Antiépileptiques en prophylaxie des crises post‐craniotomie
Résumé scientifique
Contexte
Ceci est une version mise à jour d’une revue Cochrane publiée précédemment dans le numéro 3, 2015.
L'incidence des crises convulsives après une craniotomie sus‐tentorielle pour une pathologie non‐traumatique a été estimée à entre 15 et 20 % ; toutefois, le risque de crise peut varier entre 3 et 92 % sur une période de cinq ans. Les crises post‐opératoires peuvent précipiter le développement de l'épilepsie ; ces crises surviennent plutôt au cours du premier mois suivant la chirurgie crânienne. L'utilisation de médicaments antiépileptiques (AE) administrés avant ou après l'intervention dans le but de prévenir les crises convulsives après une chirurgie crânienne a été examinée dans plusieurs essais contrôlés randomisés (ECR).
Objectifs
Déterminer l'efficacité et l'innocuité des AE lorsqu'ils sont utilisés de manière prophylactique chez des personnes subissant une craniotomie et examiner quels AE sont les plus efficaces.
Stratégie de recherche documentaire
Pour la dernière mise à jour, nous avons consulté les bases de données suivantes le 26 juin 2017 : le registre spécialisé du groupe Cochrane sur l'épilepsie, le registre Cochrane des essais contrôlés (CENTRAL), MEDLINE, ClinicalTrials.gov, et le Système d'enregistrement international des essais cliniques de l’OMS (ICTRP). Nous n'avons appliqué aucune restriction de langue.
Critères de sélection
Nous avons inclus les ECR portant sur des personnes sans antécédent d'épilepsie qui subissaient une craniotomie pour des raisons thérapeutiques ou de diagnostic. Les essais avec des méthodes de randomisation appropriées et une assignation secrète ont été inclus ; il pouvait s'agir d'essais en parallèle à l'aveugle ou ouverts. Aucune période de traitement minimale n'était stipulée, et les essais utilisant des médicaments actifs ou un placebo dans le groupe témoin ont été inclus.
Recueil et analyse des données
Trois auteurs de revue (JW, JG, YD) ont choisi indépendamment les essais à inclure et ont extrait les données et évalué les risques de biais. Les désaccords éventuels ont été résolus par la discussion. Les critères de jugement examinés incluaient le nombre de patients présentant des crises convulsives (précoces ‐ survenant au cours de la première semaine suivant la craniotomie ‐ et tardives ‐ survenant après la première semaine suivant la craniotomie), le nombre de décès et le nombre de personnes présentant une incapacité et des effets indésirables. En raison de la nature hétérogène des essais, les données issues des essais n'ont pas été combinées dans une méta‐analyse ; les résultats de la revue sont présentés sous forme narrative. Des comparaisons visuelles des critères de jugement sont présentées dans des graphiques en forêt.
Résultats principaux
Nous avons inclus 10 ECR (n = 1815) publiés entre 1983 et 2015. Trois essais ont comparé un seul AE (phénytoïne) avec un placebo ou avec une absence de traitement. Un essai à trois bras a comparé deux AE (phénytoïne, carbamazépine) avec une absence de traitement. Un deuxième essai à trois bras a comparé la phénytoïne et le phénobarbital avec une absence de traitement. Sur les cinq essais comparant les AE au placebo ou à l’absence de traitement, deux essais ont rapporté un bénéfice significatif en faveur du traitement AE comparativement au témoin pour l’occurrence précoce de crises ; toutes les autres comparaisons n'ont révélé aucune différence claire ou statistiquement significative entre les AE et le traitement témoin. Parmi les essais en face à face, aucun n'a signalé de différence statistiquement significative entre les traitements AE (phénytoïne et valproate de sodium ; phénytoïne et phénobarbital ; levetiracetam et phénytoïne ; zonisamide et phénobarbital) que ce soit pour des crises précoces ou tardives.
Seuls cinq essais ont rapporté les cas de décès. Un essai a fait état d'une diminution statistiquement significative du nombre de décès dans les groupes recevant de la carbamazépine et sans traitement comparativement au groupe recevant de la phénytoïne après 24 mois de traitement, mais pas après six mois de traitement. L'incidence des effets indésirables du traitement a été mal signalée ; toutefois, trois essais ont montré que les effets indésirables de la phénytoïne étaient significativement plus nombreux que ceux du valproate, du placebo ou de l'absence de traitement. Aucun essai n'a fait état de résultats liés à des critères de jugement fonctionnels tels que l'incapacité.
Nous avons jugé la qualité des données probantes comme étant faible pour tous les critères de jugement signalés en raison de problèmes méthodologiques et de la variabilité des comparaisons effectuées dans les essais.
Conclusions des auteurs
Seules des données probantes limitées et de faible qualité suggèrent que le traitement AE administré à titre prophylactique est soit efficace, soit inefficace dans la prévention des crises post‐craniotomie (précoces ou tardives). La base de données probantes actuelle est limitée en raison des différentes méthodologies des essais et des incohérences dans le compte‐rendu des critères de jugement, y compris les décès et les événements indésirables. Des données probantes supplémentaires provenant d'essais actuels de bonne qualité sont nécessaires pour évaluer l'efficacité clinique du traitement prophylactique par AE par rapport au placebo ou à l'absence de traitement, ou à d'autres AE en matière de prévention des crises post‐craniotomie chez cette sélection de patients.
PICO
Résumé simplifié
Utilisation de médicaments antiépileptiques pour prévenir les crises convulsives après une chirurgie du cerveau
Problématique de la revue
La présente étude Cochrane examine les données probantes sur l'efficacité et l'innocuité des médicaments antiépileptiques (AE) lorsqu'ils sont administrés à des personnes qui ne souffrent pas d'épilepsie pour prévenir les crises convulsives après une craniotomie (un type de chirurgie du cerveau couramment utilisé pour l’ablation de tumeurs cérébrales). Nous avions également prévu d'évaluer si un AE est plus efficace que les autres pour prévenir les crises après une craniotomie.
Contexte
Les personnes qui subissent une chirurgie du cerveau (craniotomie) peuvent courir un risque accru de convulsions post‐opératoires. Les AE ont été utilisés dans le cadre d'essais cliniques pour prévenir les crises convulsives survenant après une intervention chirurgicale chez des personnes n'ayant pas d'antécédents d'épilepsie. Un petit nombre d'essais cliniques ont comparé différents traitements AE entre eux, tandis que d'autres ont comparé les AE à un placebo (une pilule qui ne contient aucun médicament) ou à un groupe sans traitement.
Caractéristiques de l’étude
Les données sont à jour en juin 2017. Dix essais répondaient à nos critères d'inclusion et portaient sur un total de 1 815 personnes. Trois essais ont comparé la phénytoïne (AE) avec un placebo ou avec une absence de traitement. Un essai a comparé un AE (la phénytoïne ou la carbamazépine) avec une absence de traitement. Un essai a comparé la phénytoïne ou le phénobarbital (AE) avec une absence de traitement. Cinq autres essais en face à face (un médicament est directement comparé à un autre) on comparé les AE entre eux (phénytoïne et valproate; zonisamide et phénobarbital; levetiracetam et phénytoïne).
Résultats principaux
Nous n'avons pas trouvé de preuves cohérentes suggérant que les traitements AE préventifs seraient efficaces pour réduire le nombre de crises après la chirurgie, les décès ou les effets indésirables.
Qualité des données probantes
Tous essais confondus, nous avons considéré que la qualité des données probantes était faible en raison de problèmes potentiels liés à la conception des essais. De plus, les différences dans la conception des essais en ce qui concerne les traitements étudiés et les résultats rapportés ont rendu difficile la comparaison des résultats d'un essai à l'autre. D'autres études de bonne qualité sont nécessaires pour valider les résultats mentionnés ci‐dessus.
