Benzodiacepinas para el alivio de la disnea en enfermedades malignas y no malignas avanzadas en adultos
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double Methodological quality: 10/22 (Edwards Method Score) | |
| Participants | Disease: COPD Number (randomised): N = 5 Setting: hospital Age (years, mean): not stated (only range: 51 to 68) Sex (male/female): 4/1 Participant pool: 56 Randomised: 5; study completed: 5 Withdrawals/dropouts: 0 (intervention 2 (clorazepate 22.5 mg) with 3 dropouts; whole intervention excluded from analysis) Reason for drop‐out (intervention 2): intolerable AEs (which AEs not mentioned) Baseline parameters: FEV1 less than 50% SpO2 (mmHg): 65.36; SpCO2 (mmHg): 41.58 | |
| Interventions | Drug (dose): 1. clorazepate (7.5 mg) at bedtime; 2. clorazepate (22.5) mg at bedtime; 3. placebo Delivery: oral Duration of treatment: 2 weeks | |
| Outcomes | Breathlessness grade (1 to 6) Results: no significant difference between clorazepate and placebo regarding dyspnoea and walking test (no numbers given; dyspnoea change only in graphs) Adverse effects: none within the 5 participants in intervention 1 and placebo SpO2 and SpCO2: no significant change | |
| Notes | Author conclusion: this study failed to demonstrate that placebo or clorazepate consistently relieved breathlessness in non‐anxious people with severe COPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned (“Patients were assigned in a randomised double‐blind manner”) |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Double‐blinding stated in the abstract, but not mentioned further |
| Incomplete outcome data (attrition bias) | Unclear risk | Not mentioned |
| Selective reporting (reporting bias) | High risk | Anxiety, depression, etc. were assessed but data not reported |
| Other bias | High risk |
|
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double‐blind Methodological quality: 18/22 (Edwards Method Score) | |
| Participants | Disease: advanced cancer (12/17 lung cancer) Number (randomised): N = 26 Setting: outpatient and inpatient Age (years, SD): 67.2 (8.3) Sex (male/female): 16/1 Participant pool: 54 Randomised: 26; study completed: 17 Withdrawals/dropouts: 9 (4 drowsiness, 1 deterioration, 1 dysphagia, 2 death, 1 unclear) (excluded from analysis) | |
| Interventions | Drug (dose): lorazepam (0.5 mg twice daily = 1 mg per day) Delivery: oral Duration of treatment: 5 days (2 days wash‐out) | |
| Outcomes | Dyspnoea VAS 0 to 100 ("How much trouble has your breathing caused you over the last 24 hours?") Results (mean): baseline to 5 days after intervention: 1. lorazepam 49.18 to 44.49; 2. placebo 48.06 to 45.94 Adverse effects (number of AEs/number with withdrawals): 1. lorazepam: 5/3; 2 placebo: 4/1 No change or differences in anxiety and depression (HADS) | |
| Notes | Author conclusion: there were no differences between lorazepam and placebo in relieving breathlessness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...was randomly determined by computer prior to the study commencement" |
| Allocation concealment (selection bias) | Low risk | "A randomisation list was kept by the pharmacy" |
| Blinding (performance bias and detection bias) | Low risk | Study was blinded |
| Incomplete outcome data (attrition bias) | Low risk | All data were presented The study author sent the raw data in addition |
| Selective reporting (reporting bias) | Low risk | Study protocol is available |
| Other bias | Low risk | Study appeared to be free of other bias |
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double Methodological quality: 16/22 (Edwards Method Score) | |
| Participants | Disease: COPD Number (randomised): N = 29 Setting: outpatient Age (years, mean): 65.4 Sex (male/female): 16/8 (complete) Participant pool: not stated Randomised: 29; study completed: 24 Withdrawals/dropouts: 5 (excluded from analysis) Reason for drop‐out: 1 AE (placebo), 4 missed appointments Baseline parameters: FEV1/FVC: 54% SpO2 (mmHg): 73.4; SpCO2 (mmHg): 32.8 | |
| Interventions | Drug (dose): alprazolam 1.0 mg/day (0.5 mg twice daily) Control: placebo Delivery: oral Duration of treatment: 1 week | |
| Outcomes | Dyspnoea grade at rest (1 to 5); dyspnoea scoring at rest and exercise (VAS 0 to 10) Results (mean, baseline to after intervention): alprazolam: 3.0 to 3.0; placebo: 3.2 to 3.0 No significant change in dyspnoea scoring during rest and exercise Adverse effects: 11 reported (7/11 drowsiness), 9/11 on alprazolam Functional test (12‐minute walking test in metres; baseline to after intervention): alprazolam: 896.5 to 880.88; placebo: 902.17 to 931.29 The resting SpO2 was significantly higher with placebo and exercising SpCO2 was significantly lower with placebo | |
| Notes | Author conclusion: the subjective perception of dyspnoea was the same before and after alprazolam, at rest and during exercise | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...designed as a randomized...” Not mentioned how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | “double‐blind...using alprazolam and matching placebo” Labelled bottles with tablets (alprazolam‐placebo‐wash‐out) described in detail Probably done |
| Incomplete outcome data (attrition bias) | Unclear risk | Total screened patients not mentioned No intention‐to‐treat analysis (5/29 lost were excluded from analysis), however only one with AE (placebo) |
| Selective reporting (reporting bias) | Low risk | Broad information available, good summaries, good presentation |
| Other bias | Low risk | Pharmaceutical funding (company with alprazolam), although negative results |
| Methods | Design: RCT, parallel, multi‐arm (3), control: morphine and morphine + midazolam Blinding: single‐blind (only participant blinded) Methodological quality: 17/22 (Edwards Method Score) | |
| Participants | Disease: terminal cancer (life expectancy less than a week) Number (randomised): N = 101 Setting: hospital inpatient Age (years, mean): 57.3 Sex (male/female): 47/54 Participant pool: n = 146 Randomised: 101; study completed: 70 Withdrawals/dropouts: 31 (all deaths) (excluded from analysis) | |
| Interventions | Drug (dose): 1. morphine (Mo ‐ 10 mg/day); 2. midazolam (Mi ‐ 20 mg/day); 3. morphine + midazolam (MM ‐ Mo 10 mg/day + Mi 20 mg/day) Rescue dose: 1. Mi 5 mg; 2. Mo 2.5 mg; 3. Mo 2.5 mg (this means that all 3 treatment arms could include a combination of morphine and midazolam) Delivery: subcutaneous Duration of treatment: 48 hours | |
| Outcomes | Dyspnoea intensity: modified Borg scale 0 to 10 Results presented in the paper: baseline (mean) to after intervention (24/48 hours = median and P‐values): 1. (Mo) 7.1 to 3/2 (P=0.002/P=0.0001); 2. (Mi) 6.9 to 4/2 (P=0.018/P=0.004); 3. (MM) 6.8 to 3/2 (P=0.003/P<0.0001) (Mean and CI (95%) for 24‐ and 48‐hour measures received from the authors (data skewed): 1. (Mo) 24 hours: 3.9 (2.8 to 5.0), 48 hours: 2.8 (1.6 to 4.0); 2. (Mi) 24 hours: 4.1 (2.8 to 5.4), 48 hours: 3.1 (1.7 to 4.5); 3. (MM) 24 hours: 3.4 (2.4 to 4.4), 48 hours: 3.0 (2.0 to 4.0)) Percentages of participants with breakthrough dyspnoea (24/48 hours): 1. (Mo) 34.3%/38%; 2. (Mi) 36.4%/38.5%; 3. (MM) 21.2%/24% Numbers of breakthrough episodes of dyspnoea per participant (24/48 hours): 1. (Mo) 2/2; 2. (Mi) 1/1; 3. (MM) 1/1 Percentages of participants with dyspnoea relief after 24 hours: 1. (Mo) 69% (P=0.03); 2. (Mi) 46% (P=0.004); 3. (MM) 92% (P‐values compare to MM) Percentages of participants with persistent, uncontrolled dyspnoea after 48 hours: 1. (Mo) 12.6%; 2. (Mi) 26% (P=0.04 compare to MM); 3. (MM) 4% Adverse effects: the most frequently recorded AE was somnolence (Mo > MM > Mi) Oxygen saturation (mean; baseline to after intervention; 24/48 hours): 1. (Mo) 72% to 72%/70%; 2. (Mi) 73% to 70%/70%; 3. (MM) 73% to 73%/71.5% Anxiety: significant correlation between dyspnoea and anxiety at all times | |
