Jedna doza intravenskog paracetamola ili intravenskog propacetamola za ublažavanje boli nakon operacije
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, placebo and active‐controlled, multiple dose, over 24 h First dose administered 15 min prior to extubation | |
| Participants | Type of surgery: thyroidectomy Paracetamol group Entered/completing: 30/30 Age (mean, SD): 44.5 ± 15.1 Sex (male, %): 16.7% Placebo Entered/completing: 30/30 Age (mean, SD): 47.9 ± 11.8 Sex (male, %): 10.0% Parecoxib Entered/completing: 30/30 Age (mean, SD): 48.3 ± 14.2 Sex (male, %): 23.3% Metamizole Entered/completing: 30/30 Age (mean, SD): 43.8 ± 13.7 Sex (male, %): 16.7% | |
| Interventions | 1 g paracetamol in 100 ml normal saline over 15 min Placebo, parecoxib 40 mg, or metamizole 1 g: all in 100 ml NS over 15 min | |
| Outcomes | Primary: accumulated opioid consumption (piritramide via PCA) Secondary: Pain intensity (VAS) Pain relief (VRS) Patient satisfaction (VRS) | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | Participants with chronic pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization (http://www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelope, only opened in emergency |
| Blinding (performance bias and detection bias) | Low risk | “The study solutions were clear so that they could not be recognized by the anesthesiologists collecting the data and were prepared by one of the researchers who was not involved in the intraoperative and postoperative treatment of these patients”. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or protocol violations – complete data set obtained for all 4 groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section, but side effects not monitored adequately |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 placebo, 30 parecoxib, 30 metamizole) |
| Methods | Double‐blind, placebo and active‐controlled, multiple dose, over 24 h First dose administered 15 min prior to extubation | |
| Participants | Type of surgery: laparoscopic cholecystectomy Paracetamol group Entered/completing: 30/30 Age (mean, SD): 52.5 ± 15.8 Sex (male, %): 23.3% Placebo Entered/completing: 30/30 Age (mean, SD): 47.1 ± 13.9 Sex (male, %): 20.0% Parecoxib Entered/completing: 30/30 Age (mean, SD): 54.9 ± 13.0 Sex (male, %): 26.7% Metamizole Entered/completing: 30/30 Age (mean, SD): 52.4 ± 15.6 Sex (male, %): 30.0% | |
| Interventions | 1 g paracetamol in 100 ml normal saline over 15 min Placebo, parecoxib 40 mg, or metamizole 1 g: all in 100 ml NS over 15 min | |
| Outcomes | Primary: accumulated opioid consumption (piritramide via PCA) Secondary: Pain intensity (VAS) Pain relief (VRS) Patient satisfaction (VRS) | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | Participants with chronic pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization (http://www.randomization.com) |
| Allocation concealment (selection bias) | Low risk | The group assignment code was retained until the conclusion of the study |
| Blinding (performance bias and detection bias) | Low risk | “The study solutions were prepared by one of the researchers who were not involved in the intraoperative and postoperative treatment of these patients. Postoperative data were collected by anesthesiologists who were blinded as to the treatment used. Other caretakers were also unaware of the analgesic drug that would be used for each patient during the study”. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or protocol violations – complete data set obtained for all 4 groups |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section, but side effects not monitored adequately and satisfaction score results not fully described |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 placebo, 30 parecoxib, 30 metamizole) |
| Methods | Randomized, double‐blind, parallel, active‐controlled. Pain evaluated up to 6 h after dose administered. | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 40/unclear Age (mean, SD): 24.2 ± 1.1 Sex (male, %): 0 Control group Entered/completing: 40/unclear Age (mean, SD): 24.4 ± 1.2 Sex (male, %): 0 | |
| Interventions | Paracetamol 1 g IV over 15 min at first complaint of pain Diclofenac 75 mg IM as above | |
| Outcomes | Primary: time to first rescue analgesic (1 mg/kg IM pethidine) Secondary: VAS pain scores at 30 min, 1, 2, 4 and 6 h; adverse events | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes ‐ all P values > 0.05: demographic characteristics, week of pregnancy, newborn's weight, Apgar scores, operation time, time to first postoperative analgesic requirement | |
| Details of preoperative pain | Participants with previous continuous analgesic use were excluded | |
| Notes | Translated from Turkish using Google Translate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used envelopes to randomly divide 2 groups – no additional details given |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | No details but stated as double‐blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of participants dropping out |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 diclofenac) |
| Methods | Parallel, double‐blind, double dummy, active‐controlled study Interventions administered 5 min after perineal repair and at 6 h | |
| Participants | Type of surgery: repair of episiotomy or perineal tear post vaginal delivery using cut and suturing Paracetamol group Entered/completing: 46/41 Age (mean, SD): 24.71 ± 4.91 Sex (male, %): 0 Control group Entered/completing: 49/41 Age (mean, SD): 24.71 ± 5.83 Sex (male, %): 0 | |
| Interventions | Paracetamol 1 g/100 ml via slow infusion q6 x 2 doses Dexketoprofen 50 mg IV also via slow infusion q6 x 2 doses | |
| Outcomes | Primary: VAS (0 to 10 0 to 100?) scores at 1 h Secondary: VAS at 6 and 12 h, adverse events. VAS at 2 and 3 also measured and reported, but not listed as outcomes. | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Only difference was gravidity (P value = 0.02). No significant differences in sociodemographic data or baseline parameters (parity, age, the length of the labour, birth weight, dose of the local anesthetic used during the repair of episiotomy or perineal tears (lidocaine) and VAS 0. | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Block randomization was achieved by using a computer‐generated random number chart” |
| Allocation concealment (selection bias) | Low risk | “Consecutive numbers generated by the computer were written on the numbered opaque envelopes, which were sealed by someone other than those involved in the study” |
| Blinding (performance bias and detection bias) | Unclear risk | Described as blinded, but no details other than that participants in both of the groups had also placebo injections mimicking the active drug |
| Incomplete outcome data (attrition bias) | Unclear risk | 1 participant in paracetamol group and 2 participants in dexketoprofen group not included in final analysis due to incomplete data. 4 participants in paracetamol group and 6 in dexketoprofen group excluded due to need for extra analgesic. Despite flow chart, unclear if these participants were excluded before or after receiving interventions. |
| Selective reporting (reporting bias) | Low risk | Adverse events assessed but reported that none occurred in any participant |
| Size | High risk | Fewer than 50 participants per arm of the study (46 paracetamol, 49 dexketoprofen) |
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled Medications administered prior to skin closure | |
| Participants | Type of surgery: elective total abdominal hysterectomy by laparotomy Paracetamol group Entered/completing: 30/27 Age (mean, SD): 47.73 ± 7.20 Sex (male, %): 0 Placebo group Entered/completing: 30/27 Age (mean, SD): 49.90 ± 6.40 Sex (male, %): 0 | |
| Interventions | Intervention: paracetamol 1 g/100 ml IV at skin closure Placebo: normal saline 100 ml Third group received paracetamol preemptively (not included in analysis) | |
| Outcomes | Pain intensity at rest and movement (VAS) Opioid consumption (morphine) All other outcomes reported at 24 h only | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, height, weight, ASA status, time of operation | |
| Details of preoperative pain | Not reported | |
| Notes | Preemptive group had lower morphine consumption versus postsurgical group at all time intervals post 2 to 4 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Same number of dropouts in both groups (n = 3), but reasons for dropouts not given |
| Selective reporting (reporting bias) | Low risk | Full reporting of primary outcome data, some secondary outcomes listed only as "no significant difference" |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 placebo) |
| Methods | Double‐blind, placebo and active‐controlled, single dose, over 24 h Interventions administered at end of procedure | |
| Participants | Type of surgery: thyroidectomy Paracetamol group Entered/completing: 20/20 Age (mean, SD): 49.2 ± 13.3 Sex (male, %): 10.0% Lornoxicam group Entered/completing: 20/20 Age (mean, SD): 43.9 ± 9.5 Sex (male, %): 20.0% Placebo group Entered/completing: 20/20 Age (mean, SD): 48.7 ± 12.3 Sex (male, %): 15.0% | |
| Interventions | Paracetamol 1 g over 10 min Lornoxicam 8 mg or placebo (100 ml NS) over 10 min | |
| Outcomes | Primary: analgesic consumption (tramadol, n/N) at 0 to 6, 6 to 12 and 12 to 24 h and mg total Secondary: VAS pain scores at 15 min, and 1, 2, 4, 6, 8, 12, 18, and 24 h postoperatively Time to first request for analgesia Adverse events | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | Patients using analgesics long‐term were excluded from the study | |
| Notes | Translated from Turkish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Withdrawal of a card. No further details. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | Appears that all participants completed the study and that all data were collected |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | 60 participants (20 paracetamol, 20 placebo, 20 lornoxicam) |
| Methods | Randomized, placebo‐controlled, single dose, over 24 h Interventions administered preemptively (not included in this review) or at end of surgery | |
| Participants | Type of surgery: elective lap cholecystectomy Paracetamol group Entered/completing: 100/100 Age (mean, SD): 41.5 ± 7.8 Sex (male, %): 32 Control group Entered/completing: 100/100 Age (mean, SD): 44.5 ± 6.5 Sex (male, %): 34 | |
| Interventions | Paracetamol 1 g/100 ml IV over 10 min Placebo: saline as above | |
| Outcomes | Primary: time to first rescue dose and cumulative amount of rescue analgesic (tramadol 100 mg IV for VAS pain score > 4, up to 400 mg max) Secondary: VAS pain scores at several time points up to 24 h, numbers of participants in each group requiring rescue medication within various postoperative intervals and cumulatively, adverse events, patient satisfaction at 24 h (0 = poor to 4 = excellent) | |
| Source of funding | No funding | |
| Were treatment groups comparable at baseline? | Yes | |
| Details of preoperative pain | None – participants with history of usage of paracetamol, opioids, or NSAIDs for 3 months were excluded | |
| Notes | Third group receiving paracetamol preemptively not included in this review. All participants received fentanyl 1 µg/kg at induction of anesthesia, which had a total duration of about 100 min in all groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (100 paracetamol, 100 placebo) |
| Methods | Randomized, double‐blind, placebo‐controlled Medications administered at the end of surgery | |
| Participants | Type of surgery: elective standard bipolar diathermy tonsillectomy Paracetamol group Entered/completing: 38/38 Age (mean, SD): 27 ± 4 Sex (male, %): 50 Placebo group Entered/completing: 38/38 Age (mean, SD): 25 ± 5 Sex (male, %): 47 | |
| Interventions | Intervention: paracetamol 1 g IV in 100 ml normal saline over 15 min Placebo: 100 ml normal saline | |
| Outcomes | Pain intensity at rest and on swallowing (VAS 0 to 100) Pain relief (defined as a VAS score of < 30 mm at rest and < 50 mm on swallowing Opioid consumption (pethidine) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes ‐ patient characteristics and duration of operation | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Nurse not involved in the study prepared the study solutions. Similar appearance of the study infusions assured blinding. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts; figures suggest that all participants reported data |
| Selective reporting (reporting bias) | Low risk | Primary outcome stated and fully reported. Stated secondary outcomes fully reported. |
| Size | High risk | Fewer than 50 participants per arm of the study (38 paracetamol, 38 placebo) |
| Methods | Randomized, placebo‐controlled, double‐blind Medications administered at the beginning of skin closure | |
| Participants | Type of surgery: orthopedic, abdominal, general, or gynecological surgery Propacetamol group Entered/completing: ?/275 Age (mean, SD): 44 (range 18 to 85) Sex (male, %): 46 Placebo group Entered/completing: ?/275 Age (mean, SD): 45 (18 to 72) Sex (male, %): 42 | |
| Interventions | Intervention: 2 g propacetamol over 15 min Placebo: 125 ml saline | |
| Outcomes | Morphine related AEs Opioid consumption (morphine) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: patient characteristics, type and duration of surgery, expected postoperative pain, ASA status and type of anesthesia | |
| Details of preoperative pain | Not mentioned, but anesthetists were asked to exclude patients who were expected to have no postoperative pain and those who were expected to have very severe postoperative pain requiring prolonged epidural and/or spinal postoperative analgesia or prolonged postoperative sedation and/or patient‐controlled analgesia (PCA). | |
| Notes | Minor protocol violation occurred in 80 (15%) participants. There were no significant differences between the 2 groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified according to centers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Each vial was prepared immediately before administration by a nurse who was not involved in the care or pain assessment of the patient. Vials containing 2 g of propacetamol (yielding 1 g of acetaminophen) or saline were administered IV over 15 min. |
| Incomplete outcome data (attrition bias) | Low risk | 3% of randomized participants were not included in analysis, but reasons for exclusion suggest outcome would be unaffected |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome, morphine‐related adverse events, reported in full, except for incidence of bronchospasm. All secondary outcomes reported, but VAS and pain relief only reported as "no significant difference" between groups. |
| Size | Low risk | >/= 200 participants in each arm of the study |
| Methods | Randomized, double‐blind, double‐dummy, active‐controlled Medications administered at the beginning of wound closure | |
| Participants | Type of surgery: inguinal hernia repair Propacetamol group Entered/completing: 90/90 Age (mean, SD): 46 ± 14 Sex (male, %): 94 Parecoxib group Entered/completing: 92/90 Age (mean, SD): 42 ± 14 Sex (male, %): 91 | |
| Interventions | Intervention: 2 g propacetamol over 15 min Control: parecoxib 40 mg IV | |
| Outcomes | Primary: opioid consumption (morphine) Pain intensity at rest and while coughing (VRS, VAS) and derived summary measures | |
| Source of funding | Pfizer SA | |
| Were treatment groups comparable at baseline? | Yes: intraoperative opioid consumption and time to tracheal extubation did not differ between groups; demographics (age, sex, weight, height) similar | |
| Details of preoperative pain | Patients with chronic pain excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization performed centrally as blocks of 4 and with a 2:2 treatment ratio. In each center, treatment allocation was performed on the basis of one complete treatment block. |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy design employed. Propacetamol was administered by slow infusion over 15 min whereas parecoxib was injected by rapid bolus. Each participant received both an active product and the placebo of the other product. |
| Incomplete outcome data (attrition bias) | Low risk | For primary outcome, data reported for both modified‐ITT population and per‐protocol population. Secondary outcomes reported data for ITT population. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported with mean data or raw numbers |
| Size | Unclear risk | 50 to 199 participants per arm of the study (90 propacetamol, 92 parecoxib) |
| Methods | Prospective, double‐blind, placebo‐ and active‐controlled study Multiple‐dose study, first dose administered 30 min before the end of surgery. Outcomes assessed for at least 48 h and up to 1 week in patients not discharged. | |
| Participants | Type of surgery: plastic surgery (breast surgery, inguinal or axillary dissections), oral and maxillofacial surgery (correction of retrognathism and prognathism), gynecological (laparoscopy, breast surgery), and urological (cystoscopy, transurethral prostatectomy) surgery and orthopedic surgery (hip endoprosthesis for coxarthrosis) Paracetamol group Entered/completing: 49/45 Age (mean, SD): 50.5 ± 17.5 Sex (male, %): 26.5% Dipyrone group Entered/completing: 49/41 Age (mean, SD): 45.5 ± 17.9 Sex (male, %): 44.9% Parecoxib group Entered/completing: 49/44 Age (mean, SD): 49.4 ± 14.6 Sex (male, %): 26.5% Placebo group Entered/completing: 49/45 Age (mean, SD): 42.8 ± 16.8 Sex (male, %): 49.0% | |
| Interventions | Paracetamol 1 g/100 ml NS over 15 min every 6 h for at least 48 h Dipyrone 1 g every 6 h, parecoxib 40 mg every 12 h (saline every 6 h between doses), or placebo (0.9% saline) every 6 h as above | |
| Outcomes | Primary: dynamic VAS (0 to 100) for pain localized to the site of surgery Secondary: time to first piritramide PCA bolus and piritramide consumption as quantified by the number of boluses demanded and administered; satisfaction rated as 1, excellent; 2, good; 3, moderate; 4, insufficient; and 5, poor; adverse events (respiratory depression, N/V, sedation, itching sweating) | |
| Source of funding | Bristol‐Myers Squibb, Munich, Germany, and Pfizer Pharma GmbH, Karlsruhe, Germany | |
| Were treatment groups comparable at baseline? | Yes, with 3 exceptions: participants of group 3 parecoxib had a significantly shorter duration of anesthesia and needed significantly less intraoperative sufentanil compared to group 4 placebo, and there were more women in group 1 paracetamol and group 3 parecoxib than in group 2 dipyrone and group 4 placebo | |
| Details of preoperative pain | Surgical area (0 to 100 VAS): paracetamol 9.2 ± 17.1, dipyrone 10.8 ± 17.2, parecoxib 13.3 ± 16.6, placebo 6.0 ± 13.2 | |
| Notes | Numbers completing based on number of participants discontinued after at least 2 doses of intervention. ITT analysis employed – no participants lost to follow‐up at 42 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “assigned by random numbers” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | “All study drugs were prepared by the hospital pharmacy in identical glass bottles as infusions of 100 ml. The bottles were labelled with patient number and time of administration. Infusions were administered by a blinded attending physician”. |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. ITT analysis performed, but no mention of how missing data imputed. |
| Selective reporting (reporting bias) | Unclear risk | Secondary outcome, patient satisfaction, reported as mean across all groups and statement that all participants were satisfied with their pain treatment. No mean data for each group and no details of what point on scale was defined as satisfied. |
| Size | High risk | Fewer than 50 participants per arm of the study (49 paracetamol, 49 dipyrone, 49 parecoxib, 49 placebo) |
| Methods | Randomized, double‐blind, placebo‐controlled, over 24 h Medications administered during skin closure | |
| Participants | Type of surgery: elective lumbar laminectomy and discectomy Paracetamol group Entered/completing: 20/20 Age (mean, SD): 41 ± 10 Sex (male, %): 60 Placebo group Entered/completing: 20/20 Age (mean, SD): 44 ± 10 Sex (male, %): 55 | |
| Interventions | Intervention: paracetamol 1 g IV over 15 min Placebo: 100 ml normal saline | |
| Outcomes | Pain intensity at rest or movement (VAS) Opioid consumption (morphine) All other outcomes reported at 24 h only | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographic data (sex, age, duration of surgery, intraoperative opioid use) and vital signs | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Placebo administered in same solution over same time period |
| Incomplete outcome data (attrition bias) | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo) |
| Methods | RCT, 60 participants were randomly divided into 2 groups, with 30 participants in each group Participants in Group I were treated with 2 g propacetamol 15 min before the end of operation | |
| Participants | Type of surgery: lumbar spine surgery Propacetamol group Entered/completing: 30 Age (mean, SD): 48, 13 Sex (male, %): 13, 43% Control group Entered/completing: 30 Age (mean, SD): 53, 14 Sex (male, %): 14, 47% | |
| Interventions | 2 g propacetamol in 100 ml saline, intravenous injection, 15 min before the end of operation Placebo: 100 ml saline, intravenous injection, 15 min before the end of operation | |
| Outcomes | The authors did not point out which were primary outcomes Vomiting frequency in 48 h after the operation VAS pain score (0 to 10), Ramsay sedation score(0, 1, 2, 3), breathing frequency, heart rate and mean arterial pressure were observed at the time of 0 min, 30 min, 1 h, 2 h, 4 h, 6 h, 12 h, 24 h, 36 h, 48 h after the operation | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: sex, age, weight, height | |
| Details of preoperative pain | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) | Low risk | Reported as no incomplete outcome data in the study |
| Selective reporting (reporting bias) | High risk | There were only comparisons of VAS pain scores and Ramsay sedation scores between the 2 groups as shown in Table 2 and 3 |
| Size | High risk | Fewer than 50 participants per arm of the study (30 propacetamol, 30 placebo) |
| Methods | Randomized, blinded, active‐controlled Medications administered on request in the PACU | |
| Participants | Type of surgery: thyroidectomy Propacetamol group Entered/completing: 40/40 Age (mean, SD): 46.9 ± 2.1 Sex (male, %): 15 Tramadol group Entered/completing: 40/40 Age (mean, SD): 44.1 ± 1.8 Sex (male, %): 10 | |
| Interventions | Intervention: 2 g IV propacetamol Control: tramadol 1.5 mg/kg | |
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VAS) | |
| Source of funding | SearΙe Continental Pharma Ιnc provided the trial drugs and statistical assistance | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia; intraoperative opioid use; nausea, vomiting, and drowsiness incidence pre‐interventions | |
| Details of preoperative pain | Not reported ‐ participants with chronic opioid use were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 40 tramadol) |
| Methods | Randomized, double‐blind, placebo‐controlled Medications administered in the recovery room | |
| Participants | Type of surgery: knee ligamentoplasty Propacetamol group Entered/completing: 30/29 Age (mean, SD): 25.5 ± 5.6 Sex (male, %): 97 Placebo group Entered/completing: 30/28 Age (mean, SD): 26.3 ± 5.8 Sex (male, %): 90 | |
| Interventions | Intervention: 2 g propacetamol in 125 ml 5% dextrose over 15 min Placebo: 125 ml 5% dextrose | |
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VAS 0 to 100 and 5‐point VRS) and derived summary measures Global efficacy (5‐point VRS) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Data presented for some outcomes from all participants, but for other outcomes from only those apparently completing the study |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (30 propacetamol, 30 placebo) |
| Methods | Randomized, blinded, placebo‐controlled Medications administered 30 min before extubation | |
| Participants | Type of surgery: coronary bypass surgery Paracetamol group Entered/completing: 22/? Age (mean, SD): unclear Sex (male, %): 77 Placebo group Entered/completing: 23/? Age (mean, SD): unclear Sex (male, %): 73 | |
| Interventions | Intervention: paracetamol 1 g/100 ml IV (every 6 h x 4 doses) Placebo: 100 ml normal saline | |
| Outcomes | Inspiratory volume Pain intensity (VRS) AEs not reported All other outcomes described over duration of study (18 h) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics | |
| Details of preoperative pain | Not reported | |
| Notes | Russian language study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Described as blinded, but no mention of whether single‐ or double‐blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Size | High risk | Fewer than 50 participants per arm of the study (22 paracetamol, 23 placebo) |
| Methods | Randomized, double‐blind, active‐controlled. Evaluated up to 24 h after surgery. Medications administered prior to skin closure | |
| Participants | Type of surgery: elective abdominal hysterectomy Paracetamol group Entered/completing: 40/40 Age (mean, SD): 49.9 ± 6.9 Sex (male, %): 0 Control group Entered/completing: 40/40 Age (mean, SD): 47.2 ± 7.2 Sex (male, %): 0 | |
| Interventions | Paracetamol 15 mg/kg in 100 ml NS, single dose over 15 min Ketamine 0.15 mg/kg administered as above | |
| Outcomes | Primary: pain (VAS 0 to 10) in recovery room and at 4, 6, 12 and 24 h postop Secondary: sedation (Ramsay scale), adverse events (nausea, vomiting, respiratory complications, hemodynamic changes), rescue analgesia (pethidine 15 mg for VAS pain > 3) | |
| Source of funding | Iran University of Medical Sciences | |
| Were treatment groups comparable at baseline? | Yes: demographics and duration of surgery | |
| Details of preoperative pain | Not reported, but participants currently using opioids were excluded | |
| Notes | Low dose of ketamine used in comparator group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | “Both medication solutions were prepared by the research pharmacist in 100 ml of normal saline and were administered by the anesthesia care team within a 15‐minute time period. The administering team was blinded to the nature of the infusate”. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts; tables suggest that all participants reported data |
| Selective reporting (reporting bias) | Low risk | All planned primary and secondary outcomes reported, although total amount of rescue analgesia not reported. Confirmed on trials registry (http://www.irct.ir/searchresult.php?id=11319&number=1). |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 ketamine) |
| Methods | Randomized, double‐blind, double‐placebo, placebo‐ and active‐controlled Medication administered when baseline pain reached at least moderate intensity | |
| Participants | Type of surgery: abdominal aortic repair Propacetamol group Entered/completing: 29/15 Age (mean, SD): 64.3 ± 2.2 Sex (male, %): 3 Dipyrone group Entered/completing: 30/21 Age (mean, SD): 62.4 ± 1.7 Sex (male, %): 20 Placebo group Entered/completing: 30/15 Age (mean, SD): 64.3 ± 1.8 Sex (male, %): 3 | |
| Interventions | Intervention: 2 g propacetamol over 2 min Control: 2.5 g dipyrone plus 0.01 g pitofenone IV Placebo: not described | |
| Outcomes | Pain intensity (5‐point VRS) Requirement for rescue analgesia (morphine) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics, baseline postoperative pain score | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | "Investigator who did not know the nature of the products, infused the test products to each patient according to their number of entry into the trial. The patients were then monitored in the recovery room over six hours by the same investigator who did not know the nature of the product administered". |
| Incomplete outcome data (attrition bias) | Unclear risk | Post‐rescue assessments imputed using LOCF; imbalance amongst groups in number of participants withdrawn due to requirement for rescue analgesia |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (29 propacetamol, 30 dipyrone, 30 placebo) |
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled Medications administered at skin closure (and repeated every 6 h x 48 h) | |
| Participants | Type of surgery: surgery of one herniated lumbar disc Propacetamol group Entered/completing: 15/14 Age (mean, SD): 41.8 ± 2.7 Sex (male, %): 53 Ketoprofen group Entered/completing: 15/14 Age (mean, SD): 49.7 ± 2.9 Sex (male, %): 53 Placebo group Entered/completing: 15/15 Age (mean, SD): 41.8 ± 2.4 Sex (male, %): 60 | |
| Interventions | Intervention: 2 g propacetamol in 125 ml dextrose 5% Control: ketoprofen 50 mg Control: combination of ketoprofen with propacetamol (not included in our analysis) Placebo: 125 ml dextrose 5% | |
| Outcomes | Pain intensity at rest and with movement (VAS) Sedation (4‐point categorical scale) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; preoperative pain; duration of surgery | |
| Details of preoperative pain | Incidence of preoperative leg and/or back pain similar between groups. Mean severity of preoperative pain similar (ranged from 42 to 51/100) between groups. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before the study began, a random number table was used to generate a randomized schedule specifying the group to which each patient would be assigned upon entry into the trial |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | All drugs were administered IV after dilution in 125 ml dextrose 5% labeled with the randomization number of the participant. Participants in all groups received 2 injections to assure blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Low attrition rate balanced amongst groups |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (15 propacetamol, 15 ketoprofen, 15 placebo) |
| Methods | Randomized, double‐blinded, placebo‐controlled Medications administered after surgery and immediately before extubation | |
| Participants | Type of surgery: laparoscopic sterilization Propacetamol group Entered/completing: 15/15 Age (mean, SD): 36 ± 4 Sex (male, %): 0 Placebo group Entered/completing: 16/16 Age (mean, SD): 37 ± 4 Sex (male, %): 0 | |
| Interventions | Intervention: 40 mg/kg propacetamol Control: 20 mg/kg propacetamol (not included in our analysis) Control: 10 mg/kg propacetamol (not included in our analysis) 1 g propacetamol was dissolved in 5 ml of contained solvent and administered as bolus Placebo: normal saline | |
| Outcomes | Opioid consumption (alfentanil via PCA) Postoperative pain at rest and with movement (10 cm VAS) | |
| Source of funding | SmithKline Beecham, Denmark supported the study. The infusion pumps were supplied by Baxter, Denmark. | |
| Were treatment groups comparable at baseline? | Yes: demographics and duration of anesthesia | |
| Details of preoperative pain | Patients were excluded if they had a history of chronic pain | |
| Notes | 40 mg/kg dose chosen in analysis as, based on participant weights, this would be closest dose to standard 2 g of propacetamol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | Drugs were administered by an anesthetist who had no further contact with the participant or study personnel |
| Incomplete outcome data (attrition bias) | Low risk | Minimal number of dropouts; it appears that all other data reported for remaining participants and at all relevant time points |
| Selective reporting (reporting bias) | Unclear risk | Time points not specified in Methods section for primary outcome; data pooled for different dosing regimens post 3 h |
| Size | High risk | Fewer than 50 participants per arm of the study (15 propacetamol, 16 placebo) |
| Methods | Randomized, placebo‐controlled Medications administered at end of surgery | |
| Participants | Type of surgery: lumbar disc surgery Propacetamol group Entered/completing: 20/20 Age (mean, SD): 38.3 ± 9.4 Sex (male, %): 80 Placebo group Entered/completing: 20/20 Age (mean, SD): 38.2 ± 10.8 Sex (male, %): 55 | |
| Interventions | Intervention: propacetamol 2 g over 20 min Placebo: saline | |
| Outcomes | Opioid consumption (piritramide on request) Pain intensity (VAS) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics and duration of surgery | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | It appears that all participants completed the study and contributed data for each outcome at all relevant time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 placebo) |
| Methods | Randomized, double‐blind, active‐controlled Medications administered immediately after induction of anesthesia (surgeries averaged around 30 min) | |
| Participants | Type of surgery: elective tonsillectomy Propacetamol group Entered/completing: 26/25 Age (mean, SD): 29 ± 11 Sex (male, %): 52 Diclofenac group Entered/completing: 25/25 Age (mean, SD): 27 ± 7 Sex (male, %): 44 | |
| Interventions | Intervention: 2 g propacetamol in 100 ml normal saline Control: 75 mg diclofenac Control: 2 g propacetamol plus 75 mg diclofenac (not included in our analysis) | |
| Outcomes | Opioid consumption (oxycodone) Pain intensity at rest and on swallowing (VRS, VAS) Patient satisfaction (VAS) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery, intraoperative opioid use; blood loss | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelope method |
| Blinding (performance bias and detection bias) | Low risk | Nurse preparing solutions did not participate in the study. To maintain double‐blind design volumes infused were equal. |
| Incomplete outcome data (attrition bias) | Unclear risk | Various reasons for dropouts amongst groups, data analyzed in completers only, but minimal missing data |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not specified in Methods section; possibility of additional post‐hoc analyses cannot be ruled out |
| Size | High risk | Fewer than 50 participants per arm of the study (26 propacetamol, 25 diclofenac) |
| Methods | Randomized, double‐blind, placebo‐controlled, over 24 h Medications administered during skin closure | |
| Participants | Type of surgery: major spine (posterior and/or anterior correction) Paracetamol group Entered/completing: 18/18 Age (mean, SD): 15.1 ± 2.0 Sex (male, %): 5, 27.8% Placebo group Entered/completing: 18/18 Age (mean, SD): 14.4 ± 1.9 Sex (male, %): 6, 33.3% | |
| Interventions | Paracetamol 30 mg/kg IV (3 ml/kg) over 15 min, max dose 1.5 g, q8h x 3 doses Placebo (NS) as above | |
| Outcomes | Primary: pain scores at rest on the surgical ward q1h for 24 h as the highest VAS 0 to 10 score during the preceding hour Secondary: time to first and total PCA oxycodone dose; supplemental analgesia (for VAS ≥ 6 oxycodone 0.05 mg/kg IV, if VAS score still ≥ 6, parecoxib 20 to 40 mg IV); adverse events (nausea, vomiting, and pruritus); sedation (Michigan sedation scale: 0, awake; 4, unresponsive to painful stimulus); plasma levels of acetaminophen and metabolites | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia; intraoperative analgesia; blood loss; spinal pathology and type of procedure | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | “An unblinded anesthesia nurse who did not participate in peri‐ or postoperative care opened an envelope and prepared the study medications (acetaminophen or placebo)”. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis (method of imputation not specified); reasons for protocol violations specified and similar between groups, and results reported to be similar if these participants were excluded. |
| Selective reporting (reporting bias) | High risk | All outcomes from Methods section reported in Results section (other than number of participants with pruritus), but many only presented graphically and without SDs |
| Size | High risk | Fewer than 50 participants per arm of the study (18 paracetamol, 18 placebo) |
| Methods | Randomized, double‐blinded, double‐dummy, placebo‐ and active‐controlled Medication administered on postoperative day 1, when baseline pain reached moderate‐to‐severe intensity | |
| Participants | Type of surgery: total hip arthroplasty Propacetamol group Entered/completing: 40/40 Age (mean, SD): 65.7 ± 9.8 Sex (male, %): 40 Diclofenac group Entered/completing: 40/40 Age (mean, SD): 65.6 ± 7.6 Sex (male, %): 55 Placebo group Entered/completing: 40/40 Age (mean, SD): 66.1 ± 7.1 Sex (male, %): 45 | |
| Interventions | Intervention: 2 g propacetamol IV Control: 75 mg diclofenac IM Placebo: double‐dummy not described | |
| Outcomes | Pain intensity (VRS, VAS) Pain relief (categorical) Time to request for rescue medication Global assessment (categorical) | |
| Source of funding | Supported by Bristοl‐Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of anesthesia; baseline postoperative pain | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy technique employed |
| Incomplete outcome data (attrition bias) | Unclear risk | 11/40 missing pain assessment data at 5 h in intervention group due to lack of efficacy and administration of rescue dose (29/40 in placebo group), but all 40 included in efficacy analysis. Data were imputed using LOCF. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section, but time to rescue was instead reported as number of participants requesting rescue |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 40 diclofenac, 40 placebo) |
| Methods | Parallel, active‐controlled, randomized, double‐blind, single dose over 24 h | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 25/unclear Age (mean, SD): 30.6 ± 4.23 Sex (male, %): 0 Pethidine group Entered/completing: 25/unclear Age (mean, SD): 29.6 ± 3.51 Sex (male, %): 0 | |
| Interventions | Paracetamol 1 g/100 ml single dose over 15 min, 30 min before end of surgery pethidine 100 mg IV as above | |
| Outcomes | Primary: VAS pain intensity at 0, 1, 5, 30 min and 1, 2, 4, 6, 8 and 24 h after surgery Secondary: Side effects Total rescue analgesic use (unspecified) over 24 h for pain > 7/10 | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, height, weight, duration of surgery, duration of anesthesia | |
| Details of preoperative pain | Participants with chronic abdominal pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) | High risk | Unclear how many participants completed the study; mean pain data did not have SDs; 24 h rescue analgesic use did not specify analgesic administered |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section were reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 pethidine) |
| Methods | Randomized, double‐blind, placebo‐controlled single dose study evaluated 6 h postop Study entry occurred the day after surgery | |
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 16/16 Age (mean, SD): 74.6 (5.7) Sex (male, %): 8 (50%) Placebo Entered/completing: 17/17 Age (mean, SD): 73.9 (6.2) Sex (male, %): 5 (29.4%) | |
| Interventions | Paracetamol: 1000 mg IV as a single dose Placebo Each arm had free access to PCA (details not specified including if it could be different opioids in PCA) | |
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID6 Secondary: adverse events | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. Participants were required to have moderate pain the day after surgery. Baseline PI scores were not statistically different between groups (VAS 0 to 100). Details regarding drugs used/dosing of opioid PCA for all participants are lacking. (“…each arm having free access to PCA opioids.”) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (16 paracetamol, 17 placebo) |
| Methods | Randomized, double‐blind, placebo‐controlled, single dose study evaluated 6 h postop Study entry occurred the day after surgery. | |
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 19/19 Age (mean, SD): 52.6 (7.9) Sex (male, %): 9 (47.4%) Placebo Entered/completing: 17/17 Age (mean, SD): < 65: 57.2 (6.4) Sex (male, %): 8 (47.1%) | |
| Interventions | Paracetamol: 1000 mg IV as a single dose Placebo Each arm had free access to PCA (details not specified including if it could be different opioids in PCA) | |
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID6 Secondary: adverse events | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. Participants were required to have moderate pain the day after surgery. Baseline PI scores were not statistically different between groups (VAS 0 to 10). Details regarding drugs used/dosing of opioid PCA for all participants are lacking. (“…each arm having free access to PCA opioids.”) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (19 paracetamol, 17 placebo) |
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter repeated dose study evaluated up to 16 h postop Study entry occurred the day after surgery. Participants were required to have moderate postop pain for eligibility | |
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 15/15 Age (mean, SD): 71.4 +/‐ 4.7 Sex (male, %): 9 (60%) Placebo Entered/completing: 12/12 Age (mean, SD): 68.4 +/‐ 3.5 Sex (male, %): 6 (50%) | |
| Interventions | Paracetamol: 1000 mg IV administered at 0, 4, 10, 16 h Placebo | |
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID4, patient satisfaction Secondary: adverse events | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Only published as an abstract. Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 12 placebo) |
| Methods | Randomized, double‐blind, placebo‐controlled, multicenter repeated dose study evaluated up to 16 h postop Study entry occurred the day after surgery. Participants were required to have moderate postop pain for eligibility. | |
| Participants | Type of surgery: orthopedic (THA) Paracetamol group Entered/completing: 15/unclear Age (mean, SD): 54.1 +/‐ 6.2 Sex (male, %): 11 (73.3%) Placebo Entered/completing: 19/unclear Age (mean, SD): 53.4 +/‐ 9.3 Sex (male, %): 14 (73.7%) | |
| Interventions | Paracetamol: 1000 mg IV administered at 0, 4, 10, 16 h Placebo | |
| Outcomes | Primary: pain relief, pain intensity, total rescue medication, median time to rescue, SPID4, patient satisfaction Secondary: adverse events | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height, ASA classification, baseline PI | |
| Details of preoperative pain | Not reported | |
| Notes | Only published as an abstract. Study terminated early due to an issue unrelated to efficacy or safety of the interventions. Precipitates were found in the placebo vials. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Not described; reported as double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis: WOCF if a participant was given rescue medication within the first 4 h after dosing LOCF if a participant missed the 4‐hour mean PI assessment and had not received rescue medication or if a participant terminated the study due to an adverse event Extrapolation if a participant missed one mean PI assessment and no rescue medication was received |
| Selective reporting (reporting bias) | Unclear risk | Reported all outcomes but no data presented for pain relief |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 19 placebo) |
| Methods | Randomized, double‐blind, placebo‐ and active‐controlled Medication administered immediately after surgery in patients with at least moderate pain | |
| Participants | Type of surgery: hallux valgus Propacetamol group Entered/completing: 108/108 Age (mean, SD): 52.2 ± 13.0 Sex (male, %): 11 Placebo group Entered/completing: 109/109 Age (mean, SD): 51.9 ± 13.6 Sex (male, %): 8 | |
| Interventions | Intervention: propacetamol 2 g in 125 ml dextrose 5% over 15 min Control: oral paracetamol 1 g (not included in our analysis) Placebo: 125 ml dextrose 5% and tablet | |
| Outcomes | Pain intensity (categorical) and derived pain intensity difference, SPID and maximum pain intensity difference Time to rescue medication Global evaluation (categorical) | |
| Source of funding | Supported by UPSA Laboratories | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery; baseline postoperative pain | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Inadequately described |
| Incomplete outcome data (attrition bias) | Unclear risk | Participants requesting rescue medication had LOCF in efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (108 propacetamol, 109 placebo) |
| Methods | Randomized, double‐blind, double‐dummy, active‐ and placebo‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 6 h of surgery | |
| Participants | Type of surgery: third molar extraction Paracetamol group Entered/completing: 132/132 Age (mean, SD): 25.0 ± 2.6 Sex (male, %): 41 Placebo group Entered/completing: 33/33 Age (mean, SD): 25.2 ± 2.8 Sex (male, %): 55 | |
| Interventions | Intervention: IV paracetamol 1 g Control: IV paracetamol 2 g (not included in our analysis) Placebo: 100 ml solution All interventions administered in 100 ml solution for each 1 g of paracetamol (or placebo) over 15 min | |
| Outcomes | Pain relief (VAS and VRS) and derived TOTPAR Pain intensity (VAS and VRS) Time to request of rescue medication Global evaluation (categorical) | |
| Source of funding | Supported by Bristol‐Myers Squibb Company | |
| Were treatment groups comparable at baseline? | Yes: demographics; ASA classification; number of teeth removed; baseline postoperative pain intensity; surgical trauma No: longer duration of surgery in the IV paracetamol 1 g group in comparison with the IV paracetamol 2 g and placebo groups | |
| Details of preoperative pain | Participants with other painful physical conditions that might confound pain assessment were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization 4:4:1, each block n = 9 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | A double‐dummy method was used to assure double‐blinding |
| Incomplete outcome data (attrition bias) | Unclear risk | ITT analysis using LOCF. Unclear how many participants had data imputed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section, but SPID was only calculated using categorical pain intensity despite being measured with both categorical and VAS scales |
| Size | Unclear risk | 50 to 199 participants per arm of the study (132 paracetamol, 33 placebo) |
| Methods | Randomized, parallel, active‐controlled trial; multiple doses evaluated for 24 h | |
| Participants | Type of surgery: cesarean sections and gynecological surgeries Paracetamol group Entered/completing: 51/50 Age (mean, SD): not reported Sex (male, %): 100% female Butorphanol group Entered/completing: 50/50 Age (mean, SD): not reported Sex (male, %): 100% female | |
| Interventions | Paracetamol: 1 g IV every 8 h Butorphanol 2 mg IV every 12 h | |
| Outcomes | Primary: pain intensity Secondary: administration of rescue medication (tramadol), timing of rescue medication, adverse effects | |
| Source of funding | ICMR under STS program | |
| Were treatment groups comparable at baseline? | Not reported | |
| Details of preoperative pain | Not reported | |
| Notes | Poster presentation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described; due to the fact that the study was likely unblinded we categorized this as high risk also |
| Blinding (performance bias and detection bias) | High risk | Not described; assume to be unblinded |
| Incomplete outcome data (attrition bias) | Unclear risk | One participant was not accounted for in the presentation of graphical results |
| Selective reporting (reporting bias) | Unclear risk | Timing of rescue medication was not presented in Results. No data for pain scores reported. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (51 paracetamol, 50 placebo) |
| Methods | Randomized, double‐blind, active‐controlled Medications administered 30 min before the end of surgery | |
| Participants | Type of surgery: breast cancer (breast conserving or total mastectomy, balanced between groups) Propacetamol group Entered/completing: 20/20 Age (mean, SD): 52 ± 10.2 Sex (male, %): 0 Dipyrone group Entered/completing: 20/20 Age (mean, SD): 55.9 ± 8.7 Sex (male, %): 0 | |
| Interventions | Intervention: 1 g propacetamol in 100 ml solution over 15 min Control: 1 g dipyrone | |
| Outcomes | Pain intensity at rest and on coughing (VAS) Opioid consumption (piritramide via PCA) Patient satisfaction (categorical) | |
| Source of funding | In part supported by a grant from BristoΙ‐Myers Squibb GmbFΙ, München, Germany, with publication support provided by the Department of Anaesthesiology, University of Cologne | |
| Were treatment groups comparable at baseline? | Yes: demographics, type of procedure | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on a computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Randomization results were sealed in sequentially numbered, opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | The infusions were made to look identical; participants and investigators were blinded to the study treatment |
| Incomplete outcome data (attrition bias) | Low risk | "The data for all patients were eligible for statistical analysis." |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 dipyrone) |
| Methods | Randomized, active‐controlled study, multiple dose, evaluated up to 2 days postop Medication administered at the end of the operation, without being contingent upon pain intensity Rescue medication (meperidine/pethidine 1 mg/kg IM) given to both groups as rescue medication for VAS > 4 | |
| Participants | Type of surgery: trans‐urethral resection of prostate Paracetamol group Entered/completing: 25/25 Age (mean, SD): only median age reported (64.3) Sex (male, %): 25 (100%) Diclofenac group Entered/completing: 25/25 Age (mean, SD): only median reported (66.8) Sex (male, %): 25 (100%) | |
| Interventions | Paracetamol 1 g/100 ml IV over 15 min twice daily Diclofenac IM 75 mg at the end of the operation, followed by 75 mg IM for 24 h. Time interval between first two 75 mg doses not reported, but the authors describe this regimen as 150 mg once per day = 150 mg per 24 h. | |
| Outcomes | Primary: pain intensity (VAS) Secondary: hemoglobin levels, hemostatic variables (bleeding time PT, INR), adverse effects, rescue opioid use (pethidine) | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery; transrectal ultrasound volume | |
| Details of preoperative pain | Similar at baseline | |
| Notes | No statistically/clinically significant differences in postoperative hemoglobin, hemostatic parameters or bleeding events between placebo, paracetamol, and diclofenac groups. Diclofenac dosing (2 x 75 mg given in presumably quick succession, then repeated as a 150 mg dose 24 h later) is highly idiosyncratic, high. This regimen could bias results towards a greater diclofenac effect in the first portion of the 24 h dosing interval, and a lesser effect towards the end, compared with a more conventional dosing regimen of 75 mg IM every 12 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described. Assessed as high risk based on assumption of non‐blinding. |
| Blinding (performance bias and detection bias) | High risk | Not described; assume to be unblinded |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or protocol violations |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section; opioid use reported in Results but not specifically mentioned in Methods. No SD reported for VAS data. |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 diclofenac) |
| Methods | Randomized, double‐blind, active‐controlled study, multiple dose, 24 h Medication was administered at the end of surgery after skin closure | |
| Participants | Type of surgery: ENT surgery (nasal/sinus, otologic, head/neck) Paracetamol group Entered/completing: 30/30 Age (mean, SD): 48.5 +/‐ 12.1 Sex (male, %): 16 (53%) Dexketoprofen Entered/completing: 30/30 Age (mean, SD): 54.8 +/‐ 8.6 Sex (male, %): 16 (53%) | |
