Левоноргестрел‐высвобождающая внутриматочная система для защиты эндометрия женщин с раком молочной железы, получающих адъювантную терапию тамоксифеном
Appendices
Appendix 1. MDSG search (October 2015)
Keywords CONTAINS "IUD" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "LNG‐IUS" or "LNG20"or "Intrauterine Releasing Devices" or "Intrauterine Devices Medicated" or "intrauterine devices" or "intrauterine device" or "intrauterine contraceptive devices" or "Mirena" or Title CONTAINS "IUD" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "levonorgestrel‐releasing intrauterine system" or "Levonorgestrel‐Therapeutic‐Use" or "LNG‐IUS" or "LNG20"or "Intrauterine Releasing Devices" or "Intrauterine Devices Medicated" or "intrauterine devices" or "intrauterine device" or "intrauterine contraceptive devices" or "Mirena"
AND
Keywords CONTAINS "breast cancer" or "breast cancer incidence"or "breast changes"or "breast disease"or "breast outcomes"or "cancer risk"or "endometrial cancer"or "endometrial hyperplasia"or"endometrial pathology"or"endometrial polyps"or"endometrial proliferation"or "polyps" or Title CONTAINS "breast cancer" or "breast cancer incidence"or "breast changes"or "breast disease"or "breast outcomes"or "cancer risk"or "endometrial cancer"or "endometrial hyperplasia"or"endometrial pathology"or"endometrial polyps"or"endometrial proliferation"or "polyps"
Appendix 2. CBCG search (October 2015)
Details regarding the search strategies used by the Cochrane Breast Cancer Group for the identification of studies and procedures used to code references for the Specialised Register are outlined in the Group’s module: www.onlinelibrary.wiley.com/o/cochrane/clabout/articles/BREASTCA/frame.html
The following key words were used to identify relevant studies for consideration: "IUD", "intrauterine devices", "intrauterine system", "levonorgestrel intrauterine system", "levonorgestrel‐releasing intrauterine device", "levonorgestrel‐releasing intrauterine system", "levonorgestrel‐therapeutic use", "LNG‐IUS", "LNG20" and "Mirena".
Appendix 3. CENTRAL search (October 2015)
1 exp Breast Neoplasms/ (7208)
2 Breast Neoplasms, Male/ (23)
3 1 not 2 (7185)
4 (Breast cancer$ or Breast Neoplasm$).tw. (15233)
5 3 or 4 (16346)
6 exp Intrauterine Devices, Medicated/ (300)
7 Intrauterine Device$.tw. (350)
8 (LNG IUS or LNG IUD).tw. (133)
9 Levonorgestrel‐releasing intrauterine system$.tw. (106)
10 Levonorgestrel‐releasing intrauterine device$.tw. (43)
11 (IUD$ or Mirena).tw. (550)
12 or/6‐11 (784)
13 Intrauterine Devices, Medicated/ae [Adverse Effects] (1)
14 exp Neoplasm Recurrence, Local/ (2984)
15 exp Endometrial Hyperplasia/ (94)
16 exp Endometrial Neoplasms/ (274)
17 exp Adenocarcinoma/ (3971)
18 exp Neoplasm Metastasis/ (3273)
19 exp Antineoplastic Agents, Hormonal/ae [Adverse Effects] (346)
20 exp Tamoxifen/ae [Adverse Effects] (74)
21 breast cancer recurrence$.tw. (150)
