Intervenciones para la prevención de la mastitis después del parto
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomised controlled trial. | |
| Participants | Breastfeeding postpartum women (N = 10) with cracked nipples colonised with Staphylococcus aureus. Setting: hospitals in Melbourne, Australia. Inclusion criteria: lactating women with Staphylococcus aureus‐colonised nipples wishing to breastfeed. Exclusion criteria: cracked nipples that were not colonised with Staphylococcus aureus. | |
| Interventions | Prophylactic antibiotics (flucloxacillin capsules taken for 7 days); N = 5 versus placebo (capsules with glucose powder taken for 7 days); N = 5. Women with a positive nipple culture for Staphylococcus aureus had a follow‐up visit at 1 week. Women with negative nipple cultures had telephone follow up at 1 week. All participants had a final telephone interview at 6 weeks. | |
| Outcomes | Mastitis study aborted at 12 months due to poor intervention compliance and lack of eligible participants. | |
| Notes | After 12 months, only 10 of the planned total of 133 women had been randomised to the trial and so the trial was stopped early. The author for this trial was contacted to clarify risks of bias. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The pharmacist used a random numbers table to label the capsules (placebo or active); sequence was stratified by hospitals in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Third party (pharmacist). |
| Blinding (performance bias and detection bias) | Low risk | Capsules were of identical appearance and so participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 2 women (2/10) dropped out of the study as they did not wish to take medications ‐ both women had been allocated to the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Trial stopped early. |
| Methods | Randomised controlled trial. | |
| Participants | 211 breastfeeding mother‐infant pairs. Inclusion criteria:healthy non‐twin newborns with birthweight ≥ 2500 g. Exclusion criteria: mother‐infant pairs unable to stay together due to a health concern in either the mother or the infant. Setting: Porto Alegre, Brazil (women were recruited from June to November 2003). | |
| Interventions | Breastfeeding education session (30 minutes) with an lactation consultant and an experienced breastfeeding nurse in hospital (N = 74) versus usual care (N = 137). All women received a follow‐up home visit at day 7 and day 30. | |
| Outcomes | Measure of exclusive breastfeeding rates, breastfeeding related problems. Measure of mastitis, sore nipples and engorgement. | |
| Notes | Attempts to contact the authors were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using 2 different coloured balls from a bag, 1 colour for the intervention, 1 colour for the control. |
| Allocation concealment (selection bias) | Unclear risk | 2 different coloured balls from a bag, 1 colour for the intervention, 1 colour for the control. |
| Blinding (performance bias and detection bias) | Unclear risk | Researchers responsible for assessment blinded to intervention group assignment. Not feasible for participants and clinician. |
| Incomplete outcome data (attrition bias) | Low risk | Between 5%‐9.9%. The original number of participants in the experimental group and the control group was 74 and 137 respectively. At the time of data analysis there had been a loss of participants in both groups, 3 participants in the experimental group leaving 71 women and 5 women in the control group leaving 132 women. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | Unclear risk | No apparent evidence of other bias. |
| Methods | This study trial led basic breastfeeding advice with a combination of antibiotics and topical ointments. Mothers attending breastfeeding clinic for breastfeeding problems, cracked/sore nipples, positive Staphyloccocus aureus results. Exclusion criteria: mothers with local or system spread of infection such as cellulitis, ascending lactiferous duct infection or mastitis were excluded. | |
| Participants | N = 84. Postpartum breastfeeding women with sore or cracked nipples. | |
| Interventions | 4 intervention groups: 1. Optimal breastfeeding technique (basic breastfeeding advice) N = 23. 2. Topical 2% mupirocin ointment to nipples, N = 25. 3. Topical fusidic acid ointment to nipples, N = 17. 4. Oral antibiotics ‐ cloxacillin/erythromycin, N = 19. | |
| Outcomes | Measured nipple symptoms, breast symptoms and mastitis. | |
| Notes | 100% compliance ‐ highly‐motivated breastfeeding women. Women that presented with mastitis were excluded from the study. Intention‐to‐treat not used. This study was stopped prematurely ‐ women who did not receive antibiotic perceived to have a higher rate of mastitis. An attempt to contact this author to clarify findings was unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 100 tags were alternatively labelled A, B, C, D and placed in an envelope. |
| Allocation concealment (selection bias) | Low risk | Each case randomly assigned by drawing a tag from the envelope. |
| Blinding (performance bias and detection bias) | High risk | Open unblinded study. |
| Incomplete outcome data (attrition bias) | Low risk | No losses reported. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting. |
| Other bias | Unclear risk | Trial was stopped early. |
| Methods | Randomised controlled trial of women who planned to have caesarean sections and who were HIV infected. This study trial led antibiotics versus placebo. | |
