Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007354.pub3Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 20 octubre 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Dolor y cuidados paliativos
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
All review authors contributed to the development of the idea for this review, revised the manuscript, and approved the final version.
STS: developed and wrote the protocol, developed the search strategies and the data extraction form, searched for studies, obtained copies of the studies, extracted data from studies, entered data into RevMan, carried out analysis and meta‐analysis, drafted the review and finalised it after discussion with the other review authors. Responsible for further updates.
IJH: discussed and approved the protocol, the search strategy, and the data extraction form, discussed the outcomes and analysis with the other review authors, and provided epidemiological and wider systematic review expertise.
SB: discussed and approved the protocol, the search strategy, and the data extraction form, checked extracted information from studies, discussed the outcomes and analysis with the other review authors.
RH: discussed and approved the protocol, the search strategy, and the data extraction form, discussed the outcomes and analysis with the other review authors, and provided social science expertise.
VW: searched for studies for the update, extracted data from studies for the update, entered data into RevMan for the update, contributed to the meta‐analysis for the update, drafted the review update.
CB: supervised the protocol, contributed to the development of the search strategy and the data extraction form, searched the titles, extracted data from studies, supervised the analysis, discussed the outcomes and analysis with the other review authors, and provided wider systematic review expertise.
Sources of support
Internal sources
-
Department of Palliative Care, Policy and Rehabilitation, King's College London, UK.
-
Institute of Palliative Care, Germany.
-
Department of Palliative Medicine, University Hospital of Cologne, Germany.
External sources
-
The Werner Jackstaedt Foundation, Germany.
-
The Federal Ministry of Education and Research (BMBF; 01KG1509), Germany.
Research grant for the conduction of the review update (2015)
Declarations of interest
STS: none known. STS is a specialist in palliative care and works as a physician caring for patients with life‐limiting diseases.
IJH: none known. IJH is a specialist in palliative care and works as a researcher and physician caring for patients with life‐limiting diseases.
SB: none known. SB worked as a specialist in palliative care and and now supports patients with chronic illness.
