pethidine 150 mg (N = 105) | |
| Outcomes | Primary outcome: maternal analgesia assessed in labour as poor, fair, good, very good. Pain relief also assessed in postnatal questionnaire within 36 hrs of birth. Secondary outcomes: maternal: amnesia, restlessness, anxiety, vomiting. Neonatal: Apgar at 1 min and 5 mins. | |
| Notes | Epidural available if further analgesia required. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: 'There was no significant difference between the two groups with respect to age, parity, height, weight, pelvic size, incidence of induced labour or cervical dilation at the time of first dose of analgesia'. Funding source: Smith and Nephew (Pharmaceutics) Ltd provided the marked drug ampoules Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Code kept by hospital pharmacist and remained unbroken until trial completed. |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind, coded ampoules but no further description given. |
| Blinding of outcome assessment (detection bias) | Low risk | Code kept by hospital pharmacist and remained unbroken until trial completed. |
| Incomplete outcome data (attrition bias) | Unclear risk | 212 women randomised. Number of women analysed is not reported. |
| Selective reporting (reporting bias) | Unclear risk | MW assessed maternal side effects in labour. |
| Other bias | Unclear risk | Although baseline characteristics described as similar ‐ proportion of primips to multips not provided. Balanced for age, parity, height, weight, cx dilatation. PN maternal recollection of pain within 36 hr and unclear to whom women reported ratings. |
| Methods | RCT. 2‐arm parallel‐group design | |
| Participants | 185 randomised. analysis for 160 women in labour. Inclusion criteria: primiparous, no pregnancy complications. Exclusions: women with hypertension or pre‐eclampsia. It appeared that women who had any complications during birth (e.g. CS) were excluded after randomisation. | |
| Interventions | Intervention group: Avacan ® 25 mg IM (a spasmolytic) Control group: Fortral ® 20 mg IM (pentazocine) | |
| Outcomes | Number of requests for analgesia, infant birthweight, Apgar score (at birth). | |
| Notes | Data extraction was done from translation notes. Start and end date: June 1969 ‐ January 1971 Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Described as a double‐blind trial but methods were not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | High risk | 185 women approached, 25 were excluded and results suggest that any women who had CS were excluded from the analysis along with women who had long labours (> 24 hrs) or where no injections were given. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Assessment of risk of bias done using translation notes. |
| Methods | RCT 4‐arm parallel‐group design | |
| Participants | Setting not clear, USA 200 women admitted to hospital in the 1st stage of normal labour, mean age 24 years, women received medication if they complained of moderate or severe pain. | |
| Interventions | Experimental: (100 women) (i) IV butorphanol 1 mg (67 women) (ii) IV butorphanol 2 mg (33 women) Control: (100 women) (i) IV pethidine 40 mg (68 women) (ii) IV pethidine 80 mg (32 women) | |
| Outcomes | Pain intensity (graphs with hourly readings); pain relief (4‐point scale); neuro‐behavioural assessment 1 day after birth (Scanlon scale). | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information. Described as "double‐blind". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind but little detail of methods of allocation concealment or blinding. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind but little detail of methods of allocation concealment or blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up apparent |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Very little information on study methods. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Austria 40 women with no pregnancy complications, in spontaneous and induced labour, cx 3 cm to 5 cm dilated. 72.5% primips, 27.5% multips. | |
| Interventions | Experimental: IM tramadol 100 mg (N = 20) Control: IM pethidine 100 mg (N = 20) | |
| Outcomes | Primary: pain relief, assessed 10, 30, 60, 120 mins after injection using VAS 0‐100, 0 = pain free to 100 strongest pain experienced. Secondary: side effects, augmentation and type of birth. | |
| Notes | Not stated in 1 dose only Start and end date: unclear Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding not described |
| Incomplete outcome data (attrition bias) | Low risk | All women analysed |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Baseline characteristics stated as similar |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 100 women in labour at term gestation with uncomplicated pregnancy. | |
| Interventions | Experimental: Meptazinol 1.8 mg/kg body weight (N = 50) Control: pethidine 1.8 mg/kg body weight (N = 50) All participants received promethazine 12.5 mg with first injection. | |
| Outcomes | Primary: newborn effects: Apgar score at 1 min and 3 mins | |
| Notes | If additional analgesia required, a repeat injection could be administered 3‐hourly. 6/50 women from each arm received a second dose at a 3‐hourly interval. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but method not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but method not described |
| Incomplete outcome data (attrition bias) | High risk | 5 babies excluded from analysis due to heart defects and fetal distress. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Balanced for parity, weight and size of baby at baseline. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: Maternity Unit, Tamin Ejtemai Hospital, Iran 70 women randomised Inclusion criteria: multiparous pregnant women (gravida 2‐7); term singleton pregnancy; cephalic presentation; low‐risk pregnancy with no history of drug tolerance (addiction), medical or mental diseases. Exclusion criteria: respiratory rate < 8 or maternal bradycardia (pulse rate less than 60) and severe congenital anomalies in neonate after birth. | |
| Interventions | Experimental: Fentanyl ‐ 50 mcg fentanyl was prescribed in 2 doses with an interval of 1 hr after being diluted in 4 cc normal saline (total volume 5 cc ‐ 25 μg/5 mL during 10 mins infusion and repeated second dose an hr later 25 μg/5 mL) at zero and 60 mins. Control: no analgesia | |
| Outcomes | Outcomes: pain score, blood pressure, heart rate, FHR and maternal respiratory rate, duration of labour, maternal side effects drowsiness, dizziness, nausea/vomiting, respiratory depression, hypotension (BP < 90 mmHg or less than < 20% of baseline), bradycardia (HR < 60 beats min‐1), and pruritus.Neonatal outcomes included Apgar scores at 1 min and 5 mins and resuscitation efforts (if any). | |
| Notes | Power calculation: 'based on results from a pilot study on 10 parturients (and mean duration of the active phase), effect size was obtained at 0.4 hours with power 80% and confidence level of 95%, the sample size was then calculated to be 70 parturients'. Baseline imbalances between groups: ‘There was no statistically significant difference in mean age between the two groups. There were no significant differences in gravidity, parity, fetal heart rate, contraction duration or HR between the two groups'. Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A coin was tossed to determine the participants comprising the control and case groups (35 women per group). It was not reported if this was at the point of randomisation but no information on allocation concealment. |
| Allocation concealment (selection bias) | High risk | Coin toss to determine group. If this was at the point of randomisation this is a high‐risk method. There was no indication of allocation concealment. |
| Blinding of participants and personnel (performance bias) | High risk | Not feasible |
| Blinding of outcome assessment (detection bias) | High risk | Outcome assessor likely to be aware of allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | No reported loss to follow‐up, no denominators given in tables and no details of women requesting further analgesia and changing groups. Not clear if there was loss to follow‐up or not. |
| Selective reporting (reporting bias) | High risk | Protocol not seen, outcomes listed in methods but are not well reported. Apgar results described narratively, resuscitation measures not mentioned. |
| Other bias | Low risk | Baseline characteristics were balanced across groups. |
| Methods | RCT 3‐arm parallel‐group design | |
| Participants | Setting: hospital, Germany 66 women. 38‐41 weeks' gestation, free of complications, in active labour and requiring analgesia, excluded if analgesia received within 4 hrs of randomisation. Parity: not reported | |
| Interventions | Experimental: IM tramadol 100 mg (N = 20); IM tramadol 100 mg + triflupromazine 10 mg (N = 25) Control: IM pethidine 50 mg + triflupromazine 10 mg (N = 21) Unclear if single or multiple doses administered, and if additional analgesia administered. | |
| Outcomes | Maternal outcomes: maternal pain intensity VAS (0 to 10 cm) 30, 60, 120 and 180 mins, vomiting, drowsiness, BP, HR, cardiotocogram | |
| Notes | Tramadol 100 mg only group (A) not included in our analyses. German language, translation obtained. Start and end date: unclear Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "zulfallszahlentafel" coincidence number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Stated as double‐blind but methods not described |
| Blinding of outcome assessment (detection bias) | Unclear risk | Stated as double‐blind but methods not described |
| Incomplete outcome data (attrition bias) | Unclear risk | 2/66 women excluded due to giving birth within 1 hr of study drug administration. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital in Iran 88 primiparous women in spontaneous labour, gestation ≥ 37 weeks, and cervix 5 cm dilated. Excluded if high‐risk pregnancy, narcotic addiction. | |
| Interventions | Experimental: IM (placebo) normal saline 1.5 mL (N = 44) Control: IM pethidine 75 mg (N = 44) | |
| Outcomes | Primary: analgesic effect. Pain assessed pre and post injection using Likert Scale VAS: 10 cm line, 0% = minimum effect, 100% = maximum effect. Secondary: side effects on uterine contractions (contraction duration and interval recorded 3 times 15 to 60 mins post injection) and neonatal Apgar score at 1 min and 5 mins. | |
| Notes | Timing of maternal pain assessment not reported. Start and end date: not reported Power calculation: the required number of women was based on a pilot study and considering a power of 90%, d 7%, and error 5%, 44 women were needed in each group. Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States 'divided randomly'. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | Study agents were of identical volume and appearance |
| Blinding of outcome assessment (detection bias) | Low risk | Study agents were of identical volume and appearance |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of participants analysed and planned analysis not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The number of women allocated to each group is not reported and unclear if there are baseline imbalances in prognostic factors. |
| Methods | RCT, individual randomisation – difficult to assess abstract only | |
| Participants | Setting: not clear, Iran 48 women with term pregnancies in active labour. Exclusion criteria: not stated | |
| Interventions | Experimental group: pethidine (n = not clear) route and dose not stated Control group: acupressure (n = not clear) acupressure at spleen point 6 (SP6) | |
| Outcomes | No data ‐ abstract only | |
| Notes | Dates of study: not stated Funding: not stated Conflicts of interest: not stated 15th June 2017 ‐ email to 2nd author. Awaiting response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Subjects were randomly selected and divided”. No further description. |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Not mentioned but not possible to blind intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcomes probably assessed by caregiver |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear due to lack of information in abstract |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess |
| Other bias | Unclear risk | Unable to assess |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Turkey 59 primiparous women with uncomplicated pregnancy at term gestation, in labour with cervix 3 cm to 5 cm dilated and reporting a pain score 4 ‐ 5 according to Wong‐Baker Faces Pain Rating Scales with 0 = no pain, 5 = most intense pain. Exclusions: maternal medical disorders, history of drug or alcohol abuse | |
| Interventions | Experimental: IM tramadol 100 mg, single dose (N = 30) Control: IM pethidine 100 mg, single dose (N = 29) | |
| Outcomes | Primary: analgesic effect assessed 30, 60 and 120 mins following injection using Wong‐Baker Faces Pain Rating Scales with 0 = no pain, 5 = most intense pain. Secondary: side effects: nausea, vomiting, drowsiness, fatigue and neonatal effects (Apgar score at 1 min and 5 mins). | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding sources: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. "randomly divided into two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessor unaware of treatment group |
| Incomplete outcome data (attrition bias) | High risk | Losses to follow‐up not explained and no ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Iran 160 women. Free of complications, spontaneous and induced onset, cx 4 cm dilated, in active labour and requiring analgesia. Women excluded if cx dilated > 5 cm. Parity: not reported | |
| Interventions | Experimental: IM tramadol 100 mg (N = 80) Control: IM pethidine 50 mg (N = 80) 2nd dose on maternal request after 4 hrs but pethidine withheld if cx dilated > 8 cm and tramadol given instead. | |
| Outcomes | Maternal outcomes: maternal pain intensity VAS (0 to 10 cm) 10 mins , 30 mins and 1‐hourly intervals until birth, maternal satisfaction 24 hrs postpartum 5‐point scale (excellent, very good, good, fair, poor), drowsiness, nausea, vomiting. Neonatal outcomes: Apgar score at 1 min and 5 mins, naloxone administration, respiratory depression | |
| Notes | Start and end date: 2004 Power calculation: based on the assumption that a difference of 30 mins in duration of labour would be clinically significant, 53 women was needed in each group 80% power on a 5% significance (α = 0.05, β = 0.2). Baseline imbalances between groups: 'The two groups were comparable regarding age, parity, height, weight, period of gestation, fetal weight, cervical dilatation at initiation of analgesia and need for oxytocin use'. Funding source: not specified Conflicts of interest: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Drugs administered by clinician blind to group allocation, but does not state how this was achieved. |
| Blinding of outcome assessment (detection bias) | Low risk | Women fed back their maternal pain score to anaesthetist. |
| Incomplete outcome data (attrition bias) | Low risk | Flow chart addresses all data. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Baseline characteristics similar |
| Methods | Reported to be randomised clinical trial. Individual women randomised. | |
| Participants | Setting: labour ward of Wesley Guild Hospital, Ilesa Nigeria. 100 women who were admitted in active spontaneous labour at term with uncomplicated singleton pregnancies requesting analgesia. Exclusion criteria: mothers with chronic medical diseases. | |
| Interventions | Experimental group: IM injection of Pentazocine 30 mg (Laborate Pharmaceuticals, India) (N = 50, 44 following exclusions) Control group: IM tramadol 100 mg.(P.T Interbat, Indonesia) (n = 50, 42 following exclusions) | |
| Outcomes | Satisfaction with analgesia Pain in labour Mode of birth Maternal side effects Neonatal admission to special care Apgar scores | |
| Notes | Start and end dates: June 2005 – May 2006 Funding and COI: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers in blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were placed in sequentially‐numbered, opaque, sealed envelope. Envelope was opened when the woman requested pain relief and the drug administered by the randomising midwife. |
| Blinding of participants and personnel (performance bias) | Low risk | It is reported that the trial is double‐blind. When each woman requested pain relief, the next numbered envelope was opened and the appropriate drug administered by the randomising midwife. Not clear if this midwife cared for woman in labour. Both given IM so women should be unaware. |
| Blinding of outcome assessment (detection bias) | Low risk | The labour ward resident doctor, unaware of the type of injection given, recorded the clinical data and assess the analgesic efficacy. |
| Incomplete outcome data (attrition bias) | Unclear risk | 14 women (6 in the pentazocine group and 8 in the tramadol group) delivered within 1 hr of drug administration and were therefore excluded from further analysis. Outcome data are available for the remaining women in the respective groups. Giving birth within the hr, the drug administered could have affected the neonate. |
| Selective reporting (reporting bias) | Unclear risk | All essential outcomes are reported. Protocol not seen. |
| Other bias | Unclear risk | Women had similar characteristics at trial entry. Some lack of clarity in reporting outcomes. |
| Methods | Reported to be randomised clinical trial. Individual women randomised. | |
| Participants | Setting: hospital in Iran 120 women randomised, nulliparous women with term singleton pregnancy who had induction of labour (reasons for and methods of induction not stated in abstract). Exclusion criteria: not stated | |
| Interventions | Experimental group: single dose of 50 mg IV pethidine at 4 cm dilatation (it was not clear whether this was at maternal request or whether all women received it) (N = 60) Control group: IV normal saline (placebo) (N = 60) | |
| Outcomes | Duration of labour | |
| Notes | Start and end dates: unclear Conflict of interest: not stated Funding not stated Translation requested 15th June 2017 ‐ no data used in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information “randomly assigned” |
| Allocation concealment (selection bias) | Unclear risk | No information “randomly assigned” |
| Blinding of participants and personnel (performance bias) | Low risk | Participants: placebo‐controlled trial. Caregiver: placebo‐controlled trial. Staff may have been aware of allocation if there was sedation or other side effects. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear when group assignment revealed and staff providing care recorded outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | 120 women included. No information on dropouts or missing data. Not clear. |
| Selective reporting (reporting bias) | Unclear risk | This was a very brief abstract. Key outcomes not reported. |
| Other bias | Unclear risk | Assessment from abstract. Full paper in Arabic. Very little information on methods. Full translation requested. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Argentina: 2 hospitals 310 women of mixed parity, in labour 37‐42 weeks' gestation with cervix 4 cm to 6 cm dilated, cephalic presentation and requiring analgesia. Exclusions: maternal medical condition, evidence of fetal distress, previous caesarean section | |
| Interventions | Experimental: IM nalbuphine 20 mg, single dose (N = 152) Control: IM pethidine 100 mg, single dose (N = 158) | |
| Outcomes | Primary: neonatal Apgar score < 7 at 1 min Secondary: maternal pain assessed using VAS pre‐injection, and 30 mins and 120 mins afterwards (severe pain 75 or >), nausea, vomiting and type of birth. Neonatal side effects: condition over first 24 hrs, admission to neonatal intensive care nursery. | |
| Notes | Stratified by hospital. Start and end date: June 1991 ‐ September 1993 Power calculation: based on previous literature, mean incidence of low Apgar score was 12% in women receiving meperidine and 3% in women receiving nalbuphine, α = 0.05, β = 0.20, 152 women in each group is needed for a 75% relative risk reduction in the primary endpoint. Baseline imbalances between groups: the groups were balanced across various prognostic variables such as age, nulliparity, weeks of gestation, maternal weight, cervical dilatation at randomisation, uterine activity, number of women with induced labour, severe pain, nausea, vomiting, dizziness, and dry mouth. Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Coded ampoules, sealed and prepared by independent pharmacist and identical in appearance |
| Blinding of participants and personnel (performance bias) | Low risk | Identical ampoules |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | Few losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned if women reported pain to their caregiver |
| Other bias | Unclear risk | Data analyst unaware of coding. Balanced at baseline. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | USA: hospital setting 93 primiparous women in labour, uncomplicated pregnancy at 37 or more weeks' gestation and in pain described as moderate or severe. | |
| Interventions | Experimental: IM pentazocine 60 mg (N = 38) Control: IM pethidine 100 mg (N = 45) | |
| Outcomes | Primary: pain relief assessed at 1 hr, as good or poor. Secondary: maternal side effects, nausea or vomiting, labour progress. Neonatal Apgar score at 1 min and 5 mins. | |
| Notes | If additional analgesia was required, a second injection could be administered at the discretion of medic. Not stated if IOL onset included. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding sources: Sterling drug company and NIH Grant RR 00404 Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Identical vials with code number but no further information given. |
| Blinding of participants and personnel (performance bias) | Low risk | Identical vials with code number |
| Blinding of outcome assessment (detection bias) | Low risk | No‐one involved with the immediate care of the woman knew the drug identity. |
| Incomplete outcome data (attrition bias) | High risk | 83/93 women analysed and reasons for missing data not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear how many women randomised to each group and balance at baseline unclear. |
| Methods | (Feasibility study) RCT, 2‐arm parallel‐group design | |
| Participants | 10 primiparous women in labour requesting pain relief, and who had no made any request for alternative analgesia. | |
| Interventions | Intervention group: meptazinol (PCA IM) up to 600 mg (75 mg per mL) Comparison group: pethidine (PCA IM) up to 400 mg (50 mg per mL) Doses described as equivalent. Nitrous oxide available to women in both groups. | |
| Outcomes | Pain, drowsiness and nausea on a 100 mm VAS (0 = no pain) during labour and also rated on the day after birth; Apgar score and neonatal weight gain over 3 days. | |
| Notes | Feasibility study focusing on PCA IM administration of opioids. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding sources: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, "randomly allocated". |
| Allocation concealment (selection bias) | Unclear risk | Described as a double‐blind comparison but methods not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as a double‐blind comparison but methods not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as a double‐blind comparison but methods not described. |
| Incomplete outcome data (attrition bias) | Low risk | 10 women randomised and all accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance apparent. |
| Methods | RCT. 2‐arm parallel groups. | |
| Participants | Setting: Beijing hospital, China 60 women in early labour (cervical dilatation 2 cm to 3 cm) at term, with singleton pregnancy, vertex presentation, with no pregnancy complications. | |
| Interventions | Intervention group: 100 mg IM tramadol Comparison group: no analgesia | |
| Outcomes | Analgesic effect (not clear when measured); satisfactory, some effect or no effect. | |
| Notes | Data extraction from translation notes. Start and end date: August 1993 – October 1993 Power calculation: unclear Baseline imbalances between groups: unclear Funding sources: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were divided "at random" into groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Women in the control arm received no treatment. |
| Blinding of outcome assessment (detection bias) | High risk | Women in the control arm received no treatment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Denominators not clear. No apparent loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | It was not clear whether or not women in the comparison group were given any analgesia or whether they requested any. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Brazil 56 women No information in abstract about participant inclusion criteria or characteristics. | |
| Interventions | Experimental: IM nalbuphine 10 mg Control: IM pethidine 100 mg | |
| Outcomes | Analgesia and side effects. Neonatal: Apgar score | |
| Notes | Abstract only: insufficient information about participants. Not reported if > 1 dose given or anti‐emetic. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: not reported Funding sources: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly selected" but not explained how. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Impossible to decipher. |
| Other bias | Unclear risk | Impossible to decipher. |
| Methods | Randomised controlled trial with individual randomisation. | |
| Participants | Setting: Beijing Obstetrics and Gynecology Hospital, China. 120 women randomised who had no previous poor obstetrical outcome; no experience in Han's Acupoint Nerve Stimulator and TENS for other reasons; term pregnancy (> 37 weeks of gestation); at active phase of the first stage of labour with cervical dilatation 3 cm. Exclusion criteria: had the history of experimental drug allergy; had been diagnosed with other diseases such as preoperative presence of maternal mental, neurological diseases, affecting evaluation of pains and disease conditions; had combined with gestational hypertension, gestational diabetes, gestational thyroid diseases; had taken analgesic drugs or with a history of long‐term use of analgesic drugs; had used diazepam, piperazine hydrochloride or other sedative, analgesic drugs in the stages of labour; were overweight or low pregnancy weight, BMI < 18.5 or BMI > 25 kg/m2; were not agreeable to receive painless labour and not sign the informed consent form. | |
| Interventions | Experimental group 1: HANS (Han’s acupoint nerve stimulator) group (N = 30) received DC pulse stimulus at acupoints of Jiaji points (T 10‐L 3) and Ciliao (BL 32)The stimulus was 100 Hz with a burst frequency of 2 Hz (dense dispersed waveform) The intensity was 15 mA to 30 mA. The pulse duration was used for 30 mins. Experimental group 2: PCIA (Patient‐controlled intravenous analgesia) group (N = 30) IV infused ondansetron 8 mg; 5 mins later, 1.5 mg/kg tramadol injection was slowly dripped, connected to Baxter AP Ⅱ electronic pump with 50 mL of 0.70% tramadol + ondansetron 8 mg, background infusion 2 mL/hr, PCA dose of 2 mL, lockout interval of 10 mins. Experimental group 3: PCEA (patient‐controlled epidural analgesia) group (N = 30) L2‐3 combined spinal‐ epidural puncture, intrathecal injection of 3 mg ropivacaine, epidural catheter connected to Baxter AP electronic pump, with 100 mL 0.1% ropivacaine and 50 ug sufentanil, background infusion 5 mL, PCA dose of 5 mL, lockout interval of 10 mins when the cervix was fully dilated (10 cm). N =30. All treatments were stopped at the point of complete cervical dilatation. Control group (N = 30) did not receive analgesia. Only experimental groups 1 and 2 are included in this review as per methods. | |
| Outcomes | Outcomes Maternal side effects Oxytocin use Neonatal asphyxia Pain scores Duration of labour Apgar (mean, SD) | |
| Notes | Trial dates: August 2010 – November 2013 Funding: The Scientific Achievement and Appropriate Technology Extension Project of Beijing Municipal Commission of Health and Family Planning (TG‐2014‐12). Conflict of interest: not reported 120 women were randomised, so the number of women in each group should be 30, this is what reported in tables 1‐4, but different in trial profile. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table method |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | It is not feasible to implement blinding. |
| Blinding of outcome assessment (detection bias) | High risk | It is not feasible to implement blinding. |
| Incomplete outcome data (attrition bias) | Unclear risk | No reports of loss to follow‐up or women requesting other analgesia and changing groups. Not clear if women in the control group requested analgesia at all. Denominators given in the tables are lower than in flowchart. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods are reported. Protocol not seen. |
| Other bias | Unclear risk | There was no statistical difference in the basic information between 4 groups (P> 0.05). Generally reporting is unclear. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, USA 80 women at term gestation, in spontaneous and induced labour with moderate to severe pain. Exclusions: drug abuse history, systemic disease and women who planned to breastfeed their babies. | |
| Interventions | Experimental: IM butorphanol 1 or 2 mg (N = 40) Control: IM pethidine 40 or 80 mg (N = 40) | |
| Outcomes | Primary: pain intensity assessed 30 and 60 mins post injection. Described as 1 = slight relief, 2 = moderate relief, 3 = good relief, 4 = complete relief. Maternal satisfaction of overall drug effect assessed postnatally as 1 = poor, 2 = fair, 3 = very good, 4 = excellent Secondary: neonatal Apgar score at 1 and 5 mins, resuscitation. Maternal nausea and vomiting. | |
| Notes | If additional analgesia was required, a second dose of original drug could be administered. Maternal parity not reported but different drug dosage depending on parity. Almost all (77/80) participants were non‐Caucasion and all were delivered with local or regional anaesthesia. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: Quote: "There was little difference among test groups with respect to type of labour, age, sex, type of delivery, and anaesthetic agent administered. Butorphanol 1 mg group slightly lower mean body weight.'' Funding source: Bristol laboratories, Syracuse, New York Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Drugs in consecutively‐numbered, identical vials prepared by independent laboratory. |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind, drugs in identical vials. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | All women analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Balanced at baseline for type of labour, weight, age, type of birth and anaesthetic agent. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: Germany 40 women. Term pregnancy, cx dilated 2 cm to 3 cm, spontaneous labour onset, in active labour and requiring analgesia. Parity: not reported | |
| Interventions | Experimental: IM nalbuphine 0.1 mg/kg (N = 20) Control: IM pethidine 0.8 mg/kg (N = 20) States dosing was 'on demand'. Unclear if single or multiple doses administered, and if additional analgesia administered. | |
| Outcomes | Maternal outcomes: maternal pain relief VAS (0 cm to 20 cm) 30, 60, 90 and 120 mins, opinion of pain relief 12 hrs postpartum, sedation 4‐point scale (awake, tired, sleeping but will wake if spoken to, sleeping but will wake if shaken, asleep not possible to wake up) 30, 60, 90 and 120 mins, 'side effects', blood pressure, heart rate, CTG. Neonatal outcomes: Apgar score at 10 mins, respiratory depression. | |
| Notes | German language ‐ translation obtained Start and end date: unclear Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 4/40 women excluded due to insufficient pain relief. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | Randomised clinical trial, individually randomised | |
| Participants | Setting: hospital in Iran. 100 women randomised in spontaneous labour pain along with appropriate maternal and fetal indications for vaginal delivery. Exclusion criteria: presence of a personality disorder, an addiction, a complicated pregnancy, diabetes mellitus, macrosomia, chronic obstructive pulmonary disease, an unconfident fetal heart rate, valvular heart disease, an upper respiratory tract infection or sinus obstruction, a history of asthma, and contraindications for Entonox and pethidine usage. | |
| Interventions | Experimental group: pethidine (50 women) The pethidine group received an intramuscular injection of 0.5 mg/kg of pethidine. If a patient’s pain rated higher than 5 VAS, 0.25 mg/kg of pethidine was injected. Not clear if pethidine was limited to 2 doses. Control group: Entonox (50 women) Patients were taught to use an Entonox face mask at the beginning of uterine contractions and to continue deep inspirations at times when there was pain and cramps. Use of Entonox could be started or cut at any moment during labour according to the needs and preferences of the woman. | |
| Outcomes | Pain scores after analgesia. Duration of first and second stage of labour. | |
| Notes | Dates: 2015 Funding: Ardabil Medical Sciences University Conflict of interest: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By using random numbers, the participants were randomly allocated into 2 groups. Equal groups and no further detail. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Infeasible to blind. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned, but likely to be caregiver carrying out assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | No loss to follow‐up reported, and no denominators given in results tables. Demographic data do not add up to total number of participants. Difficult to assess due to reporting. Also not clear if anyone changed intervention. |
| Selective reporting (reporting bias) | Unclear risk | No protocol seen and few outcomes pre‐specified in the methods. |
| Other bias | Unclear risk | Parity is not reported in each group. There were 16/50 under 20 year olds in the Entonox group and 9/50 in the pethidine group. These could be more likely to be nulliparous. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 206 mixed parity healthy women, in spontaneous or induced labour, at > 35 weeks' gestation, cephalic presentation and in pain described as severe, moderate or slight. | |
| Interventions | Experimental: IM pentazocine 40 mg (N = 73) Control: IM pethidine 100 mg or 50 mg (N = 133) | |
| Outcomes | Primary: pain intensity assessed at 30, 60 and 90 mins post injection, described as severe, moderate or slight. Asked at 12 to 24 hr postnatal if drug had helped. Secondary: neonatal Apgar score at 1 min and 5 mins, maternal side effects of nausea or vomiting. | |
| Notes | If additional analgesia required, a maximum of 3 further doses of study drug could be administered at 2‐ to 3‐hourly intervals. Women could also use nitrous oxide and some had a paracervical block. > 35 weeks' gestation therefore preterm babies may be included. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: the age, physical and obstetric characteristics were similar between groups except in fetal presentation. Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Coded ampoules but no further information given. |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind. Coded ampoules but not stated if identical in appearance. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind. Coded ampoules but not stated if identical in appearance. |
| Incomplete outcome data (attrition bias) | High risk | 29/206 excluded because delivered or had paracervical block. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: labour ward of a university health centre in Canada. 23 women randomised when they requested analgesia, 83% primips, gestational age > 32 weeks, weight < 100 kg or > 50 kg, able to speak English, no history of opioid abuse and normal FHR tracing. (Women recruited to the study had medical contraindications to epidural although it was no specified what these were.) | |
| Interventions | Experimental: fentanyl, PCA 10 mcg per mL, initial bolus dose 1 mL, basal infusion rate of 2 mL per hr with PCA bolus 2 mL. Control: alfentanil, PCA 100 micro g per mL, initial bolus dose 1 mL, basal infusion rate of 2 mL per hr with PCA bolus 2 mL. Doses described as equivalent. Drugs were discontinued in both groups when the attending midwife estimated that birth was likely to take place within 15 mins. | |
| Outcomes | Pain (rated on a 100 mm VAS, recorded at baseline and every 30 mins thereafter); sedation (nurse rated hourly); side effects; satisfaction with pain relief (good, adequate, inadequate); Apgar scores at 5 and 10 mins; cord blood gases; naloxone dose; neonatal neuro‐behavioural score at 4 and 24 hrs. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: quote: "The two groups were similar in age, weight, gestational age, parity, inductions, type of delivery, baseline pain scores, rate of cervical dilatation, duration of PCA use. The only difference was that the opioid dose‐ to delivery time was shorter in the alfentanil group." Funding sources: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule prepared by pharmacy. |
| Allocation concealment (selection bias) | Low risk | Plain, numbered vials prepared by pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Plain vials prepared by pharmacy. |
| Blinding of outcome assessment (detection bias) | Low risk | Stated that assessment was carried out by staff blind to group assignment. |
| Incomplete outcome data (attrition bias) | Unclear risk | 25 women were randomised. 2 did not follow the protocol and were not followed up. There were missing data for some variables. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Small sample size and the onset of analgesia varied. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 1100 women. 37‐42 weeks' gestation, in active labour and requiring analgesia. Parity: 44% primips, 56% multips. | |
| Interventions | Experimental: IM meptazinol 100 mg ≤ 70 kg, 150 mg > 70 kg (N = 513) Control: IM pethidine 1100 mg ≤ 70 kg, 150 mg > 70 kg (N = 522) Second dose, epidural or inhalation analgesia at maternal request. | |
| Outcomes | Maternal outcomes: maternal pain at 30, 60, 90 and 120 mins VAS (0 to 100 mm), nausea, vomiting, sleepiness, use of supplementary analgesia, method of birth, opinion of analgesic effect assessed 3‐5 days postpartum (rated excellent, good, poor but just able to cope, no effect and required additional analgesia). Neonatal outcomes: Apgar at 1 min and 5 mins, resuscitation, naloxone administration, fetal distress, type of feeding. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: 'The groups were comparable with regard to age, maternal weight, parity and gestation' Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Coded drug containers prepared at a site remote from the trial. |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind and used coded drug containers. |
| Blinding of outcome assessment (detection bias) | Low risk | States double‐blind and used coded drug containers. |
| Incomplete outcome data (attrition bias) | High risk | 65 women excluded due to clerical errors or administration of wrong drug. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Women were balanced at baseline for age, weight, parity and gestation. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 94 women. > 35 weeks' gestation, age ≥ 18 years, excluded if diabetic, history of renal or hepatic impairment or taking monoamine oxidase inhibitors, in active labour and requiring analgesia. Parity: ≤ 3 | |
| Interventions | Experimental: IM pentazocine 60 ≤ mg (N = 46) Control: IM pethidine 15 ≤ 0 mg (N = 48) Up to 3 injections > 3 hrs apart at maternal request. | |
| Outcomes | Maternal outcomes: satisfied with analgesia, nausea, vomiting, sleepiness, use of additional analgesia (study drug), method of birth. Neonatal outcomes: Apgar at 1 min and 5 mins. | |
| Notes | Data for some outcomes available after first dose. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: the groups were similar with respect to age, and number of previous pregnancies. Funding source: Sterling Winthrop Research Division supplied the drugs. Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but how achieved not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but how achieved not reported. |
| Incomplete outcome data (attrition bias) | High risk | Exclusions from most analyses. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | Balanced at baseline for age, parity, induced labour onset. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, South Africa 75 women. Healthy with no clinically detectable abnormality, in active labour, spontaneous and induced, and requiring analgesia. Excluded if history of hypersensitivity to any drug, previous caesarean, preterm labour, cardiac, pulmonary or renal disease and significant hypertension. Parity: mixed | |
| Interventions | Experimental: IM meptazinol 100 mg (N = 37) Control: IM pethidine 100 mg (N = 38) No concomitant analgesia given, metoclopramide 10 mg as required for nausea. | |
