Interventions pour le traitement des mamelons douloureux chez la femme allaitante
Résumé scientifique
Contexte
Les principales autorités sanitaires recommandent toutes l'allaitement exclusif jusqu'à six mois post‐partum. Alors que la plupart des femmes commencent à allaiter, beaucoup l'interrompent en raison de difficultés rencontrées plutôt que par choix maternel. Une difficulté courante de l'allaitement sont les mamelons douloureux. Les recherches ont identifié le mauvais positionnement de l'enfant ou la prise incorrecte du sein comme une cause fréquente des mamelons douloureux. Alors que de nombreuses interventions différentes visant à réduire les douleurs des mamelons chez les femmes allaitantes ont été évaluées, on ne sait pas quelle intervention constitue le traitement le plus efficace. Une bonne compréhension des douleurs des mamelons et des options de traitement est nécessaire pour améliorer la durée de l'allaitement et les taux d'exclusivité et pour adresser de façon systématique l'une des difficultés les plus fréquemment rencontrées par les femmes allaitantes.
Objectifs
Évaluer les effets de toute intervention dans la résolution ou la réduction des douleurs du mamelon et l'impact des interventions sur d'autres critères de jugement tels que les traumatismes et infections du mamelon, la mastite du sein, la durée de l'allaitement maternel, l'exclusivité de l'allaitement maternel et la satisfaction maternelle.
Stratégie de recherche documentaire
Nous avons effectué des recherches dans le registre des essais cliniques du groupe Cochrane sur la grossesse et l'accouchement (30 septembre 2014) et avons examiné des références secondaires.
Critères de sélection
Tous les essais contrôlés randomisés ou quasi randomisés visant à évaluer toute intervention pour traiter les douleurs des mamelons chez la femme allaitante. Les essais à randomisation par grappes étaient éligibles à l'inclusion. Les essais croisés ne l'étaient pas. Les interventions suivantes étaient admissibles pour inclusion, en comparaison les unes avec les autres ou avec les soins habituels (c.‐à‐d. l'éducation seule) : interventions pharmacologiques (ex. crèmes antifongiques) ; traitements topiques non pharmacologiques (ex. lanoline) ; pansements (ex. pansements hydrogels) ; dispositifs de protection du mamelon (ex. coquilles d'allaitement), photothérapie et lait maternel exprimé. Chez les femmes qui nourrissent leur bébé avec du lait maternel exprimé (c.‐à‐d. les femmes dont les nourrissons sont dans des unités de soins néonatals), les douleurs des mamelons sont associées à d'autres méthodes d'expression de lait du sein de la mère, comme l'expression manuelle et différents types de tire‐lait. Dans cette population maternelle unique, les douleurs des mamelons et leur traitement subséquent sont différents. C'est pourquoi nous avons exclu de cette revue les femmes nourrissant leur bébé uniquement avec du lait maternel exprimé.
Recueil et analyse des données
Deux auteurs de la revue ont indépendamment évalué les essais à inclure, extrait les données, évalué la qualité méthodologique et vérifié l’exactitude des données. Nous avons recherché des informations supplémentaires auprès de plusieurs chercheurs impliqués dans les essais.
Résultats principaux
Nous avons inclus dans cette revue quatre essais de bonne qualité méthodologique portant sur un total de 656 femmes. Les quatre essais inclus évaluaient cinq interventions différentes, à savoir les coussinets de glycérine, la lanoline avec les coquilles d'allaitement, la lanoline seul, le lait maternel exprimé et une pommade tout usage pour les mamelons. Toutes les études comprenaient l'éducation pour positionner l'enfant au sein correctement dans le cadre des soins post‐partum de routine pour les groupes de traitement et témoin.
Des données regroupées existaient seulement pour la comparaison de la lanoline par rapport aux soins habituels. Nous n'avons pas regroupé les données pour les autres critères en raison de l'hétérogénéité dans les mesures des résultats ou d'interventions différentes.
Aucune preuve n'indique que les coussinets de gel à la glycérine ou les coquilles d'allaitement associées à la lanoline aient amélioré de façon significative les douleurs des mamelons. Un essai n'a observé aucune différence claire dans les douleurs des mamelons (à un à trois jours, quatre à cinq jours, ou six à sept jours post‐traitement) entre les femmes ayant appliqué de la lanoline ou n'ayant rien appliqué sur les mamelons. En revanche, le même essai a constaté que les femmes ayant appliqué du lait maternel exprimé sur les mamelons avaient des perceptions significativement plus faibles de la douleur des mamelons après quatre à cinq jours de traitement que les femmes ayant appliqué de la lanoline. Toutefois, cet effet bénéfique n'était pas maintenu après six à sept jours de traitement. Aucune différence entre les groupes n'a été observée dans la perception de la douleur du mamelon lors des évaluations entre les femmes ayant appliqué du lait maternel exprimé sur les mamelons et les femmes n'ayant rien appliqué. Les femmes ayant appliqué une pommade « tout usage » sur les mamelons n'ont ressenti aucune amélioration dans les douleurs des mamelons après sept jours de traitement par rapport aux femmes ayant appliqué de la lanoline. Les preuves étaient insuffisantes pour affirmer que les coussinets de gel à la glycérine, la lanoline associée aux coquilles d'allaitement, la lanoline seule, le lait maternel exprimé, ou une pommade tout usage pour les mamelons aient amélioré la perception maternelle des douleurs du mamelon.
Dans l'ensemble, les preuves sont insuffisantes pour recommander une intervention donnée pour le traitement des douleurs du mamelon. Cependant, une conclusion importante est que, quel qu'ait été le traitement utilisé, pour la plupart des femmes, les douleurs des mamelons ont diminué à des niveaux faibles approximativement sept à dix jours post‐partum. La fourniture de conseils préventifs sur le temps habituel avant la réduction des douleurs peut être une stratégie utile pour aider les femmes à continuer à allaiter et à le faire exclusivement. La qualité globale des preuves pour le critère de jugement principal des douleurs du mamelon était faible selon GRADE, principalement parce que des études individuelles avec peu de participants on fourni des données pour l'analyse.
Conclusions des auteurs
Les preuves sont insuffisantes pour affirmer que les coussinets de gel à la glycérine, les coquilles d'allaitement associées à la lanoline, la lanoline seule ou la pommade tout usage pour les mamelons aient amélioré de façon significative la perception maternelle des douleurs du mamelon. Les résultats de ces quatre études de qualité méthodologique élevée suggèrent que l'application de rien ou juste de lait maternel exprimé pourrait être aussi ou plus avantageux que l'application d'une pommade telle que la lanoline dans l'expérience à court terme de douleurs des mamelons.
La qualité des preuves dans cette revue n'a pas abouti à des conclusions solides concernant les objectifs évalués. Nous n'avons inclus dans la revue que quatre petits essais portant sur 656 femmes, et tous variaient en termes d'interventions comparées, de participants, de mesures de résultats et de normes de soins habituels. La qualité méthodologique des études incluses était élevée, mais la qualité globale des preuves pour le critère de jugement principal des douleurs du mamelon était faible, principalement parce que études individuelles avec peu de participants ont fourni des données pour l'analyse.
PICO
Résumé simplifié
Interventions pour le traitement des mamelons douloureux chez les femmes qui allaitent
Contexte
Bien que les avantages pour la santé de l'allaitement maternel soient bien établis, de nombreuses femmes arrêtent l'allaitement dans les premières semaines après l'accouchement. Une raison courante pour l'arrêt de l'allaitement sont les mamelons douloureux.
Caractéristiques des études
Nous avons recherché en septembre 2014 dans la base de données du groupe Cochrane sur la grossesse et l'accouchement des essais cliniques évaluant des méthodes (interventions) pour améliorer les douleurs des mamelons chez les femmes allaitantes. Nous avons également examiné la guérison et l'infection des mamelons, la durée de l'allaitement, les enfants nourris exclusivement au lait maternel et la satisfaction des mères vis‐à‐vis des traitements pour les problèmes de mamelons et l'allaitement en général. Les interventions comprenaient les traitements médicamenteux (traitements contre les bactéries administrés par voie orale, en spray ou en pommade ; traitements contre les infections fongiques), les traitements non médicamenteux (lanoline, vaseline, huile de menthe poivrée, glycérine), les pansements, les protège‐mamelons (coupelles ou coquilles), le traitement par la lumière ou l'application de lait maternel exprimé. Les interventions étaient comparées les unes aux autres ou aux soins habituels (témoin).
Principaux résultats
Nous avons trouvé quatre essais de bonne qualité méthodologique portant sur un total de 656 femmes et évaluant cinq interventions différentes, à savoir les coussinets de glycérine, la lanoline avec les coquilles d'allaitement, la lanoline seul, le lait maternel exprimé et une pommade tout usage pour les mamelons. Toutes les études comprenaient l'éducation pour positionner l'enfant au sein correctement dans le cadre des soins de routine pour les groupes d'intervention et témoin.
Actuellement, il n'y a pas suffisamment de preuves pour recommander un type spécifique de traitement pour les mamelons douloureux chez les femmes qui allaitent. Ces résultats suggèrent que l'application de rien ou du lait maternel exprimé pourrait être aussi ou plus avantageux que l'application d'une pommade telle que la lanoline dans l'expérience à court terme de douleurs des mamelons. Une conclusion importante de cette étude est que, quel qu'ait été le traitement utilisé, pour la plupart des femmes, les douleurs des mamelons ont diminué à des niveaux faibles environ sept à dix jours après l'accouchement (post‐partum).
