Sistema intrauterino de levonorgestrel para la protección del endometrio en mujeres con cáncer de mama bajo tratamiento adyuvante con tamoxifeno
Referencias
References to studies included in this review
Ir a:
Additional references
Ir a:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial | |
| Participants | Pre‐ and postmenopausal women who required adjuvant tamoxifen for breast cancer after completion of postoperative radiotherapy and chemotherapy. 129 women randomised. Exclusion criteria included contraindication for intrauterine device, such as pelvic inflammatory disease, congenital uterine anomaly or uterine cavity length > 10 cm. | |
| Interventions | Two interventions compared:
| |
| Outcomes |
| |
| Notes | Study funding: The Chinese University of Hong Kong Department of Obstetrics and Gynaecology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number series. |
| Allocation concealment (selection bias) | Low risk | Allocation in serially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | The pathologist was blinded to the randomisation. Even though the provider and participant were not blinded given the clinical intervention (insertion of the LNG‐IUS), the blinding of providers and participants is considered very unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | At 12 months of follow‐up, 16/129 (12%) participants (7 in the control group and 9 in the treatment group) were lost to follow up or dropped out. 113 women (58 in the control and 55 in the treatment group) were analysed. At 60 months of follow up, 35/129 (27%) participants (17 in the control group and 18 in the treatment group) were lost to follow up. 94 women (48 in the control and 46 in the treatment group) were analysed. There is no description of the population who dropped out or comparison of drop‐outs to participants who remained in the study; as such this is judged as unclear risk of bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned. |
| Other bias | Low risk | No additional biases to report. |
| Methods | Randomised controlled trial | |
| Participants | Postmenopausal women who had been on adjuvant tamoxifen for at least 12 months. Postmenopause was defined by serum estradiol < 50 pmol/L. 122 women randomised; 9 were excluded after randomization (6 were premenopausal, 3 with unsatisfactory hysteroscopy). Additional exclusion criteria included suspected pelvic inflammatory disease, active liver disease, history of malignant disease other than breast cancer, grade 3 submucous fibroid, endometrial polyps, and refusal to receive the levonorgestrel intrauterine system. | |
| Interventions | Two interventions compared:
| |
| Outcomes |
| |
| Notes | Study funding: A grant from Trent NHS Research and Development, with support from The University of Leicester and The University Hospitals of Leicester NHS Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided on random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Pre‐prepared serially‐numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | The pathologist was blinded to the randomization. Even though the provider and participant were not blinded given the clinical intervention (insertion of the LNG‐IUS), the blinding of providers and participants is considered very unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) | Unclear risk | At 12 months of follow‐up, 23/122 (19%) of participants (6 in control group and 17 in treatment group) were lost to follow‐up or dropped out. 99 women (52 in control and 47 in treatment) were included in the analyses. There were no evidence of differences in baseline data between women who completed and did not complete the study. These data are at low risk of attrition bias. The 24, 36 and 48 months follow up data are considered at high risk of attrition bias. At 24 months of follow‐up, 62/122 (51%) of participants were not included in the analyses as they were lost to follow‐up or dropped out. At 36 months of follow‐up, 83/122 (68%) of participants were lost to follow‐up or dropped out. At 48 months of follow‐up, only 15 women were included in the analyses, due to 107/122 (88%) of participants lost to follow‐up or dropped out. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned. |
| Other bias | Low risk | No additional bias to report. |
| Methods | Randomised controlled trial | |
| Participants | Postmenopausal women who had been on adjuvant tamoxifen for more than 12 months. 148 women randomised; 6 were excluded after randomisation (2 refused LNG‐IUS, and 4 in whom the LNG‐IUS could not be fitted). Exclusion criteria included contraindication for intrauterine device (such as pelvic inflammatory disease), progestogen treatment since diagnosis of breast cancer, history of malignant disease other than breast cancer, allergy to polyethylene, and refusal to receive the levonorgestrel intrauterine system. | |
| Interventions | Two interventions compared:
| |
| Outcomes |
| |
| Notes | Study funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by computer‐aided numbering of sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Pre‐prepared numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | The pathologist was blinded to the randomization. Even though the provider and participant were not blinded given the clinical intervention (insertion of the LNG‐IUS), the blinding of providers and participants is considered very unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | After randomization, 6/148 (4%) of women were excluded (2 refused LNG‐IUS, and 4 in whom the LNG‐IUS could not be fitted). At 36 months of follow‐up, 0 participants were lost to follow‐up or dropped out. 142 women were included in the analyses. These data are at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned. |
| Other bias | Low risk | No additional bias to report. |
| Methods | Randomised controlled trial | |
| Participants | Pre‐ and postmenopausal women with early stage breast cancer who required adjuvant tamoxifen after completion of postoperative radiation and chemotherapy. 150 women randomised; 18 were excluded after randomization (8 from the control and 10 from the treatment group declined participation). At baseline, 9 women (4 from control and 5 from treatment) were excluded due to an unsuccessful hysteroscopy. Exclusion criteria included age > 60 years, contraindications for intrauterine device (such as pelvic inflammatory disease, uterine cavity > 8 cm), active liver disease, history of progestogen treatment since diagnosis of breast cancer, history of malignant disease other than breast cancer, allergy to polyethylene, and refusal to receive the levonorgestrel intrauterine system. | |
| Interventions | Two interventions compared:
| |
| Outcomes |
| |
| Notes | Study funding: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation by computer‐generated random number series. |
| Allocation concealment (selection bias) | Low risk | Pre‐prepared serially‐numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | The pathologist was blinded to the randomization. Even though the provider and participant were not blinded given the clinical intervention (insertion of the LNG‐IUS), the blinding of providers and participants is considered very unlikely to influence the outcomes. |