| Outcomes | Maternal outcomes: pain at 1 hr 5‐point VAS scale, drug‐related side effects. Neonatal outcomes: Apgar at 1 min and 5 mins, paediatrician assessment at 24 hrs | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but does not describe how blinding achieved. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but does not describe how blinding achieved. |
| Incomplete outcome data (attrition bias) | High risk | Number of women randomised not reported only number analysed, not same numbers analysed for all outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Women requiring caesarean or epidural were excluded from further study, unclear if this is pre‐ or post‐randomisation. |
| Methods | RCT, 3‐arm parallel‐group design | |
| Participants | Setting: hospital in North Carolina USA. 45 healthy women with singleton pregnancies requesting analgesia. Women with allergies to the study medication, those that had already had medication and those taking opioids for chronic conditions were excluded, along with those with any signs of fetal distress. | |
| Interventions | Experimental: (15 women) IV butorphanol, 1 mg bolus Control: (15 women) IV pethidine, 50 mg bolus (A second control group received IV pethidine 25 mg plus 0.5 mg butorphanol; this group has not been included in the analyses in this review.) | |
| Outcomes | Pain (measured on a 0 ‐10 numerical rating scale); sedation and nausea, Apgar scores at 1 min and 5 mins. | |
| Notes | Results for pain outcomes were reported on bar charts and are difficult to interpret. We have not included these results in the analyses in this review. Start and end date: not reported Power calculation: 'the study was powered based on the increased variability in pain'. Baseline imbalances between groups: 'Groups did not differ in demographic or labor characteristics'. Funding source: National Institutes of Health, Bethesda, Maryland (grant No. NS41386) Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated balanced block design". Block size not stated. |
| Allocation concealment (selection bias) | Unclear risk | Study described as double‐blind but not details on allocation concealment provided. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "drug was prepared by an anaesthesiologist not involved with the treatment of the patient or obtaining study measures". Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | High risk | It was not clear how many women were randomised. Any women undergoing ARM, commencing oxytocin or requesting epidural were excluded after randomisation and were replaced Quote: "their randomization was re‐entered for another patient". Women who reached 10 cm dilation within 1 hr of drug administration were also excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | Randomised trial (methods unclear) | |
| Participants | 30 women Inclusion criteria: Quote: "co‐operative patients" with no drug dependency. Various ages and social groups. Exclusion criteria: unclear | |
| Interventions | 5 study groups:
| |
| Outcomes | Pain intensity (grades 1 ‐ 6 ‐ no pain, light, bearable, heavy, very heavy, unbearable) over 70‐min period. Satisfaction with analgesia 1 day after the birth "Reaction of the subjects the day after the birth to analgesia ‐ rated as "good", "inadequate analgesia" or "none" ‐ table 2. Progress in labour. | |
| Notes | Paper in German. Translation notes used for data extraction. We were unable to use the data from this paper in the review. We had intended comparing outcomes for women receiving IV pethidine versus no treatment. The only outcome reported in the paper was the amount of relief obtained from the analgesia and no outcomes were reported for the control group (no treatment). 5 women received pethidine and 5 women no treatment. It was reported that 2/5 women receiving pethidine had "good relief", 3 had insufficient or no relief. All women in the control group were reported as having an increase in pain. Results ‐ categories for pain relief (good, insufficient, none) do not correspond with pain scale ‐ 6 perceptions reported in the translation. Start and end date: not reported Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described ‐ "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | 1 group received no treatment. TENS groups ‐ 1 without current and 1 where it was applied to wrong positions were blinded to the TENS intervention. Pethidine group presumably were not blinded. blinding of personnel is unclear. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not clear |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 woman was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Small study and results were difficult to interpret. |
| Other bias | Unclear risk | Translation, so difficult to evaluate other bias. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 450 women. Healthy women with no obstetric complications, full‐term pregnancy, in active labour and requiring analgesia. Excluded if history of hypersensitivity to any drug, previous caesarean, preterm labour, cardiac, pulmonary or renal disease and significant hypertension. Parity: not reported | |
| Interventions | Experimental: IM meptazinol (N = 186 analysed). Control: IM pethidine (N = 172 analysed). Both given according to body weight. 75 mg if 38 kg to 50 kg, 100 mg if 51 kg to 69 kg or 150 mg if 70‐85 kg. Each patient received up to 2 injections of study drug, and if analgesia still inadequate epidural given. | |
| Outcomes | Maternal outcomes: maternal assessment of pain relief at 15, 30, 45, 60, 90 and 120 mins (rated none, poor, satisfactory, good, very good or complete), type of birth, epidural, sleepiness, nausea and vomiting. Neonatal outcomes: Apgar at 1 min and 5 mins, apnoea, resuscitation, and lethargy, muscle tone, irritability success of feeding within first 24‐hr period. | |
| Notes | Does not report number randomised to each group. Start and end date:not reported Power calculation: not reported Baseline imbalances between groups: not reported Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but does not describe methods used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but does not describe methods used. |
| Incomplete outcome data (attrition bias) | High risk | 79.5% follow‐up but no ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 100 women. Age > 18 years, > 35 weeks' gestation, uncomplicated singleton, vaginal birth expected, in active labour and requiring analgesia. Parity: 9% primips, 76% multips, 15% grand multips. | |
| Interventions | Experimental: IM pentazocine 30 mg (N = 48 analysed) Control: IM Pethilorfan ®100 mg (N = 50 analysed) Second injection possible after 2 hr, each patient could receive up to 4 injections of study drug, and nitrous oxide or trilene to supplement analgesia if required. | |
| Outcomes | Maternal outcomes: maternal assessment of pain relief (numbers obtaining or not obtaining pain relief), type of birth, additional analgesia required (study drug). Neonatal outcomes: Apgar at 1 min and 5 mins, naloxone administration. | |
| Notes | Does not state actual number randomised to each group. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: the group was balanced in most of the variables such as age, number of previous pregnancies, and cervical dilatation Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind but does not describe methods used. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind but does not describe methods used. |
| Incomplete outcome data (attrition bias) | High risk | 31/98 excluded from primary outcome as delivered within 1 hr of administration of study drug, and 16 babies excluded from Apgar assessment as study drug administered more than 4 hrs before birth. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Balanced at baseline for age, parity, contractions and vital signs. |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Stockholm, Sweden 20 healthy nulliparous women in active labour after spontaneous rupture of the membranes, cephalic presentation. No exclusion criteria specified. | |
| Interventions | Experimental: 0.05 mg/kg IV morphine up to 3 doses (max 0.15 mg/kg body weight) Control: 0.5 mg/kg IV pethidine up to 3 doses (max 1.5 mg/kg body weight) Both groups had continuous FHR monitoring. | |
| Outcomes | Sedation rates; CS, nausea and vomiting. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: the demographic variables were balanced between the groups Funding source: Karolinska Institute foundations and the Swedish Medical Research Council Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "assigned at random." |
| Allocation concealment (selection bias) | Low risk | Coded ampoules provided by pharmacy. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind; pharmacy provided identical coded ampoules. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | No apparent loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Small sample and no clear information that groups were comparable at baseline. Range of cervical dilations at recruitment between 4 cm and 9 cm. |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: Washington, USA 194 women in established labour. Analgesia was given at approximately 4 cm to 5 cm cervical dilatation. | |
| Interventions | Experimental: IV phenazocine 1 mg Control: IV pethidine 50 mg Both groups received promethazine 50 mg, and for both groups "birth was accomplished under pudendal nerve block anaesthesia with terminal self‐administered trichloroethylene". | |
| Outcomes | Pain relief (recorded by women on the first postpartum day); nausea and vomiting; adverse effects; progress in labour; Apgar scores at 1 min and 5 mins. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Drugs were prepared by pharmacy in identical coded vials and the code was not broken by the pharmacist until the study had been completed. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Drugs in identical vials.Pharmacy prepared identical coded drugs. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Low risk | Missing data for some outcomes (approximately 5% for maternal postpartum outcomes, and 10% for nurse recorded evaluations in labour). |
| Selective reporting (reporting bias) | Unclear risk | None apparent, protocol not seen. |
| Other bias | Low risk | None apparent |
| Methods | RCT 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Denmark 199 women. Spontaneous or induced labour onset, in active labour and requiring analgesia. Parity: 78% nullips, 22% multips | |
| Interventions | Experimental: IM meptazinol 100 mg (N = 100). Control: IM pethidine 750 mg (N = 99). Each patient could receive up to 3 injections of study drug with an interval of not less than 2 hrs between doses. | |
| Outcomes | Maternal outcomes: maternal assessment of pain relief 5, 15, 30, 60, 90, 120 mins (rated complete, good, satisfactory, unsatisfactory), type of birth, additional analgesia required, epidural, adverse effects. Neonatal outcomes: Apgar at 1 min and 5 mins, neonatal distress, admission to SCBU. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: 'There were no differences between the two groups in age, body weight or height, or number of previous deliveries. Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind but no methods described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double blind but no methods described. |
| Incomplete outcome data (attrition bias) | Low risk | All women analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance in age, weight, height or number of previous deliveries. |
| Methods | RCT 3‐arm parallel‐group design | |
| Participants | Setting: hospital, Thailand 135 women. 37 to 42 weeks' gestation, cx ≥ 3 cm, in active labour and requiring analgesia. Parity: not reported | |
| Interventions | Experimental: IM tramadol 100 mg (N = 45); IM morphine 100 mg (N = 45). Control: IM pethidine 100 mg (N = 45). Second injection possible after 1 hr of half original study dose, each participant could receive maximum of 2 doses. | |
| Outcomes | Maternal outcomes: pain severity/relief 30 mins, 1, 2, 3, and 4 hrs (rated good, satisfactory, no response), drowsiness, nausea, vomiting. Neonatal outcomes: Apgar at 1 min and 5 mins, neonatal resuscitation. | |
| Notes | Start and end date: 1 February 1986 ‐ 28 February 1986 Power calculation: not reported Baseline imbalances between groups: unclear Funding sources: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States blind but does not describe the method. |
| Blinding of outcome assessment (detection bias) | Low risk | Medical students unaware of group allocation assessed outcome. |
| Incomplete outcome data (attrition bias) | Low risk | All participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | Age and maternal weight balanced at baseline. |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting not clear (hospital in USA) 100 women in good health in active labour, with no addiction to or tolerance to drugs and complaining of moderate to severe pain. Women who "planned to nurse" were excluded. | |
| Interventions | Experimental: (50 women) IV butorphanol 1 mg to 2 mg (44 women had an initial dose of 1 mg and 6 an initial dose of 2 mg, after 1 hr or more a 2nd dose was given if requested). Control: (50 women) IV pethidine 40 mg to 80 mg (45 women had an initial dose of 40 mg and 5 an initial dose of 80 mg, a 2nd dose was given after 1 hr or more if requested). | |
| Outcomes | Pain (5‐point scale 0 ‐ no pain, 4 ‐ very severe pain); pain relief (5‐point scale 0 ‐ none, 4 ‐ complete relief); FHR; Apgar scores at 1 min and 5 mins. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding sources: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind study but no details provided. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Outcome data were available for all women randomised. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance was apparent although 8 women in the butorphanol group were induced compared with 1 woman in the pethidine group. |
| Methods | RCT. 2‐arm parallel groups | |
| Participants | Setting: Nebraska university hospital, USA 105 women in early active labour (3 cm to 4 cm cervical dilation); at or beyond 37 weeks' gestation with no medical or obstetric complications, with no signs of fetal distress and requesting narcotic analgesia rather than an epidural. (Intervention group: 55% nulliparous, 71% non‐white race, mean age 23 years; control group: 48% nulliparous, 70% non‐white race, mean age 23 years.) | |
| Interventions | Experimental: (49 women) IV fentanyl 50 µg to 100 µg per hr Control: (56 women) IV pethidine 25 mg to 50 mg per hr | |
| Outcomes | Pain (measured on 10‐point VAS recorded by labour ward nurses); nausea and vomiting; sedation; itching; FHR changes. | |
| Notes | Women were recruited only between 8 am and 3 pm on weekdays. Start and end date: January 1988 ‐ August 1988 Power calculation: not reported Baseline imbalances between groups: 'There were no statistically significant differences in maternal demographic characteristics and need for oxytocin augmentation.' Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacy randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | High risk | Staff not blinded to group allocation. |
| Blinding of outcome assessment (detection bias) | High risk | Staff not blinded to group allocation. |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised seem to be included in the results. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Women were recruited only on weekdays between 8am and 3pm so may not represent the population attending the study hospital. |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Norway 85 women. Healthy women at term, expected to have a normal birth in active labour and requiring analgesia. Parity: not reported | |
| Interventions | Experimental: IM pentazocine 45 mg (N = 43) Control: IM pethidine 100 mg (N = 42) Half dose repeated after 1 hr if required and further full dose after 3 hrs if labour prolonged. All women received promazine 25 mg IM before 1st injection, nitrous oxide or pudendal block or both allowed at end of 2nd stage. | |
| Outcomes | Maternal outcomes: pain relief at 1 hr (0 = no pain, 1 = slight pain, 2 = moderate pain, 3 = severe pain), type of birth, additional analgesia required. Neonatal outcomes: Apgar at 1 min and 5 mins, naloxone administration, FHR changes. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: Sterling‐Winthrop company supplied trial drugs Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | High risk | No blinding |
| Blinding of outcome assessment (detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | High risk | 25/85 women excluded from analysis as delivered within 1 hr of 1st dose of study drug. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Nitrous oxide or pudendal block permitted during second stage. |
| Methods | Reported to be a randomised clinical trial with individual randomisation. | |
| Participants | Setting: hospital in Iran 150 women Healthy women, with singleton cephalic presentation pregnancy in spontaneous labour, 3 cm or more cervical dilatation requesting analgesia. Exclusion criteria: pethidine allergy, contraindication to vaginal delivery, fetal death or distress, fetal congenital heart malformation or obstetric complications such as antepartum haemorrhage. | |
| Interventions | Experimental group: pethidine IM 50 mg, with 25 mg after 4 hrs if women requested. (N = 75) Control group: placebo, IM saline. (N = 75) Women in both groups received routine care which included FHR surveillance and 2‐hourly vaginal examinations, with a protocol for oxytocin augmentation for delay. | |
| Outcomes | Apgar scores Fetal heart rate changes Oxytocin administration | |
| Notes | Start and end date: October 2004 to September 2005 It was reported that the study was not supported by any pharmaceutical company. Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was reported that women were allocated “randomly (using a randomized consecutive numbered chart)”. It was not clear whether the chart had random numbers or that numbers were ordered consecutively. |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | Low risk | Participants: study was placebo controlled. Caregiver: study was placebo controlled – staff may have been aware of allocation as some women received no analgesia. |
| Blinding of outcome assessment (detection bias) | Low risk | Reported to be blind to allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | 150 women were randomised, 75 in each group. There was no mention of dropouts or any missing data. |
| Selective reporting (reporting bias) | High risk | There was little information. The outcome reported were FHR only. Mode of birth was not reported, some outcomes were reported to be “no different” but raw data were not reported. No protocol available. No power calculation. |
| Other bias | Unclear risk | There was little information on methods so it was not possible to assess whether other risk of bias was present. |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 205 women. Healthy women 38 to 41 weeks' gestation, uncomplicated pregnancy, spontaneous or induced labour onset, in active labour and requiring analgesia. Excluded if epidural or forceps birth likely. Parity: mixed | |
| Interventions | Experimental: IM meptazinol 100 mg (N = 98) Control: IM pethidine 100 mg (N = 99) Additional doses of test drug allowed at intervals no less than 2 hrs if required to a maximum of 3 doses. All women could receive nitrous oxide if required and prochlorperazine 12.5 mg IM for nausea and vomiting. Epidural at midwife discretion. | |
| Outcomes | Maternal outcomes: pain intensity 30 mins and then hourly intervals until birth (rated none, mild, moderate, severe), pain relief (rated none, slight, moderate, strong or complete), type of birth, additional analgesia required, nausea and vomiting. Neonatal outcomes: Apgar at 1 min and 5 mins, resuscitation. Within 72 hrs postpartum feeding problems, irritability and muscle tone. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: both the groups were balanced for age, body weight, and parity. Funding sources: Wyeth laboratories supplied the coded ampoules of the trial drugs Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Coded ampoules kept at a site remote from trial. |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind, used coded ampoules and states that identity of drug unknown. |
| Blinding of outcome assessment (detection bias) | Low risk | Blind outcome assessor for all bar 15% of women. |
| Incomplete outcome data (attrition bias) | Low risk | 8 women excluded from analysis as delivered within 30 mins of administration. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Balanced at baseline for age and weight, but imbalance in parity. 43/98 multip meptazinol group versus 34/99 in pethidine group. |
| Methods | RCT, 3‐arm parallel‐group design | |
| Participants | Setting: hospital, South Africa 196 women. Healthy women at term, uncomplicated labour, in active labour expected to deliver in next 4 hrs and requiring analgesia. Excluded if likely to deliver within 30 mins and had received analgesia within previous 6 hrs. Parity: mixed | |
| Interventions | Experimental: IM dihydrocodeine 50 mg (N = 80) Control: IM pethidine 100 mg (N = 58), placebo (saline) (N = 58) Single dose of study drug. | |
| Outcomes | Maternal outcomes: pain relief at 1 hr (rated good, fair, poor), sedation (rated drowsy, alert but calm, restless), nausea, vomiting. Neonatal outcomes: Modified Apgar at 1 min and 5 mins (minus colour). | |
| Notes | Women excluded after randomisation if delivered more than 4 hrs after injection of study drug. Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: all the groups were balanced for age, race, and parity. Funding sources: BDH (South Africa) Pty Ltd supplied dihydrocodeine bitartrate Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | States double‐blind. Not reported how blinding was achieved. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind. Not reported how blinding was achieved. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of women randomised not reported, authors only report the number of women analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unequal number of women in each treatment group due to post‐randomisation exclusions. Exclusions included women who delivered < 30 mins or > 4 hrs after administration of study agents. |
| Methods | RCT: methods not clear | |
| Participants | Setting: Egypt 90 primiparous women with normal presentation and position and expected to deliver normally. | |
| Interventions | Intervention: pethidine 50 mg IM 4‐ to 5‐hourly Comparison: TENS applied to back. The position arranged to suit the mother and moved to lower abdomen if preferred. Both groups were given 10 mg diazepam IM. Both groups had artificial rupture of membranes at 5 cm and oxytocin augmentation. | |
| Outcomes | Pain intensity (scored as being: severe = 3; moderate = 2; mild = 1) ‐ only measured before intervention; pain relief scored (complete = 4, excellent = 3, good = 2, slight (satisfactory) = 1) at 30 mins, 5 cm and at full cervical dilatation; patient's opinion on the technique ‐ satisfaction (during whole period of delivery), scored as (excellent = 3, good = 2, satisfactory = 1); Apgar score; side effects (drowsiness, nausea, vomiting). | |
| Notes | Start and end date: Funding sources: Conflicts of interest: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided between 2 groups." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) | High risk | Not reported ‐ but not feasible with nature of interventions. |
| Blinding of outcome assessment (detection bias) | High risk | Not reported ‐ but not feasible with nature of interventions. |
| Incomplete outcome data (attrition bias) | Low risk | No apparent loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described within the methods are reported upon within the results. However, the study protocol was not evaluated. |
| Other bias | Unclear risk | Unbalanced groups; 35 in the intervention group and 55 in the comparison group. |
| Methods | RCT | |
| Participants | Setting: Indore, India 300 women in established labour attending for care in a hospital in India. The participants were described as being predominantly from low socio‐economic groups and from urban areas. Inclusion criteria: term pregnancy (37 to 42 weeks), vertex presentation, cervical dilatation 3 cm or more with contractions. Exclusion criteria: previous uterine scar, malpresentation, multiple pregnancy, cephalo‐pelvic disproportion, antepartum haemorrhage, pre‐eclampsia or other medical disorders. | |
| Interventions | Interventions group: TENS to back Comparison group 1: 100 mg IM tramadol Comparison group 2: no intervention | |
| Outcomes | Maternal pain score measured on a verbal response scale during labour "degree of analgesia" (degree of pain relief: no relief, mild relief, moderate relief, complete relief ‐ dichotomised as a percentage); mean time for onset and duration of analgesia; duration of stages of labour; mode of delivery (normal, forceps, CS); mean Apgar score of neonates; side effects for mothers. | |
| Notes | Start and end date: not reported in translation. Funding sources: not reported in translation. Conflicts of interest: not reported in translation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly allocated" but groups were of identical size with identical numbers of primiparous and multiparous women in each group. |
| Allocation concealment (selection bias) | Unclear risk | Method of randomisation not described. |
| Blinding of participants and personnel (performance bias) | High risk | No blinding reported ‐ but not possible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) | High risk | No blinding reported ‐ but not possible due to nature of intervention. |
| Incomplete outcome data (attrition bias) | Low risk | Apparently there was no loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described within the methods are reported upon within the results. However, the study protocol was not evaluated. |
| Other bias | Unclear risk | Groups were unusually similar and it was not clear that there had been stratification to achieve such balanced groups. |
| Methods | RCT. 2‐arm parallel groups | |
| Participants | 200 nulliparous women in labour. Inclusion criteria: at term (37 to 42 weeks) spontaneous labour, in active labour, vertex presentation. Exclusions: age < 16 or > 35, weight < 50 kg or > 75 kg, infant birthweight estimated < 2500 g or > 4000 g, medical or surgical complication or unable to understand VAS. | |
| Interventions | Intervention group: IM buprenorphine 300 µg Comparison group: IM pethidine 75 mg | |
| Outcomes | Analgesic effect at 1, 2, 3, 4 hrs, side effects (nausea, drowsiness, use of antidote) | |
| Notes | Data extraction from translation notes. Start and end date: January 1996 ‐ December 1996 Power calculation: unclear Baseline imbalances between groups: unclear Funding source: unclear Conflicts of interest: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Unclear risk | Treatment described as blinded. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Denominators in tables not clear. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital, Hong Kong 50 women. Healthy women in early active labour and requiring analgesia. Uncomplicated singleton term pregnancy, cephalic presentation. Spontaneous and induced labour onset. Excluded if epidural already requested. Parity: 3:2 nullip:multip ratio | |
| Interventions | Experimental: IM pethidine 100 mg (N = 25) Control: placebo (saline) (N = 25) Single dose of study drug. Rescue analgesia allowed after 30 mins nitrous oxide or epidural for women in pethidine group and pethidine for women in placebo group. | |
| Outcomes | Maternal outcomes: pain intensity at 15 mins and 30 mins VAS (0 to 100), maternal assessment of sedation at 15 mins and 30 mins VAS (0 to 100), type of birth, additional analgesia required, vomiting, maternal satisfaction at 30 mins 5‐point scale (1 = totally dissatisfied to 5 = very satisfied). Neonatal outcomes: Apgar at 1 min and 5 mins, resuscitation and admission to SCBU. | |
| Notes | Study terminated after 50 women recruited as interim analysis demonstrated benefit for pethidine. Stratified by parity. Start and end date: September 2000 to May 2001. Power calculation: Using published and unpublished data, a sample size of 56 women per group was needed to have 90% power at 5% significant level to detect a mean difference of 13 mm in VAS pain score between groups. Baseline imbalances between groups: Table 1 provides this information, but it is unclear if the groups were balanced or not. Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind and women blind to contents of syringe. |
| Blinding of outcome assessment (detection bias) | Unclear risk | States double‐blind and women blind to contents of syringe. No further detail given. |
| Incomplete outcome data (attrition bias) | Low risk | All women accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | High risk | 20/25 women in pethidine group versus 12/25 women in placebo group had labour induced which may affect maternal and neonatal outcomes. |
| Methods | RCT, 3‐arm parallel‐group design | |
| Participants | Setting: hospital, Singapore 90 women. Women aged 18 to 35 years in active labour and requiring analgesia, cx 3 cm to 5 cm, uncomplicated term pregnancy with uncomplicated birth expected, spontaneous or induced labour onset. Excluded if preterm labour. Parity: 100% nullips | |
| Interventions | Experimental: IM tramadol 50 mg (N = 30), tramadol 100 mg (N = 30) Control: IM pethidine 75 mg (N = 30) Single dose of study drug. | |
| Outcomes | Maternal outcomes: pain relief at 10, 20, 30, 45 mins and 1 hr 4‐point scale (0 = none, 1 = insufficient, 2 = sufficient, 3 = complete pain relief), type of birth, drowsiness, nausea, vomiting. Neonatal outcomes: Apgar at 1 min and 5 mins, resuscitation and admission to SCBU. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: Table 1 suggests that the groups were balanced Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind, identical syringes prepared separately from clinical observer. |
| Blinding of outcome assessment (detection bias) | Low risk | States double‐blind, identical syringes prepared separately from clinical observer. |
| Incomplete outcome data (attrition bias) | Low risk | All participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Unclear |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital in Surrey, UK 17 healthy women 36 to 40 weeks' gestation requesting pethidine for pain relief in labour, ASA I or II. Women with a contraindication to pethidine or remifentanil or requesting epidural were excluded. | |
| Interventions | Experimental: IV PCA remifentanil, 0.5 µg bolus per kg (based on antenatal booking weight) with 2 mins lock‐out, no hourly max. Control: IV PCA pethidine, 10 mg bolus, 5 mins lock‐out, 100 mg hourly max. All women were given 10 mg metoclopramide IV over 8 hrs. | |
| Outcomes | Maternal: pain on 10 cm VAS recorded hourly; nausea recorded on a 10 cm VAS; itching; BP pulse and resps. Neonate: 1 min and 5 mins Apgar scores. | |
| Notes | Start and end date: not reported Power calculation: 'Power analysis (β = 0.2) revealed that 17 women would be required in each group, assuming 10 mm change in visual analogue pain score to be clinically significant.' Baseline imbalances between groups: the groups were balanced for baseline characteristics Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described "randomly allocated". |
| Allocation concealment (selection bias) | Low risk | Quote: "by selecting the next in a series of sealed envelopes prepared by pharmacy." |
| Blinding of participants and personnel (performance bias) | Low risk | Women were described as blinded. Quote: "One investigator selected the envelope and prepared the PCA pump. the pump was covered so that the other investigator, the observer, was unable to see which drug the woman was receiving." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "One investigator selected the envelope and prepared the PCA pump. the pump was covered so that the other investigator, the observer, was unable to see which drug the woman was receiving." |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up apparent although for some outcomes it was not clear what the denominators were. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Low risk | None apparent |
| Methods | RCT. 4‐arm parallel groups Start and end date: May 1984 to November 1985 | |
| Participants | Setting: hospital in Cairo, Egypt 80 multiparous women at term (39 to 41 weeks), 19 to 27 years (parity 2 to 6), in the first stage of labour following uncomplicated pregnancies, spontaneous labour. Women with respiratory or cardiac disease were excluded. | |
| Interventions | Group 1: IM nalbuphine 0.13 mg/kg Group 2: IM butorphanol 0.16 mg/kg Group 3: IM pentazocine 0.4 mg/kg Group 4: IM placebo | |
| Outcomes | Pain relief 0 = complete relief, 3 = no relief. Apgar score at 1 min and 5 mins. Maternal and fetal blood gases. | |
| Notes | Data were reported as means and have not been included in data tables. We describe findings briefly in the text. Start and end date: May 1984 ‐ September 1985 Power calculation: not reported Baseline imbalances between groups: the groups were balanced for baseline characteristics Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described "randomly divided". |
| Allocation concealment (selection bias) | Unclear risk | Not described "four equal groups". |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Not clear when randomisation took place and denominators in tables not clear. |
| Other bias | Unclear risk | The equal division into groups suggests that there may not have been true random allocation. |
| Methods | Double‐blind randomised trial | |
| Participants | 231 women with term, singleton pregnancy in cephalic position in the active stage of labour. | |
| Interventions | IM 100 mg tramadol (114 women) versus IM 30 mg pentazocine (117 women). | |
| Outcomes | Pain at 30 and 60 mins, maternal satisfaction, side effects, neonatal outcomes. | |
| Notes | No raw data were reported in this brief abstract. We have contacted the author for more information (27th June 2017). No data are included in the analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) | Low risk | Reported to be double‐blind. |
| Blinding of outcome assessment (detection bias) | Low risk | Reported to be double‐blind. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information to assess (not clear how many women were randomised or if there were missing data). |
| Selective reporting (reporting bias) | Unclear risk | Brief abstract so unable to assess. |
| Other bias | Unclear risk | Unable to assess. The trial author has been contacted to provide more information on methods. |
| Methods | Prospective, parallel‐arm 2‐centre RCT. Block randomisation. Blocks of 2 to 10. Women randomised individually. | |
| Participants | Setting: 2 large hospitals in the UK. 484 women Nulliparous and multiparous women aged 16 years or older who had given written informed consent, who were in active labour defined as regular uterine contractions of at least 2 in 10 mins, with a singleton pregnancy, cervical dilatation of at least 3 cm, with gestation of 37to 42 weeks, and weight between 60 kg and 120 kg. The weight eligibility criterion was reduced from 70 kg to 60 kg with a substantial amendment in June 2009 approximately 3 months after the start of recruitment. Exclusion criteria: allergy or previous adverse reaction to opioids or opioid dependency, use of parenteral opioids within the previous 24 hrs or presence of severe systemic disease. | |
| Interventions | Experimental group 1: diamorphine 7.5 mg group Given into the muscles of the gluteus or lateral thigh by the midwife looking after the women from the trial syringes provided by the research midwife. IM. (244 women). Experimental group 2: pethidine 150 mg group Given into the muscles of the gluteus or lateral thigh by the midwife looking after the women from the trial syringes provided by the research midwife. IM. (240 women). A maximum of 2 doses of opioid were given with a minimum interval of 2 hrs if the women requested additional analgesia. Women also received metoclopramide 10 mg with the first dose. Regional analgesia or Entonox were available as rescue analgesia. | |
| Outcomes | Satisfaction with analgesia Severe pain Mode of birth Additional analgesia required Naloxone admin Neonatal resuscitation Admission to special care Breastfeeding problems Apgar scores Abnormal CTG Umbilical cord gases | |
| Notes | Dates of study: not stated Funding: independent research funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0407‐13170) with additional support costs funded by the Western Comprehensive Local Research Network. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Conflict of interest: all authors have completed the Unified Competing Interest form and there are no competing interests. 3 authors received travel expenses for meetings in relation to the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial statistician provided the computer‐generated block randomisation using block sizes between 2 and 10 to ensure approximately equal group sizes, and stratified by centre. |
| Allocation concealment (selection bias) | Low risk | The pharmacies of both trial centres prepared batches of 2 identical syringes labelled only with the trial number to conceal group allocation and to ensure that if 2 doses were given, the same opioid was given both times. |
| Blinding of participants and personnel (performance bias) | Low risk | Women, researchers, maternity unit staff and trial statistician were blinded to allocation. The actual identities of the 2 groups were not revealed until after full analysis and discussion of the results. |
| Blinding of outcome assessment (detection bias) | Low risk | Women, researchers, maternity unit staff and trial statistician were blinded to allocation. The actual identities of the 2 groups were not revealed until after full analysis and discussion of the results. |
| Incomplete outcome data (attrition bias) | Unclear risk | No loss to follow‐up reported except Quote: “from the 60‐minute measurement onwards there was significantly more missing data in the pethidine group than the diamorphine group (for example 19% versus 10% at 60 minutes, 53% versus 34% at 120 minutes). The difference in quantity of missing data was largely because the women in the pethidine group tended to deliver earlier.”. The study recruited over their target recruitment to account for the missing data. ITT analysis adhered to. Not all denominators reported in tables. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per protocol |
| Other bias | Low risk | Similar baseline characteristics |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 47 women. Women in active labour and requiring analgesia, 37 to 42 weeks' gestation, singleton pregnancies with no known disorders, spontaneous or induced labour onset. Parity: mixed | |
| Interventions | Experimental: IM meptazinol (N = 17). Control: IM pethidine (N = 17). Study dose dependent on woman's weight: 100 mg if weight < 70 kg, 150 mg if weight ≥ 70 kg. Additional analgesia at discretion of caregiver, either 2nd dose of study drug, epidural or nitrous oxide, metoclopramide as required for nausea and vomiting. | |
| Outcomes | Maternal outcomes: type of birth, additional analgesia, epidural. Neonatal outcomes: Apgar at 1 min and 5 mins, FHR changes. | |
| Notes | Open non‐randomised control arm Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: Medical Research Council and Wyeth Research (UK) Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Unclear risk | Described as double‐blind but methods not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind but methods not described. |
| Incomplete outcome data (attrition bias) | Low risk | All patients analysed in an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Balanced at baseline for height, weight, age, socioeconomic group, gestation, cervical dilation, parity and smoking. |
| Methods | RCT, 2‐arm parallel‐group design | |
| Participants | Setting: hospital, UK 80 women. Healthy women in active labour and requiring analgesia, ≥ 38 weeks' gestation, uncomplicated pregnancy. Parity: 4 or less | |
| Interventions | Experimental: IM nalbuphine 20 mg (N = 37). Control: IM pethidine 100 mg (N = 35). Additional doses of test drug allowed at intervals no less than 2 hrs if required to a maximum of 3 doses. Epidural if analgesia inadequate at discretion of caregiver and subsequently removed from trial. | |
| Outcomes | Maternal outcomes: pain intensity at peak of contraction at 30, 60 and 90 mins (rated very severe, severe, moderate, slight) and with VAS (0 to 100), type of birth, sleepiness, nausea and vomiting. Neonatal outcomes: Apgar at 1 min and 5 mins, naloxone administration, Scanlon score (neuro‐behavioural score) at 2 to 4 hrs and 24 hrs. | |