Authors' conclusions
Summary of findings
| Antiepileptic drugs as prophylaxis for postcraniotomy seizures | ||||||
| Patient or population: people with postcraniotomy seizures Control: another antiepileptic drug, placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antiepileptic drugs | |||||
| Early seizures Follow‐up: up to 1 week | See comment | See comment | Not estimable | 1539 | ⊕⊕⊝⊝ | 7 trials found no significant differences across comparisons examined: phenytoin vs no treatment, phenobarbital or phenytoin vs no treatment, phenytoin vs valproate, levetiracetam vs phenytoin and zonisamide vs phenobarbital. 2 trials found a significantly lower number of seizures following use of phenytoin vs no treatment.c |
| Late seizures Follow‐up: 1 week up to 4 years (median) | See comment | See comment | Not estimable | 798 | ⊕⊕⊝⊝ | All trials found no significant differences across comparisons examined; phenytoin vs placebo or no treatment, phenobarbital or phenytoin vs no treatment, phenytoin vs valproate, zonisamide vs phenobarbital. |
| Death Follow‐up: up to 4 years (median) | See comment | See comment | Not estimable | 1016 | ⊕⊕⊝⊝ | 4 trials found no significant differences over comparisons: phenytoin vs valproate, zonisamide vs phenobarbital; levetiracetam vs phenytoin and phenytoin vs placebo. 1 trial found significantly fewer deaths in the carbamazepine and the no‐treatment group at 24 months compared to phenytoin.d This trial showed no significant difference between the interventions at 6 months. |
| Functional outcome (number of people with disabilities) Follow‐up: NA | See comment | See comment | Not estimable | NA | NA | No included studies reported a functional outcome. |
| Adverse effects Follow‐up: up to 12 months | See comment | See comment | Not estimable | 1165 | ⊕⊕⊝⊝ | Most trials found low numbers of adverse effects, and five trials found that no significant differences across comparisons were reported. Two trials found that significantly more adverse events were reported on phenytoin compared to placebo or no treatmente and one trial found that significantly more adverse events were reported on phenytoin compared valproate.f |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once due to risk of bias: methodological biases identified in trials (no allocation concealment, one study unblinded, unclear methods of dealing with missing data). | ||||||
Background
This is an updated version of the Cochrane Review previously published in Issue 3, 2015.
Description of the condition
The incidence of epilepsy following supratentorial craniotomy for non‐traumatic pathology has been estimated to be 15% to 20% (Foy 1981); however, due to the nature of the underlying disease for which surgery is undertaken, the risk of postcraniotomy seizures appears to vary from 3% to 92% over a five‐year period. It is likely that such seizures may cause epilepsy in previously unaffected people. The probability of de novo seizures occurring in people who have no history of epilepsy decreases over time after surgery. The highest incidence of postoperative epilepsy (two‐thirds of the seizures) occurs within the first month after cranial surgery (North 1983), and 75% of those who develop epilepsy do so within one year of surgery. Few people (approximately 8%) have their first seizure more than two years after surgery. The risk of seizures for particular groups of people is higher for some groups than others; for example, people who suffer from an abscess continue to run a high risk of developing epilepsy (92%) after five years, whilst for those with an arteriovenous malformation who have had a spontaneous intracerebral haematoma, the overall risk does not fall below 10% between year two and year five after surgery (Shaw 1991).
Description of the intervention
Due to the risk of postoperative seizures, the prophylactic use of antiepileptic drugs (AEDs) has been advocated for people undergoing cranial surgery. However, it is also argued that AEDs should not be used prophylactically, but should only be administered following at least one seizure (Temkin 2002). Other investigators maintain that early postoperative seizures do not justify the diagnosis of epilepsy and only late seizures are considered to be true epilepsy (Manaka 2003).
How the intervention might work
Uncontrolled retrospective trials support the use of AED treatment in people with a predisposition towards developing postoperative seizures (Matthew 1980), and data from pathological trials suggest that certain AEDs could have a neuro‐protective action on damaged cerebral cortex (Calabresi 2003).
Why it is important to do this review
To inform decision making regarding the prophylactic use of AEDs for people undergoing craniotomy, reliable high‐quality evidence is required. Benefits and harms and any trade‐offs between these need to be examined carefully. Potential benefits include reduced short‐term seizure recurrence, reduced long‐term epilepsy rates, and better surgical outcome and quality of life. Harms include adverse effects and poorer surgical outcome. This Cochrane Review will provide a summary of the currently available evidence from randomised controlled trials (RCTs) regarding the prophylactic use of AEDs for people undergoing craniotomy by examining the following outcomes: occurrence of early and late seizures, occurrence of death, functional disability and occurrence of adverse events.
Objectives
To determine the efficacy and safety of AEDs when used prophylactically in people undergoing craniotomy and to examine which AEDs are most effective.
Methods
Criteria for considering studies for this review
Types of studies
-
RCTs
-
Double‐blinded, single‐blinded or unblinded trials
-
Placebo‐controlled, active drug‐control group or no‐treatment control group
Types of participants
People of any age and either gender undergoing a supratentorial or infratentorial craniotomy for either therapeutic or diagnostic reasons for all pathologies, who have had no history of seizures or prior exposure to AEDs. We excluded people with traumatic brain injuries from this review.
Types of interventions
-
The active treatment groups received treatment with any AED administered prior to or immediately postcraniotomy
-
The control groups received matched placebo, different AEDs or no treatment
Types of outcome measures
Primary outcomes
1. Early seizures
The proportion of people experiencing seizures occurring within the first week following craniotomy.
2. Late seizures
The proportion of people experiencing seizures occurring after the first week following craniotomy, including follow‐up period of one, two and five years postoperatively.
Secondary outcomes
1. Death
The proportion of deaths that occurred within the treatment period or during follow‐up.
2. Functional outcome
The proportion of people who experienced disability (partially or fully dependent on others in normal activities of daily living).
3. Adverse effects
The proportion of people who experienced any of the following adverse events.
-
Skin irritation
-
Dizziness
-
Fatigue
-
Nausea
-
Headache
In addition, we decided to look at the proportion of people who experienced the five most common adverse effects mentioned in the included trials if these differed from the list above.
Search methods for identification of studies
Electronic searches
We ran searches for the original review in January 2012 and subsequent searches in September 2012, August 2014, August 2016, and June 2017. For the latest update we searched:
-
The Cochrane Epilepsy Group Specialized Register (26 June 2017) using the search strategy outlined in Appendix 1.
-
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017 issue 6) via the Cochrane Register of Studies Online (CRSO), using the search strategy outlined in Appendix 2.
-
MEDLINE (Ovid, 1946 to 26 June 2017) using the search strategy outlined in Appendix 3.
-
ClinicalTrials.gov (26 June 2017) using the search strategy outlined in Appendix 4.
-
WHO International Clinical Trials Registry Platform (ICTRP, 26 June 2017) using the search strategy outlined in Appendix 5.
We did not impose any language restrictions.
Searching other resources
We reviewed the reference lists of retrieved trials to check for additional reports of relevant studies.
Data collection and analysis
Selection of studies
Three review authors (JW, JG and YD) independently assessed articles for inclusion. We resolved any disagreements through discussion, and failing this, we sought the opinion of a fourth review author (AM). The same review authors independently carried out data extraction and assessed risk of bias. Again, we resolved any disagreements through discussion. Failing this, we sought the opinion of the fourth review author (AM).
Data extraction and management
We extracted the following information for each trial using a data extraction sheet.
Methodology/trial design
-
Method of randomisation and concealment
-
Method of blinding
-
Number of people excluded from analyses
-
Duration of baseline, treatment and follow‐up periods
-
Type of AED and dose tested
-
Time of treatment commencement
Participant demographics
-
Total number of people randomised to each group
-
Age/gender
-
Pathological group
-
Type of surgery
-
Site of lesion
-
Number of people with previous acute symptomatic seizures
Results
-
Sample size
-
Summary data for each intervention
For all trials we attempted to confirm the above information with trial authors or researchers, and sponsors.
Assessment of risk of bias in included studies
Three review authors (JW, JG and YD) independently assessed the risk of bias for each trial using the Cochrane 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We rated the included trials as low, high or unclear on six domains applicable to RCTs: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting and other sources of bias (Assessment of risk of bias in included studies).
Measures of treatment effect
We have presented treatment effects as they were reported in the original reports. In this latest update, where data for each trial are entered into Data and analyses tables to allow for visual comparisons of results across trials, we have presented results for all dichotomous outcomes as risk ratios (RR) and 95% confidence intervals (CI).
Unit of analysis issues
The unit of allocation and analysis had to be the individual for all included trials, therefore cluster‐RCTs were not an eligible design. Due to the acute nature of postcraniotomy seizures, cross‐over designs were also not a suitable design.
For included trials with more than two treatment arms (e.g. AED1 versus AED2 versus placebo), we considered pairs of interventions in separate head‐to‐head comparisons (see Effects of interventions).