| Incomplete outcome data (attrition bias) | Low risk | After randomization, 18/150 (12%) of women were excluded (8 from the control and 10 from the treatment group declined participation). At baseline, 9/150 (6%) of women (4 from control and 5 from treatment) were excluded due to an unsuccessful hysteroscopy. At 12 months of follow‐up, 2/123 (2%) of participants [1 in control group (breast cancer‐related death) and 1 in treatment group (hysterectomy)] were lost to follow up. At 24 months of follow‐up (62 in the control group and 59 in the treatment group), 0 participants were lost to follow up. At both follow‐up time points, 121 women were included in the analyses. There were no evidence of differences in baseline data between women who completed and did not complete the study. These data are at low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned. |
| Other bias | Low risk | No additional bias to report. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometrial Polyps Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 1 Endometrial Polyps. | ||||
| 1.1 Short term follow‐up (12 months) | 2 | 212 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.08, 0.64] |
| 1.2 Long term follow‐up (24 to 60 months) | 4 | 417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.13, 0.39] |
| 2 Endometrial Hyperplasia Show forest plot | 4 | 417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.03, 0.67] |
| Analysis 1.2  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 2 Endometrial Hyperplasia. | ||||
| 3 Endometrial Cancer Show forest plot | 2 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 1.3  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 3 Endometrial Cancer. | ||||
| 4 Fibroids Show forest plot | 3 | 314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.16, 1.46] |
| Analysis 1.4  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 4 Fibroids. | ||||
| 5 Abnormal Vaginal Bleeding or Spotting Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 5 Abnormal Vaginal Bleeding or Spotting. | ||||
| 5.1 12 months | 3 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [3.37, 15.66] |
| 5.2 24 months | 2 | 233 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [1.04, 7.10] |
| 5.3 45 months | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 60 months | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Breast Cancer Recurrence Show forest plot | 2 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.64, 4.74] |
| Analysis 1.6  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 6 Breast Cancer Recurrence. | ||||
| 7 Breast Cancer‐related Death Show forest plot | 3 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.36, 2.84] |
| Analysis 1.7  Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 7 Breast Cancer‐related Death. | ||||
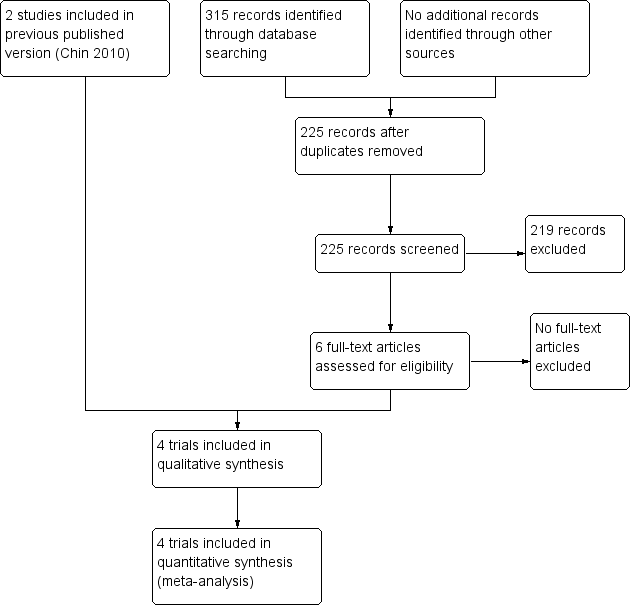
Study flow diagram
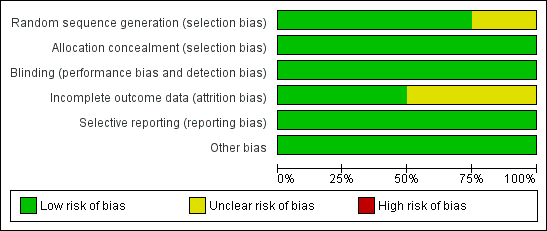
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors’ judgements about each risk of bias item for each included study

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.1 Endometrial Polyps.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.2 Endometrial Hyperplasia.
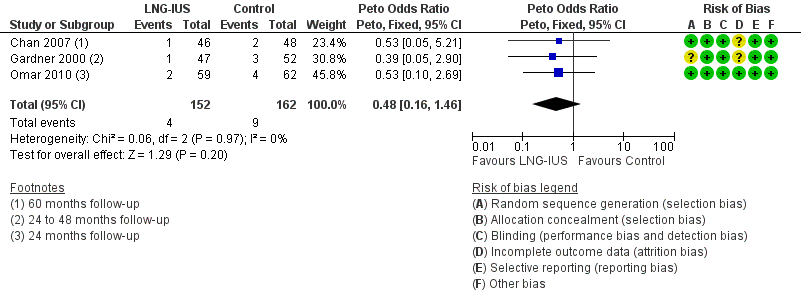
Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.4 Fibroids.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.5 Abnormal Vaginal Bleeding or Spotting.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.6 Breast Cancer Recurrence.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.7 Breast Cancer‐related Death.
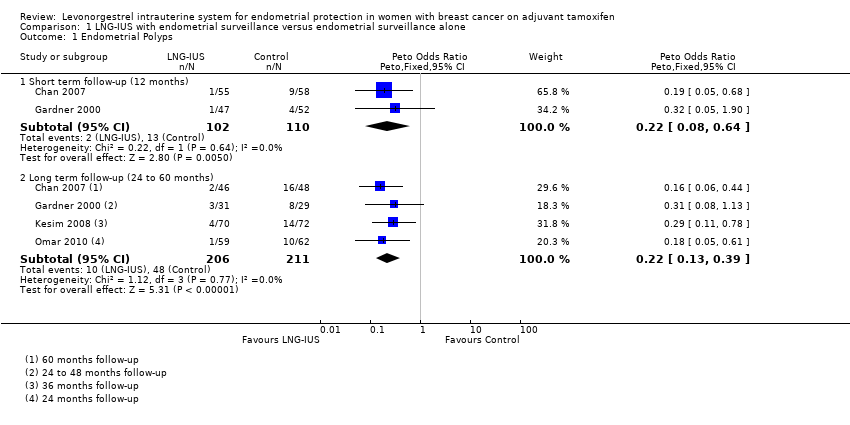
Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 1 Endometrial Polyps.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 2 Endometrial Hyperplasia.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 3 Endometrial Cancer.