| Notes | Start and end date: not reported Power calculation: not reported Baseline imbalances between groups: unclear Funding source: not reported Conflicts of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) | Low risk | States double‐blind and study drugs were dispensed in coded ampoules. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | High risk | 8/80 excluded from analyses due to inadequate pain relief. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Does not report actual number randomised per group. Broadly comparable at baseline with respect to physical and obstetric characteristics. |
| Methods | RCT with individual randomisation. | |
| Participants | Setting: Islamic International Medical college trust – Rawalpindi (Punjab province) and Islamabad, Pakistan. 150 women in early labour (3 cm to 6 cm) (spontaneous or induced) with uncomplicated singleton term pregnancy and cephalic presentation. Exclusion criteria: women, who requested for other forms of analgesia, had a complicated pregnancy (e.g. pre‐eclampsia, antepartum haemorrhage)/pre‐existing medical disease, had any contraindication to vaginal delivery, or contraindication to opioids. | |
| Interventions | Experimental group 1 (n = 50) (conventional group) received a single intramuscular injection of 1 mL of pentazocine (30 mg) and oral placebo. Experimental group 2 (n = 50) (homeopathy group) received 1 mL of saline injection and oral homeopathic medicine prescribed by a qualified homeopath. The homeopathic medicine used wasChamomilla recutita with strength of 1 M, manufactured by William Schwabe Karlsruhe (Schwabe) and origin was from Germany. It was used in a dose of 3 drops. This medicine comes in a dilution of _30, _200 and 1 M. Control group (n = 50) (placebo group) received oral placebo and 1 mL of saline injection. | |
| Outcomes | Mode of birth Side effects Pain intensity Duration of labour | |
| Notes | Start and end dates: August 2008 to September 2009 Funding: the funding for this project was provided by the Higher Education Commission Pakistan. Conflict of interest: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated through computer. |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were placed in sequential numbers in sealed envelopes. Women were asked to pick from a shuffled deck of cards with a number that was assigned to an envelope. The selected envelope containing the treatment was opened up by a health worker who prepared the study drugs and had no further involvement with women’s assessment. |
| Blinding of participants and personnel (performance bias) | Low risk | The study drugs and the placebo were dispensed in similar packing to ensure blinding of patients as well as dispensers. Caregiver: a health worker, who was blinded to the contents of the drug injected the medicine and dispensed oral preparation of small, white sugar pellets. |
| Blinding of outcome assessment (detection bias) | Low risk | Not mentioned though if caregiver recorded outcomes, assessors were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 women in placebo group opted for epidural and was withdrawn from the study. 3 women form conventional group, 8 women from homeopathy group, and 8 women from placebo group were lost to follow‐up. It is reported that ‘the missing values were observed as some women delivered before any pain assessment or the observations were not recorded.’ There were no further reasons provided. |
| Selective reporting (reporting bias) | Unclear risk | All essential outcomes are reported, protocol not seen and outcomes not clearly specified in text. |
| Other bias | Unclear risk | The baseline demographic characteristics, age, weight and height, were similar in the 3 groups. However Camomilla group had fewer primips, and fewer > para 3. Denominators not clearly specified. Abstract reports 99 women randomised, full‐text reports 150 before exclusions. |
| Methods | Randomised trial with individual randomisation. | |
| Participants | 150 full‐term primiparous women intending to have normal vaginal birth. No exclusion criteria (abstract only) | |
| Interventions | Group 1 (50 women): fentanyl‐droperidol mixed liquor via acupoint injection at different time stages: BL 23 in active phase and BL 32 in second stage. Group 2 (50 women): fentanyl‐droperidol mixed liquor via subcutaneous injection Group 3 (50 women): NaCl 0.9% via subcutaneous injection | |
| Outcomes | VAS score level or norepinephrine Blood pressure | |
| Notes | Dates: not in abstract Funding: not reported Conflict of interest: not reported ABSTRACT ONLY ‐ no data. Full text in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly divided". Method not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) | High risk | Women receiving the subcutaneous injections may have been blinded, unlikely that blinding would have been maintained for staff. |
| Blinding of outcome assessment (detection bias) | High risk | Not mentioned, likely to have outcomes collected by staff. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unable to assess ‐ abstract only |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess ‐ abstract only |
| Other bias | Unclear risk | Unable to assess ‐ abstract only |
ARM: artificial rupture of the membranes
ASA: American Society of Anesthesiologists Classification
BMI: body mass index
BP: blood pressure
CS: caesarean section
CTG: cardiotocograph
cx: cervix
FGR: fetal growth restriction
FHR: fetal heart rate
GA: gestational age
HR: heart rate
IM: intramuscular
IOL: induction of labour
ITT: intention‐to‐treat
hr(s): hour(s)
IV: intravenous
min(s): minute(s)
multips: multiparous women
MW: midwife
NACS: Neurologic and Adaptive Capacity Score
nullips: nulliparous women
PCA: patient controlled analgesia
PN: postnatal
primips: primiparous women
RCT: randomised controlled trial
resps: respirations
SC: subcutaneous
SCBU: special care baby unit
SD: standard deviation
TENS: transcutaneous electrical nerve stimulation
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| The intervention was IV paracetamol, which is not an opiate. | |
| IM pethidine was compared with IV paracetamol. The comparison of opiates with non‐opioid drugs is not a relevant comparison for this review. | |
| This study compares the use of diazepam versus a placebo. Both groups had pethidine. | |
| IM pethidine was compared with IV paracetamol. The comparison of opiates with non‐opioid drugs is not a relevant comparison for this review. | |
| IV morphine was compared with IV paracetamol. The comparison of opiates with non‐opioid drugs is not a relevant comparison for this review. | |
| In this study group allocation was by order of hospital admission (alternate allocation). Not an RCT. | |
| In this study both groups received the same drug (remifentanil) by PCA. The focus of the study was on variation in the bolus size versus variation in the background infusion rate. Studies that examine variation in mode of administration will be considered in a separate related Cochrane review. | |
| In this study both groups had opioids (remifentanil administration in the form of either an infusion or PCA demand bolus (intravenous injection of a single dose over a short period of time). | |
| There was no evidence that this study was an RCT. There were 3 study groups and all 3 received pethidine (1 after 1‐hour delay). The aim of the study was to monitor uterine activity over 60 minutes. | |
| This study examined the effects of hydroxine hydrochloride, an antihistamine. None of the study groups received an opioid analgesic drug. | |
| IM tramadol was compared with IV paracetamol. The comparison of opiates with non‐opioid drugs is not a relevant comparison for this review. | |
| This study was not an RCT. Alternate allocation to groups. | |
| This was a quasi‐randomised study with group allocation by month of birth. The aim of the study was to look at hydroxine as an adjunct to pethidine. both study groups had pethidine. | |
| This trial compares different routes of administration as well as different drugs. | |
| In this study, 1 group received pethidine with promethazine and 1 received no treatment. As the opioid group received a combination of drugs any differences between groups may have been due to the effect of the add‐on drug. | |
| This study had 3 groups: SC pethidine, SC benzethidine and SC flurethidine. We are not aware that, apart from pethidine, these drugs are used any longer for pain relief in labour. | |
| In this study, 1 group received IV remifentanil and 1 group received IM pethidine with haloperidol. With 1 group receiving an add‐on drug it would not be possible to compare the effects of the 2 opioids. | |
| In this study pethidine was compared with the use of a sedative. It was not clear that this was an RCT. | |
| This study compared IV sufentanil with epidural analgesia. Epidural analgesia in labour is covered in a related Cochrane review. | |
| This study was for pain relief during second trimester labour for termination of pregnancy and so not for pain relief for labour of childbirth. | |
| This study had 4 groups: pethidine IM, anileridine IM, pethidine + perphenazine IM and anileridine + perphenazine IM. We are not aware that anileridine is used any longer in obstetric practice. | |
| It was not clear whether or not this was a randomised trial. Women were divided into 2 equal‐sized groups but there was no indication that allocation was random. | |
| It was not clear that participants in this trial were all in labour. The aim of the study was to examine fetal acid balance, with maternal and fetal blood sampling 30 and 60 minutes after administering the drugs. No other outcomes were recorded. | |
| Brief conference abstract. It was not clear that this was an RCT. We attempted to trace the authors for more information without success. | |
| This was not an RCT. Groups were divided into groups according to date of hospital attendance. | |
| IM tramadol was compared with IV paracetamol. The comparison of opiates with non‐opioid drugs is not a relevant comparison for this review. | |
| In this study 1 group received IV nalbuphine and the other pethidine with promethazine, as the pethidine group had an add‐on drug it is not possible to compare the 2 opioids. | |
| This study was excluded for methodological reasons; there was extremely high attrition for some outcomes (> 50%). SC pethidine and placebo were compared in this study; however, it appeared that the drugs were administered very late in labour. Of 224 women included in the analysis, it appeared that more than half had given birth within an hour of drug administration. There were data on pain relief for only approximately 103 women at 1 hour. Results were very difficult to interpret. | |
| All study groups received pethidine. The aim of the study was to examine the effects of tranquillisers as adjuncts to analgesics. | |
| This study had 2 groups: pethidine 100 mg IM and oxymorphone 1.5 mg IM. Oxymorphone is no longer used for pain relief in labour. | |
| The trial registration refers to "crossover assignment" in the methods. Cross‐over trials are not eligible for inclusion in this review. | |
| This study compares pethidine with an NSAID; this is not a eligible comparison for this review. | |
| This study compared opioids with epidural analgesia. Epidural analgesia in labour is covered in a related Cochrane review. | |
| IV pethidine was compared with IV paracetamol. The comparison of opiates with non‐opioid drugs is not a relevant comparison for this review. | |
| This study examines ketamine which is not an opioid. | |
| There was no evidence that there was random allocation in this study. There were 2 study groups and both received pethidine, the aim of the study was to compare drugs administered as an adjunct to the opioid analgesia (diazepam vs promazine). | |
| In this study 2 different drugs using different modes of administration were compared. IV pethidine (with dummy PCA) was compared with PCA remifentanil (with dummy background IV infusion). With both the drug and method being different in each arm of the trial results from this study are very difficult to interpret. | |
| PCA IV pethidine was compared with epidural analgesia. | |
| In this study with 4 different treatment arms, 1 group received IV remifentanil, the remaining 3 received epidural analgesia. Epidurals are covered in a separate Cochrane review. | |
| In this study pethidine was given with haloperidol compared with a birth ball. The addition of haloperidol means this comparison is not relevant for this review. | |
| In this study all the 3 groups received fentanyl but in different doses and by different routes of administration. | |
| PCA remifentanil was compared with epidural (this comparison is eligible for inclusion in a related review). | |
| This study compared IV pethidine versus a combined spinal epidural. | |
| Study examining IV versus epidural fentanyl. | |
| Entry in trials register. It is not clear that this study was completed. We attempted to contact the author and searched for any published results relating to this trial without success. | |
| Study examining IV versus epidural analgesia. | |
| The study evaluated the effects of the interventions on platelet function in the newborn. | |
| This study is looking at IV paracetamol ad an adjunct to PCA epidural analgesia. | |
| This study examine an opioid compared with paracetamol. This is not a relevant comparison for this review. | |
| In this study both randomised groups received pethidine. One group also received naloxone. A third, non‐randomised "matched" group received no narcotic drugs. | |
| In this study both groups received the same drug (pethidine). The focus of the study was on variation in route of administration; IM was compared with PCA (IV) pethidine. Studies that examine variation in mode of administration will be considered in a separate related Cochrane review. | |
| Study examining cortisol levels in women receiving IV opioid vs epidural. This comparison is eligible for a related review. | |
| This was a cross‐over study which is not eligible for inclusion in this review. The study was examining different bolus doses of PCA remifentanil. | |
| No results were reported in this brief abstract. We attempted to contact the author without success. | |
| Only women in preterm labour were recruited to this study. This study was excluded for methodological reasons: there was no information about the number of women randomised and women who received any additional non‐study medications were excluded post randomisation. Under these circumstances interpreting the findings of this study are very difficult. | |
| IV PCA opioid vs epidural. This comparison is eligible for inclusion in a related review. | |
| IM opioid (tramadol) was compared with non opioid (IV paracetamol). This comparison is not eligible for inclusion in this review. | |
| Intervention and control were both IV remifentanil, comparing different regimens. | |
| Study participants were not women in labour. | |
| IM opioid (tramadol) was compared with non opioid (IV paracetamol). This comparison is not eligible for inclusion in this review. | |
| In this study, 2 opioid drugs were compared (tramadol and dihydroetorphine hydrochloride). However, the drugs were administered by different routes (sublingual versus oral) and results are therefore very difficult to interpret. | |
| This study compared PCA remifentanil with epidural; this comparison is not eligible for this review. | |
| Not an RCT; consecutive allocation to groups. Study examining the sedative effects of drugs and their effects on memory. | |
| In this study both groups received pethidine. The focus of the trial was on the use of promethazine versus hydroxyzine as add‐on drugs. | |
| This study compared and opiate (not clear what drug, route or dose) with epidural. This comparison is not eligible for this review. | |
| This study included 5 study arms and focused specifically on neonatal serum bilirubin, an outcome not relevant to this review. | |
| A study examining epidural versus IV analgesia. | |
| In this study both groups received the same drug (diamorphine) either by PCA or IM. Studies that examine variation in mode of administration will be considered in a separate related Cochrane review. | |
| This study focused on promethazine, promazine and propiomazine ad adjuncts to pethidine. All study groups received pethidine. | |
| It was not clear that this was a randomised trial. Women were paired and then allocated in sequence to 4 study arms. | |
| This was a pilot study reported as an abstract only and there was too little information on methods and results to assess risk of bias and results did not include outcomes relevant to this review. | |
| Study focusing on IV versus epidural fentanyl. | |
| Study comparing IV pethidine versus epidural. | |
| Although both the groups received different opioids, the mode of administration was not the same. | |
| In this study, women in the 2 arms of the trials were given different drugs with different routes of administration. PCA IV fentanyl was compared with paracervical blockade; 10 mL 0.25% bupivacaine injected into 4 locations in the cervix. | |
| This study comparing sublingual diamorphine with IM pethidine was reported in a brief abstract; no denominators for study groups were provided. We attempted to contact the study author for more information without success. | |
| In this study, women received either IM tramadol or IM pethidine. It was not clear that this was an RCT. | |
| This study compared IV vs epidural fentanyl (epidural analgesia is the subject of separate Cochrane reviews). | |
| In this study both groups received pethidine; the focus of the study was on a narcotic antagonist (levallorphan) as an adjunct to pethidine. | |
| All 3 groups in this study received pethidine. The aim of the study was to examine the effects of promethazine and propiomazine as adjuncts to pethidine. | |
| This study compared the use of IV PCA remifentanil versus epidural. | |
| This was a cross‐over study. This design is not eligible for inclusion in the review. | |
| This study had 2 groups: pethidine 125 mg IM and oxymorphone 1.25 mg IM. | |
| In this study both groups received the same drug (pethidine) by PCA versus nurse administered (IV). Studies that examine variation in mode of administration will be considered in a separate related Cochrane review. | |
| In this study both groups received the same drug (fentanyl) 1 group by PCA and 1 nurse administered (IV). Studies that examine variation in mode of administration will be considered in a separate related Cochrane review. | |
| In this study a mood‐enhancing drug (methylpentonol) was compared with an analgesic (pethidine). The outcome was not pain relief but fetal expiratory volume. There was no comparison of analgesic drugs in labour. We are not aware that methylpentonol is any longer used during childbirth. | |
| In this study both groups received the same IM opioid analgesia (alphaprodine). The study examined the effects of a narcotic antagonist (levallorphan) as an adjunct to the opioid. | |
| This study compared different ways of administering pethidine (IM vs IV); the IM group received an anti‐emetic the IV group did not. 386 women were randomised but there appears to have been serious attrition with complete data for only approximately a third of women randomised. Attrition was mainly due to protocol deviations. With these methodological problems findings from this study are very difficult to interpret. | |
| Study examining the value of promethazine as an adjunct to pethidine. The study did include a placebo group but the only result reported was maternal blood pressure 10 minutes after injection of the drug/ placebo. | |
| This was a quasi‐randomised study. The outcomes collected in this study were neonate bilirubin levels. | |
| In this study the comparison group received epidural. This comparison is examined in a related review. | |
| In this study the comparison group received epidural. This comparison is examined in a related review. | |
| Quasi‐randomised study with alternate allocation. | |
| This study had 4 groups: pethidine 50 mg, 75 mg or 100 mg IM, oxymorphone 0.75 mg, 1.125 mg or 1.5 mg, pethidine + noroxymorphone IM and oxymorphone + noroxymorphone IM. Oxymorphone is no longer used in clinical practice. | |
| In this study IV remifentanil was compared with IM pethidine. As both the drug and the route were different, we excluded this study as results are difficult to interpret. | |
| Not an RCT. | |
| This study compared opioids with epidural analgesia. Epidural analgesia in labour is covered in a related Cochrane review. | |
| This was a quasi‐randomised study and allocation could be anticipated. | |
| This study focused on women with dystocia and the use of pethidine to promote progress in labour. Women requiring pain relief were excluded. | |
| All study groups received pethidine; the aim of the study was to look at the effects of adjuncts. | |
| In this study the comparison group received epidural. This comparison is examined in a related review. | |
| In this study the comparison group received epidural. This comparison is examined in a related review. | |
| In this study comparing IM pethidine and IM tramadol the report states that the sample was randomly selected, but there was no indication that there was random allocation to groups. | |
| In this study the focus was on the rate of cervical dilatation rather than pain relief. The study was reported in a brief abstract; we attempted to contact the authors for more information without success. | |
| In this study 2 different drugs with different modes of administration were compared. IM pethidine (with an antiemetic) was compared with PCA remifentanil. In view of the different modes of administration we decided to exclude this study as results are very difficult to interpret. | |
| It was not clear that the women included in this study were in labour; women were recruited in the third trimester admitted to hospital following complications or "awaiting caesarean section or the birth of multiple pregnancies". | |
| This study, otherwise eligible for the review, focused on the effect of pethidine on the frequency and intensity of uterine contractions and the rate of cervical dilatation; no other outcomes were reported. | |
| This study did not focus on pain relief in labour; rather, it examined the effects of different drugs on progress in labour for women with dystocia (oxytocin, chlorpromazine, ritodine and pethidine were compared). | |
| Quasi‐randomised study with alternate allocation. | |
| There was no evidence that this was an RCT. | |
| This study compares IV remifentanil with inhaled 50% nitrous oxide in a cross‐over trial. Results were not reported separately for the first stage of this trial. | |
| This study compared IV remifentanil versus epidural analgesia. | |
| This study reported on different regimens of IVPCA remifentanil. | |
| This study focused on speeding up progress in labour. In this group study groups received oxytocin as well as analgesics and women in the control arm received an higher dose of oxytocin. | |
| In this study pethidine was compared with a NSAID ketorolac. Ketorolac is not used nowadays in obstetric analgesia. | |
| Both study groups received pethidine; the aim of the study was to look at the effects of a sedative as an adjuvant therapy. | |
| The comparison group in this study received epidural; this is not a relevant comparison in this review. | |
| In this study epidural analgesia was compared with IM pethidine. It was not clear that this was an RCT. | |
| Both groups in this study received pethidine. The aim of the study was to examine the effects of a narcotic antagonist (levallorphan) as an adjunct to pethidine. | |
| In this study different opioids were compared but the route of administration was also different. | |
| This study is reported in a series of papers and conference abstracts. The study examined the use of an intrathecal opioid as part of a combined spinal epidural compared to a systemic opioid. Epidural analgesia is covered in a separate related Cochrane review. |
IM: intramuscular
IV: intravenous
NSAID: non‐steroidal anti‐inflammatory drug
PCA: patient controlled analgesia
RCT: randomised controlled trial
SC: subcutaneous
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | This report is awaiting classification pending further investigation. |
| Methods | RCT with individual randomisation |
| Participants | 120 women randomised. |
| Interventions | Group 1: massage Group 2: intramuscular pethidine Group 3: standard care |
| Outcomes | Pain intensity Duration of labour only |
| Notes | Setting: Valiasr hospital in Broojen, Iran Abstract only, full‐text awaiting translation. |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | The effect of oxycodone to placental and fetal circulation during the phase I of labour and the efficacy, safety and neonatal effects of oxycodone |
| Methods | Clinical trial (methods not clear) |
| Participants | Women at the onset of labour |
| Interventions | IV oxycodone versus placebo |
| Outcomes | Fetal circulation and condition of the newborn. |
| Starting date | Not clear |
| Contact information | Dr Kokki at Kuopio University Hospital, Finland. [email protected] Author contacted 26th June 2017. |
| Notes |
| Trial name or title | Tramadol for labour analgesia in low‐risk women: a placebo controlled randomised trial |
| Methods | Placebo controlled RCT with parallel assignment |
| Participants | 86 women in labour |
| Interventions | 50 mg IM tramadol vs placebo (IV water) |
| Outcomes | Pain (VAS), satisfaction (1‐5 Likert), fetal distress, mode of birth, duration of labour |
| Starting date | December 2018 (completion planned for May 2018 |
| Contact information | [email protected] Dr Aastha Raheja, Maulana Azad Medical College |
| Notes |
| Trial name or title | Tramadol for labour analgesia in low‐risk primiparous women |
| Methods | Double‐blind randomised trial |
| Participants | Primiparous women with singleton pregnancy in labour with intact membranes. |
| Interventions | Subcutaneous 100 mg tramadol vs placebo |
| Outcomes | Pain in labour, duration of labour, neonatal outcomes, side effects, oxytocin |
| Starting date | October 2012. (Reported to be completed by June 2013) |
| Contact information | Osvaldo A. Reyes T., Saint Thomas Hospital, Panama |
| Notes | No email address and unable to contact author. |
| Trial name or title | Study of the effectiveness of administration of meperidine on the length of active phase of labour in women |
| Methods | Clinical trial |
| Participants | Not clear |
| Interventions | Not clear |
| Outcomes | Not clear |
| Starting date | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than 2 years. |
| Contact information | This study was due for completion in 2012. There is no email address.Orhan SAHIN, M.D., Kanuni Sultan Suleyman Training and Research |
| Notes |
| Trial name or title | Intravenous Remifentanil for Labour Analgesia (IRELAN) |
| Methods | Reported to be parallel RCT. |
| Participants | Planned enrolment 1000 nulliparous women in spontaneous labour requesting analgesia. |
| Interventions | IV PCA remifentanil versus IV intermittent hydromorphone 1 mg (on request) |
| Outcomes | Pain (VAS) during labour, mode of birth, maternal satisfaction with analgesia, use of other analgesia, use of oxytocin, breastfeeding at 6 weeks, neonatal outcomes. |
| Starting date | July 2008, planned completion September 2009. There is no evidence that this study was completed. No email address. The record has not been updated since 2009. |
| Contact information | XiaoFeng Shen, Nanjing Medical University |
| Notes | ClinicalTrials.gov: NCT00710086 |
IV: intravenous
RCT: randomised controlled trial:
PCA: patient‐controlled analgesia
VAS: visual analogue scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| Analysis 1.1  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 1 Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes). | ||||
| 2 Maternal pain score or pain measured in labour (described as good or fair after 1 hour) Show forest plot | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.24, 2.47] |
| Analysis 1.2  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 2 Maternal pain score or pain measured in labour (described as good or fair after 1 hour). | ||||
| 3 Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.0 [1.56, 400.54] |
| Analysis 1.3  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 3 Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes). | ||||
| 4 Additional analgesia required Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.94] |
| Analysis 1.4  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 4 Additional analgesia required. | ||||
| 5 Epidural Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.14, 1.78] |
| Analysis 1.5  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 5 Epidural. | ||||
| 6 Nausea and vomiting Show forest plot | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.31] |
| Analysis 1.6 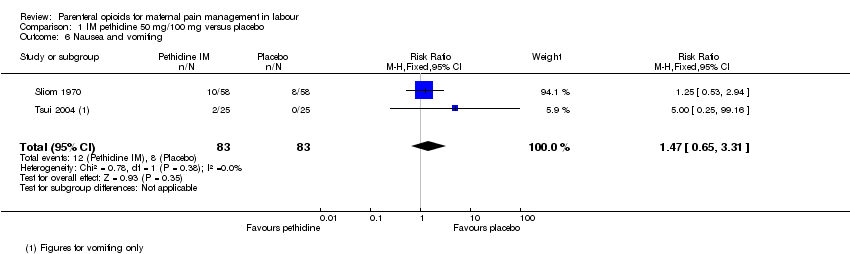 Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 6 Nausea and vomiting. | ||||
| 7 Maternal sleepiness Show forest plot | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [2.43, 8.95] |
| Analysis 1.7 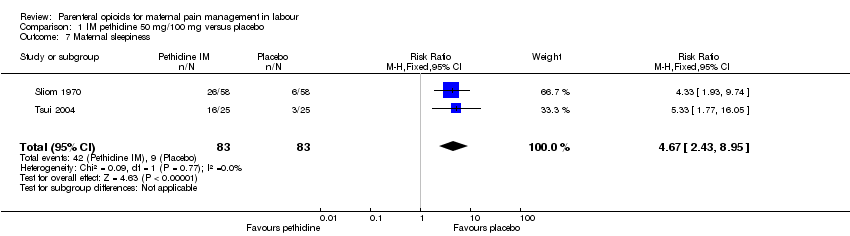 Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 7 Maternal sleepiness. | ||||
| 8 Assisted vaginal delivery Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.34, 2.19] |
| Analysis 1.8  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 8 Assisted vaginal delivery. | ||||
| 9 Caesarean section Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.37] |
| Analysis 1.9 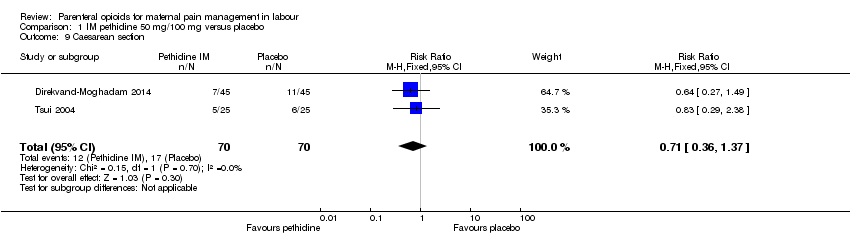 Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 9 Caesarean section. | ||||
| 10 Neonatal resuscitation Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.45, 6.24] |
| Analysis 1.10  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 10 Neonatal resuscitation. | ||||
| 11 Low Apgar score (≤ 7) at 1 and 5 minutes Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.11 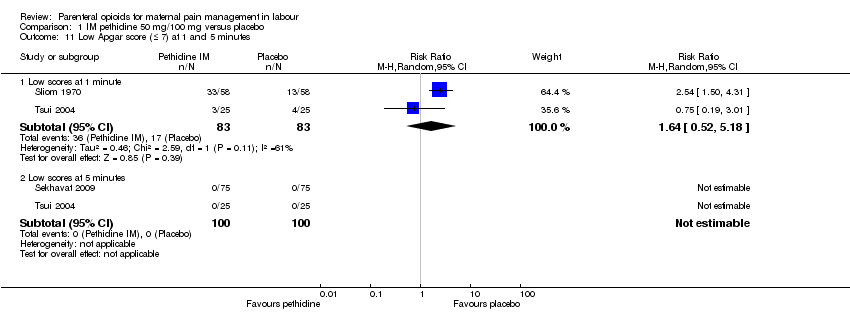 Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 11 Low Apgar score (≤ 7) at 1 and 5 minutes. | ||||
| 11.1 Low scores at 1 minute | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.52, 5.18] |
| 11.2 Low scores at 5 minutes | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Admission to NICU Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| Analysis 1.12  Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 12 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐9.91, 2.71] |
| Analysis 2.1  Comparison 2 IM pentazocine versus placebo, Outcome 1 Maternal pain score measured during labour. | ||||
| 2 Nausea and vomiting Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 2.2 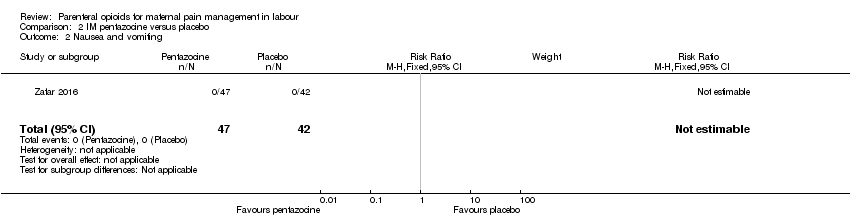 Comparison 2 IM pentazocine versus placebo, Outcome 2 Nausea and vomiting. | ||||
| 3 Caesarean section Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.24, 3.35] |
| Analysis 2.3 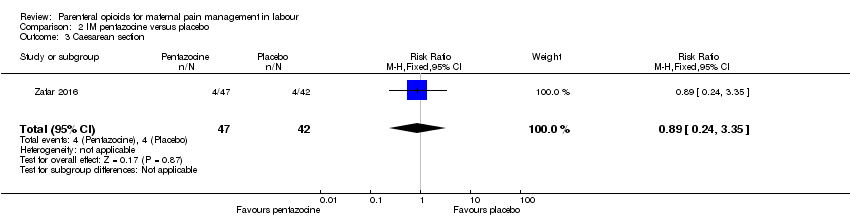 Comparison 2 IM pentazocine versus placebo, Outcome 3 Caesarean section. | ||||
| 4 Assisted vaginal birth Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.10, 3.39] |
| Analysis 2.4  Comparison 2 IM pentazocine versus placebo, Outcome 4 Assisted vaginal birth. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| Analysis 3.1  Comparison 3 IM tramadol versus no treatment, Outcome 1 Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)) Show forest plot | 1 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.12] |
| Analysis 4.1  Comparison 4 IM meptazinol versus pethidine, Outcome 1 Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)). | ||||
| 2 Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)) Show forest plot | 2 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.69, 1.80] |
| Analysis 4.2 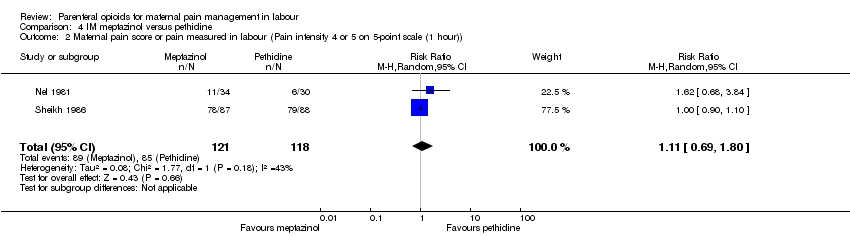 Comparison 4 IM meptazinol versus pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)). | ||||
| 3 Additional analgesia required Show forest plot | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| Analysis 4.3  Comparison 4 IM meptazinol versus pethidine, Outcome 3 Additional analgesia required. | ||||
| 4 Epidural Show forest plot | 4 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.29] |
| Analysis 4.4  Comparison 4 IM meptazinol versus pethidine, Outcome 4 Epidural. | ||||
| 5 Maternal sleepiness Show forest plot | 3 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.07] |
| Analysis 4.5  Comparison 4 IM meptazinol versus pethidine, Outcome 5 Maternal sleepiness. | ||||
| 6 Nausea and vomiting Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.6  Comparison 4 IM meptazinol versus pethidine, Outcome 6 Nausea and vomiting. | ||||
| 6.1 Nausea | 3 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.28] |
| 6.2 Vomiting | 3 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.47] |
| 7 Caesarean section Show forest plot | 3 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.16, 2.00] |
| Analysis 4.7 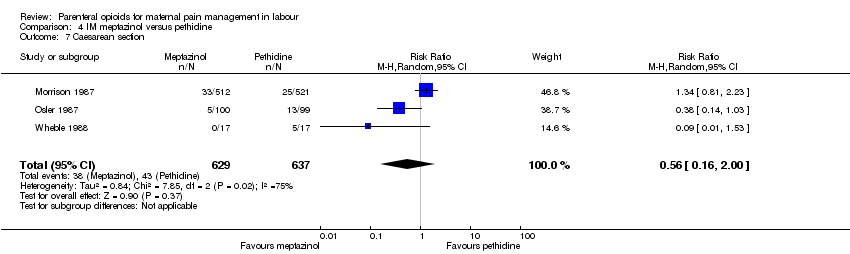 Comparison 4 IM meptazinol versus pethidine, Outcome 7 Caesarean section. | ||||
| 8 Assisted vaginal birth Show forest plot | 3 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.22] |
| Analysis 4.8 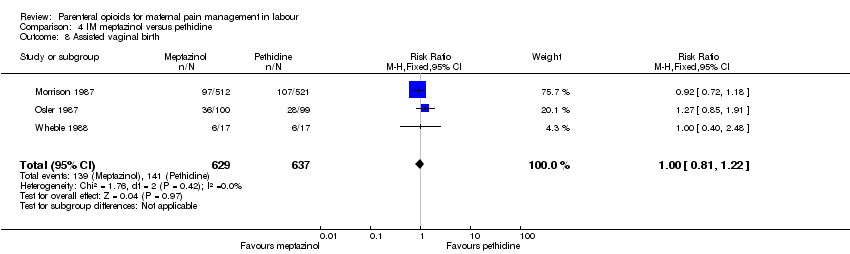 Comparison 4 IM meptazinol versus pethidine, Outcome 8 Assisted vaginal birth. | ||||
| 9 Breastfeeding at discharge (problems) Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.30] |
| Analysis 4.9  Comparison 4 IM meptazinol versus pethidine, Outcome 9 Breastfeeding at discharge (problems). | ||||
| 10 Fetal heart rate changes (decelerations) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.92, 1.64] |
| Analysis 4.10 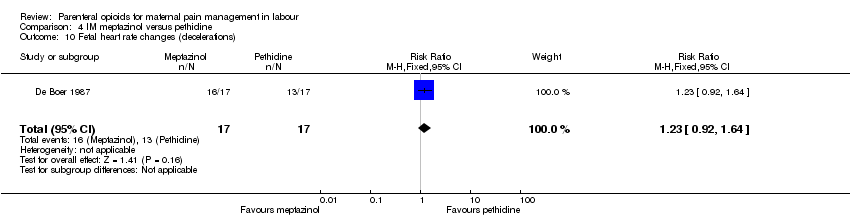 Comparison 4 IM meptazinol versus pethidine, Outcome 10 Fetal heart rate changes (decelerations). | ||||
| 11 Naloxone administration Show forest plot | 1 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| Analysis 4.11 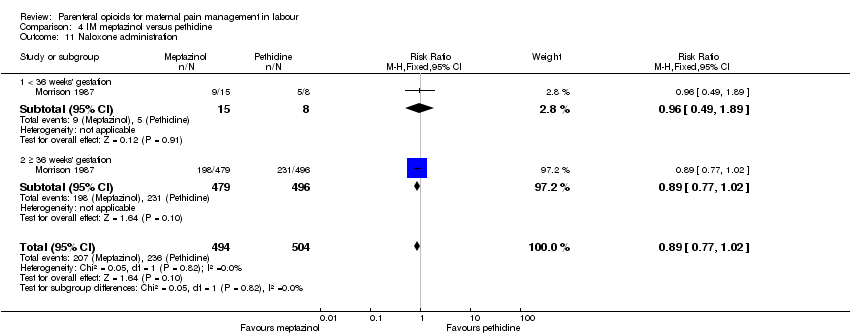 Comparison 4 IM meptazinol versus pethidine, Outcome 11 Naloxone administration. | ||||
| 11.1 < 36 weeks' gestation | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.49, 1.89] |
| 11.2 ≥ 36 weeks' gestation | 1 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 12 Neonatal resuscitation (by gestation) Show forest plot | 2 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| Analysis 4.12  Comparison 4 IM meptazinol versus pethidine, Outcome 12 Neonatal resuscitation (by gestation). | ||||
| 12.1 < 36 weeks' gestation | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.16] |
| 12.2 ≥ 36 weeks' gestation | 2 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 13 Neonatal resuscitation Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.60] |
| Analysis 4.13  Comparison 4 IM meptazinol versus pethidine, Outcome 13 Neonatal resuscitation. | ||||
| 14 Apgar score ≤ 7 at 1 minute Show forest plot | 6 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.11] |
| Analysis 4.14  Comparison 4 IM meptazinol versus pethidine, Outcome 14 Apgar score ≤ 7 at 1 minute. | ||||
| 15 Apgar score ≤ 7 at 5 minutes Show forest plot | 3 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.37] |
| Analysis 4.15  Comparison 4 IM meptazinol versus pethidine, Outcome 15 Apgar score ≤ 7 at 5 minutes. | ||||
| 16 Admission to NICU Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.63] |
| Analysis 4.16 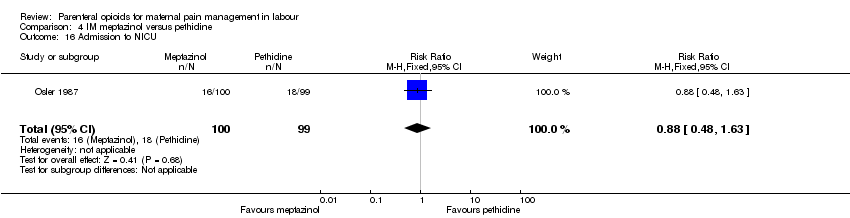 Comparison 4 IM meptazinol versus pethidine, Outcome 16 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| Analysis 5.1  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours). | ||||
| 2 Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| Analysis 5.2  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)). | ||||
| 3 Additonal analgesia required Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.53, 3.40] |
| Analysis 5.3 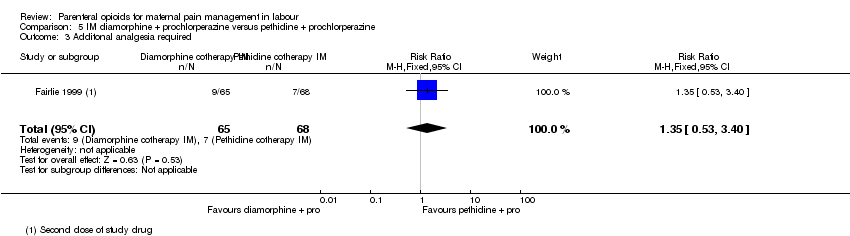 Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 3 Additonal analgesia required. | ||||
| 4 Epidural Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.72, 2.07] |
| Analysis 5.4  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 4 Epidural. | ||||
| 5 Maternal sleepiness during labour Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.66] |
| Analysis 5.5 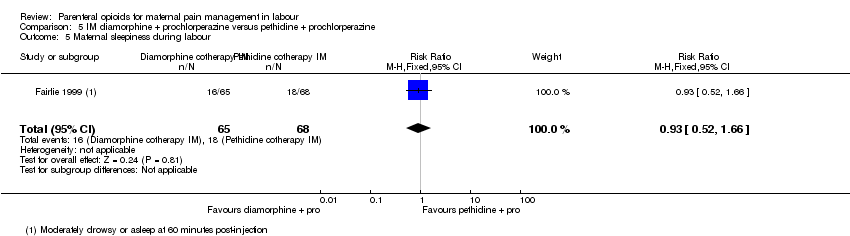 Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 5 Maternal sleepiness during labour. | ||||
| 6 Vomiting in labour Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.17, 0.86] |
| Analysis 5.6  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 6 Vomiting in labour. | ||||
| 7 Caesarean section Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.76] |
| Analysis 5.7  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 7 Caesarean section. | ||||
| 8 Assisted vaginal birth Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.46, 2.02] |
| Analysis 5.8  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 8 Assisted vaginal birth. | ||||
| 9 Neonatal resuscitation Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.73, 2.02] |
| Analysis 5.9  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 9 Neonatal resuscitation. | ||||
| 10 Apgar < 7 at 1 minute Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.91] |
| Analysis 5.10 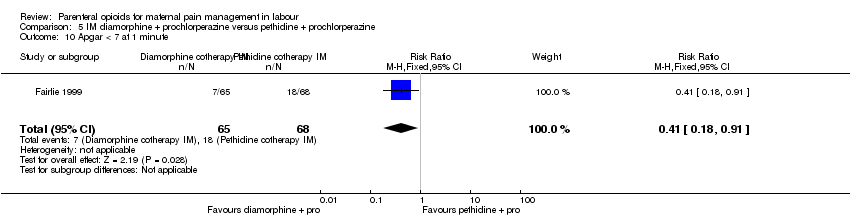 Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 10 Apgar < 7 at 1 minute. | ||||
| 11 Apgar < 7 at 5 minutes Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.27] |
| Analysis 5.11  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 11 Apgar < 7 at 5 minutes. | ||||
| 12 Admission to NICU Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.64] |
| Analysis 5.12  Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 12 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief) Show forest plot | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.10, 2.21] |
| Analysis 6.1 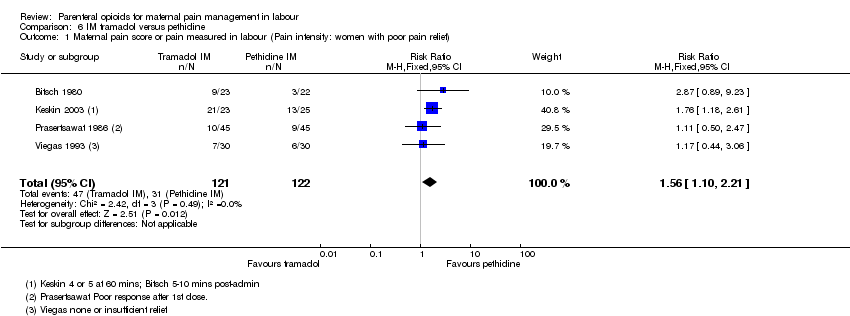 Comparison 6 IM tramadol versus pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief). | ||||
| 2 Additional analgesia required Show forest plot | 3 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.91] |
| Analysis 6.2  Comparison 6 IM tramadol versus pethidine, Outcome 2 Additional analgesia required. | ||||
| 3 Maternal sleepiness in labour Show forest plot | 5 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.97] |
| Analysis 6.3 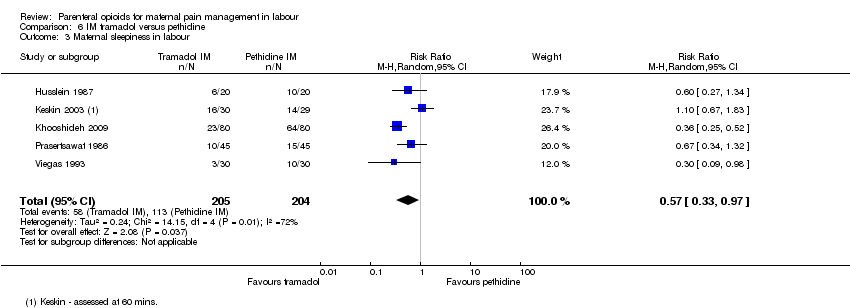 Comparison 6 IM tramadol versus pethidine, Outcome 3 Maternal sleepiness in labour. | ||||
| 4 Nausea and vomiting in labour Show forest plot | 6 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.34, 2.76] |
| Analysis 6.4  Comparison 6 IM tramadol versus pethidine, Outcome 4 Nausea and vomiting in labour. | ||||
| 5 Caesarean section Show forest plot | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| Analysis 6.5  Comparison 6 IM tramadol versus pethidine, Outcome 5 Caesarean section. | ||||
| 6 Assisted vaginal birth Show forest plot | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.56] |
| Analysis 6.6 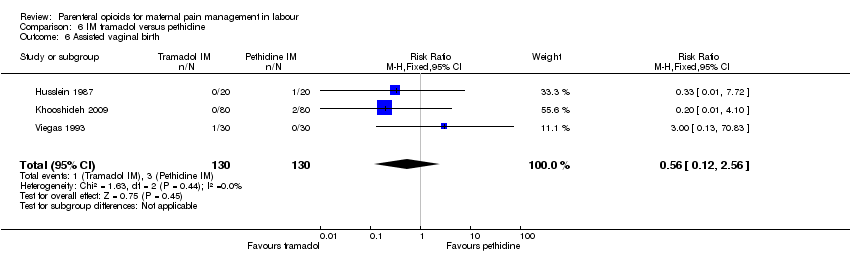 Comparison 6 IM tramadol versus pethidine, Outcome 6 Assisted vaginal birth. | ||||
| 7 Neonatal resuscitation Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 6.7  Comparison 6 IM tramadol versus pethidine, Outcome 7 Neonatal resuscitation. | ||||
| 8 Apgar scores ≤ 7 at 1 and 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.8  Comparison 6 IM tramadol versus pethidine, Outcome 8 Apgar scores ≤ 7 at 1 and 5 minutes. | ||||
| 8.1 Less than 7 at 1 minute | 2 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Less than 7 at 5 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neonatal respiratory distress Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| Analysis 6.9  Comparison 6 IM tramadol versus pethidine, Outcome 9 Neonatal respiratory distress. | ||||
| 10 Admission to NICU Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| Analysis 6.10  Comparison 6 IM tramadol versus pethidine, Outcome 10 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal sleepiness in labour Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.68, 12.12] |
| Analysis 7.1 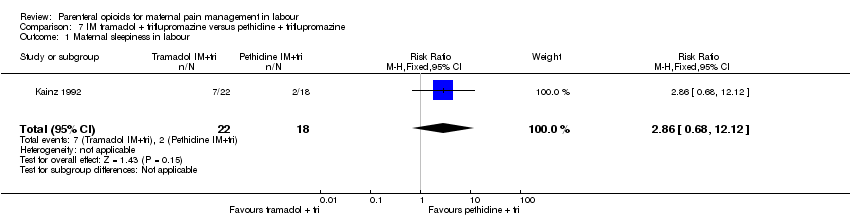 Comparison 7 IM tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 1 Maternal sleepiness in labour. | ||||
| 2 Nausea and vomiting in labour Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.2 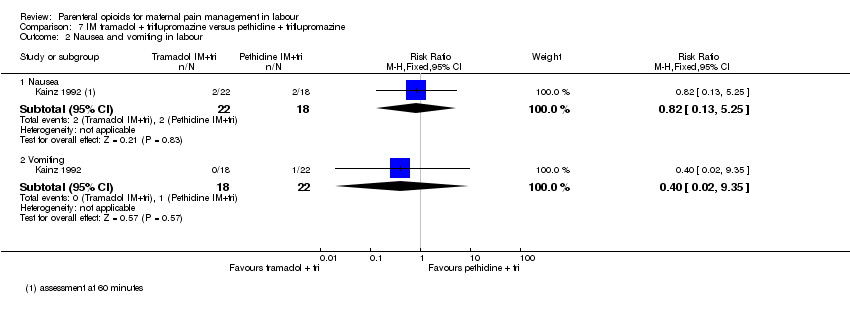 Comparison 7 IM tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 2 Nausea and vomiting in labour. | ||||
| 2.1 Nausea | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.13, 5.25] |
| 2.2 Vomiting | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour) Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.64, 1.86] |
| Analysis 8.1  Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 1 Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour). | ||||
| 2 Maternal sleepiness in labour Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.04] |
| Analysis 8.2  Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 2 Maternal sleepiness in labour. | ||||
| 3 Nausea and vomiting in labour Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.40, 1.88] |
| Analysis 8.3  Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 3 Nausea and vomiting in labour. | ||||
| 4 Apgar ≤ 7 at 1 minute Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| Analysis 8.4  Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 4 Apgar ≤ 7 at 1 minute. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery) Show forest plot | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.27] |
