Однократная доза кетопрофена или декскетопрофена для приема внутрь при острой послеоперационной боли у взрослых
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of at least moderate intensity. Pain assessed at baseline and every 15 min to 2 h, then hourly to 8 h. | |
| Participants | Surgical removal of 1 or 2 impacted third molars. N = 82 (84 cases; 2 participants had 2 operations), 76 (78 cases) assessed (4 protocol violations, 2 inadequate pain). M 31, F 45. Mean age: 23 years. | |
| Interventions | Ketoprofen 100 mg, n = 20. Paracetamol 1000 mg, n = 18. Ketoprofen 100 mg + paracetamol 1000 mg, n = 20. Placebo, n = 20. | |
| Outcomes | PI: standard 4‐point scale. Use of rescue medication. Time to use of rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. Participants asked to wait ≥ 1 h before using rescue medication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly assigned", "computer‐generated allocation schedule". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐dummy design, "patients were given identical sealed containers of study medication". |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐dummy design, "patients were given identical sealed containers of study medication". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h. | |
| Participants | General surgery (including gynaecological and orthopaedic). N = 59. M 35, F 24. Age: 22‐70 years. | |
| Interventions | Ketoprofen 25 mg, n = 14. Ketoprofen 100 mg, n = 16. Ibuprofen 400 mg, n = 15. Placebo, n = 14. | |
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: standard 5‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. | |
| Notes | Oxford Quality Score: R1, DB2, W0. 4‐h analgesic and anti‐inflammatory washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization chart". |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐dummy design. |
| Blinding of outcome assessment (detection bias) | Low risk | Double‐dummy design. |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, 2 parallel groups, multiple dose study. Medication administered when baseline pain was of moderate to severe intensity, then every 8 h for total of 3 days. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5 ,6, 8 h, then daily. | |
| Participants | Surgical removal of impacted third molars. N = 60. M 37, F 23. Mean age: approximately 37 years. | |
| Interventions | Ketoprofen lysine 80 mg, n = 30. Placebo, n = 30. | |
| Outcomes | PI: 0‐100‐mm VAS. PGE: standard 5‐point scale. AE: any, serious. Withdrawals. Tolerability 4‐point scale at end of study. | |
| Notes | Oxford Quality Score: R1, DB2, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "placebo indistinguishable". |
| Blinding of outcome assessment (detection bias) | Low risk | "placebo indistinguishable". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 181 (153 analysed). M 48, F 105. Mean age: 23 years. | |
| Interventions | Ketoprofen 25 mg, n = 30. Ketoprofen 50 mg, n = 31. Ketoprofen 100 mg, n = 31. Aspirin 650 mg, n = 31. Placebo, n = 30. | |
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: standard 5‐point scale. Time to use of rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. 6‐h analgesic, anti‐inflammatory, or sedative washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not reported. |
| Allocation concealment (selection bias) | Low risk | "each study medication bottle was identified only by a sequential code number". |
| Blinding of participants and personnel (performance bias) | Low risk | "all capsules in each bottle appeared identical". |
| Blinding of outcome assessment (detection bias) | Low risk | "all capsules in each bottle appeared identical". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 1, 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 181 (161 analysed). M 59, F 102. Mean age: 23 years. | |
| Interventions | Ketoprofen 25 mg, n = 42. Ketoprofen 100 mg, n = 39. Ibuprofen 400 mg, n = 37. Placebo, n = 43. | |
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: standard 5‐point scale. Numbers of participants using rescue medication. Time to use of rescue medication. Numbers with any AE Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "units of medication appeared identical". |
| Blinding of outcome assessment (detection bias) | Low risk | "units of medication appeared identical". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45 min, and 1, 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 177. M 75, F 102. Mean age: 23 years. | |
| Interventions | Dexketoprofen 25 mg, n = 50. Dexketoprofen 100 mg, n = 51. Paracetamol 1000 mg, n = 50. Placebo, n = 26. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. Minimum 4‐h analgesic, caffeine, and sedative washout before surgery. Rescue medication permitted after 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "treatments were randomly allocated", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "study medications all appeared identical" |
| Blinding of outcome assessment (detection bias) | Low risk | "study medications all appeared identical" |
| Size | High risk | < 50 participants in 3 of 4 treatment arms (range 26 to 51). |
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 206 (204 analysed). M 85, F 119. Mean age: 24 years. | |
