Uso limitado de chupetes en recién nacidos a término que reciben lactancia para aumentar la duración de la lactancia materna
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007202.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 30 agosto 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Embarazo y parto
- Copyright:
-
- Copyright © 2016 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Sharifah Halimah is the main author and guarantor for the review. She wrote the first draft of the protocol; provided a clinical and policy perspective as well as providing general advice on the development of the protocol. For the review she assessed studies for inclusion, assessed trial quality and extracted and analysed the data, and wrote the review. For this update she rewrote the background and updated other sections of the review.
Jacqueline Ho provided general comments and advice from the protocol development to the completion of the review and provided extensive input for this update. She assessed trial quality where disagreement arose in the decision to include or exclude trials. She revised the Plain language summary for the update and prepared the 'Summary of findings' table.
Shayesteh Jahanfar provided input into the protocol development as well as the review. She independently assessed the quality of the trials, extracted and analysed the data. She also wrote the Plain language summary of the review. She critically evaluated the update and made recommendations.
Mubashir Angolkar provided general comment, proof read the draft of the protocol as well as the review.
Sources of support
Internal sources
-
University Kuala Lumpur Royal College of Medicine Perak, Malaysia.
-
Penang Medical College, Malaysia.
-
Jawaharlal Nehru Medical College Campus, Belgaum, India.
-
Ipoh Specialist Hospital, Perak, Malaysia.
External sources
-
SEA ORCHID, Malaysia.
-
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
Declarations of interest
None known.
Acknowledgements
We would like to acknowledge the contributions of the SEA‐ORCHID group and members of the Cochrane Australasian Centre for their role in the development of the protocol and first version of the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
This research was supported by a grant from the Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization. The findings, interpretations and conclusions expressed in this paper are entirely those of the authors and should not be attributed in any manner whatsoever to WHO.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Aug 30 | Effect of restricted pacifier use in breastfeeding term infants for increasing duration of breastfeeding | Review | Sharifah Halimah Jaafar, Jacqueline J Ho, Shayesteh Jahanfar, Mubashir Angolkar | |
| 2012 Jul 11 | Effect of restricted pacifier use in breastfeeding term infants for increasing duration of breastfeeding | Review | Sharifah Halimah Jaafar, Shayesteh Jahanfar, Mubashir Angolkar, Jacqueline J Ho | |
| 2011 Mar 16 | Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding | Review | Sharifah Halimah Jaafar, Shayesteh Jahanfar, Mubashir Angolkar, Jacqueline J Ho | |
| 2008 Jul 16 | Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding | Protocol | Halimah Sharifah, Mubashir Angolkar, Shayesteh Jahanfar, Jacqueline J Ho | |
Differences between protocol and review
The methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Pregnancy and Childbirth Group's methodological guidelines. We are no longer excluding studies from analysis based on high attrition rates, but are instead planning on conducting sensitivity analysis to explore the effects of high attrition.
In this update, it has been clarified that the intervention is "restricted pacifier use" and the control is "unrestricted pacifier use".
The title has been changed from "Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding" to "Effect of restricted pacifier use on breastfeeding duration", as detailed in the authors' response to feedback ‐ see Feedback 1.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Infant; Infant, Newborn;
PICO
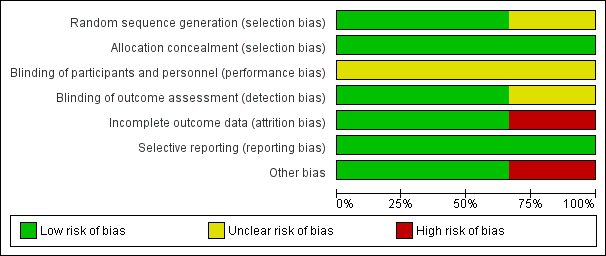
Figure 1: 'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
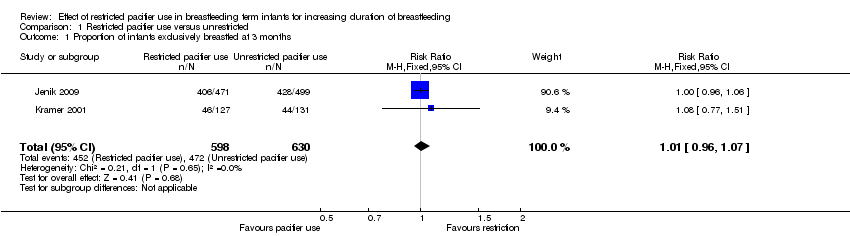
Comparison 1 Restricted pacifier use versus unrestricted, Outcome 1 Proportion of infants exclusively breastfed at 3 months.

Comparison 1 Restricted pacifier use versus unrestricted, Outcome 2 Proportion of infants partially breastfed at 3 months.
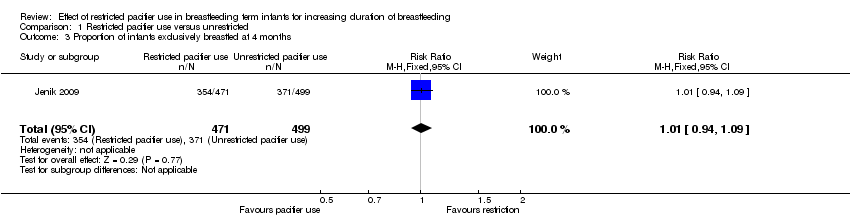
Comparison 1 Restricted pacifier use versus unrestricted, Outcome 3 Proportion of infants exclusively breastfed at 4 months.

Comparison 1 Restricted pacifier use versus unrestricted, Outcome 4 Proportion infants partially breastfed at 4 months.
| Pacifier use versus pacifier restriction for increasing duration of breastfeeding | ||||||
| Patient or population: healthy full‐term newborns whose mothers have initiated breastfeeding and intend to exclusively breastfeed Comparison: no restriction in pacifier use | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Pacifier use versus pacifier restriction | |||||
| Proportion of infants exclusively breastfed at 4‐6 months | Study population | RR 1.01 | 970 | ⊕⊕⊕⊝ | Not downgraded for study limitations (lack of blinding of the intervention as there was blinding of the outcome assessor and outcome is objective) | |
| 743 per 1000 | 751 per 1000 | |||||
| Duration of full or exclusive breastfeeding | Outcome not reported | |||||
| Breastfeeding difficulties | Outcome not reported | |||||
| Maternal satisfaction and level of confidence in parenting | Outcome not reported | |||||
| Infant otitis media | Outcome not reported | |||||
| Infant dental malocclusion | Outcome not reported | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) taken from the included studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Evidence obtained from only one study and so downgraded for imprecision | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion of infants exclusively breastfed at 3 months Show forest plot | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.96, 1.07] |
| 2 Proportion of infants partially breastfed at 3 months Show forest plot | 2 | 1228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.02] |
| 3 Proportion of infants exclusively breastfed at 4 months Show forest plot | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.09] |
| 4 Proportion infants partially breastfed at 4 months Show forest plot | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.97, 1.02] |