| Analysis 9.1  Comparison 9 IM pentazocine versus pethidine, Outcome 1 Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery). | ||||
| 2 Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.2  Comparison 9 IM pentazocine versus pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)). | ||||
| 2.1 No add‐on drugs | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.74, 2.05] |
| 2.2 With promazine | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.66, 3.58] |
| 3 Additional analgesia required Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.3 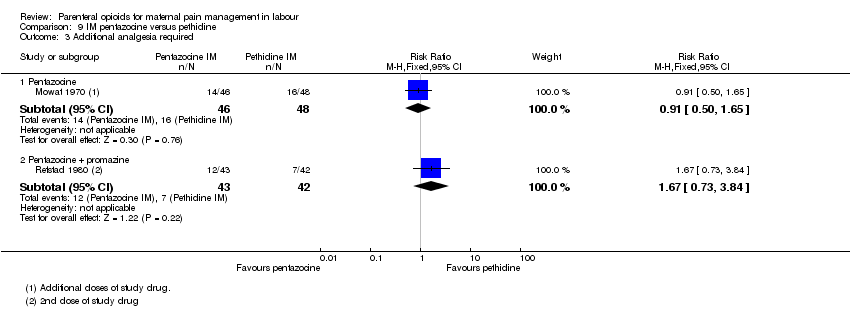 Comparison 9 IM pentazocine versus pethidine, Outcome 3 Additional analgesia required. | ||||
| 3.1 Pentazocine | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.65] |
| 3.2 Pentazocine + promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.73, 3.84] |
| 4 Maternal sleepiness in labour Show forest plot | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| Analysis 9.4 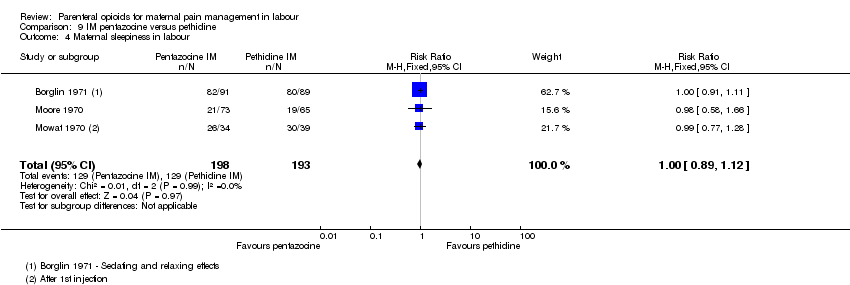 Comparison 9 IM pentazocine versus pethidine, Outcome 4 Maternal sleepiness in labour. | ||||
| 5 Nausea and vomiting in labour Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.5  Comparison 9 IM pentazocine versus pethidine, Outcome 5 Nausea and vomiting in labour. | ||||
| 5.1 Nausea | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 5.2 Vomiting | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.27, 3.14] |
| 6 Assisted vaginal birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.6  Comparison 9 IM pentazocine versus pethidine, Outcome 6 Assisted vaginal birth. | ||||
| 6.1 No add‐on drugs | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.63, 42.97] |
| 6.2 With promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.23, 2.71] |
| 7 Naloxone administration Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| Analysis 9.7  Comparison 9 IM pentazocine versus pethidine, Outcome 7 Naloxone administration. | ||||
| 7.1 With promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| 8 Apgar score ≤ 7 at 1 minute Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 9.8  Comparison 9 IM pentazocine versus pethidine, Outcome 8 Apgar score ≤ 7 at 1 minute. | ||||
| 8.1 No add‐on drugs | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.06, 32.97] |
| 8.2 With promazine | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.07, 17.30] |
| 9 Apgar score ≤ 7 at 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.9 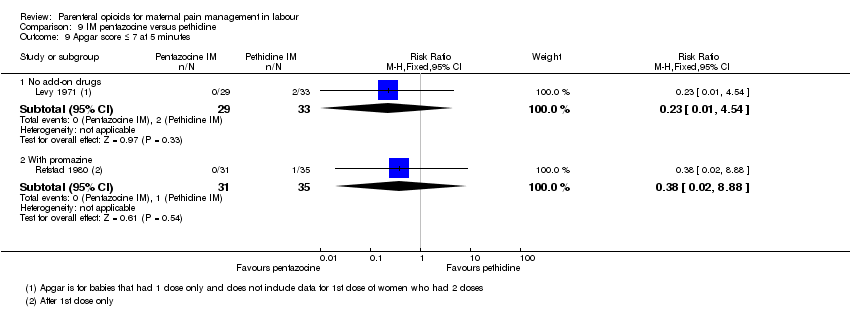 Comparison 9 IM pentazocine versus pethidine, Outcome 9 Apgar score ≤ 7 at 5 minutes. | ||||
| 9.1 No add‐on drugs | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.54] |
| 9.2 With promazine | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.96] |
| Analysis 10.1 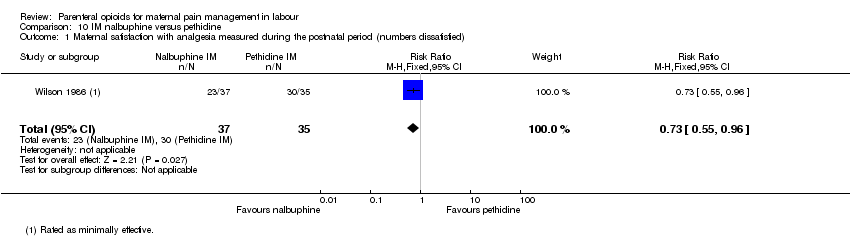 Comparison 10 IM nalbuphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied). | ||||
| 2 Maternal satisfaction with analgesia measured during labour (Pain free) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.79, 45.42] |
| Analysis 10.2  Comparison 10 IM nalbuphine versus pethidine, Outcome 2 Maternal satisfaction with analgesia measured during labour (Pain free). | ||||
| 3 Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain) Show forest plot | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| Analysis 10.3  Comparison 10 IM nalbuphine versus pethidine, Outcome 3 Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain). | ||||
| 4 Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)) Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐18.55, 2.55] |
| Analysis 10.4  Comparison 10 IM nalbuphine versus pethidine, Outcome 4 Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)). | ||||
| 5 Additional analgesia required Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.49, 3.27] |
| Analysis 10.5 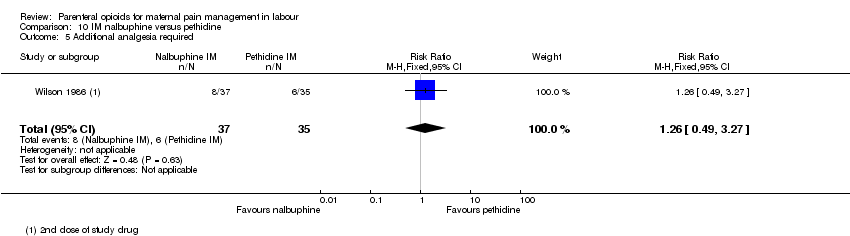 Comparison 10 IM nalbuphine versus pethidine, Outcome 5 Additional analgesia required. | ||||
| 6 Epidural Show forest plot | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.55, 4.94] |
| Analysis 10.6 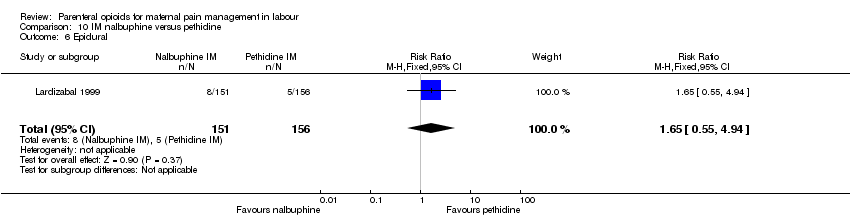 Comparison 10 IM nalbuphine versus pethidine, Outcome 6 Epidural. | ||||
| 7 Maternal sleepiness in labour Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.86, 16.60] |
| Analysis 10.7  Comparison 10 IM nalbuphine versus pethidine, Outcome 7 Maternal sleepiness in labour. | ||||
| 8 Nausea and vomiting in labour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.8 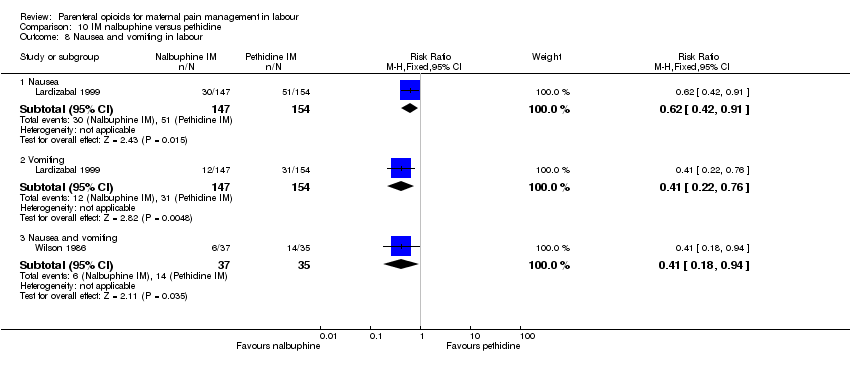 Comparison 10 IM nalbuphine versus pethidine, Outcome 8 Nausea and vomiting in labour. | ||||
| 8.1 Nausea | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.91] |
| 8.2 Vomiting | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.22, 0.76] |
| 8.3 Nausea and vomiting | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.94] |
| 9 Caesarean section Show forest plot | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.69] |
| Analysis 10.9  Comparison 10 IM nalbuphine versus pethidine, Outcome 9 Caesarean section. | ||||
| 10 Assisted vaginal birth Show forest plot | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.25, 3.85] |
| Analysis 10.10 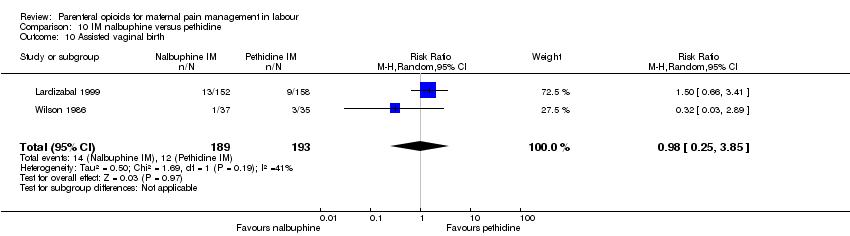 Comparison 10 IM nalbuphine versus pethidine, Outcome 10 Assisted vaginal birth. | ||||
| 11 Naloxone administration Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [0.35, 123.93] |
| Analysis 10.11  Comparison 10 IM nalbuphine versus pethidine, Outcome 11 Naloxone administration. | ||||
| 12 Apgar score ≤ 7 at 1 and 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 10.12  Comparison 10 IM nalbuphine versus pethidine, Outcome 12 Apgar score ≤ 7 at 1 and 5 minutes. | ||||
| 12.1 Low score at 1 minute | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.95] |
| 12.2 Low score at 5 minutes | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 4.99] |
| 13 Admission to NICU Show forest plot | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.61, 1.89] |
| Analysis 10.13  Comparison 10 IM nalbuphine versus pethidine, Outcome 13 Admission to NICU. | ||||
| 14 Neonatal neurobehavioural (Scanlon) 2‐4 hours PN Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐6.14, ‐1.26] |
| Analysis 10.14 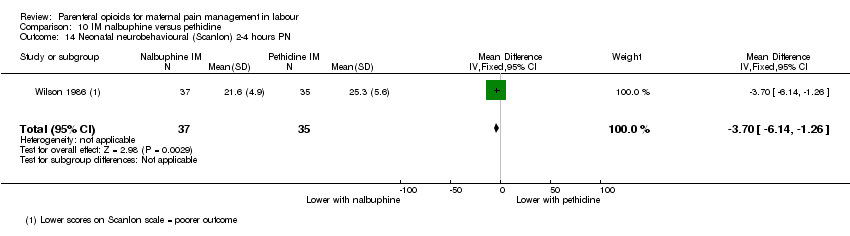 Comparison 10 IM nalbuphine versus pethidine, Outcome 14 Neonatal neurobehavioural (Scanlon) 2‐4 hours PN. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Epidural Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.58, 2.97] |
| Analysis 11.1 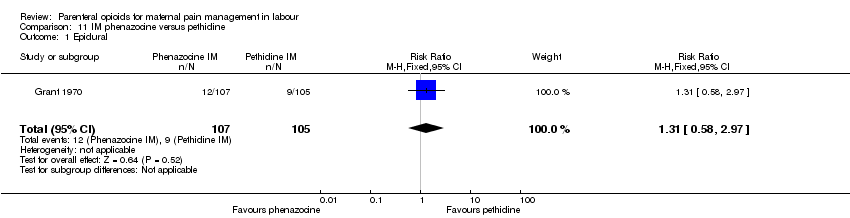 Comparison 11 IM phenazocine versus pethidine, Outcome 1 Epidural. | ||||
| 2 Vomiting Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.20, 0.78] |
| Analysis 11.2 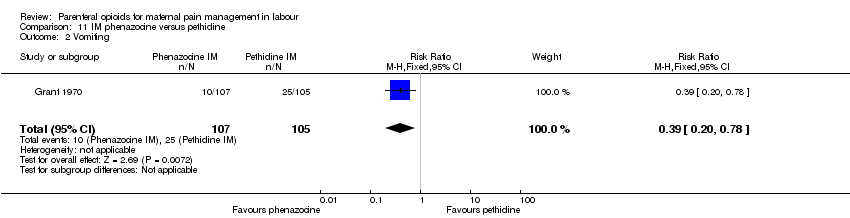 Comparison 11 IM phenazocine versus pethidine, Outcome 2 Vomiting. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (number of women satisfied or very satisfied) Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.26] |
| Analysis 12.1  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia (number of women satisfied or very satisfied). | ||||
| 2 Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor) Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.66] |
| Analysis 12.2 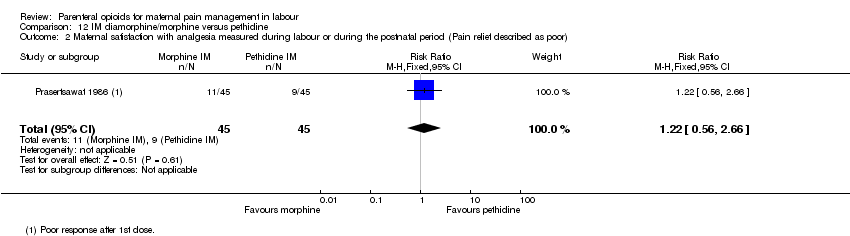 Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 2 Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor). | ||||
| 3 Maternal pain score or pain measured in labour (pain relief at 30 mins) Show forest plot | 1 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.24, ‐0.36] |
| Analysis 12.3  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 3 Maternal pain score or pain measured in labour (pain relief at 30 mins). | ||||
| 4 Maternal pain score or pain measured in labour (pain relief at 60 mins) Show forest plot | 1 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.26, ‐0.34] |
| Analysis 12.4 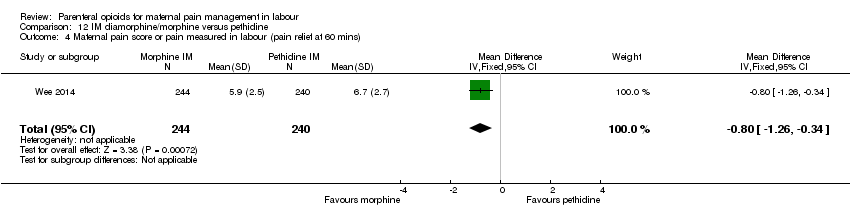 Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 4 Maternal pain score or pain measured in labour (pain relief at 60 mins). | ||||
| 5 Additional analgesia required Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.10] |
| Analysis 12.5  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 5 Additional analgesia required. | ||||
| 6 Maternal sleepiness Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.29, 1.23] |
| Analysis 12.6  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 6 Maternal sleepiness. | ||||
| 7 Nausea and vomiting Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.69] |
| Analysis 12.7  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 7 Nausea and vomiting. | ||||
| 8 Caesarean section Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.35] |
| Analysis 12.8 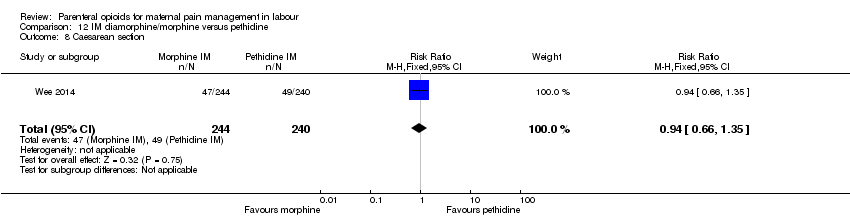 Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 8 Caesarean section. | ||||
| 9 Assisted vaginal birth Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.91, 1.80] |
| Analysis 12.9 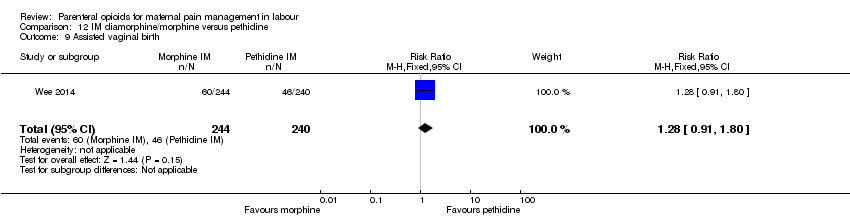 Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 9 Assisted vaginal birth. | ||||
| 10 Naloxone administration Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.83] |
| Analysis 12.10 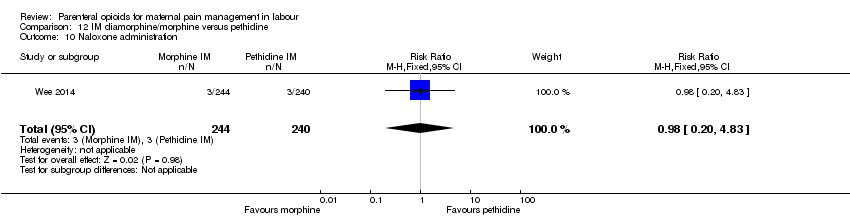 Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 10 Naloxone administration. | ||||
| 11 Neonatal resuscitation Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| Analysis 12.11  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 11 Neonatal resuscitation. | ||||
| 12 Apgar < 7 at 1 minute Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.73] |
| Analysis 12.12  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 12 Apgar < 7 at 1 minute. | ||||
| 13 Admission to NICU Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.34, 2.23] |
| Analysis 12.13  Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 13 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| Analysis 13.1  Comparison 13 IM butorphanol versus pethidine, Outcome 1 Additional analgesia required. | ||||
| 2 Nausea Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.04] |
| Analysis 13.2  Comparison 13 IM butorphanol versus pethidine, Outcome 2 Nausea. | ||||
| 3 Vomiting Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| Analysis 13.3 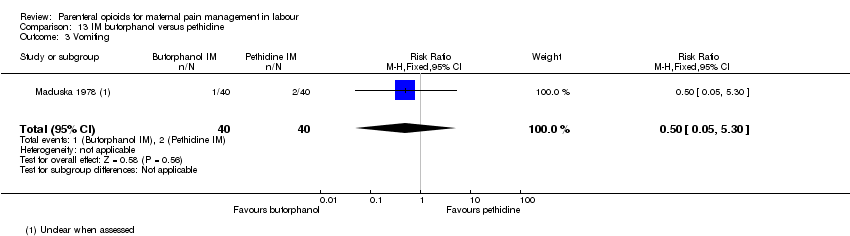 Comparison 13 IM butorphanol versus pethidine, Outcome 3 Vomiting. | ||||
| 4 Neonatal resuscitation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Analysis 13.4  Comparison 13 IM butorphanol versus pethidine, Outcome 4 Neonatal resuscitation. | ||||
| 5 Naloxone administration (neonatal) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Analysis 13.5 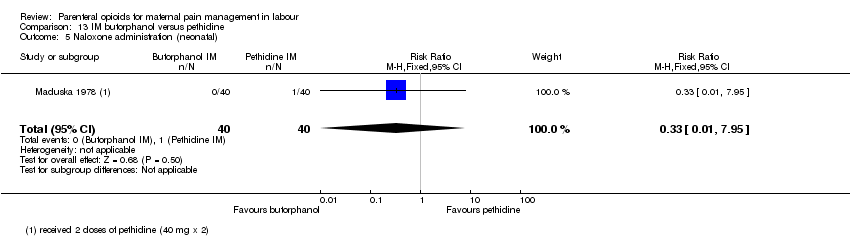 Comparison 13 IM butorphanol versus pethidine, Outcome 5 Naloxone administration (neonatal). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required ‐ Entonox Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.63] |
| Analysis 14.1 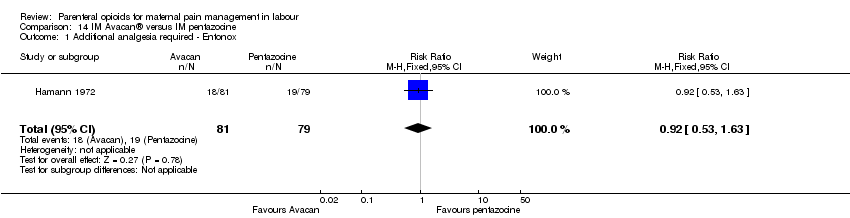 Comparison 14 IM Avacan® versus IM pentazocine, Outcome 1 Additional analgesia required ‐ Entonox. | ||||
| 2 Additional analgesia required ‐ pudendal‐paracervical block Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.16, 3.53] |
| Analysis 14.2 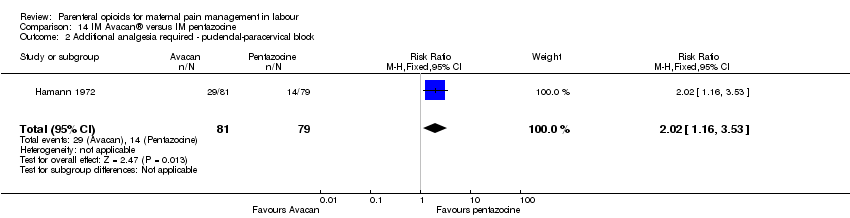 Comparison 14 IM Avacan® versus IM pentazocine, Outcome 2 Additional analgesia required ‐ pudendal‐paracervical block. | ||||
| 3 Caesarean section Show forest plot | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.84] |
| Analysis 14.3 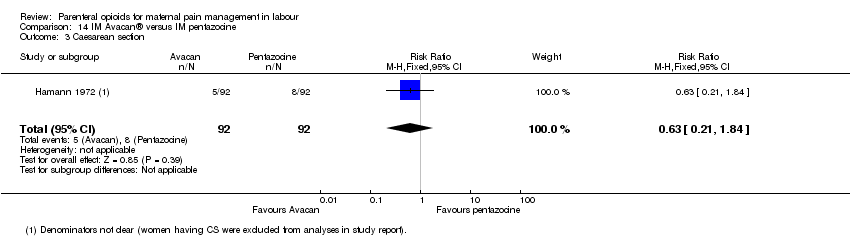 Comparison 14 IM Avacan® versus IM pentazocine, Outcome 3 Caesarean section. | ||||
| 4 Low Apgar score (< 7) "at birth" Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
| Analysis 14.4  Comparison 14 IM Avacan® versus IM pentazocine, Outcome 4 Low Apgar score (< 7) "at birth". | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour (Pain relief (women NOT obtaining pain relief) at 1 hour) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.77, 1.95] |
| Analysis 15.1  Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 1 Maternal pain score measured during labour (Pain relief (women NOT obtaining pain relief) at 1 hour). | ||||
| 2 Additional analgesia required Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| Analysis 15.2 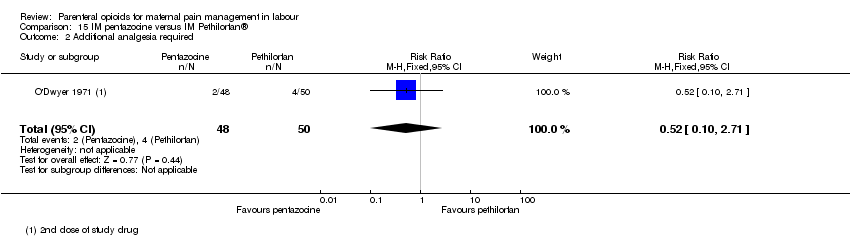 Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 2 Additional analgesia required. | ||||
| 3 Assisted vaginal birth Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.19] |
| Analysis 15.3  Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 3 Assisted vaginal birth. | ||||
| 4 Apgar < 8 at 1 minute (non pre‐specified) Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.71 [0.72, 45.39] |
| Analysis 15.4 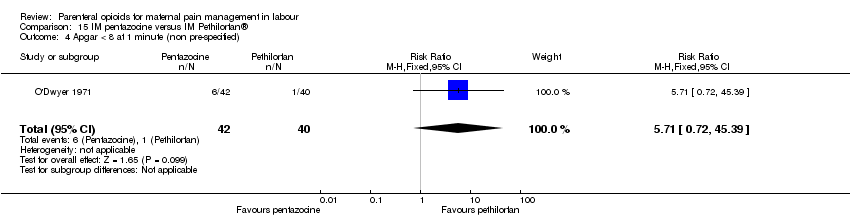 Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 4 Apgar < 8 at 1 minute (non pre‐specified). | ||||
| 5 Apgar < 8 at 5 minutes (non pre‐specified) Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 15.5  Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 5 Apgar < 8 at 5 minutes (non pre‐specified). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐7.61, 6.81] |
| Analysis 16.1  Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 1 Maternal pain score measured during labour. | ||||
| 2 Nausea and vomiting Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.14] |
| Analysis 16.2 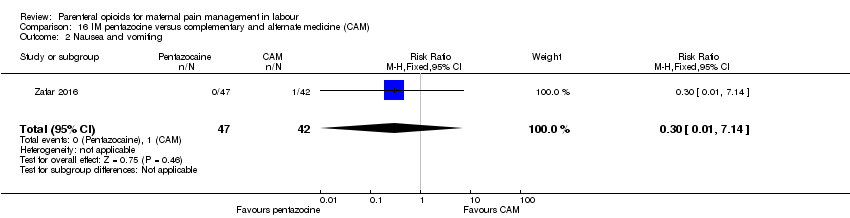 Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 2 Nausea and vomiting. | ||||
| 3 Caesarean section Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.24, 3.35] |
| Analysis 16.3 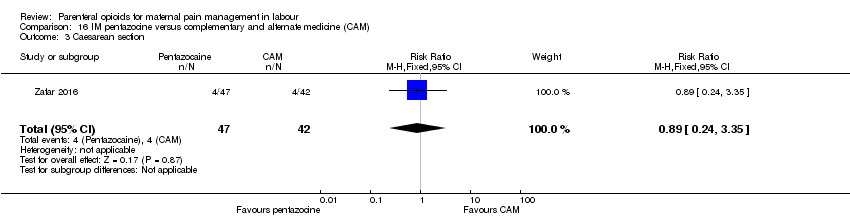 Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 3 Caesarean section. | ||||
| 4 Assisted vaginal delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.13, 6.07] |
| Analysis 16.4  Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 4 Assisted vaginal delivery. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (pain relief after 30 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.28, 4.48] |
| Analysis 17.1  Comparison 17 IM pentazocine versus IM tramadol, Outcome 1 Maternal satisfaction with analgesia measured during labour (pain relief after 30 mins). | ||||
| 2 Maternal satisfaction with analgesia measured during labour (pain after 60 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.91, 2.86] |
| Analysis 17.2 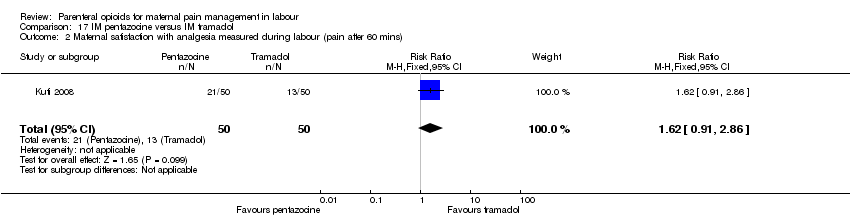 Comparison 17 IM pentazocine versus IM tramadol, Outcome 2 Maternal satisfaction with analgesia measured during labour (pain after 60 mins). | ||||
| 3 Maternal pain score or pain measured in labour (moderate or severe at 30 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| Analysis 17.3  Comparison 17 IM pentazocine versus IM tramadol, Outcome 3 Maternal pain score or pain measured in labour (moderate or severe at 30 mins). | ||||
| 4 Maternal pain score or pain measured in labour (moderate or severe at 60 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.08] |
| Analysis 17.4  Comparison 17 IM pentazocine versus IM tramadol, Outcome 4 Maternal pain score or pain measured in labour (moderate or severe at 60 mins). | ||||
| 5 Maternal sleepiness during labour Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.66, 4.24] |
| Analysis 17.5 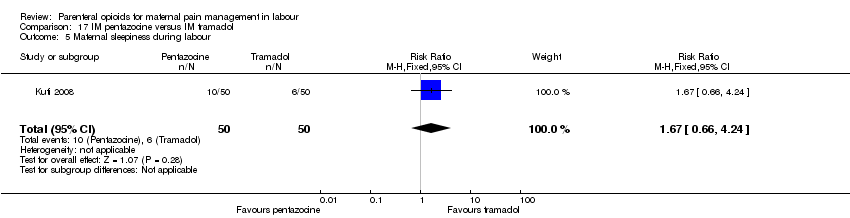 Comparison 17 IM pentazocine versus IM tramadol, Outcome 5 Maternal sleepiness during labour. | ||||
| 6 Nausea and vomiting Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| Analysis 17.6 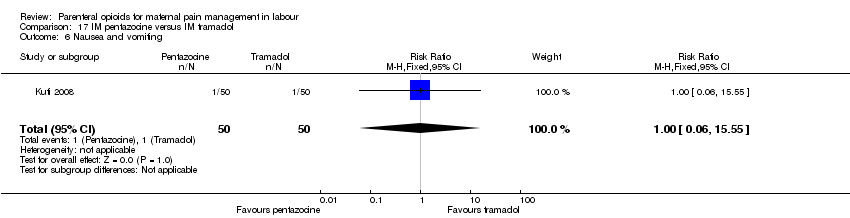 Comparison 17 IM pentazocine versus IM tramadol, Outcome 6 Nausea and vomiting. | ||||
| 7 Caesarean section Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.45, 4.99] |
| Analysis 17.7  Comparison 17 IM pentazocine versus IM tramadol, Outcome 7 Caesarean section. | ||||
| 8 Assisted vaginal delivery Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| Analysis 17.8  Comparison 17 IM pentazocine versus IM tramadol, Outcome 8 Assisted vaginal delivery. | ||||
| 9 Apgar score < 7 at 1 minute Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.60] |
| Analysis 17.9 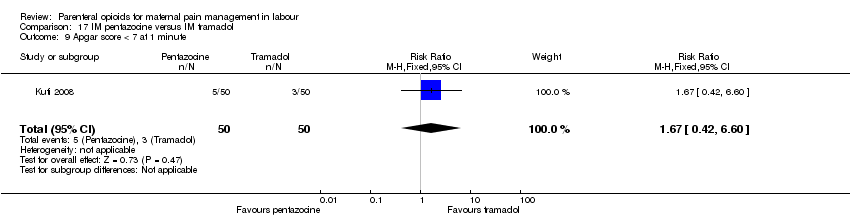 Comparison 17 IM pentazocine versus IM tramadol, Outcome 9 Apgar score < 7 at 1 minute. | ||||
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| Analysis 17.10 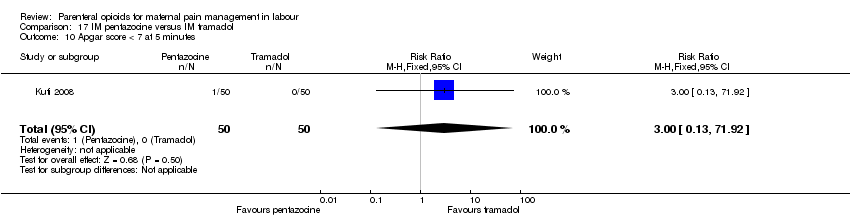 Comparison 17 IM pentazocine versus IM tramadol, Outcome 10 Apgar score < 7 at 5 minutes. | ||||
| 11 Admission to NICU Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 68.47] |
| Analysis 17.11 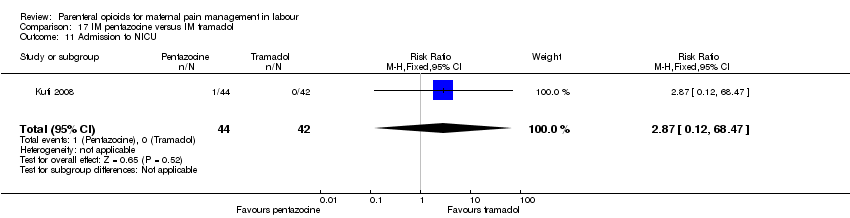 Comparison 17 IM pentazocine versus IM tramadol, Outcome 11 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (after 30 mins) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [1.17, 2.15] |
| Analysis 18.1  Comparison 18 IM pethidine versus Entonox, Outcome 1 Maternal pain score or pain measured in labour (after 30 mins). | ||||
| 2 Maternal pain score or pain measured in labour (after 60 mins) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.85, 0.13] |
| Analysis 18.2  Comparison 18 IM pethidine versus Entonox, Outcome 2 Maternal pain score or pain measured in labour (after 60 mins). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia) Show forest plot | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.56, ‐3.64] |
| Analysis 19.1  Comparison 19 IV pethidine versus placebo, Outcome 1 Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia). | ||||
| 2 Nausea and vomiting Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.05, 5.64] |
| Analysis 19.2  Comparison 19 IV pethidine versus placebo, Outcome 2 Nausea and vomiting. | ||||
| 3 Caesarean section Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.68] |
| Analysis 19.3 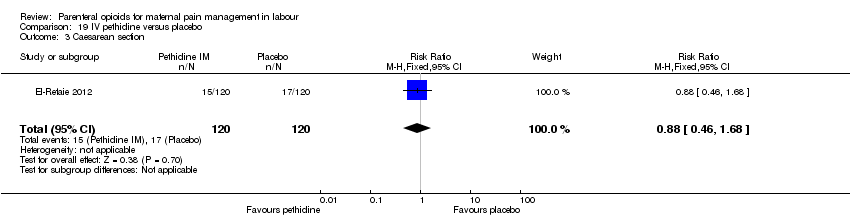 Comparison 19 IV pethidine versus placebo, Outcome 3 Caesarean section. | ||||
| 4 Assisted vaginal birth Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.33, 1.71] |
| Analysis 19.4  Comparison 19 IV pethidine versus placebo, Outcome 4 Assisted vaginal birth. | ||||
| 5 Admission to NICU Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.92] |
| Analysis 19.5  Comparison 19 IV pethidine versus placebo, Outcome 5 Admission to NICU. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia) Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐5.47, ‐4.53] |
| Analysis 20.1  Comparison 20 IV fentanyl versus no treatment, Outcome 1 Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia). | ||||
| 2 Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour) Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.25] |
| Analysis 20.2  Comparison 20 IV fentanyl versus no treatment, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour). | ||||
| 3 Caesarean section Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.43] |
| Analysis 20.3  Comparison 20 IV fentanyl versus no treatment, Outcome 3 Caesarean section. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.18, 0.78] |
| Analysis 21.1  Comparison 21 IV fentanyl versus IV pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration). | ||||
| 2 Mean doses of analgesia (non pre‐specified) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| Analysis 21.2  Comparison 21 IV fentanyl versus IV pethidine, Outcome 2 Mean doses of analgesia (non pre‐specified). | ||||
| 3 Maternal sleepiness in labour (sedation) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.82] |
| Analysis 21.3 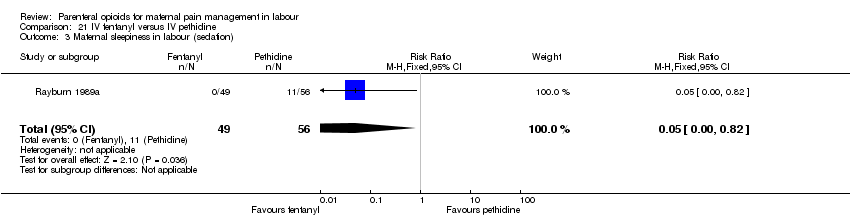 Comparison 21 IV fentanyl versus IV pethidine, Outcome 3 Maternal sleepiness in labour (sedation). | ||||
| 4 Nausea and/or vomiting Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.17, 1.55] |
| Analysis 21.4 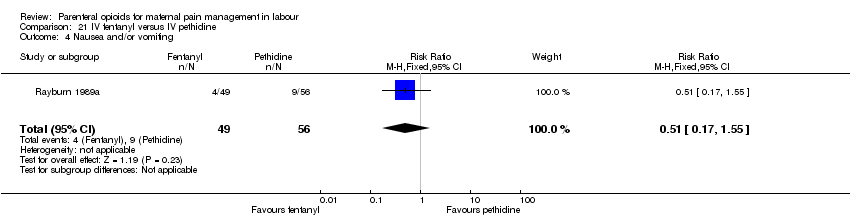 Comparison 21 IV fentanyl versus IV pethidine, Outcome 4 Nausea and/or vomiting. | ||||
| 5 Anti‐emetic required (non pre‐specified) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.52] |
| Analysis 21.5  Comparison 21 IV fentanyl versus IV pethidine, Outcome 5 Anti‐emetic required (non pre‐specified). | ||||
| 6 Caesarean section Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.24, 5.40] |
| Analysis 21.6 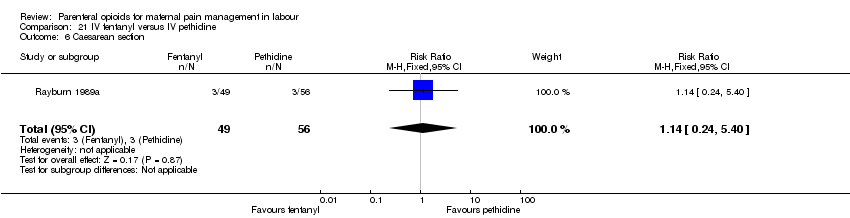 Comparison 21 IV fentanyl versus IV pethidine, Outcome 6 Caesarean section. | ||||
| 7 Naloxone administered Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| Analysis 21.7  Comparison 21 IV fentanyl versus IV pethidine, Outcome 7 Naloxone administered. | ||||
| 8 Babies requiring resuscitation/ventilatory support Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.32] |
| Analysis 21.8  Comparison 21 IV fentanyl versus IV pethidine, Outcome 8 Babies requiring resuscitation/ventilatory support. | ||||
| 9 Apgar score < 7 at 1 minute Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 21.9  Comparison 21 IV fentanyl versus IV pethidine, Outcome 9 Apgar score < 7 at 1 minute. | ||||
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 21.10  Comparison 21 IV fentanyl versus IV pethidine, Outcome 10 Apgar score < 7 at 5 minutes. | ||||
| 11 Neurobehavioural score (1 ‐ 2 hours after delivery) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.15, 2.45] |
| Analysis 21.11 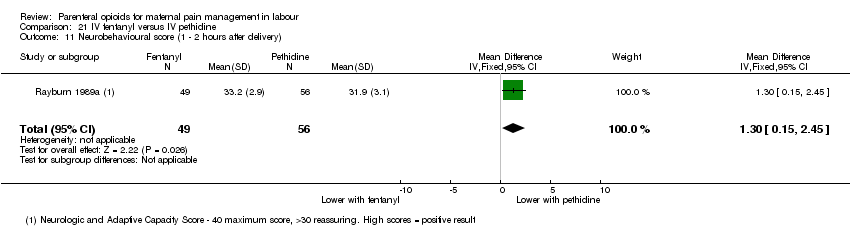 Comparison 21 IV fentanyl versus IV pethidine, Outcome 11 Neurobehavioural score (1 ‐ 2 hours after delivery). | ||||
| 12 Neurobehavioural score (2 hours ‐ 24 hours) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.42, 2.22] |
| Analysis 21.12  Comparison 21 IV fentanyl versus IV pethidine, Outcome 12 Neurobehavioural score (2 hours ‐ 24 hours). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 95.61] |
| Analysis 22.1 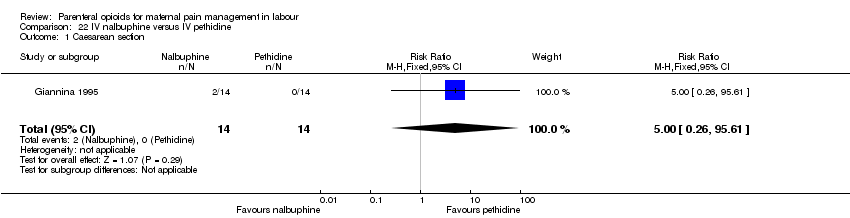 Comparison 22 IV nalbuphine versus IV pethidine, Outcome 1 Caesarean section. | ||||
| 2 Apgar score < 7 at 1 minute Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 22.2  Comparison 22 IV nalbuphine versus IV pethidine, Outcome 2 Apgar score < 7 at 1 minute. | ||||
| 3 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 22.3  Comparison 22 IV nalbuphine versus IV pethidine, Outcome 3 Apgar score < 7 at 5 minutes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (women with fair or poor relief) Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.10] |
| Analysis 23.1  Comparison 23 IV phenazocine versus IV pethidine, Outcome 1 Maternal satisfaction with analgesia measured during labour (women with fair or poor relief). | ||||
| 2 Nausea with vomiting Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 2.01] |
| Analysis 23.2  Comparison 23 IV phenazocine versus IV pethidine, Outcome 2 Nausea with vomiting. | ||||
| 3 Perinatal death Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 23.3  Comparison 23 IV phenazocine versus IV pethidine, Outcome 3 Perinatal death. | ||||
| 4 Apgar score < 7 at 1 minute Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 23.4  Comparison 23 IV phenazocine versus IV pethidine, Outcome 4 Apgar score < 7 at 1 minute. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain relief score) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.25, 1.09] |
| Analysis 24.1 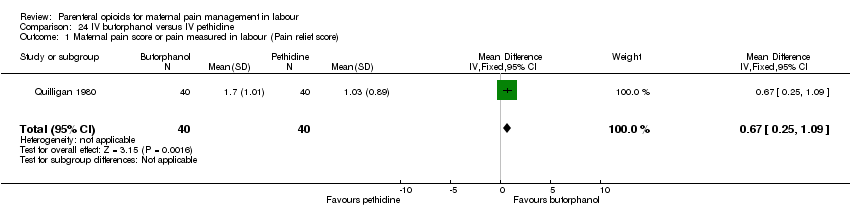 Comparison 24 IV butorphanol versus IV pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain relief score). | ||||
| 2 Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.02, ‐0.18] |
| Analysis 24.2  Comparison 24 IV butorphanol versus IV pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)). | ||||
| 3 Additional analgesia required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.63, 1.45] |
| Analysis 24.3  Comparison 24 IV butorphanol versus IV pethidine, Outcome 3 Additional analgesia required. | ||||
| 4 Epidural Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.35] |
| Analysis 24.4  Comparison 24 IV butorphanol versus IV pethidine, Outcome 4 Epidural. | ||||
| 5 Nausea and/or vomiting Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| Analysis 24.5  Comparison 24 IV butorphanol versus IV pethidine, Outcome 5 Nausea and/or vomiting. | ||||
| 6 Caesarean section Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.89] |
| Analysis 24.6 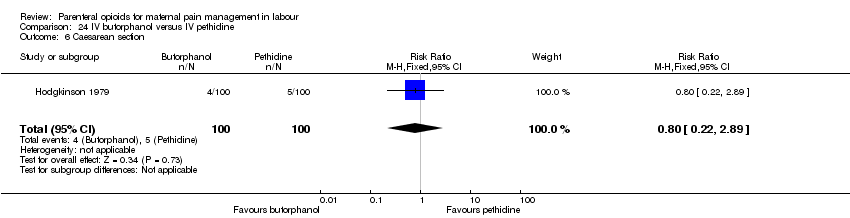 Comparison 24 IV butorphanol versus IV pethidine, Outcome 6 Caesarean section. | ||||
| 7 Assisted vaginal birth Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.83] |
| Analysis 24.7  Comparison 24 IV butorphanol versus IV pethidine, Outcome 7 Assisted vaginal birth. | ||||
| 8 Apgar score < 7 at 1 minute Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 24.8 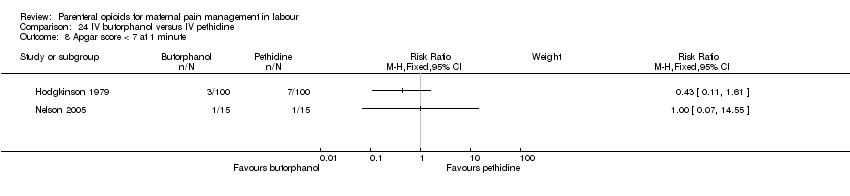 Comparison 24 IV butorphanol versus IV pethidine, Outcome 8 Apgar score < 7 at 1 minute. | ||||
| 9 Apgar score < 7 at 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 24.9  Comparison 24 IV butorphanol versus IV pethidine, Outcome 9 Apgar score < 7 at 5 minutes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (assessed 3 days postpartum) Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.98] |
| Analysis 25.1 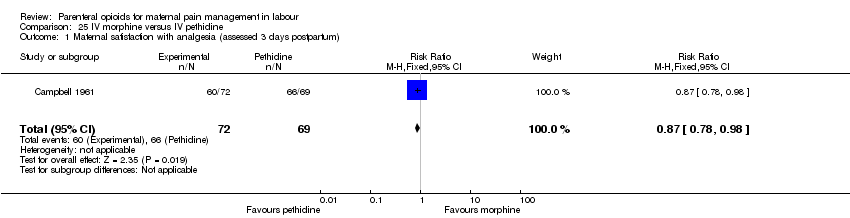 Comparison 25 IV morphine versus IV pethidine, Outcome 1 Maternal satisfaction with analgesia (assessed 3 days postpartum). | ||||
| 2 Additional analgesia required Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.90, 6.12] |
| Analysis 25.2  Comparison 25 IV morphine versus IV pethidine, Outcome 2 Additional analgesia required. | ||||
| 3 Nausea and vomiting Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 25.3  Comparison 25 IV morphine versus IV pethidine, Outcome 3 Nausea and vomiting. | ||||
| 3.1 Nausea | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.14] |
| 3.2 Vomiting | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 4 Caesarean section Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 25.4  Comparison 25 IV morphine versus IV pethidine, Outcome 4 Caesarean section. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea and vomiting Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 26.1  Comparison 26 IV Nisentil versus IV pethidine, Outcome 1 Nausea and vomiting. | ||||
| 1.1 Nausea | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 1.2 Vomiting | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.66] |
| 2 Neonatal resuscitation/ventilatory support Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.85, 4.63] |
| Analysis 26.2  Comparison 26 IV Nisentil versus IV pethidine, Outcome 2 Neonatal resuscitation/ventilatory support. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.05, 1.85] |
| Analysis 27.1  Comparison 27 IV fentanyl versus IV butorphanol, Outcome 1 Additional analgesia required. | ||||
| 2 Epidural Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.00, 4.02] |
| Analysis 27.2  Comparison 27 IV fentanyl versus IV butorphanol, Outcome 2 Epidural. | ||||
| 3 Matenal sleepiness (required tactile rousing) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.64, 14.16] |
| Analysis 27.3  Comparison 27 IV fentanyl versus IV butorphanol, Outcome 3 Matenal sleepiness (required tactile rousing). | ||||
| 4 Caesarean section Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.81] |
| Analysis 27.4  Comparison 27 IV fentanyl versus IV butorphanol, Outcome 4 Caesarean section. | ||||
| 5 Naloxone required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.81, 3.80] |
| Analysis 27.5 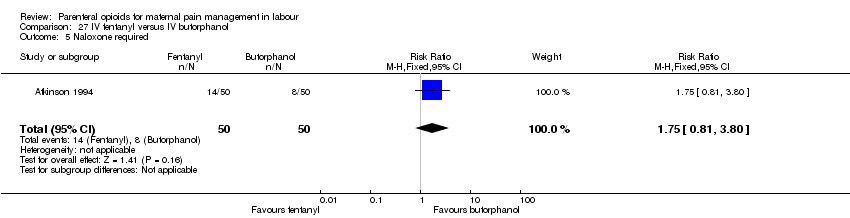 Comparison 27 IV fentanyl versus IV butorphanol, Outcome 5 Naloxone required. | ||||
| 6 Neonatal resuscitation (Babies requiring ventilatory support) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.62, 193.80] |
| Analysis 27.6 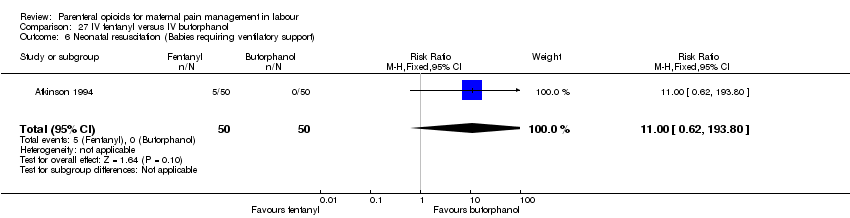 Comparison 27 IV fentanyl versus IV butorphanol, Outcome 6 Neonatal resuscitation (Babies requiring ventilatory support). | ||||
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.39, 3.68] |
| Analysis 27.7  Comparison 27 IV fentanyl versus IV butorphanol, Outcome 7 Apgar score < 7 at 5 minutes. | ||||
| 8 Newborn neurobehavioural score at 2‐4 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.61, 1.61] |
| Analysis 27.8 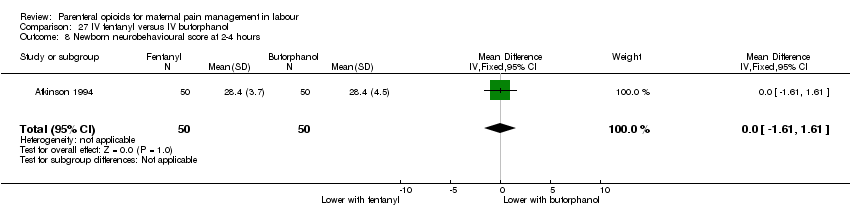 Comparison 27 IV fentanyl versus IV butorphanol, Outcome 8 Newborn neurobehavioural score at 2‐4 hours. | ||||
| 9 Newborn neurobehavioural score at 24‐36 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.62, 0.62] |
| Analysis 27.9  Comparison 27 IV fentanyl versus IV butorphanol, Outcome 9 Newborn neurobehavioural score at 24‐36 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pan score or pain measured in labour Show forest plot | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.96, 0.06] |
| Analysis 28.1 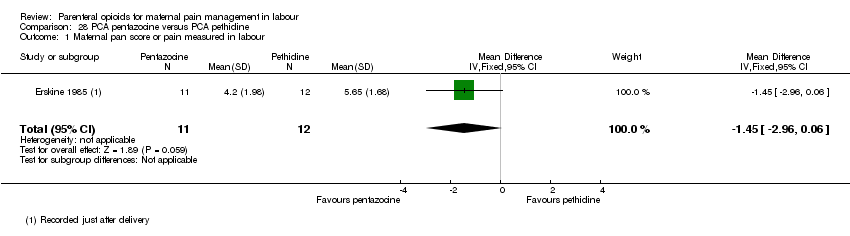 Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 1 Maternal pan score or pain measured in labour. | ||||
| 2 Maternal pan score or pain measured in labour (rated as good one day after birth) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.51, 1.32] |
| Analysis 28.2  Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 2 Maternal pan score or pain measured in labour (rated as good one day after birth). | ||||
| 3 Epidural Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.65] |
| Analysis 28.3  Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 3 Epidural. | ||||
| 4 Nausea and vomiting Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| Analysis 28.4  Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 4 Nausea and vomiting. | ||||
| 5 Maternal sleepiness during labour (Sedation) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.09] |
| Analysis 28.5 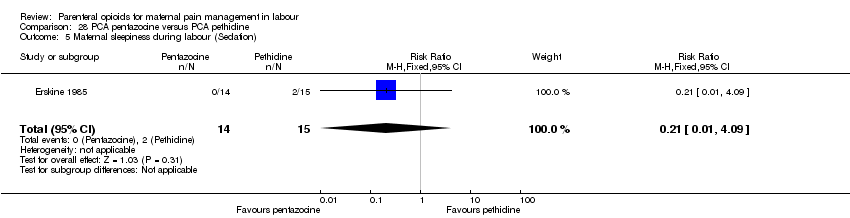 Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 5 Maternal sleepiness during labour (Sedation). | ||||
| 6 Caesarean section Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.07] |
| Analysis 28.6  Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 6 Caesarean section. | ||||
| 7 Breastfeeding at discharge Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.85, 1.17] |
| Analysis 28.7 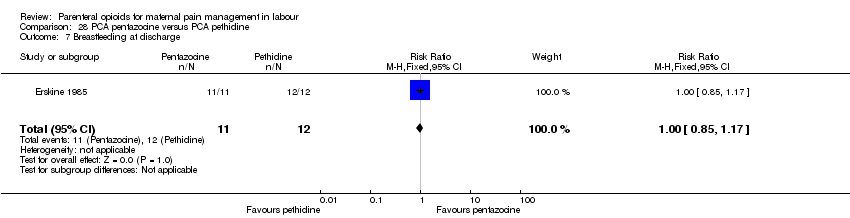 Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 7 Breastfeeding at discharge. | ||||
| 8 Apgar score < 7 at 5 minutes Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 28.8  Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 8 Apgar score < 7 at 5 minutes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score in labour Show forest plot | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐8.59 [‐27.61, 10.44] |
| Analysis 29.1  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 1 Maternal pain score in labour. | ||||
| 2 Additional analgesia required Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| Analysis 29.2  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 2 Additional analgesia required. | ||||
| 3 Epidural Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| Analysis 29.3  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 3 Epidural. | ||||
| 4 Maternal sleepiness during labour Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| Analysis 29.4  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 4 Maternal sleepiness during labour. | ||||
| 5 Nausea and vomiting Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.49] |
| Analysis 29.5  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 5 Nausea and vomiting. | ||||
| 6 Caesarean section Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.60, 5.46] |
| Analysis 29.6 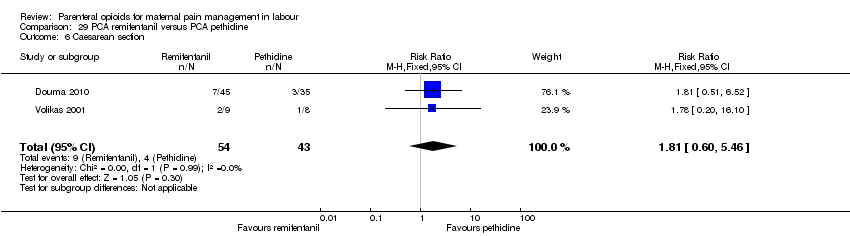 Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 6 Caesarean section. | ||||
| 7 Assisted vaginal birth Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.46, 2.00] |
| Analysis 29.7  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 7 Assisted vaginal birth. | ||||
| 8 Satisfaction with childbirth experience Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.46, 1.74] |
| Analysis 29.8  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 8 Satisfaction with childbirth experience. | ||||
| 9 Naloxone administered Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| Analysis 29.9 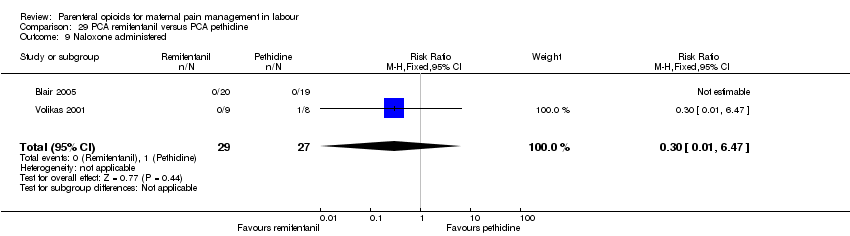 Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 9 Naloxone administered. | ||||
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.16] |
| Analysis 29.10  Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 10 Apgar score < 7 at 5 minutes. | ||||
| 11 Admission to NICU Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| Analysis 29.11 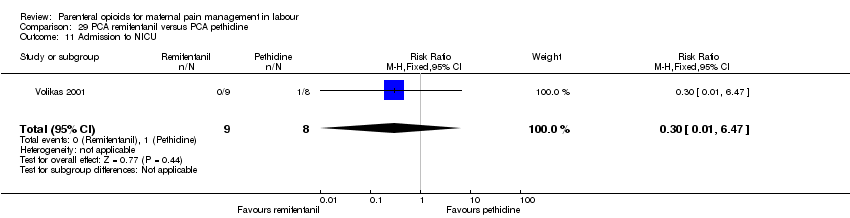 Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 11 Admission to NICU. | ||||
| 12 Newborn neurobehavioural score (15 minutes post delivery) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.93, 1.33] |
| Analysis 29.12 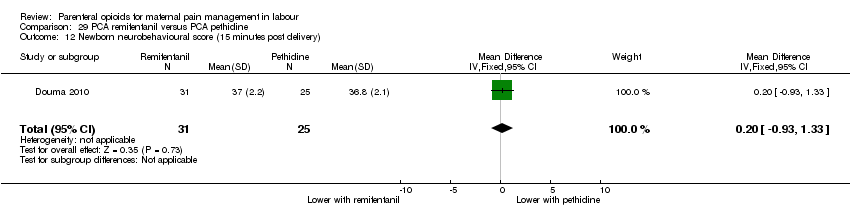 Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 12 Newborn neurobehavioural score (15 minutes post delivery). | ||||
| 13 Newborn neurobehavioural score (2 hours post delivery) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.66, 1.86] |
| Analysis 29.13 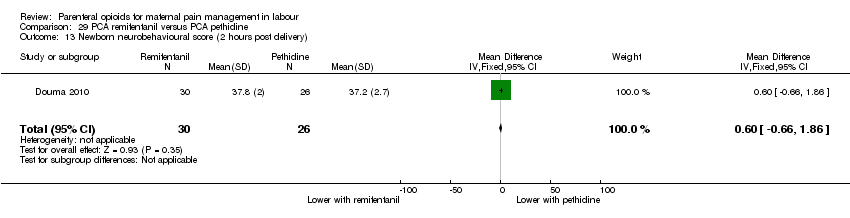 Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 13 Newborn neurobehavioural score (2 hours post delivery). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.88, 1.89] |
| Analysis 30.1 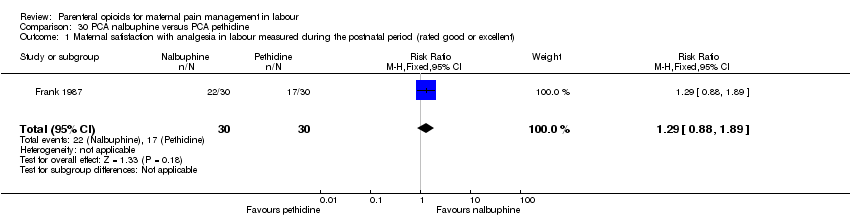 Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent). | ||||
| 2 Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again) Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| Analysis 30.2 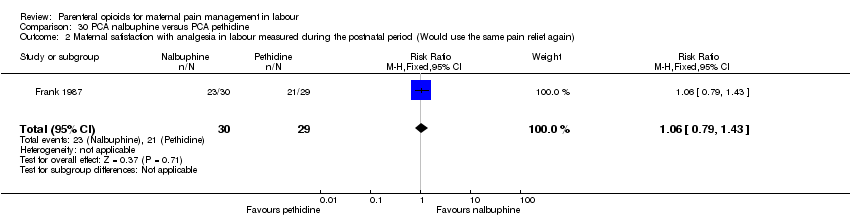 Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 2 Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again). | ||||
| 3 Maternal pain score or pain measured in labour Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.79, ‐0.01] |
| Analysis 30.3  Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 3 Maternal pain score or pain measured in labour. | ||||
| 4 Additional analgesia required Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.48] |
| Analysis 30.4 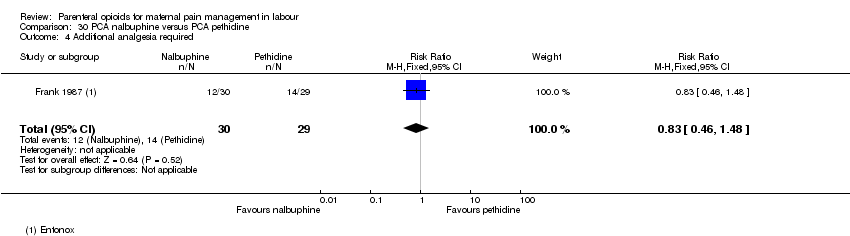 Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 4 Additional analgesia required. | ||||
| 5 Nausea and vomiting Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.30, 1.54] |
| Analysis 30.5 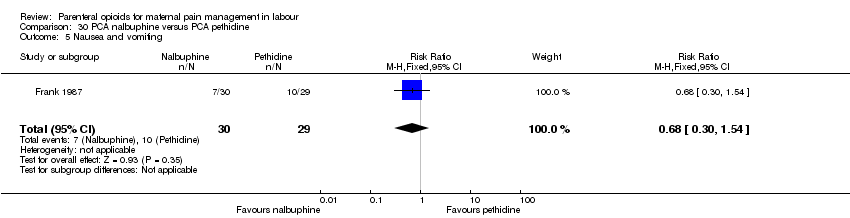 Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 5 Nausea and vomiting. | ||||
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.76] |
| Analysis 30.6 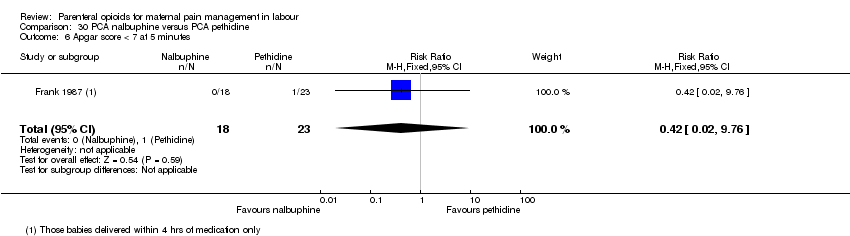 Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 6 Apgar score < 7 at 5 minutes. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (described as adequate) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.93, 2.60] |
| Analysis 31.1  Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (described as adequate). | ||||
| 2 Maternal pain score or pain measured in labour (Pain score at 4‐6 cm cervical dilatation) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐12.80 [‐32.12, 6.52] |
| Analysis 31.2  Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 2 Maternal pain score or pain measured in labour (Pain score at 4‐6 cm cervical dilatation). | ||||
| 3 Nausea Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.66, 11.30] |
| Analysis 31.3 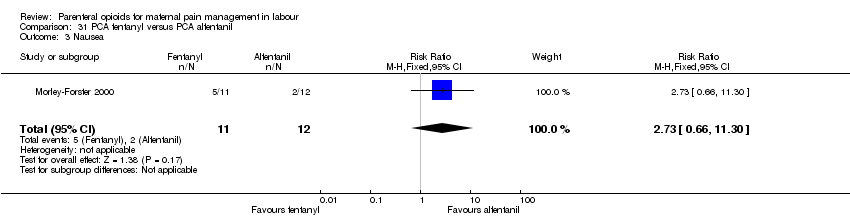 Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 3 Nausea. | ||||
| 4 Caesarean section Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.33, 8.03] |
| Analysis 31.4  Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 4 Caesarean section. | ||||
| 5 Naloxone required Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.53, 10.55] |
| Analysis 31.5 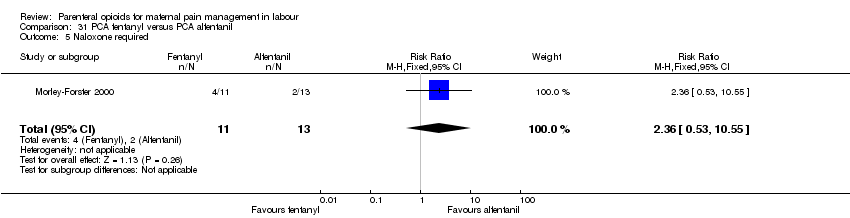 Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 5 Naloxone required. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternla pain score measured in labour Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.56, 0.26] |
| Analysis 32.1 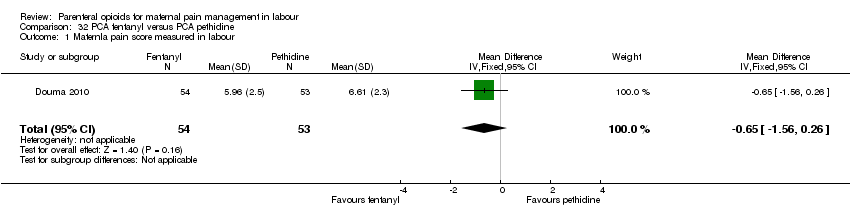 Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 1 Maternla pain score measured in labour. | ||||
| 2 Epidural Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.92] |
| Analysis 32.2 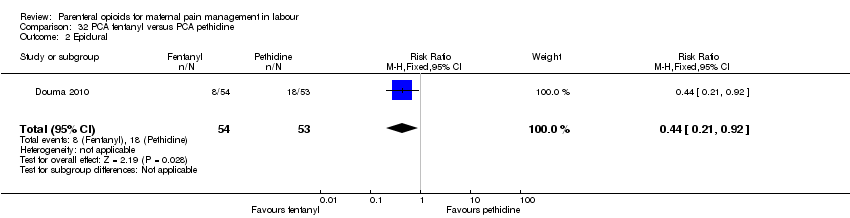 Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 2 Epidural. | ||||
| 3 Maternal sleepiness during labour Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.25, 0.13] |
| Analysis 32.3  Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 3 Maternal sleepiness during labour. | ||||
| 4 Nausea and vomiting Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| Analysis 32.4  Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 4 Nausea and vomiting. | ||||
| 5 Caesarean section Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.34] |
| Analysis 32.5 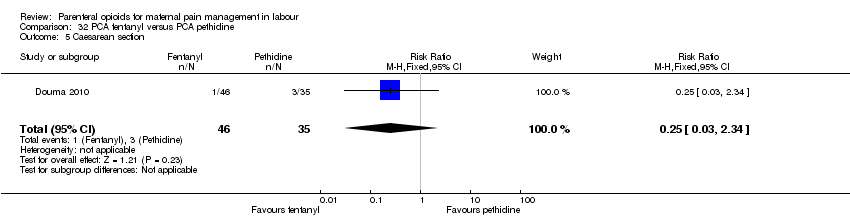 Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 5 Caesarean section. | ||||
| 6 Assisted vaginal birth Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.49] |
| Analysis 32.6  Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 6 Assisted vaginal birth. | ||||
| 7 Newborn neurobehavioural score (15 minutes post delivery) Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.31, 0.51] |
| Analysis 32.7 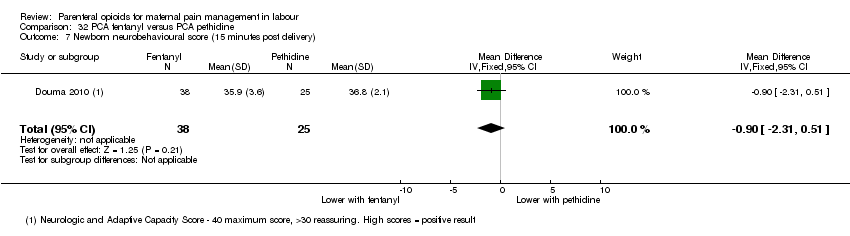 Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 7 Newborn neurobehavioural score (15 minutes post delivery). | ||||
| 8 Newborn neurobehavioural score (2 hours post delivery) Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.95, 0.95] |
| Analysis 32.8  Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 8 Newborn neurobehavioural score (2 hours post delivery). | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (measured 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐17.60 [‐49.93, 14.73] |
| Analysis 33.1  Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 1 Maternal pain score or pain measured in labour (measured 1 day after delivery). | ||||
| 2 Satisfied with mode of administration (PCA IM) (non pre‐specified) Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| Analysis 33.2 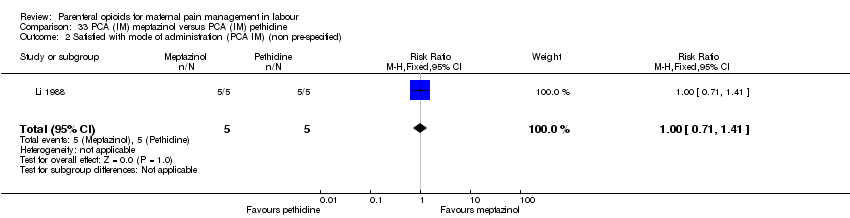 Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 2 Satisfied with mode of administration (PCA IM) (non pre‐specified). | ||||
| 3 Epidural Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.15, 59.89] |
| Analysis 33.3  Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 3 Epidural. | ||||
| 4 Maternal sleepiness in labour (Drowsiness score in labour rated 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐28.19, 39.39] |
| Analysis 33.4 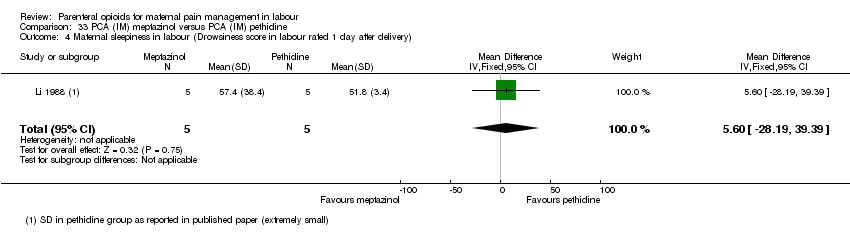 Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 4 Maternal sleepiness in labour (Drowsiness score in labour rated 1 day after delivery). | ||||
| 5 Nausea (score in labour rated 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐48.70, 32.70] |
| Analysis 33.5 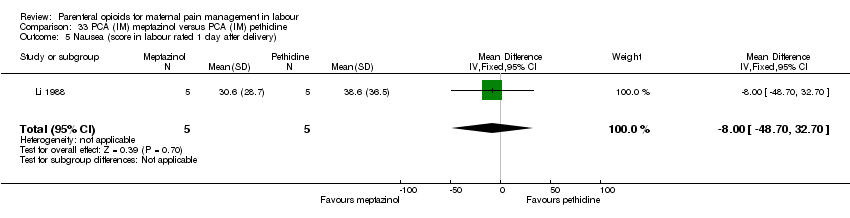 Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 5 Nausea (score in labour rated 1 day after delivery). | ||||
| 6 Naloxone administered Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.08, 11.93] |
| Analysis 33.6  Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 6 Naloxone administered. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured post delivery (rated as good) Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.79, 1.92] |
| Analysis 34.1 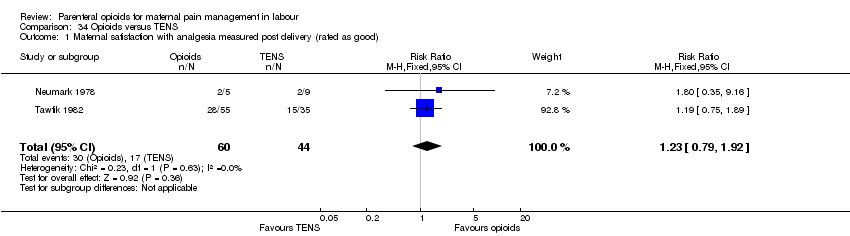 Comparison 34 Opioids versus TENS, Outcome 1 Maternal satisfaction with analgesia measured post delivery (rated as good). | ||||
| 2 Maternal pain score measured during labour Show forest plot | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.81, 1.61] |
| Analysis 34.2  Comparison 34 Opioids versus TENS, Outcome 2 Maternal pain score measured during labour. | ||||
| 3 Maternal pain score in labour Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 34.3  Comparison 34 Opioids versus TENS, Outcome 3 Maternal pain score in labour. | ||||
| 3.1 Pain score (after 30 minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐26.09, ‐13.91] |
| 3.2 Pain score (after 60 minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐25.16, ‐14.84] |
| 4 Maternal sleepiness during labour (Drowsiness) Show forest plot | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.96 [1.13, 71.07] |
| Analysis 34.4 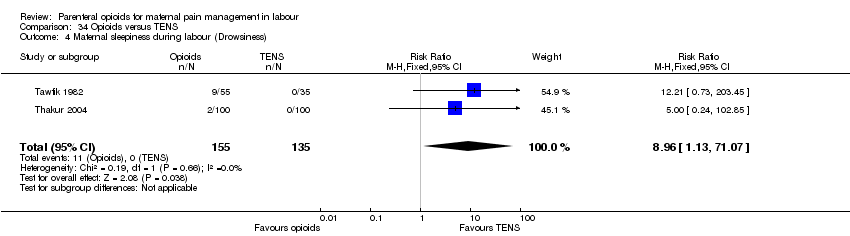 Comparison 34 Opioids versus TENS, Outcome 4 Maternal sleepiness during labour (Drowsiness). | ||||
| 5 Nausea and vomiting Show forest plot | 3 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.73 [2.72, 69.24] |
| Analysis 34.5  Comparison 34 Opioids versus TENS, Outcome 5 Nausea and vomiting. | ||||
| 6 Caesarean section Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| Analysis 34.6  Comparison 34 Opioids versus TENS, Outcome 6 Caesarean section. | ||||
| 7 Assisted vaginal birth Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.40, 8.18] |
| Analysis 34.7 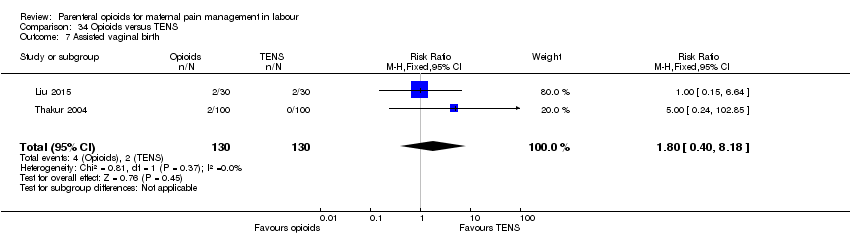 Comparison 34 Opioids versus TENS, Outcome 7 Assisted vaginal birth. | ||||
| 8 Fetal heart rate changes in labour (Fetal distress) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
| Analysis 34.8  Comparison 34 Opioids versus TENS, Outcome 8 Fetal heart rate changes in labour (Fetal distress). | ||||
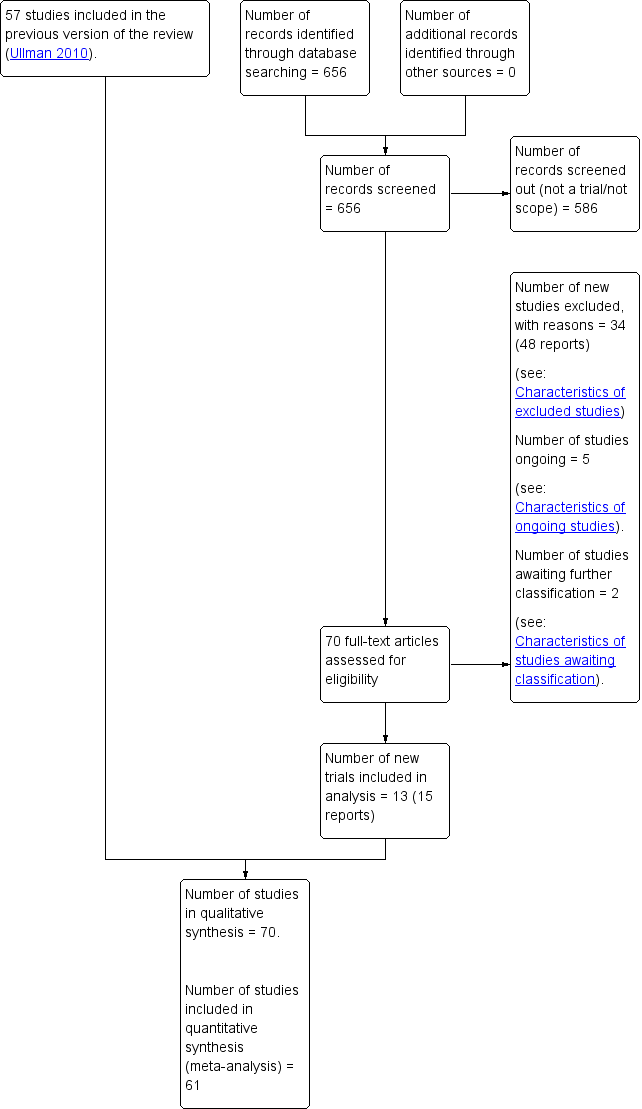
Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 1 Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes).