| Interventions | Dexketoprofen tromethamine 5 mg, n = 41. Dexketoprofen tromethamine 10 mg, n = 42. Dexketoprofen tromethamine 20 mg, n = 41. Ibuprofen 400 mg, n = 41. Placebo, n = 41. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "study medication was identical in appearance to maintain blinding". |
| Blinding of outcome assessment (detection bias) | Low risk | "study medication was identical in appearance to maintain blinding". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 3 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 10, 20, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 141 (137 in efficacy analysis). M 63, F 78. Mean age: 26 years. | |
| Interventions | Dexketoprofen tromethamine 12.5 mg, n = 49. Dexketoprofen tromethamine 25 mg, n = 46. Placebo, n = 46. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R2, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization list was generated by computer program" |
| Allocation concealment (selection bias) | Low risk | Generation of sequence, and preparation of code envelopes and study medication performed by third party; participants assigned consecutively. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double‐blind conditions", "tablets of identical size, colour and weight". |
| Blinding of outcome assessment (detection bias) | Low risk | "Double‐blind conditions", "tablets of identical size, colour and weight". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45 min, and 1, 2, 3, 4, 5, 6, 7, 8, 24 h. | |
| Participants | Surgical removal of impacted third molars. N = 123 (120 analysed). M 39, F 81. Mean age: 29 years. | |
| Interventions | Dexketoprofen trometamol 25 mg, n = 42. Rofecoxib 50 mg, n = 37. Placebo, n = 41. | |
| Outcomes | PI: standard 4‐point scale and 100‐mm VAS PR: standard 5‐point scale and 100‐mm VAS PGE: standard 5‐point scale. Time use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. Rescue medication permitted after 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation carried out by third party, but method of allocation not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "Double blind", "All study drugs identical [in appearance] with patient numbers only on the packaging". |
| Blinding of outcome assessment (detection bias) | Low risk | "Double blind", "All study drugs identical [in appearance] with patient numbers only on the packaging". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 10, 20, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 210 (200 in efficacy analysis). M 88, F 122. Mean age: 28 years. | |
| Interventions | Dexketoprofen trometamol 12.5 mg, n = 44. Dexketoprofen trometamol 25 mg, n = 41. Dexketoprofen trometamol 50 mg, n = 43. Ketoprofen 50 mg (racemic), n = 43. Placebo, n = 39. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R2, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomization list generated by a computer program in blocks of five patients". |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "tablets of identical appearance to ensure double‐blind conditions". |
| Blinding of outcome assessment (detection bias) | Low risk | "tablets of identical appearance to ensure double‐blind conditions". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | Multicentre, RCT, DB (double‐dummy), multiple dose, placebo‐controlled (first dose), and active comparator, 4 parallel groups. Medication administered orally every 8 h over 5‐day period. First dose administered after cessation of postoperative analgesia once participants able to take oral medication and PI ≥ 40/100. Pain assessed at 30 min, and 1, 1.5, 2, 3, 4, 6, 8 h following first dose. | |
| Participants | Standard unilateral total hip arthroplasty due to osteoarthritis. Age 18 to 80 years; moderate to severe pain at rest on day after surgery. N = 641. M 295, F 346. Mean age: 62 years (range 29 to 80). Baseline PI: moderate in 324, severe in 315. | |
| Interventions | Single dose phase. Dexketoprofen 25 mg, n = 161. Tramadol 100 mg, n = 160. Dexketoprofen 25 mg + tramadol 75 mg, n = 159. Placebo, n = 161. | |
| Outcomes | PI: 100‐mm VAS. PR: standard 5‐point VRS (0 = none, 4 = complete). PGE: standard 5‐point VRS (1 = poor, 5 = excellent) at 24 h or use of rescue medication/withdrawal. Use of rescue medication. Time to use of rescue medication. AEs. Withdrawals. | |
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication: metamizole 500 mg. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence stratified by baseline PI‐VAS categories [moderate pain (40 to 60) and severe pain (> 60) with an imbalanced 1:3:1:3:1:3 ratio, using block size of 12]". |
| Allocation concealment (selection bias) | Low risk | "Interactive Voice/Web Response (IVR/IWR) system". |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐dummy design". |
| Blinding of outcome assessment (detection bias) | Low risk | "double‐dummy design". |
| Size | Unclear risk | 50‐199 participants per treatment arm (range 159 to 161). |
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 138 (129 analysed). M/F not given. Mean age: 26 years. | |
| Interventions | Ketoprofen 25 mg, n = 24. Ketoprofen 50 mg, n = 27. Ketoprofen 100 mg, n = 27. Codeine 90 mg, n = 27. Placebo, n = 24. | |
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: 5‐point scale (1 to 5 and reverse order). Number using rescue medication. AEs: any. | |
