شیمیدرمانی پروفیلاکتیک برای مول هیداتیدیفرم بهمنظور پیشگیری از نئوپلازی تروفوبلاستیک بارداری
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT conducted in Japan. Participants recruited between 1963 and 1977. Stated as a prospective study with participants "selected at random" to receive prophylaxis This may not be a true RCT. | |
| Participants | 420 women with molar pregnancy (low and high risk). Excluded women who were referred longer than 3 weeks after evacuation; those who had received other drugs for prophylaxis (see 'Risk of bias' table below); women who had undergone hysterectomy; and women diagnosed as having partial mole or hydropic degeneration. | |
| Interventions | Arm 1: methotrexate 10 mg daily (IM or oral) for 7 days, within 3 weeks of evacuation (293 women). Arm 2: no P‐Chem (127 women). Women were followed up weekly with urine hCG measurements. | |
| Outcomes | GTN diagnosed by histology or Ishizuka score (a risk rating system used in Japan); side effects and subsequent pregnancy. | |
| Notes | 5‐ to 15‐year follow‐up reported. Time to invasive mole diagnosis was 56.8 days in P‐Chem group and 42.7 days in control group (SD not given; P = 0.6). No attrition occurred for primary outcomes. Only reported adverse effects in the P‐Chem group: 27.3% experienced drug‐related side effects including stomatitis (10.3%), nausea/vomiting (6.8%) and leukopenia (4.4%). Grades of toxicity were not reported but the report states that there were no severe complications or drug‐related deaths. Baseline characteristics were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | |
| Selective reporting (reporting bias) | High risk | |
| Other bias | High risk | |
| Methods | RCT conducted in Korea. Participants recruited between 1978 and 1984. | |
| Participants | 133 women with complete hydatidiform mole (both high and low risk) were randomised into 2 groups, but 62 were excluded (36 lost to follow‐up, 7 had "insufficient length of follow‐up" and 19 had hysterectomy) and only 71 completed this trial (39 in the treatment group and 32 in the untreated group). | |
| Interventions | Arm 1: methotrexate 1.0 mg/kg/day IM on days 1, 3, 5 and 7 and citrovorum factor rescue 0.1 mg/kg/day IM on days 2, 4, 6 and 8 (39/71 women including 18/31 low‐risk and 21/40 high‐risk women). ERPC was done on the third or fourth day of P‐Chem. Arm 2: no treatment other than ERPC (32 women including 13/31 low‐risk and 19/40 high‐risk women). | |
| Outcomes | Efficacy: incidence of GTN. Adverse effects: incidence of gastrointestinal toxicity, myelotoxicity, epithelial toxicity including rash, hair loss and mouth ulcers. The number of courses required to achieve remission in cases of GTN. Time to GTN diagnosis. Subsequent pregnancy. | |
| Notes | Baseline characteristics were similar between the groups, including the proportion of low‐ and high‐risk lesions. ERPC was done on the third or fourth day of P‐Chem. Women were followed up weekly until hCG was normal for 3 consecutive weeks, then monthly for 6 months, then bimonthly for 6 months, then every 6 months. The mean duration of follow‐up was 19 months (SD 9.7; range 6 to 50). All women were in complete remission at study closure. Pregnancy rates after molar pregnancy were similar between the 2 groups (93% vs 94%). P‐Chem had little effect on the rate of subsequent GTN in the low‐risk group; only 2/31 low‐risk women developed GTN (1 women in each study group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | High risk | Of 133 women treated, 62 were excluded from the study (36 were lost to follow‐up, 7 had insufficient length of follow‐up and 19 had a hysterectomy). Therefore the outcome data were extracted from the 71 women (39 in the treatment group and 32 in the untreated group). |
| Selective reporting (reporting bias) | High risk | All the pre‐specified outcomes were reported. However, certain women were excluded from the analyses (those who underwent hysterectomy and those with insufficient follow‐up) therefore the analyses were not by intention‐to‐treat. |
| Other bias | High risk | It is unclear on what basis the participants were initially diagnosed as having CM. If prophylaxis was given based on a clinical diagnosis before ERPC, this may have resulted in women with hydropic degeneration or PM being included in the study. |
| Methods | RCT conducted in Thailand. Participants were recruited between 1989 and 1994. | |
