Interventionen zur Behandlung schmerzender Brustwarzen bei Stillenden
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Quasi‐randomised trial | |
| Participants | 94 breastfeeding Caucasian Latvian women with sore nipples or nipple trauma who delivered in the previous 10 days. Excluded women with mastitis, abscess, fungal infections, or other pain related conditions. Women were identified and assessed by 12 different hospital‐based midwives who received training to establish inter‐user consistency regarding latch assessment | |
| Interventions | Intervention group 1: lanolin + breast shell (n = 31): women air‐dried nipples after breastfeeding, applied lanolin with washed hands and wore breast shells until the next feeding. Treatment continued for 10 days or until the resolution of symptoms Intervention group 2: glycerine gel (n = 33): with clean hands, women applied glycerine gel dressing to the breast and continued for 10 days or until resolution of symptoms Control group: (n = 30) breastfeeding assessment, education, and corrective interventions. Measurement continued for 10 days or until resolution of symptoms Both intervention groups received the same breastfeeding assessment, education, and corrective interventions that were provided to women in the control group | |
| Outcomes | At follow‐up visits that occurred a maximum of 3 times in 10 days, midwives ranked signs and symptoms of wound healing (women's pain reports and midwives' assessment of skin surface) 1. Nipple pain: maternal self report measured with a 5‐point verbal descriptor scale comparing pain at first visit with pain at last visit 2. Nipple trauma: midwife assessment based on a scale of 1 to 3 where 1 = better/resolved, 2 = no change, and 3 = worse 3. Satisfaction with treatment: maternal self report ranging from very or somewhat satisfied to very or somewhat dissatisfied | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants allocated to whichever pre‐packaged instruction kit was next in the queue |
| Allocation concealment (selection bias) | High risk | The order of kits may easily be altered in the queue |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel not blinded and outcome potentially influenced due to the lack of blinding |
| Blinding of outcome assessment (detection bias) | High risk | No indication that outcome assessors were blinded to group allocation and outcome potentially influenced due to the lack of blinding |
| Incomplete outcome data (attrition bias) | Low risk | 95 women approached, 94 consented, 1 dropped out, 0 excluded due to confounding problems or co‐morbidities |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis completed |
| Other bias | Low risk | One minor note ‐ the causes of nipple pain in the first week may be clinically different from nipple pain in the second week of life. The age of the infant at trial commencement was unknown but was within the first 10 days' postpartum |
| Methods | Double‐blind randomised controlled trial | |
| Participants | 151 Canadian women who were breastfeeding with painful, damaged nipples within the first 2 weeks' postpartum. Excluded women who were using finger feeding or lactation devices to give artificial milk, using a breast shield, had a history of breast reduction surgery or had breast abnormalities that precluded exclusive breastfeeding. Potential participants were identified by the hospital lactation consultants or staff nurses and were recruited from a large teaching hospital in Canada | |
| Interventions | Intervention group: all‐purpose nipple ointment (n = 75): women applied ointment sparingly after each feeding and did not wash off the ointment prior to next feeding. Treatment continued after each feeding for the first 10 days of the trial, then ointment was applied every other feeding for the remaining 4 days of the trial Control group: lanolin (n = 76): women applied lanolin sparingly after each feeding and did not wash it off prior to next feeding. Treatment continued after every feeding for the first 10 days of trial, then ointment was applied every other feeding for the remaining 4 days of the trial | |