| Notes | Oxford Quality Score: R1, DB2, W0. Minimum 3‐h analgesic, anti‐inflammatory, and psychotropic washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random code", method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "double blind", "identical capsules". |
| Blinding of outcome assessment (detection bias) | Low risk | "double blind", "identical capsules". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | Multicentre, RCT, DB (double‐dummy), placebo‐controlled and active comparator, 10 parallel groups. Medication administered within 4 h of surgery when PI ≥ 40/100 and 4‐point VRS ≥ 2. Pain assessed at 0, 15, 30, 45 min, and 1, 1.5, 2, 2.5, 3, 3.5, 4, 5, 6, 8, 12, 24 h. | |
| Participants | Outpatient surgical removal, under local anaesthesia, of ≥ 1 third molar (≥ fully or partially impacted in mandibular bone). N = 606 for efficacy, 611 for safety. M 247, F 359. Mean age: 27 years (range 18 to 64). Baseline PI: 64% moderate, 35% severe (3 mild, 2 missing data). | |
| Interventions | Dexketoprofen 12.5 mg, n = 60. Dexketoprofen 25 mg, n = 60. Tramadol 37.5 mg, n = 59. Tramadol 75 mg, n = 59. Dexketoprofen 12.5 mg + tramadol 37.5 mg, n = 60. Dexketoprofen 12.5 mg + tramadol 75 mg, n = 62. Dexketoprofen 25 mg + tramadol 37.5 mg, n = 63. Dexketoprofen 25 mg + tramadol 75 mg, n = 61. Ibuprofen 400 mg, n = 60. Placebo, n = 62. | |
| Outcomes | PI: standard 4‐point VRS (0 = none, 3 = severe). PR: standard 5‐point VRS (0 = none, 4 = complete). Use of rescue medication. Time to use of rescue medication. | |
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication: paracetamol 1000 mg (maximum 4 doses in 24 h) after ≥ 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated" "blocked randomisation procedure, with block size of 10". |
| Allocation concealment (selection bias) | Low risk | "Interactive Voice/Web Response (IVR/IWR) system". |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐dummy design". |
| Blinding of outcome assessment (detection bias) | Low risk | "double‐dummy design". |
| Size | Unclear risk | 50‐199 participants per treatment arm (range 59 to 63). |
| Methods | Multicentre, RCT, DB, placebo‐controlled and active control, 4 parallel groups. Single and multiple dose phases. Medication administered orally every 8 h over 3‐day period. First dose administered after cessation of postoperative analgesia once participants able to take oral medication and PI ≥ 40/100. Pain assessed at 30 min, and 1, 1.5, 2, 3, 4, 6, 8 h. | |
| Participants | Abdominal hysterectomy for benign conditions. N = 606. All F. Mean age: 48 years (range 25 to 73). Baseline PI: moderate 38%, severe 62%. | |
| Interventions | Dexketoprofen 25 mg, n = 151. Tramadol 100 mg, n = 150. Dexketoprofen 25 mg + tramadol 75 mg, n = 152. Placebo, n = 153. | |
| Outcomes | PI: 100‐mm VAS. PR: standard 5‐point VRS (0 = none, 4 = complete). PGE: standard 5‐point VRS (1 = poor, 5 = excellent) at 8 h or use of rescue medication/withdrawal. Use of rescue medication. Time to use of rescue medication. AEs. Withdrawals. | |
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication: metamizole 500 mg. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization sequence stratified by baseline PI‐VAS categories [moderate pain (40 to 60) and severe pain (> 60)] with an imbalanced 3:3:3:1:1:1 ratio, using block size of 12]". |
| Allocation concealment (selection bias) | Low risk | "Interactive Voice/Web Response (IVR/IWR) system". |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐dummy design". |
| Blinding of outcome assessment (detection bias) | Low risk | "double‐dummy design". |
| Size | Unclear risk | 50‐199 participants per treatment arm (range 150 to 153). |
| Methods | RCT, DB, single dose oral liquid formulation of ketoprofen, 4 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 15, 30, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Episiotomy. N = 108 (terminated early, recruitment target N = 276). All F. Mean age: 24 years. | |
| Interventions | Ketoprofen 25 mg liquid formulation, n = 28. Ketoprofen 50 mg liquid formulation, n = 26. Dipyrone 500 mg liquid formulation, n = 27. Placebo, n = 27. | |
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs; any, severe. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB1, W1. 2 women entered with 2nd degree vaginal tears. Minimum 6‐h washout before surgery for any medication that could confound results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported, medication assignment in sealed envelopes. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study medications not identical in appearance. Nurse preparing study medication also administered it. A second nurse, blinded to the medication given, observed the woman. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study medications not identical in appearance. Nurse preparing study medication also administered it. A second nurse, blinded to the medication given, observed the woman. |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, triple dummy, single oral dose, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 10, 20, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 239. M 76, F 163. Mean age: 23 years. | |
| Interventions | Ketoprofen 25 mg, n = 67. Ibuprofen liquigel 400 mg, n = 67. Paracetamol 1000 mg, n = 66. Placebo, n = 39. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. Analgesic and anti‐inflammatory washout before surgery (5 × half‐life). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomization schedule generated by the sponsor", method not described. |