| Notes | Author conclusion: the data demonstrate that the beneficial effects of morphine in controlling baseline levels of dyspnoea could be improved with the addition of midazolam to the treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “...using a random number generator in 1:1:1 ratio in blocks of nine” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how it was done |
| Blinding (performance bias and detection bias) | Low risk | “Drug administrations were performed in a single‐blind fashion.” “One potential limitation of our study is the single‐blinded nature of the design. The treating physicians’ knowledge of which schedule of drugs the patient received could influence their need for administering rescue medications. A double‐blind design can avoid this, but was considered not appropriate for our study population by the Ethics Committee at our institution. Nevertheless, the risk for underestimation of rescue needs was minimized by a double assessment of breakthrough episodes carried out by caregivers and research physicians.” |
| Incomplete outcome data (attrition bias) | Unclear risk | 45/146 excluded with statement of reasons Attrition (deaths) clearly mentioned Missing data not stated Unclear if participants who experienced relief of dyspnoea was assessed on the whole number of participants or only on participants alive at the end of the study (30% died) |
| Selective reporting (reporting bias) | High risk | Unclear which other symptoms were measured (only anxiety‐dyspnoea is reported). Results of ECOG and MMSE not reported. Dyspnoea relief (only after 24 hours) and uncontrolled dyspnoea (only after 48 hours) |
| Other bias | High risk | Using cross‐over rescue medication (midazolam for the morphine group and vice versa) could produce confusion for separate analysis |
| Methods | Design: RCT, parallel, control: morphine Blinding: single‐blind (only participant and caregiver blinded) Methodological quality: 21/22 (Edwards Method Score) | |
| Participants | Disease: advanced cancer Number (randomised): N = 63 Setting: outpatient clinic Age (years, median, intervention group 1/2): 59/55 Sex (male/female): not mentioned Participant pool: not mentioned Randomised: 63; study completed: 61* Withdrawals/dropouts: 2* (unable or unwilling to comply with the programmed follow‐up visits) (excluded from analysis) *Data at day 5 for the morphine group were only available for 29 participants, therefore all calculations at day 5 were done with 29 participants | |
| Interventions | Drug (starting dose within fast titration phase): 2 mg for oral midazolam or 3 mg for oral morphine with incremental steps of 25% of the preceding dose every 30 min until dyspnoea was alleviated 50% or more ("effective dose" used in follow‐up assessment) Delivery: oral Duration of follow‐up treatment: 5 days using the "effective dose" every 4 hours (except the sleep hours) | |
| Outcomes | Dyspnoea relief (fast titration phase): 5‐category scale (0% none, 25% slight, 50% moderate, 75% a lot, 100% complete) Dyspnoea intensity for the chronic component of dyspnoea (baseline and follow‐up assessment): NRS 0 to 10 Proportion of participants with BTD episodes Number of BTD episodes per day Results presented in the published paper: In the fast titration phase dyspnoea relief of at least 50% was achieved in all participants in both arms (after starting dose: midazolam 21/32 vs morphine 11/31 (P = 0.023), after dosing step 1: 9/32 vs 11/31 (P = 0.59), and after dosing step 2: 2/32 vs 9/31 (P = 0.022)); in the follow‐up phase, mean (95% CI) baseline dyspnoea intensity is presented in a table: midazolam 8.8 (±0.3) vs morphine 8.7 (±0.3) (P = 0.62), but follow‐up results on dyspnoea intensity are only presented in figure with box plots (participants receiving midazolam maintained a significantly lower dyspnoea intensity level in comparison with the morphine group, during the 4 days of follow‐up) and as median at the second day in text: midazolam 6 vs morphine 4.5 (P = 0.003); number of participants with 1 or more BTD episodes at baseline was 25 in both arms, and the proportion of participants with BTD episodes was significantly different at days 3 to 5, favouring the midazolam arm (data only presented by a figure); therapeutic failure (i.e. NRS 8 to 10 by day 5) midazolam 0/31 vs morphine 6/30; AE (n during fast titration phase): mild somnolence midazolam 18/32 vs morphine 15/31, mild agitation 2/32 vs 2/31, mild and moderate nausea only morphine 2/31 and 1/31; AE (n during follow‐up): somnolence (time spent sleeping during daytime) 3 h to 5 h midazolam 4/31 vs morphine 5/30, 6.11 h only morphine 1/30; agitation grade 1/2 only in morphine arm 2/1/30; nausea grade 1 only morphine 1/30; constipation grade 2 only morphine 2/30; others midazolam 1/31 (cognitive disturbance) vs morphine 2/31 (cough g1, pruritus g2, xerostomia g1, flushing g1); dose reduction (because of excessive somnolence): midazolam 1 vs morphine 2; Oxygen saturation: no change in either group Additional results received from the authors: Mean (95% CI) dyspnoea intensity for baseline and day 5 measures (data skewed): 1. (midazolam) baseline: 8.84 (8.50 to 9.19), day 5: 3.23 (2.51 to 3.94); 2. (morphine) baseline: 8.74 (8.44 to 9.04), day 5: 6.00 (5.31 to 6.69) | |
| Notes | Author conclusion: the data demonstrate the beneficial effect of midazolam versus morphine in the relief of chronic dyspnoea intensity and the number of episodes of breathlessness (breakthrough dyspnoea), while adverse events occurred and were comparable between both arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number generator in 1:1 ratio in blocks of six" |
| Allocation concealment (selection bias) | Low risk | "Numbered envelopes that were used to implement the randomization were concealed until interventions were assigned. The researchers had final responsibility for patient enrollment" |
| Blinding (performance bias and detection bias) | High risk | Only single‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No information on dealing with missing data or presence of it |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting |
| Other bias | Unclear risk | None |
| Methods | Design: RCT, cross‐over, placebo‐controlled Blinding: double Methodological quality: 15/22 (Edwards Method Score) | |
| Participants | Disease: COPD Number (randomised): N = 12 Setting: unclear Age (years, mean): 64.9 Sex (male/female): 8/0 Participant pool: not stated Randomised: 12; study completed: 8 Withdrawals/dropouts: 4 (excluded from analysis) Reason for drop‐out: all on placebo (3/4 increasing dyspnoea and drowsiness, 1/4 acute exacerbation) Baseline parameters: FEV1/FVC: all less than 65% SpO2 (mmHg): 76.0; SpCO2 (mmHg): 38.0 | |