| Participants | N = 615, HIV infected women > 18 years, ≥ 36 weeks' gestation with anticipated vaginal delivery at King Edward VIII and Addington Hospital in Durban, South Africa between February 2003 and May 2005. 675 delivered at the hospitals in the study and were eligible for randomisation. 60 had a planned caesarean section and were excluded. 305 were randomised and received cefoxitin and 310 were randomly assigned the placebo. Following this, a further 92 women from the intervention group and 99 from the placebo group were excluded because they had an emergency caesarean delivery. | |
| Interventions | 2 gm dose of cefoxitin intravenously over 20 minutes during active labour versus a water placebo administered over the same period of time. | |
| Outcomes | Of the 213 women assigned randomly to the cefoxitin group, 182 (85%) returned for the follow‐up evaluation at 1 week and 184 (86%) returned at 2 weeks. Of the 212 women assigned the placebo, 180 (85%) returned for the follow up at 1 week and 178 (84%) returned at the 2 week for follow up. | |
| Notes | Clinicians blinded to intervention. Women were excluded if they had an emergency caesarean delivery after randomisation. The randomised groups were comparable with regards age, parity, gestational age at delivery and most baseline haematology. An attempt to contact this author to clarify findings was unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated by statistician. Syringes labelled D001‐D686; participants were given the drug during labour according to the next available number. |
| Allocation concealment (selection bias) | Low risk | The statistician generated a computer‐based allocation of each study numbered 1‐686 into either group 1 or 2 which represented either cefoxitin or placebo. Only the pharmacist was aware of the drug code for the duration of the study. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinded to clinicians. |
| Incomplete outcome data (attrition bias) | Unclear risk | Incomplete outcome data > 20%. The original number of participants in the study was 716, of which 675 delivered within the study premises. 60 of these women were not randomised. Finally 615 women were randomised, with 305 women in the experimental group and 310 in the control group. The 1‐week follow up resulted in 182 participants in the experimental group and 180 in the control group. At the 2‐week follow up there were 184 in the experimental group and 178 in the control group. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | Unclear risk | No apparent evidence of other bias. |
| Methods | This study trial led the use of anti‐secretory factor (AF) in cereal to prevent mastitis. Data collected from April to August 2002. All mothers were Swedish or raised in Sweden. Duration of follow up 5 weeks. | |
| Participants | N = 40 postpartum breastfeeding women that had normal deliveries and have healthy full ferm infants, were randomly divided into 2 groups. Participants were breastfeeding or intended to breastfeed. | |
| Interventions | Anti‐secretory factor in cereal versus similar cereal without the AF. Experimental group N = 12, control group N = 16 (11 mothers dropped out and one mother failed to give a milk sample from the control group). | |
| Outcomes | Incidence of mastitis between groups. | |
| Notes | Participants requested to eat 50 g of cereal for a period of 5 weeks. Duration of follow up was 5 weeks. No difference between the groups, regarding background, obstetric data, age, education, parity, type of anaesthesia used during the delivery, child sex and birth rate. Loss of participants to follow up > 20%. To date, attempts to contact the authors have been unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (sealed envelopes that were opened consecutively) to 1 of 2 groups. (further information not available at this stage, unable to contact the author). |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes that were opened consecutively. |
| Blinding (performance bias and detection bias) | Low risk | Blinding to participants, cereal used looked the same. Researchers and participants blinded to which cereal was allocated. Randomising sequence concealed until data were collected and laboratory analysis was performed. |
| Incomplete outcome data (attrition bias) | Low risk | Incomplete outcome data > 20%. The original number of participants in the study was 40. 11 of these participants dropped out in the first 2 weeks. 7 mothers in the experimental group and 3 in the control group. 1 mother was excluded because of incorrect compliance of the intervention. The final number for the experimental group was 12 mothers and 17 for the control group. 1 of the mothers in the control group failed to provide a milk sample at the end of the study. Final data analysis was on 12 mothers from the experimental group and 17 from the control group. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | Unclear risk | No apparent evidence of other bias. |
GP: general practitioner
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This study is not a randomised controlled trial and does not meet the requirements necessary for random allocation, concealment or blinding. | |
| This is not a trial of mastitis prevention. It is an RCT on the effect of different postnatal ward practices on lactation performance. | |
| This is not a trial of mastitis prevention. It is an RCT on treating sore nipples. | |