| Participants | Women diagnosed with high‐risk CM (with histological diagnosis) within 1 week of evacuation of molar tissue. Women were considered 'high risk' if they had at least 1 of the following characteristics: initial serum hCG > 100,000 mIU/mL; uterine size larger than dates; theca lutein cysts > 6 cm; age > 40 years; or associated medical and epidemiological factors including previous GTD, toxaemia, hyperthyroidism, trophoblast embolisation or disseminated intravascular coagulation. 60 participants were randomised into 2 groups (30:30). | |
| Interventions | Arm 1: IV actinomycin D (10 µg/kg) for 5 days, within 1 week of evacuation of molar tissue. Arm 2: IV fluids and analgesic drugs for 5 days within 1 week of evacuation of molar tissue. | |
| Outcomes | Efficacy: incidence of GTN. Adverse effects: incidence of gastrointestinal toxicity, myelotoxicity, hair loss, mouth ulcers. Time to diagnosis of GTN. | |
| Notes | The gestational age at diagnosis of HM was 13.8 ± 3.0 weeks in the intervention group and 13.6 ± 4.2 weeks in the control group. Women were followed up for 1 year with hCG assays every 2 weeks for 3 months, then monthly for 3 months, then every 2 months up to 1 year. The diagnosis of GTN was made in all women in the P‐Chem group (4/4) and 12/15 women in the control group according to the following criteria: rising hCG levels for 2 weeks or a plateau for 3 weeks; persistent or recurrent vaginal bleeding with detectable hCG levels; clinical or histological evidence of invasive mole, choriocarcinoma or metastases with persistently high or rising hCG values. Histology was obtained for 3 participants. 2 out of 4 women in the P‐Chem group and 3 out of 15 in the control group were lost to follow‐up after diagnosis of GTN; therefore 5 women with GTN received no subsequent treatment and data were insufficient to compare the number of chemotherapy courses received in each group. Side effects were reported as percentages and only recorded for the P‐Chem group, as follows: stomatitis (10%), nausea/vomiting (10%), oral ulcers (3.3%) and hair loss (13.3%). All adverse effects were grade 1 except for 2 patients with patchy alopecia (grade 2). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by lot‐drawing (information obtained by e‐mail correspondence with Dr Limpongsanurak). |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (information obtained by e‐mail correspondence with Dr Limpongsanurak). |
| Blinding of participants and personnel (performance bias) | Low risk | Described as double‐blind. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Described as double‐blind, but the details of outcome assessment are unclear. |
| Incomplete outcome data (attrition bias) | Low risk | 1 woman in the P‐Chem group was lost to follow‐up 1 month after treatment and not included in the primary outcome analysis. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | Baseline characteristics and risk factors for disease progression were similar between the groups. |
CM: complete mole; ERPC: evacuation of retained products of conception; GTD: gestational trophoblastic disease; GTN: gestational trophoblastic neoplasia; hCG: β‐human chorionic gonadotrophin; IM: intramuscular; IV: intravenous; P‐Chem: prophylactic chemotherapy; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| A prospective randomised clinical trial comparing intravenous (IV) MTX and IV Act‐D in the treatment of low‐risk gestational trophoblastic neoplasia, invasive mole, and choriocarcinoma. | |
| A retrospective study evaluating characteristics and outcomes for 23 women with high‐risk HM who received prophylactic chemotherapy (5‐FU or dactinomycin). | |
| A RCT of 75 patients with low‐risk GTD (FIGO stage I, II, or III disease, a WHO risk score of 6 or less), 50 receiving pulsed actinomycin D and 25 receiving 5‐day methotrexate. | |
| A retrospective study evaluating a bolus dose of dactinomycin for prevention of persistent GTD in 29 adolescents with high‐risk molar pregnancy compared with a similar control group of 31 adolescents. | |
| A retrospective study evaluating the effect of a bolus dose of dactinomycin, given 1 hour before ERPC to women with high‐risk HM, on the rate of malignant transformation to GTN. |
5‐FU: 5‐fluorouracil; ERPC: evacuation of retained products of conception; GTD: gestational trophoblastic disease; GTN: gestational trophoblastic neoplasia; HM: hydatidiform mole.