Dealing with missing data
We recorded attrition rates reported in each trial and if appropriate, attempted to contact original trial authors where the extent of missing data was unclear. In order to allow an intention‐to‐treat analysis within this review, we collected data by allocated treatment groups, irrespective of compliance, later exclusion (regardless of cause) or loss to follow‐up.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the differences in trial characteristics in order to inform decisions regarding the combination of trial data (Higgins 2002). Due to high levels of clinical heterogeneity, we did not synthesis any outcome data, If we had performed meta‐analysis, we would have estimated heterogeneity statistically using a Chi2 test for heterogeneity (with a conservative judgement of P value < 0.1 suggesting heterogeneity) and the I2 statistic, interpreted as follows (Deeks 2011):
-
might not be important (I2 values 0% to 40%);
-
may represent moderate heterogeneity (I2 values 30% to 60%);
-
may represent substantial heterogeneity (I2 values 50% to 90%); and
-
considerable heterogeneity (I2 values 75% to 100%).
Assessment of reporting biases
We examined reporting biases, such as publication bias, by identifying specific aspects of each trial (e.g. sponsors of the research, research teams involved).
Data synthesis
It was not possible to synthesise outcome data as we considered meta‐analysis to be inappropriate given the differences across trials in AED treatment, trial intervention characteristics and control groups (see Table 1).
| Study | Intervention and daily dose (N) | Comparator(s) and daily dose (N) | Time of administration | Treatment duration | Measurement period reported ‐ early | Measurement period reported ‐ late | Analysis |
| PHT 300 mg (N = 50) | VAL 1500 mg/day | Post‐op | 12 months | 1 week | 2 weeks to 12 months | ITT | |
| PHT 300 mg (N = 55) 24‐months (N = 56) | CBZ 600 mg (N = 50) (N = 56) No treatment (N = 59) | Pre‐op (pre‐ and post‐op doses differed) | 6 months | Not reported | 4 years (median) | ITT | |
| PHT 5 mg/kg (N = 16) | PB 2 mg/kg | Pre‐op (pre‐ and post‐op doses differed) | Unclear | 1 week | Unclear | No ITT 24 participants lost to follow‐up (for late seizure) | |
| LEV 250‐500 mg daily (N = 39) | PHT 300 mg daily (N = 42) | Pre‐op and post‐op | 90 days | 3 days | 90 days | Not ITT Only participants receiving 1 dose were analysed | |
| LEV 500 mg daily (no prior seizure subgroup = 52) | PHT 15‐18 mg/kg IV daily and 250 mg single oral dose (no prior seizure subgroup = 58) | After anaesthesia induction and post‐op | 7 days | 7 days | Not measured | Not ITT. 1 participant was excluded from the analysis postrandomisation. Lesion was found to be not neoplastic. | |
| PHT 5‐6 mg/kg | Placebo | Pre‐op (pre‐ and post‐op doses differed) | 3 days | 3 days | Not measured | ITT unclear Randomised = 400 but 26 deaths prior to treatment | |
| ZNS 200 mg | PB 80 mg | Pre‐op (doses changed across course of trial) | 12 months | Not reported | 1‐12 months | ITT for 255 participants who received treatment 23 randomised participants were excluded prior to treatment | |
| PHT 300 mg | Placebo | Post‐op | 12 months | 1 week | 12 months | ITT | |
| PHT 300 mg (N = 62) | No treatment (N = 61) | Pre‐op and post‐op | 7 days | 7 days | > 30 days | ITT | |
| PHT 10 mg/kg 3 x daily (oral) or 5 mg/kg IV (N = 72) | VAL 30 mg/kg 3 x daily (oral) or 20 mg/kg (IV) (N = 80) | Pre‐op and post‐op | 1 month | 7 days | > 3 months | ITT unclear Numbers included in final analyses not reported |
CBZ: carbamazepine
ITT: intention‐to‐treat
LEV: levetiracetam
PB: phenobarbital
PHT: phenytoin
VAL: valproate
ZNS: zonisamide
We have presented study‐specific results for the following comparisons.
-
Treatment group versus control group on early seizures
-
Treatment group versus control group on late seizures
-
Treatment group versus control group on number of deaths
-
Treatment group versus control group on functional outcome
-
Treatment group versus control group on adverse effects (for each adverse effect see Types of outcome measures)
We stratified each comparison by type of drug and control group (i.e. placebo, other AED or no treatment) and presented the study‐specific results for comparison without synthesising in Data and analyses.
Subgroup analysis and investigation of heterogeneity
We did not plan any subgroup analyses a priori. The main sources of heterogeneity anticipated were the different trial interventions and control groups (considered within separate comparisons in this review) and different time points of measures (considered within different outcomes of this review).
Sensitivity analysis
We considered a sensitivity analysis of the primary outcomes of the review (where possible) based on the methodological quality of the studies, restricting meta‐analysis to only studies with a globally low risk of bias. However, given the minimal amount of data available for each comparison and the fact that we considered only two studies to have a low risk of bias due to lack of blinding, we did not deem this sensitivity analysis appropriate.
Summary of findings and quality of the evidence (GRADE)
Due to the variability of interventions and control groups within the included studies in this review, we have presented a single 'Summary of findings' table for all comparisons considered within this review (Schünemann 2011; summary of findings Table for the main comparison).
We have included all primary and secondary outcomes in the 'Summary of findings' table. We determined the quality of the evidence using the GRADE approach (GRADE Working Group 2004), and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results and high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
Our searches identified 157 records from the databases outlined in the Electronic searches section. We identified 10 additional records through the reference lists of the included trials. Eighty‐seven records remained after we removed duplicates, and we screened all for inclusion in the review. We excluded 60 records at this point, leaving 27 full‐text articles to be assessed for eligibility. Following this, we excluded 15 full‐text articles (see Figure 1 and Characteristics of excluded studies for reasons for exclusion). We included 10 trials from 12 reports in a narrative synthesis.

Study flow diagram
Included studies
We identified 10 parallel RCTs (Beenen 1999; Foy 1992; Franceschetti 1990; Fuller 2013; Iuchi 2015; Lee 1989; Nakamura 1999; North 1983; Wu 2013; Zhang 2000), examining the effectiveness of AEDs on postcraniotomy seizures in 1815 people. We have presented treatment protocols of the 10 included trials in Table 1.
The treatment periods varied across trials from three days to 24 months; in one trial the treatment period was unclear (Franceschetti 1990). People were excluded from six of the trials if they were taking AEDs already or if they had a history of epilepsy (Beenen 1999; Foy 1992; Lee 1989; Nakamura 1999; North 1983; Zhang 2000). One trial (Franceschetti 1990), included both people who had pre‐operative seizures (Group A) and those who did not (Group B). They analysed Group A and Group B separately compared to controls. We only extracted data pertaining to Group B to be included within this Cochrane Review, as Group A did not meet our inclusion criteria. One trial (Iuchi 2015), included people who had pre‐operative seizures, but the trial authors provided the results of a subgroup analysis for participants with no pre‐operative seizures.
Beenen 1999 was a single‐centre trial with a treatment period of 12 months. People aged between 18 and 80 years who were undergoing supratentorial craniotomy were eligible for inclusion in the trial. 100 patients were randomised: 50 to phenytoin 100 mg and 50 to valproate 500 mg treatment. They administered both treatments intravenously immediately postoperatively in a recovery room. Outcomes reported included early and late seizures, death and adverse effects. They did not report any data for functional outcome.
Foy 1992 was a single‐centre, head‐to‐head, three‐armed trial with a treatment period of either six or 24 months, and follow‐up of three years to a maximum of eight years. People aged over 16 years undergoing supratentorial craniotomy were eligible for inclusion in the trial. The trial authors randomised 276 patients: 55 to phenytoin for a six‐month treatment period, 56 to phenytoin for a 24‐month treatment period, 50 to carbamazepine for a six‐month treatment period, 56 to carbamazepine for a 24‐month treatment period and 59 to no treatment. Phenytoin (15 mg/kg) was administered 24 hours pre‐operation and increased to 100 mg eight‐hourly thereafter. Administration of carbamazepine (200 mg) was every six hours for the 24 hours immediately pre‐operatively and every eight hours thereafter. Outcomes reported included number of participants with seizures and death. The trial did not differentiate between early and late seizures, and no data were reported for functional outcome or adverse effects. All data were reported at six months into the treatment.