22 recurrent breast cancer.tw. (119)
23 Local Neoplasm Recurrence$.tw. (0)
24 secondary breast cancer$.tw. (24)
25 secondary neoplasm$.tw. (11)
26 secondary cancer$.tw. (30)
27 Neoplasm Metastasis.tw. (2)
28 cancer metastasis.tw. (35)
29 advanced breast cancer.tw. (1815)
30 breast cancer survival.tw. (57)
31 Endometrial Hyperplasia.tw. (192)
32 Endometri$ patholog$.tw. (44)
33 Endometri$ polyp$.tw. (86)
34 Endometrial adenocarcinoma$.tw. (30)
35 endometrial cancer.tw. (458)
36 or/13‐35 (11723)
37 5 and 12 and 36 (6)
Appendix 4. The Cochrane Library (October 2015)
#1 "Clinical Trial" or "Phase I Clinical Trial" or "Phase II Clinical Trial" or "Phase III Clinical Trial" or "Phase IV Clinical Trial" or "Controlled Clinical Trial" or "Multicenter Study" or "Randomized Controlled Trial" or "Pragmatic Clinical Trial" in Cochrane Reviews (Reviews and Protocols) and Trials
#2 mh "Breast Neoplasms" not mh "Breast Neoplasms, Male" or "Breast cancer" or "Breast Neoplasms"
#3 mh "Intrauterine Devices" or mh "Levonorgestrel" or "Intrauterine Devices" or "IUD" or "Medicated Intrauterine Devices" or "LNG IUS" or "Levonorgestrel‐releasing intrauterine system" or "Mirena" or "Levonorgestrel"
#4 mh "Intrauterine Devices/adverse effects" or mh "Levonorgestrel/adverse effects" or mh "Neoplasm Recurrence, Local" or mh "Breast Neoplasms/secondary" or mh "Neoplasms/secondary" or mh "Endometrial Hyperplasia" or mh "Neoplasm Metastasis" or "breast cancer recurrence" or "recurrent breast cancer" or "Local Neoplasm Recurrence" or "secondary breast cancer" or "secondary neoplasms" or "secondary cancers" or "Neoplasm Metastasis" or "cancer metastasis" or "breast cancer metastasis" or "advanced breast cancer" or "breast cancer survival" or "Endometrial Hyperplasia" or "Endometrial pathology" or "Endometrial polyps" or "Endometrial adenocarcinoma" or "endometrial cancer"
#5 #1 and #2 and #3 and #4
Appendix 5. MEDLINE search (1946 to October 2015)
1 exp Breast Neoplasms/ (239438)
2 Breast Neoplasms, Male/ (2450)
3 1 not 2 (236988)
4 (Breast cancer$ or Breast Neoplasm$).tw. (200308)
5 3 or 4 (285644)
6 exp Intrauterine Devices, Medicated/ (2884)
7 Intrauterine Device$.tw. (4350)
8 (LNG IUS or LNG IUD).tw. (607)
9 Levonorgestrel‐releasing intrauterine system$.tw. (494)
10 Levonorgestrel‐releasing intrauterine device$.tw. (164)
11 (IUD$ or Mirena).tw. (8243)
12 or/6‐11 (11321)
13 Intrauterine Devices, Medicated/ae [Adverse Effects] (383)
14 exp Neoplasm Recurrence, Local/ (91623)
15 exp Endometrial Hyperplasia/ (3118)
16 exp Endometrial Neoplasms/ (16799)
17 exp Adenocarcinoma/ (308090)
18 exp Neoplasm Metastasis/ (168719)
19 exp Antineoplastic Agents, Hormonal/ae [Adverse Effects] (16203)
20 exp Tamoxifen/ae [Adverse Effects] (2944)
21 breast cancer recurrence$.tw. (1065)
22 recurrent breast cancer.tw. (1245)
23 Local Neoplasm Recurrence$.tw. (5)