| Allocation concealment (selection bias) | Low risk | "Numbers were assigned to subjects in sequential order within the appropriate strata". |
| Blinding of participants and personnel (performance bias) | Low risk | Placebo capsules and caplets matched the active treatments; "all unit doses were identical in appearance". |
| Blinding of outcome assessment (detection bias) | Low risk | Placebo capsules and caplets matched the active treatments; "all unit doses were identical in appearance". |
| Size | High risk | < 50 participants in 1 of 4 treatment arms (range 39 to 67). |
| Methods | RCT, DB, single and multiple oral dose phases, 4 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 30 min, and 1, 2, 4 h after the 1st dose. | |
| Participants | Knee (meniscus or ligament reconstruction) or ankle surgery. N = 230. M 110, F 103. Mean age: 40 years. | |
| Interventions | Dexketoprofen tromethamine 12.5 mg, n = 52. Dexketoprofen tromethamine 25 mg, n = 52. Ketoprofen 50 mg, n = 54. Placebo, n = 55. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Number using rescue medication. Withdrawals. | |
| Notes | Oxford Quality Score: R2, DB2, W1. 12‐h analgesic and anti‐inflammatory washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation list", in blocks of 8. Judged low risk as computer randomisation described in related studies carried out by same sponsor. |
| Allocation concealment (selection bias) | Low risk | Generation of sequence and preparation of code envelopes and study medication performed by third party; participants assigned consecutively. |
| Blinding of participants and personnel (performance bias) | Low risk | "tablets of identical size, colour and weight", packaging indistinguishable except for randomisation number. |
| Blinding of outcome assessment (detection bias) | Low risk | "tablets of identical size, colour and weight", packaging indistinguishable except for randomisation number. |
| Size | Unclear risk | 50 to 199 participants per treatment arm (range 52 to 55) |
| Methods | R, DB, double dummy, single oral dose, 5 parallel groups. Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 206. M 66, F 140. Mean age: 25 years. | |
| Interventions | Ketoprofen 12.5 mg, n = 42. Ketoprofen 25 mg, n = 41. Paracetamol 500 mg, n = 41. Paracetamol 1000 mg, n = 41. Placebo, n = 41. | |
| Outcomes | PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. 12‐h analgesic washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized trial", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐dummy technique", "ketoprofen dosages identical in appearance and standard paracetamol tablets were used. Matched placebos were prepared for both medications". |
| Blinding of outcome assessment (detection bias) | Low risk | "double‐dummy technique", "ketoprofen dosages identical in appearance and standard paracetamol tablets were used. Matched placebos were prepared for both medications". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 45, 60, 90 min, and 2, 3, 4, 5, 6 h. | |
| Participants | Surgical removal of impacted third molars. N = 180. M 58, F 122. Mean age: 27 years. | |
| Interventions | Buffered ketoprofen 12.5 mg, n = 61. Ibuprofen 200 mg, n = 59. Placebo, n = 60. | |
| Outcomes | PI: standard 4‐point scale. PR: 100‐mm VAS and standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. 12‐h analgesic washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐dummy technique", "both tablets and dragees were of identical appearance, irrespective of their contents". |
| Blinding of outcome assessment (detection bias) | Low risk | "double‐dummy technique", "both tablets and dragees were of identical appearance, irrespective of their contents". |
| Size | Unclear risk | 50‐199 participants per treatment arm. |
| Methods | RCT, DB, single dose, parallel groups. Medication administered when baseline pain was of at least moderate intensity. Pain assessed at 0, 30, 60 min then hourly to 6 h. | |
| Participants | Study 3. 'General surgery' procedures (details not reported). N = 123. All M (Veterans Administration hospital). Age: not reported. | |
| Interventions | Ketoprofen 50 mg, n = 32. Ketoprofen 150 mg, n = 31. Paracetamol 650 mg + codeine 60 mg, n = 28. Placebo, n = 32. | |
| Outcomes | PI: 4‐point VRS. PR: 5‐point VRS. PGE: 4‐point medication rating (0 = no help, 3 = excellent) and 7‐point VRS overall rating (1 = very much worse, 7 = very much better). | |
| Notes | Oxford Quality Score: R1, DB1, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation not specifically mentioned, but used the same methods as other studies described as randomised. Method of sequence generation not described. "The same general methods were used in all of the studies under discussion. All studies met current standards of well‐controlled trials" |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind", method not reported. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind", method not reported. |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single and multiple oral dose, parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 30, 60 min then hourly to 8 h. | |
| Participants | Caesarean section. N = 250. All F. Mean age: 26 years. | |
| Interventions | Ketoprofen 50 mg, n = 48. Ketoprofen 100 mg, n = 48. Paracetamol 650 mg, n = 48. Paracetamol 650 mg + oxycodone 10 mg, n = 48. Placebo, n = 48. | |