| Outcomes | 1. Nipple pain: maternal self report measured at the initial assessment and at 1 week' post‐randomisation with the Short Form McGill Pain Questionnaire, VAS (adapted into a Likert scale 0‐10) and the Present Pain Index 2. Mastitis symptoms: maternal self report at 12 weeks' postpartum measured using diagnostic criteria adapted from another study (Fetherston 1998) 3. Breastfeeding duration: assessed at 1 week' post‐randomisation and 12 weeks' postpartum by asking women if their infant received any breast milk during the past 24 hours 4. Breastfeeding exclusivity: assessed at 1 week' post‐randomisation and 12 weeks' postpartum using the levels of infant feeding suggested by Labbok 1990 5. Maternal satisfaction with infant feeding method measured by using an adaptation of the Maternal Satisfaction with Infant Feeding Questionnaire (Dennis 2002b) 6. Maternal satisfaction with treatment: measured using questions related to satisfaction with the effectiveness of the ointment. 1 item was rated on a 5‐point Likert‐type scale where 1 = definitely satisfied to 5 = definitely not satisfied | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was centrally controlled by the hospital pharmacy using standard procedures for drug trials. Randomisation numbers were generated in blocks of 20 |
| Allocation concealment (selection bias) | Low risk | 160 identical containers were filled with either lanolin or all‐purpose nipple ointment by the hospital pharmacy in accordance with the standards of a double‐blinded trial and sequentially numbered. Because of a slight difference in the appearance of the 2 ointments, inert food colouring was added to the all‐purpose nipple ointment to make it look similar to lanolin. The ointments were placed in identical unmarked, opaque containers by the hospital pharmacy, where the randomisation schedule was kept until data collection was complete |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel blinded to group allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Research assistant blinded to group allocation collected all outcome data |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up at the 1 week' post‐randomisation and 3% (n = 5) loss to follow‐up at the final assessment (12 weeks' postpartum) |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis completed |
| Other bias | Low risk | 1 minor note ‐ the age of the infant at trial commencement was unknown but was within the first 2 weeks' postpartum |
| Methods | Randomised controlled trial | |
| Participants | 186 in‐hospital breastfeeding women who had given birth to a term infant and were within 4 days' postpartum. All women presented with complaints of nipple pain with signs of trauma. Excluded women with the following: 1. infant not expected to be discharged home with mother; 2. infant with congenital abnormalities that would impair breastfeeding, and 3. maternal allergy to lanolin Potential participants were identified by hospital staff nurses and were recruited from a large teaching hospital in Canada | |
| Interventions | Intervention group: lanolin (n = 93): women were provided with a tube of lanolin and a handout with instructions for its use. Participants were instructed to wash their hands, and to then gently apply a pea‐sized amount of lanolin to the nipple and the areola following every feed until resolution of symptoms or the end of the intervention period Control group: usual care (n = 93): women were instructed to apply nothing to their nipples for the trial period | |