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 2 Maternal pain score or pain measured in labour (described as good or fair after 1 hour).
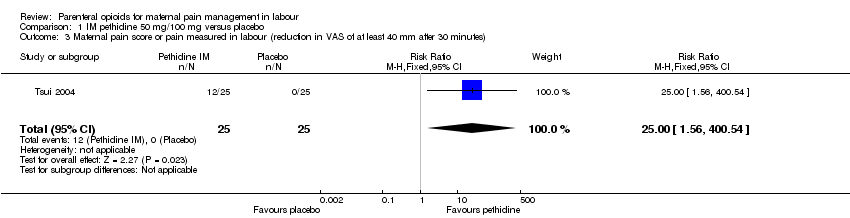
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 3 Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes).

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 4 Additional analgesia required.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 5 Epidural.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 6 Nausea and vomiting.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 7 Maternal sleepiness.
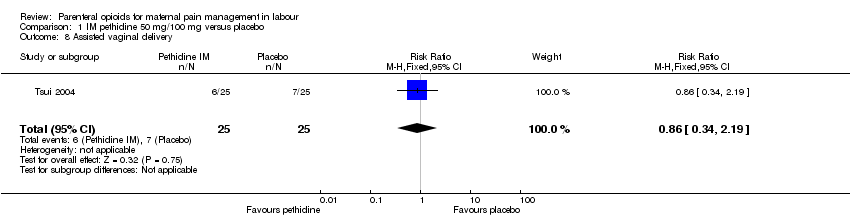
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 8 Assisted vaginal delivery.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 9 Caesarean section.
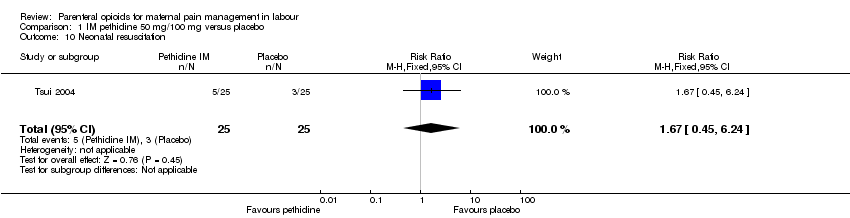
Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 10 Neonatal resuscitation.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 11 Low Apgar score (≤ 7) at 1 and 5 minutes.