| Outcomes | PI: standard 4‐point scale. PR: 100‐mm VAS and standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Low risk | "All study medication was identical in appearance". |
| Blinding of outcome assessment (detection bias) | Low risk | "All study medication was identical in appearance". |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 5 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 15, 30 min, and 1, 1.5, 2, 3, 3.5, 4, 5, 6 h. | |
| Participants | Surgical removal of ≥ 1 impacted third molars. N = 179 (175 analysed for efficacy). M 58, F 117. Mean age: 22 years. | |
| Interventions | Ketoprofen 6.25 mg, n = 35. Ketoprofen 12.5 mg, n = 35. Ketoprofen 25 mg, n = 35. Ibuprofen 200 mg, n = 35. Placebo, n = 35. | |
| Outcomes | PI: standard 4‐point scale. PR: 100‐mm VAS and standard 5‐point scale. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. 24‐h analgesic washout before surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", randomisation method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "all study medication identical in appearance". |
| Blinding of outcome assessment (detection bias) | Low risk | "all study medication identical in appearance". |
| Size | Unclear risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single oral dose, 3 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 30 min, and 1, 2, 3, 4, 5, 6 h | |
| Participants | Elective surgery (113 orthopaedic, 23 abdominal, 11 gynaecology, 8 urology, and 6 miscellaneous procedures). N = 161 (160 analysed). M 81, F 81. Mean age: 47 years. | |
| Interventions | Ketoprofen 50 mg, n = 41. Ketoprofen 150 mg, n = 39. Paracetamol 650 mg + codeine 60 mg, n = 39. Placebo, n = 42. | |
| Outcomes | PI: standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. AEs: any, serious. Withdrawals. | |
| Notes | Oxford Quality Score: R1, DB2, W1. 3‐h analgesic and anti‐inflammatory washout before surgery. Rescue medication permitted after 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | "double‐blind", method not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | "double‐blind", method not described. |
| Size | High risk | < 50 participants per treatment arm. |
| Methods | RCT, DB, single and multiple oral dose phases, 4 parallel groups. Medication administered when baseline pain was of severe intensity. Pain assessed at 0, 15, 30, 45 min, and 1, 2, 3, 4, 5, 6 h for single dose phase. | |
| Participants | Hallux vagus (bunion) surgery. N = 188 (172 analysed). M 25, F 163. Mean age: 54 years. | |
| Interventions | Dexketoprofen trometamol 12.5 mg, n = 47. Dexketoprofen trometamol 25 mg, n = 47. Ketoprofen 50 mg, n = 47. Placebo, n = 47. | |
| Outcomes | PI: 100‐mm VAS and standard 4‐point scale. PR: standard 5‐point scale. PGE: non‐standard 4‐point scale. Time to use of rescue medication. Number using rescue medication. Withdrawals. | |
| Notes | Oxford Quality Score: R2, DB2, W1. Rescue medication via PCA morphine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation "by computer program" for each centre. |
| Allocation concealment (selection bias) | Low risk | Generation of sequence, and preparation of code envelopes and study medication performed by third party; allocation "in chronological order of inclusion in each centre". |
| Blinding of participants and personnel (performance bias) | Low risk | "All the treatments .... were tablets of identical size, colour and weight". |
| Blinding of outcome assessment (detection bias) | Low risk | "All the treatments .... were tablets of identical size, colour and weight". |
| Size | High risk | < 50 participants per treatment arm. |
AE: adverse event; DB: double blind; F: female; h: hour; M: male; min: minute; N: number of participants in study; n: number of participants in treatment arm; PCA: patient‐controlled analgesia; PGE: Patient Global Evaluation of efficacy; PI: pain intensity; PR: pain relief; R: randomised (Oxford Quality Score); RCT: randomised controlled trial; VAS: visual analogue scale (see 'Glossary'; Appendix 4); VRS: verbal rating scale; W: withdrawal (Oxford Quality Score).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No placebo, no baseline pain. | |
| No placebo. | |
| No placebo, preoperative administration. | |
| No relevant control for postoperative administration, intervention given irrespective of baseline pain intensity. | |
| 3‐hour study period, no 4‐hour data. | |
| No placebo. | |
| No placebo. | |
| Included women with uterine cramps. | |
| No placebo. | |
| 3‐hour study period, no 4‐hour data. | |
| No 4‐ to 6‐hour data reported. | |
| No placebo. | |
| No placebo. | |
| Included women with uterine cramps. | |
| Study not randomised or double blind. Intravenous route. | |
| No placebo. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Japanese ‐ unable to obtain copy. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [2.68, 6.63] |
| Analysis 1.1  Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours. | ||||
| 2 Participants using rescue medication over 6 hours Show forest plot | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| Analysis 1.2  Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours. | ||||
| 3 Participants with any adverse event Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.64] |