| Interventions | Drug (dose): alprazolam 0.75 mg/day (0.25 mg 3 times a day) Control: placebo Delivery: oral Duration of treatment: 2 weeks | |
| Outcomes | Dyspnoea (modified Borg scale 0 to 10) Results* (baseline to after intervention): alprazolam: 3.6 to 3.6; placebo: 3.6 to 3.0 (*not explicitly stated if mean or median, but must be mean because of decimal numbers) Adverse effects: none within the 8 participants SpO2 and SpCO2: no significant change | |
| Notes | Author conclusion: alprazolam did not alter the sensation of breathlessness | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were started on a double‐blind, randomized crossover regimen” “The patients then received either placebo or alprazolam 0.25 mg in a double‐blind fashion” Not mentioned how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how it was done |
| Blinding (performance bias and detection bias) | Low risk | “The medication code was known only to the hospital pharmacist.” “Patients were started on a double‐blind, randomized crossover regimen” “The patients then received either placebo or alprazolam 0.25 mg in a double‐blind fashion” |
| Incomplete outcome data (attrition bias) | Unclear risk | Described the attrition and reasons for it Excluded from analysis, but stated that they did not differ with regard to spirometric measures Demographics only from included participants (8/12) No predicted FEV1 and FVC mentioned |
| Selective reporting (reporting bias) | Low risk | No indication for selective reporting |
| Other bias | High risk | Only men (Veterans Affairs Medical Center) |
| Methods | Design: RCT, placebo‐controlled, cross‐over design Blinding: double Methodological quality: 16/22 (Edwards Method Score) | |
| Participants | Disease: COPD (stages III to IV) Number (randomised/analysed): n = 17/14 Setting: outpatient center of a respiratory medicine department Age (years, mean, SD): 61.6 ∓ 8.0 Sex (male/female): 10/4 Participant pool: 199 Randomised: 17; study completed: 14 Withdrawals/dropouts: 3 (excluded from analysis) Reason for drop‐out: 1/3 on intervention: (exacerbation of COPD), 2/3 on placebo (1 obstructive sleep apnoea‐hypopnoea syndrome, 1 withdrew due to burden of the measurements) Baseline parameters: FEV1/FVC (mean, SD) 32.7 ∓ 13, PaCO2, kPa 5.4 ∓ 0.4, PaO2, kPa 9.6 ∓ 0.7 Baseline sleep‐related complaints: difficulty maintaining sleep (experienced by 8 participants), a prolonged sleep‐onset latency (experienced by 7 participants), extensive daytime sleepiness (experienced by 6 participants), and nocturnal dyspnoea (experienced by 2 participants) Baseline medication: inhaled corticosteroids 14/14, anticholinergics 13/14, β2‐antagonists 9/14, oral steroids 4/14, proton pump inhibitors 4/14, anticoagulants 4/14, other antihypertensives 4/14, theophylline 3/14, diuretics 3/14, acetylcysteine 1/14 | |
| Interventions | Drug (dose): temazepam 10 mg/day (30 min before bedtime) Control: placebo Delivery: oral Duration of treatment: 1 week each, with 1‐week wash‐out time | |
| Outcomes | Subjective dyspnoea (10‐point VAS) Results (mean (SD)): subjective dyspnoea: baseline 3.8 (2.6), temazepam 4.2 (2.9), placebo 4.1 (2.5), P = 0.90; transcutaneous carbon dioxide (PtcCO2) during sleep: baseline 6.2 (0.6), temazepam 5.9 (1.0), placebo 6.3 (1.4), P = 0.27; oxygen saturation (SpO2) during sleep: baseline 92 (2), temazepam 92 (3), placebo 92 (2), P = 0.31; total sleep time, h (mean (SD)): baseline 5.7 (1.2), temazepam 6.3 (1.0), placebo 5.4 (1.1), P = 0.03; sleep latency (10‐point VAS): baseline 4.4 (3.2), temazepam 3.3 (2.8), placebo 4.6 (3.2), P = 0.03; amount of stage 2 sleep, minutes (non‐rapid eye movement sleep): baseline 130.8 (54.5), temazepam 168.8 (34.4), placebo 140.0 (44.6), P = 0.03; no statistically significant changes for the other secondary outcomes; Adverse effects: none reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized after the baseline measurements to use 10 mg temazepam or placebo once a day orally, both during one week, separated by a washout‐period of one week. (...) Randomization was done by the hospital pharmacy." Not mentioned of how this was done |
| Allocation concealment (selection bias) | Unclear risk | "Subjects were randomized after the baseline measurements to use 10 mg temazepam or placebo once a day orally, both during one week, separated by a washout‐period of one week. Randomization was done by the hospital pharmacy." |
| Blinding (performance bias and detection bias) | Low risk | "Subjects were randomized after the baseline measurements to use 10 mg temazepam or placebo once a day orally, both during one week, separated by a washout‐period of one week. Randomization was done by the hospital pharmacy. Subjects were instructed to take the study medication 30 min before they went to bed. (…) Sleep was manually staged according to standard methods by two qualified sleep technicians blinded to the subject’s treatment status." |
| Incomplete outcome data (attrition bias) | Unclear risk | Described the attrition (3/17) and the reasons for it, but did not describe participant characteristics of dropouts. No intention‐to‐treat analysis (14 of 17 enrolled participants analysed) |
| Selective reporting (reporting bias) | Unclear risk | The article addresses only respiratory adverse events; it is unclear if other than respiratory events had occurred |
| Other bias | Unclear risk |
|
| Methods | Design: RCT, cross‐over, placebo‐controlled, multi‐arm (3) Blinding: double Methodological quality: 15/22 (Edwards Method Score) | |
| Participants | Disease: COPD Number (randomised): N = 18 Setting: outpatient Age (years, mean): 60.5 Sex (male/female): 15/3 Participant pool: not stated Randomised: 18; study completed: 15 Withdrawals/dropouts: 3 (excluded from analysis) Reason for drop‐out: 1 death (diazepam), 1 intolerable drowsiness (diazepam), 1 hypercapnia (placebo) Baseline parameters: FEV1: 25.3%; FEV1/FVC: 0.38 SpO2 (kPa): 9.5 (= 71.25 mmHg); SpCO2 (kPa): 4.6 (= 34.5 mmHg) | |
| Interventions | Drug (dose): 1. diazepam 25 mg/day (5 mg 3 times a day plus 10 mg at bedtime); 2. promethazine 125 mg/day (25 mg 3 times a day plus 50 mg at bedtime); 3. placebo Delivery: oral Duration of treatment: 2 weeks | |
| Outcomes | Dyspnoea grade (1 to 5) after each intervention and daily dyspnoea by VAS (0 to 10) at rest and after exercise (only by graph) Results (mean): dyspnoea grade: 1. 3.46 (diazepam); 2. 3.29 (P < 0.05) (promethazine); 3. 4.00 (placebo) Adverse effects (6 ‐ reduce dosage): all drowsiness: 5/6 diazepam; 1/6 promethazine; 5/5 drowsiness incidents (like falling down stairs) with diazepam Functional test (12‐minute walking test in metres): 1. 642 (P < 0.05) (diazepam); 2. 707 (P < 0.05) (promethazine); 3. 675 (placebo) SpO2 and SpCO2: no significant change No significant change in anxiety and depression | |
| Notes | Author conclusion: diazepam had no significant effect on breathlessness and noticeably reduced exercise tolerance. Promethazine reduced breathlessness and improved exercise tolerance without altering lung function. Review author: however, there is a beneficial effect of diazepam, although not significant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The treatments were given in a randomized order.” Not mentioned how this was done |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | “Double‐blind” procedure was described |