24 secondary breast cancer$.tw. (72)
25 secondary neoplasm$.tw. (452)
26 secondary cancer$.tw. (853)
27 Neoplasm Metastasis.tw. (74)
28 cancer metastasis.tw. (6717)
29 advanced breast cancer.tw. (6880)
30 breast cancer survival.tw. (1012)
31 Endometrial Hyperplasia.tw. (2536)
32 Endometri$ patholog$.tw. (646)
33 Endometri$ polyp$.tw. (1202)
34 Endometrial adenocarcinoma$.tw. (2770)
35 endometrial cancer.tw. (11955)
36 or/13‐35 (546313)
37 5 and 12 and 36 (56)
Appendix 6. EMBASE search (1980 to October 2015)
1 exp breast tumor/ (388566)
2 (Breast cancer$ or Breast Neoplasm$).tw. (266249)
3 breast tumor$.tw. (19642)
4 or/1‐3 (416695)
5 exp intrauterine contraceptive device/ (14353)
6 Intrauterine Device$.tw. (4807)
7 (LNG IUS or LNG IUD).tw. (926)
8 Levonorgestrel‐releasing intrauterine system$.tw. (647)
9 Levonorgestrel‐releasing intrauterine device$.tw. (202)
10 (IUD$ or Mirena).tw. (7409)
11 or/5‐10 (16901)
12 intrauterine contraceptive device/ae [Adverse Drug Reaction] (1458)
13 exp tumor recurrence/ (43045)
14 exp endometrium hyperplasia/ (6121)
15 exp endometrium tumor/ (44939)
16 exp breast adenocarcinoma/ or exp adenocarcinoma/ (84246)
17 exp metastasis/ (435415)
18 "antineoplastic hormone agonists and antagonists"/ae [Adverse Drug Reaction] (541)
19 exp tamoxifen/ae [Adverse Drug Reaction] (6669)
20 breast cancer recurrence$.tw. (1657)
21 recurrent breast cancer$.tw. (1589)
22 Local Neoplasm Recurrence$.tw. (5)
23 secondary breast cancer$.tw. (132)
24 secondary neoplasm$.tw. (602)
25 secondary cancer$.tw. (1265)
26 Neoplasm Metastasis.tw. (83)
27 cancer metastasis.tw. (8860)
28 advanced breast cancer.tw. (9170)
29 breast cancer survival.tw. (1254)
30 Endometrial Hyperplasia.tw. (3303)
31 Endometri$ patholog$.tw. (961)
32 Endometri$ polyp$.tw. (1881)
33 Endometrial adenocarcinoma$.tw. (3393)
34 endometrial cancer.tw. (16312)
35 or/12‐34 (589755)
36 4 and 11 and 35 (164)
37 Clinical Trial/ (851926)
38 Randomized Controlled Trial/ (386357)
39 exp randomization/ (68465)
40 Single Blind Procedure/ (21150)
41 Double Blind Procedure/ (124284)
42 Crossover Procedure/ (44751)
43 Placebo/ (264658)
44 Randomi?ed controlled trial$.tw. (125251)
45 Rct.tw. (18496)
46 random allocation.tw. (1460)
47 randomly allocated.tw. (23462)
48 allocated randomly.tw. (2066)
49 (allocated adj2 random).tw. (739)
50 Single blind$.tw. (16466)
51 Double blind$.tw. (155581)
52 ((treble or triple) adj blind$).tw. (494)
53 placebo$.tw. (222116)
54 prospective study/ (310814)
55 or/37‐54 (1512736)
56 case study/ (34158)
57 case report.tw. (292453)
58 abstract report/ or letter/ (941185)
59 or/56‐58 (1261247)
60 55 not 59 (1472739)
61 exp Meta Analysis/ (100433)
62 ((meta adj analy$) or metaanalys$).tw. (108700)
63 (systematic adj (review$1 or overview$1)).tw. (89312)
64 review.ti. (340162)
65 or/61‐64 (470737)
66 60 or 65 (1842276)
67 36 and 66 (69)
Appendix 7. CINAHL search (1982 to October 2015)
| # | Query | Results |
| S14 | S6 AND S13 | 35 |
| S13 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 | 2,313 |
| S12 | TX(IUD* or Mirena*) | 744 |
| S11 | TX (Levonorgestrel‐releasing intrauterine) | 175 |
| S10 | TX (LNG IUD) | 15 |
| S9 | TX (LNG IUS) | 78 |
| S8 | TX Intrauterine Device* | 2,065 |
| S7 | (MM "Intrauterine Devices") | 1,107 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 61,738 |
| S5 | TX (Breast cancer* or Breast Neoplasm*) | 61,494 |
| S4 | TX (Breast cancer* or Breast Neoplasm*).tw | 1 |
| S3 | TX breast tumour* | 451 |
| S2 | TX breast tumor* | 2,002 |
| S1 | (MM "Breast Neoplasms+") | 43,927 |
Appendix 8. PyscINFO (October 2015)
1 exp Intrauterine Devices/ (91)
2 Levonorgestrel.tw. (62)
3 intrauterine device$.tw. (174)
4 iud.tw. (131)
5 mirena.tw. (9)
6 intrauterine system$.tw. (26)
7 exp Breast Neoplasms/ (7066)
8 breast neoplasm$.tw. (40)
9 breast tumor$.tw. (73)
10 (breast adj5 ca).tw. (1)
11 (breast adj5 cancer$).tw. (9414)
12 or/1‐6 (303)
13 or/7‐11 (9639)
14 12 and 13 (1)
Appendix 9. PubMed search (1946 to October 2015)
(("Clinical Trial"[Publication Type]) OR ("Phase I Clinical Trial" OR "Phase II Clinical Trial" OR "Phase III Clinical Trial" OR "Phase IV Clinical Trial" OR "Controlled Clinical Trial" OR "Multicenter Study" OR "Randomized Controlled Trial" OR "Pragmatic Clinical Trial"))
AND
(("Breast Neoplasms"[Mesh] NOT "Breast Neoplasms, Male"[Mesh]) OR ("Breast cancer" OR "Breast Neoplasms"))
AND
(("Intrauterine Devices, Medicated"[Mesh]) OR ("Intrauterine Devices" OR "IUD" OR "Medicated Intrauterine Devices" OR "LNG IUS" OR "Levonorgestrel‐releasing intrauterine system" OR "Mirena"))
AND
(("Intrauterine Devices, Medicated/adverse effects"[Mesh]) OR ("Neoplasm Recurrence, Local"[Mesh] OR "Breast Neoplasms/secondary"[Mesh] OR "Neoplasms/secondary"[Mesh] OR "Endometrial Hyperplasia"[Mesh] OR "Endometrial Neoplasms"[Mesh] OR "Adenocarcinoma" [Mesh] OR "Neoplasm Metastasis"[Mesh] OR "Antineoplastic Agents, Hormonal/adverse effects"[Mesh] OR "Tamoxifen/adverse effects"[Mesh]) OR ("breast cancer recurrence" OR "recurrent breast cancer" OR "Local Neoplasm Recurrence" OR "secondary breast cancer" OR "secondary neoplasms" OR "secondary cancers" OR "Neoplasm Metastasis" OR "cancer metastasis" OR "breast cancer metastasis" OR "advanced breast cancer" OR "breast cancer survival" OR "Endometrial Hyperplasia" OR "Endometrial pathology" OR "Endometrial polyps" OR "Endometrial adenocarcinoma" OR "endometrial cancer"))
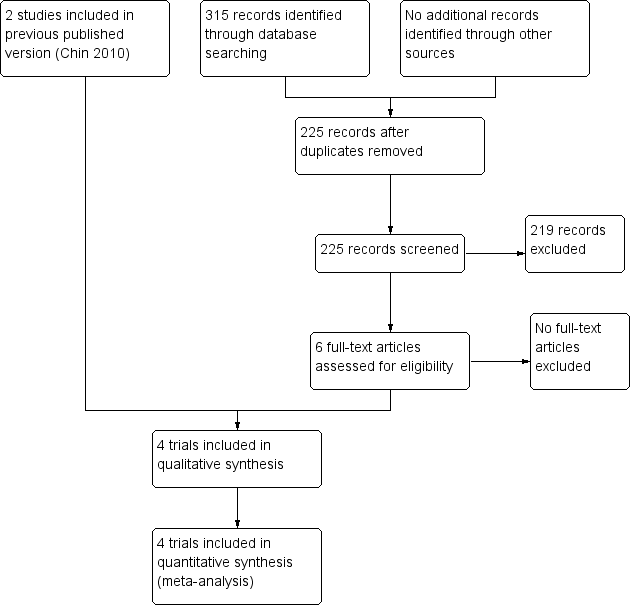
Study flow diagram
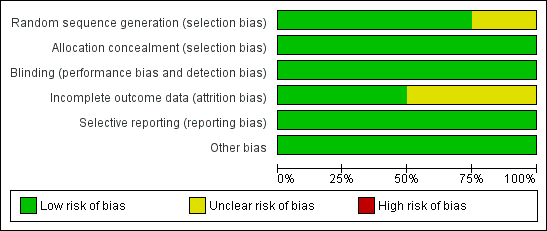
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors’ judgements about each risk of bias item for each included study

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.1 Endometrial Polyps.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.2 Endometrial Hyperplasia.