RH: none known. RH is a reader in palliative care and works as a researcher in palliative care with focus on HIV/AIDS, Sub‐Saharan Africa and Global Health.
VW received reimbursement of travel costs from Teva Pharmaceutical Industries Ltd. for the 8th World Research Congress of the European Association of Palliative Care (Lleida, Spain, 2014) outside the submitted work. VW is a health economist with experience in research in palliative care (2011‐2015) and works as a research associate at an institute of quality and efficiency in health care (since 2015).
CB: none known. CB is a specialist in palliative care and works as a physician caring for patients with life‐limiting diseases.
Acknowledgements
We are grateful to John Plummer (statistician of the Cochrane Review Group) for his very helpful comments and discussions regarding statistical methods and analysis. We are also grateful to Dr Yoshie Shizusawa, who helped with the extraction of data from the Japanese paper. Many thanks to Dr Fliss Murtagh for comments on an earlier draft of this review. We thank Joanne Abbott from the Cochrane Pain, Palliative and Supportive Care Review Group for her great support with the literature searches for the update.
The following peer referees contributed feedback to initial drafts of the review: Fiona Cramp, Ollie Minton, and Paul Perkins.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Oct 20 | Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults | Review | Steffen T Simon, Irene J Higginson, Sara Booth, Richard Harding, Vera Weingärtner, Claudia Bausewein | |
| 2010 Jan 20 | Benzodiazepines for the relief of breathlessness in advanced malignant and non‐malignant diseases in adults | Review | Steffen T Simon, Irene J Higginson, Sara Booth, Richard Harding, Claudia Bausewein | |
| 2008 Oct 08 | Benzodiazepines for the relief of breathlessness in malignant and advanced non‐malignant diseases in adults | Protocol | Steffen T Simon, Claudia Bausewein, Sara Booth, Richard Harding, Irene J Higginson | |
Differences between protocol and review
For the previous version of the review, we changed the title slightly by inserting 'advanced' in front of 'malignant'. This resulted in no changes to the included and excluded studies.
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Adult; Humans;
PICO
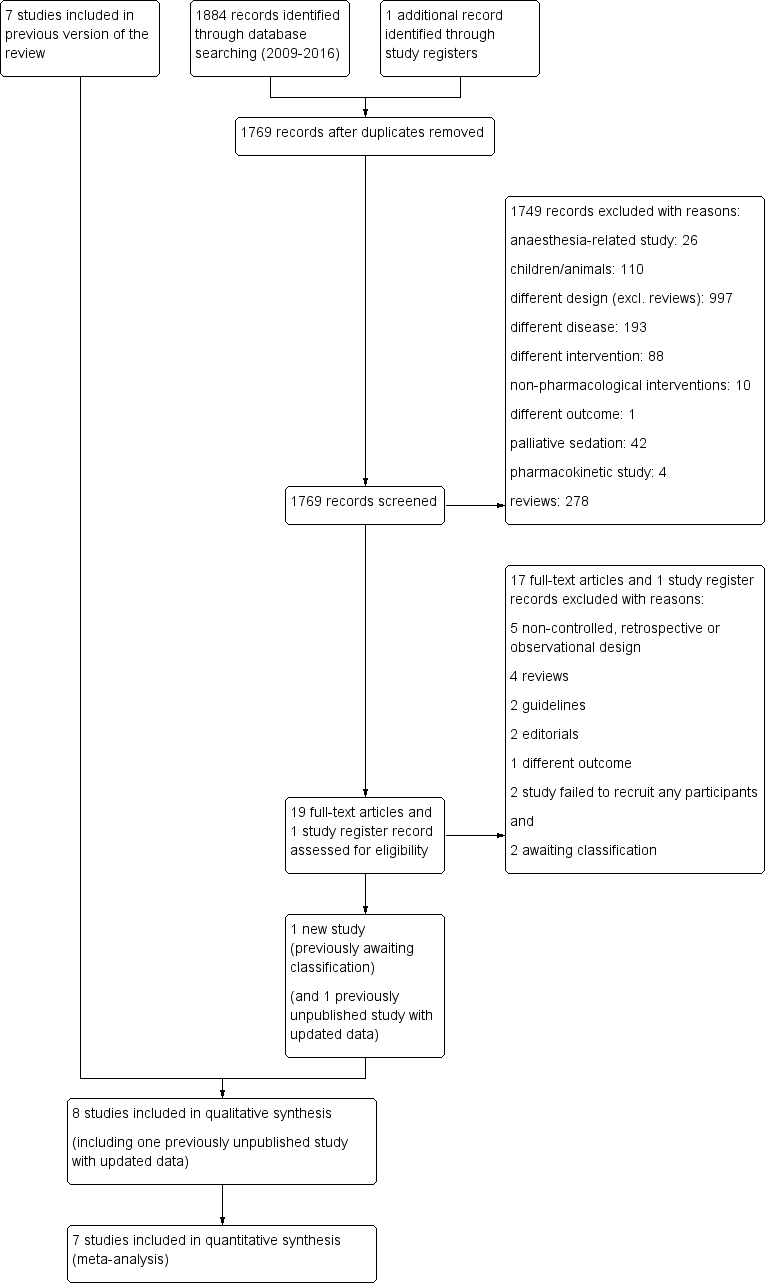
Study flow diagram.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
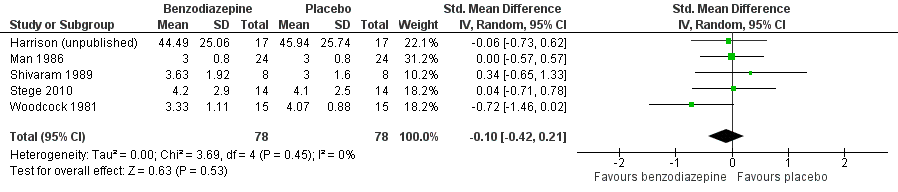
Forest plot of comparison: 1 Overall, outcome: 1.1 Placebo‐controlled/cross‐over design.
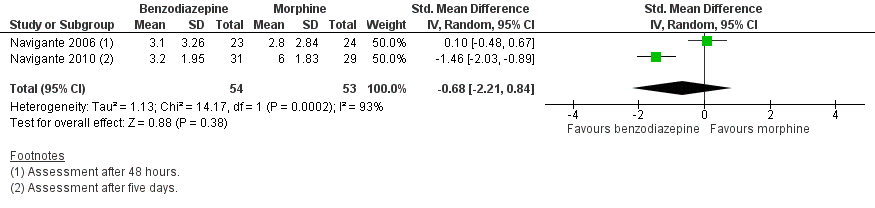
Forest plot of comparison: 1 Overall, outcome: 1.2 Morphine‐controlled/parallel design.
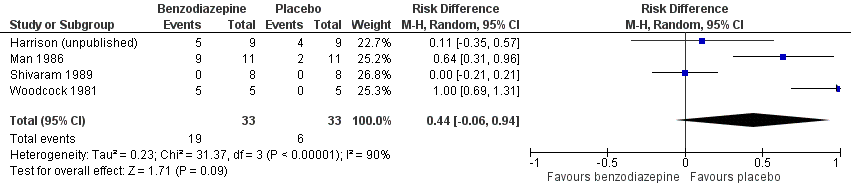
Forest plot of comparison: 5 Secondary outcomes, outcome: 5.1 Adverse effects (placebo‐controlled).