Comparison 1 IM pethidine 50 mg/100 mg versus placebo, Outcome 12 Admission to NICU.

Comparison 2 IM pentazocine versus placebo, Outcome 1 Maternal pain score measured during labour.

Comparison 2 IM pentazocine versus placebo, Outcome 2 Nausea and vomiting.

Comparison 2 IM pentazocine versus placebo, Outcome 3 Caesarean section.

Comparison 2 IM pentazocine versus placebo, Outcome 4 Assisted vaginal birth.
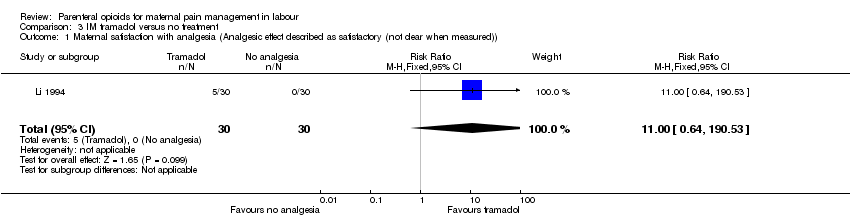
Comparison 3 IM tramadol versus no treatment, Outcome 1 Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)).

Comparison 4 IM meptazinol versus pethidine, Outcome 1 Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)).

Comparison 4 IM meptazinol versus pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)).

Comparison 4 IM meptazinol versus pethidine, Outcome 3 Additional analgesia required.

Comparison 4 IM meptazinol versus pethidine, Outcome 4 Epidural.

Comparison 4 IM meptazinol versus pethidine, Outcome 5 Maternal sleepiness.
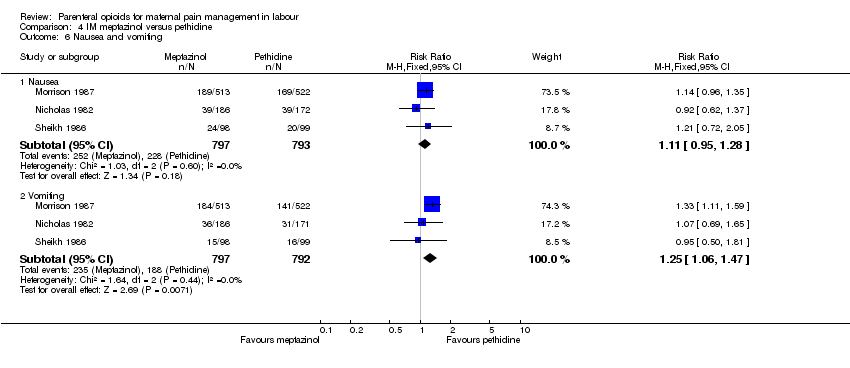
Comparison 4 IM meptazinol versus pethidine, Outcome 6 Nausea and vomiting.
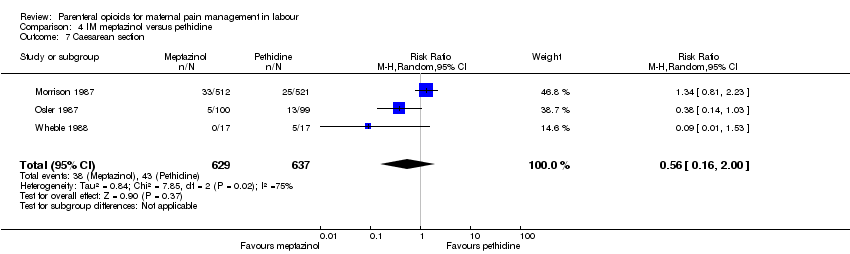
Comparison 4 IM meptazinol versus pethidine, Outcome 7 Caesarean section.
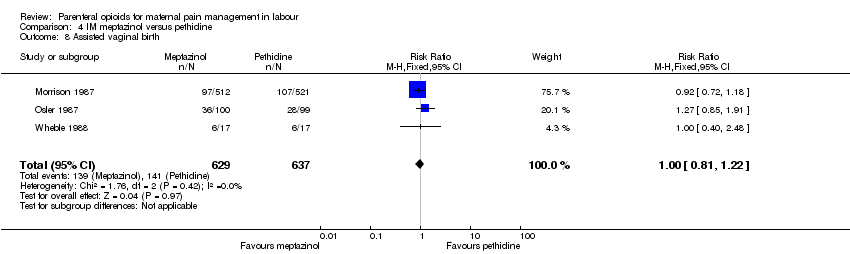
Comparison 4 IM meptazinol versus pethidine, Outcome 8 Assisted vaginal birth.
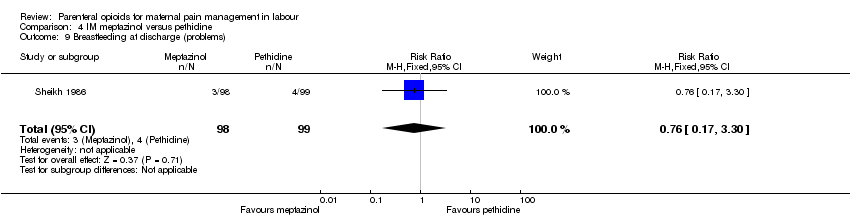
Comparison 4 IM meptazinol versus pethidine, Outcome 9 Breastfeeding at discharge (problems).

Comparison 4 IM meptazinol versus pethidine, Outcome 10 Fetal heart rate changes (decelerations).

Comparison 4 IM meptazinol versus pethidine, Outcome 11 Naloxone administration.

Comparison 4 IM meptazinol versus pethidine, Outcome 12 Neonatal resuscitation (by gestation).

Comparison 4 IM meptazinol versus pethidine, Outcome 13 Neonatal resuscitation.

Comparison 4 IM meptazinol versus pethidine, Outcome 14 Apgar score ≤ 7 at 1 minute.

Comparison 4 IM meptazinol versus pethidine, Outcome 15 Apgar score ≤ 7 at 5 minutes.

Comparison 4 IM meptazinol versus pethidine, Outcome 16 Admission to NICU.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours).

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)).

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 3 Additonal analgesia required.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 4 Epidural.
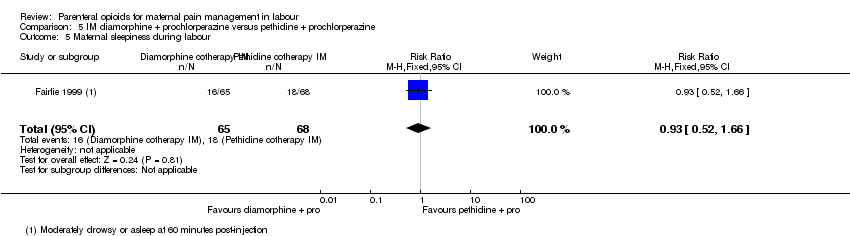
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 5 Maternal sleepiness during labour.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 6 Vomiting in labour.
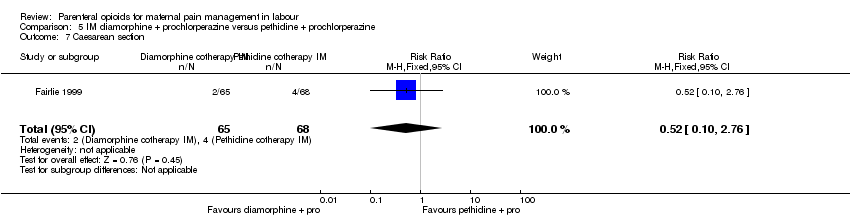
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 7 Caesarean section.
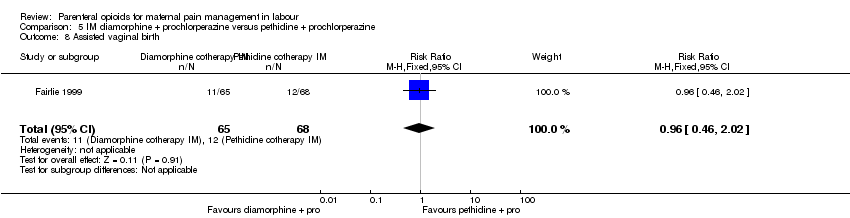
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 8 Assisted vaginal birth.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 9 Neonatal resuscitation.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 10 Apgar < 7 at 1 minute.
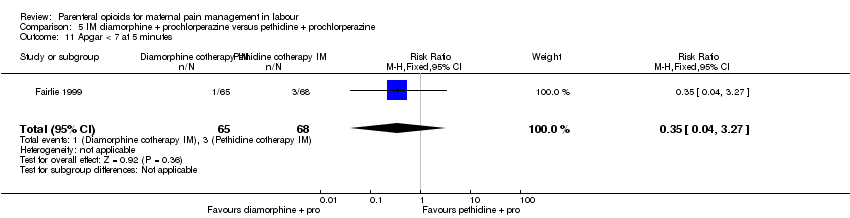
Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 11 Apgar < 7 at 5 minutes.

Comparison 5 IM diamorphine + prochlorperazine versus pethidine + prochlorperazine, Outcome 12 Admission to NICU.
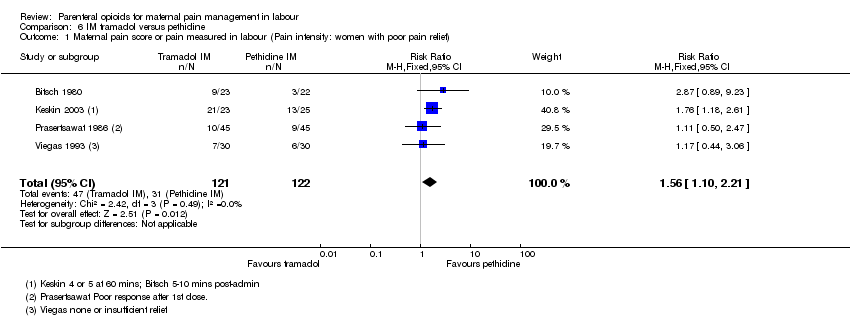
Comparison 6 IM tramadol versus pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief).
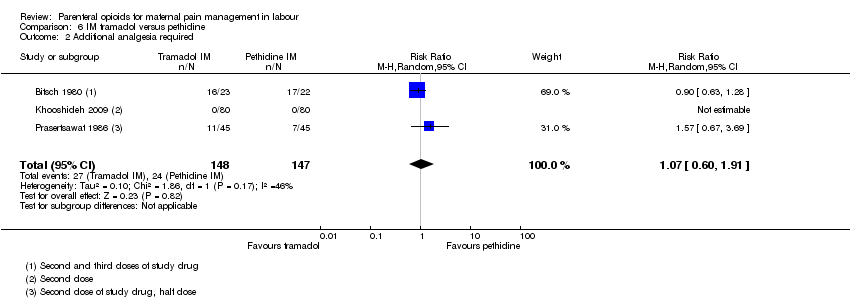
Comparison 6 IM tramadol versus pethidine, Outcome 2 Additional analgesia required.
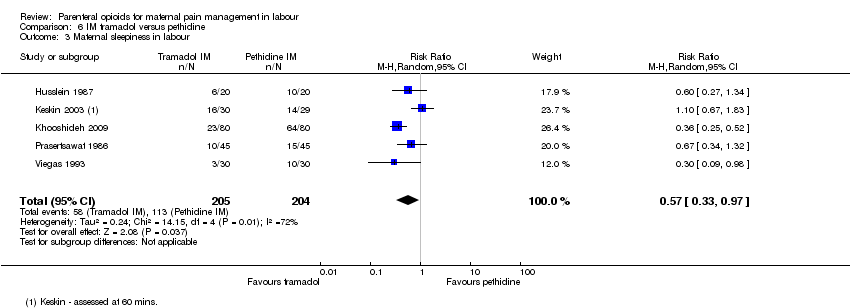
Comparison 6 IM tramadol versus pethidine, Outcome 3 Maternal sleepiness in labour.

Comparison 6 IM tramadol versus pethidine, Outcome 4 Nausea and vomiting in labour.
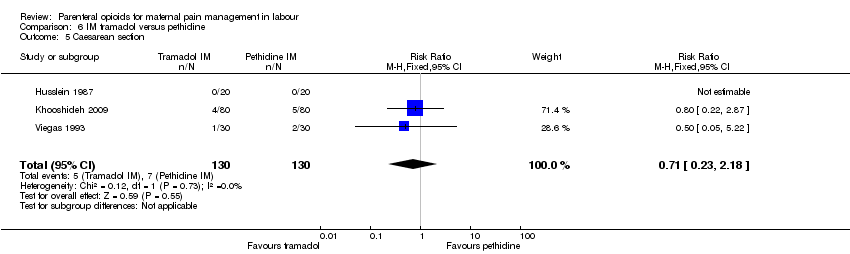
Comparison 6 IM tramadol versus pethidine, Outcome 5 Caesarean section.

Comparison 6 IM tramadol versus pethidine, Outcome 6 Assisted vaginal birth.
Comparison 6 IM tramadol versus pethidine, Outcome 7 Neonatal resuscitation.

Comparison 6 IM tramadol versus pethidine, Outcome 8 Apgar scores ≤ 7 at 1 and 5 minutes.

Comparison 6 IM tramadol versus pethidine, Outcome 9 Neonatal respiratory distress.
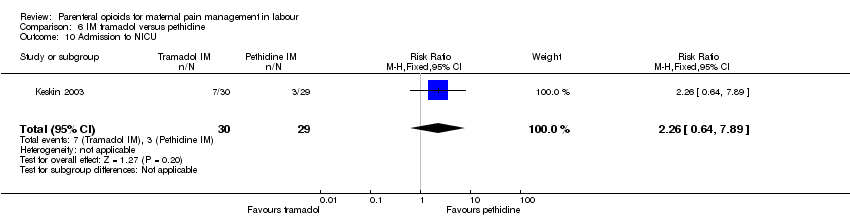
Comparison 6 IM tramadol versus pethidine, Outcome 10 Admission to NICU.

Comparison 7 IM tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 1 Maternal sleepiness in labour.

Comparison 7 IM tramadol + triflupromazine versus pethidine + triflupromazine, Outcome 2 Nausea and vomiting in labour.

Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 1 Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour).
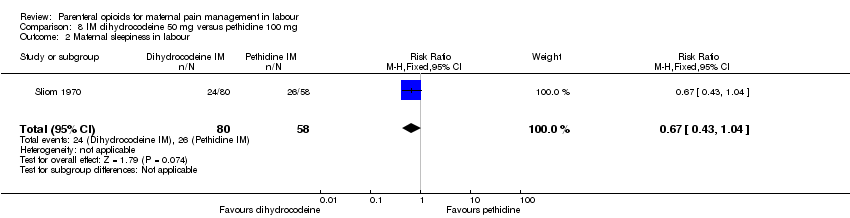
Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 2 Maternal sleepiness in labour.
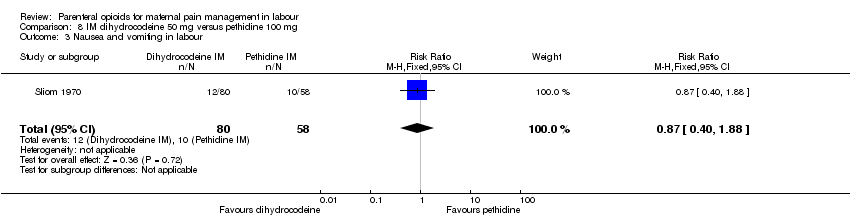
Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 3 Nausea and vomiting in labour.
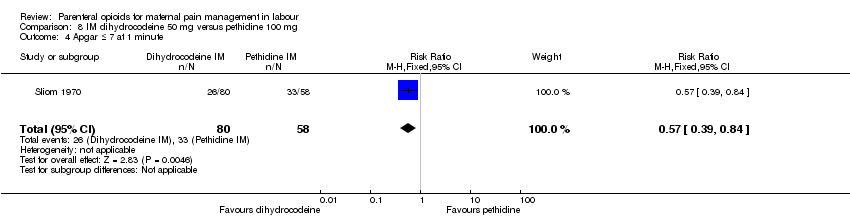
Comparison 8 IM dihydrocodeine 50 mg versus pethidine 100 mg, Outcome 4 Apgar ≤ 7 at 1 minute.

Comparison 9 IM pentazocine versus pethidine, Outcome 1 Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery).
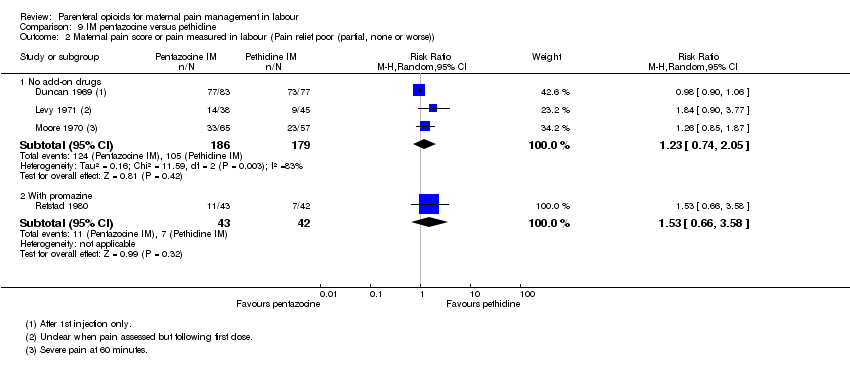
Comparison 9 IM pentazocine versus pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)).

Comparison 9 IM pentazocine versus pethidine, Outcome 3 Additional analgesia required.
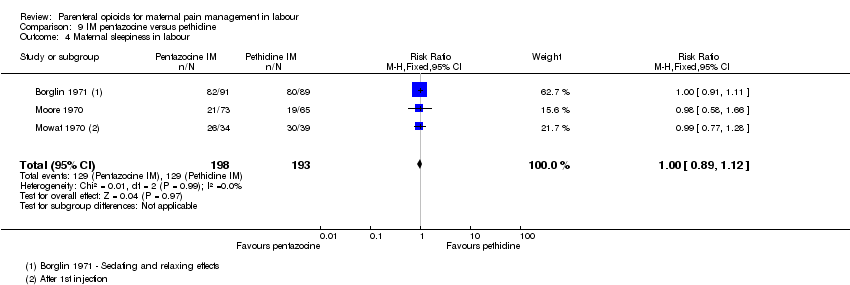
Comparison 9 IM pentazocine versus pethidine, Outcome 4 Maternal sleepiness in labour.
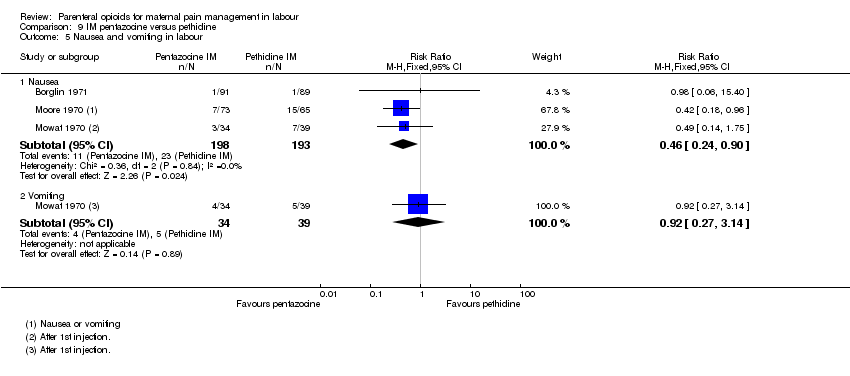
Comparison 9 IM pentazocine versus pethidine, Outcome 5 Nausea and vomiting in labour.

Comparison 9 IM pentazocine versus pethidine, Outcome 6 Assisted vaginal birth.
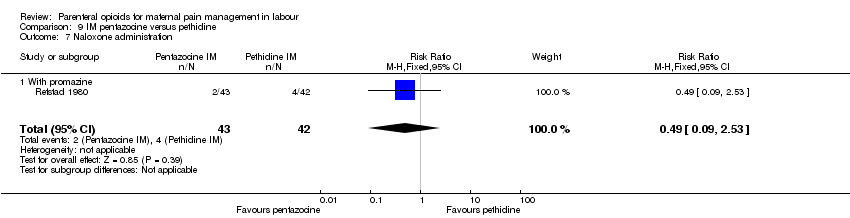
Comparison 9 IM pentazocine versus pethidine, Outcome 7 Naloxone administration.

Comparison 9 IM pentazocine versus pethidine, Outcome 8 Apgar score ≤ 7 at 1 minute.
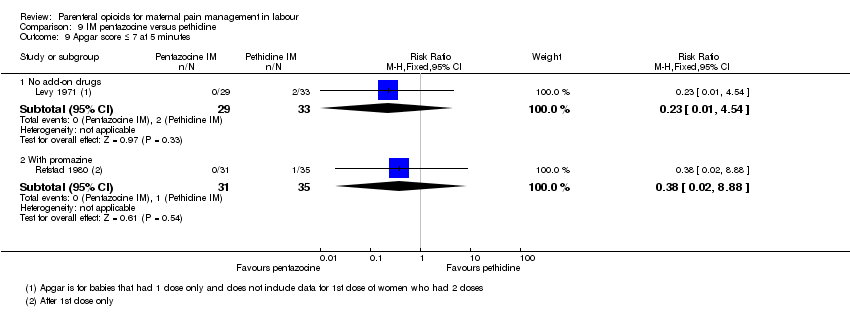
Comparison 9 IM pentazocine versus pethidine, Outcome 9 Apgar score ≤ 7 at 5 minutes.

Comparison 10 IM nalbuphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied).

Comparison 10 IM nalbuphine versus pethidine, Outcome 2 Maternal satisfaction with analgesia measured during labour (Pain free).

Comparison 10 IM nalbuphine versus pethidine, Outcome 3 Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain).
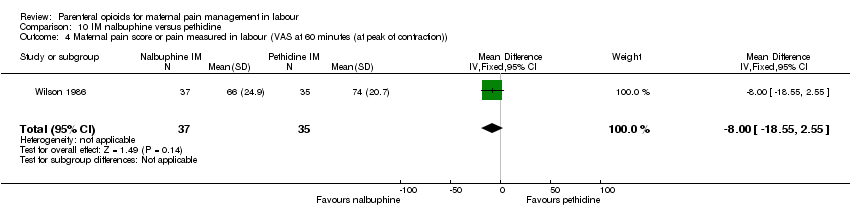
Comparison 10 IM nalbuphine versus pethidine, Outcome 4 Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)).
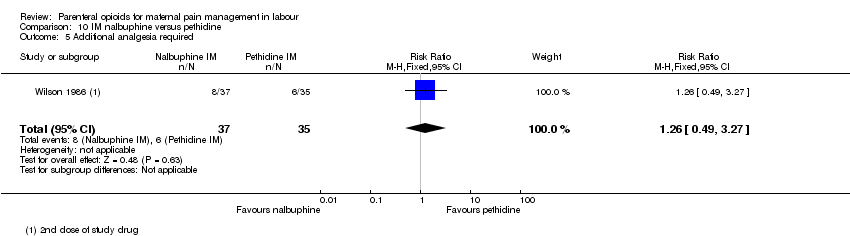
Comparison 10 IM nalbuphine versus pethidine, Outcome 5 Additional analgesia required.
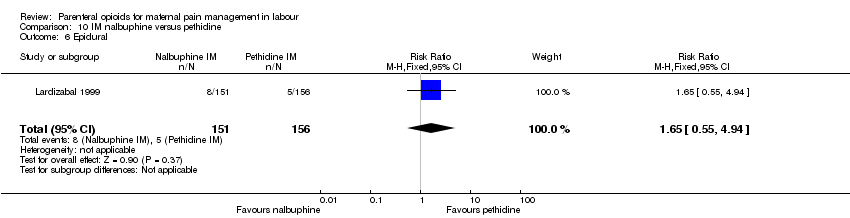
Comparison 10 IM nalbuphine versus pethidine, Outcome 6 Epidural.

Comparison 10 IM nalbuphine versus pethidine, Outcome 7 Maternal sleepiness in labour.
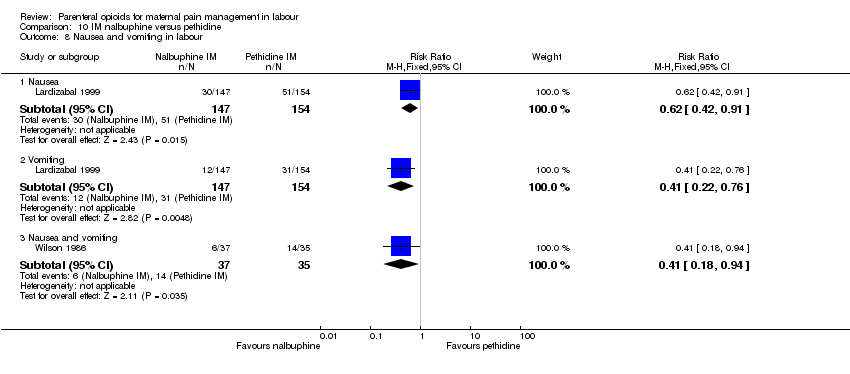
Comparison 10 IM nalbuphine versus pethidine, Outcome 8 Nausea and vomiting in labour.
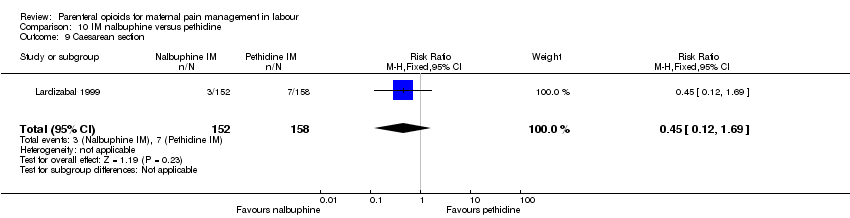
Comparison 10 IM nalbuphine versus pethidine, Outcome 9 Caesarean section.
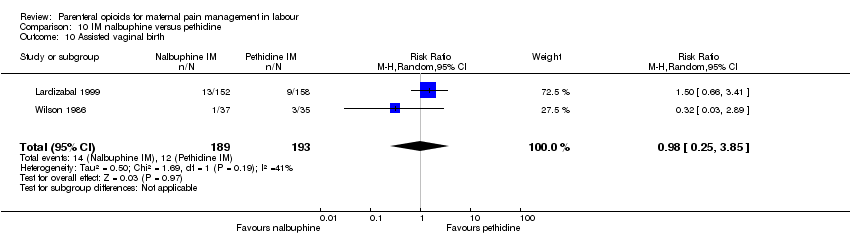
Comparison 10 IM nalbuphine versus pethidine, Outcome 10 Assisted vaginal birth.

Comparison 10 IM nalbuphine versus pethidine, Outcome 11 Naloxone administration.

Comparison 10 IM nalbuphine versus pethidine, Outcome 12 Apgar score ≤ 7 at 1 and 5 minutes.
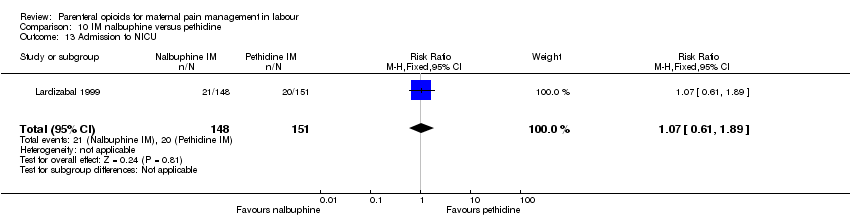
Comparison 10 IM nalbuphine versus pethidine, Outcome 13 Admission to NICU.

Comparison 10 IM nalbuphine versus pethidine, Outcome 14 Neonatal neurobehavioural (Scanlon) 2‐4 hours PN.

Comparison 11 IM phenazocine versus pethidine, Outcome 1 Epidural.

Comparison 11 IM phenazocine versus pethidine, Outcome 2 Vomiting.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 1 Maternal satisfaction with analgesia (number of women satisfied or very satisfied).

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 2 Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor).

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 3 Maternal pain score or pain measured in labour (pain relief at 30 mins).