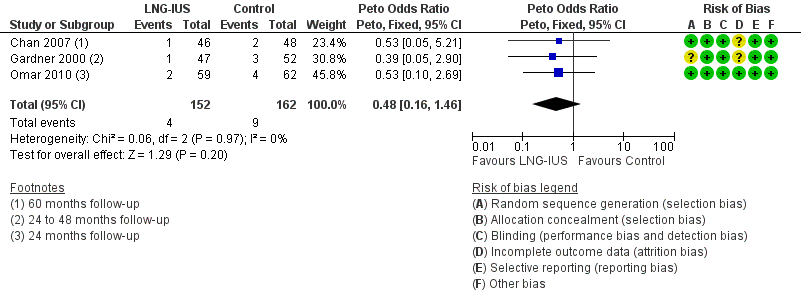
Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.4 Fibroids.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.5 Abnormal Vaginal Bleeding or Spotting.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.6 Breast Cancer Recurrence.

Forest plot of comparison: 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, outcome: 1.7 Breast Cancer‐related Death.
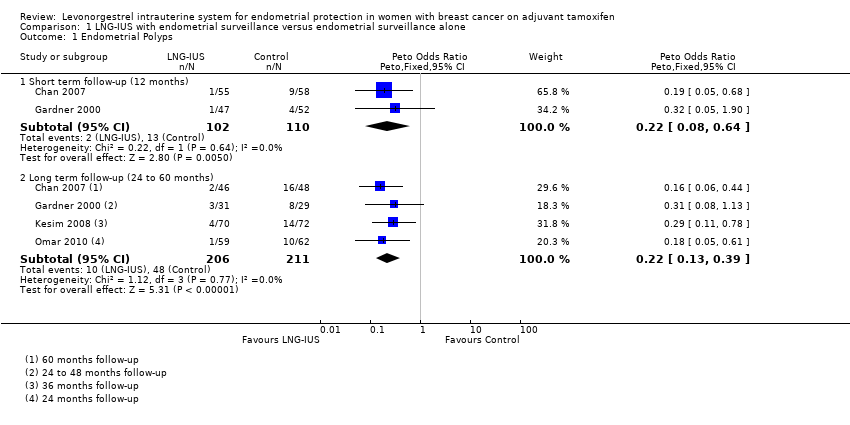
Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 1 Endometrial Polyps.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 2 Endometrial Hyperplasia.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 3 Endometrial Cancer.
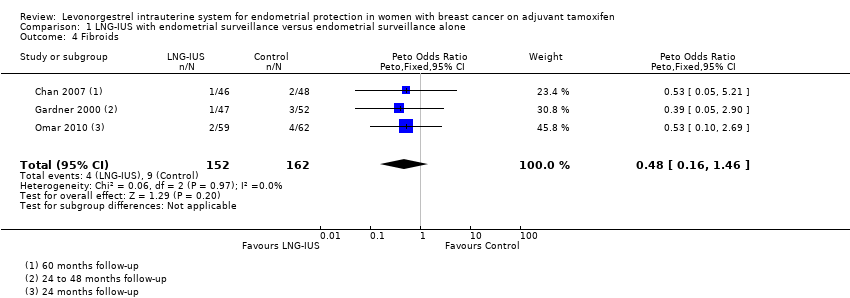
Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 4 Fibroids.