Comparison 1 Overall, Outcome 1 Placebo‐controlled/cross‐over design.

Comparison 1 Overall, Outcome 2 Morphine‐controlled/parallel design.

Comparison 2 Disease, Outcome 1 COPD.

Comparison 2 Disease, Outcome 2 Cancer ‐ placebo‐controlled.
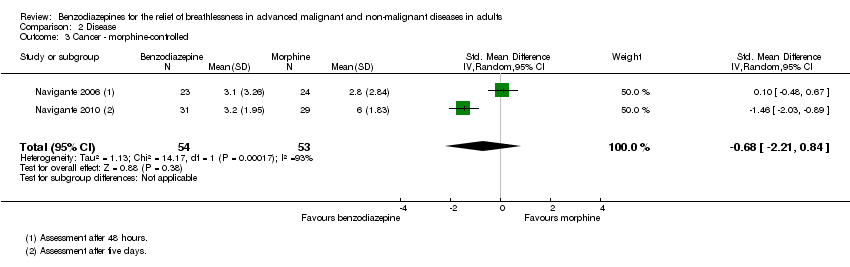
Comparison 2 Disease, Outcome 3 Cancer ‐ morphine‐controlled.
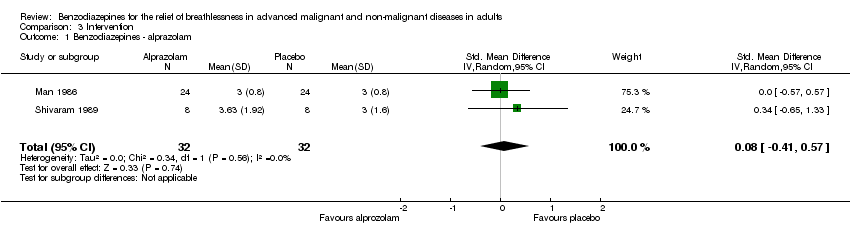
Comparison 3 Intervention, Outcome 1 Benzodiazepines ‐ alprazolam.

Comparison 3 Intervention, Outcome 2 Benzodiazepines ‐ diazepam.

Comparison 3 Intervention, Outcome 3 Benzodiazepines ‐ midazolam.
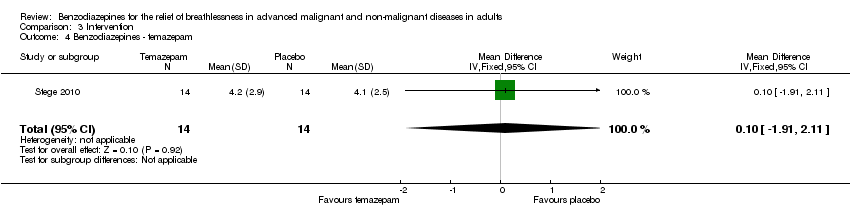
Comparison 3 Intervention, Outcome 4 Benzodiazepines ‐ temazepam.

Comparison 3 Intervention, Outcome 5 Benzodiazepines ‐ ultra short‐acting.

Comparison 3 Intervention, Outcome 6 Benzodiazepines ‐ intermediate‐acting.

Comparison 3 Intervention, Outcome 7 Benzodiazepines ‐ long‐acting.

Comparison 3 Intervention, Outcome 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours).

Comparison 3 Intervention, Outcome 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days).

Comparison 3 Intervention, Outcome 10 Benzodiazepines ‐ morphine + midazolam‐controlled.