| Trial stopped. | |
| This study is not a randomised controlled trial and does not meet the requirements necessary for random allocation, concealment or blinding. | |
| This is not a trial of mastitis prevention. It is an RCT of postpartum maternal vitamin A supplementation. | |
| This is an RCT of strategies to increase breastfeeding initiation and duration. | |
| This is not a trial of mastitis prevention. It is an RCT of discharge packs and counselling to increase breastfeeding duration. | |
| This is a micronutrient RCT looking at preventing 'subclinical' mastitis. | |
| This trial did not evaluate interventions for preventing mastitis ‐ it is a trial comparing early postnatal check up with a GP (at one week) with the usual 6 week check up. | |
| Treatment of mastitis, not prevention. | |
| This is an RCT/quasi‐RCT for preventing sore nipples. | |
| This is an RCT for treating nipple trauma. | |
| This is a continuity of care RCT. | |
| This is an RCT of breastfeeding promotion. | |
| This is a treatment trial. | |
| This is a treatment trial. | |
| This is not an RCT. | |
| This is an RCT of resources, information and support for postpartum women. | |
| This is an RCT of antibiotic prophylaxis for caesarean section. | |
| This is an RCT of ultrasound treatment for breast engorgement. | |
| This is not an RCT. It is a letter re Kramer 2001. | |
| This is not an RCT. | |
| This is an RCT of treating cracked nipples. | |
| This is not an RCT. | |
| This is an RCT for preventing breast engorgement. | |
| This is an RCT for preventing breast engorgement. | |
| This is an RCT for treating breast engorgement. | |
| This is an RCT for treating breast engorgement. | |
| This is a quasi‐randomised trial (women were allocated by the first letter of their surname). | |
| This is an RCT of lactation suppression (breast binding). | |
| Treatment of mastitis, not prevention. | |
| This trial did not evaluate interventions for preventing mastitis ‐ it is an RCT comparing birth centre care versus usual obstetric care. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised controlled trial. |
| Participants | 45 lactating mothers. |
| Interventions | Lanolin cream versus breast milk. |
| Outcomes | Mastitis observed in breast milk group. |
| Notes | Author has been contacted with regards this study. It is currently with a review committee and is not available. We will reassess this study at a later date |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Antibiotic versus no antibiotic, Outcome 1 Mastitis. | ||||
| 1.1 Antibiotic versus no antibiotic | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.61] |
| 1.2 Antibiotic versus placebo | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.15, 6.68] |
| 1.3 Antibiotic versus mupirocin | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.05, 3.89] |
| 1.4 Antibiotic versus fusidic acid | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.81] |
| 1.5 Antibiotic versus breastfeeding advice | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 1 Mastitis. | ||||
| 1.1 at hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.35, 40.70] |
| 1.3 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.17, 4.95] |
| 2 Sore nipples Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 2 Sore nipples. | ||||
| 2.1 at hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 2.2 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.22] |
| 2.3 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.37] |
| 3 Breast engorgement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 3 Breast engorgement. | ||||
| 3.1 at hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.03, 14.87] |
| 3.2 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.53] |
| 3.3 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
| 4 Exclusive breastfeeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.4  Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 4 Exclusive breastfeeding. | ||||
| 4.1 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 4.2 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
| 5 Breastfeeding problems, mean per mother Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.5  Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 5 Breastfeeding problems, mean per mother. | ||||
| 5.1 at hospital discharge | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.27, 0.67] |
| 5.2 at 30 days | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 1.72] |
| Analysis 3.1  Comparison 3 Anti‐secretory factor in cereal versus standard cereal, Outcome 1 Mastitis. | ||||
| 2 Mastitis recurrence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Anti‐secretory factor in cereal versus standard cereal, Outcome 2 Mastitis recurrence. | ||||
| 2.1 First recurrence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.51] |
| 2.2 Second recurrence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.02, 10.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.35] |
| Analysis 4.1  Comparison 4 Mupirocin ointment versus breastfeeding advice alone, Outcome 1 Mastitis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.27, 2.22] |
| Analysis 5.1  Comparison 5 Fusidic acid ointment versus breastfeeding advice alone, Outcome 1 Mastitis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 2.00] |
| Analysis 6.1  Comparison 6 Mupirocin ointment+BF advice versus fusidic acid ointment+BF advice, Outcome 1 Mastitis. | ||||

Summary of quality assessment of included studies. Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
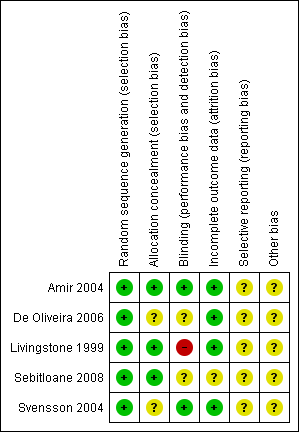
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Antibiotic versus no antibiotic, Outcome 1 Mastitis.

Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 1 Mastitis.

Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 2 Sore nipples.

Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 3 Breast engorgement.

Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 4 Exclusive breastfeeding.

Comparison 2 Breastfeeding education (specialist) versus usual care, Outcome 5 Breastfeeding problems, mean per mother.

Comparison 3 Anti‐secretory factor in cereal versus standard cereal, Outcome 1 Mastitis.

Comparison 3 Anti‐secretory factor in cereal versus standard cereal, Outcome 2 Mastitis recurrence.

Comparison 4 Mupirocin ointment versus breastfeeding advice alone, Outcome 1 Mastitis.

Comparison 5 Fusidic acid ointment versus breastfeeding advice alone, Outcome 1 Mastitis.

Comparison 6 Mupirocin ointment+BF advice versus fusidic acid ointment+BF advice, Outcome 1 Mastitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Antibiotic versus no antibiotic | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.61] |
| 1.2 Antibiotic versus placebo | 2 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.15, 6.68] |
| 1.3 Antibiotic versus mupirocin | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.05, 3.89] |
| 1.4 Antibiotic versus fusidic acid | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.03, 1.81] |
| 1.5 Antibiotic versus breastfeeding advice | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.02, 1.28] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 at hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.35, 40.70] |
| 1.3 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.17, 4.95] |
| 2 Sore nipples Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 at hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.36] |
| 2.2 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.22] |
| 2.3 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.37] |
| 3 Breast engorgement Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 at hospital discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.03, 14.87] |
| 3.2 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.53] |
| 3.3 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.49] |
| 4 Exclusive breastfeeding Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 at 7 days | 1 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.18] |
| 4.2 at 30 days | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
| 5 Breastfeeding problems, mean per mother Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 at hospital discharge | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.27, 0.67] |
| 5.2 at 30 days | 1 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.61, 0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 1.72] |
| 2 Mastitis recurrence Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 First recurrence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 3.51] |
| 2.2 Second recurrence | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.02, 10.45] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.27, 2.22] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mastitis Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.13, 2.00] |