| Analysis 1.3 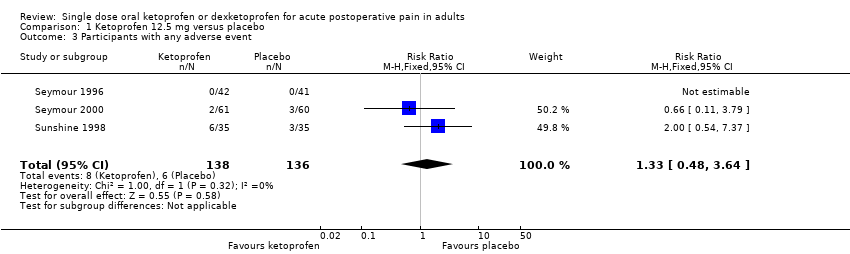 Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 8 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [3.48, 6.85] |
| Analysis 2.1 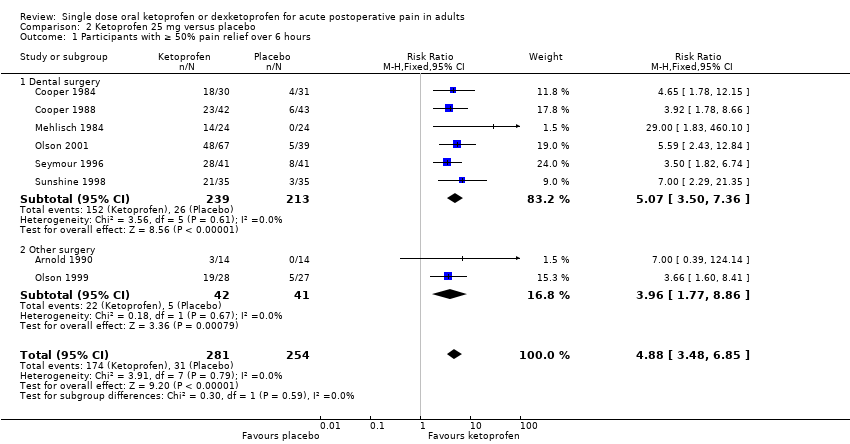 Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours. | ||||
| 1.1 Dental surgery | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [3.50, 7.36] |
| 1.2 Other surgery | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.77, 8.86] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 6 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.69] |
| Analysis 2.2 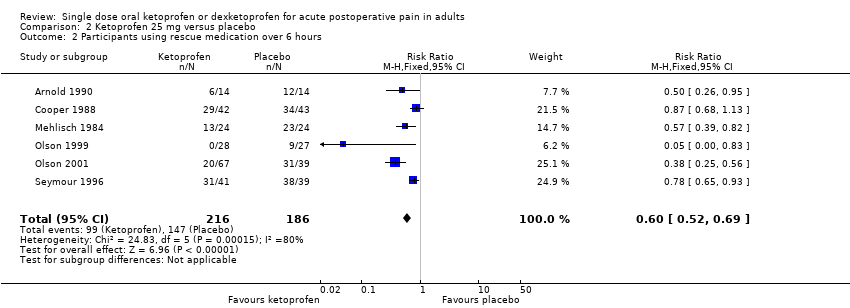 Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours. | ||||
| 3 Participants with any adverse event Show forest plot | 7 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.96] |
| Analysis 2.3  Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.97, 3.14] |
| Analysis 3.1 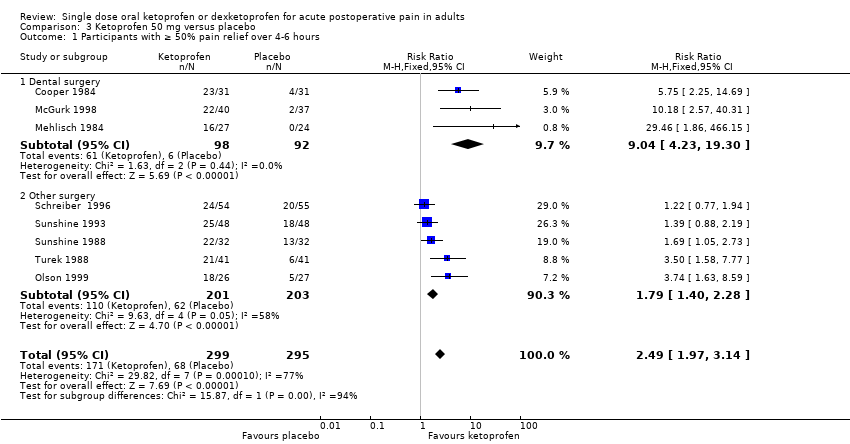 Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours. | ||||
| 1.1 Dental surgery | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.04 [4.23, 19.30] |
| 1.2 Other surgery | 5 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.40, 2.28] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 6 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| Analysis 3.2 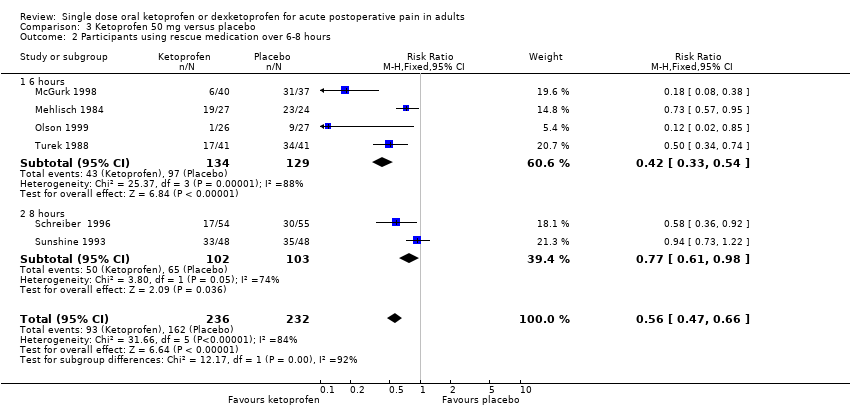 Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours. | ||||
| 2.1 6 hours | 4 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.2 8 hours | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 3 Participants with any adverse event Show forest plot | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.98, 2.75] |
| Analysis 3.3  Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 6 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [3.02, 6.08] |
| Analysis 4.1  Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief. | ||||
| 1.1 Dental surgery | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [4.67, 14.86] |
| 1.2 Other surgery | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 3.00] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 4 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.67] |
| Analysis 4.2  Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours. | ||||
| 2.1 6 hours | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.65] |
| 2.2 8 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.89] |
| 3 Participants with any adverse event Show forest plot | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.16] |
| Analysis 4.3 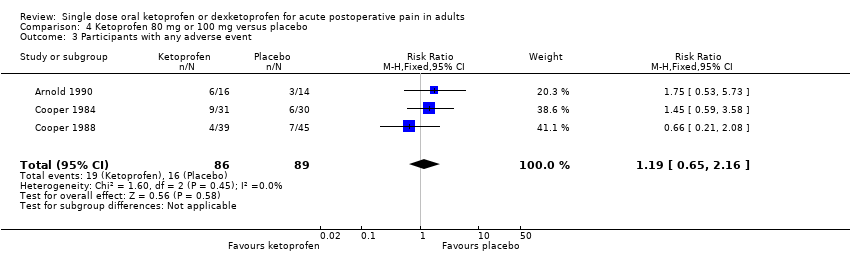 Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.79, 3.28] |
| Analysis 5.1 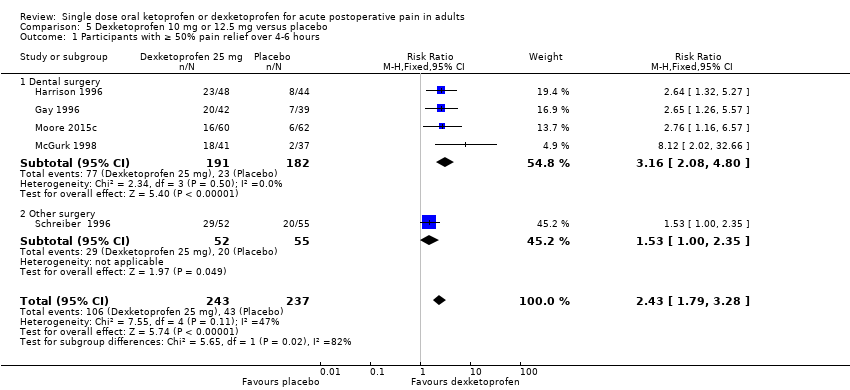 Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours. | ||||
| 1.1 Dental surgery | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.08, 4.80] |
| 1.2 Other surgery | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.00, 2.35] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.81] |
| Analysis 5.2  Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours. | ||||
| 2.1 6 hours | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.86] |
| 2.2 8 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.82] |
| 3 Participants with any adverse event Show forest plot | 4 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.35] |
| Analysis 5.3  Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.68, 2.28] |
| Analysis 6.1 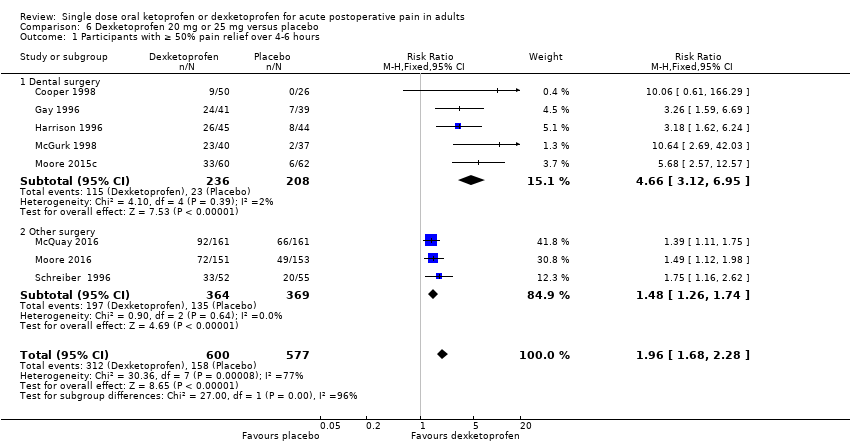 Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours. | ||||
| 1.1 Dental surgery | 5 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.12, 6.95] |
| 1.2 Other surgery | 3 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.26, 1.74] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 7 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.77] |
| Analysis 6.2  Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours. | ||||
| 2.1 6 hours | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 2.2 8 hours | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.89] |
| 3 Participants with any adverse event Show forest plot | 6 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.23] |
| Analysis 6.3 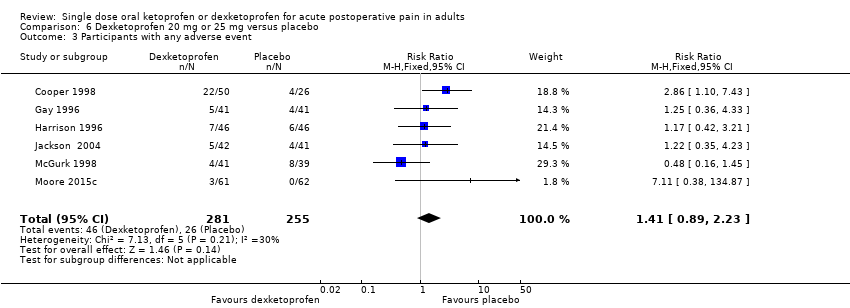 Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 3 Participants with any adverse event. | ||||

Study flow diagram.
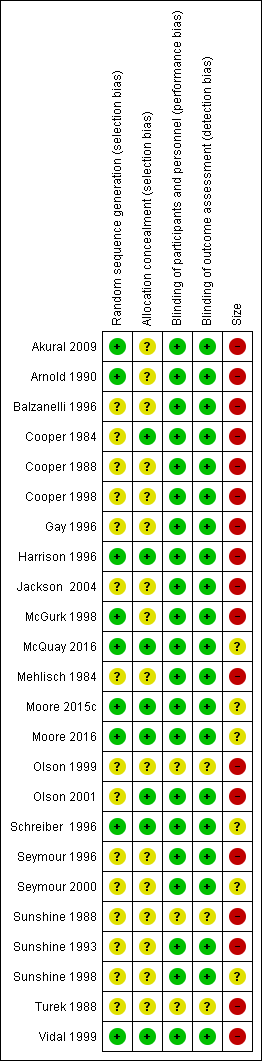
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 3 Ketoprofen 50 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over four to six hours.
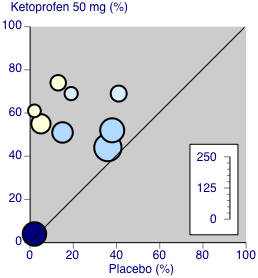
Ketoprofen 50 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.
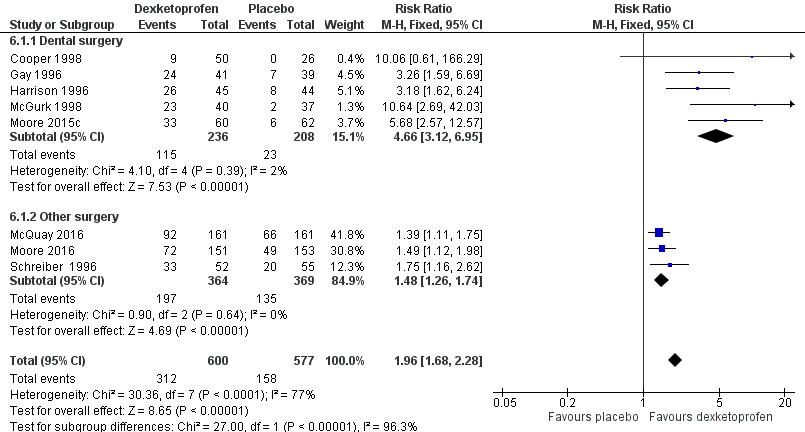
Forest plot of comparison: 6 Dexketoprofen 20 mg or 25 mg versus placebo, outcome: 6.1 Participants with at least 50% pain relief over four to six hours.

Dexketoprofen 20/25 mg: percent of participants with at least 50% pain relief over four to six hours. Size of circle is proportional to size of study (inset scale). Dental studies: yellow; bunionectomy study: dark blue; other non‐dental studies: light blue.

Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.
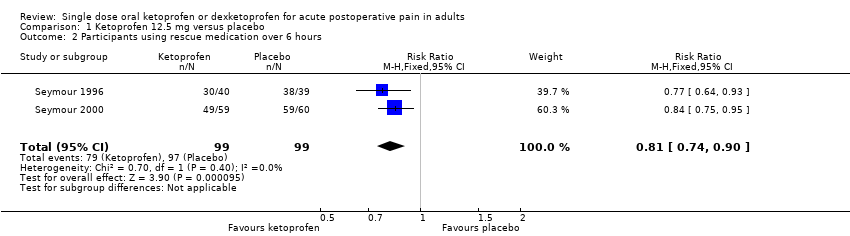
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
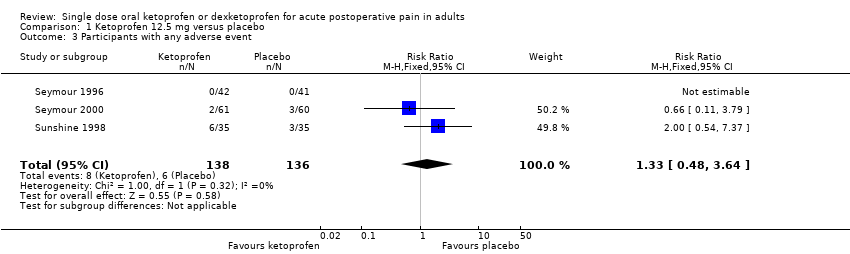
Comparison 1 Ketoprofen 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 6 hours.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.