Franceschetti 1990 was a single‐centre, head‐to‐head, three‐armed trial that included a no‐treatment group. The duration of treatment is unclear. The trial randomised people undergoing surgery for supratentorial neoplasms; those with a history of seizures formed Group A and those with no history of seizures formed Group B. Group A participants were not eligible for inclusion in this review but there were 63 people randomised to Group B: 25 to phenobarbital, 16 to phenytoin and 22 to no treatment. The phenobarbital (4 mg/kg) was intravenously administered daily for five days and then decreased to 2 mg/kg daily via oral administration. Phenytoin (10 mg/kg) was intravenously administered daily for five days and then decreased to 5 mg/kg daily via oral administration. Outcomes reported included early and late seizures. Minimal data on adverse effects were presented.
Fuller 2013 was a single‐centre, head‐to‐head, two‐arm trial with a treatment period of 90 days. People aged 18 years and over undergoing craniotomy were eligible for inclusion in the trial. The trial randomised 81 people, 39 to levetiracetam and 42 to phenytoin. They administered levetiracetam (250 mg to 500 mg) twice daily, either intravenously or orally (one pre‐operative dose was required) and phenytoin (1000 mg loading dose followed by 300 mg) daily. Outcomes measured included discontinuation of treatment due to side effects, and clinically undesirable event and seizure occurrence. Outcomes were reported at three days and at 90 days.
Iuchi 2015 was a single‐centre, head‐to‐head, two‐arm trial with a treatment period and follow‐up of seven days. People aged 16 years and over with supratentorial tumours undergoing craniotomy were eligible for inclusion in the trial. The trial randomised a total of 147 people, including 110 people with no history of seizures. Of these, 52 people received levetiracetam and 58 people received phenytoin; levetiracetam (500 mg) was administered twice daily after anaesthesia induction either by suppository or orally, and phenytoin (15 to 18 mg/kg intravenously after induction of anaesthesia and continued at 5 mg/kg to 7.5 mg/kg daily intravenously or 250 mg orally). Outcomes measured included seizure occurrence and adverse events. Outcome data were reported at seven days. No data on functional outcomes were collected.
Lee 1989 was a placebo‐controlled trial with a treatment period of three days. The number of participating treatment centres is unclear. Adults receiving intracranial, supratentorial surgery were eligible to take part in the trial. The trial authors selected and randomised 400 patients for participation, however, 26 early deaths occurred, leaving 189 people randomised to phenytoin and 185 people to placebo. Phenytoin (15 mg/kg) was administered 15 to 20 minutes prior to wound closure followed by intravenous phenytoin (5 mg/kg to 6 mg/kg) three times daily for the first three postoperative days. Outcomes measured included number of seizures occurring within the three days of the trial. Data for late seizures, death, functional outcome and adverse effects were not recorded.
Nakamura 1999 was a multi‐centre, head‐to‐head trial with a treatment phase of one year and a follow‐up after two years postmedication. Adults undergoing craniotomy for cerebral tumours, cerebrovascular disease and head trauma were selected for eligibility. The trial randomised 278 people: 129 to zonisamide (100 mg twice daily) and 126 to phenobarbital (40 mg twice daily). However, 23 participants (12 randomised to zonisamide and 11 randomised to phenobarbital) were excluded from the final analysis due to protocol violations. Both drugs were administered orally, at least one week before surgery and then increased (zonisamide to 100 mg three or four times daily and phenobarbital to 40 mg three or four times daily) for one year followed by a tapering period of six months (three months at 100 mg (zonisamide) or 40 mg (phenobarbital) twice daily then three months at 100 mg (zonisamide) or 40 mg (phenobarbital) once daily). Outcomes reported were seizure frequency, death (during follow‐up period only) and adverse effects. No data were collected on functional outcome.
North 1983 was a single‐centre, placebo‐controlled trial with a treatment period of 12 months. People undergoing supratentorial operation (either burr hole, craniectomy or osteoplastic flap procedures) were eligible for inclusion in the trial. The trial authors randomised 281 people: 140 to phenytoin and 141 to placebo. Phenytoin (250 mg twice daily) was administered in a recovery room intravenously, and then continued with oral medication (100 mg three times daily) for one year. Outcomes reported were early and late seizures, death and adverse effects. No data were collected on functional outcomes.
Wu 2013 was a single‐centre, no‐treatment controlled trial with a treatment period of seven days. People with supratentorial tumours were eligible for inclusion in the trial. The trial authors randomised 123 people, 62 to phenytoin and 61 to a no‐treatment control group . Following a pre‐operative loading dose of 15 mg/kg, phenytoin (100 mg) was administered every eight hours to the treatment group. Outcomes reported were seizure occurrence and adverse reactions. No data relevant to functional outcomes were reported.
Zhang 2000 was a single‐centre, head‐to‐head trial with a treatment period of one month. The trial randomised 152 people undergoing craniotomy for differing pathologies, 72 to phenytoin and 80 to valproate. Treatment with phenytoin (10 mg/kg) and valproate (30 mg/kg) was given orally three times daily for seven days before surgery. Outcome measures included seizure occurrence and adverse effects of treatment. No data relevant to functional outcomes were reported.
Excluded studies
Overall we excluded 15 full‐text articles for the following reasons: seven were not RCTs (Baker 1995; Boarini 1985; De Santis 1996; Grobelny 2009; Hayashi 1999; Murri 1992; Notani 1984), two were review articles (Manaka 2003; Shaw 1991), and six studies had participants that did not meet our inclusion criteria (De Santis 2002; Levati 1996; Lim 2009; Temkin 1990; Temkin 1999; Tsolaki 1987).
Risk of bias in included studies
See Characteristics of included studies tables and Figure 2 for 'Risk of bias' judgements.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials
Allocation
For sequence generation, we rated two trials at low risk of bias (Beenen 1999; Foy 1992), and eight trials at unclear risk of bias (Franceschetti 1990; Fuller 2013; Iuchi 2015Lee 1989; Nakamura 1999; North 1983; Wu 2013; Zhang 2000). We did not rate any trials at high risk of bias.
For allocation concealment, we rated one study at low risk of bias (Beenen 1999), and nine trials (Foy 1992; Franceschetti 1990; Fuller 2013; Iuchi 2015Lee 1989; Nakamura 1999; North 1983; Wu 2013; Zhang 2000), at unclear risk of bias due to the lack of detail of these methods.
Blinding
We rated four trials at low risk of bias due to the methods of blinding employed (Beenen 1999; Lee 1989; Nakamura 1999; North 1983). We rated three trials at unclear risk of bias (Franceschetti 1990; Fuller 2013; Zhang 2000), and three trials at high risk of bias, as only the outcome assessor appeared to be blinded in two trials (Foy 1992; Wu 2013), and the other trial was unblinded (Iuchi 2015).
Incomplete outcome data
We rated four trials at low risk of bias due to having no missing data (Beenen 1999; Iuchi 2015; North 1983; Wu 2013). We rated six trials at unclear risk of bias due to lack of detail regarding the analysis (Foy 1992; Franceschetti 1990; Fuller 2013; Lee 1989; Nakamura 1999; Zhang 2000). We did not rate any trials at high risk of bias.
Selective reporting
We rated all of the included trials at unclear risk of bias due to the lack of protocols available for comparison. We requested protocols from the trial authors if contact details were available, however we did not receive any responses.
Other potential sources of bias
We rated seven trials at low risk of bias as we did not identify any other bias (Beenen 1999; Franceschetti 1990; Fuller 2013; Iuchi 2015; Lee 1989; Nakamura 1999; North 1983). We rated three trials at unclear risk of bias (Foy 1992; Wu 2013; Zhang 2000).
Effects of interventions
Due to the variety of head‐to‐head drug comparisons within the included trials (see Table 1 for a comparison of treatment protocols), we have presented the effects of the interventions by outcome measure as opposed to comparisons under trial.
Seizures
See Table 2 for individual trial results, and see Analysis 1.1 for comparative results for all seizures, Analysis 1.2 for early seizures and Analysis 1.3 for late seizures.
| Trial | All seizuresa | Early seizuresa | Late seizuresa | ||||||
| AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | |
| Beenen 1999b (PHT vs VAL) | PHT: 7/50 (14%) | VAL: 7/50 (14%) | ‐ | PHT: 4/50 (8%) | VAL: 2/50 (4%) | ‐ | PHT: 3/50 (6%) | VAL: 5/50 (10%) | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 6 months) | CBZ: 21/50 (42%) | PHT: 21/55 (38%) | NT: 25/59 (42%) | NR | NR | ‐ | NR | NR | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 24 months) | CBZ: 20/56 (36%) | PHT: 16/56 (29%) | NT: 25/59 (42%) | NR | NR | ‐ | NR | NR | ‐ |
| Franceschetti 1990c (PB vs PHT vs NT) | Total in the PB and PHT groups: 6/41 (15%) | NT: 7/22 (32%) | Total in the PB and PHT groups: 3/41 (17%) | NT: 4/22 (18%) | PB 2/15 (13%) | PHT 1/10 (10%) | NT: 3/14 (21%) | ||
| Fuller 2013 (LEV vs PHT) | LEV: 0/39 (0%) | PHT: 6/42 (14%) | ‐ | LEV: 0/39 (0%) | PHT: 6/42 (14%) | ‐ | NR | NR | ‐ |
| Iuchi 2015 (LEV vs PHT) | LEV: 1/53 (2%) | PHT: 8/58 (14%) | ‐ | LEV: 1/53 (2%) | PHT: 8/58 (14%) | ‐ | NR | NR | ‐ |
| Lee 1989b (PHT vs placebo) | PHT: 2/189 (1%) | ‐ | Placebo: 9/185 (5%) | PHT: 2/189 (1%) | Placebo: 9/185 (5%) | NR | NR | ‐ | |
| Nakamura 1999c (ZNS vs PB) | ZNS: 13/129 (10%) | PB: 11/126 (9%) | ‐ | ZNS: 6/129 (5%) | PB: 3/126 (2%) | ‐ | ZNS: 7/129 (5%) | PB: 8/126 (6%) | ‐ |
| North 1983b (PHT vs placebo) | PHT: 18/140 (13%) | ‐ | Placebo: 26/141 (18%) | PHT: 4/140 (3%) | ‐ | Placebo: 14/141 (10%) | PHT: 14/140 (10%) | Placebo: 12/141 (9%) | |
| Wu 2013 (PHT vs NT) | PHT: 15/62 (24%) | ‐ | NT: 11/61 (18%) | PHT: 2/62 (3%) | ‐ | NT: 5/61 (8%) | PHT: 13/62 (21%) | ‐ | NT: 6/61 (10%) |
| Zhang 2000 (PHT vs VAL) | PHT: 6/72 (8%) | VAL: 9/80 (11%) | ‐ | PHT: 6/72 (8%) | VAL: 9/80 (11%) | ‐ | NR | NR | |
AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; NR: not reported; NT: no treatment; PB: phenobarbital; PHT: phenytoin; VAL: valproate; ZNS: zonisamide
aSee Analysis 1.1 for comparative results for all seizures, Analysis 1.2 for early seizures and Analysis 1.3 for late seizures.
bResults from these trials reported the number of participants who had seizures out of the number of participants randomised. However loss to follow‐up during the trial was unclear.
cResults from the trials only reported the number of participants who had seizures out of the number of participants who were followed up. Foy 1992 followed up 39 participants for late seizures. Franceschetti 1990 reported combination of PB and PHT results, it is not possible to differentiate between groups on seizure outcome for all seizures and early seizures.
Any seizures
All 10 trials, with a total of 1815 participants, reported results for the proportion of people experiencing any seizures. Foy 1992 reported only occurrence of seizures (without the time frame); they found no statistically significant differences between any of the treatment groups. All other trials reported whether the seizures were early (i.e. within one week) or late (i.e. after one week).
Early seizures
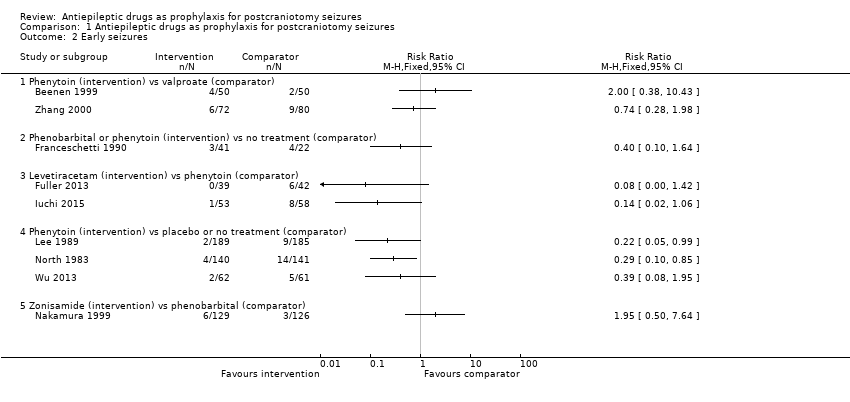
Nine trials (N = 1539) reported the number of people experiencing early seizures.
Phenytoin versus placebo or no treatment
Lee 1989 reported two seizures in 189 participants (1%) in the phenytoin group compared to nine seizures in 185 participants (5%) in the placebo group. North 1983 reported four early seizures in 140 participants (3%) in the phenytoin group compared to 14 seizures in 141 participants (10%) in the placebo group. Wu 2013 reported two early seizures in 62 participants (3%) in the phenytoin group compared to five in 61 participants (8%) in the no‐treatment group. Lee 1989 and North 1983 reported a statistically significant difference in favour of treatment with phenytoin. Within the Lee 1989 published paper, the trial authors reported no statistically significant difference between phenytoin and placebo, however, they applied a Yates correction to their analysis methods, which may have led to the difference in the results between the published paper and this review.
Phenobarbital or phenytoin versus no treatment
Franceschetti 1990 reported three early seizures occurring in 41 participants (17%) in the phenobarbital and phenytoin groups and four in 22 participants (18%) in the no‐treatment group. The difference in early seizures between the two groups was not statistically significant.
Phenytoin versus valproate
Beenen 1999 reported four early seizures in 50 participants (8%) in the phenytoin group compared to two in 50 participants (14%) in the valproate group. Zhang 2000 reported six early seizures in 72 participants (8%) in the phenytoin group compared to nine in 80 participants (11%) in the valproate group. Neither trial found a statistically significant difference in early seizures between the two treatment groups.
Levetiracetam versus phenytoin
Fuller 2013 reported no early seizures in 39 participants in the levetiracetam group and six in 42 participants (14%) in the phenytoin group. Iuchi 2015 reported one early seizure in 53 participants (2%) in the levetiracetam group and eight in 58 participants (14%) in the phenytoin group. Within this Cochrane Review, neither result was statistically significant. However, within both of the published reports of the trials, the trial authors noted a statistically significant advantage of treatment with levetiracetam. We believe that the differences between the published reports and this review are due to different measures being used; this review uses risk ratios, whilst Fuller 2013 used log‐rank methods and Iuchi 2015 reported odds ratios.
Zonisamide versus phenobarbital
Nakamura 1999 reported six early seizures in 129 participants (5%) in the zonisamide group compared to three in 126 participants (2%) in the phenobarbital group. The difference in early seizures between the two groups was not statistically significant.
Summary
Overall, the quality of the evidence for this outcome was low due to unclear risk of bias and variability of treatment protocols in the included trials. Two trials reported a significant difference between AED treatment and no treatment or placebo for early seizure occurrence (Lee 1989; North 1983). No significant differences between the treatments were reported in the other trials (Analysis 1.2).
Late seizures
Five trials, with a total of 798 participants, reported the number of people experiencing late seizures.
Phenytoin versus placebo or no treatment
North 1983 reported 14 late seizures in 140 participants (10%) in the phenytoin group compared to 12 late seizures in 141 participants (9%) in the control group at 12 months. Wu 2013 reported 13 late seizures in 62 participants (21%) in the phenytoin group compared to six late seizures in 61 participants (10%) in the control group beyond 30 days. The difference in late seizures between the two groups was not statistically significant.
Phenobarbital or phenytoin versus no treatment
The Franceschetti 1990 trial only followed up 39 participants, and reported two late seizures in 25 participants (13%) in the phenobarbital group, one late seizure in 10 participants (10%) in the phenytoin group and three late seizures in 14 participants (21%) in the no‐treatment group. The timing of the follow‐up is unclear. The difference in late seizures between the treatment and no‐treatment groups was not statistically significant.
Phenytoin versus valproate
Beenen 1999 reported three late seizures in 50 participants (6%) in the phenytoin group compared with five late seizures in 50 participants (10%) in the valproate group at up to 12 months.
Zonisamide versus phenobarbital
Nakamura 1999 reported seven late seizures in 129 participants (5%) in the zonisamide group and eight late seizures in 126 participants (6%) in the phenobarbital group at 12 months. The difference in late seizures between the two groups was not statistically significant.
Summary
Overall, the quality of the evidence for this outcome was low due to risk of bias and variability of treatment protocols in the included trials. None of the trials that reported data for late seizures found any statistically significant differences between treatment and controls (Analysis 1.3).
Deaths
Five trials, with a total of 1016 participants, reported the number of deaths that occurred during the trials. See Table 3 for individual trial results and see Analysis 1.4 for comparative results for deaths.
| Trial | Deathsa | Adverse eventsa | ||||
| AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | |
| Beenen 1999b (PHT vs VAL) | PHT: 13/50 (26%) | VAL: 10/50 (20%) | ‐ | PHT: 9/50 (18%) | VAL: 4/50 (8%) | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 6 months) | CBZ: 9/50 (18%) | PHT: 15/55 (27%) | NT: 13/59 (22%) | NR | NR | NR |
| Foy 1992b (CBZ vs PHT vs NT for 24 months) | CBZ: 10/56 (18%) | PHT: 27/56 (48%) | NT: 13/59 (22%) | NR | NR | NR |
| Franceschetti 1990c (PB vs PHT vs NT) | NR | NR | NR | PB: 1/15 (7%) | PHT: 3/10 (30%) | NR |
| Fuller 2013 (LEV vs PHT) | LEV: 3/39 (8%) | PHT: 5/42 (12%) | ‐ | LEV: 22/39 (56%) | PHT: 18/42 (43%) | ‐ |
| Iuchi 2015 (LEV vs PHT) | NR | NR | ‐ | LEV: 3/73 (4%) | PHT: 8/73 (11%) | ‐ |
| Lee 1989b (PHT vs placebo) | NR | NR | ‐ | NR | NR | ‐ |
| Nakamura 1999c (ZNS vs PB) | ZNS: 8/112 (7%) | PB: 13/107 (12%) | ‐ | ZNS: 28/129 (22%) | PB: 30/126 (24%) | ‐ |
| North 1983b (PHT vs placebo) | PHT: 20/140 (14%) | ‐ | Placebo: 24/141 (17%) | PHT: 12/140 (9%) | Placebo: 3/141 (2%) | |
| Wu 2013 (PHT vs NT) | NR | ‐ | NR | PHT: 11/62 (18%) | ‐ | NT: 0/61 (0%) |
| Zhang 2000 (PHT vs VAL) | NR | NR | ‐ | PHT: 11/72 (15%) | VAL: 2/80 (3%) | ‐ |
AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; NR: not reported; NT: no treatment; PB: phenobarbital; PHT: phenytoin; VAL: valproate; ZNS: zonisamide.
aSee Analysis 1.4 for comparative results for deaths and Analysis 1.5 for adverse events.
bResults from these trials reported the number of participants who died or experienced adverse events out of the number of participants randomised. However, loss to follow‐up during the trial was unclear.
cResults from the trials only reported the number of participants who died or experienced adverse events out of the number of participants who were followed up.
Five trials did not present data for the outcome of death (Franceschetti 1990; Iuchi 2015; Lee 1989; Wu 2013; Zhang 2000).
Phenytoin versus placebo or no treatment
North 1983 reported 20 deaths in 140 participants (14%) in the phenytoin group and 24 deaths in 141 participants (17%) in the placebo group.
Carbamazepine versus phenytoin versus no treatment
Foy 1992 reported nine deaths in 50 participants (18%) in the carbamazepine group, 15 deaths in 55 participants (27%) in the phenytoin group and 13 deaths in 59 participants (22%) in the no‐treatment group at six months. At 24 months, Foy 1992 reported 10 deaths in 56 participants (18%) in the carbamazepine group, 27 deaths in 56 participants (48%) in the phenytoin group and 13 deaths in 59 participants (22%) in the no‐treatment group. The number of deaths in the phenytoin group was significantly higher than the other treatment groups.
Phenytoin versus valproate
Beenen 1999 reported 13 deaths in 50 participants (26%) in the phenytoin group and 10 deaths in 50 participants (20%) in the valproate group.
Levetiracetam versus phenytoin
Fuller 2013 reported three deaths in 39 participants (8%) in the levetiracetam group and five deaths in 42 participants (12%) in the phenytoin group.
Zonisamide versus phenobarbital
Nakamura 1999 reported eight deaths in 112 participants (7%) in the zonisamide group and 13 deaths in 107 participants (12%) in the phenobarbital group.
Summary
Overall, the quality of the evidence for this outcome was low due to risk of bias and variability of treatment protocols in the included trials. One trial (Foy 1992), found significantly fewer deaths in the carbamazepine and the no‐treatment group at 24 months compared to phenytoin. This trial showed no significant difference between the interventions at six months (Analysis 1.4).
Functional outcome
No included trials reported any data or results for a functional outcome.
Adverse effects
Eight trials, with a total of 1165 participants, reported the number of people experiencing adverse events during the trials. See Table 3 for individual trial results and see Analysis 1.5 for comparative results for adverse events. No adverse effects data from the two remaining trials were provided (Foy 1992; Lee 1989).
Phenytoin versus valproate
Beenen 1999 reported that four out of 50 participants experienced a skin reaction, three out of 50 participants experienced liver dysfunction, one out of 50 participants experienced thrombopenia, and there was one case of nausea within the phenytoin group (N = 50). In the valproate group there were three cases of liver dysfunction and one case of a rise in liver enzymes (N = 50). Zhang 2000 reported eight cases of rash, one case of poisoning and two cases of liver damage in the phenytoin group, whilst in the valproate group, there was one case of rash and one case of mild liver damage.
Phenytoin versus placebo or no treatment
North 1983 reported eight cases of rash, one case of involuntary movements, one hirsutism, one headache and one case of discomfort of the face in the phenytoin group (N = 140) compared to one case of rash, one dizziness and one nausea in the placebo group (N = 141). Wu 2013 reported 11 participants with adverse effects of treatment in the phenytoin group (N = 62) and no participants in the NT group. The reported events included four cases of rash, four cases of increased liver function test values, two cases each of thrombocytopenia, confusion and aphasia, and one case each of decreased level of consciousness, nausea, vomiting, dry itchy skin, ataxia and photophobia.
Phenobarbital or phenytoin versus no treatment
Franceschetti 1990 reported minimal data on adverse effects, only that three out of 10 participants in the phenytoin group and one out of 10 participants in the phenobarbital group experienced neurological side effects.
Levetiracetam versus phenytoin
Fuller 2013 reported that a total of 22 out of 39 people taking levetiracetam experienced adverse events, eight experienced lethargy/tiredness or asthenia, four people experienced rash, one person had delirium, one had headache, one had pruritus and seven experienced mood/irritability problems. In the phenytoin group a total of 18 out of 42 people experienced adverse events, with five cases of rash/itch, three cases each of thrombophlebitis and mood/irritability problems, two cases each of drug intoxication and anaphylaxis, and one case each of ataxia, nausea, and lethargy/tiredness/asthenia.
Iuchi 2015 reported adverse effects for the overall trial population (N = 146) rather than the subgroup of participants with no prior history of seizures (N = 110). In the levetiracetam group, three participants experienced haematological toxicity, two participants in the phenytoin group experienced haematological toxicity, two people experienced Grade 3 hyponatraemia, two people experienced Grade 3 skin eruption and two people experienced atrial fibrillation.
Zonisamide versus phenobarbital
Nakamura 1999 reported two cases of somnolence and six cases of nausea in the zonisamide group (N = 129), and seven cases of somnolence and two cases of nausea in the phenobarbital group (N = 126). Overall they reported 28 adverse effects in 129 participants in the zonisamide group and 30 adverse effects out of 126 participants in the phenobarbital group.