Qualité des preuves
La qualité des preuves dans cette revue ne permet pas de formuler des conclusions solides concernant le traitement des douleurs du mamelon. Nous n'avons trouvé que quatre petits essais et tous variaient en termes d'interventions comparées, de participants, de variables mesurés et de normes de soins habituels. Bien que la qualité méthodologique des études incluses ait été élevée, la qualité globale des preuves pour le critère principal des douleurs du mamelon était faible, principalement en raison des études individuelles avec peu de participants ayant fourni des données pour l'analyse.
Authors' conclusions
Summary of findings
| Glycerine gel dressing versus breastfeeding education only (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breastfeeding education | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 63 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Breast shells with lanolin versus usual care for treating sore nipples in breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | breast shells with lanolin | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 61 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Glycerine gel dressing versus breast shells with lanolin for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breast shells with lanolin | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 64 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Lanolin versus no intervention (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Lanolin | |||||
| Nipple pain | Study population | RR 0.97 | 147 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 943 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 944 per 1000 | |||||
| Nipple pain | Study population | RR 1.30 | 312 | ⊕⊕⊝⊝ | ‐ | |
| 563 per 1000 | 732 per 1000 | |||||
| Moderate | ||||||
| 543 per 1000 | 706 per 1000 | |||||
| Nipple pain | Study population | RR 0.85 | 297 | ⊕⊕⊝⊝ | ‐ | |
| 315 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 310 per 1000 | 264 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). 3 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). | ||||||
| Expressed breast milk versus no intervention for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Expressed breast milk | |||||
| Nipple pain | Study population | RR 0.96 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 934 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 934 per 1000 | |||||
| Nipple pain | Study population | RR 1.17 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 274 per 1000 | 321 per 1000 | |||||
| Moderate | ||||||
| 274 per 1000 | 321 per 1000 | |||||
| Nipple pain | Study population | RR 0.62 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 41 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 41 per 1000 | 25 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (unclear selection bias; high risk of performance/detection bias). | ||||||
| Lanolin versus expressed milk for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples Comparison: expressed breast milk | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | lanolin | |||||
| Nipple pain | Study population | RR 1.01 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 936 per 1000 | 945 per 1000 | |||||
| Medium risk population | ||||||
| 936 per 1000 | 945 per 1000 | |||||
| Nipple pain | Study population | RR 1.56 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 321 per 1000 | 501 per 1000 | |||||
| Medium risk population | ||||||
| 321 per 1000 | 501 per 1000 | |||||
| Nipple pain | Study population | RR 1.58 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 26 per 1000 | 41 per 1000 | |||||
| Medium risk population | ||||||
| 26 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Insufficient information available regarding sequence generation, allocation generation method, and allocation concealment. | ||||||
| Lanolin versus all‐purpose nipple ointment for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lanolin | All‐purpose nipple ointment | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small number of participants. | ||||||
Background
Description of the condition
Leading health authorities, such as the World Health Organization (WHO 2009), the Canadian Paediatric Society (Canadian Pediatric Society 2013), and the American Academy of Pediatrics (American Academy of Pediatrics 2012), recommend breastfeeding as the optimal method of infant feeding. These recommendations suggest that infants be breastfed exclusively for the first six months of life and then, once other foods have been introduced, continue to be breastfed until two years and beyond. This strong endorsement is based on compelling evidence that breastfeeding offers important infant health benefits such as decreased incidence of childhood infections; lower sudden infant death syndrome and post‐neonatal mortality rates; and reduced incidence of childhood obesity, diabetes, and certain childhood cancers. Women also benefit from breastfeeding and may experience decreased postpartum bleeding and lower risks of breast and ovarian cancers. Breastfeeding has a significant economic impact. One cost‐analysis study reported that if 90% of families complied with the recommendation to breastfeed exclusively to six months, the US would save USD13 billion a year in medical healthcare costs and prevent an excess of 911 infant deaths (Bartick 2010).
Despite these significant individual and societal salutary effects and a breastfeeding initiation rate of 90% in Canada, one 2006/2007 nationwide telephone interview conducted by Statistics Canada on behalf of the Public Health Agency of Canada (8244 women) found a marked decline in exclusive breastfeeding in the early postpartum period (Public Health Agency of Canada 2009). At three months, 51.7% of women were exclusively breastfeeding decreasing to 14.4% at six months. At three months, 67.6% of women were offering some breast milk. This figure decreased to 53.9% at six months. Supplementation (provision of liquids other than breast milk) was initiated on average at 12 weeks' postpartum, with 25% of women supplementing by two weeks' postpartum. Data from 2011 to 2012 indicated that 89% of Canadian women initiated breastfeeding with approximately 26% exclusively breastfeeding to six months (Statistics Canada 2013). According to the Centers for Disease Control and Prevention, 74.6% of women in the US initiate breastfeeding. However, only 35% of these women were exclusively breastfeeding at three months' postpartum and only 44.6% continued any breastfeeding to six months' postpartum with only 14.8% doing so exclusively (CDC 2011). While still low, the initiation of breastfeeding has continued to increase across Great Britain. Breastfeeding rates rose from 78% to 83% between 2005 and 2010 in England, from 67% to 71% in Wales, and from 70% to 74% in Scotland. In Northern Ireland, the rate changed from 63% to 64% (NHS Information Centre 2011). Overall, these sub‐optimal breastfeeding initiation, duration, and exclusivity rates suggest that women and their infants are not receiving the maximum health benefits that breastfeeding provides.
While the reasons for early discontinuation are variable and complex, it is clear that many women discontinue breastfeeding due to difficulties encountered rather than maternal choice (Cooke 2003; Dennis 2002a; McLeod 2002). One common difficulty that many women experience is painful nipples (Cooke 2003; Henderson 2001; Kearney 1990; Livingstone 1996; NHS Information Centre 2011; Ziemer 1992). The reported incidence of nipple pain and trauma varies between 34% and 96% of breastfeeding women (Duffy 1997; Head 1995; Hewat 1987; Humenick 1983; Lavergne 1997; Tait 2000; Walker 1997; Ziemer 1992; Ziemer 1995). Characteristics associated with nipple pain include cracked, sore, bleeding, blistered nipples that may have fissures and abrasions present (Morland‐Schultz 2004). For many women, nipple pain appears to have the greatest intensity between the third and seventh day postpartum, with a peak in severity on the third day postpartum (Hewat 1987; Ziemer 1990). Several researchers have identified poor infant positioning or latch as the most common cause of persistent nipple pain within the first 10 days' postpartum (Amir 1996a; Ziemer 1990). Similarly, poor infant positioning has been identified as a significant factor related to nipple trauma (Enkin 2000). Others researchers have suggested that nipple pain may be related to the use of nipple shields, lack of nipple exposure to light and air, breast engorgement, and the frequency and duration of feedings (Morland‐Schultz 2004; Walker 1989). Unfortunately, healing damaged nipples in breastfeeding women is complicated due to repeated trauma from infant sucking and exposure to maternal skin and infant oral flora predisposing the nipple to infection (Brent 1998). In particular, any break in the skin surface may lead to a predisposition to secondary bacterial and fungal infection. As such, damaged nipples have been associated with an increased presence of infection with Candida, most commonly Candida albicans (Amir 1991; Amir 1996b; Fetherston 1998; Tanguay 1994; Tomassen 1998), and Staphylococcus aureus (Amir 1996b; Livingstone 1996; Livingstone 1999; Thomsen 1983; Thomsen 1984). Clinically, it is believed that most cases of persistent nipple pain with minimal trauma can be resolved by altering the positioning and latch of the infant to the breast, whereas women with visible nipple trauma may benefit from being treated with antibacterial or antifungal medication. As such, when considering treatment it is important to note clinically that painful nipples may be a symptom of a problem, a problem in and of itself, and it can be a risk factor for bacterial of fungal infection (for which painful nipples is a symptom). Unfortunately, nipple pain and trauma have been associated with decreased breastfeeding duration (Evans 1995; Gulick 1982; Rentschler 1991; Schwartz 2002), introduction of artificial infant milks (Goodine 1984), and increased levels of stress (Amir 1996b). Nipple pain can also decrease breastfeeding self efficacy (Dennis 1999), a variable that has been demonstrated internationally to influence breastfeeding duration and exclusivity rates. It is also known that pain has an inhibitory effect on the release of oxytocin, a hormone that causes the small muscles around the milk ducts to contract and release milk (Morland‐Schultz 2004). Despite documentation of the many detrimental outcomes associated with nipple pain, there is very little information describing the characteristics and effect of pain experienced by breastfeeding women (McClellan 2012).
Description of the intervention
Many different interventions designed to reduce nipple pain in breastfeeding women have been evaluated. These include pharmacological topical treatments (e.g. antibiotic/antifungal cream), non‐pharmacological topical treatments (e.g. lanolin, peppermint oil), dressings (e.g. warm compresses, hydrogel dressings, tea bags), nipple protection devices (e.g. breast shells), light emitting diode (LED) phototherapy, and expressed breast milk (EBM) (Morland‐Schultz 2004; Page 2003).
How the intervention might work
Due to the various causes of nipple pain, diverse treatment interventions have been proposed. The main purpose of these interventions is to treat the underlying cause of the pain (e.g. tissue trauma or infection) and promote wound healing if necessary. The most common treatment is maternal education focused on proper latch and positioning to address the underlying causes of nipple pain such as friction and compression. Seminal work on the outcomes of moist wound healing, such as glycerine pads, was introduced by George Winter (Winter 1962). Winter theorised that the skin becomes partially dehydrated during normal wound healing, which causes white blood cells to become trapped at the skin surface, slowing the rate of healing. Since Winter's discovery, the advantages of moist wound healing have become more widely recognised and include reduced dehydration and cell death (Keast 1998), increased healing (Haimowitz 1997; Knighton 1981), and decreased pain (Keast 1998). Lanolin is a waxy secretion produced by the sebaceous glands of sheep. Medical‐grade lanolin, such as Lansinoh® HPA®, is a single‐ingredient ointment processed to be free of alcohols, detergent and pesticide residues, colour, and odour‐forming impurities. It is thought to provide a bacteriostatic, semi‐occlusive barrier to the skin that allows for moisture retention and enhances healing. An "all‐purpose nipple ointment" has been used by Canadian women with painful or damaged nipples since approximately 2001 and contains an antibacterial cream (mupirocin 2% ointment ‐ 15 g), an antifungal cream (miconazole powder to give a 2% concentration), and a hydrocortisone cream (betamethasone 0.1% ointment ‐ 15 g). The ointment is thought to treat underlying bacterial and fungal infections while treating inflammation. EBM is used to treat painful nipples due to its anti‐infective and antiviral properties. Breast shells are hollow plastic discs worn over the nipple and aerola and are thought to reduce pain by protecting the nipple from contact and stimulation. Glycerine‐based gel dressings ("Smoothies" by Puronxy Inc.) are absorbent, non‐adhesive pads that can be lifted from the skin without disintegrating or causing pain or trauma to new, sensitive skin. LED phototherapy is thought to increase blood supply and cell proliferation and function to assist with wound healing.
Why it is important to do this review
It is unclear which intervention is the most appropriate treatment in the resolution or reduction of nipple pain. To improve breastfeeding duration and exclusivity rates and to address one of the most common difficulties encountered by breastfeeding women systematically, a good understanding of nipple pain and a corresponding effective treatment is needed.
Objectives
To assess the effects of all interventions in the resolution or reduction of nipple pain and the impact of the interventions on other outcomes such as nipple trauma, nipple infections, breast mastitis, breastfeeding duration, breastfeeding exclusivity, and maternal satisfaction.
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials (including cluster‐randomised trials) designed to evaluate diverse interventions to treat nipple pain (variously defined) among breastfeeding women. Cross‐over trials and trials where the unit of randomisation was the individual breast, rather than the individual mother, were not eligible for inclusion.
Types of participants
Participants were breastfeeding women identified with nipple pain (variously defined). We excluded women with nipple pain due solely to manual expression of milk using various types of breast pumps (e.g. women with infants in the neonatal intensive care unit) in this review, as the aetiology of the damage and required treatment is different. We also excluded trials that only recruited women with infants who had ankyloglossia (e.g. tongue‐tied) where the purpose was to evaluate the effect of frenotomy on nipple pain relief.
Types of interventions
Any intervention designed to reduce nipple pain in breastfeeding women. This included pharmacological oral and topical treatments, non‐pharmacological topical treatments, dressings, nipple protection devices, and EBM.
We compared the following types of interventions with each other or usual care (i.e. education only):
-
pharmacological interventions (e.g. oral antibiotics, antibiotic sprays, antibiotic ointments, antifungal ointments);
-
non‐pharmacological topical interventions (e.g. lanolin, petroleum jelly, peppermint oil, glycerine gel, proprietary ointments);
-
dressing interventions (e.g. warm water compresses, hydrogel dressings, polyethylene film dressings);
-
nipple protection interventions (e.g. breast shells, silicone nipple shields);
-
LED phototherapy; and
-
EBM.
Types of outcome measures
Primary outcomes
-
Nipple pain (as defined by trial authors).
Secondary outcomes
-
Nipple trauma (healing) (as defined by trial authors).
-
Nipple infection (as defined by trial authors).
-
Mastitis (as defined by trial authors).
-
Breastfeeding duration.
-
Breastfeeding exclusivity.
-
Maternal satisfaction with treatment and with breastfeeding.
Search methods for identification of studies
We based the following methods section of this review on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (30 September 2014).
The Cochrane Pregnancy and Childbirth Group's Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
-
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
-
weekly searches of MEDLINE (Ovid);
-
weekly searches of Embase (Ovid);
-
handsearches of 30 journals and the proceedings of major conferences;
-
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Specialized Register' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We scanned reference lists of retrieved studies; we did not apply any language or date restrictions.
Data collection and analysis
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
The primary author reviewed and retrieved titles and abstracts identified as a result of the search strategy. Three review authors independently assessed for inclusion all the potential studies. We resolved any disagreements through discussion.
Data extraction and management
We designed a form to extract data. Three review authors extracted the data using the agreed form, ensuring that authors did not extract data from any trial they were principal investigators for. We resolved discrepancies through discussion. We entered data into Review Manager 5 software (RevMan 2014), and checked them for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We developed 'Summary of findings' tables using the GRADEpro program.
1. Sequence generation (checking for possible selection bias)
For each study that met inclusion criteria, we described the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
-
low risk (e.g. random number table, computer random‐number generator);
-
high risk (odd or even date of birth, hospital or clinic record number); or
-
unclear risk.
2. Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
-
low risk (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
-
high risk (e.g. open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
-
unclear risk (e.g. insufficient information to permit judgement of 'low risk' or 'high risk').
3. Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described all the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We also provided any information relating to whether the intended blinding was effective. We noted where there has been partial blinding (e.g. where it has not been possible to blind participants but where outcome assessment was carried out without knowledge of group assignment).
We assessed the methods as:
-
low risk (e.g. no blinding but review authors judged that the outcome was not likely to be influenced by lack of blinding or blinding of participants and key study personnel ensured and unlikely to have been broken);
-
high risk (e.g. no blinding or incomplete blinding and outcome was likely to be influenced by lack of blinding or blinding attempted but likely to have been broken and outcome was likely to have been influenced by lack of blinding);
-
unclear (e.g. insufficient information to permit judgement of 'low risk' or 'high risk').
4. Blinding of outcome assessment (checking for possible performance bias)
For each included study, we described all the methods used, if any, to blind outcome assessment from knowledge of which intervention a participant received.
We assessed the methods as:
-
low risk (e.g. no blinding of outcome assessment but review authors judged that the outcome was not likely to be influenced by lack of blinding or blinding of outcome assessment ensured and unlikely to have been broken);
-
high risk (e.g. no blinding of outcome assessment and outcome assessment was likely to be influenced by lack of blinding or blinding attempted but likely to have been broken and outcome was likely to have been influenced by lack of blinding);
-
unclear (e.g. insufficient information to permit judgement of 'low risk' or 'high risk'.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, drop‐outs, protocol deviations)
For each included study, we described the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and any re‐inclusions in analyses which we undertook.
We assessed the methods as:
-
low risk (e.g. where there were no missing data or where similar reasons for missing data were reported between the two groups);
-
high risk (e.g. where missing data were likely to be related to outcomes or were not balanced across groups, or where high levels of missing data were likely to introduce serious bias or make the interpretation of results difficult);
-
unclear risk (e.g. where there was insufficient reporting of attrition or exclusions to permit a judgement to be made).
6. Selective reporting bias
For each included study, we described how the possibility of selective outcome reporting bias was examined by us and what we found.
We assessed the methods as:
-
low risk (where it was clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
-
high risk (where not all the study's pre‐specified outcomes had been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study did not include results of a key outcome that would have been expected to have been reported);
-
unclear risk (e.g. insufficient information to permit judgement of risk).
7. Other sources of bias
For each included study, we described any important concerns we had about other possible sources of bias. For example, was there a potential source of bias related to the specific study design? Was the trial stopped early due to some data‐dependent process? Was there extreme baseline imbalance? Had the study been claimed to be fraudulent?
We assessed whether each study was free of other problems that could put it at risk of bias:
-
low risk (e.g. the study appeared free of other sources of bias);
-
high risk (e.g. there was at least one important risk of bias related to the specific study design);
-
unclear risk (e.g. there was insufficient information to permit judgement of risk).
8. Overall risk of bias
We made explicit judgements about risk of bias for important outcomes within studies. With reference to 1. to 7. above, we assessed the likely magnitude and direction of the bias and whether we considered it as likely to impact on the findings. We would have explored the impact of the level of bias through undertaking sensitivity analyses if needed.
We assessed the quality of the evidence using the GRADE approach to assess the quality of the body of evidence relating to the primary outcome of nipple pain for all comparisons (Schunemann 2009).
-
Glycerine gel dressing versus usual care.
-
Breast shells with lanolin versus usual care.
-
Glycerine gel dressing versus breast shells with lanolin.
-
Lanolin versus no intervention.
-
EBM versus no intervention.
-
Lanolin versus EBM.
-
Lanolin versus all‐purpose nipple ointment.
We used GRADEprofiler (GRADE 2008) to import data from Review Manager 5 (RevMan 2014) in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we presented results as mean difference (MD) if outcomes were measured in the same way between trials and as standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this review. If we identify any cluster‐randomised trials for inclusion in future updates of this review we will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population (Higgins 2011). If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and we consider the interaction between the effect of intervention and the choice of randomisation unit to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We deemed cross‐over trials ineligible for inclusion, as they do not allow the use of outcomes that require some time to develop such as resolution of infection or breastfeeding duration.
Dealing with missing data
We noted levels of attrition when we evaluated the risk of bias. In future updates, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using a sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis (i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention). The denominator for each outcome in each trial was the number or women randomised minus any women whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity using the Tau2, I2, and Chi2 statistics. We regarded heterogeneity as substantial if I2 was greater than 30% and either Tau2 was greater than zero or there was a low P value (less than 0.10) in the Chi2 test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate possible reporting biases (such as publication bias) using funnel plots. We will assess funnel plots visually, and, if there is any obvious asymmetry apparent, we will seek statistical advice on carrying out formal tests for funnel plot asymmetry.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect (i.e. where trials were examining the same intervention and we judged the trials' populations and methods sufficiently similar). If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials or if we detected substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary if we considered a mean treatment effect across trials clinically meaningful. Random‐effects summaries were treated as the mean range of possible treatment effects. If the mean treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, we presented the results as the mean treatment effect with 95% CIs and the estimates of Tau2 and I2 statistics were provided.
Subgroup analysis and investigation of heterogeneity
We did not perform subgroup analysis due to insufficient data. In future updates, we plan to carry out the following pre‐specified subgroup analyses:
-
oral versus topical pharmacological interventions.
We will restrict subgroup analysis to the primary outcome. We will assess subgroup differences by interaction tests available within Review Manager 5 (RevMan 2014).
Sensitivity analysis
We did not conduct a sensitivity analysis. In future updates, we will carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies (high risk of bias for these domains) excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
See: Figure 1.

Study flow diagram.
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register identified 40 reports related to the treatment of nipple pain. Following application of eligibility criteria, we included four trials (involving 656 women) in the review (Cadwell 2004; Dennis 2012; Jackson 2013; Mohammadzadeh 2005). We excluded 35 studies and one study is awaiting translation (Tafazoli 2010).
Included studies
See Characteristics of included studies table.
Participants
All studies included postpartum women who had initiated breastfeeding and presented with complaints of sore nipples or nipple trauma, or both. Nipple pain in the breastfeeding women was associated with the transfer of milk from the mother's breast to the infant via the infant's mouth. While three trials specified a timeframe for onset of nipple pain and trauma (all within 14 days' postpartum) (Cadwell 2004;Dennis 2012;Jackson 2013), one trial did not (Mohammadzadeh 2005). However, the mean time for onset of symptoms in the Mohammadzadeh 2005 study was between three and four days' postpartum. While the participants' ethnic backgrounds were not specified, the studies included were conducted in Iran (Mohammadzadeh 2005), Latvia (Cadwell 2004), and Canada (Dennis 2012; Jackson 2013). All trials were hospital‐based. One trial recruited women from the neonatal intensive care unit (Mohammadzadeh 2005).
Three trials did not consider breastfeeding exclusivity as inclusion criteria even though all participants in these three trials were exclusively breastfeeding (Cadwell 2004; Jackson 2013; Mohammadzadeh 2005). Conversely, one trial excluded participants using finger feeding or lactation devices to give artificial infant milk (Dennis 2012). This trial also excluded women with any breast condition that would preclude exclusive breastfeeding (Dennis 2012).
Description of nipple pain, nipple trauma, and other trial outcomes
The conceptualisation of nipple pain and trauma varied among the four studies. Two trials included women with nipple pain or the presence of nipple trauma (e.g. cracked, bleeding, blistered nipples, or a combination) that may have fissures and abrasions present) or both (Cadwell 2004; Jackson 2013). One trial included women with sore nipples and fissures on or around the nipple (Mohammadzadeh 2005). Similarly, one trial included women with painful nipples having an open area of the skin on either the nipple or areola (Dennis 2012).
While all studies used various measures to assess the outcome of nipple pain, only one study assessed for nipple trauma (Mohammadzadeh 2005). Cadwell 2004 used 12 hospital‐based midwives for a maximum of four follow‐up visits to assess for nipple pain and wound healing. During the 10‐day trial period, women rated their nipple pain at each follow‐up using a 5‐point verbal descriptor scale where: 1 = no pain, 2 = minor discomfort, 3 = moderate pain, 4 = severe pain, and 5 = the worst pain imaginable. At each follow‐up visit, midwives ranked the signs and symptoms of wound healing on a scale of 1 to 3 where: 1 = better or resolved (no pain and skin intact), 2 = no change (persistent pain or no wound healing), and 3 = worse (persistent pain with purulent exudates and extension of lesions). Conversely, scant details were provided by Mohammadzadeh 2005 regarding how nipple pain or trauma was assessed. Participants were interviewed and physically examined on days one, three, five, seven, and 10 post‐randomisation to monitor for improvement in pain and wound healing. Improvement of pain was defined as maternal report of the absence of irritation. There was no definition of wound healing reported. Neither Cadwell 2004 or Mohammadzadeh 2005 provided details regarding reliability or validity of measurement tools. However, Cadwell 2004 reported test‐re‐test reliability on a similar verbal descriptor scale used in another nipple pain trial (Ziemer 1990). In the trial by Dennis 2012, a research assistant blinded to group allocation assessed outcomes pertaining to nipple pain, breastfeeding duration, breastfeeding exclusivity, and maternal satisfaction with infant feeding method and nipple pain treatment. In Dennis 2012, nipple pain was measured at baseline and at one week' post‐randomisation using the Short Form McGill Pain Questionnaire (SF‐MPQ), which contains 15 adjectives that describe pain rated on a 4‐point intensity scale (scores ranged from 0 to 45). The Present Pain Intensity (PPI) was also included from the long‐form McGill Pain Questionnaire (scores ranged from 0 to 5), and the visual analogue scale (VAS) of the SF‐MPQ that was modified into a Likert‐type scale (scores ranged from 0 to 10). The SF‐MPQ has well documented reliability and validity. Dennis 2012 also reported the incidence of mastitis symptoms assessed at 12 weeks' postpartum via telephone interview. Women were considered to have symptoms of mastitis based on criteria suggested by Fetherston 1998: one or more systemic symptoms (fever, chills, ache) and one or more localised symptoms (redness, swelling, pain, lump, or nipple crusts). For breastfeeding duration, during the one‐week post‐randomisation and 12‐week postpartum follow‐up assessments, women were asked if their infants had received any breast milk in the preceding 24 hours. A positive response was indicative of continued breastfeeding. Breastfeeding exclusivity was determined at one week' post‐randomisation and 12 weeks' postpartum using the infant feeding categories defined by Labbok 1990 (exclusive breastfeeding, almost exclusive breastfeeding, high breastfeeding, partial breastfeeding, token breastfeeding, and bottle feeding). The Maternal Satisfaction with Infant Feeding Questionnaire was administered via telephone at the 12‐week postpartum follow‐up assessment to determine women's satisfaction with their chosen infant feeding method (Dennis 2002b). Participants responded to each of the 12 items by rating their feelings on a 5‐point Likert‐type scale where 1 = strongly disagree and 5 = strongly agree; items are summed to produce a total score ranging from 12 to 60 with higher scores representing increased maternal satisfaction. Maternal satisfaction with treatment at 12 weeks' postpartum was assessed via two 5‐point Likert‐type scales (scores ranged from 0 to 5). While Cadwell 2004 also assessed maternal satisfaction with treatment, no specific details were provided. In the trial by Jackson 2013, a research assistant, blinded to group allocation, assessed outcomes pertaining to nipple pain, breastfeeding duration and exclusivity, and maternal satisfaction with treatment. Nipple pain was measured during the initial assessment and at four and seven days' post‐randomisation using the VAS of the SF‐MPQ that was modified into a Likert‐type scale (scores range from 0 to 10). Breastfeeding duration and exclusivity were assessed at four and 12 weeks' postpartum via a telephone interview by a research assistant blinded to group allocation. Measures of breastfeeding duration and exclusivity were similar to Dennis 2012. Maternal satisfaction with treatment was also assessed at 12 weeks' postpartum.
Interventions
Interventions to treat nipple pain varied across the four included studies. However, all trials used the application of lanolin (with or without another treatment) to the nipples as either the intervention group or the control group.
Cadwell 2004 conducted a three‐arm trial evaluating the following interventions over a 10‐day period: 1. the application of glycerine gel dressing between feeds according to the manufacturer's instructions (glycerine gel group); 2. air drying the nipples after feeding then apply lanolin cream and wearing breast shells until the next feed (breast shell and lanolin group); or 3. provision of breastfeeding assessment and corrective education (control group). All women received breastfeeding assessment and corrective educational intervention by trained midwives. Videos, live models, photographs, and post tests were used to ensure that the midwives correctly and consistently completed the assessment and documentation tool.
Mohammadzadeh 2005 conducted a three‐arm trial evaluating the following interventions over the course of seven days: 1. application of lanolin three times daily (lanolin group); 2. application of breast milk after each feed (EBM group); or 3. application of nothing (control group). Similar to the Cadwell 2004 trial, all women received education to correct their breastfeeding technique.
Jackson 2013 conducted a two‐arm trial evaluating the following interventions for seven days: (1) application of lanolin after each feeding (Lanolin group) or (2) the application of nothing (control group). Similar to Cadwell 2004 and Mohammadzadeh 2005, all women received education to correct their breastfeeding technique.
Dennis 2012 conducted a two‐arm trial where all women received an unmarked container and were instructed to apply the ointment sparingly to nipples and areolas after each feed for 10 days. They were then instructed to add the ointment after every other feed for four more days. In one group, the unmarked container had lanolin (lanolin group) and in the other group the unmarked container had an all‐purpose nipple ointment tinted with an inert food colouring to give it an appearance similar to lanolin (all‐purpose nipple ointment group).
Excluded studies
In total, 35 studies did not meet the inclusion criteria; the primary reason for exclusion (14 trials, 40%) was that the trial evaluated a prevention rather than a treatment intervention (see Characteristics of excluded studies). Other reasons for exclusion included: purpose of the study was not the treatment of painful nipples (Amir 2004; Berry 2012; Coca 2008; Eryilmaz 2005; Hogan 2005; Nicholson 1985; Woolridge 1980), insufficient information about the study with no author response from contact (Afshariani 2006; Brent 1998; Gensch 2006; Kuscu 2002; Posso 2007), methodologically weak (Chaves 2012; Gosha 1988; Gunther 1945; Lavergne 1997; Livingstone 1999), frenotomy (Buryk 2011; Dollberg 2006; Emond 2014), and no usable data (Abou‐Dakn 2011).
Risk of bias in included studies
The review included two quasi‐randomised studies (Cadwell 2004; Mohammadzadeh 2005), and two randomised controlled trials (Dennis 2012; Jackson 2013). The overall methodological quality of the included studies was good with few areas of high risk of bias. See Figure 2 and Figure 3 for a summary of risk of bias assessments.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Two trials had low risk of bias related to allocation concealment (Dennis 2012; Jackson 2013). In the Dennis 2012 trial, the hospital pharmacy used the centrally controlled standardised procedures for drug trials and provided identical containers filled with lanolin or the all‐purpose nipple ointment that was coloured using an inert yellow food colouring to ensure there was no difference in appearance. These containers were sequentially numbered and distributed consecutively. In the Jackson 2013 trial, women were randomly allocated using consecutively numbered, sealed opaque envelopes developed by a research assistant not involved in the trial. One trial had unclear methods of sequence generation and allocation concealment with no details specified (Mohammadzadeh 2005). One trial had high risk of bias related to randomisation methods (Cadwell 2004). Groups were assigned according to whichever pre‐packaged instruction kit was next in the queue or by using hospital bed numbers.
Blinding
In one trial, women and the outcome assessors were blinded to group allocation and thus the risk for performance and detection bias was low (Dennis 2012). The nature of the intervention in the other trials precluded blinding women to group allocation, and thus we deemed all three trials high risk for performance bias (Cadwell 2004; Jackson 2013; Mohammadzadeh 2005). It was unknown if the outcome assessor in the Cadwell 2004 trial was blinded to group allocation so we deemed this trial high risk for detection bias. Similarly, Mohammadzadeh 2005 indicated the "examiner" was unaware of treatment method, yet the role of the examiner was not clearly defined. In the Jackson 2013 trial, the outcome assessor was blinded to group allocation and was classified as low risk for detection bias.
Incomplete outcome data
Two trials reported no losses to follow‐up (Cadwell 2004; Mohammadzadeh 2005), and one trial reported no loss to follow‐up at the one‐week assessment and a 3% (five women) loss to follow‐up at the final assessment (12 weeks' post‐randomisation) (Dennis 2012). One trial recruited 186 women of which data were available for 165 (88.7%) on day four and 150 (80.6%) on day seven post‐randomisation (Jackson 2013). At four weeks' postpartum, 160 (86%) women had follow‐up data and at 12 weeks' postpartum, 122 (65.6%) women had follow‐up data.
Selective reporting
All trials completed intention‐to‐treat data analyses.
Other potential sources of bias
While not a critical source of bias, it is relevant to note that nipple pain in the first week' postpartum might be clinically different to nipple pain in the second week' postpartum. Thus, infant age at randomisation may affect treatment outcomes. Fortunately, infant age at recruitment varied slightly between trials from just a few days up to two weeks' postpartum. In Cadwell 2004, infants could be between one to 10 days of age at study entry. Dennis 2012 did not report the exact age of the infant at trial recruitment, but it was in the first week' postpartum. Jackson 2013 collected data for the primary outcome for infants between four and seven days of age, so infants were only one or two days old at the time of recruitment. All the preceding trials were rated low risk for other sources of bias. However, in Mohammadzadeh 2005, how "improvement time" and "healing time" were measured was not reported. Further, the age of the infant at recruitment was not known. As such, we rated this trial unclear risk for other sources of bias.
Effects of interventions
See: Summary of findings for the main comparison Glycerine gel dressing versus usual care; Summary of findings 2 Breast shells with lanolin versus usual care; Summary of findings 3 Glycerine gel dressing versus breast shells with lanolin; Summary of findings 4 Lanolin versus no intervention (control group); Summary of findings 5 Expressed breast milk versus no intervention (control group); Summary of findings 6 Lanolin versus expressed breast milk; Summary of findings 7 Lanolin versus all‐purpose nipple ointment
We included four trials, involving 656 women, in this review. We presented the results in sequential order, starting with the primary outcome nipple pain (variously defined). There were no infant or family outcomes reported in any of the included trials. With the exception of comparison four (lanolin versus no intervention), we were unable to pool the review data, so there is no sensitivity or subgroup analysis. It is important to note that if a trial measured an outcome using several measures, we included these in the review. None of the studies used a composite measure. We also acknowledge that results of an assessment at one time point is not truly independent of an assessment completed at another time point by the same women.
Comparison one: glycerine gel dressing versus breastfeeding education only (control group)
Maternal outcomes
Primary outcome ‐ nipple pain
There was no significant difference in the mean pain rating between women who applied a glycerine gel dressing to their nipples and women who received individualised breastfeeding education and corrective instruction and applied nothing to their nipples (MD 0.22, 95% CI ‐0.32 to 0.76, one trial, 63 women, low quality evidence; Analysis 1.1).
Secondary outcomes
Nipple trauma
There was no significant difference between glycerine gel dressing and control in nipple healing at the final visit with the midwife (RR 0.98, 95% CI 0.78 to 1.23, one trial, 63 women; Analysis 1.2).
Maternal satisfaction with treatment
Overall, most women were satisfied with their treatment and there was no difference in satisfaction between the two groups (RR 1.11, 95% CI 0.97 to 1.27, one trial, 63 women; Analysis 1.3).
Comparison two: breast shells with lanolin versus breastfeeding education (control group)
Maternal outcomes
Primary outcome ‐ nipple pain
There was no significant difference in the mean pain rating between women who used breast shells with lanolin and women who received individualised breastfeeding education and corrective instructions and applied nothing to their nipples (MD ‐0.20, 95% CI ‐0.60 to 0.20, one trial, 61 women, low quality evidence; Analysis 2.1).
Secondary outcomes
Nipple trauma
There was no significant difference in nipple healing between breast shells with lanolin and control (RR 0.58, 95% CI 0.15 to 2.22, one trial, 61 women; Analysis 2.2).
Maternal satisfaction with treatment
There was no difference in the rates of satisfaction between breast shells with lanolin and control (RR 1.00, 95% CI 0.85 to 1.18, one trial, 61 women; Analysis 2.3).
Comparison three: glycerine gel dressing versus breast shells with lanolin
Maternal outcomes
Primary outcome ‐ nipple pain
There was no significant difference in mean pain ratings between women who applied a glycerine gel dressing and women who used breast shells with lanolin (MD 0.42, 95% CI ‐0.09 to 0.93, one trial, 64 women, low quality evidence; Analysis 3.1).
Secondary outcomes
Nipple trauma
There was no significant difference between glycerine gel dressings and breast shell with lanolin (RR 1.88, 95% CI 0.51 to 6.87, one trial, 64 women; Analysis 3.2).
Maternal satisfaction with treatment
There was no significant difference in the rates of maternal satisfaction between glycerine gel dressings and breast shell with lanolin (RR 1.11, 95% CI 0.97 to 1.26, one trial, 64 women; Analysis 3.3).
Comparison four: lanolin versus no intervention (control group)
Maternal outcomes
Primary outcome ‐ nipple pain
There was no difference in the number of women reporting nipple pain among women in the lanolin group and women in the control group who were instructed to apply nothing to their nipples at one to three days' post‐treatment initiation (mean RR 0.97, 95% CI 0.91 to 1.04, one trial, 147 women); at four to five days' post‐treatment (mean RR 1.30, 95% CI 0.63 to 2.66, two trials, 312 women); or at six to seven days' post‐treatment (mean RR 0.85, 95% CI 0.63 to 1.14, two trials, 297 women) (see Analysis 4.1). Due to substantial heterogeneity at the four‐ to five‐day assessment (Tau2 = 0.24, I2 = 90%), we used a random‐effects model for this outcome. We graded all evidence as low quality.
Secondary outcomes
Nipple trauma
No women in either group had any improvement in their nipple condition at one to three days' post‐treatment initiation (RR 1.00, 95% CI 0.97 to 1.03, one trial, 147 women). While there were improvements at four to five days' post‐treatment for some women, there were no differences between the two groups (RR 1.07, 95% CI 0.91 to 1.27, one trial, 147 women). However, at six to seven days' post‐treatment, women in the control group had significantly improved nipple healing than women in the lanolin group (RR 1.81, 95% CI 1.13 to 2.91, one trial, 147 women; Analysis 4.2).
Breastfeeding duration and exclusivity
There were no differences between groups in the number of women breastfeeding at four weeks' postpartum (RR 1.11, 95% CI 0.95 to 1.29, one trial, 162 women) and at 12 weeks' postpartum (RR 1.15, 95% CI 0.94 to 1.43, one trial, 165 women; Analysis 4.3). There were also no differences between groups in rates of exclusive breastfeeding at four weeks' postpartum (RR 1.06, 95% CI 0.84 to 1.33, one trial, 162 women) and 12 weeks' postpartum (RR 1.06, 95% CI 0.79 to 1.43, one trial, 165 women; Analysis 4.4).
Maternal satisfaction with treatment
Women in the lanolin group were more satisfied with their nipple pain treatment than women in the control group (RR 1.14, 95% CI 1.04 to 1.25, one trial, 160 women; Analysis 4.5).
Comparison five: expressed breast milk versus no intervention (control group)
Maternal outcomes
Primary outcome ‐ nipple pain
There was no difference in reports of nipple pain between women who applied EBM or women who applied nothing at one to three days' post‐treatment initiation (RR 0.96, 95% CI 0.90 to 1.03, one trial, 151 women), four to five days' post‐treatment initiation (RR 1.17, 95% CI 0.71 to 1.92, one trial, 151 women), and six to seven days' post‐treatment initiation (RR 0.62, 95% CI 0.11 to 3.63, one trial, 151 women) (see Analysis 5.1). We graded all evidence as low quality.
Secondary outcomes
Nipple trauma
Similarly, there was no difference in reports of nipple healing between EBM and control at one to three days' post‐treatment initiation (RR 0.99, 95% CI 0.95 to 1.02, one trial, 151 women), four to five days' post‐treatment initiation (RR 0.87, 95% CI 0.71 to 1.06, one trial, 151 women), and six to seven days' post‐treatment initiation (RR 1.30, 95% CI 0.78 to 2.18, one trial, 151 women) (see Analysis 5.2).
Comparison six: lanolin versus expressed breast milk
Maternal outcomes
Primary outcome ‐ nipple pain
While there was no difference in maternal reports of nipple pain at one to three days' post‐treatment among women in the lanolin group and women in the EBM group (RR 1.01, 95% CI 0.93 to 1.09, one trial, 152 women), there was a decrease in pain among women in the EBM group at four to five days' post‐treatment (RR 1.56, 95% CI 1.05 to 2.32, one trial, 152 women). However, a this benefit was not apparent at six to seven days' post‐treatment (RR 1.58, 95% CI 0.27 to 9.20, one trial, one trial, 152 women) (see Analysis 6.1). We graded all evidence as low quality.
Secondary outcomes
Nipple trauma
Similarly, while there was no difference in nipple healing at one to three days' post‐treatment among women in the lanolin group and women in the EBM group (RR 1.01, 95% CI 0.98 to 1.05, one trial, 152 women), there was a significant improvement in nipple healing among women in the EBM group at four to five days' post‐treatment (RR 1.24, 95% CI 1.02 to 1.49, one trial, 152 women). However, this difference in nipple healing was not apparent at six to seven days' post‐treatment (RR 1.39, 95% CI 0.92 to 2.10, one trial, 152 women) (see Analysis 6.2).
Comparison seven: lanolin versus all‐purpose nipple ointment
Maternal outcomes
Primary outcome ‐ nipple pain
There were no differences in mean scores between lanolin and all‐purpose nipple ointment one week' post‐randomisation using the PPI (MD 0.12, 95% CI ‐0.24 to 0.48, one trial, 150 women) or the Pain Scale (MD 0.14, 95% CI ‐0.67 to 0.95, one trial, 150 women) (see Analysis 7.1). There was a difference in pain scores favouring the all‐purpose nipple ointment group at one week' post‐randomisation using the McGill pain measure (MD 2.51, 95% CI 0.61 to 4.41, one trial, 150 women; Analysis 7.1). We graded all evidence as moderate quality.
Secondary outcomes
Mastitis
There was no difference in the incidence of mastitis between lanolin and all‐purpose nipple ointment at 12 weeks' post‐randomisation (RR 0.66, 95% CI 0.11 to 3.82, one trial, 145 women; Analysis 7.2).
Breastfeeding duration
There was no difference between lanolin and all‐purpose nipple ointment in the practice of any breastfeeding at one week' post‐randomisation (RR 0.99, 95% CI 0.93 to 1.05, one trial, 150 women). At 12 weeks' post‐randomisation, although there were more women in the lanolin group breastfeeding than women in the all‐purpose nipple ointment group, this was not statistically significant (RR 1.18, 95% CI 0.99 to 1.40, one trial, 145 women (see Analysis 7.3).
Breastfeeding exclusivity
There was no difference between lanolin and all‐purpose nipple ointment in the practice of exclusive breastfeeding at one week' postpartum (RR 0.89, 95% CI 0.71 to 1.11, one trial, 150 women) or 12 weeks' postpartum (RR 1.32, 95% CI 0.96 to 1.80, one trial, 145 women) (see Analysis 7.4).
Maternal satisfaction with breastfeeding
Women in the lanolin group were more satisfied with their infant feeding experience at 12 weeks' postpartum than women in the all‐purpose nipple ointment group (RR 3.12, 95% CI 0.85 to 5.39, one trial, 142 women; Analysis 7.5).
Maternal satisfaction with treatment
There was no difference between lanolin and all‐purpose nipple ointment in the number of women satisfied with their treatment (RR 1.03, 95% CI 0.94 to 1.14, one trial, 145 women; Analysis 7.6).
Discussion
Summary of main results
Primary outcome: nipple pain
This review summarised the results of four trials involving 656 women conducted in three different counties (Canada, Iran, and Latvia) under a variety of circumstances. Currently, there is no evidence that glycerine gel dressings or breast shells with lanolin significantly improve maternal perceptions of nipple pain (Cadwell 2004). Two trials found that women who applied nothing to their breasts had the same level of perceived nipple pain at all time periods post‐treatment initiation as women who applied lanolin (Jackson 2013; Mohammadzadeh 2005). Mohammadzadeh 2005 also found women who applied EBM had significantly lower perceptions of nipple pain at four to five days' post‐treatment initiation than women who applied lanolin. However, this beneficial effect was not seen at six to seven days' post‐treatment initiation. There were no group differences in nipple pain perceptions at any assessment period between women who applied EBM and women who applied nothing. In the trial by Dennis 2012, women who applied an all‐purpose nipple ointment, in comparison to women who applied lanolin, had no differences in nipple pain perceptions on two of three validated pain measures. As such, there was no clear beneficial effect of the all‐purpose nipple ointment on nipple pain. Based on these review results, there was insufficient evidence that glycerine gel dressings, breast shells with lanolin, lanolin alone, or all‐purpose nipple ointment significantly improved maternal perceptions of nipple pain. These findings suggest that applying nothing or EBM may be equally beneficial in short‐term nipple pain as the application of lanolin. For most women, regardless of treatment used, nipple pain reduced to mild levels after approximately seven to 10 days postpartum.
Secondary outcomes
Two trials examined nipple trauma (Cadwell 2004; Mohammadzadeh 2005). There was no evidence to suggest that glycerine gel dressings or the application of lanolin and the use of breast shells improved nipple healing. In one trial, women who applied nothing had significantly improved nipple healing at six to seven days' post‐treatment initiation than women who applied lanolin (Mohammadzadeh 2005). Furthermore, women who applied EBM had significantly improved nipple healing at four to five days' post‐treatment initiation than women who applied lanolin, although the differences between groups were no longer seen at six to seven days' post‐treatment initiation. There were no differences in nipple healing time between women who applied EBM and women who applied nothing. These limited results suggested that applying nothing or EBM may be equally or more beneficial in relation to nipple wound healing as applying an ointment such as lanolin.
Two trials followed women to 12 weeks' postpartum (Dennis 2012; Jackson 2013). The trial by Dennis 2012 found no difference in symptoms of mastitis at 12 weeks' postpartum among women who applied lanolin and women who applied the all‐purpose nipple ointment. However, the rate of mastitis in this trial was at 2.8%, suggesting that there may have been insufficient power to detect differences between the two groups. This trial also examined breastfeeding duration and exclusivity at one week' post‐randomisation and 12 weeks' postpartum and found women who applied lanolin were equally likely to be breastfeeding at both time periods as women who applied the all‐purpose nipple ointment. There was no difference between the groups in relation to breastfeeding exclusivity (Dennis 2012). Similarly, there were no differences in breastfeeding duration and exclusivity rates at four and 12 weeks' postpartum between women who applied lanolin versus women who applied nothing to their nipples (Jackson 2013).
Three trials evaluated maternal satisfaction with nipple pain treatment (Cadwell 2004; Dennis 2012; Jackson 2013). In the trial by Cadwell 2004, all women were satisfied with the glycerine gel dressing and all but three were satisfied with the breast shell with lanolin intervention. In the trial by Dennis 2012, women were equally satisfied with the lanolin and all‐purpose nipple ointment treatments. Conversely, in the trial by Jackson 2013, more women were satisfied with applying lanolin than nothing to their painful nipples despite it not decreasing their perceptions of pain. Only one trial examined women's satisfaction with infant feeding at 12 weeks' postpartum and found that women who used lanolin were more satisfied with their breastfeeding experience than women who applied the all‐purpose nipple ointment (Dennis 2012).
Overall completeness and applicability of evidence
The primary objective of this review was to assess the effects of all interventions in the resolution or reduction of nipple pain. Interventions eligible to be part of this review included pharmacological oral and topical treatments, non‐pharmacological topical treatments, dressings, nipple protection devices, and EBM. While we included only four studies in the review, these trials evaluated diverse interventions including pharmacological topical ointments (Dennis 2012), non‐pharmacological topical ointments (Cadwell 2004; Dennis 2012; Jackson 2013; Mohammadzadeh 2005), dressings (Cadwell 2004), nipple protection devices (Cadwell 2004), and EBM (Mohammadzadeh 2005). No included trials evaluated pharmacological oral treatments. One trial did not include a "no treatment" control group (Dennis 2012), and one trial combined breast shells with lanolin as one intervention rendering us unable to determine whether one component or both interventions were responsible for any treatment effect (Cadwell 2004). While three trials recruited women from postpartum units or breastfeeding clinics (Cadwell 2004; Dennis 2012; Jackson 2013), one trial recruited women from a neonatal intensive care unit, which included a maternal population with unique breastfeeding challenges (Mohammadzadeh 2005). No trial recruited women from multiple sites. All four trials evaluated nipple pain as an outcome to be included in this review. Only two trials examined nipple trauma (Cadwell 2004; Mohammadzadeh 2005). Two trials included breastfeeding duration and exclusivity (Dennis 2012; Jackson 2013), and three trials examined treatment satisfaction (Cadwell 2004; Dennis 2012; Jackson 2013). No infant outcomes were included in any trial. Thus, the results of this review are limited in completeness of the treatment interventions evaluated, the types of participants included, and the study outcomes assessed. The applicability of evidence from this review was not strong and the results should be interpreted with caution.