Comparison 2 Ketoprofen 25 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
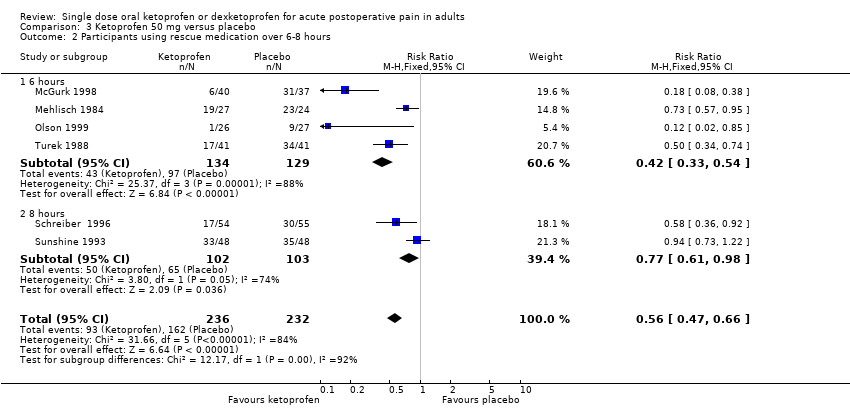
Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 3 Ketoprofen 50 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 4 Ketoprofen 80 mg or 100 mg versus placebo, Outcome 3 Participants with any adverse event.
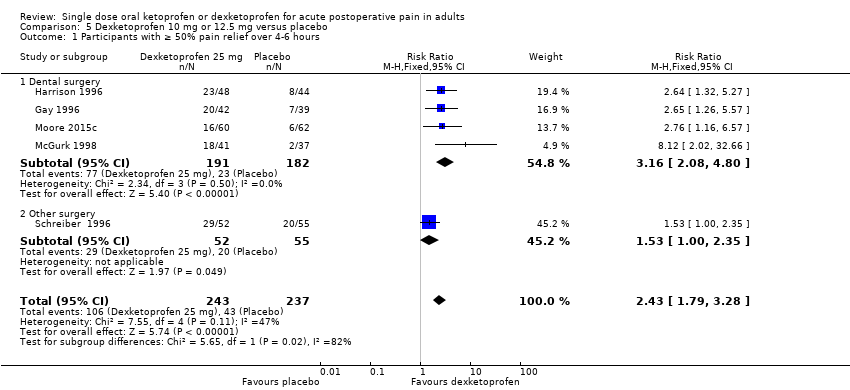
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
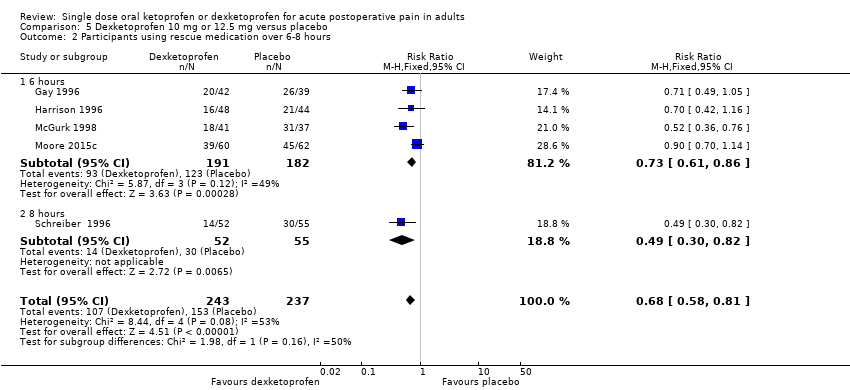
Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.

Comparison 5 Dexketoprofen 10 mg or 12.5 mg versus placebo, Outcome 3 Participants with any adverse event.

Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 1 Participants with ≥ 50% pain relief over 4‐6 hours.
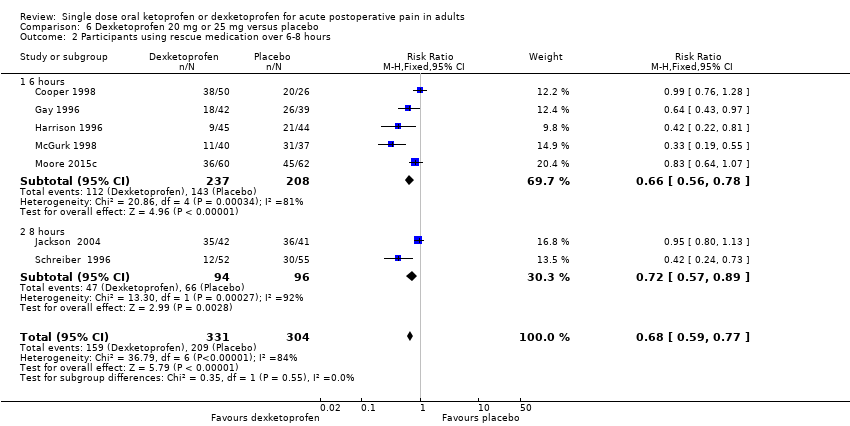
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 2 Participants using rescue medication over 6‐8 hours.