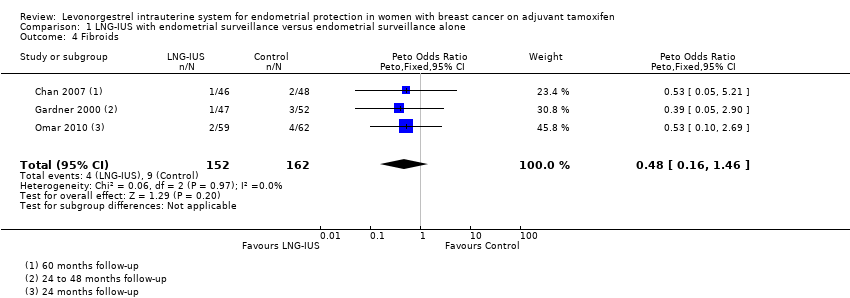
Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 4 Fibroids.
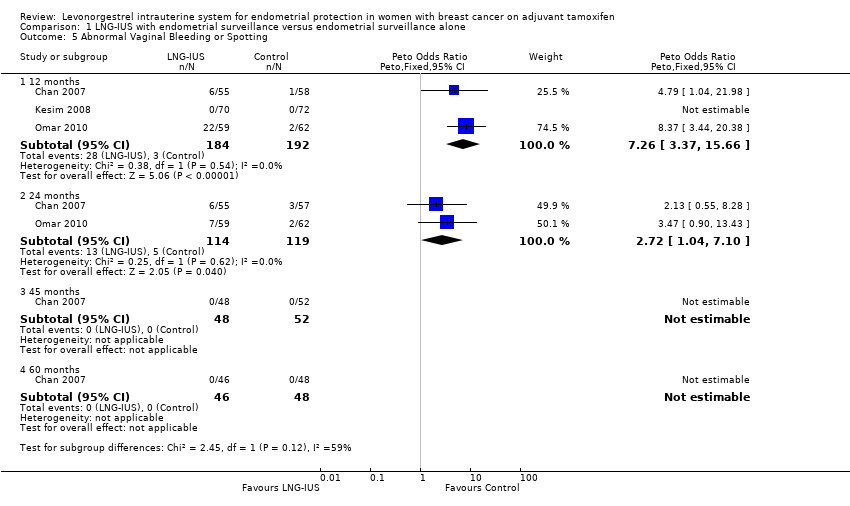
Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 5 Abnormal Vaginal Bleeding or Spotting.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 6 Breast Cancer Recurrence.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 7 Breast Cancer‐related Death.
| The LNG‐IUS with endometrial surveillance compared to endometrial surveillance alone for endometrial protection in women with breast cancer on adjuvant tamoxifen | ||||||
| Patient or population: endometrial protection in women with breast cancer on adjuvant tamoxifen | ||||||
| Outcomes | Illustrated comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed Risk | Corresponding Riks | |||||
| Endometrial surveillance alone | LNG‐IUS with endometrial surveillance | |||||
| Endometrial Polyps | Moderate | OR 0.22 | 417 | ⊕⊕⊕⊝ | ||
| 235 per 1000 | 63 per 1000 | |||||
| Endometrial Hyperplasia | Moderate | OR 0.13 | 417 | ⊕⊕⊕⊝ | ||
| 28 per 1000 | 4 per 1000 | |||||
| Endometrial Cancer | Moderate | not estimable | 154 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Fibroids | Moderate | OR 0.48 | 314 | ⊕⊕⊕⊝ | ||
| 58 per 1000 | 29 per 1000 | |||||
| Abnormal Vaginal Bleeding or Spotting | Moderate | OR 7.26 | 376 | ⊕⊕⊕⊝ | ||
| 17 per 1000 | 113 per 1000 | |||||
| Abnormal Vaginal Bleeding or Spotting | Moderate | OR 2.72 | 233 | ⊕⊕⊕⊝ | ||
| 42 per 1000 | 107 per 1000 | |||||
| Abnormal Vaginal Bleeding or Spotting | Moderate | not estimable | 94 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Breast Cancer Recurrence | Moderate | OR 1.74 | 154 | ⊕⊕⊕⊝ | ||
| 80 per 1000 | 131 per 1000 | |||||
| Breast Cancer‐related Death | Moderate | OR 1.02 | 277 | ⊕⊕⊕⊝ | ||
| 69 per 1000 | 70 per 1000 | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 limited sample size and low event rate | ||||||
| Treatment Group | Control | P value | |
| 6 months follow‐up | |||
| Randomised | 64 | 65 | |
| Completed | 55 | 58 | |
| Abnormal vaginal bleeding or spotting | 20 | 1 | <0.001 |
| 12 months follow‐up | |||
| Completed | 55 | 58 | |
| Abnormal vaginal bleeding or spotting | 6 | 1 | 0.06 |
| Endometrial polyps | 1 | 9 | 0.02 |
| Endometrial hyperplasia | 0 | 0 | |
| Fibroids | 1 | 2 | 1.0 |
| 24 months follow‐up | |||
| Completed | 55 | 57 | |
| Abnormal vaginal bleeding or spotting | 6 | 3 | 0.45 |
| 45 months follow‐up | |||
| Completed | 48 | 52 | |
| Abnormal vaginal bleeding or spotting | 0 | 0 | |
| 60 months follow‐up | |||
| Completed | 46 | 48 | |
| Abnormal vaginal bleeding or spotting | 0 | 0 | |
| Endometrial polyps | 2 | 16 | < 0.001 |
| Endometrial hyperplasia | 0 | 1 | 1.0 |
| Endometrial cancer | 0 | 0 | |
| Fibroids | 1 | 2 | 1.0 |
| Breast cancer recurrence | 10 | 6 | 0.25 |