Quality of the evidence
The quality of the evidence for this review does not lead to robust conclusions regarding the objectives assessed. We included only four trials, incorporating 656 women, in the review and all four compared varying interventions, participants, study outcome measures, and standards of usual care. The methodological quality of the included studies was good. In two trials, the outcome assessors were blinded to group allocation decreasing the possibility of performance bias (Dennis 2012; Jackson 2013). For three of the trials, there was no loss to follow‐up for the primary outcome measure (Cadwell 2004; Dennis 2012; Mohammadzadeh 2005). Jackson 2013 reported a 12% loss to follow‐up for the primary outcome. All data were analysed according to intention‐to‐treat principles and there was no obvious evidence of selective reporting bias or any other sources of bias (e.g. trial discontinued early due to some data‐dependent process or extreme baseline imbalances). We assessed the overall quality of the evidence for the primary outcome of nipple pain using GRADE as low quality (GRADE 2008), mainly because single studies with few participants contributed data for analysis.
We completed a comprehensive electronic search according to the Cochrane Pregnancy and Childbirth Group's detailed search strategy and identified all known relevant studies. However, we could not obtain all trial data and some potentially relevant studies many have been excluded from this review. We excluded some studies due to insufficient study information and no response from trial authors to our email correspondence (Afshariani 2006; Brent 1998; Gensch 2006; Kuscu 2002; Posso 2007). We excluded two studies that evaluated a treatment for cracked nipples but did not include data for the primary outcome of nipple pain (Coca 2008; Nicholson 1985). One study is awaiting translation (Tafazoli 2010).
Agreements and disagreements with other studies or reviews
Several reviews have been completed regarding the management of nipple pain and trauma associated with breastfeeding. While three reviews were descriptive (Braund 2001; Morland‐Schultz 2004; Tait 2000), one was systematic in its approach (Page 2003). Various studies aimed at treating nipple pain or trauma (or both) that we excluded from our review were included in these reviews. For example, in relation to pharmacological oral and topical treatments, one small Canadian trial (84 women) evaluated four treatment regimens for nipples infected with Staphylococcus aureus (Livingstone 1999). Treatments included 1. the review of basic breastfeeding techniques, 2. topical treatment with 2% mupirocin ointment to nipples after each feed, 3. treatment with topical fusidic acid ointment to nipples after each feed, and 4. treatment with oral cloxacillin/erythromycin 500 mg every six hours for 10 days. Significantly more women had improved nipple damage in the oral antibiotic group than the other groups and the trial was discontinued early. However, this trial had several weaknesses including small sample size, poor randomisation method, and no nipple pain data presented by study group. One small trial (65 women) randomised primiparous women reporting nipple soreness to one of six groups with one of three regimens (tea bag compress, water compress, no compress) and found that water and tea bag compresses were equally effective in alleviating nipple soreness (Lavergne 1997). However, the study reported an unclear randomisation process, used a combined nipple pain and trauma measure that was unvalidated, provided unusable raw data, and had a high attrition rate (44.9%). Large, multi‐site methodologically strong trials are need to evaluate diverse treatments for nipple pain.

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
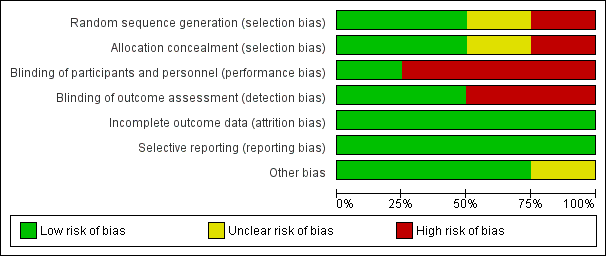
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 1 Nipple pain.

Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 2 Nipple trauma.
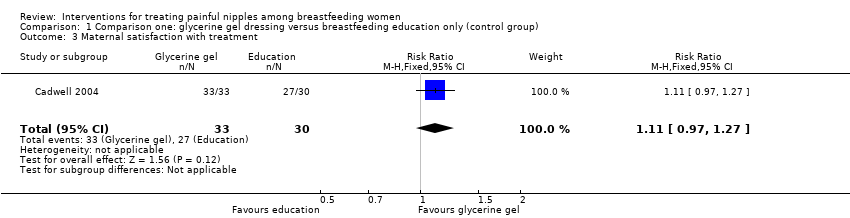
Comparison 1 Comparison one: glycerine gel dressing versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment.
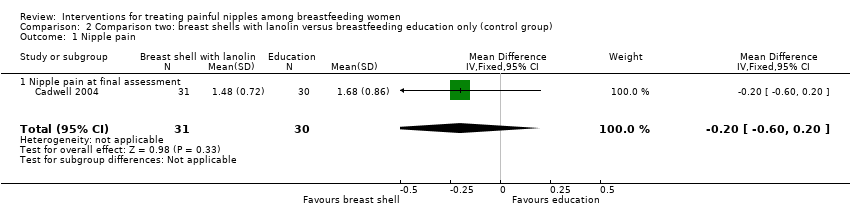
Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 1 Nipple pain.

Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 2 Nipple trauma.

Comparison 2 Comparison two: breast shells with lanolin versus breastfeeding education only (control group), Outcome 3 Maternal satisfaction with treatment.

Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 1 Nipple pain.

Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 2 Nipple trauma.
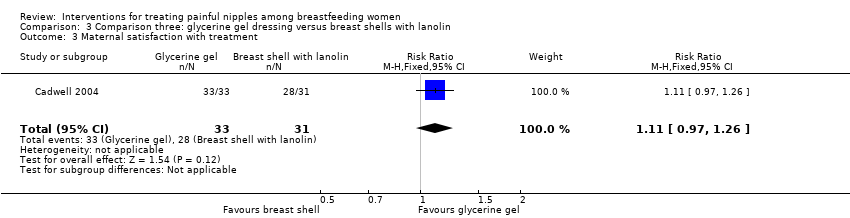
Comparison 3 Comparison three: glycerine gel dressing versus breast shells with lanolin, Outcome 3 Maternal satisfaction with treatment.

Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 1 Nipple pain.
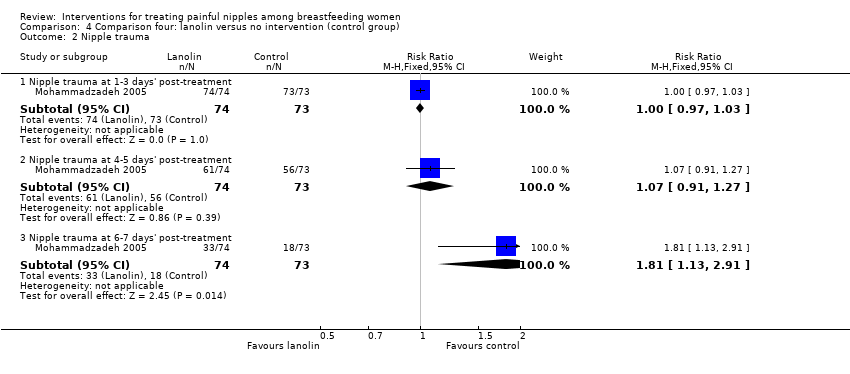
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 2 Nipple trauma.

Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 3 Breastfeeding duration.
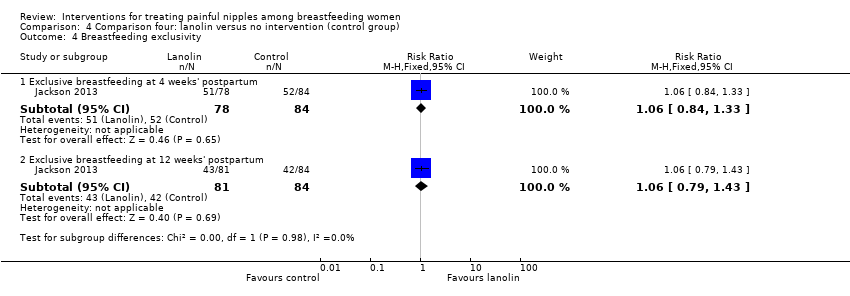
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 4 Breastfeeding exclusivity.
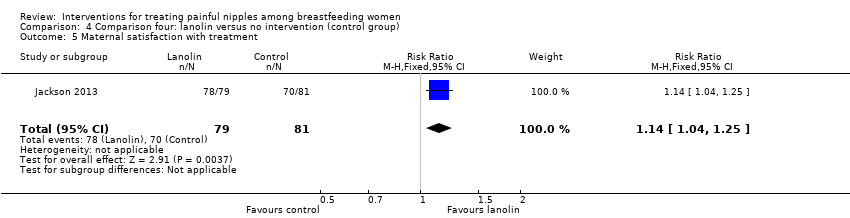
Comparison 4 Comparison four: lanolin versus no intervention (control group), Outcome 5 Maternal satisfaction with treatment.