| Outcomes | 1. Nipple pain: maternal self report measured at initial assessment and at 4 and 7 days' post‐randomisation using a Numeric Rating Scale (0‐10) (primary outcome) Exploratory analyses (not included in the review) were completed with the Sensory Pain Rating Index (0‐33), Affective Pain Rating Index (0‐12), Total Pain Rating Index (0‐45), Present Pain Intensity (0‐5), and Short Form McGill Pain Questionnaire (0‐60) 2. Breastfeeding duration: measured at 4 and 12 weeks' postpartum by asking women if their infant received any breast milk during the past 24 hours 3. Breastfeeding exclusivity: measured at 4 and 12 weeks' postpartum using the levels of infant feeding suggested by Labbok 1990 4. Maternal satisfaction with treatment: measured at 12 weeks' postpartum using questions related to satisfaction with the effectiveness of the ointment. 1 item was rated on a 5‐point Likert‐type scale where 1 = definitely satisfied to 5 = definitely not satisfied | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Small pieces of paper were labelled either 'control group' or 'intervention group' and folded in half and then randomly placed in envelopes by a research assistant not involved in the trial |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Participants could not be blinded to group allocation |
| Blinding of outcome assessment (detection bias) | Low risk | Research assistant blinded to group allocation collected outcome data |
| Incomplete outcome data (attrition bias) | Low risk | 186 women were randomised of which data were available for 165 (88.7%) at 4 and 7 days' post‐randomisation, 162 (87.1%) at 4 weeks' postpartum, and 165 (88.7%) at 12 weeks' postpartum; 160 women completed the maternal satisfaction questionnaire at 12 weeks' postpartum |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis completed |
| Other bias | Low risk | None noted |
| Methods | Quasi‐randomized trial | |
| Participants | 225 breastfeeding women from a single site in Iran presenting with fissure on the nipple surface or around the nipples. Potential participants were identified by the 'ward specialists' where the infants were in the unit's neonatal intensive care unit | |
| Interventions | Intervention group 1: expressed breast milk (n = 78): women rubbed hind milk on their nipples at the end of each feeding for a treatment period of 7 days Intervention group 2: lanolin (n = 74): women applied lanolin to nipples 3 times daily and cleansed breasts with a wet cloth prior to feeding for a treatment period of 7 days Control group: usual care (n = 73): women were instructed to apply nothing to their nipples for the trial period For all women in the trial, breastfeeding technique was assessed and, if necessary, corrected and all Infants were fed on demand and breastfed exclusively | |
| Outcomes | Nipple pain: maternal self report measured on days 1, 3, 5, 7, and 10 post‐treatment initiation and defined as the absence of irritation Nipple trauma: determined subjectively as wound healing by an outcome assessor on days 1, 3, 5, 7, and 10 post‐treatment initiation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available regarding sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available regarding allocation generation method and allocation concealment |
| Blinding of participants and personnel (performance bias) | High risk | Participants could not be blinded to group allocation |
| Blinding of outcome assessment (detection bias) | High risk | It is unknown if the outcome assessor was blinded to group allocation |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis completed |
| Other bias | Unclear risk | Measurement tools not described, e.g. how 'improvement time' and 'healing time' were measured was not reported |
