Intervenciones para el tratamiento del dolor del pezón en mujeres que lactan
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007366.pub2Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 15 diciembre 2014see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Cindy‐Lee Dennis and conceived and designed the protocol (Dennis 2008).
C‐L Dennis wrote the protocol and review with assistance from Kim Jackson (nee Allen).
Jo Watson contributed to the assessment of studies included and excluded in this review and co‐wrote the draft of the review and final revisions.
Sources of support
Internal sources
-
University of Toronto, Canada.
-
University of York, UK.
External sources
-
No sources of support supplied
Declarations of interest
There are no potential financial conflicts of interest; however, there may be potential perceived secondary conflicts of interest (personal conflict). Co‐author C‐L Dennis is the principal investigator for the lanolin versus all‐purpose nipple ointment trial included in this review (Dennis 2012). Co‐author K Jackson was a doctoral student who focused her dissertation on the topic of nipple pain in breastfeeding women. For her dissertation, Jackson was the principal investigator for a randomised controlled trial evaluating the effect of the application of lanolin versus nothing among breastfeeding women. This trial is included in the review (Jackson 2013). The contact author, C‐L Dennis, was K Jackson's PhD supervisor, who provided guidance throughout the duration of her studies and the planning and conduct of the aforementioned clinical trial. Decisions relating to the Dennis 2012 and Jackson 2013 studies (assessment of the studies for inclusion and trial quality, data extraction) were carried out by other members of the review team who were not directly involved in these trials.
Acknowledgements
We would like to acknowledge the contribution of the Cochrane Pregnancy and Childbirth Group's editorial team for their assistance with this review.
We thank Felicia McCormick, Mary Renfrew for their contribution to the development of the protocol for this review (Dennis 2008).
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers, and the Group's statistical adviser.
Version history
| Published | Title | Stage | Authors | Version |
| 2014 Dec 15 | Interventions for treating painful nipples among breastfeeding women | Review | Cindy‐Lee Dennis, Kim Jackson, Jo Watson | |
| 2008 Oct 08 | Interventions for treating painful nipples among breastfeeding women | Protocol | Cindy‐Lee Dennis, Kim Allen, Felicia M McCormick, Mary J Renfrew | |
Differences between protocol and review
The methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not include mothers with nipple pain due solely to manual expression of milk using various types of breast pumps (e.g. mothers with infants in the neonatal intensive care unit) in this review as the aetiology of the damage and required treatment is different.
We added LED phototherapy as an intervention for inclusion.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans;
PICO

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
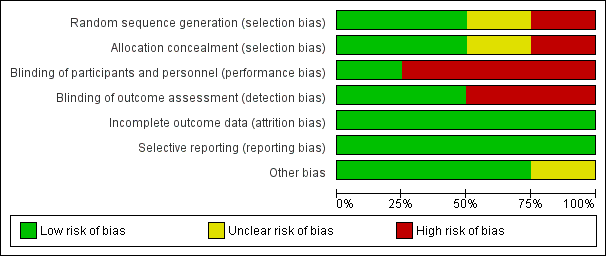
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 1 Nipple pain.

Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 2 Nipple trauma.
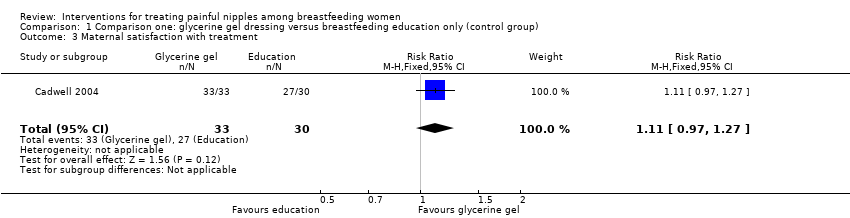
Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment.
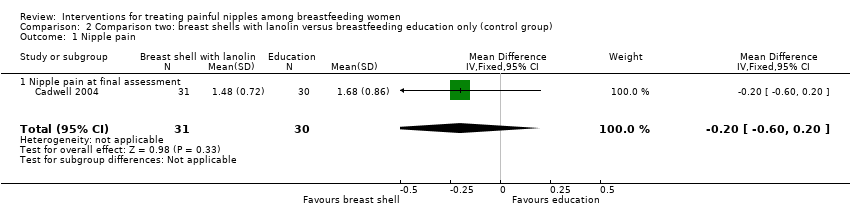
Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 1 Nipple pain.

Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 2 Nipple trauma.

Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment.

Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 1 Nipple pain.

Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 2 Nipple trauma.
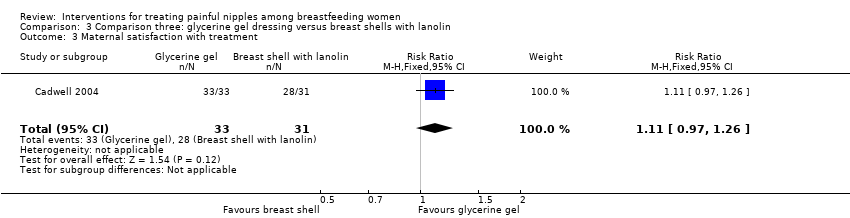
Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 3 Maternal satisfaction with treatment.

Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 1 Nipple pain.
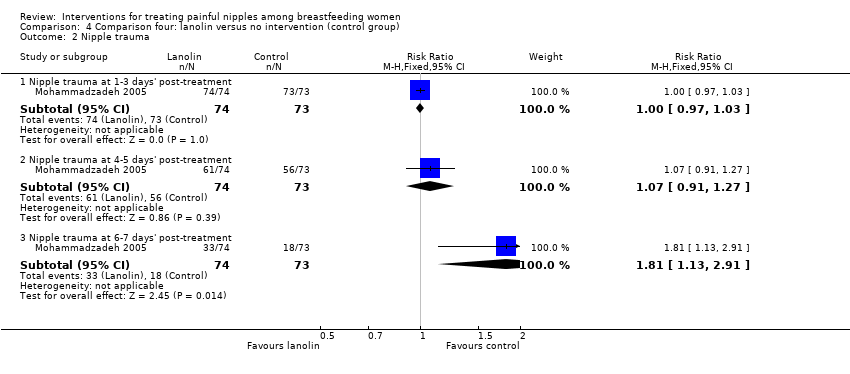
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 2 Nipple trauma.

Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 3 Breastfeeding duration.
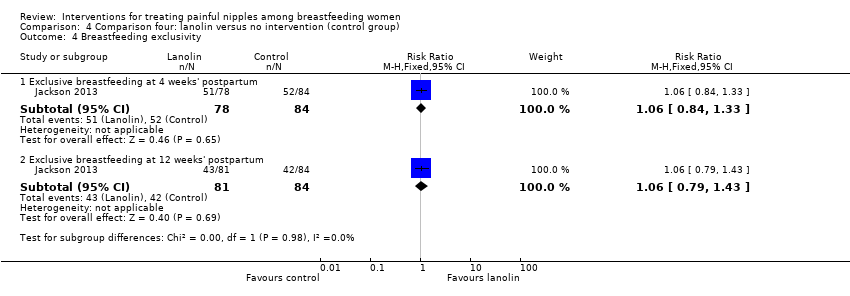
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 4 Breastfeeding exclusivity.
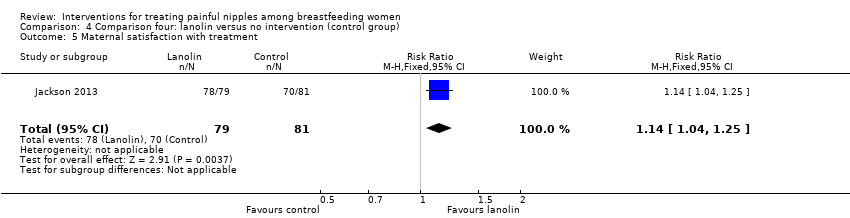
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 5 Maternal satisfaction with treatment.
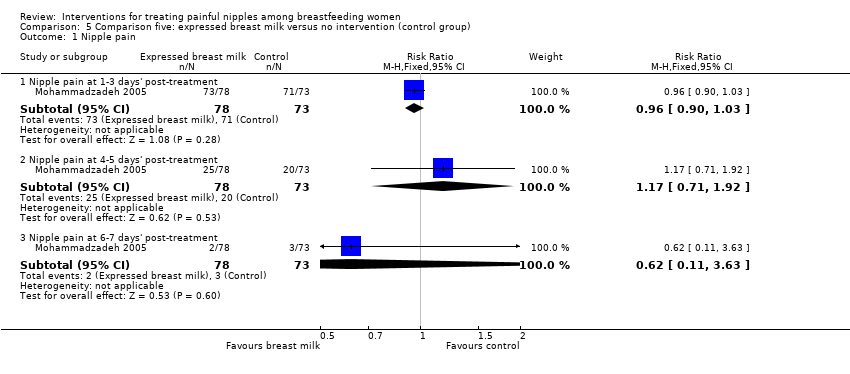
Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 1 Nipple pain.

Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 2 Nipple trauma.

Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 1 Nipple pain.

Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 2 Nipple trauma.
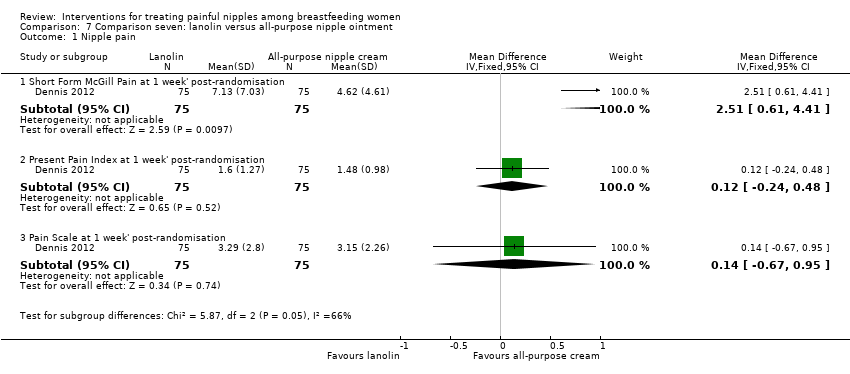
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 1 Nipple pain.
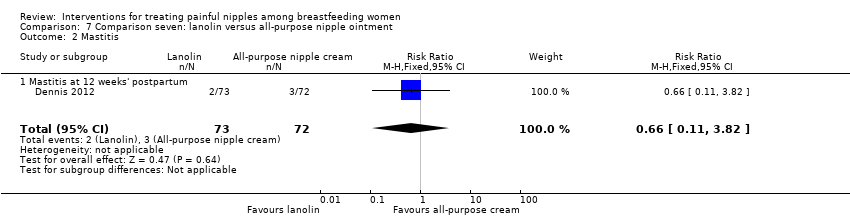
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 2 Mastitis.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 3 Breastfeeding duration.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 4 Breastfeeding exclusivity.
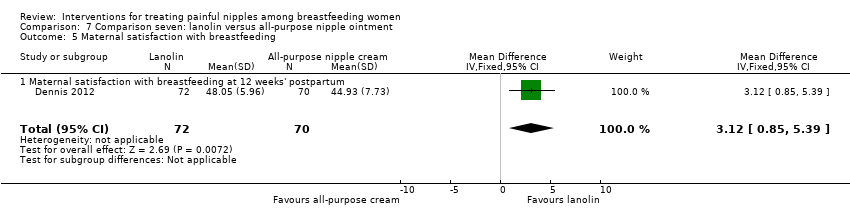
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 5 Maternal satisfaction with breastfeeding.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 6 Maternal satisfaction with treatment.
| Glycerine gel dressing versus breastfeeding education only (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breastfeeding education | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 63 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Breast shells with lanolin versus usual care for treating sore nipples in breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | breast shells with lanolin | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 61 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Glycerine gel dressing versus breast shells with lanolin for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breast shells with lanolin | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 64 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Lanolin versus no intervention (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Lanolin | |||||
| Nipple pain | Study population | RR 0.97 | 147 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 943 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 944 per 1000 | |||||
| Nipple pain | Study population | RR 1.30 | 312 | ⊕⊕⊝⊝ | ‐ | |
| 563 per 1000 | 732 per 1000 | |||||
| Moderate | ||||||
| 543 per 1000 | 706 per 1000 | |||||
| Nipple pain | Study population | RR 0.85 | 297 | ⊕⊕⊝⊝ | ‐ | |
| 315 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 310 per 1000 | 264 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). 3 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). | ||||||
| Expressed breast milk versus no intervention for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Expressed breast milk | |||||
| Nipple pain | Study population | RR 0.96 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 934 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 934 per 1000 | |||||
| Nipple pain | Study population | RR 1.17 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 274 per 1000 | 321 per 1000 | |||||
| Moderate | ||||||
| 274 per 1000 | 321 per 1000 | |||||
| Nipple pain | Study population | RR 0.62 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 41 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 41 per 1000 | 25 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (unclear selection bias; high risk of performance/detection bias). | ||||||
| Lanolin versus expressed milk for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples Comparison: expressed breast milk | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | lanolin | |||||
| Nipple pain | Study population | RR 1.01 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 936 per 1000 | 945 per 1000 | |||||
| Medium risk population | ||||||
| 936 per 1000 | 945 per 1000 | |||||
| Nipple pain | Study population | RR 1.56 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 321 per 1000 | 501 per 1000 | |||||
| Medium risk population | ||||||
| 321 per 1000 | 501 per 1000 | |||||
| Nipple pain | Study population | RR 1.58 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 26 per 1000 | 41 per 1000 | |||||
| Medium risk population | ||||||
| 26 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Insufficient information available regarding sequence generation, allocation generation method, and allocation concealment. | ||||||
| Lanolin versus all‐purpose nipple ointment for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lanolin | All‐purpose nipple ointment | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small number of participants. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 1.1 Nipple pain at final assessment | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 2 Nipple trauma Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 2.1 Nipple trauma at final assessment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 1.1 Nipple pain at final assessment | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 2 Nipple trauma Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 2.1 Nipple trauma at final assessment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 1.1 Nipple pain at final assessment | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 2 Nipple trauma Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 2.1 Nipple trauma at final assessment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.63, 2.66] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.14] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.03] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.27] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.13, 2.91] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.43] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Exclusive breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.33] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 5 Maternal satisfaction with treatment Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.04, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.92] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.11, 3.63] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.02] |
| 2.2 Nipple trauma 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 2.3 Nipple trauma 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.78, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.32] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.27, 9.20] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.05] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.49] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short Form McGill Pain at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [0.61, 4.41] |
| 1.2 Present Pain Index at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.24, 0.48] |
| 1.3 Pain Scale at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.67, 0.95] |
| 2 Mastitis Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 2.1 Mastitis at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Exclusive breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.80] |
| 5 Maternal satisfaction with breastfeeding Show forest plot | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 5.1 Maternal satisfaction with breastfeeding at 12 weeks' postpartum | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 6 Maternal satisfaction with treatment Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.14] |