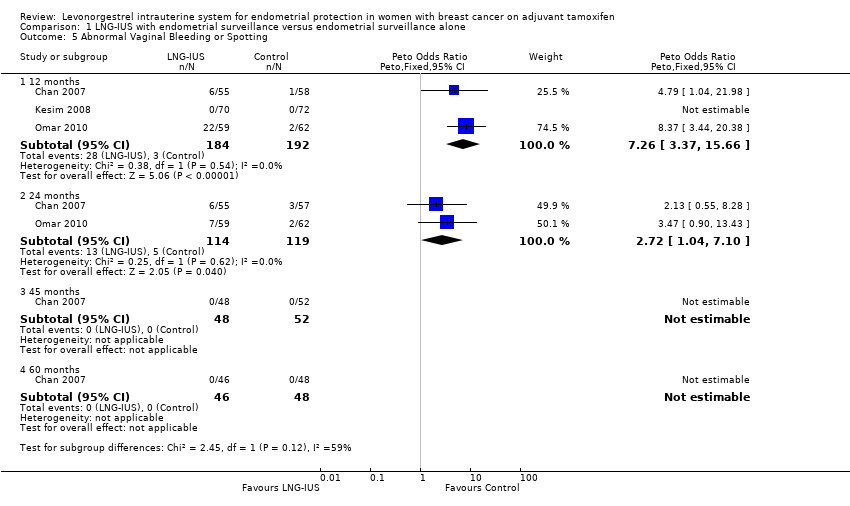
Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 5 Abnormal Vaginal Bleeding or Spotting.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 6 Breast Cancer Recurrence.

Comparison 1 LNG‐IUS with endometrial surveillance versus endometrial surveillance alone, Outcome 7 Breast Cancer‐related Death.
| The LNG‐IUS with endometrial surveillance compared to endometrial surveillance alone for endometrial protection in women with breast cancer on adjuvant tamoxifen | ||||||
| Patient or population: endometrial protection in women with breast cancer on adjuvant tamoxifen | ||||||
| Outcomes | Illustrated comparative risks* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Assumed Risk | Corresponding Riks | |||||
| Endometrial surveillance alone | LNG‐IUS with endometrial surveillance | |||||
| Endometrial Polyps | Moderate | OR 0.22 | 417 | ⊕⊕⊕⊝ | ||
| 235 per 1000 | 63 per 1000 | |||||
| Endometrial Hyperplasia | Moderate | OR 0.13 | 417 | ⊕⊕⊕⊝ | ||
| 28 per 1000 | 4 per 1000 | |||||
| Endometrial Cancer | Moderate | not estimable | 154 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Fibroids | Moderate | OR 0.48 | 314 | ⊕⊕⊕⊝ | ||
| 58 per 1000 | 29 per 1000 | |||||
| Abnormal Vaginal Bleeding or Spotting | Moderate | OR 7.26 | 376 | ⊕⊕⊕⊝ | ||
| 17 per 1000 | 113 per 1000 | |||||
| Abnormal Vaginal Bleeding or Spotting | Moderate | OR 2.72 | 233 | ⊕⊕⊕⊝ | ||
| 42 per 1000 | 107 per 1000 | |||||
| Abnormal Vaginal Bleeding or Spotting | Moderate | not estimable | 94 | ⊕⊕⊕⊝ | ||
| 0 per 1000 | 0 per 1000 | |||||
| Breast Cancer Recurrence | Moderate | OR 1.74 | 154 | ⊕⊕⊕⊝ | ||
| 80 per 1000 | 131 per 1000 | |||||
| Breast Cancer‐related Death | Moderate | OR 1.02 | 277 | ⊕⊕⊕⊝ | ||
| 69 per 1000 | 70 per 1000 | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 limited sample size and low event rate | ||||||
| Treatment Group | Control | P value | |
| 6 months follow‐up | |||
| Randomised | 64 | 65 | |
| Completed | 55 | 58 | |
| Abnormal vaginal bleeding or spotting | 20 | 1 | <0.001 |
| 12 months follow‐up | |||
| Completed | 55 | 58 | |
| Abnormal vaginal bleeding or spotting | 6 | 1 | 0.06 |
| Endometrial polyps | 1 | 9 | 0.02 |
| Endometrial hyperplasia | 0 | 0 | |
| Fibroids | 1 | 2 | 1.0 |
| 24 months follow‐up | |||
| Completed | 55 | 57 | |
| Abnormal vaginal bleeding or spotting | 6 | 3 | 0.45 |
| 45 months follow‐up | |||
| Completed | 48 | 52 | |
| Abnormal vaginal bleeding or spotting | 0 | 0 | |
| 60 months follow‐up | |||
| Completed | 46 | 48 | |
| Abnormal vaginal bleeding or spotting | 0 | 0 | |
| Endometrial polyps | 2 | 16 | < 0.001 |
| Endometrial hyperplasia | 0 | 1 | 1.0 |
| Endometrial cancer | 0 | 0 | |
| Fibroids | 1 | 2 | 1.0 |
| Breast cancer recurrence | 10 | 6 | 0.25 |
| Breast cancer‐related deaths | 6 | 5 | 0.71 |
| Treatment Group | Control | P value | |
| 12 months follow‐up | |||
| Randomised | 64 | 58 | |
| Completed | 47 | 52 | |
| Endometrial polyps | 1 | 4 | 0.4 |
| Endometrial hyperplasia | 0 | 1 | |
| Fibroids | 1 | 3 | 0.2 |
| Final follow‐up (24, 36, or 48 months) | |||
| Completed at 24 months | 31 | 29 | |
| Completed at 36 months | 19 | 20 | |
| Completed at 48 months | 6 | 9 | |
| Endometrial polyps | 3 | 8 | |
| Endometrial hyperplasia | 0 | 1 | |
| Endometrial cancer | 0 | 0 | |
| Breast cancer recurrence | 1 | 1 | |
| Breast cancer‐related deaths | 2 | 2 |
| Treatment Group | Control | P value | |
| 5 months follow‐up | |||
| Randomised | 70 | 72 | |
| Completed | 70 | 72 | |
| Abnormal vaginal bleeding or spotting | 7 | 0 | |
| 12 months follow‐up | |||
| Randomised | 70 | 72 | |
| Completed | 70 | 72 | |
| Abnormal vaginal bleeding or spotting | 0 | 0 | |
| 36 months follow‐up | |||
| Randomised | 70 | 72 | |
| Completed | 70 | 72 | |
| Endometrial polyps | 4 | 14 | < 0.05 |
| Endometrial hyperplasia | 0 | 4 | < 0.05 |
| Treatment Group | Control | P value | |
| 12 months follow‐up | |||
| Randomised | 75 | 75 | |
| Completed | 60 | 63 | |
| Abnormal vaginal bleeding or spotting | 22 | 2 | <0.001 |
| Breast cancer‐related deaths | 0 | 1 | |
| 24 months follow‐up | |||
| Completed | 59 | 62 | |
| Abnormal vaginal bleeding or spotting | 7 | 2 | 0.08 |
| Endometrial polyps | 1 | 10 | 0.02 |
| Endometrial hyperplasia | 0 | 0 | |
| Fibroids | 2 | 4 | 1.0 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Endometrial Polyps Show forest plot | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short term follow‐up (12 months) | 2 | 212 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.08, 0.64] |
| 1.2 Long term follow‐up (24 to 60 months) | 4 | 417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.13, 0.39] |
| 2 Endometrial Hyperplasia Show forest plot | 4 | 417 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.03, 0.67] |
| 3 Endometrial Cancer Show forest plot | 2 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Fibroids Show forest plot | 3 | 314 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.16, 1.46] |
| 5 Abnormal Vaginal Bleeding or Spotting Show forest plot | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 12 months | 3 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.26 [3.37, 15.66] |
| 5.2 24 months | 2 | 233 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.72 [1.04, 7.10] |
| 5.3 45 months | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 60 months | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Breast Cancer Recurrence Show forest plot | 2 | 154 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.74 [0.64, 4.74] |
| 7 Breast Cancer‐related Death Show forest plot | 3 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.36, 2.84] |