| Incomplete outcome data (attrition bias) | Low risk | Although 3/18 participants were lost and excluded from the analysis, they would underline the presented results rather than bias them |
| Selective reporting (reporting bias) | Unclear risk | All main outcomes are presented in detail The effect of diazepam in the relief of breathlessness is nearly statistically significant, but was discussed as "diazepam had no effect on breathlessness" |
| Other bias | Unclear risk | It is not explicitly stated if a wash‐out phase was used (on contacting the author, there was no wash‐out) Results of compliance test are not mentioned Screening method and numbers are not mentioned |
AEs = adverse effects
BTD = breakthrough dyspnoea
CI = confidence interval
COPD = chronic obstructive pulmonary disease
ECOG = Eastern Cooperative Oncology Group
FEV1 = forced expiratory volume in one second
FVC = forced vital capacity
HADS = Hospital Anxiety and Depression Scale
Mi = midazolam
MM = midazolam + morphine
MMSE = Mini‐Mental State Exam
Mo = morphine
NRS = numeric rating scale
RCT = randomised controlled trial
SD = standard deviation
VAS = visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Non‐controlled study (phase II) | |
| Review | |
| Review | |
| Review | |
| No subjective measurement of breathlessness; different drug (flumazenil) | |
| Different drug (buspirone) | |
| Review | |
| No subjective measurement of breathlessness | |
| Different drug (nortriptyline) | |
| No subjective measurement of breathlessness; observational design | |
| Systematic review | |
| No subjective measurement of breathlessness | |
| No subjective measurement of breathlessness | |
| Guideline | |
| No subjective measurement of breathlessness; case series | |
| Non‐controlled study (before‐after design) | |
| No subjective measurement of breathlessness | |
| Study protocol; study was cancelled before any participants were enrolled | |
| No subjective measurement of breathlessness; letter/observational design | |
| No subjective measurement of breathlessness | |
| No subjective measurement of breathlessness; healthy participants | |
| Review | |
| Review | |
| Guideline | |
| No subjective measurement of breathlessness; healthy participants | |
| Review | |
| Review | |
| No subjective measurement of breathlessness | |
| Non‐controlled, retrospective study | |
| Non‐controlled experimental study (case report) | |
| No subjective measurement of breathlessness; observational design | |
| Review | |
| No subjective measurement of breathlessness; sedation for artificial ventilation | |
| No subjective measurement of breathlessness; different disease (sleep apnoea) | |
| Review | |
| Non‐advanced disease stage; a few participants with a different disease (asthma, tuberculosis) | |
| Different drug (laevomepromazine) | |
| Review | |
| Different disease (psychosomatic disorder) | |
| No subjective measurement of breathlessness; observational design | |
| Different disease (healthy participants) | |
| Editorial | |
| Review | |
| No subjective measurement of breathlessness; observational design | |
| No subjective measurement of breathlessness; healthy participants | |
| No drug intervention (secondary analysis) | |
| No subjective measurement of breathlessness; case report; no benzodiazepine | |
| Benzodiazepine only in combination (oxazepam + orciprenaline) | |
| Different drug (promethazine) | |
| No drug intervention (retrospective study) | |
| Different drug (chlorpromazine) | |
| No subjective measurement of breathlessness; no control group; no standardised or systematic design | |
| No drug intervention | |
| No subjective measurement of breathlessness; case report | |
| No subjective measurement of breathlessness | |
| No subjective measurement of breathlessness | |
| Benzodiazepine only in combination (midazolam + morphine) | |
| Benzodiazepine only in combination (midazolam + morphine) | |
| Study failed to recruit any participants | |
| Review | |
| No drug intervention (observational study) | |
| Review | |
| Review | |
| Different drug (chlorpromazine) | |
| No subjective measurement of breathlessness | |
| No subjective measurement of breathlessness; healthy participants | |
| Review | |
| Different drug (promethazine) | |
| Editorial | |
| Review | |
| No subjective measurement of breathlessness | |
| Review | |
| Review | |
| No subjective measurement of breathlessness; no control group | |
| Different drug (promethazine) | |
| Review | |
| Different disease (healthy participants) | |
| Different disease (healthy participants) | |
| Different drug (dihydrocodeine) | |
| Review | |
| No subjective measurement of breathlessness | |
| Non‐controlled, retrospective study; congress abstract | |
| No subjective measurement of breathlessness | |
| Observational design | |
| Review | |
| Review | |
| Review | |
| No subjective measurement of breathlessness; different drug (oxygen, morphine, barbiturate) | |
| Different drug (dihydrocodeine, alcohol, caffeine) | |
| Different drug (oxygen) |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: multicentre, placebo‐controlled, cross‐over design, blinded (masking used) RCT |
| Participants | Inclusion criteria: adults with dyspnoea related to life‐limiting disease (malignant and non‐malignant) or its treatment, dyspnoea score > 3/10 on at least 3 occasions during the previous week, English speaking or have an interpreter available, AKPS scale > 30, able to operate a nasal spray device, able to understand all trial requirements and complete a dyspnoea diary, no changes in any medication likely to affect dyspnoea (e.g. steroids, opioids) within 48 hours of starting the study Target sample size: 200 > terminated after interim analysis including 75 participants |
| Interventions | Intranasal midazolam (3 inhalations (total dose of 1.5 mg active drug) vs placebo (citric acid 7.65 mg/ml in normal saline placebo nasal spray) |
| Outcomes | Primary outcome: dyspnoea intensity at 15 minutes compared to baseline Secondary outcomes: DID (dyspnoea intensity difference) at 5, 30, and 60 mins, sedation (NRS 0 = not at all drowsy to 10 = extremely drowsy), anxiety (NRS 0 = not at all anxious to 10 = extremely anxious) |
| Notes | The study has been published just before publication of this review update The study is registered at the Australian New Zealand Clinical Trials Registry (ACTRN), trial ID: ACTRN12609000506291, Title: Midazolam nasal spray for the treatment of breathlessness in patients with life‐limiting disease Contact information: Clare Randall, Arohanui Hospice 1 Heretaunga St Palmerston North 4414, New Zealand, [email protected] |
| Methods | Design: randomised, double‐blind, double‐dummy, placebo‐controlled pilot study |
| Participants | 30 people with severe respiratory disease (MRC dyspnoea score 4 or 5) |
| Interventions | Lorazepam tablets 0.5 mg twice daily with dummy nasal spray up to 4 times daily or intranasal midazolam (dose 400 mg) 2 sprays up to 4 times daily with placebo tablets |
| Outcomes | Primary outcome measures were designed to evaluate quality of life measures incorporating change in:
|
| Notes | The authors conclude in the abstract that intranasal midazolam is no less effective in this setting than oral lorazepam and suggest that intranasal midazolam is another useful tool for managing dyspnoea. However, only conference abstract is available; we contacted two of the authors asking for further details, but did not receive an answer until the review was published |
AKPS = Australia‐modified Karnofsky Performance Status
DID = dyspnoea intensity difference
MRC = Medical Research Council
NRS = numeric rating scale
RCT = randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placebo‐controlled/cross‐over design Show forest plot | 5 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| Analysis 1.1  Comparison 1 Overall, Outcome 1 Placebo‐controlled/cross‐over design. | ||||
| 2 Morphine‐controlled/parallel design Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Analysis 1.2  Comparison 1 Overall, Outcome 2 Morphine‐controlled/parallel design. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 COPD Show forest plot | 4 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.52, 0.29] |
| Analysis 2.1  Comparison 2 Disease, Outcome 1 COPD. | ||||
| 2 Cancer ‐ placebo‐controlled Show forest plot | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.73, 0.62] |
| Analysis 2.2  Comparison 2 Disease, Outcome 2 Cancer ‐ placebo‐controlled. | ||||
| 3 Cancer ‐ morphine‐controlled Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Analysis 2.3  Comparison 2 Disease, Outcome 3 Cancer ‐ morphine‐controlled. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepines ‐ alprazolam Show forest plot | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.41, 0.57] |
| Analysis 3.1  Comparison 3 Intervention, Outcome 1 Benzodiazepines ‐ alprazolam. | ||||
| 2 Benzodiazepines ‐ diazepam Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| Analysis 3.2  Comparison 3 Intervention, Outcome 2 Benzodiazepines ‐ diazepam. | ||||
| 3 Benzodiazepines ‐ midazolam Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Analysis 3.3  Comparison 3 Intervention, Outcome 3 Benzodiazepines ‐ midazolam. | ||||
| 4 Benzodiazepines ‐ temazepam Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.91, 2.11] |
| Analysis 3.4  Comparison 3 Intervention, Outcome 4 Benzodiazepines ‐ temazepam. | ||||
| 5 Benzodiazepines ‐ ultra short‐acting Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Analysis 3.5  Comparison 3 Intervention, Outcome 5 Benzodiazepines ‐ ultra short‐acting. | ||||
| 6 Benzodiazepines ‐ intermediate‐acting Show forest plot | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.38] |
| Analysis 3.6  Comparison 3 Intervention, Outcome 6 Benzodiazepines ‐ intermediate‐acting. | ||||
| 7 Benzodiazepines ‐ long‐acting Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| Analysis 3.7  Comparison 3 Intervention, Outcome 7 Benzodiazepines ‐ long‐acting. | ||||
| 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours) Show forest plot | 2 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.74, 0.01] |
| Analysis 3.8  Comparison 3 Intervention, Outcome 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours). | ||||
| 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days) Show forest plot | 5 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| Analysis 3.9  Comparison 3 Intervention, Outcome 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days). | ||||
| 10 Benzodiazepines ‐ morphine + midazolam‐controlled Show forest plot | 1 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.54, 0.61] |
| Analysis 3.10  Comparison 3 Intervention, Outcome 10 Benzodiazepines ‐ morphine + midazolam‐controlled. | ||||
| 11 Benzodiazepines ‐ promethazine‐controlled Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.72, 0.72] |
| Analysis 3.11  Comparison 3 Intervention, Outcome 11 Benzodiazepines ‐ promethazine‐controlled. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breathlessness ‐ no relief (placebo‐controlled) Show forest plot | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.39] |
| Analysis 4.1  Comparison 4 Primary outcome, Outcome 1 Breathlessness ‐ no relief (placebo‐controlled). | ||||
| 2 Breathlessness ‐ no relief (morphine‐controlled) Show forest plot | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.91, 3.32] |
| Analysis 4.2  Comparison 4 Primary outcome, Outcome 2 Breathlessness ‐ no relief (morphine‐controlled). | ||||
| 3 Breathlessness ‐ episodic after 48 hours Show forest plot | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.09] |
| Analysis 4.3  Comparison 4 Primary outcome, Outcome 3 Breathlessness ‐ episodic after 48 hours. | ||||
| 4 Breathlessness ‐ episodic after 24 hours Show forest plot | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.34] |
| Analysis 4.4  Comparison 4 Primary outcome, Outcome 4 Breathlessness ‐ episodic after 24 hours. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects (placebo‐controlled) Show forest plot | 4 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [‐0.06, 0.94] |
| Analysis 5.1  Comparison 5 Secondary outcomes, Outcome 1 Adverse effects (placebo‐controlled). | ||||
| 2 Adverse effects (morphine‐controlled) Show forest plot | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.31, ‐0.04] |
| Analysis 5.2  Comparison 5 Secondary outcomes, Outcome 2 Adverse effects (morphine‐controlled). | ||||
| 3 Adverse effects ‐ clinical relevance only (morphine‐controlled) Show forest plot | 2 | 54 | Risk Difference (M‐H, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.25] |
| Analysis 5.3  Comparison 5 Secondary outcomes, Outcome 3 Adverse effects ‐ clinical relevance only (morphine‐controlled). | ||||
| 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled) Show forest plot | 3 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.74 [0.37, 1.11] |
| Analysis 5.4  Comparison 5 Secondary outcomes, Outcome 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled). | ||||
| 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled) Show forest plot | 2 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
| Analysis 5.5  Comparison 5 Secondary outcomes, Outcome 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled). | ||||
| 6 Attrition (placebo‐controlled) Show forest plot | 4 | 146 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| Analysis 5.6  Comparison 5 Secondary outcomes, Outcome 6 Attrition (placebo‐controlled). | ||||
| 7 Attrition (morphine‐controlled) Show forest plot | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| Analysis 5.7  Comparison 5 Secondary outcomes, Outcome 7 Attrition (morphine‐controlled). | ||||
| 8 Deaths (placebo‐controlled) Show forest plot | 4 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| Analysis 5.8  Comparison 5 Secondary outcomes, Outcome 8 Deaths (placebo‐controlled). | ||||
| 9 Deaths (morphine‐controlled) Show forest plot | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.06] |
| Analysis 5.9  Comparison 5 Secondary outcomes, Outcome 9 Deaths (morphine‐controlled). | ||||
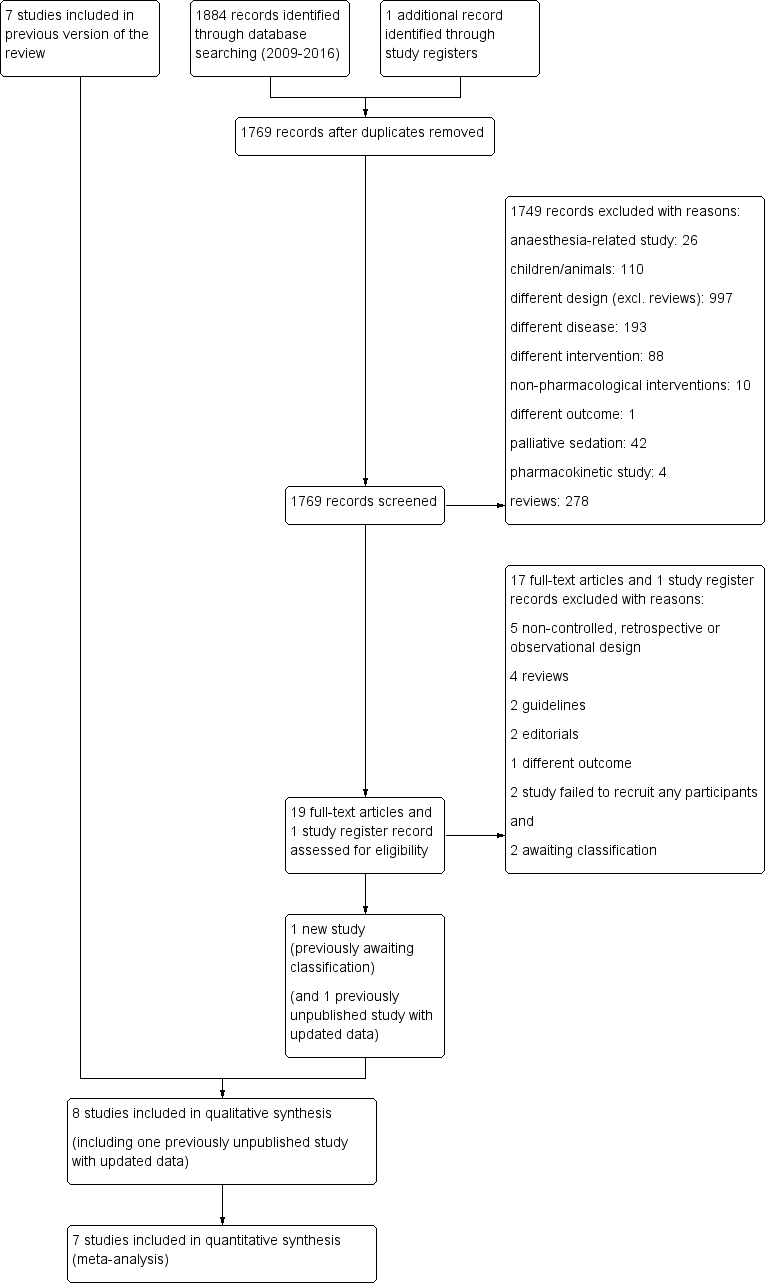
Study flow diagram.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
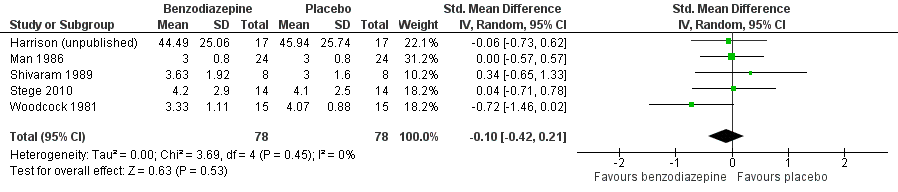
Forest plot of comparison: 1 Overall, outcome: 1.1 Placebo‐controlled/cross‐over design.
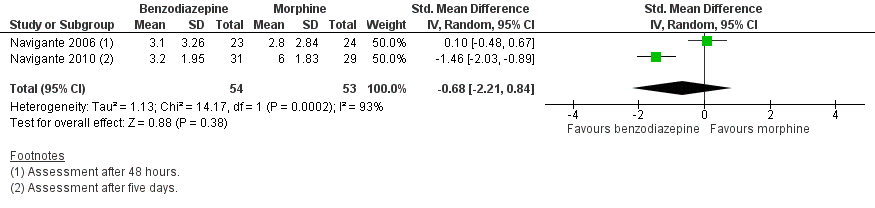
Forest plot of comparison: 1 Overall, outcome: 1.2 Morphine‐controlled/parallel design.
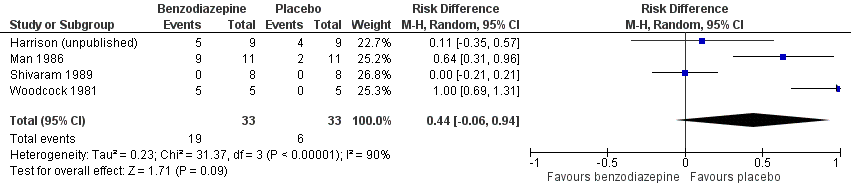
Forest plot of comparison: 5 Secondary outcomes, outcome: 5.1 Adverse effects (placebo‐controlled).