n: number of women; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No usable data ‐ data presented by breast not per woman so uncertain of denominators for primary outcome and secondary outcome data were presented in figure format with no specific numbers. Emailed authors but no response received | |
| Insufficient information regarding the study. Emailed authors but no response received | |
| Aim of study was not treatment of nipple pain ‐ primary purpose was to discuss implementation problems when conducting a trial to evaluate the effect of antibiotic use in the prevention of mastitis | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| Aim of the study was not the treatment of nipple pain ‐ primary purpose was to evaluate the effect of tongue‐tie division on breastfeeding outcomes among women with various breastfeeding problems | |
| Insufficient information regarding the study. Emailed authors but no response received | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| Primary purpose of study was to examine the effect of frenotomy on nipple pain relief among infants with ankyloglossia | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| Methodologically weak: small sample size (10 women), participants up to 5 months' postpartum recruited resulting in different aetiology for nipple trauma, no information regarding compliance and attendance of the 8 phototherapy sessions, sessions occurred twice per week for 4 weeks during a time period in which natural nipple healing would be expected independent of phototherapy intervention, high loss to follow‐up, and no usable data ‐ only median nipple pain data per group per session provided | |
| Aim of study was not the treatment of nipple pain ‐ primary purpose was the promotion of nipple healing. No data available related to the primary outcome of this review, nipple pain | |
| A prevention trial ‐ nipple pain not part of the inclusion criteria | |
| Primary purpose of study was to examine the effect of frenotomy on nipple pain relief among infants with ankyloglossia | |
| Primary purpose of study was to examine the effect of immediate frenotomy on breastfeeding outcomes | |
| Aim of study was not the treatment of nipple pain ‐ primary purpose was to compare incision and drainage against needle aspiration for the treatment of breast abscesses | |
| Insufficient information regarding the study. Emailed authors but no response received | |
| Methodologically weak: convenience sample, women served as their own controls, and small sample size (15 women) | |
| Methodologically weak: not an experimental study | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| Aim of study was not treatment of nipple pain ‐ primary purpose was to evaluate the effect of a surgical intervention to treat tongue‐tied breastfeeding infants | |
| Insufficient information regarding the study. Emailed authors but no response received | |
| Methodologically weak: unknown randomisation method (just state envelope), combined unvalidated nipple pain and trauma measure, high attrition rate (44.9%), and no usable data | |
| Methodologically weak: small sample size for a 4‐arm trial, poor randomisation method (tags pulled out of an envelope), trial stopped early with uneven groups, and no usable data | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| A prevention trial ‐ nipple pain not part of inclusion criteria | |
| Aim of study was not the treatment of nipple pain ‐ primary purpose was the treatment of cracked nipples. No data available related to the primary outcome of this review, nipple pain | |
| Insufficient information regarding the study. Emailed authors but no response received | |
| A prevention trial ‐ nipple pain not part of the inclusion criteria | |
| A prevention trial ‐ nipple pain not part of the inclusion criteria | |