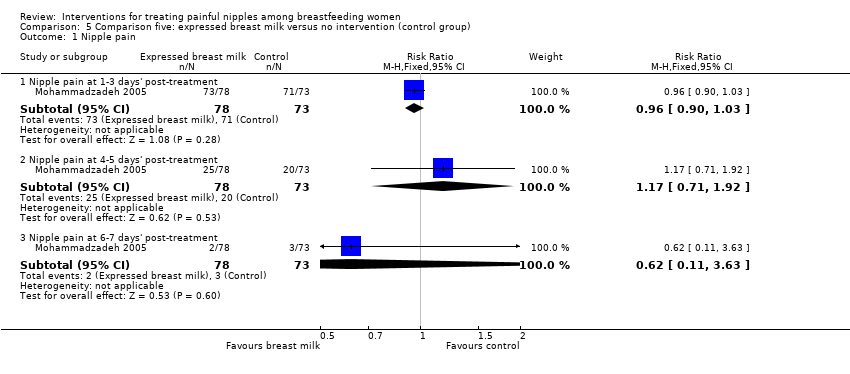
Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 1 Nipple pain.

Comparison 5 Comparison five: expressed breast milk versus no intervention (control group), Outcome 2 Nipple trauma.

Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 1 Nipple pain.

Comparison 6 Comparison six: lanolin versus expressed breast milk, Outcome 2 Nipple trauma.
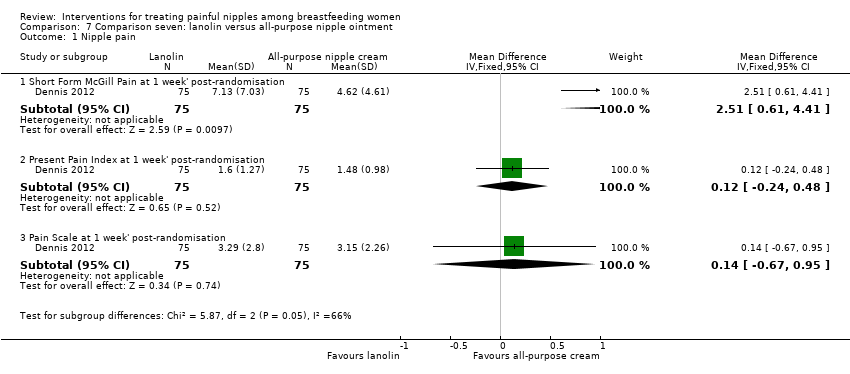
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 1 Nipple pain.
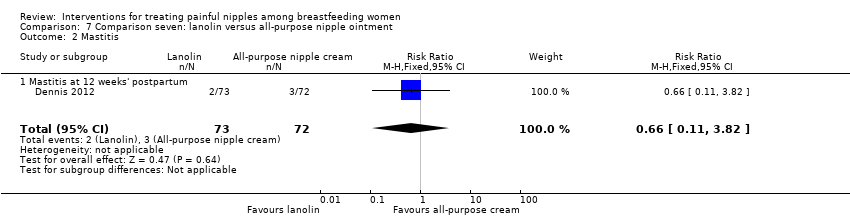
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 2 Mastitis.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 3 Breastfeeding duration.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 4 Breastfeeding exclusivity.
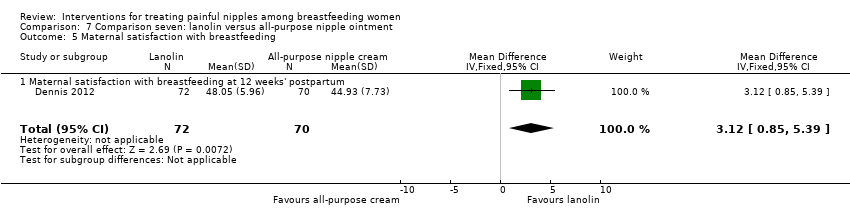
Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 5 Maternal satisfaction with breastfeeding.

Comparison 7 Comparison seven: lanolin versus all‐purpose nipple ointment, Outcome 6 Maternal satisfaction with treatment.
| Glycerine gel dressing versus breastfeeding education only (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breastfeeding education | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 63 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Breast shells with lanolin versus usual care for treating sore nipples in breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | breast shells with lanolin | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 61 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Glycerine gel dressing versus breast shells with lanolin for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breast shells with lanolin | Glycerine gel dressing | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 64 | ⊕⊕⊝⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (high risk of selection bias). | ||||||
| Lanolin versus no intervention (control group) for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Lanolin | |||||
| Nipple pain | Study population | RR 0.97 | 147 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 943 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 944 per 1000 | |||||
| Nipple pain | Study population | RR 1.30 | 312 | ⊕⊕⊝⊝ | ‐ | |
| 563 per 1000 | 732 per 1000 | |||||
| Moderate | ||||||
| 543 per 1000 | 706 per 1000 | |||||
| Nipple pain | Study population | RR 0.85 | 297 | ⊕⊕⊝⊝ | ‐ | |
| 315 per 1000 | 268 per 1000 | |||||
| Moderate | ||||||
| 310 per 1000 | 264 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). 3 Limitations in study design (high risk of bias for blinding; unclear risk for 1 study for selection bias). | ||||||
| Expressed breast milk versus no intervention for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Expressed breast milk | |||||
| Nipple pain | Study population | RR 0.96 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 973 per 1000 | 934 per 1000 | |||||
| Moderate | ||||||
| 973 per 1000 | 934 per 1000 | |||||
| Nipple pain | Study population | RR 1.17 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 274 per 1000 | 321 per 1000 | |||||
| Moderate | ||||||
| 274 per 1000 | 321 per 1000 | |||||
| Nipple pain | Study population | RR 0.62 | 151 | ⊕⊕⊝⊝ | ‐ | |
| 41 per 1000 | 25 per 1000 | |||||
| Moderate | ||||||
| 41 per 1000 | 25 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Study limitations (unclear selection bias; high risk of performance/detection bias). | ||||||
| Lanolin versus expressed milk for treating sore nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples Comparison: expressed breast milk | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | lanolin | |||||
| Nipple pain | Study population | RR 1.01 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 936 per 1000 | 945 per 1000 | |||||
| Medium risk population | ||||||
| 936 per 1000 | 945 per 1000 | |||||
| Nipple pain | Study population | RR 1.56 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 321 per 1000 | 501 per 1000 | |||||
| Medium risk population | ||||||
| 321 per 1000 | 501 per 1000 | |||||
| Nipple pain | Study population | RR 1.58 | 152 | ⊕⊕⊝⊝ | ‐ | |
| 26 per 1000 | 41 per 1000 | |||||
| Medium risk population | ||||||
| 26 per 1000 | 41 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Insufficient information available regarding sequence generation, allocation generation method, and allocation concealment. | ||||||
| Lanolin versus all‐purpose nipple ointment for treating painful nipples among breastfeeding women | ||||||
| Patient or population: breastfeeding women with sore nipples | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Lanolin | All‐purpose nipple ointment | |||||
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| Nipple pain | ‐ | The mean nipple pain in the intervention groups was | ‐ | 150 | ⊕⊕⊕⊝ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Small number of participants. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 1.1 Nipple pain at final assessment | 1 | 63 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.32, 0.76] |
| 2 Nipple trauma Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 2.1 Nipple trauma at final assessment | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.78, 1.23] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 1.1 Nipple pain at final assessment | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.60, 0.20] |
| 2 Nipple trauma Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 2.1 Nipple trauma at final assessment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.15, 2.22] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 1.1 Nipple pain at final assessment | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.09, 0.93] |
| 2 Nipple trauma Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 2.1 Nipple trauma at final assessment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.51, 6.87] |
| 3 Maternal satisfaction with treatment Show forest plot | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.26] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.91, 1.04] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 2 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.63, 2.66] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.63, 1.14] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.03] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.91, 1.27] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.13, 2.91] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.29] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.43] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Exclusive breastfeeding at 4 weeks' postpartum | 1 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.33] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.43] |
| 5 Maternal satisfaction with treatment Show forest plot | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.04, 1.25] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.90, 1.03] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.71, 1.92] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.11, 3.63] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma 1‐3 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.95, 1.02] |
| 2.2 Nipple trauma 4‐5 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.71, 1.06] |
| 2.3 Nipple trauma 6‐7 days' post‐treatment | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.78, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Nipple pain at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 1.2 Nipple pain at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.05, 2.32] |
| 1.3 Nipple pain at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.27, 9.20] |
| 2 Nipple trauma Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nipple trauma at 1‐3 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.98, 1.05] |
| 2.2 Nipple trauma at 4‐5 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.49] |
| 2.3 Nipple trauma at 6‐7 days' post‐treatment | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.92, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nipple pain Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short Form McGill Pain at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 2.51 [0.61, 4.41] |
| 1.2 Present Pain Index at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.24, 0.48] |
| 1.3 Pain Scale at 1 week' post‐randomisation | 1 | 150 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.67, 0.95] |
| 2 Mastitis Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 2.1 Mastitis at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.11, 3.82] |
| 3 Breastfeeding duration Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.05] |
| 3.2 Any breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.99, 1.40] |
| 4 Breastfeeding exclusivity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Exclusive breastfeeding at 1 week' post‐randomisation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
| 4.2 Exclusive breastfeeding at 12 weeks' postpartum | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.96, 1.80] |
| 5 Maternal satisfaction with breastfeeding Show forest plot | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 5.1 Maternal satisfaction with breastfeeding at 12 weeks' postpartum | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | 3.12 [0.85, 5.39] |
| 6 Maternal satisfaction with treatment Show forest plot | 1 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.94, 1.14] |