Comparison 1 Overall, Outcome 1 Placebo‐controlled/cross‐over design.

Comparison 1 Overall, Outcome 2 Morphine‐controlled/parallel design.

Comparison 2 Disease, Outcome 1 COPD.

Comparison 2 Disease, Outcome 2 Cancer ‐ placebo‐controlled.
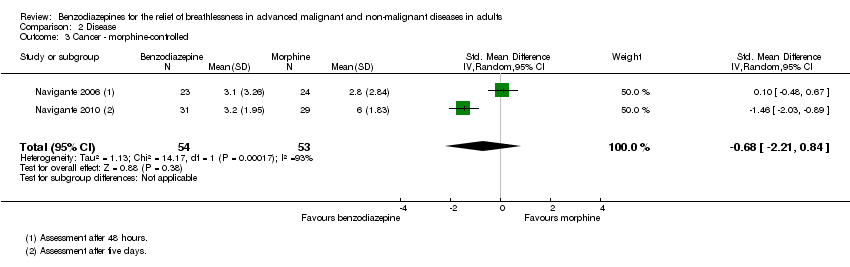
Comparison 2 Disease, Outcome 3 Cancer ‐ morphine‐controlled.
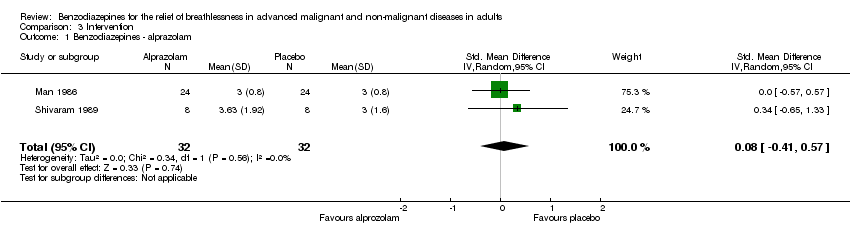
Comparison 3 Intervention, Outcome 1 Benzodiazepines ‐ alprazolam.