| A prevention trial ‐ nipple pain not part of the inclusion criteria | |
| A prevention trial ‐ nipple pain not part of the inclusion criteria | |
| Aim of study was not the treatment of nipple pain ‐ the primary purpose was to evaluate the effect of a traditional (Mexican Hat) and new (Thin Latex) nipple shield on the sucking patterns and milk intake of 5‐ to 8‐day‐old infants | |
| A prevention trial ‐ nipple pain not part of the inclusion criteria |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial |
| Participants | 100 Iranian women with sore nipples |
| Interventions | Intervention group 1: lanolin (n = 50) Intervention group 2: aloe vera gel (n = 50) |
| Outcomes | Nipple pain at day 3 and 7 |
| Notes | Trial report awaiting translation to English |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| Analysis 1.1  Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 1 Nipple pain. | ||||
| 1.1 Nipple pain at final assessment | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 2 Nipple trauma Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| Analysis 1.2 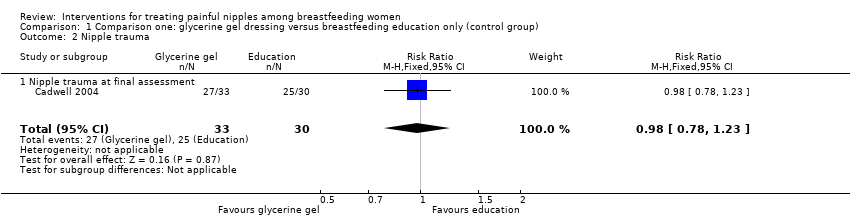 Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 2 Nipple trauma. | ||||
| 2.1 Nipple trauma at final assessment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.27] |
| Analysis 1.3 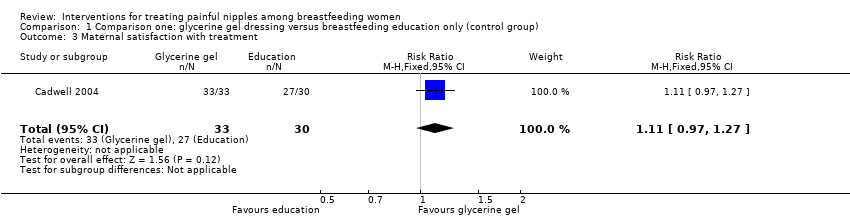 Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| Analysis 2.1  Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 1 Nipple pain. | ||||
| 1.1 Nipple pain at final assessment | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 2 Nipple trauma Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| Analysis 2.2  Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 2 Nipple trauma. | ||||
| 2.1 Nipple trauma at final assessment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| Analysis 2.3 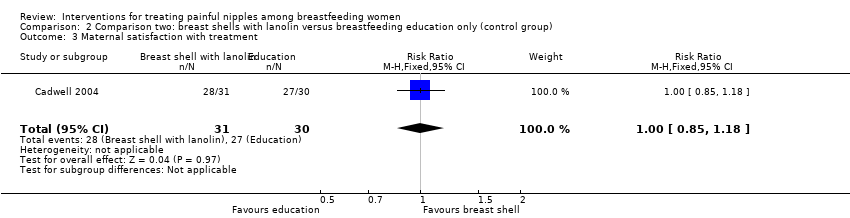 Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| Analysis 3.1  Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 1 Nipple pain. | ||||
| 1.1 Nipple pain at final assessment | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 2 Nipple trauma Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| Analysis 3.2 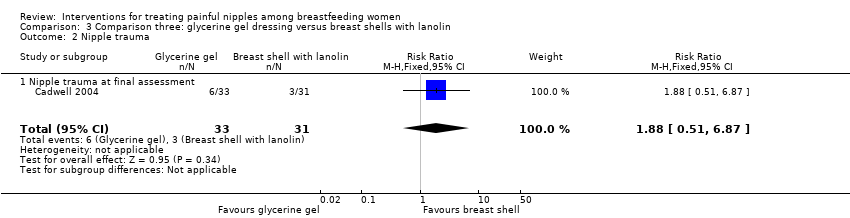 Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 2 Nipple trauma. | ||||
| 2.1 Nipple trauma at final assessment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.26] |
| Analysis 3.3  Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 3 Maternal satisfaction with treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 4.1 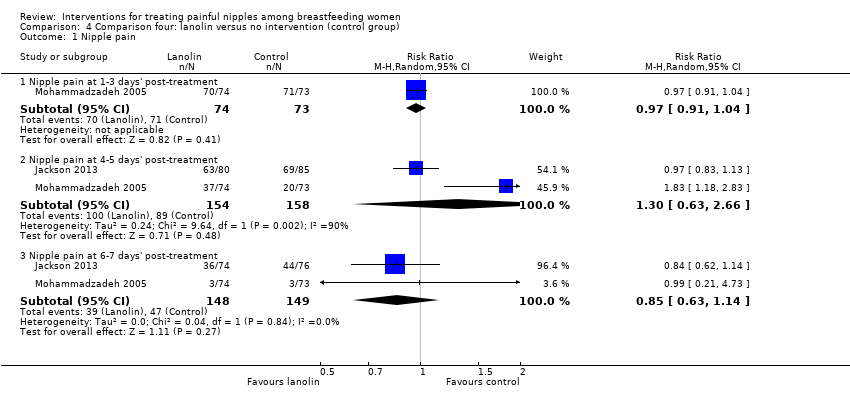 Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 1 Nipple pain. | ||||
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.63, 2.66] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.14] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.2  Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 2 Nipple trauma. | ||||
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.03] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.27] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.13, 2.91] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.3 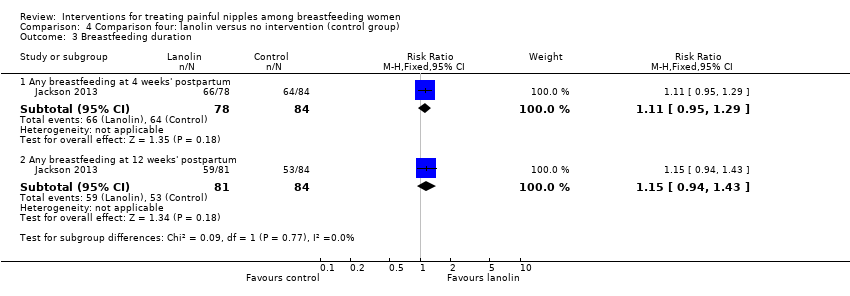 Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 3 Breastfeeding duration. | ||||
| 3.1 Any breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.43] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.4  Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 4 Breastfeeding exclusivity. | ||||
| 4.1 Exclusive breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.33] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 5 Maternal satisfaction with treatment Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.04, 1.25] |