Comparison 3 Intervention, Outcome 11 Benzodiazepines ‐ promethazine‐controlled.
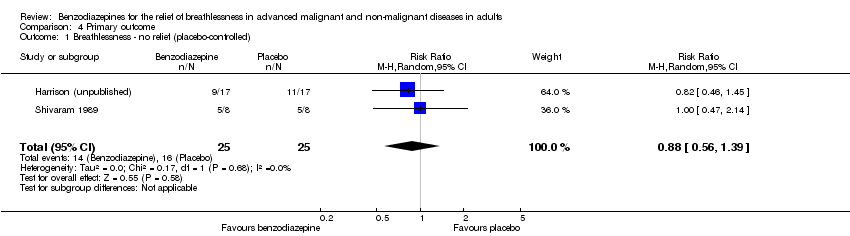
Comparison 4 Primary outcome, Outcome 1 Breathlessness ‐ no relief (placebo‐controlled).

Comparison 4 Primary outcome, Outcome 2 Breathlessness ‐ no relief (morphine‐controlled).
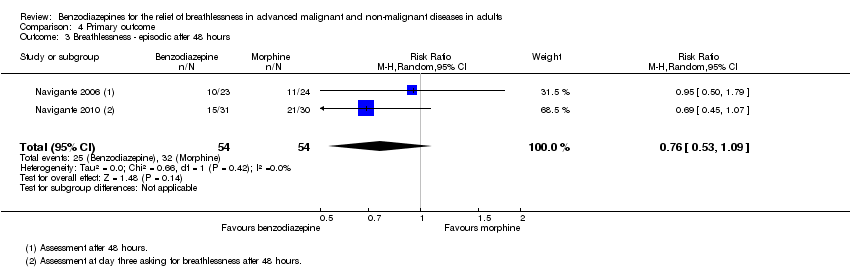
Comparison 4 Primary outcome, Outcome 3 Breathlessness ‐ episodic after 48 hours.

Comparison 4 Primary outcome, Outcome 4 Breathlessness ‐ episodic after 24 hours.
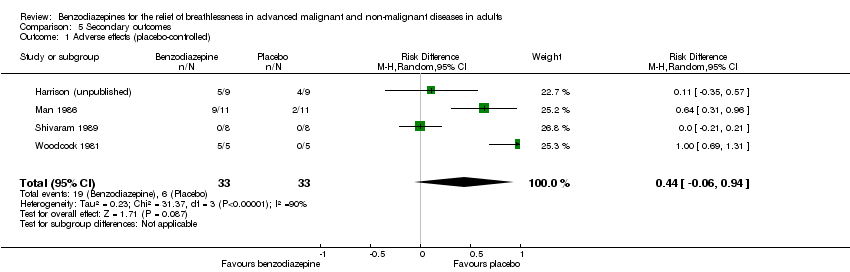
Comparison 5 Secondary outcomes, Outcome 1 Adverse effects (placebo‐controlled).

Comparison 5 Secondary outcomes, Outcome 2 Adverse effects (morphine‐controlled).

Comparison 5 Secondary outcomes, Outcome 3 Adverse effects ‐ clinical relevance only (morphine‐controlled).
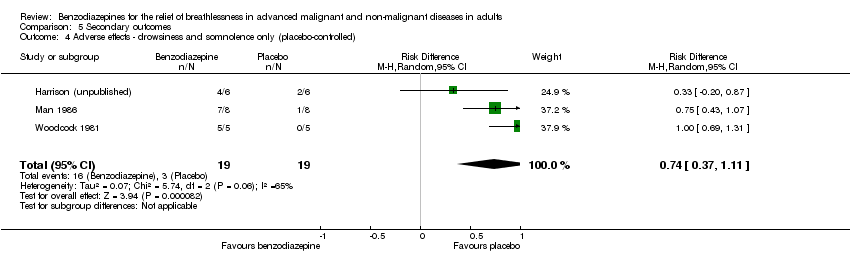
Comparison 5 Secondary outcomes, Outcome 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled).

Comparison 5 Secondary outcomes, Outcome 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled).
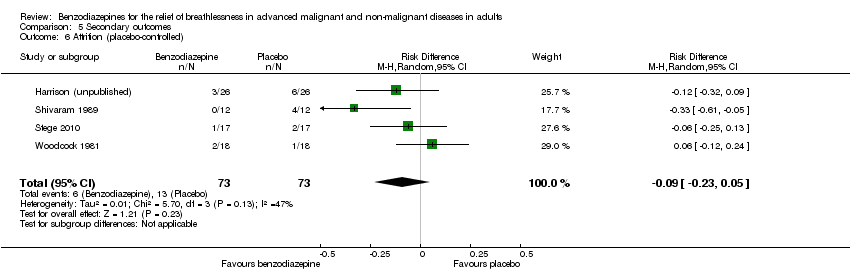
Comparison 5 Secondary outcomes, Outcome 6 Attrition (placebo‐controlled).
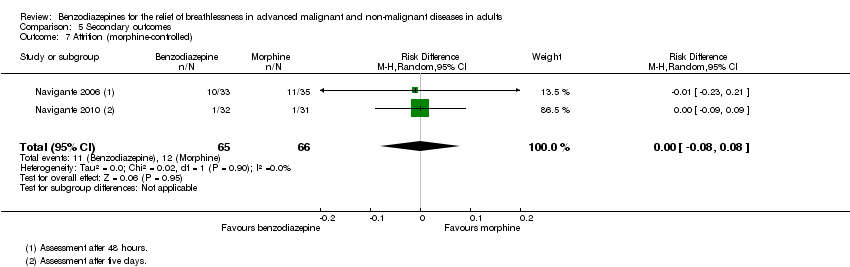
Comparison 5 Secondary outcomes, Outcome 7 Attrition (morphine‐controlled).

Comparison 5 Secondary outcomes, Outcome 8 Deaths (placebo‐controlled).
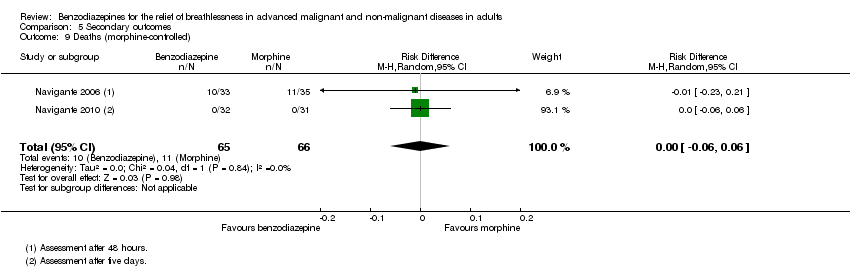
Comparison 5 Secondary outcomes, Outcome 9 Deaths (morphine‐controlled).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placebo‐controlled/cross‐over design Show forest plot | 5 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| 2 Morphine‐controlled/parallel design Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 COPD Show forest plot | 4 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.52, 0.29] |
| 2 Cancer ‐ placebo‐controlled Show forest plot | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.73, 0.62] |
| 3 Cancer ‐ morphine‐controlled Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepines ‐ alprazolam Show forest plot | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.41, 0.57] |
| 2 Benzodiazepines ‐ diazepam Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| 3 Benzodiazepines ‐ midazolam Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| 4 Benzodiazepines ‐ temazepam Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.91, 2.11] |
| 5 Benzodiazepines ‐ ultra short‐acting Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| 6 Benzodiazepines ‐ intermediate‐acting Show forest plot | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.38] |
| 7 Benzodiazepines ‐ long‐acting Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours) Show forest plot | 2 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.74, 0.01] |
| 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days) Show forest plot | 5 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| 10 Benzodiazepines ‐ morphine + midazolam‐controlled Show forest plot | 1 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.54, 0.61] |
| 11 Benzodiazepines ‐ promethazine‐controlled Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.72, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breathlessness ‐ no relief (placebo‐controlled) Show forest plot | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.39] |
| 2 Breathlessness ‐ no relief (morphine‐controlled) Show forest plot | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.91, 3.32] |
| 3 Breathlessness ‐ episodic after 48 hours Show forest plot | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.09] |
| 4 Breathlessness ‐ episodic after 24 hours Show forest plot | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects (placebo‐controlled) Show forest plot | 4 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [‐0.06, 0.94] |
| 2 Adverse effects (morphine‐controlled) Show forest plot | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.31, ‐0.04] |
| 3 Adverse effects ‐ clinical relevance only (morphine‐controlled) Show forest plot | 2 | 54 | Risk Difference (M‐H, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.25] |
| 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled) Show forest plot | 3 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.74 [0.37, 1.11] |
| 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled) Show forest plot | 2 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
| 6 Attrition (placebo‐controlled) Show forest plot | 4 | 146 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 7 Attrition (morphine‐controlled) Show forest plot | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| 8 Deaths (placebo‐controlled) Show forest plot | 4 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| 9 Deaths (morphine‐controlled) Show forest plot | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.06] |