Comparison 3 Intervention, Outcome 2 Benzodiazepines ‐ diazepam.

Comparison 3 Intervention, Outcome 3 Benzodiazepines ‐ midazolam.
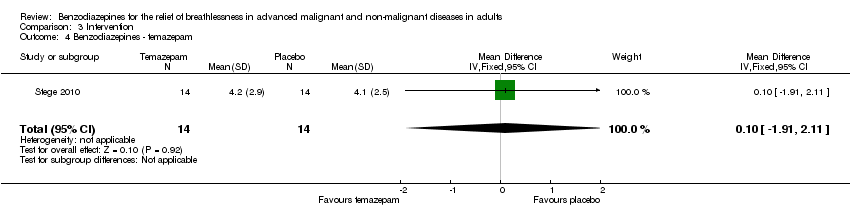
Comparison 3 Intervention, Outcome 4 Benzodiazepines ‐ temazepam.

Comparison 3 Intervention, Outcome 5 Benzodiazepines ‐ ultra short‐acting.

Comparison 3 Intervention, Outcome 6 Benzodiazepines ‐ intermediate‐acting.

Comparison 3 Intervention, Outcome 7 Benzodiazepines ‐ long‐acting.

Comparison 3 Intervention, Outcome 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours).

Comparison 3 Intervention, Outcome 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days).

Comparison 3 Intervention, Outcome 10 Benzodiazepines ‐ morphine + midazolam‐controlled.

Comparison 3 Intervention, Outcome 11 Benzodiazepines ‐ promethazine‐controlled.
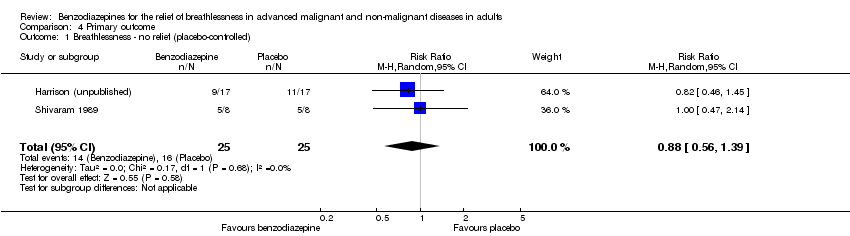
Comparison 4 Primary outcome, Outcome 1 Breathlessness ‐ no relief (placebo‐controlled).

Comparison 4 Primary outcome, Outcome 2 Breathlessness ‐ no relief (morphine‐controlled).
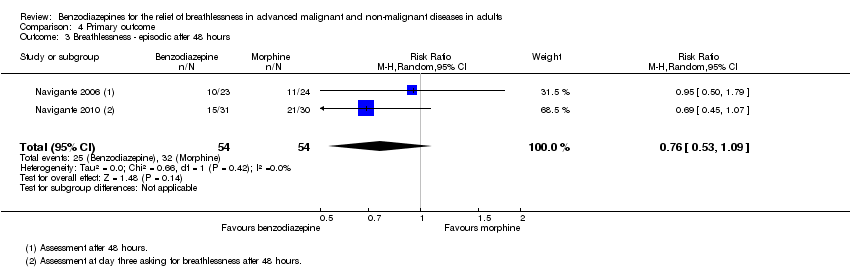
Comparison 4 Primary outcome, Outcome 3 Breathlessness ‐ episodic after 48 hours.

Comparison 4 Primary outcome, Outcome 4 Breathlessness ‐ episodic after 24 hours.
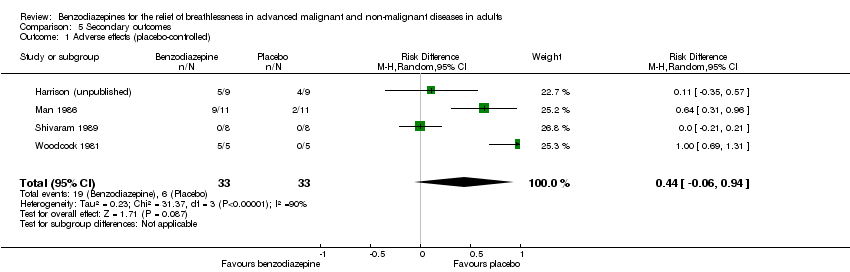
Comparison 5 Secondary outcomes, Outcome 1 Adverse effects (placebo‐controlled).

Comparison 5 Secondary outcomes, Outcome 2 Adverse effects (morphine‐controlled).