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 4 Maternal pain score or pain measured in labour (pain relief at 60 mins).
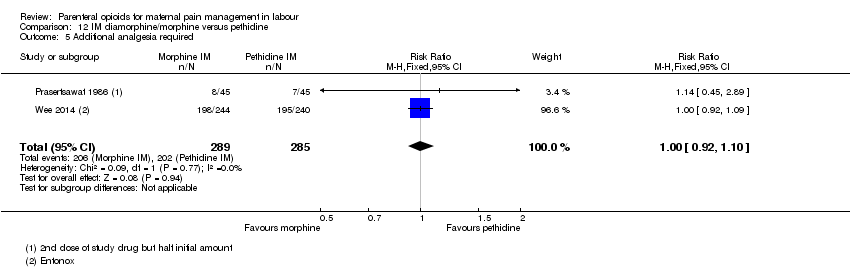
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 5 Additional analgesia required.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 6 Maternal sleepiness.
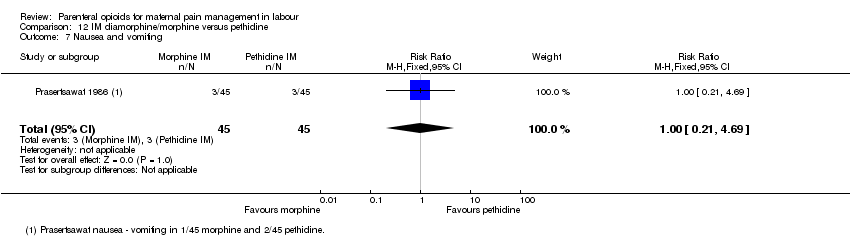
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 7 Nausea and vomiting.
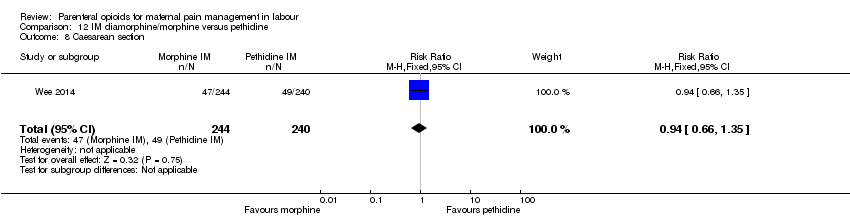
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 8 Caesarean section.
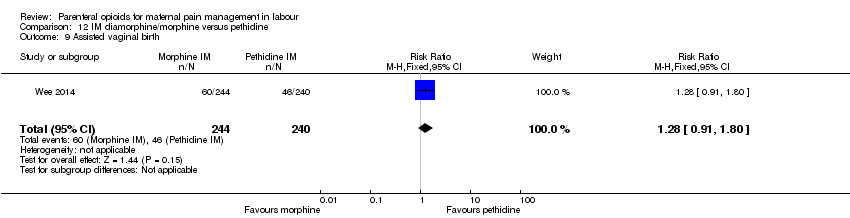
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 9 Assisted vaginal birth.
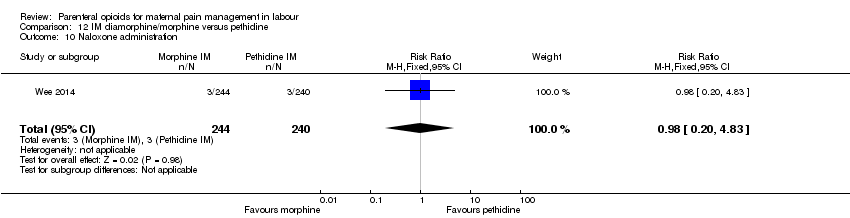
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 10 Naloxone administration.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 11 Neonatal resuscitation.
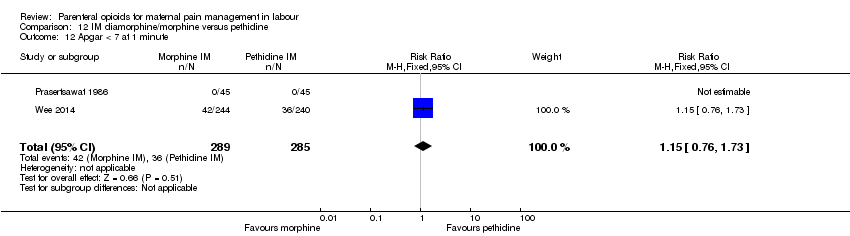
Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 12 Apgar < 7 at 1 minute.

Comparison 12 IM diamorphine/morphine versus pethidine, Outcome 13 Admission to NICU.
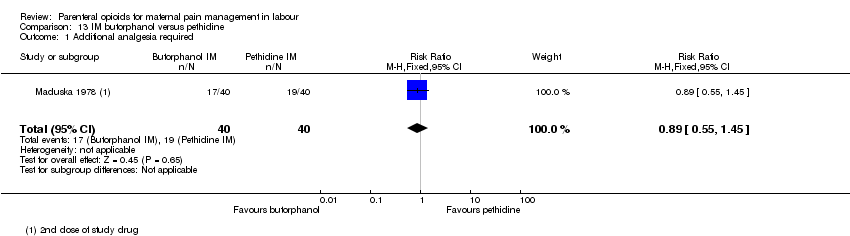
Comparison 13 IM butorphanol versus pethidine, Outcome 1 Additional analgesia required.

Comparison 13 IM butorphanol versus pethidine, Outcome 2 Nausea.
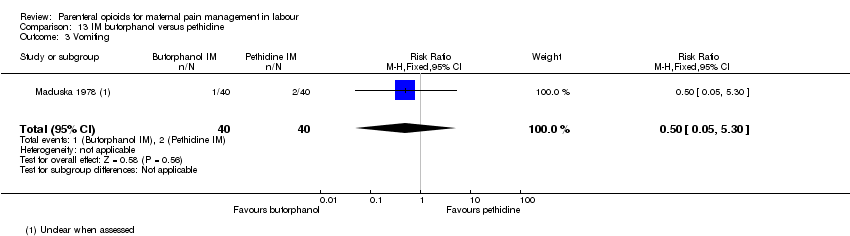
Comparison 13 IM butorphanol versus pethidine, Outcome 3 Vomiting.

Comparison 13 IM butorphanol versus pethidine, Outcome 4 Neonatal resuscitation.
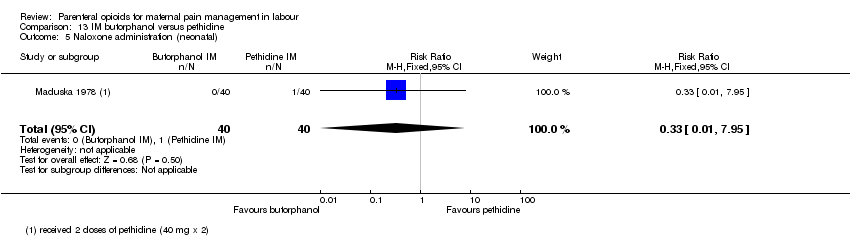
Comparison 13 IM butorphanol versus pethidine, Outcome 5 Naloxone administration (neonatal).

Comparison 14 IM Avacan® versus IM pentazocine, Outcome 1 Additional analgesia required ‐ Entonox.

Comparison 14 IM Avacan® versus IM pentazocine, Outcome 2 Additional analgesia required ‐ pudendal‐paracervical block.
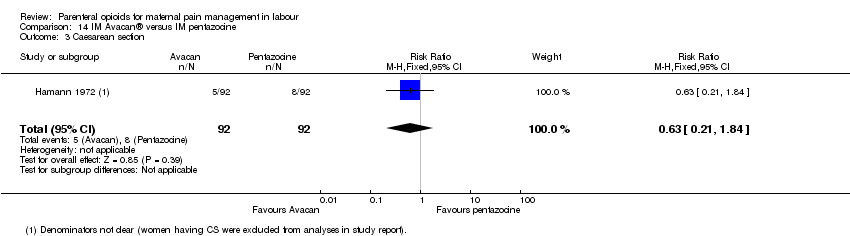
Comparison 14 IM Avacan® versus IM pentazocine, Outcome 3 Caesarean section.

Comparison 14 IM Avacan® versus IM pentazocine, Outcome 4 Low Apgar score (< 7) "at birth".
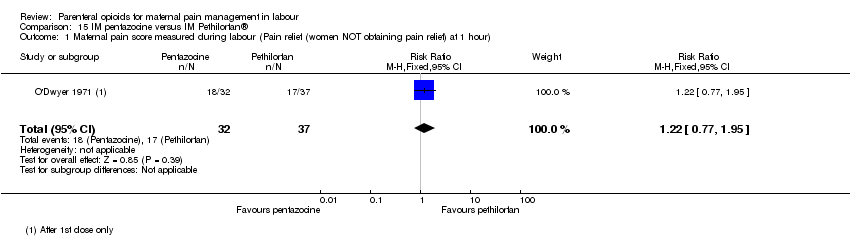
Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 1 Maternal pain score measured during labour (Pain relief (women NOT obtaining pain relief) at 1 hour).

Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 2 Additional analgesia required.

Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 3 Assisted vaginal birth.
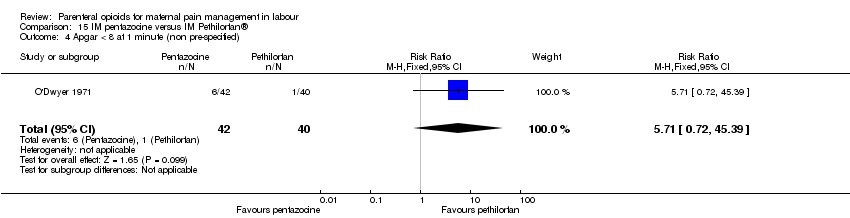
Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 4 Apgar < 8 at 1 minute (non pre‐specified).
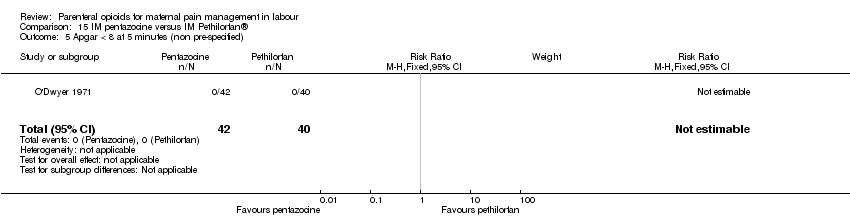
Comparison 15 IM pentazocine versus IM Pethilorfan®, Outcome 5 Apgar < 8 at 5 minutes (non pre‐specified).

Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 1 Maternal pain score measured during labour.

Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 2 Nausea and vomiting.

Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 3 Caesarean section.
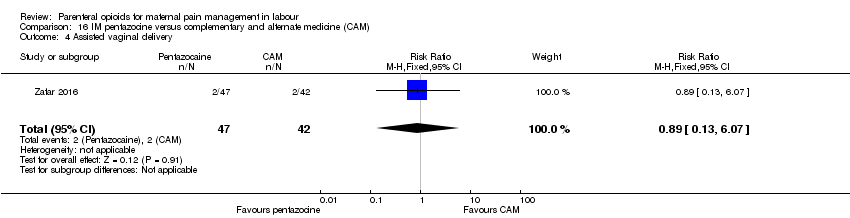
Comparison 16 IM pentazocine versus complementary and alternate medicine (CAM), Outcome 4 Assisted vaginal delivery.

Comparison 17 IM pentazocine versus IM tramadol, Outcome 1 Maternal satisfaction with analgesia measured during labour (pain relief after 30 mins).

Comparison 17 IM pentazocine versus IM tramadol, Outcome 2 Maternal satisfaction with analgesia measured during labour (pain after 60 mins).

Comparison 17 IM pentazocine versus IM tramadol, Outcome 3 Maternal pain score or pain measured in labour (moderate or severe at 30 mins).

Comparison 17 IM pentazocine versus IM tramadol, Outcome 4 Maternal pain score or pain measured in labour (moderate or severe at 60 mins).

Comparison 17 IM pentazocine versus IM tramadol, Outcome 5 Maternal sleepiness during labour.
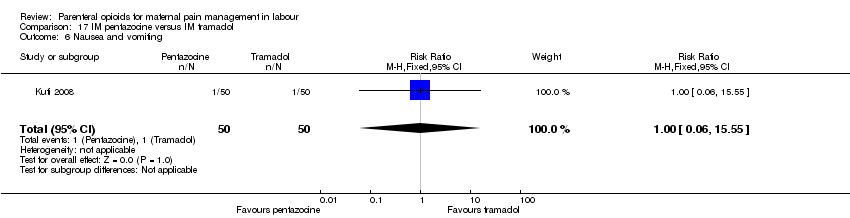
Comparison 17 IM pentazocine versus IM tramadol, Outcome 6 Nausea and vomiting.
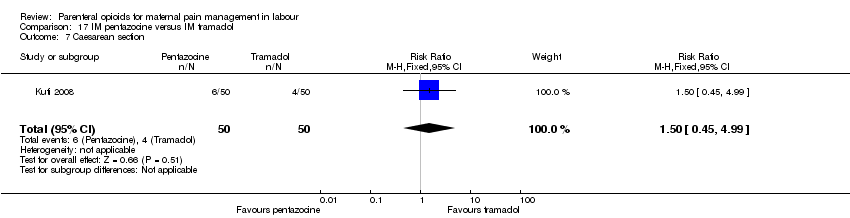
Comparison 17 IM pentazocine versus IM tramadol, Outcome 7 Caesarean section.
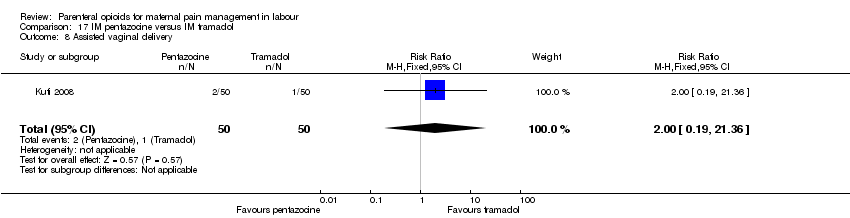
Comparison 17 IM pentazocine versus IM tramadol, Outcome 8 Assisted vaginal delivery.

Comparison 17 IM pentazocine versus IM tramadol, Outcome 9 Apgar score < 7 at 1 minute.

Comparison 17 IM pentazocine versus IM tramadol, Outcome 10 Apgar score < 7 at 5 minutes.
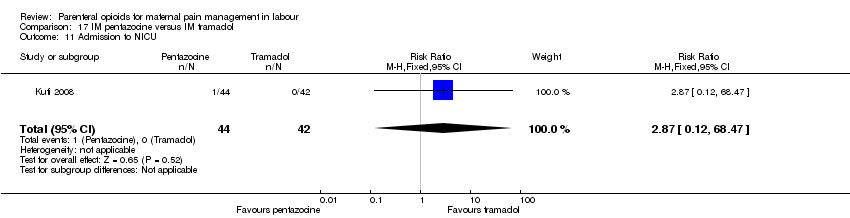
Comparison 17 IM pentazocine versus IM tramadol, Outcome 11 Admission to NICU.

Comparison 18 IM pethidine versus Entonox, Outcome 1 Maternal pain score or pain measured in labour (after 30 mins).
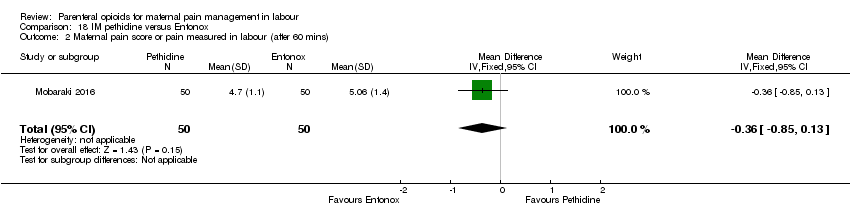
Comparison 18 IM pethidine versus Entonox, Outcome 2 Maternal pain score or pain measured in labour (after 60 mins).

Comparison 19 IV pethidine versus placebo, Outcome 1 Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia).
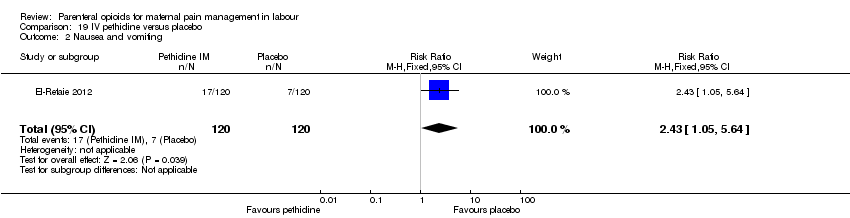
Comparison 19 IV pethidine versus placebo, Outcome 2 Nausea and vomiting.

Comparison 19 IV pethidine versus placebo, Outcome 3 Caesarean section.

Comparison 19 IV pethidine versus placebo, Outcome 4 Assisted vaginal birth.
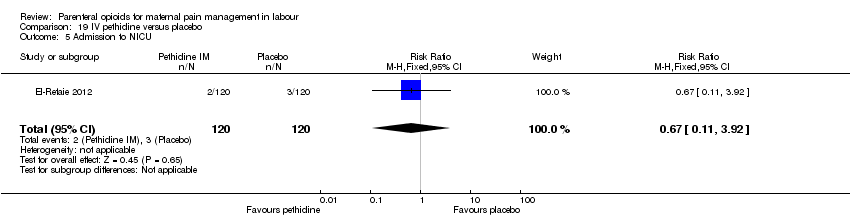
Comparison 19 IV pethidine versus placebo, Outcome 5 Admission to NICU.

Comparison 20 IV fentanyl versus no treatment, Outcome 1 Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia).

Comparison 20 IV fentanyl versus no treatment, Outcome 2 Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour).
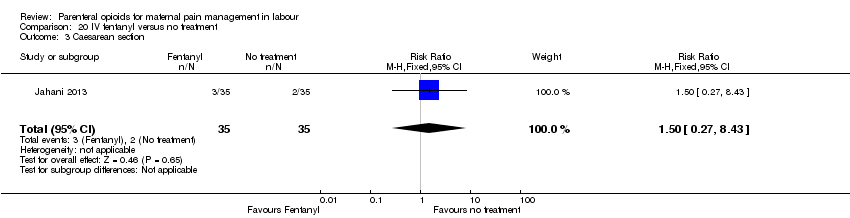
Comparison 20 IV fentanyl versus no treatment, Outcome 3 Caesarean section.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration).
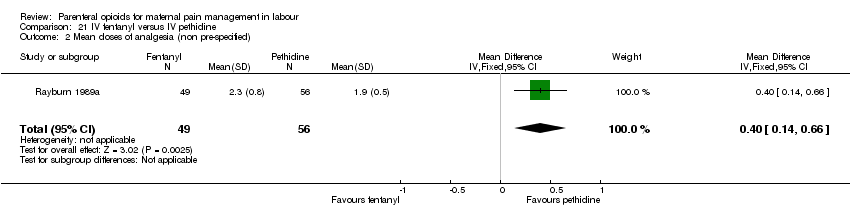
Comparison 21 IV fentanyl versus IV pethidine, Outcome 2 Mean doses of analgesia (non pre‐specified).
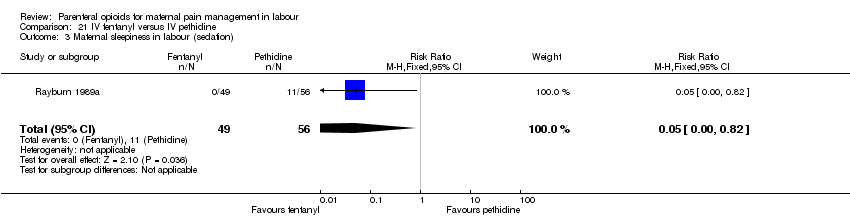
Comparison 21 IV fentanyl versus IV pethidine, Outcome 3 Maternal sleepiness in labour (sedation).
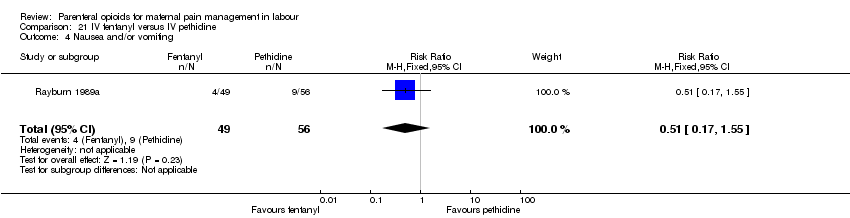
Comparison 21 IV fentanyl versus IV pethidine, Outcome 4 Nausea and/or vomiting.
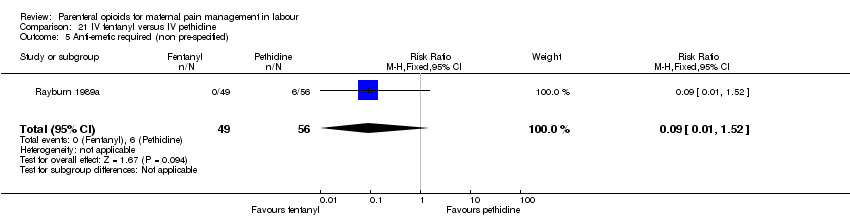
Comparison 21 IV fentanyl versus IV pethidine, Outcome 5 Anti‐emetic required (non pre‐specified).

Comparison 21 IV fentanyl versus IV pethidine, Outcome 6 Caesarean section.
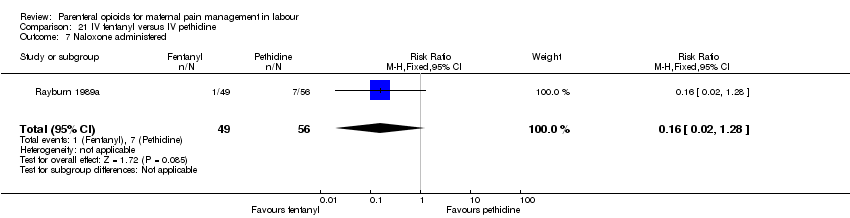
Comparison 21 IV fentanyl versus IV pethidine, Outcome 7 Naloxone administered.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 8 Babies requiring resuscitation/ventilatory support.
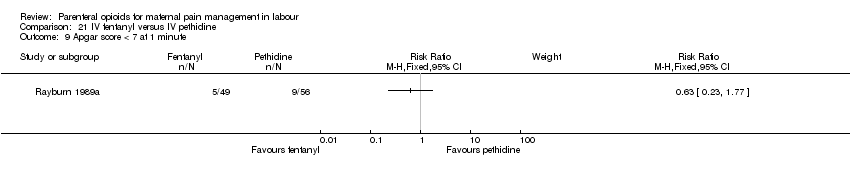
Comparison 21 IV fentanyl versus IV pethidine, Outcome 9 Apgar score < 7 at 1 minute.

Comparison 21 IV fentanyl versus IV pethidine, Outcome 10 Apgar score < 7 at 5 minutes.
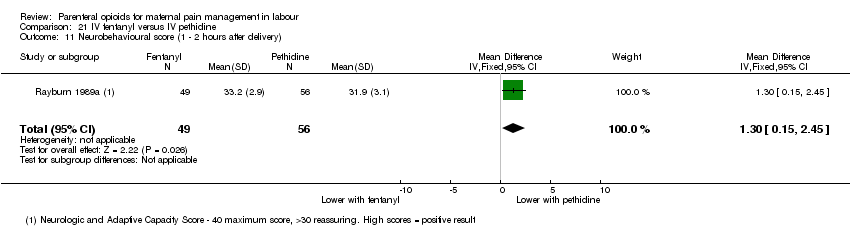
Comparison 21 IV fentanyl versus IV pethidine, Outcome 11 Neurobehavioural score (1 ‐ 2 hours after delivery).

Comparison 21 IV fentanyl versus IV pethidine, Outcome 12 Neurobehavioural score (2 hours ‐ 24 hours).
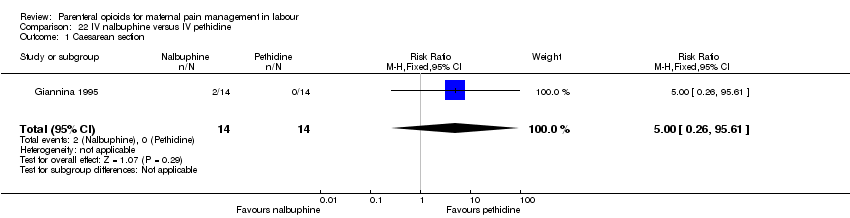
Comparison 22 IV nalbuphine versus IV pethidine, Outcome 1 Caesarean section.
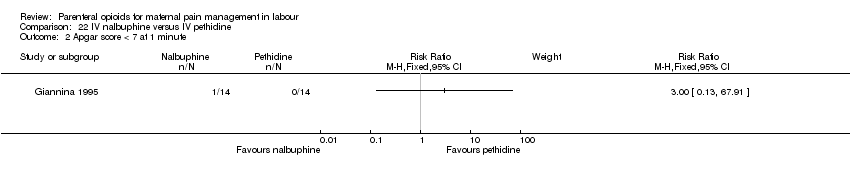
Comparison 22 IV nalbuphine versus IV pethidine, Outcome 2 Apgar score < 7 at 1 minute.

Comparison 22 IV nalbuphine versus IV pethidine, Outcome 3 Apgar score < 7 at 5 minutes.

Comparison 23 IV phenazocine versus IV pethidine, Outcome 1 Maternal satisfaction with analgesia measured during labour (women with fair or poor relief).

Comparison 23 IV phenazocine versus IV pethidine, Outcome 2 Nausea with vomiting.

Comparison 23 IV phenazocine versus IV pethidine, Outcome 3 Perinatal death.
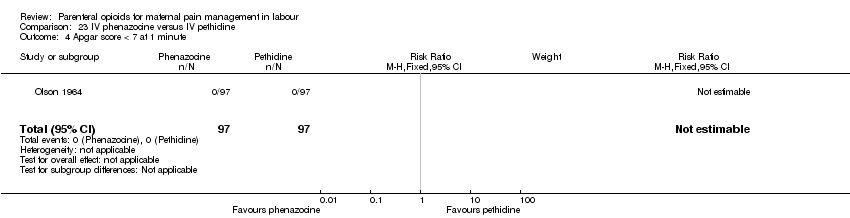
Comparison 23 IV phenazocine versus IV pethidine, Outcome 4 Apgar score < 7 at 1 minute.
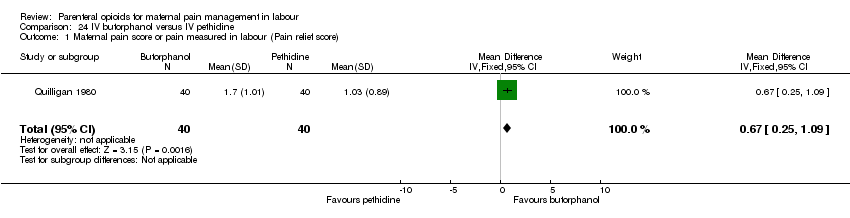
Comparison 24 IV butorphanol versus IV pethidine, Outcome 1 Maternal pain score or pain measured in labour (Pain relief score).

Comparison 24 IV butorphanol versus IV pethidine, Outcome 2 Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)).

Comparison 24 IV butorphanol versus IV pethidine, Outcome 3 Additional analgesia required.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 4 Epidural.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 5 Nausea and/or vomiting.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 6 Caesarean section.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 7 Assisted vaginal birth.

Comparison 24 IV butorphanol versus IV pethidine, Outcome 8 Apgar score < 7 at 1 minute.
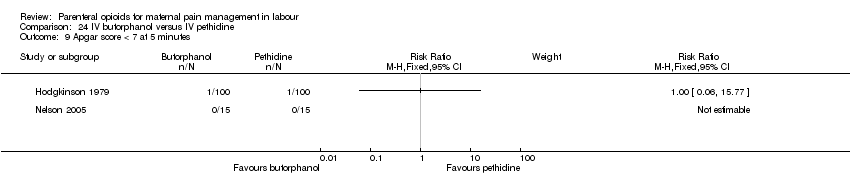
Comparison 24 IV butorphanol versus IV pethidine, Outcome 9 Apgar score < 7 at 5 minutes.

Comparison 25 IV morphine versus IV pethidine, Outcome 1 Maternal satisfaction with analgesia (assessed 3 days postpartum).

Comparison 25 IV morphine versus IV pethidine, Outcome 2 Additional analgesia required.

Comparison 25 IV morphine versus IV pethidine, Outcome 3 Nausea and vomiting.

Comparison 25 IV morphine versus IV pethidine, Outcome 4 Caesarean section.

Comparison 26 IV Nisentil versus IV pethidine, Outcome 1 Nausea and vomiting.

Comparison 26 IV Nisentil versus IV pethidine, Outcome 2 Neonatal resuscitation/ventilatory support.

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 1 Additional analgesia required.
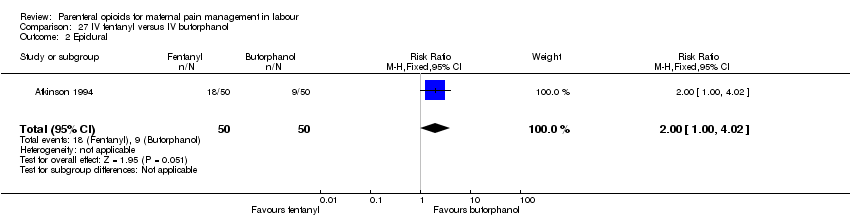
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 2 Epidural.

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 3 Matenal sleepiness (required tactile rousing).
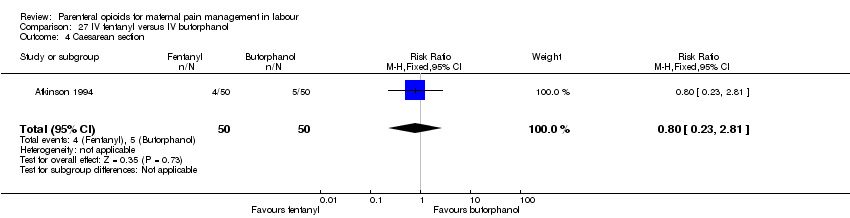
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 4 Caesarean section.

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 5 Naloxone required.
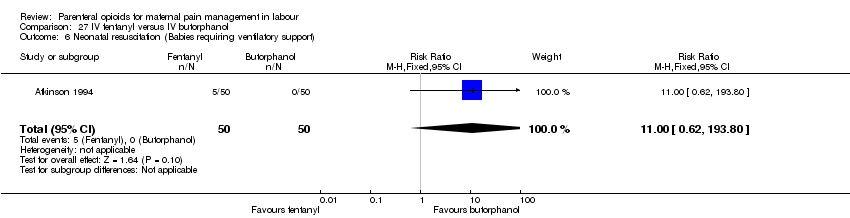
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 6 Neonatal resuscitation (Babies requiring ventilatory support).

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 7 Apgar score < 7 at 5 minutes.
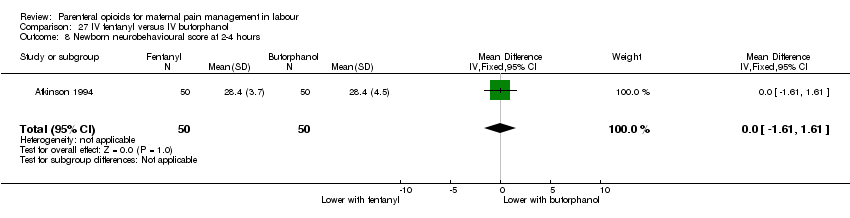
Comparison 27 IV fentanyl versus IV butorphanol, Outcome 8 Newborn neurobehavioural score at 2‐4 hours.

Comparison 27 IV fentanyl versus IV butorphanol, Outcome 9 Newborn neurobehavioural score at 24‐36 hours.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 1 Maternal pan score or pain measured in labour.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 2 Maternal pan score or pain measured in labour (rated as good one day after birth).
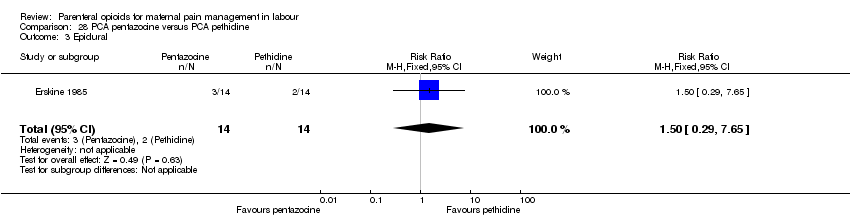
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 3 Epidural.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 4 Nausea and vomiting.

Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 5 Maternal sleepiness during labour (Sedation).
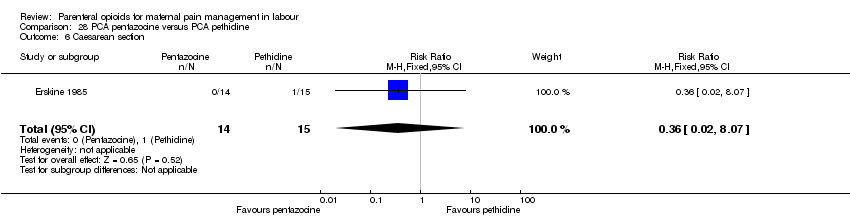
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 6 Caesarean section.
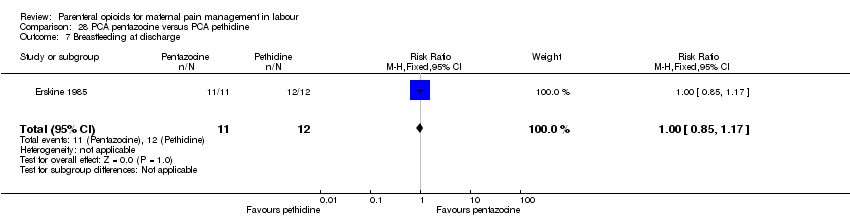
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 7 Breastfeeding at discharge.
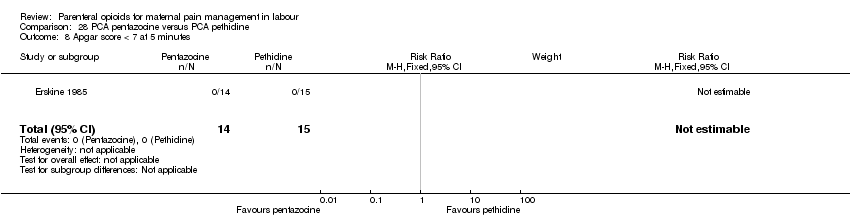
Comparison 28 PCA pentazocine versus PCA pethidine, Outcome 8 Apgar score < 7 at 5 minutes.
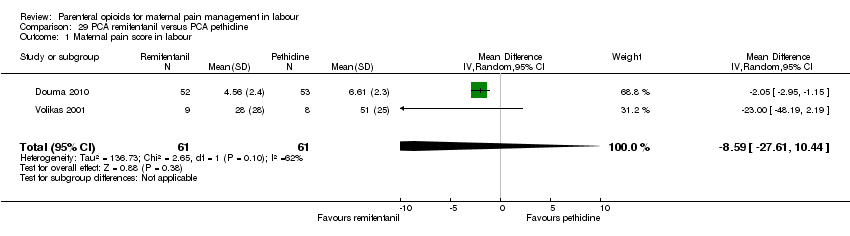
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 1 Maternal pain score in labour.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 2 Additional analgesia required.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 3 Epidural.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 4 Maternal sleepiness during labour.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 5 Nausea and vomiting.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 6 Caesarean section.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 7 Assisted vaginal birth.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 8 Satisfaction with childbirth experience.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 9 Naloxone administered.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 10 Apgar score < 7 at 5 minutes.
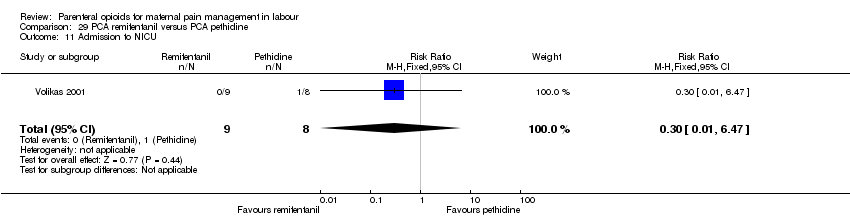
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 11 Admission to NICU.

Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 12 Newborn neurobehavioural score (15 minutes post delivery).
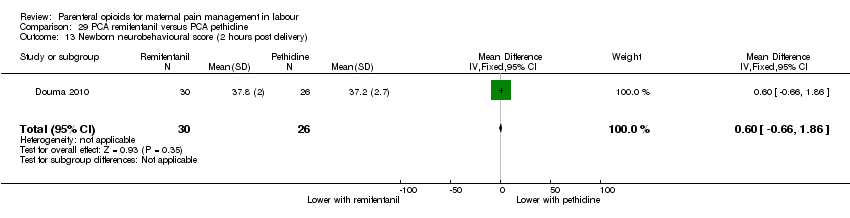
Comparison 29 PCA remifentanil versus PCA pethidine, Outcome 13 Newborn neurobehavioural score (2 hours post delivery).

Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent).

Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 2 Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again).
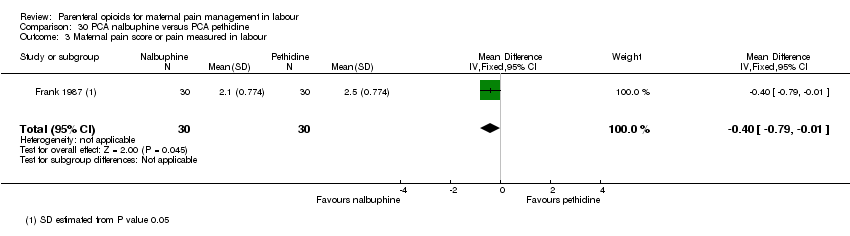
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 3 Maternal pain score or pain measured in labour.
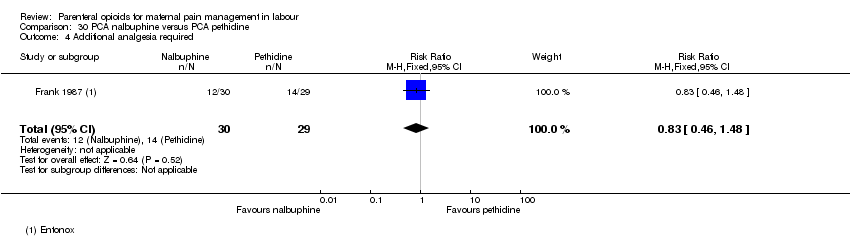
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 4 Additional analgesia required.
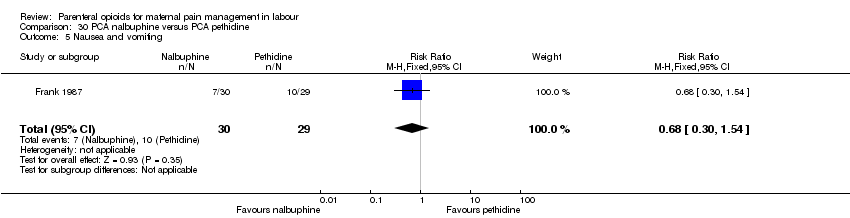
Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 5 Nausea and vomiting.