Summary
Overall, the quality of the evidence for this outcome was low due to risk of bias and variability of treatment protocols in the included trials. Two trials (North 1983; Wu 2013) found that significantly more adverse events were reported on phenytoin compared to placebo or no treatment and one trial (Zhang 2000) found that significantly more adverse events were reported in the phenytoin group compared with participants treated with valproate (Analysis 1.5).
Discussion
Summary of main results
The 10 trials included in this Cochrane Review were all RCTs investigating the effects of a range of AEDs given either immediately before or after a craniotomy procedure to people with no previous history of seizures or exposure to AEDs.
For the outcome of incidence of seizures, overall most trials reported no significant difference between treatment with AEDs and no treatment, or between treatment with different AEDs. Only two trials reported any statistically significant findings. In Lee 1989 and North 1983, the incidence of early seizures was reduced in the AED group (phenytoin) compared to placebo (P = 0.05 and P = 0.02 respectively). Overall, the majority of results from the individual trials showed few significant differences between AED treatment participants and control participants for outcomes relevant to the number of deaths and adverse effects. However, one trial (Foy 1992), showed that significantly more deaths occurred on phenytoin than carbamazepine or no treatment at 24 months (P = 0.001 and P = 0.005 respectively) and three trials did show significant differences for adverse event outcomes (Fuller 2013; Wu 2013; Zhang 2000). None of the included trials examined participants' functional outcomes.
Overall completeness and applicability of evidence
The underlying pathologies for craniotomy surgery were mixed within the trials (e.g. tumour, abscess, meningioma), with a small percentage of participants having surgery as a result of head injuries. One study included a substantial proportion (210/374) of people with head‐injury (Lee 1989). This is a major limitation of this review as the objective is to examine outcomes for people undergoing craniotomy presenting with non‐trauma pathology. We acknowledge the possibility of differences in the risk of seizure postsurgery depending on the underlying pathology of the participant.
We were unable to meta‐analyse any of the data and structuring a narrative summary was difficult for a number of reasons: few trials were available under each comparison examined (see Data synthesis for list of comparisons under investigation) and the interventions varied substantially with regards to duration of treatment period, dose and method of drug administration, country, methodological rigour and underlying pathologies. Trials differed regarding their reporting of outcomes, one trial did not differentiate between early and late seizures, and information about adverse effects of treatment was very limited. Most trials had similar inclusion and exclusion criteria. People undergoing supratentorial craniotomy were randomised in seven of the 10 included trials, but Fuller 2013, Iuchi 2015 and Nakamura 1999 did not specify the type of surgery.
Quality of the evidence
The outcomes of the risk of bias assessments conducted for each trial are noteworthy. We rated most trials as unclear on several of the criteria due to lack of published information regarding methodological trial design. We rated only two of the 10 trials at low risk of bias due to the method used to generate the randomisation sequence (Beenen 1999; Foy 1992), and only one trial used adequate methods for concealing the allocation of intervention (Beenen 1999). Most trials used adequate methods for blinding participants and outcome assessors; however, one trial was unblinded (Foy 1992), and therefore we rated it at high risk of bias for this criteria. There were no protocols available for any of the trials, therefore assessing selective reporting across trials was rated as unclear. We rated several trials as unclear on how they managed missing data within their analyses. In most cases trials reported attrition and described the reasons for withdrawal.
Furthermore, variability of treatment protocols, particularly AED interventions examined and control groups used prevented us from conducting data synthesis and comparison of interventions and controls was difficult. Therefore, we rated the overall quality of the evidence for all outcomes provided by this review to be low.
Potential biases in the review process
We did not identify any biases in the review process. We conducted this review in line with Cochrane MECIR standards (MECIR 2016), and presented results in the most appropriate way possible, given the heterogeneity of the evidence.
Agreements and disagreements with other studies or reviews
A systematic review published in 1996 (Kuijlen 1996), assessed the effectiveness of prophylactic AED use in people undergoing supratentorial craniotomies. The review included three trials (Foy 1992; Lee 1989; North 1983), that were considered to be of satisfactory methodological quality. Kuijlen 1996 calculated odds ratios as a means of assessing the degree of association between treatment and the incidences of convulsions. The results of pooling the data from these three trials demonstrated no statistically significant difference between prophylaxis with AEDs and no treatment. The authors of Kuijlen 1996 noted that there were only a small number of trials available in this area. A systematic review published in 2017 (Islim 2017), assessed the use of prophylactic AEDs for people undergoing surgery for meningioma. It included 11 cohort trials (1143 participants) and the authors reported that there was no statistically significant difference between prophylaxis with AEDs and no treatment. They advised that good‐quality RCTs are needed for robust conclusions to be drawn.

Study flow diagram

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials

Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 1 All seizures.
Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 2 Early seizures.

Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 3 Late seizures.

Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 4 Deaths.

Comparison 1 Antiepileptic drugs as prophylaxis for postcraniotomy seizures, Outcome 5 Adverse events.
| Antiepileptic drugs as prophylaxis for postcraniotomy seizures | ||||||
| Patient or population: people with postcraniotomy seizures Control: another antiepileptic drug, placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antiepileptic drugs | |||||
| Early seizures Follow‐up: up to 1 week | See comment | See comment | Not estimable | 1539 | ⊕⊕⊝⊝ | 7 trials found no significant differences across comparisons examined: phenytoin vs no treatment, phenobarbital or phenytoin vs no treatment, phenytoin vs valproate, levetiracetam vs phenytoin and zonisamide vs phenobarbital. 2 trials found a significantly lower number of seizures following use of phenytoin vs no treatment.c |
| Late seizures Follow‐up: 1 week up to 4 years (median) | See comment | See comment | Not estimable | 798 | ⊕⊕⊝⊝ | All trials found no significant differences across comparisons examined; phenytoin vs placebo or no treatment, phenobarbital or phenytoin vs no treatment, phenytoin vs valproate, zonisamide vs phenobarbital. |
| Death Follow‐up: up to 4 years (median) | See comment | See comment | Not estimable | 1016 | ⊕⊕⊝⊝ | 4 trials found no significant differences over comparisons: phenytoin vs valproate, zonisamide vs phenobarbital; levetiracetam vs phenytoin and phenytoin vs placebo. 1 trial found significantly fewer deaths in the carbamazepine and the no‐treatment group at 24 months compared to phenytoin.d This trial showed no significant difference between the interventions at 6 months. |
| Functional outcome (number of people with disabilities) Follow‐up: NA | See comment | See comment | Not estimable | NA | NA | No included studies reported a functional outcome. |
| Adverse effects Follow‐up: up to 12 months | See comment | See comment | Not estimable | 1165 | ⊕⊕⊝⊝ | Most trials found low numbers of adverse effects, and five trials found that no significant differences across comparisons were reported. Two trials found that significantly more adverse events were reported on phenytoin compared to placebo or no treatmente and one trial found that significantly more adverse events were reported on phenytoin compared valproate.f |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded once due to risk of bias: methodological biases identified in trials (no allocation concealment, one study unblinded, unclear methods of dealing with missing data). | ||||||