Comparison 5 Secondary outcomes, Outcome 3 Adverse effects ‐ clinical relevance only (morphine‐controlled).
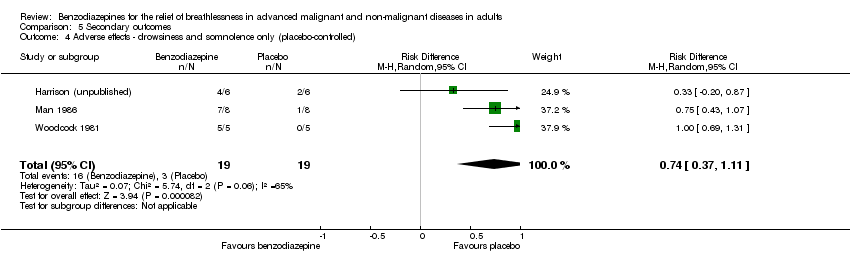
Comparison 5 Secondary outcomes, Outcome 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled).

Comparison 5 Secondary outcomes, Outcome 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled).
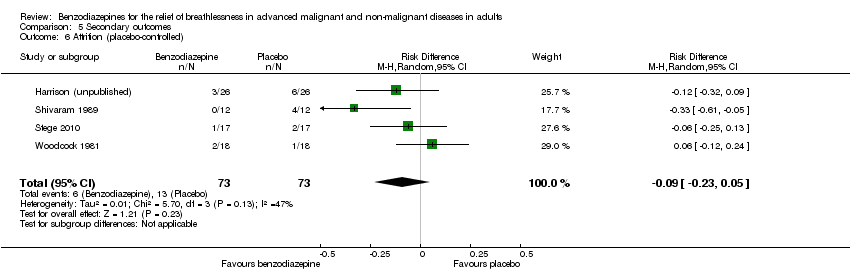
Comparison 5 Secondary outcomes, Outcome 6 Attrition (placebo‐controlled).
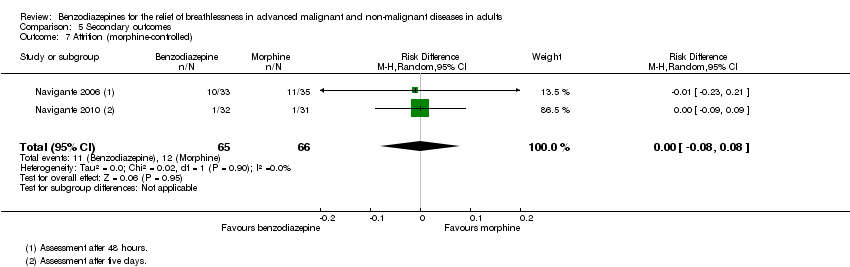
Comparison 5 Secondary outcomes, Outcome 7 Attrition (morphine‐controlled).

Comparison 5 Secondary outcomes, Outcome 8 Deaths (placebo‐controlled).
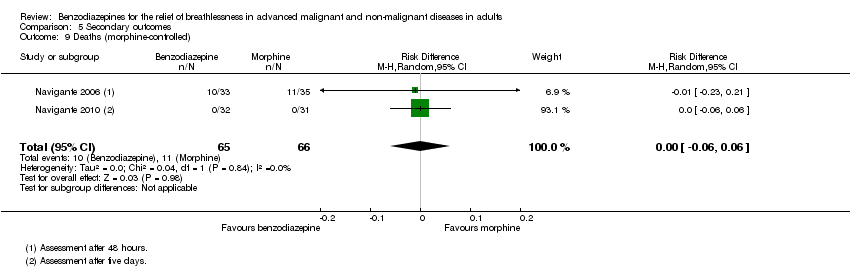
Comparison 5 Secondary outcomes, Outcome 9 Deaths (morphine‐controlled).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placebo‐controlled/cross‐over design Show forest plot | 5 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| 2 Morphine‐controlled/parallel design Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 COPD Show forest plot | 4 | 122 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.52, 0.29] |
| 2 Cancer ‐ placebo‐controlled Show forest plot | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.73, 0.62] |
| 3 Cancer ‐ morphine‐controlled Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Benzodiazepines ‐ alprazolam Show forest plot | 2 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.41, 0.57] |
| 2 Benzodiazepines ‐ diazepam Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| 3 Benzodiazepines ‐ midazolam Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| 4 Benzodiazepines ‐ temazepam Show forest plot | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐1.91, 2.11] |
| 5 Benzodiazepines ‐ ultra short‐acting Show forest plot | 2 | 107 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐2.21, 0.84] |
| 6 Benzodiazepines ‐ intermediate‐acting Show forest plot | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.31, 0.38] |
| 7 Benzodiazepines ‐ long‐acting Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.72 [‐1.46, 0.02] |
| 8 Benzodiazepines ‐ short duration of treatment (≦ 24 hours) Show forest plot | 2 | 116 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.74, 0.01] |
| 9 Benzodiazepines ‐ long duration of treatment (5 to 14 days) Show forest plot | 5 | 156 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.42, 0.21] |
| 10 Benzodiazepines ‐ morphine + midazolam‐controlled Show forest plot | 1 | 46 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.54, 0.61] |
| 11 Benzodiazepines ‐ promethazine‐controlled Show forest plot | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.72, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Breathlessness ‐ no relief (placebo‐controlled) Show forest plot | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.56, 1.39] |
| 2 Breathlessness ‐ no relief (morphine‐controlled) Show forest plot | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.91, 3.32] |
| 3 Breathlessness ‐ episodic after 48 hours Show forest plot | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.53, 1.09] |
| 4 Breathlessness ‐ episodic after 24 hours Show forest plot | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.71, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Adverse effects (placebo‐controlled) Show forest plot | 4 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.44 [‐0.06, 0.94] |
| 2 Adverse effects (morphine‐controlled) Show forest plot | 2 | 194 | Risk Difference (M‐H, Random, 95% CI) | ‐0.18 [‐0.31, ‐0.04] |
| 3 Adverse effects ‐ clinical relevance only (morphine‐controlled) Show forest plot | 2 | 54 | Risk Difference (M‐H, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.25] |
| 4 Adverse effects ‐ drowsiness and somnolence only (placebo‐controlled) Show forest plot | 3 | 38 | Risk Difference (M‐H, Random, 95% CI) | 0.74 [0.37, 1.11] |
| 5 Adverse effects ‐ drowsiness and somnolence only (morphine‐controlled) Show forest plot | 2 | 122 | Risk Difference (M‐H, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
| 6 Attrition (placebo‐controlled) Show forest plot | 4 | 146 | Risk Difference (M‐H, Random, 95% CI) | ‐0.09 [‐0.23, 0.05] |
| 7 Attrition (morphine‐controlled) Show forest plot | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.08, 0.08] |
| 8 Deaths (placebo‐controlled) Show forest plot | 4 | 120 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| 9 Deaths (morphine‐controlled) Show forest plot | 2 | 131 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.06, 0.06] |