| Analysis 4.5  Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 5 Maternal satisfaction with treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1 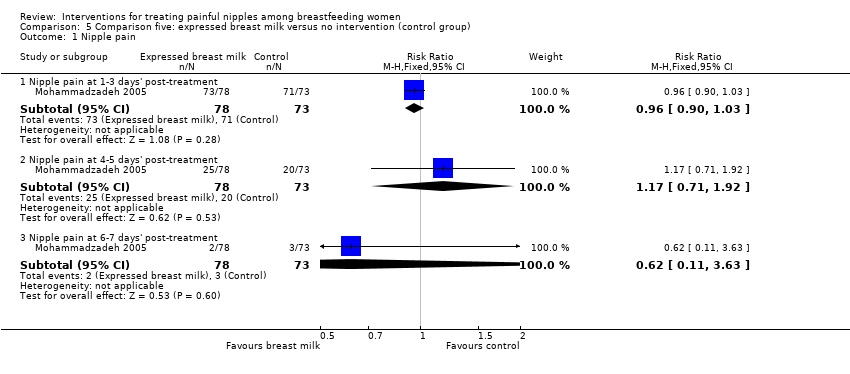 Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 1 Nipple pain. | ||||
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.92] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.11, 3.63] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.2  Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 2 Nipple trauma. | ||||
| 2.1 Nipple trauma 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.02] |
| 2.2 Nipple trauma 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 2.3 Nipple trauma 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.78, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1 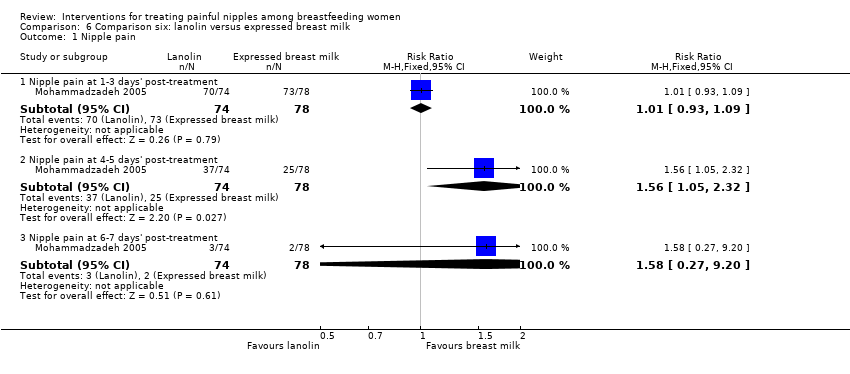 Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 1 Nipple pain. | ||||
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.32] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.27, 9.20] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.2  Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 2 Nipple trauma. | ||||
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.05] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.49] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.1  Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 1 Nipple pain. | ||||
| 1.1 Short Form McGill Pain at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [0.61, 4.41] |
| 1.2 Present Pain Index at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.24, 0.48] |
| 1.3 Pain Scale at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.67, 0.95] |
| 2 Mastitis Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| Analysis 7.2  Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 2 Mastitis. | ||||
| 2.1 Mastitis at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.3  Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 3 Breastfeeding duration. | ||||
| 3.1 Any breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.4 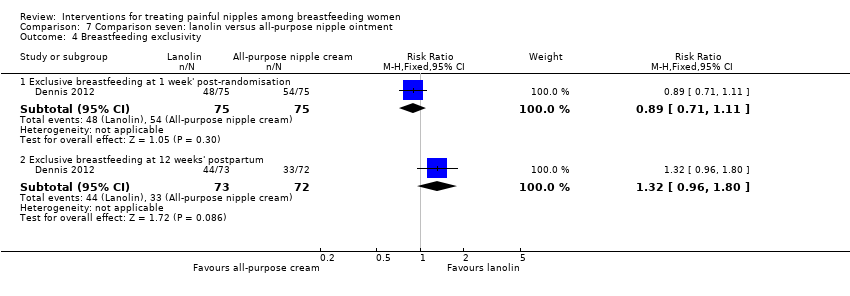 Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 4 Breastfeeding exclusivity. | ||||
| 4.1 Exclusive breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.80] |
| 5 Maternal satisfaction with breastfeeding Show forest plot | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| Analysis 7.5  Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 5 Maternal satisfaction with breastfeeding. | ||||
| 5.1 Maternal satisfaction with breastfeeding at 12 weeks' postpartum | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 6 Maternal satisfaction with treatment Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.14] |
| Analysis 7.6  Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 6 Maternal satisfaction with treatment. | ||||

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
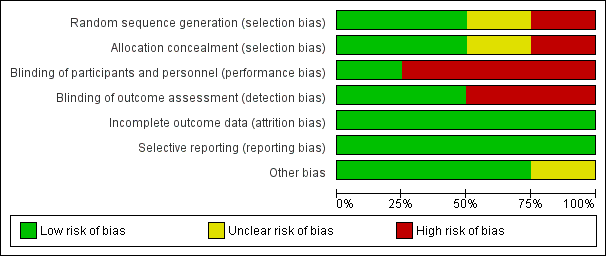
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 1 Nipple pain.

Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 2 Nipple trauma.
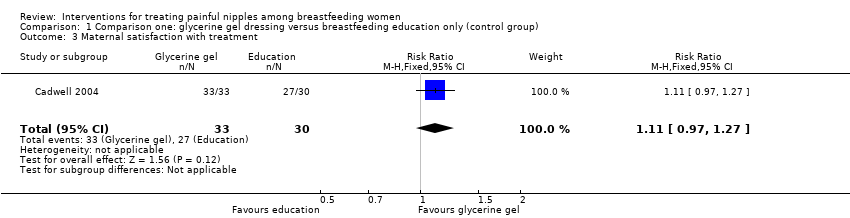
Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment.
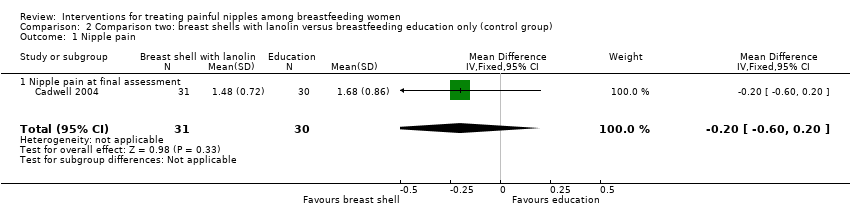
Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 1 Nipple pain.

Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 2 Nipple trauma.

Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment.

Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 1 Nipple pain.

Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 2 Nipple trauma.
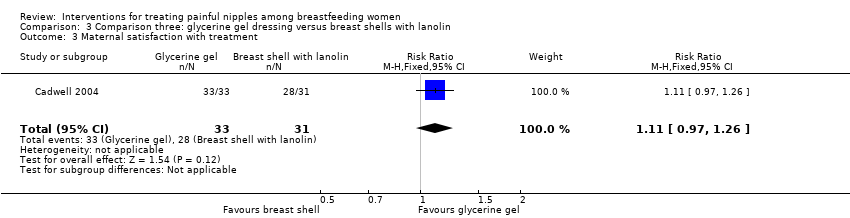
Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 3 Maternal satisfaction with treatment.

Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 1 Nipple pain.
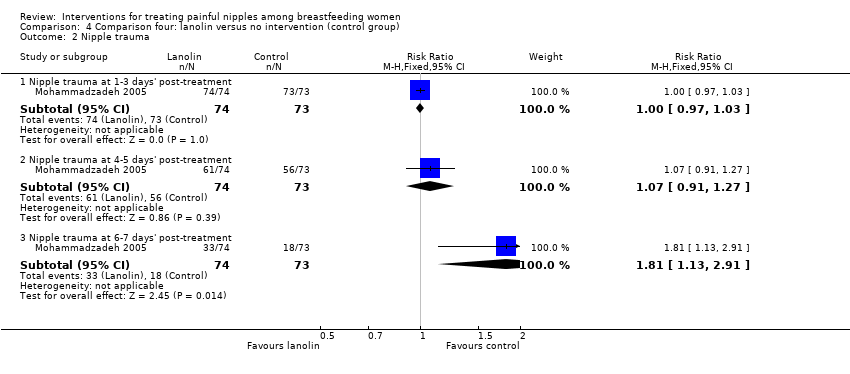
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 2 Nipple trauma.

Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 3 Breastfeeding duration.
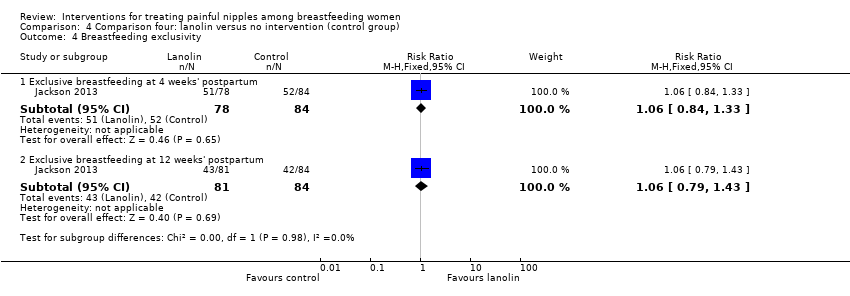
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 4 Breastfeeding exclusivity.
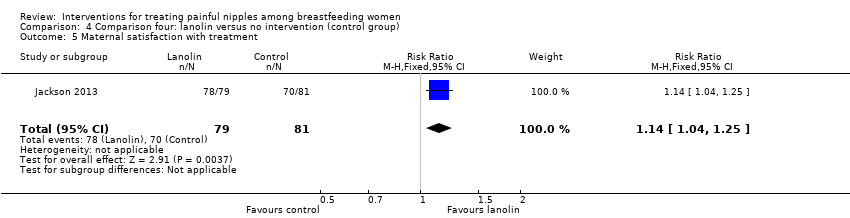
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 5 Maternal satisfaction with treatment.
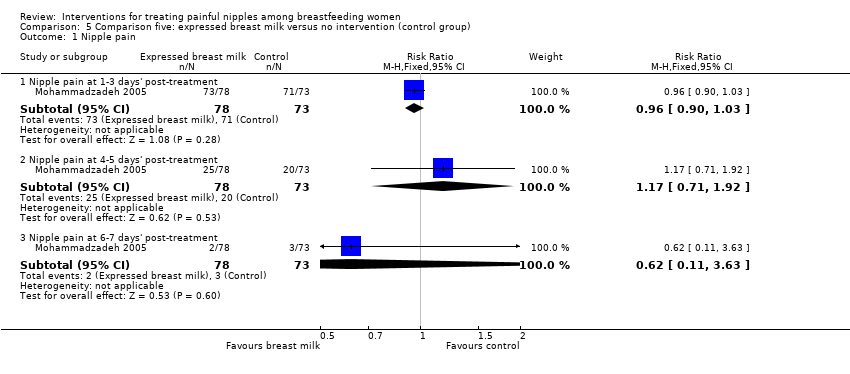
Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 1 Nipple pain.

Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 2 Nipple trauma.

Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 1 Nipple pain.

Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 2 Nipple trauma.
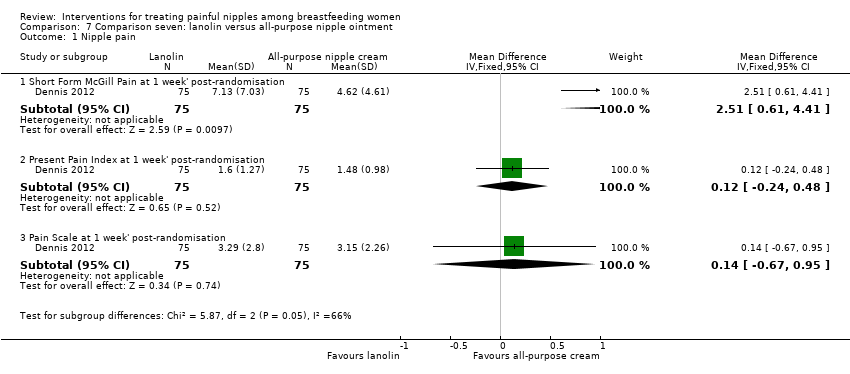
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 1 Nipple pain.
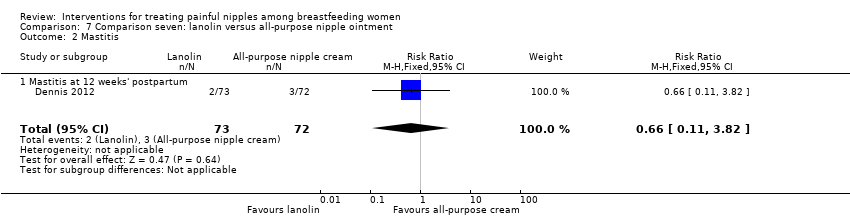
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 2 Mastitis.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 3 Breastfeeding duration.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 4 Breastfeeding exclusivity.