Comparison 30 PCA nalbuphine versus PCA pethidine, Outcome 6 Apgar score < 7 at 5 minutes.

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (described as adequate).
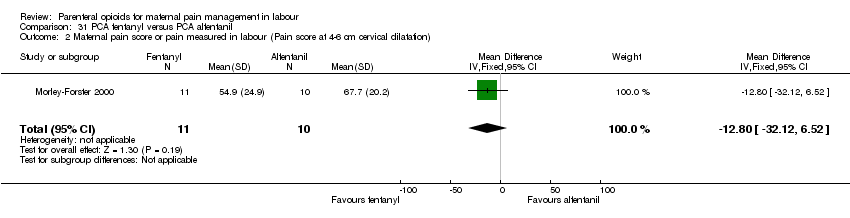
Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 2 Maternal pain score or pain measured in labour (Pain score at 4‐6 cm cervical dilatation).

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 3 Nausea.

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 4 Caesarean section.

Comparison 31 PCA fentanyl versus PCA alfentanil, Outcome 5 Naloxone required.

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 1 Maternla pain score measured in labour.

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 2 Epidural.

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 3 Maternal sleepiness during labour.
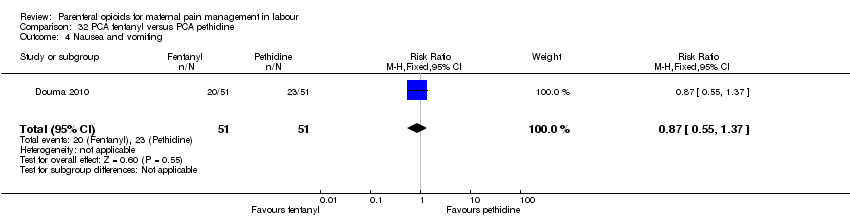
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 4 Nausea and vomiting.
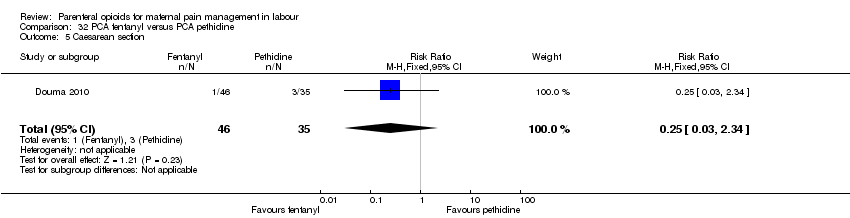
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 5 Caesarean section.
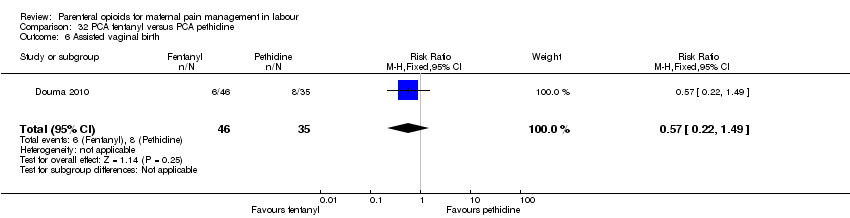
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 6 Assisted vaginal birth.
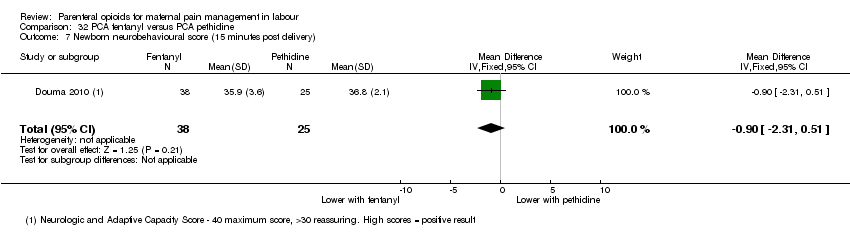
Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 7 Newborn neurobehavioural score (15 minutes post delivery).

Comparison 32 PCA fentanyl versus PCA pethidine, Outcome 8 Newborn neurobehavioural score (2 hours post delivery).
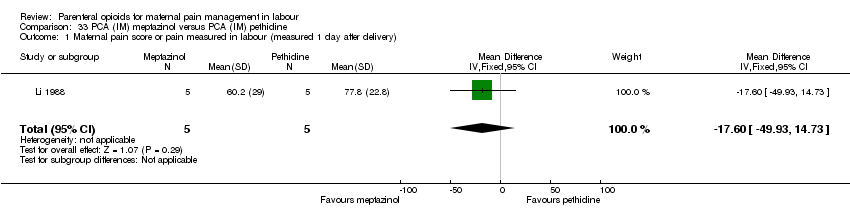
Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 1 Maternal pain score or pain measured in labour (measured 1 day after delivery).

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 2 Satisfied with mode of administration (PCA IM) (non pre‐specified).

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 3 Epidural.

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 4 Maternal sleepiness in labour (Drowsiness score in labour rated 1 day after delivery).

Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 5 Nausea (score in labour rated 1 day after delivery).
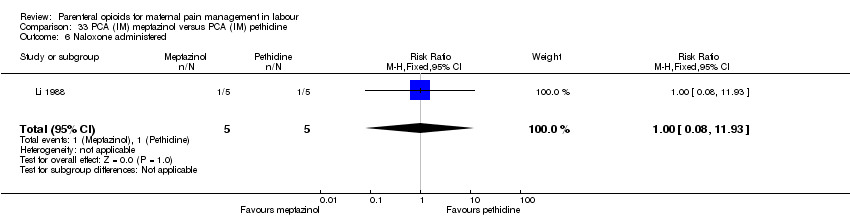
Comparison 33 PCA (IM) meptazinol versus PCA (IM) pethidine, Outcome 6 Naloxone administered.
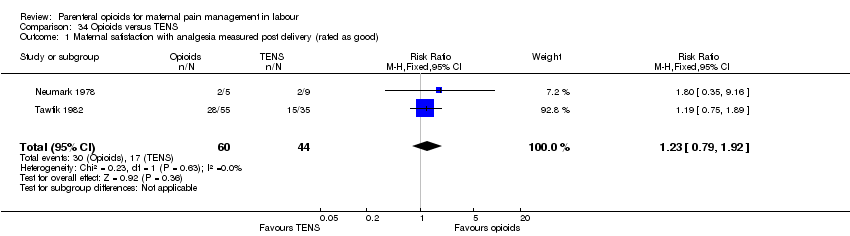
Comparison 34 Opioids versus TENS, Outcome 1 Maternal satisfaction with analgesia measured post delivery (rated as good).

Comparison 34 Opioids versus TENS, Outcome 2 Maternal pain score measured during labour.

Comparison 34 Opioids versus TENS, Outcome 3 Maternal pain score in labour.
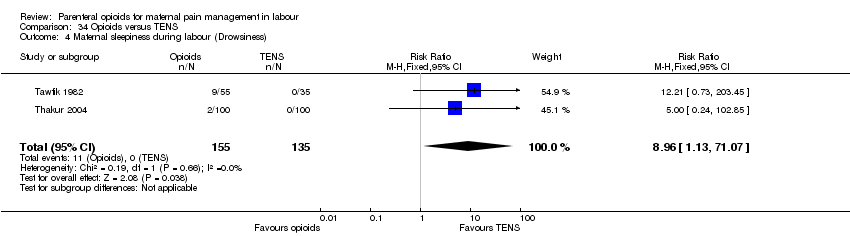
Comparison 34 Opioids versus TENS, Outcome 4 Maternal sleepiness during labour (Drowsiness).

Comparison 34 Opioids versus TENS, Outcome 5 Nausea and vomiting.

Comparison 34 Opioids versus TENS, Outcome 6 Caesarean section.

Comparison 34 Opioids versus TENS, Outcome 7 Assisted vaginal birth.

Comparison 34 Opioids versus TENS, Outcome 8 Fetal heart rate changes in labour (Fetal distress).
| IM pethidine compared to placebo for pain management for women in labour | ||||||
| Patient or population: women in labour | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with IM pethidine 50 mg/100 mg | Risk with placebo | |||||
| Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) | Study population | RR 7.00 | 50 | ⊕⊝⊝⊝ | ||
| 0 per 1000 (0 to 0) | 0 per 1000 | |||||
| Maternal pain score or pain measured in labour (described as good or fair after 1 hour) | Study population | RR 1.75 | 116 | ⊕⊕⊝⊝ | ||
| 724 per 1000 | 414 per 1000 | |||||
| Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) | Study population | RR 25.00 | 50 | ⊕⊕⊝⊝ | ||
| 0 per 1000 (0 to 0) | 0 per 1000 | |||||
| Additional analgesia required (epidural, pethidine and Entonox) | Study population | RR 0.71 | 50 | ⊕⊕⊝⊝ | ||
| 682 per 1000 | 960 per 1000 | |||||
| Epidural | Study population | RR 0.50 | 50 | ⊕⊝⊝⊝ | ||
| 120 per 1000 | 240 per 1000 | |||||
| *SEE ADDITIONAL Table 1FOR FURTHER GRADE COMPARISONS* | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: serious (effect estimate from single study with design limitations) 2 Imprecision: very serious (wide confidence interval crossing the line of no effect, few events, and small sample size) 3 Imprecision: serious (small sample size) 4 Imprecision: serious (small sample size and few events) | ||||||
| OUTCOME | N STUDIES (n women) | EFFECT | CERTAINTY OF EVIDENCE | |
| Relative | Absolute | |||
| IM pethidine 50 mg/100 mg versus placebo | ||||
| Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) | 1 (50) | RR 7.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (described as good or fair after 1 hour) | 1 (118) | RR 1.75 | 310 more per 1000 | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) | 1 (50) | RR 25.00 | 0 fewer per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (50) | RR 0.71 | 278 fewer per 1000 | ⊕⊕⊝⊝ |
| Epidural | 1 (50) | RR 0.50 | 120 fewer per 1000 (from 187 more to 206 fewer) | ⊕⊝⊝⊝ |
| IM pentazocine versus placebo | ||||
| Maternal pain score measured during labour | 1 (89) | ‐ | MD 3.60 lower | ⊕⊕⊝⊝ |
| IM tramadol versus no treatment | ||||
| Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)) | 1 (60) | RR 11.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| IM meptazinol versus pethidine | ||||
| Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)) | 1 (801) | RR 1.01 | 6 more per 1000 | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)) | 2 (239) | RR 1.11 | 79 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 2 (233) | RR 1.03 | 20 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 4 (788) | RR 0.96 | 7 fewer per 1000 | ⊕⊝⊝⊝ |
| IM diamorphine + prochlorperazine versus pethidine + prochlorperazine | ||||
| Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours) | 1 (133) | RR 0.88 | 78 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)) | 1 (133) | RR 0.85 | 130 fewer per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 1 (133) | RR 1.35 | 36 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (133) | RR 1.22 | 58 more per 1000 | ⊕⊝⊝⊝ |
| IM tramadol versus pethidine | ||||
| Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief) | 4 (243) | RR 1.56 | 142 more per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 3 (295) | RR 1.07 | 11 more per 1000 | ⊕⊝⊝⊝ |
| IM dihydrocodeine 50 mg versus pethidine 100 mg | ||||
| Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour) | 1 (138) | RR 1.09 | 25 more per 1000 | ⊕⊝⊝⊝ |
| IM pentazocine versus pethidine | ||||
| Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery) | 2 (253) | RR 1.08 | 51 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) ‐ No add‐on drugs | 3 (365) | Average RR 1.23 | 135 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) ‐ With promazine | 1 (85) | RR 1.53 | 88 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required ‐ pentazocine | 1 (94) | RR 0.91 | 30 fewer per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required ‐ pentazocine + promazine | 1 (85) | RR 1.67 | 112 more per 1000 | ⊕⊝⊝⊝ |
| IM nalbuphine versus pethidine | ||||
| Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied) | 1 (72) | RR 0.73 | 231 fewer per 1000 | ⊕⊕⊝⊝ |
| Maternal satisfaction with analgesia measured during labour (Pain free) | 1 (40) | RR 6.00 | 250 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain) | 1 (295) | RR 0.86 | 40 fewer per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)) | 1 (72) | ‐ | MD 8.00 lower | ⊕⊝⊝⊝ |
| Additional analgesia required | 1 (72) | RR 1.26 | 45 more per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (307) | RR 1.65 | 21 more per 1000 | ⊕⊕⊝⊝ |
| IM phenazocine versus pethidine | ||||
| Epidural | 1 (212) | RR 1.31 (0.58 to 2.97) | 27 more per 1000 (from 36 fewer to 169 more) | ⊕⊝⊝⊝ |
| IM diamorphine/morphine versus pethidine | ||||
| Maternal satisfaction with analgesia (number of women satisfied or very satisfied) | 1 (484) | RR 1.13 | 92 more per 1000 | ⊕⊕⊕⊕ |
| Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor) | 1 (90) | RR 1.22 | 44 more per 1000 | ⊕⊝⊝⊝ |
| Additional analgesia required | 2 (574) | RR 1.00 | 0 fewer per 1000 | ⊕⊕⊕⊝ |
| Maternal pain relief at 30 mins | 1 (484) | ‐ | MD 0.80 lower | ⊕⊕⊕⊕ |
| Maternal pain relief at 60 mins | 1 (484) | ‐ | MD 0.80 lower | ⊕⊕⊕⊕ |
| IM butorphanol versus pethidine | ||||
| Additional analgesia required | 1 (80) | RR 0.89 | 52 fewer per 1000 | ⊕⊝⊝⊝ |
| IM pethidine versus Entonox | ||||
| Maternal pain score or pain measured in labour (after 30 mins) | 1 (100) | ‐ | MD 1.66 higher | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (after 60 mins) | 1 (100) | ‐ | MD 0.36 lower | ⊕⊝⊝⊝ |
| IV pethidine versus placebo | ||||
| Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia) | 1 (240) | ‐ | MD 4.10 lower | ⊕⊕⊕⊝ |
| IV fentanyl versus no treatment | ||||
| Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia) | 1 (70) | ‐ | MD 5.00 lower | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour) | 1 (70) | RR 0.02 | 868 fewer per 1000 | ⊕⊝⊝⊝ |
| IV fentanyl versus IV pethidine | ||||
| Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration) | 1 (105) | ‐ | MD 0.20 lower | ⊕⊕⊝⊝ |
| Mean doses of analgesia (non pre‐specified) | 1 (105) | ‐ | MD 0.40 higher | ⊕⊕⊝⊝ |
| IV phenazocine versus IV pethidine | ||||
| Maternal satisfaction with analgesia measured during labour (women with fair or poor relief) | 1 (194) | RR 0.72 | 104 fewer per 1000 | ⊕⊝⊝⊝ |
| IV butorphanol versus IV pethidine | ||||
| Maternal pain score or pain measured in labour (Pain relief score) | 1 (80) | ‐ | MD 0.67 higher | ⊕⊕⊝⊝ |
| Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)) | 1 (80) | ‐ | MD 0.60 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (100) | RR 0.96 | 19 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (200) | RR 1.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| IV morphine versus pethidine | ||||
| Maternal satisfaction with analgesia (assessed 3 days postpartum) | 1 (141) | RR 0.87 | 124 fewer per 1000 | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (143) | RR 3.41 | 373 more per 1000 | ⊕⊕⊝⊝ |
| IV Nisentil versus IV pethidine | ||||
| Maternal satisfaction with analgesia, maternal pain score or pain measured in labour, additional analgesia, epidural | 1 (395) | ‐ | ‐ | No trial reported these outcomes. |
| PCA pentazocine versus PCA pethidine | ||||
| Maternal pan score or pain measured in labour | 1 (23) | ‐ | SMD 0.76 lower | ⊕⊝⊝⊝ |
| Maternal pan score or pain measured in labour (rated as good one day after birth) | 1 (28) | RR 0.82 | 141 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (28) | RR 1.50 | 71 more per 1000 | ⊕⊝⊝⊝ |
| PCA remifentanil versus PCA pethidine | ||||
| Maternal pain score in labour | 2 (122) | ‐ | MD 8.59 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 2 (56) | RR 0.86 | 124 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 2 (122) | RR 0.42 | 181 fewer per 1000 | ⊕⊕⊕⊝ |
| PCA nalbuphine versus PCA pethidine | ||||
| Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent) | 1 (60) | RR 1.29 | 164 more per 1000 | ⊕⊝⊝⊝ |
| Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again) | 1 (59) | RR 1.06 | 43 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score or pain measured in labour | 1 (60) | ‐ | MD 0.40 lower | ⊕⊕⊝⊝ |
| Additional analgesia required | 1 (59) | RR 0.83 | 82 fewer per 1000 | ⊕⊝⊝⊝ |
| PCA fentanyl versus PCA pethidine | ||||
| Maternla pain score measured in labour | 1 (107) | ‐ | MD 0.65 lower | ⊕⊕⊝⊝ |
| Epidural | 1 (107) | RR 0.44 | 190 fewer per 1000 | ⊕⊕⊕⊝ |
| PCA (IM) meptazinol versus PCA (IM) pethidine | ||||
| Maternal pain score or pain measured in labour (measured 1 day after delivery) | 1 (10) | ‐ | MD 17.60 lower | ⊕⊝⊝⊝ |
| Satisfied with mode of administration (PCA IM) | 1 (10) | RR 1.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Epidural | 1 (10) | RR 3.00 | 0 fewer per 1000 | ⊕⊝⊝⊝ |
| Opioids versus TENS | ||||
| Maternal satisfaction with analgesia measured post delivery (rated as good) | 2 (104) | RR 1.23 | 89 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score measured during labour | 2 (290) | Average RR 1.15 | 97 more per 1000 | ⊕⊝⊝⊝ |
| Maternal pain score measured during labour (after 30 minutes) | 1 (60) | ‐ | MD 20 lower | ⊕⊕⊝⊝ |
| Maternal pain score measured during labour (after 60 minutes) | 1 (60) | ‐ | MD 20.00 lower | ⊕⊕⊝⊝ |
| CI: confidence interval; RR: risk ratio; MD: mean difference aRisk of bias: serious (Effect estimate from single study with design limitations) bImprecision: very serious (Wide confidence interval crossing the line of no effect, few events, and small sample size) cImprecision: serious (Small sample size) dImprecision: serious (Small sample size and few events) eImprecision: very serious (Wide confidence interval crossing the line of no effect, and small sample size) fRisk of bias: very serious (Effect estimate from single study with serious design limitations) gImprecision: serious (Wide confidence interval crossing the line of no effect) hRisk of bias: serious (Pooled effect provided by studies with design limitations) iRisk of bias: very serious (Pooled effect provided by studies with serious design limitations) jRisk of bias: serious (Pooled effect estimate mainly from studies with design limitations) kInconsistency: serious (unexplained substantial heterogeneity) lImprecision: very serious (Wide confidence interval crossing the line of no effect, and few events) | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (number of women satisfied or very satisfied after 30 minutes) Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| 2 Maternal pain score or pain measured in labour (described as good or fair after 1 hour) Show forest plot | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.24, 2.47] |
| 3 Maternal pain score or pain measured in labour (reduction in VAS of at least 40 mm after 30 minutes) Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.0 [1.56, 400.54] |
| 4 Additional analgesia required Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.94] |
| 5 Epidural Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.14, 1.78] |
| 6 Nausea and vomiting Show forest plot | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.65, 3.31] |
| 7 Maternal sleepiness Show forest plot | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.67 [2.43, 8.95] |
| 8 Assisted vaginal delivery Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.34, 2.19] |
| 9 Caesarean section Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.36, 1.37] |
| 10 Neonatal resuscitation Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.45, 6.24] |
| 11 Low Apgar score (≤ 7) at 1 and 5 minutes Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Low scores at 1 minute | 2 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.52, 5.18] |
| 11.2 Low scores at 5 minutes | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Admission to NICU Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐3.60 [‐9.91, 2.71] |
| 2 Nausea and vomiting Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.24, 3.35] |
| 4 Assisted vaginal birth Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.10, 3.39] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (Analgesic effect described as satisfactory (not clear when measured)) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 190.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Maternal pain relief poor or none (3‐5 PN)) Show forest plot | 1 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.12] |
| 2 Maternal pain score or pain measured in labour (Pain intensity 4 or 5 on 5‐point scale (1 hour)) Show forest plot | 2 | 239 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.69, 1.80] |
| 3 Additional analgesia required Show forest plot | 2 | 233 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 4 Epidural Show forest plot | 4 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.29] |
| 5 Maternal sleepiness Show forest plot | 3 | 1590 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.28, 1.07] |
| 6 Nausea and vomiting Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 3 | 1590 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.28] |
| 6.2 Vomiting | 3 | 1589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.06, 1.47] |
| 7 Caesarean section Show forest plot | 3 | 1266 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.16, 2.00] |
| 8 Assisted vaginal birth Show forest plot | 3 | 1266 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.22] |
| 9 Breastfeeding at discharge (problems) Show forest plot | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.30] |
| 10 Fetal heart rate changes (decelerations) Show forest plot | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.92, 1.64] |
| 11 Naloxone administration Show forest plot | 1 | 998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 11.1 < 36 weeks' gestation | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.49, 1.89] |
| 11.2 ≥ 36 weeks' gestation | 1 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 12 Neonatal resuscitation (by gestation) Show forest plot | 2 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 12.1 < 36 weeks' gestation | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.16] |
| 12.2 ≥ 36 weeks' gestation | 2 | 1333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.95, 1.05] |
| 13 Neonatal resuscitation Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.60] |
| 14 Apgar score ≤ 7 at 1 minute Show forest plot | 6 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.56, 1.11] |
| 15 Apgar score ≤ 7 at 5 minutes Show forest plot | 3 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.37] |
| 16 Admission to NICU Show forest plot | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (Global assessment of pain relief at 24 hours) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.16] |
| 2 Maternal pain score or pain measured in labour (Pain intensity at 1 hour (moderate or severe)) Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.01] |
| 3 Additonal analgesia required Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.53, 3.40] |
| 4 Epidural Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.72, 2.07] |
| 5 Maternal sleepiness during labour Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.66] |
| 6 Vomiting in labour Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.17, 0.86] |
| 7 Caesarean section Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.76] |
| 8 Assisted vaginal birth Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.46, 2.02] |
| 9 Neonatal resuscitation Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.73, 2.02] |
| 10 Apgar < 7 at 1 minute Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.91] |
| 11 Apgar < 7 at 5 minutes Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.27] |
| 12 Admission to NICU Show forest plot | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain intensity: women with poor pain relief) Show forest plot | 4 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.10, 2.21] |
| 2 Additional analgesia required Show forest plot | 3 | 295 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.60, 1.91] |
| 3 Maternal sleepiness in labour Show forest plot | 5 | 409 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.33, 0.97] |
| 4 Nausea and vomiting in labour Show forest plot | 6 | 454 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.34, 2.76] |
| 5 Caesarean section Show forest plot | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.18] |
| 6 Assisted vaginal birth Show forest plot | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.56] |
| 7 Neonatal resuscitation Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Apgar scores ≤ 7 at 1 and 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Less than 7 at 1 minute | 2 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Less than 7 at 5 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Neonatal respiratory distress Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| 10 Admission to NICU Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.64, 7.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal sleepiness in labour Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [0.68, 12.12] |
| 2 Nausea and vomiting in labour Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.13, 5.25] |
| 2.2 Vomiting | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.02, 9.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Maternal pain relief poor at 1 hour) Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.64, 1.86] |
| 2 Maternal sleepiness in labour Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.04] |
| 3 Nausea and vomiting in labour Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.40, 1.88] |
| 4 Apgar ≤ 7 at 1 minute Show forest plot | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (Pain relief (good or very good) at delivery) Show forest plot | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.92, 1.27] |
| 2 Maternal pain score or pain measured in labour (Pain relief poor (partial, none or worse)) Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 No add‐on drugs | 3 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.74, 2.05] |
| 2.2 With promazine | 1 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.66, 3.58] |
| 3 Additional analgesia required Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pentazocine | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.50, 1.65] |
| 3.2 Pentazocine + promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.73, 3.84] |
| 4 Maternal sleepiness in labour Show forest plot | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.12] |
| 5 Nausea and vomiting in labour Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.24, 0.90] |
| 5.2 Vomiting | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.27, 3.14] |
| 6 Assisted vaginal birth Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 No add‐on drugs | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.63, 42.97] |
| 6.2 With promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.23, 2.71] |
| 7 Naloxone administration Show forest plot | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| 7.1 With promazine | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.53] |
| 8 Apgar score ≤ 7 at 1 minute Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 No add‐on drugs | 2 | 242 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.06, 32.97] |
| 8.2 With promazine | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.07, 17.30] |
| 9 Apgar score ≤ 7 at 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 No add‐on drugs | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.54] |
| 9.2 With promazine | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.02, 8.88] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during the postnatal period (numbers dissatisfied) Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.96] |
| 2 Maternal satisfaction with analgesia measured during labour (Pain free) Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.79, 45.42] |
| 3 Maternal pain score or pain measured in labour (Pain intensity at 30 minutes: women with severe pain) Show forest plot | 1 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.59, 1.26] |
| 4 Maternal pain score or pain measured in labour (VAS at 60 minutes (at peak of contraction)) Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐18.55, 2.55] |
| 5 Additional analgesia required Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.49, 3.27] |
| 6 Epidural Show forest plot | 1 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.55, 4.94] |
| 7 Maternal sleepiness in labour Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.86, 16.60] |
| 8 Nausea and vomiting in labour Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Nausea | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.42, 0.91] |
| 8.2 Vomiting | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.22, 0.76] |
| 8.3 Nausea and vomiting | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.94] |
| 9 Caesarean section Show forest plot | 1 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.69] |
| 10 Assisted vaginal birth Show forest plot | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.25, 3.85] |
| 11 Naloxone administration Show forest plot | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.63 [0.35, 123.93] |
| 12 Apgar score ≤ 7 at 1 and 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Low score at 1 minute | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.95] |
| 12.2 Low score at 5 minutes | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 4.99] |
| 13 Admission to NICU Show forest plot | 1 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.61, 1.89] |
| 14 Neonatal neurobehavioural (Scanlon) 2‐4 hours PN Show forest plot | 1 | 72 | Mean Difference (IV, Fixed, 95% CI) | ‐3.70 [‐6.14, ‐1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Epidural Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.58, 2.97] |
| 2 Vomiting Show forest plot | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.20, 0.78] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (number of women satisfied or very satisfied) Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [1.02, 1.26] |
| 2 Maternal satisfaction with analgesia measured during labour or during the postnatal period (Pain relief described as poor) Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.56, 2.66] |
| 3 Maternal pain score or pain measured in labour (pain relief at 30 mins) Show forest plot | 1 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.24, ‐0.36] |
| 4 Maternal pain score or pain measured in labour (pain relief at 60 mins) Show forest plot | 1 | 484 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐1.26, ‐0.34] |
| 5 Additional analgesia required Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.10] |
| 6 Maternal sleepiness Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.29, 1.23] |
| 7 Nausea and vomiting Show forest plot | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.69] |
| 8 Caesarean section Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.35] |
| 9 Assisted vaginal birth Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.91, 1.80] |
| 10 Naloxone administration Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.83] |
| 11 Neonatal resuscitation Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 12 Apgar < 7 at 1 minute Show forest plot | 2 | 574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.73] |
| 13 Admission to NICU Show forest plot | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.34, 2.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.55, 1.45] |
| 2 Nausea Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.04] |
| 3 Vomiting Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 4 Neonatal resuscitation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 5 Naloxone administration (neonatal) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required ‐ Entonox Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.63] |
| 2 Additional analgesia required ‐ pudendal‐paracervical block Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.16, 3.53] |
| 3 Caesarean section Show forest plot | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.21, 1.84] |
| 4 Low Apgar score (< 7) "at birth" Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour (Pain relief (women NOT obtaining pain relief) at 1 hour) Show forest plot | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.77, 1.95] |
| 2 Additional analgesia required Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| 3 Assisted vaginal birth Show forest plot | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.07, 16.19] |
| 4 Apgar < 8 at 1 minute (non pre‐specified) Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.71 [0.72, 45.39] |
| 5 Apgar < 8 at 5 minutes (non pre‐specified) Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score measured during labour Show forest plot | 1 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐7.61, 6.81] |
| 2 Nausea and vomiting Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.14] |
| 3 Caesarean section Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.24, 3.35] |
| 4 Assisted vaginal delivery Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.13, 6.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (pain relief after 30 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.4 [1.28, 4.48] |
| 2 Maternal satisfaction with analgesia measured during labour (pain after 60 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.91, 2.86] |
| 3 Maternal pain score or pain measured in labour (moderate or severe at 30 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.55, 1.02] |
| 4 Maternal pain score or pain measured in labour (moderate or severe at 60 mins) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.60, 1.08] |
| 5 Maternal sleepiness during labour Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.66, 4.24] |
| 6 Nausea and vomiting Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 7 Caesarean section Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.45, 4.99] |
| 8 Assisted vaginal delivery Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 9 Apgar score < 7 at 1 minute Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.60] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 11 Admission to NICU Show forest plot | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.87 [0.12, 68.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (after 30 mins) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.66 [1.17, 2.15] |
| 2 Maternal pain score or pain measured in labour (after 60 mins) Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.85, 0.13] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 30 mins post analgesia) Show forest plot | 1 | 240 | Mean Difference (IV, Fixed, 95% CI) | ‐4.1 [‐4.56, ‐3.64] |
| 2 Nausea and vomiting Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.05, 5.64] |
| 3 Caesarean section Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.68] |
| 4 Assisted vaginal birth Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.33, 1.71] |
| 5 Admission to NICU Show forest plot | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 1 hour post‐analgesia) Show forest plot | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐5.47, ‐4.53] |
| 2 Maternal pain score or pain measured in labour (Pain intensity (Severe) after 1 hour) Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.25] |
| 3 Caesarean section Show forest plot | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain score 1 hour after drug administration) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.18, 0.78] |
| 2 Mean doses of analgesia (non pre‐specified) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| 3 Maternal sleepiness in labour (sedation) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.82] |
| 4 Nausea and/or vomiting Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.17, 1.55] |
| 5 Anti‐emetic required (non pre‐specified) Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.52] |
| 6 Caesarean section Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.24, 5.40] |
| 7 Naloxone administered Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| 8 Babies requiring resuscitation/ventilatory support Show forest plot | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.46, 2.32] |
| 9 Apgar score < 7 at 1 minute Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11 Neurobehavioural score (1 ‐ 2 hours after delivery) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.15, 2.45] |
| 12 Neurobehavioural score (2 hours ‐ 24 hours) Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.42, 2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Caesarean section Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 95.61] |
| 2 Apgar score < 7 at 1 minute Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Apgar score < 7 at 5 minutes Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured during labour (women with fair or poor relief) Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.48, 1.10] |
| 2 Nausea with vomiting Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 2.01] |
| 3 Perinatal death Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Apgar score < 7 at 1 minute Show forest plot | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (Pain relief score) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.25, 1.09] |
| 2 Maternal pain score or pain measured in labour (Pain score (1 hour after drug administration)) Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.02, ‐0.18] |
| 3 Additional analgesia required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.63, 1.45] |
| 4 Epidural Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.35] |
| 5 Nausea and/or vomiting Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.67] |
| 6 Caesarean section Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.89] |
| 7 Assisted vaginal birth Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.60, 2.83] |
| 8 Apgar score < 7 at 1 minute Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9 Apgar score < 7 at 5 minutes Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia (assessed 3 days postpartum) Show forest plot | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.78, 0.98] |
| 2 Additional analgesia required Show forest plot | 1 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.90, 6.12] |
| 3 Nausea and vomiting Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.14] |
| 3.2 Vomiting | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.86] |
| 4 Caesarean section Show forest plot | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea and vomiting Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nausea | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.33, 1.52] |
| 1.2 Vomiting | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.22, 0.66] |
| 2 Neonatal resuscitation/ventilatory support Show forest plot | 1 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.85, 4.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Additional analgesia required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.05, 1.85] |
| 2 Epidural Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [1.00, 4.02] |
| 3 Matenal sleepiness (required tactile rousing) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.64, 14.16] |
| 4 Caesarean section Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.81] |
| 5 Naloxone required Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.81, 3.80] |
| 6 Neonatal resuscitation (Babies requiring ventilatory support) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.62, 193.80] |
| 7 Apgar score < 7 at 5 minutes Show forest plot | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.39, 3.68] |
| 8 Newborn neurobehavioural score at 2‐4 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.61, 1.61] |
| 9 Newborn neurobehavioural score at 24‐36 hours Show forest plot | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.62, 0.62] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pan score or pain measured in labour Show forest plot | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.45 [‐2.96, 0.06] |
| 2 Maternal pan score or pain measured in labour (rated as good one day after birth) Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.51, 1.32] |
| 3 Epidural Show forest plot | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.65] |
| 4 Nausea and vomiting Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.61] |
| 5 Maternal sleepiness during labour (Sedation) Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.09] |
| 6 Caesarean section Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.07] |
| 7 Breastfeeding at discharge Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.85, 1.17] |
| 8 Apgar score < 7 at 5 minutes Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score in labour Show forest plot | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐8.59 [‐27.61, 10.44] |
| 2 Additional analgesia required Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.69, 1.08] |
| 3 Epidural Show forest plot | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| 4 Maternal sleepiness during labour Show forest plot | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.14, 0.66] |
| 5 Nausea and vomiting Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.49] |
| 6 Caesarean section Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.60, 5.46] |
| 7 Assisted vaginal birth Show forest plot | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.46, 2.00] |
| 8 Satisfaction with childbirth experience Show forest plot | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.46, 1.74] |
| 9 Naloxone administered Show forest plot | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| 10 Apgar score < 7 at 5 minutes Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.16] |
| 11 Admission to NICU Show forest plot | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.01, 6.47] |
| 12 Newborn neurobehavioural score (15 minutes post delivery) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.93, 1.33] |
| 13 Newborn neurobehavioural score (2 hours post delivery) Show forest plot | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.66, 1.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (rated good or excellent) Show forest plot | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.88, 1.89] |
| 2 Maternal satisfaction with analgesia in labour measured during the postnatal period (Would use the same pain relief again) Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 3 Maternal pain score or pain measured in labour Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.79, ‐0.01] |
| 4 Additional analgesia required Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.48] |
| 5 Nausea and vomiting Show forest plot | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.30, 1.54] |
| 6 Apgar score < 7 at 5 minutes Show forest plot | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.76] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia in labour measured during the postnatal period (described as adequate) Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.93, 2.60] |
| 2 Maternal pain score or pain measured in labour (Pain score at 4‐6 cm cervical dilatation) Show forest plot | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐12.80 [‐32.12, 6.52] |
| 3 Nausea Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.66, 11.30] |
| 4 Caesarean section Show forest plot | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.33, 8.03] |
| 5 Naloxone required Show forest plot | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.53, 10.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternla pain score measured in labour Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐1.56, 0.26] |
| 2 Epidural Show forest plot | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.21, 0.92] |
| 3 Maternal sleepiness during labour Show forest plot | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.25, 0.13] |
| 4 Nausea and vomiting Show forest plot | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 5 Caesarean section Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.34] |
| 6 Assisted vaginal birth Show forest plot | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.49] |
| 7 Newborn neurobehavioural score (15 minutes post delivery) Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.31, 0.51] |
| 8 Newborn neurobehavioural score (2 hours post delivery) Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.95, 0.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal pain score or pain measured in labour (measured 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐17.60 [‐49.93, 14.73] |
| 2 Satisfied with mode of administration (PCA IM) (non pre‐specified) Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| 3 Epidural Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.15, 59.89] |
| 4 Maternal sleepiness in labour (Drowsiness score in labour rated 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐28.19, 39.39] |
| 5 Nausea (score in labour rated 1 day after delivery) Show forest plot | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐48.70, 32.70] |
| 6 Naloxone administered Show forest plot | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.08, 11.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Maternal satisfaction with analgesia measured post delivery (rated as good) Show forest plot | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.79, 1.92] |
| 2 Maternal pain score measured during labour Show forest plot | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.81, 1.61] |
| 3 Maternal pain score in labour Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pain score (after 30 minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐26.09, ‐13.91] |
| 3.2 Pain score (after 60 minutes) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐20.0 [‐25.16, ‐14.84] |
| 4 Maternal sleepiness during labour (Drowsiness) Show forest plot | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.96 [1.13, 71.07] |
| 5 Nausea and vomiting Show forest plot | 3 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.73 [2.72, 69.24] |
| 6 Caesarean section Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 7 Assisted vaginal birth Show forest plot | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.40, 8.18] |
| 8 Fetal heart rate changes in labour (Fetal distress) Show forest plot | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |