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Second Curettage in Treating Patients With Persistent Non‐metastatic Gestational Trophoblastic Tumors |
| Methods | Mutlicentre Phase II study (NCT00521118) |
| Participants | Women with histologically confirmed gestational trophoblastic neoplasia (GTN) (complete or partial hydatidiform mole) with no histologically confirmed choriocarcinoma, placental site trophoblastic tumour (PSTT), or epithelioid trophoblastic tumour (ETT) on the first curettage. Persistent, low‐risk disease (based on FIGO/WHO 2002 staging and risk‐scoring criteria), as defined by 1 of the following criteria: less than 10% decline in β‐human chorionic gonadotropin (hCG) levels, based on 4 consecutive measurements over a 3‐week period (plateau); greater than 20% rise in β‐hCG levels, based on 3 consecutive measurements over a 2‐week period; β‐hCG level remains elevated above normal for ≥ 6 months. WHO risk score ≤ 6. Must have a clinically significant elevated β‐hCG level of > 20 miu/mL. No evidence of metastatic disease beyond the uterus by pelvic examination, pelvic ultrasound, and chest x‐ray. No previously treated, persistent or recurrent GTN (same gestation) that have been treated with chemotherapy. |
| Interventions | Women undergo a second curettage rather than standard treatment (immediate chemotherapy). Women whose disease has transformed into choriocarcinoma, placental site trophoblastic tumour, or epithelioid trophoblastic tumour (histologically diagnosed at the second curettage) are removed from the study. All other women undergo weekly β‐human chorionic gonadotropin (hCG) testing beginning 14 days after the second curettage and continuing until the β‐hCG level is normal. Women then undergo further β‐hCG testing weekly for 4 weeks and then monthly for 5 months. If the level does not regress to normal, or rises, or if metastatic disease is identified, the participant is removed from the study. |
| Outcomes | Frequency of surgical cure, defined as a normal β‐human chorionic gonadotropin (hCG) level documented for 6 consecutive months AND no chemotherapy. Development of choriocarcinoma, placental site trophoblastic tumour (PSTT), or epithelioid trophoblastic tumour (ETT) histologically diagnosed at second curettage. Development of “second persistent” disease, defined as failure to achieve or maintain a normal assay, or a plateau, or a rise in the assay level after second curettage. Frequency and severity of adverse effects of second curettage, specifically uterine operative injury, haemorrhage, and infection (pelvis, fallopian tubes, and ovaries), as assessed by CTCAE version 3.0. |
| Starting date | October 2007 |
| Contact information | Philip J. DiSaia, Gynecologic Oncology Group |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of GTN (overall) Show forest plot | 3 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.24, 0.57] |
| Analysis 1.1 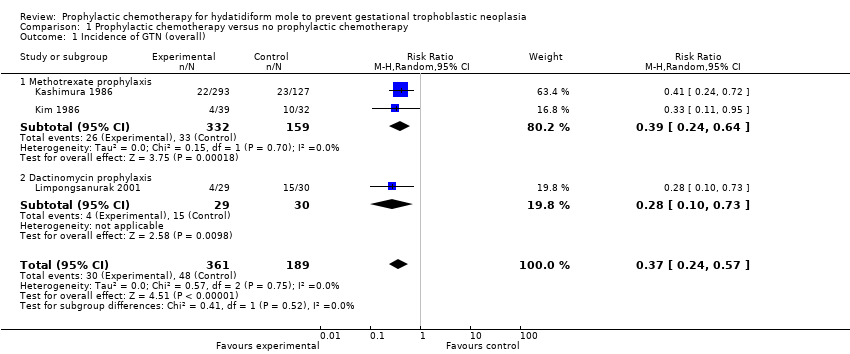 Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 1 Incidence of GTN (overall). | ||||
| 1.1 Methotrexate prophylaxis | 2 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.64] |
| 1.2 Dactinomycin prophylaxis | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 2 Incidence of GTN (high‐risk HM only) Show forest plot | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.60] |
| Analysis 1.2  Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 2 Incidence of GTN (high‐risk HM only). | ||||
| 2.1 Methotrexate prophylaxis | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.95] |
| 2.2 Dactinomycin prophylaxis | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 3 Time to GTN diagnosis Show forest plot | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 28.72 [13.19, 44.24] |
| Analysis 1.3 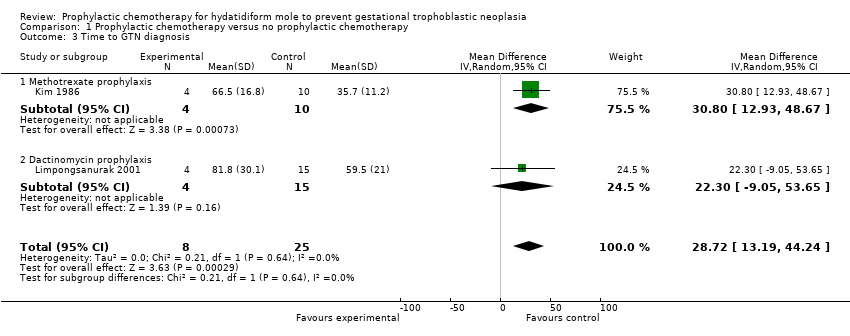 Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 3 Time to GTN diagnosis. | ||||
| 3.1 Methotrexate prophylaxis | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 30.80 [12.93, 48.67] |
| 3.2 Dactinomycin prophylaxis | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 22.30 [‐9.05, 53.65] |
| 4 Number of courses of chemotherapy to cure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.4 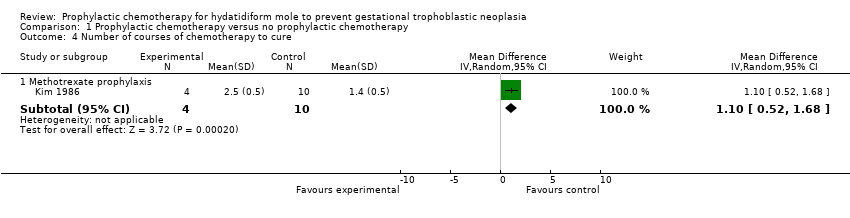 Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 4 Number of courses of chemotherapy to cure. | ||||
| 4.1 Methotrexate prophylaxis | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.1 [0.52, 1.68] |
| 5 Mortality rate Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 5 Mortality rate. | ||||

Study flow diagram.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.

Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 1 Incidence of GTN (overall).

Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 2 Incidence of GTN (high‐risk HM only).

Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 3 Time to GTN diagnosis.

Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 4 Number of courses of chemotherapy to cure.

Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 5 Mortality rate.
| Prophylactic chemotherapy compared with no prophylactic chemotherapy for hydatidiform mole | ||||||
| Patient or population: women with a molar pregnancy Settings: inpatient Intervention: methotrexate or dactinomycin Comparison: placebo or no prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No prophylaxis | P‐Chem | |||||
| Incidence of GTN (including low‐quality studies) | Mixed‐risk population | RR 0.37 (0.24 to 0.57) | 550 women | ⊕⊕⊝⊝ | The NNTB to prevent 1 woman developing GTN after evacuation of HM was 6 (95% CI 5 to 10). We downgraded this evidence because this meta‐analysis included 2 studies that we considered to be of poor methodological quality. | |
| 254 per 1000 | 94 per 1000 (61 to 145) | |||||
| High‐risk population | RR 0.29 (0.14 to 0.60) | 99 women (2 studies) | ⊕⊕⊝⊝ | The NNTB for women with high‐risk HM was 3 (95% CI 2 to 5). We downgraded this evidence because the meta‐analysis included 2 small studies, 1 of which was of a poor methodological quality. | ||
| 490 per 1000 | 142 per 1000 (69 to 294) | |||||
| Incidence of GTN (excluding low‐quality studies) | High‐risk population | RR 0.28 (0.10 to 0.73) | 59 women (1 study) | ⊕⊕⊝⊝ | The NNTB to prevent 1 woman developing GTN after evacuation of high‐risk HM was 3 (95% CI 2 to 20). We downgraded this evidence because only 1 small study (Limpongsanurak 2001) contributed data, giving an imprecise result. | |
| 500 per 1000 | 140 per 1000 (50 to 365) | |||||
| Time to GTN diagnosis (days) | The mean time to GTN diagnosis ranged across control groups from 35.7 days to 59.5 days. | The mean time to GTN diagnosis in the intervention groups was 65.5 days to 81.8 days (higher). | MD 28.72 (13.19 to 44.24) | 33 women | ⊕⊕⊝⊝ | We downgraded this evidence because the meta‐analysis included 1 study of poor methodological quality (Kim 1986). When this study was excluded, the results of the remaining study (Limpongsanurak 2001; 19 women) were: MD 22.30; 95% CI −9.05 to 53.65. |
| Number of courses of chemotherapy to cure | The mean number of courses of chemotherapy required to cure subsequent GTN was 1.4 courses (10 women). | The mean number of courses of chemotherapy required to cure subsequent GTN was 2.5 courses (4 women). | MD 1.10 (0.52 to 1.68) | 14 women | ⊕⊝⊝⊝ | This analysis only included 1 study, and we considered to be of a poor methodological quality (Kim 1986). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| The assumed risk for the mixed‐risk population was calculated by using the weighted mean risk across the control group for this outcome. The assumed risk for the high‐risk population was based on the control group of Limpongsanurak 2001, which was the only study to evaluate a high‐risk population only. | ||||||
| Prognostic factor | Score | |||
| 0 | 1 | 2 | 3 | |
| U/S diagnosis | Partial | Complete | Recurrent | |
| Uterine size for GA (months) | not more than 1 | > 1 | > 2 | > 3 |
| hCG level (mIU/mL) | < 50,000 | > 50,000 to < 100,000 | > 100,000 to < 1,000,000 | > 1,000,000 |
| Diameter of theca lutein cysts (cm) | ‐ | < 6 | < 6 to < 10 | > 10 |
| Patient age (years) | ‐ | < 20 | ≥ 40 | > 50 |
| Medical complications** | ‐ | ≥ 1 | ‐ | ‐ |
| *From Berkowitz 1987 Low risk is defined as a score of < 4; high risk is defined as a score ≥ 4 U/S: ultrasound; GA: gestational age, hCG: β‐human chorionic gonadotrophin. ** hyperemesis, hyperthyroidism, pre‐eclampsia, trophoblastic embolisation, disseminated intravascular coagulation. | ||||
| Study | Design | Participants (P‐Chem) | Participants (control/no P‐Chem) | Intervention | Rate of GTN (P‐Chem) | Rate of GTN (control) | Comments |
| Case‐control | 107 women (HM) | 42 women (HM) | Methotrexate 10 mg/day PO × 7 days given within 3 weeks of ERPC. | 2/107 (2%) | 4/42 (10%) | No choriocarcinoma observed in the P‐Chem group vs 3/42 in the control group. Toxic side effects occurred in 84/107 women, including stomatitis (34/107) and myelosuppression (22/107). | |
| Prospective case‐control | 73 women (CM) | 116 women (CM) | 3 intervention arms: methotrexate 0.3 mg/kg/day × 5 days (20 women); or dactinomycin 9 to 12 μg/kg/day × 5 days (53 women); ERPC on day 3. | 6/73 (8%) | 23/116 (20%) | No metastatic disease observed in the P‐Chem groups. P‐Chem well tolerated with minor side effects. | |
| Prospective case‐control | 100 women (HM) | 100 women (HM) | Dactinomycin 12 μg/kg/day × 5 days. ERPC on day 3. | 2/100 (2%) | 16/100 (16%) | No metastatic disease observed in the P‐Chem group vs 4/100 in the control group (4%). Reversible alopecia occurred in 32% of the P‐Chem group. No serious toxic reactions. | |
| Prospective case‐control | 174 women (CM) | 858 women (CM) | Dactinomycin 12 μg/kg/day × 5 days. ERPC on day 3. | 10/247 (4%) | 160/858 (19%) | No metastatic disease observed in the P‐Chem group vs 34/858 (4%) in the control group. This report includes data from Goldstein 1974. | |
| Retrospective case‐control | 104 women (92% CM) | 250 women (CM) | Methotrexate 10 mg/day PO × 5 days every 3 weeks for 3 cycles. | 3/104 (3%) | 23/250 (9%) | Significantly fewer high‐risk women in the P‐Chem group (1/47) vs the control group (18/126) developed GTN (2% vs 14%; P < 0.05). 2 women had severe myelosuppression and 1 had severe alopecia. | |
| RCT (?) | 293 women (CM) | 127 women (CM) | Methotrexate 10 mg/day (IM or PO) for 7 days, within 3 weeks of evacuation. | 22/293 (7%) | 23/127 (18%) | There were 5 cases of metastatic disease in each group (1.7% vs 3.9%, respectively) 27.3% of the P‐Chem group experienced drug‐related side effects including stomatitis (10.3%), nausea/vomiting (6.8%) and leukopenia (4.4%). However none were reported to be severe. | |
| RCT | 39/71 women (CM; 18/31 low‐risk and 21/40 high‐risk women) | 32 women (CM) | Methotrexate 1.0 mg/kg/day IM (days 1, 3, 5, 7) and citrovorum factor rescue 0.1 mg/kg/day IM (days 2, 4, 6, 8). ERPC on day 3. | 4/39 (10%) | 10/32 (31%) | Significantly fewer high‐risk women in the P‐Chem group (14%) vs the control group (47%) developed GTN. There was no significant difference in the GTN rates of low‐risk women between groups. | |
| Retrospective case‐control | 52 women (14 low‐risk, 21 medium‐risk and 17 high‐risk HM) | 88 women (38 low‐risk, 25 medium‐risk and 25 high‐risk HM) | Methotrexate 1 mg/kg (days 1, 3, 5, 7) and citrovorum factor (0.1 mg/kg (days 2, 4, 6, 8); or dactinomycin 12 μg/kg/day × 5 days started at the time of ERPC. | 8/52 (15.4%) | 28/88 (31.8%) | Significantly fewer high‐risk women in the P‐Chem group (7/17) vs the control group (22/25) developed GTN (41% vs 88%; P < 0.01). There was no significant difference in the GTN rates in low‐ and medium‐risk women between groups. The time to achieve normal hCG levels was shorter in high‐risk women in the P‐Chem group. | |
| Double‐blind RCT | 30 women (high‐risk CM) | 30 women (high risk CM) | Dactinomycin 10 µg/kg for 5 days, within 1 week after ERPC and histology. | 4/29 (15.4%) | 15/30 (50%) | Mild, reversible side effects reported including stomatitis (10%), nausea/vomiting (10%), oral ulcers (3.3%) and hair loss (13.3%) ‒ all grade 1 except for 2 women with grade 2 patchy alopecia. | |
| Retrospective case‐control | 29 adolescents (high‐risk CM) | 31 adolescents (high‐risk CM) | Dactinomycin 1.25 mg/m² IV given 1 hour before ERPC. | 2/29 (6.9%) | 9/31 (29%) | Mean risk scores and hCG levels were significantly higher and gestational age was significantly lower in the P‐Chem group than the control group. Mild and transient side effects included hepatotoxicity (10%) and mild alopecia (6.8%). | |
| Retrospective case‐control | 163 women (high risk, > 90% CM) | 102 women (high risk, > 90% CM) | Dactinomycin 1.25 mg/m² IV given 1 hour before ERPC. | 30/163 (18.4%) | 35/102 (34.3%) | Mild and transient side effects including nausea (8%), raised liver enzymes (3.7%), stomatitis (3.1%), rash (2.4%) diarrhoea (2.4%), alopecia (1.2%) and neutropenia (0.6%) were seen in 21% of the P‐Chem group. Time to GTN diagnosis, subsequent drug resistance and the number of chemotherapy course to cure was similar in the 2 groups. | |
| * Three studies administered P‐Chem after ERPC including Koga 1968, Kashimura 1986 and Limpongsanurak 2001. CM; complete mole; ERPC: evacuation of retained products of conception; GTN: gestational trophoblastic neoplasia; HM: hydatidiform mole; IM: intramuscular; IV: intravenous; P‐Chem; prophylactic chemotherapy; PO: per os; RCT: randomised controlled trial. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of GTN (overall) Show forest plot | 3 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.24, 0.57] |
| 1.1 Methotrexate prophylaxis | 2 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.64] |
| 1.2 Dactinomycin prophylaxis | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 2 Incidence of GTN (high‐risk HM only) Show forest plot | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.60] |
| 2.1 Methotrexate prophylaxis | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.95] |
| 2.2 Dactinomycin prophylaxis | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 3 Time to GTN diagnosis Show forest plot | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 28.72 [13.19, 44.24] |
| 3.1 Methotrexate prophylaxis | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 30.80 [12.93, 48.67] |
| 3.2 Dactinomycin prophylaxis | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 22.30 [‐9.05, 53.65] |
| 4 Number of courses of chemotherapy to cure Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Methotrexate prophylaxis | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.1 [0.52, 1.68] |
| 5 Mortality rate Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |