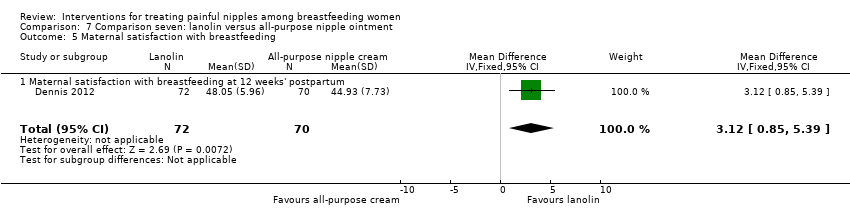
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 5 Maternal satisfaction with breastfeeding.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 6 Maternal satisfaction with treatment.
| Glycerine gel dressing versus breastfeeding education only (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breastfeeding education | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 63 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Breast shells with lanolin versus usual care for treating sore nipples in breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | breast shells with lanolin | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 61 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Glycerine gel dressing versus breast shells with lanolin for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breast shells with lanolin | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 64 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Lanolin versus no intervention (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Lanolin | |||||
| Nipple pain | Study population | RR 0.97 | 147 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 943 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 944 per 1000 | |||||
| Nipple pain | Study population | RR 1.30 | 312 | ⊕⊕⊝⊝ | ‐ | |
| 563 per 1000 | 732 per 1000 | |||||
| Moderate | ||||||
| 543 per 1000 | 706 per 1000 | |||||
| Nipple pain | Study population | RR 0.85 | 297 | ⊕⊕⊝⊝ | ‐ | |
| 315 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 310 per 1000 | 264 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). 3 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). | ||||||
| Expressed breast milk versus no intervention for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Expressed breast milk | |||||
| Nipple pain | Study population | RR 0.96 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 934 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 934 per 1000 | |||||
| Nipple pain | Study population | RR 1.17 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 274 per 1000 | 321 per 1000 | |||||
| Moderate | ||||||
| 274 per 1000 | 321 per 1000 | |||||
| Nipple pain | Study population | RR 0.62 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 41 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 41 per 1000 | 25 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (unclear selection bias; high risk of performance/detection bias). | ||||||
| Lanolin versus expressed milk for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples Comparison: expressed breast milk | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | lanolin | |||||
| Nipple pain | Study population | RR 1.01 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 936 per 1000 | 945 per 1000 | |||||
| Medium risk population | ||||||
| 936 per 1000 | 945 per 1000 | |||||
| Nipple pain | Study population | RR 1.56 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 321 per 1000 | 501 per 1000 | |||||
| Medium risk population | ||||||
| 321 per 1000 | 501 per 1000 | |||||
| Nipple pain | Study population | RR 1.58 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 26 per 1000 | 41 per 1000 | |||||
| Medium risk population | ||||||
| 26 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Insufficient information available regarding sequence generation, allocation generation method, and allocation concealment. | ||||||
| Lanolin versus all‐purpose nipple ointment for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lanolin | All‐purpose nipple ointment | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small number of participants. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 1.1 Nipple pain at final assessment | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 2 Nipple trauma Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 2.1 Nipple trauma at final assessment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 1.1 Nipple pain at final assessment | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 2 Nipple trauma Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 2.1 Nipple trauma at final assessment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 1.1 Nipple pain at final assessment | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 2 Nipple trauma Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 2.1 Nipple trauma at final assessment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.63, 2.66] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.14] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.03] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.27] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.13, 2.91] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.43] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Exclusive breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.33] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 5 Maternal satisfaction with treatment Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.04, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.92] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.11, 3.63] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.02] |
| 2.2 Nipple trauma 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 2.3 Nipple trauma 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.78, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.32] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.27, 9.20] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.05] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.49] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short Form McGill Pain at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [0.61, 4.41] |
| 1.2 Present Pain Index at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.24, 0.48] |
| 1.3 Pain Scale at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.67, 0.95] |
| 2 Mastitis Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 2.1 Mastitis at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Exclusive breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.80] |
| 5 Maternal satisfaction with breastfeeding Show forest plot | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 5.1 Maternal satisfaction with breastfeeding at 12 weeks' postpartum | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 6 Maternal satisfaction with treatment Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.14] |