| Breast cancer‐related deaths | 6 | 5 | 0.71 |
| Treatment Group | Control | P value | |
| 12 months follow‐up | |||
| Randomised | 64 | 58 | |
| Completed | 47 | 52 | |
| Endometrial polyps | 1 | 4 | 0.4 |
| Endometrial hyperplasia | 0 | 1 | |
| Fibroids | 1 | 3 | 0.2 |
| Final follow‐up (24, 36, or 48 months) | |||
| Completed at 24 months | 31 | 29 | |
| Completed at 36 months | 19 | 20 | |
| Completed at 48 months | 6 | 9 | |
| Endometrial polyps | 3 | 8 | |
| Endometrial hyperplasia | 0 | 1 | |
| Endometrial cancer | 0 | 0 | |
| Breast cancer recurrence | 1 | 1 | |
| Breast cancer‐related deaths | 2 | 2 |
| Treatment Group | Control | P value | |
| 5 months follow‐up | |||
| Randomised | 70 | 72 | |
| Completed | 70 | 72 | |
| Abnormal vaginal bleeding or spotting | 7 | 0 | |
| 12 months follow‐up | |||
| Randomised | 70 | 72 | |
| Completed | 70 | 72 | |
| Abnormal vaginal bleeding or spotting | 0 | 0 | |
| 36 months follow‐up | |||
| Randomised | 70 | 72 | |
| Completed | 70 | 72 | |
| Endometrial polyps | 4 | 14 | < 0.05 |
| Endometrial hyperplasia | 0 | 4 | < 0.05 |
| Treatment Group | Control | P value | |
| 12 months follow‐up | |||
| Randomised | 75 | 75 | |
| Completed | 60 | 63 | |
| Abnormal vaginal bleeding or spotting | 22 | 2 | <0.001 |
| Breast cancer‐related deaths | 0 | 1 | |
| 24 months follow‐up | |||
| Completed | 59 | 62 | |
| Abnormal vaginal bleeding or spotting | 7 | 2 | 0.08 |
| Endometrial polyps | 1 | 10 | 0.02 |
| Endometrial hyperplasia | 0 | 0 | |
| Fibroids | 2 | 4 | 1.0 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometrial Polyps Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short term follow‐up (12 months) | 2 | 212 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.08, 0.64] |
| 1.2 Long term follow‐up (24 to 60 months) | 4 | 417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.13, 0.39] |
| 2 Endometrial Hyperplasia Show forest plot | 4 | 417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.03, 0.67] |
| 3 Endometrial Cancer Show forest plot | 2 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fibroids Show forest plot | 3 | 314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.16, 1.46] |
| 5 Abnormal Vaginal Bleeding or Spotting Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 12 months | 3 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [3.37, 15.66] |
| 5.2 24 months | 2 | 233 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [1.04, 7.10] |
| 5.3 45 months | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 60 months | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Breast Cancer Recurrence Show forest plot | 2 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.64, 4.74] |
| 7 Breast Cancer‐related Death Show forest plot | 3 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.36, 2.84] |