| Study | Intervention and daily dose (N) | Comparator(s) and daily dose (N) | Time of administration | Treatment duration | Measurement period reported ‐ early | Measurement period reported ‐ late | Analysis |
| PHT 300 mg (N = 50) | VAL 1500 mg/day | Post‐op | 12 months | 1 week | 2 weeks to 12 months | ITT | |
| PHT 300 mg (N = 55) 24‐months (N = 56) | CBZ 600 mg (N = 50) (N = 56) No treatment (N = 59) | Pre‐op (pre‐ and post‐op doses differed) | 6 months | Not reported | 4 years (median) | ITT | |
| PHT 5 mg/kg (N = 16) | PB 2 mg/kg | Pre‐op (pre‐ and post‐op doses differed) | Unclear | 1 week | Unclear | No ITT 24 participants lost to follow‐up (for late seizure) | |
| LEV 250‐500 mg daily (N = 39) | PHT 300 mg daily (N = 42) | Pre‐op and post‐op | 90 days | 3 days | 90 days | Not ITT Only participants receiving 1 dose were analysed | |
| LEV 500 mg daily (no prior seizure subgroup = 52) | PHT 15‐18 mg/kg IV daily and 250 mg single oral dose (no prior seizure subgroup = 58) | After anaesthesia induction and post‐op | 7 days | 7 days | Not measured | Not ITT. 1 participant was excluded from the analysis postrandomisation. Lesion was found to be not neoplastic. | |
| PHT 5‐6 mg/kg | Placebo | Pre‐op (pre‐ and post‐op doses differed) | 3 days | 3 days | Not measured | ITT unclear Randomised = 400 but 26 deaths prior to treatment | |
| ZNS 200 mg | PB 80 mg | Pre‐op (doses changed across course of trial) | 12 months | Not reported | 1‐12 months | ITT for 255 participants who received treatment 23 randomised participants were excluded prior to treatment | |
| PHT 300 mg | Placebo | Post‐op | 12 months | 1 week | 12 months | ITT | |
| PHT 300 mg (N = 62) | No treatment (N = 61) | Pre‐op and post‐op | 7 days | 7 days | > 30 days | ITT | |
| PHT 10 mg/kg 3 x daily (oral) or 5 mg/kg IV (N = 72) | VAL 30 mg/kg 3 x daily (oral) or 20 mg/kg (IV) (N = 80) | Pre‐op and post‐op | 1 month | 7 days | > 3 months | ITT unclear Numbers included in final analyses not reported | |
| CBZ: carbamazepine | |||||||
| Trial | All seizuresa | Early seizuresa | Late seizuresa | ||||||
| AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | |
| Beenen 1999b (PHT vs VAL) | PHT: 7/50 (14%) | VAL: 7/50 (14%) | ‐ | PHT: 4/50 (8%) | VAL: 2/50 (4%) | ‐ | PHT: 3/50 (6%) | VAL: 5/50 (10%) | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 6 months) | CBZ: 21/50 (42%) | PHT: 21/55 (38%) | NT: 25/59 (42%) | NR | NR | ‐ | NR | NR | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 24 months) | CBZ: 20/56 (36%) | PHT: 16/56 (29%) | NT: 25/59 (42%) | NR | NR | ‐ | NR | NR | ‐ |
| Franceschetti 1990c (PB vs PHT vs NT) | Total in the PB and PHT groups: 6/41 (15%) | NT: 7/22 (32%) | Total in the PB and PHT groups: 3/41 (17%) | NT: 4/22 (18%) | PB 2/15 (13%) | PHT 1/10 (10%) | NT: 3/14 (21%) | ||
| Fuller 2013 (LEV vs PHT) | LEV: 0/39 (0%) | PHT: 6/42 (14%) | ‐ | LEV: 0/39 (0%) | PHT: 6/42 (14%) | ‐ | NR | NR | ‐ |
| Iuchi 2015 (LEV vs PHT) | LEV: 1/53 (2%) | PHT: 8/58 (14%) | ‐ | LEV: 1/53 (2%) | PHT: 8/58 (14%) | ‐ | NR | NR | ‐ |
| Lee 1989b (PHT vs placebo) | PHT: 2/189 (1%) | ‐ | Placebo: 9/185 (5%) | PHT: 2/189 (1%) | Placebo: 9/185 (5%) | NR | NR | ‐ | |
| Nakamura 1999c (ZNS vs PB) | ZNS: 13/129 (10%) | PB: 11/126 (9%) | ‐ | ZNS: 6/129 (5%) | PB: 3/126 (2%) | ‐ | ZNS: 7/129 (5%) | PB: 8/126 (6%) | ‐ |
| North 1983b (PHT vs placebo) | PHT: 18/140 (13%) | ‐ | Placebo: 26/141 (18%) | PHT: 4/140 (3%) | ‐ | Placebo: 14/141 (10%) | PHT: 14/140 (10%) | Placebo: 12/141 (9%) | |
| Wu 2013 (PHT vs NT) | PHT: 15/62 (24%) | ‐ | NT: 11/61 (18%) | PHT: 2/62 (3%) | ‐ | NT: 5/61 (8%) | PHT: 13/62 (21%) | ‐ | NT: 6/61 (10%) |
| Zhang 2000 (PHT vs VAL) | PHT: 6/72 (8%) | VAL: 9/80 (11%) | ‐ | PHT: 6/72 (8%) | VAL: 9/80 (11%) | ‐ | NR | NR | |
| AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; NR: not reported; NT: no treatment; PB: phenobarbital; PHT: phenytoin; VAL: valproate; ZNS: zonisamide aSee Analysis 1.1 for comparative results for all seizures, Analysis 1.2 for early seizures and Analysis 1.3 for late seizures. | |||||||||
| Trial | Deathsa | Adverse eventsa | ||||
| AED 1 | AED 2 | NT or placebo | AED 1 | AED 2 | NT or placebo | |
| Beenen 1999b (PHT vs VAL) | PHT: 13/50 (26%) | VAL: 10/50 (20%) | ‐ | PHT: 9/50 (18%) | VAL: 4/50 (8%) | ‐ |
| Foy 1992b (CBZ vs PHT vs NT for 6 months) | CBZ: 9/50 (18%) | PHT: 15/55 (27%) | NT: 13/59 (22%) | NR | NR | NR |
| Foy 1992b (CBZ vs PHT vs NT for 24 months) | CBZ: 10/56 (18%) | PHT: 27/56 (48%) | NT: 13/59 (22%) | NR | NR | NR |
| Franceschetti 1990c (PB vs PHT vs NT) | NR | NR | NR | PB: 1/15 (7%) | PHT: 3/10 (30%) | NR |
| Fuller 2013 (LEV vs PHT) | LEV: 3/39 (8%) | PHT: 5/42 (12%) | ‐ | LEV: 22/39 (56%) | PHT: 18/42 (43%) | ‐ |
| Iuchi 2015 (LEV vs PHT) | NR | NR | ‐ | LEV: 3/73 (4%) | PHT: 8/73 (11%) | ‐ |
| Lee 1989b (PHT vs placebo) | NR | NR | ‐ | NR | NR | ‐ |
| Nakamura 1999c (ZNS vs PB) | ZNS: 8/112 (7%) | PB: 13/107 (12%) | ‐ | ZNS: 28/129 (22%) | PB: 30/126 (24%) | ‐ |
| North 1983b (PHT vs placebo) | PHT: 20/140 (14%) | ‐ | Placebo: 24/141 (17%) | PHT: 12/140 (9%) | Placebo: 3/141 (2%) | |
| Wu 2013 (PHT vs NT) | NR | ‐ | NR | PHT: 11/62 (18%) | ‐ | NT: 0/61 (0%) |
| Zhang 2000 (PHT vs VAL) | NR | NR | ‐ | PHT: 11/72 (15%) | VAL: 2/80 (3%) | ‐ |
| AED: antiepileptic drug; CBZ: carbamazepine; LEV: levetiracetam; NR: not reported; NT: no treatment; PB: phenobarbital; PHT: phenytoin; VAL: valproate; ZNS: zonisamide. aSee Analysis 1.4 for comparative results for deaths and Analysis 1.5 for adverse events. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All seizures Show forest plot | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Phenytoin (intervention) vs valproate (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Carbamazepine (intervention) vs phenytoin (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Carbamazepine (intervention) vs phenytoin (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Carbamazepine (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Carbamazepine (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Phenytoin (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Phenytoin (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Phenobarbital or phenytoin (intervention) vs no treatment (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Levetiracetam (intervention) vs phenytoin (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Phenytoin (intervention) vs placebo or no treatment (comparator) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Early seizures Show forest plot | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Phenytoin (intervention) vs valproate (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Phenobarbital or phenytoin (intervention) vs no treatment (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Levetiracetam (intervention) vs phenytoin (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Phenytoin (intervention) vs placebo or no treatment (comparator) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Late seizures Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Phenytoin (intervention) vs valproate (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Phenytoin (intervention) vs placebo or no treatment (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Deaths Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Phenytoin (intervention) vs valproate (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Carbamazepine (intervention) vs phenytoin (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Carbamazepine (intervention) vs phenytoin (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Carbamazepine (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Carbamazepine (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Phenytoin (intervention) vs no treatment (comparator) for 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Phenytoin (intervention) vs no treatment (comparator) for 24 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Levetiracetam (intervention) vs phenytoin (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Phenytoin (intervention) vs placebo (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Phenytoin (intervention) vs valproate (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Phenobarbital (intervention) vs phenytoin (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Levetiracetam (intervention) vs phenytoin (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Phenytoin (intervention) vs placebo or no treatment (comparator) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Zonisamide (intervention) vs phenobarbital (comparator) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