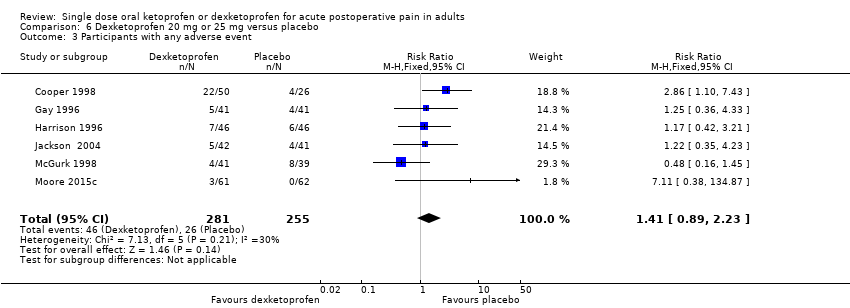
Comparison 6 Dexketoprofen 20 mg or 25 mg versus placebo, Outcome 3 Participants with any adverse event.
| Ketoprofen 25 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 6 hours | 620 in 1000 | 120 in 1000 | RR 4.9 (3.5 to 6.9) NNT 2.0 (1.8 to 2.3) | 8 studies 535 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 5.3 hours (4.6 hours) | 1.6 hours (2.5 hours) | Not estimated | 2 studies 188 participants (5 studies 277 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 460 in 1000 | 79 in 1000 | RR 0.60 (0.52 to 0.69) NNTp 3.0 (2.4 to 4.1) | 6 studies 402 participants | Moderate | Modest numbers of participants and events. |
| Participants with ≥ 1 adverse event following a single dose | 100 in 1000 | 91 in 1000 | RR 1.2 (0.68 to 2.0) NNH not calculated | 7 studies 490 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 8 studies 535 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Ketoprofen 50 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: ketoprofen 50 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 570 in 1000 | 230 in 1000 | RR 2.5 (2.0 to 3.1) NNT 2.9 (2.4 to 3.7) | 8 studies 594 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | Approximately 5 hours (3.4 hours) | Approximately 3 hours (2.5 hours) | Not estimated | 1 study 77 participants (5 studies, 342 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 320 in 1000 | 750 in 1000 | RR 0.42 (0.33 to 0.52) NNTp 2.3 (1.8 to 3.1) | 4 studies 263 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 180 in 1000 | 110 in 1000 | RR 1.6 (0.98 to 2.8) NNH not calculated | 5 studies 342 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 688 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Dexketoprofen 10 mg‐12.5 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 10 mg‐12.5 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 440 in 1000 | 180 in 1000 | RR 2.4 (1.8 to 3.3) NNT 3.9 (3.0 to 5.7) | 5 studies 480 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Median (mean) time to use of rescue medication | 3.6 hours (4.9 hours) | 1.4 hours (3.6 hours) | Not estimated | 1 study 122 participants (3 studies 253 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 490 in 1000 | 680 in 1000 | RR 0.73 (0.61 to 0.86) NNTp 5.3 (3.5 to 11) | 4 studies 373 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 68 in 1000 | 96 in 1000 | RR 0.70 (0.36 to 1.4) NNH not calculated | 4 studies 380 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 6 studies 574 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for an additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Dexketoprofen 20 mg or 25 mg compared with placebo for acute postoperative pain | ||||||
| Patient or population: adults with moderate or severe acute postoperative pain Settings: clinic or hospital Intervention: dexketoprofen 20 mg or 25 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with placebo | RR, NNT, NNTp, or NNH (95% CI) | Number of studies, participants, or events | Quality of the evidence | Comments |
| Participants with ≥ 50% pain relief over 4‐6 hours | 520 in 1000 | 270 in 1000 | RR 2.0 (1.6 to 2.2) NNT 4.1 (3.3 to 5.2) | 8 studies 1177 participants | High quality | Good quality studies, important outcome available, robust numbers |
| Median (mean) time to use of rescue medication | 4.7 hours (5.2 hours) | 1.8 hours (3.6 hours) | Not estimated | 3 studies 281 participants (3 studies, 251 participants) | Very low quality | Small numbers of participants. |
| Participants using rescue medication over 6 hours | 470 in 1000 | 690 in 1000 | RR 0.66 (0.56 to 0.78) NNTp 4.7 (3.3 to 8.0) | 5 studies 445 participants | High quality | Reasonable numbers of participants and high event rate. |
| Participants with ≥ 1 adverse event following a single dose | 160 in 1000 | 100 in 1000 | RR 1.4 (0.89 to 2.2) NNH not calculated | 6 studies 536 participants | High quality | Good quality studies, important outcome available, robust numbers. |
| Participants with a serious adverse event following a single dose | No serious adverse events reported | Not estimated | 9 studies 1271 participants | Very low quality | No events in single dose studies not designed to evaluate serious but rare adverse events. | |
| CI: confidence interval; NNH: number needed to treat for an additional harmful outcome; NNT: number needed for one additional beneficial outcome; NNTp: number needed to treat to prevent an additional outcome: RR: risk ratio. | ||||||
| We used the following descriptors for levels of evidence (EPOC 2015).
a Substantially different: a large enough difference that it might affect a decision. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [2.68, 6.63] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 3 Participants with any adverse event Show forest plot | 3 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.48, 3.64] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 6 hours Show forest plot | 8 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.88 [3.48, 6.85] |
| 1.1 Dental surgery | 6 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.07 [3.50, 7.36] |
| 1.2 Other surgery | 2 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.96 [1.77, 8.86] |
| 2 Participants using rescue medication over 6 hours Show forest plot | 6 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.52, 0.69] |
| 3 Participants with any adverse event Show forest plot | 7 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.68, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 594 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.97, 3.14] |
| 1.1 Dental surgery | 3 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.04 [4.23, 19.30] |
| 1.2 Other surgery | 5 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.40, 2.28] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 6 | 468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.47, 0.66] |
| 2.1 6 hours | 4 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.33, 0.54] |
| 2.2 8 hours | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.61, 0.98] |
| 3 Participants with any adverse event Show forest plot | 5 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.98, 2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief Show forest plot | 6 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [3.02, 6.08] |
| 1.1 Dental surgery | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.33 [4.67, 14.86] |
| 1.2 Other surgery | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.26, 3.00] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 4 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.44, 0.67] |
| 2.1 6 hours | 3 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.38, 0.65] |
| 2.2 8 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.44, 0.89] |
| 3 Participants with any adverse event Show forest plot | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [1.79, 3.28] |
| 1.1 Dental surgery | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.08, 4.80] |
| 1.2 Other surgery | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.00, 2.35] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 5 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.81] |
| 2.1 6 hours | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.86] |
| 2.2 8 hours | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.30, 0.82] |
| 3 Participants with any adverse event Show forest plot | 4 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.36, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with ≥ 50% pain relief over 4‐6 hours Show forest plot | 8 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.68, 2.28] |
| 1.1 Dental surgery | 5 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [3.12, 6.95] |
| 1.2 Other surgery | 3 | 733 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.26, 1.74] |
| 2 Participants using rescue medication over 6‐8 hours Show forest plot | 7 | 635 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.59, 0.77] |
| 2.1 6 hours | 5 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 2.2 8 hours | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.89] |
| 3 Participants with any adverse event Show forest plot | 6 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.89, 2.23] |