| Interventions | Paracetamol: 1 g IV at the end of surgery then at 6, 12, 18 h (4 g total) Dexketoprofen: 50 mg IV at the end of surgery then repeated twice at an 8‐h interval (150 mg total) Metamizol: 1 g IV at the end of surgery then repeated twice at an 8‐h interval (3 g total, not included in our analysis) | |
| Outcomes | Primary: VAS (0 to 10) and VRS (0 to 3) pain intensity Secondary: adverse events, sedation score, use of rescue medication (pethidine 1 mg/kg for VAS ≥ 30 mm) | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery | |
| Details of preoperative pain | Not reported ‐ participants were excluded if they had received analgesics within 12 h before surgery | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Participants and anesthetists were unaware of treatment assignments. All outcome measurements were recorded by the same anesthesia resident who was blinded to assignments. “All medicines were prepared by a nurse who had no other involvement in the study” |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or protocol violations – complete data set obtained for all groups |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section although no data provided for sedation assessment |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 dexketoprofen) |
| Methods | Randomized, double‐blind, placebo‐controlled Medications administered at completion of surgery over 15 min | |
| Participants | Type of surgery: endoscopic sinus Paracetamol group Entered/completing: 36/36 Age (mean, SD): not reported Sex (male, %): not reported Placebo group Entered/completing: 38/38 Age (mean, SD): not reported Sex (male, %): not reported | |
| Interventions | Intervention: paracetamol 1 g IV Placebo: 100 ml normal saline | |
| Outcomes | Pain intensity (NRS) Time to rescue medication Opioid consumption (oxycodone) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No details | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Low risk | Opaque envelope method |
| Blinding (performance bias and detection bias) | Low risk | The nurse preparing the infusions did not participate in the study. Preparation of infusions of identical volumes (100 ml) to assure blinding. |
| Incomplete outcome data (attrition bias) | Low risk | "All the patients asked agreed to participate and there were no dropouts during the study". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (36 paracetamol, 38 placebo) |
| Methods | Randomized, double‐blind, active‐controlled Medications administered directly before skin closure | |
| Participants | Type of surgery: renal transplant Paracetamol group Entered/completing: 15/15 Age (mean, SD): 40.47 ± 11.2 Sex (male, %): 53 Morphine group Entered/completing: 15/15 Age (mean, SD): 40.2 ± 11.6 Sex (male, %): 60 | |
| Interventions | Intervention: propacetamol 2 g IV over 10 min Control: morphine 5 mg IV | |
| Outcomes | Pain intensity (VRS) Pain relief (VRS) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No statistical analysis, but groups appear balanced for demographics | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Anesthesiologist blinded to the drug administered assessed pain score, blood pressure, heart rate, lab tests, etc. No description of blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Table suggests that all participants (15 in each group) reported data at all time points |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 15 morphine) |
| Methods | Randomized, double‐blind placebo‐controlled, single dose, over 24 h IV acetaminophen was administered before skin closure versus a control group that received normal saline as placebo; preemptive group receiving 15 mg/kg 0.5 h preoperatively, not reported here | |
| Participants | Type of surgery: orthopedic, lower extremity Paracetamol group Entered/completing: 25/25 Age (mean, SD): 36.8 +/‐ 14.8 Sex (male, %): 21 (84%) Placebo group Entered/completing: 25/25 Age (mean, SD): 37.8 +/‐ 12.9 Sex (male, %): 17 (68%) | |
| Interventions | Paracetamol: 15 mg/kg in 100 ml of IV normal saline prior to skin closure Placebo (normal saline) 100 ml prior to skin closure | |
| Outcomes | Primary: pain intensity according to VRS Secondary: Timing, # participants requesting and dose of rescue medication (pethidine) Adverse effects (sedation, hypotension, etc.) | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery; site of surgery; postoperative VRS scores | |
| Details of preoperative pain | Patients with a history of opioid use in the past 48 h or chronic pain were excluded; baseline pain scores not significantly different | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized (random number generator) |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Participants and anesthesiologists were blinded by “creating treatments that looked identical” |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts or protocol violations – complete data set obtained for both groups |
| Selective reporting (reporting bias) | High risk | Not all outcomes from Methods section reported in Results section (e.g., sedation scores, patient satisfaction) |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 placebo) |
| Methods | Parallel, single dose, active comparator, quasi‐randomized, double‐blinded over 4 h Dose administered just before reversal of general anesthesia | |
| Participants | Type of surgery: diagnostic knee arthroscopic procedures Paracetamol group Entered/completing: 43/unclear (appears to be 43 from Figures) Age (mean, SD): range (entire population) 18 to 69 years Sex (male, %): 90% (entire population) Morphine group Entered/completing: 41/unclear (appears to be 41 from Figures) Age (mean, SD): see above Sex (male, %): see above | |
| Interventions | Paracetamol 1 g IV over 15 min Morphine 0.1 mg/kg IV bolus | |
| Outcomes | Primary: VRS pain intensity at 0, 1, 2, 3, and 4 h Secondary: adverse effects | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No data presented | |
| Details of preoperative pain | Not reported | |
| Notes | Both interventions given along with 0.5% bupivacaine 20 ml intra‐articular injection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization via last number of medical record number, with odd receiving paracetamol and even receiving morphine |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding (performance bias and detection bias) | Unclear risk | Study mentioned once it was double‐blinded without further description |
| Incomplete outcome data (attrition bias) | High risk | No SDs for pain scores, unclear how many participants completed study |
| Selective reporting (reporting bias) | Low risk | All outcomes in Methods section reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (43 paracetamol, 41 morphine) |
| Methods | Paracetamol Randomized, placebo‐controlled, multiple dose study evaluated 24 h postop Medication was administered 15 min before the end of surgery | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 25/25 Age (mean, SD): 28.8 +/‐ 4.8 Sex (male, %): 0 (100% female) Placebo Entered/completing: 25/25 Age (mean, SD): 27.6 +/‐ 5.4 Sex (male, %): 0 (100% female) | |
| Interventions | Paracetamol: 1 g in 100 ml 15 min before the end of surgery and every 6 h x 24 h Placebo: saline 100 ml 15 min before the end of surgery and every 6 h x 24 h All participants received IV PCA (tramadol) – 20 mg bolus; 10 min lockout | |
| Outcomes | Primary: pain score (VAS 0 to 10), tramadol consumption Secondary: sedation scores, nausea/vomiting scores, adverse effects | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia; weeks pregnant | |
| Details of preoperative pain | Participants with chronic pain were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding (performance bias and detection bias) | Unclear risk | Anaesthesiologists and participants were blinded. No other description provided. |
| Incomplete outcome data (attrition bias) | Low risk | Complete data provided for all participants. No one was excluded from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in Methods are discussed in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 placebo) |
| Methods | Randomized, double‐blind, controlled, multiple dose study; evaluated outcomes over at least 3 days. Medication was administered immediately after surgery upon arrival in the PACU. | |
| Participants | Type of surgery: hip replacement or surgery of the femoral shaft Paracetamol group Entered/completing: 27/25 Age (mean, SD): 76.7 +/‐ 8.9 Sex (male, %): 15 (55.6%) Parecoxib group Entered/completing: 28/25 Age (mean, SD): 76 +/‐ 8 Sex (male, %): 11 (39.3%) Placebo group (saline) Entered/completing: 28/25 Age (mean, SD): 76.7 +/‐ 8.6 Sex (male, %): 12 (42.9%) | |
| Interventions | Paracetamol: IV infusion of 1000 mg paracetamol (Perfalgan) over 10 min; admin at 6‐h intervals for at least 3 days Parecoxib: 40 mg IV over 10 min (Dynastat); admin at 12‐h intervals for at least 3 days Placebo: saline IV over 10 min | |
| Outcomes | Primary: renal function: blood samples (serum Cystatin C, creatinine, blood urea nitrogen, liver biochemistry); urine samples (creatinine clearance, urinary excretion of sodium, potassium, albumin, alpha1‐microglobulin; fluid balance (CVP) Secondary: pain intensity (NRS 0 to 10), rescue medication usage including morphine equianalgesic dosages | |
| Source of funding | Supported by Bristol‐Meyers Squibb | |
| Were treatment groups comparable at baseline? | “All groups were comparable with regard to age, weight, height, distribution of sex, preexisting diseases, and ASA status…. Type and lengths of surgical and anesthetic procedures across the treatment groups were similar… Furthermore, consumption of crystalloids and colloids were similar.” | |
| Details of preoperative pain | Not reported | |
| Notes | “If a patient had received NSAIDs or COX‐2 inhibitors, there was a washout period of at least 72 h and the weak opioid, tramadol, was provided as a substitute.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization of the study medication (parecoxib versus paracetamol versus saline) was performed by computer‐generated codes maintained in sequentially numbered, opaque envelopes. Additional envelopes were provided if participants had to be excluded after recruitment and randomization.” |
| Allocation concealment (selection bias) | High risk | Despite use of sequentially numbered envelopes, participants and nursing staff on ward were unblinded |
| Blinding (performance bias and detection bias) | High risk | Anesthesiologist, nursing staff, and investigators were blinded. All study medication solutions were prepared by a hospital pharmacist who was not involved in the data collection. On the ward, participants and nursing staff were unblinded. How blinding was maintained was not described. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. All participants that did not complete the study were accounted for. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (27 paracetamol, 28 parecoxib, 28 placebo) |
| Methods | Randomized, double‐blind, active‐ and placebo‐controlled, multiple dose, 24 h Medications administered at time of wound closure | |
| Participants | Type of surgery: lumbar disc Paracetamol group Entered/completing: 20/20 Age (mean, SD): 46.0 ± 11.0 Sex (male, %): 11, 55% Lornoxicam group Entered/completing: 20/20 Age (mean, SD): 46.7 ± 12.8 Sex (male, %): 12, 60% Placebo group Entered/completing: 20/19 Age (mean, SD): 44.5 ± 14.4 Sex (male, %): 9, 47.4% | |
| Interventions | Paracetamol 1 g in 100 ml NS over 15 min every 6 h Metamizole 1 g (not included in our analysis), lornoxicam 8 mg or saline. All administered as above (lornoxicam every 12 h). Placebo: normal saline 100 ml infused over 15 min every 6 h | |
| Outcomes | Primary: VAS (0 to 10) pain intensity over 24 h Secondary: sedation (Ramsay score); morphine consumption via PCA, other adverse events, vital signs | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; smoking status; history of postoperative nausea and vomiting; duration of anesthesia; type of surgery; number of herniated discs; experience of surgeon | |
| Details of preoperative pain | Not reported | |
| Notes | All participants received morphine PCA 100 mg in 100 ml normal saline for 24 h postop (1 mg bolus, lockout 7 min) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | “The study solutions were prepared by a nurse, whereas postoperative data were collected by a blinded anaesthesiologist. The colour of lornoxicam solution is yellow; to maintain blinding, all solutions were covered by aluminium foil during administration”. |
| Incomplete outcome data (attrition bias) | Low risk | Per‐protocol analysis only, but number of dropouts small and apparently unrelated to interventions |
| Selective reporting (reporting bias) | High risk | Adverse events only reported as similar between groups, with no accompanying data. Morphine requirements were reported in Results and not specifically mentioned in Methods. Blood pressure, heart rate, and sedation not reported in Results. |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 lornoxicam, 20 placebo) |
| Methods | Randomized, double‐blinded, placebo‐controlled Medications administered immediately after arrival in the PACU | |
| Participants | Type of surgery: cardiac Propacetamol group Entered/completing: unclear/40 Age (mean, SD): 59 ± 6 Sex (male, %): 85 Placebo group Entered/completing: unclear/39 Age (mean, SD): 58 ± 7 Sex (male, %): 90 | |
| Interventions | Intervention: 2 g propacetamol in 100 ml normal saline Placebo: 100 ml normal saline | |
| Outcomes | Opioid consumption (oxycodone via PCA and rescue) Pain intensity at rest and during deep breath (VAS) Patient satisfaction (categorical) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of anesthesia and surgery; intraoperative opioid use | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers and a balanced design with a computer program |
| Allocation concealment (selection bias) | Low risk | Randomization/blinding performed in pharmacy |
| Blinding (performance bias and detection bias) | Low risk | The propacetamol and placebo ampoules were supplied in identical packages. The code remained blinded until the end of the study. |
| Incomplete outcome data (attrition bias) | Unclear risk | 9/88 participants withdrew for various reasons ‐ unclear which arm participants withdrew from and if this was after receiving intervention |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 39 placebo) |
| Methods | Randomized, double‐blinded, placebo‐ and active‐controlled Medications administered 30 min before arrival in the recovery area | |
| Participants | Type of surgery: retinal Propacetamol group Entered/completing: 12/12 Age (mean, SD): 52 ± 18 Sex (male, %): 67 Metamizole group Entered/completing: 13/13 Age (mean, SD): 60 ± 19 Sex (male, %): 31? (data reported in error) Placebo group Entered/completing: 13/13 Age (mean, SD): 58 ± 22 Sex (male, %): 69 | |
| Interventions | Intervention: 1 g paracetamol in 100 ml over 15 min Active control: 1 g metamizol Placebo: 100 ml normal saline | |
| Outcomes | Pain intensity at rest and on coughing (VRS, VAS) Opioid consumption (tilidine) Patient satisfaction (categorical) | |
| Source of funding | The study was in part financed by a grant from Bristol‐Myers Squibb GmbH, München, Germany | |
| Were treatment groups comparable at baseline? | Yes: demographics | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Code prepared at a remote site and sealed in sequentially numbered, opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Infusions were made to look identical |
| Incomplete outcome data (attrition bias) | Low risk | 5 participants in placebo group had incomplete data, imputed by LOCF. It appears that all other participants contributed data at all time points for all outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section. Some adverse event data listed only as P values. |
| Size | High risk | Fewer than 50 participants per arm of the study (12 propacetamol, 13 metamizole, 13 placebo) |
| Methods | Randomized, controlled, single dose study evaluating outcomes 6 h postop Medication was administered 30 min before the end of surgery | |
| Participants | Type of surgery: thyroidectomy Paracetamol group Entered/completing: 20/20 Age (mean, SD): 44.7 +/‐ 7.3 Sex (male, %): 0 Control (normal saline) Entered/completing: 20/20 Age (mean, SD): 46.3 +/‐ 9.5 Sex (male, %): 0 Ketorolac Entered/completing: 20/20 Age (mean, SD): 46.1 +/‐ 9.9 Sex (male, %): 0 | |
| Interventions | Paracetamol: 1 g IV over 15 min administered 30 min before the end of surgery Normal saline; ketorolac 30 mg; paracetamol 700 mg/morphine 3 mg (not included in our analysis). All administered 30 min before end of surgery. | |
| Outcomes | Primary: degree of pain (VAS 0 to 10) Secondary: side effects, respiratory depression, degree of satisfaction, incidence of rescue medication | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: demographics; duration of surgery and anesthesia | |
| Details of preoperative pain | Participants excluded if medicated with drugs that could affect analgesic effect before the operation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Anesthesiologists were blinded. Manuscript did not specifically state if participants were blinded. Also, no description of blinding methods. |
| Incomplete outcome data (attrition bias) | Low risk | No dropouts ‐ complete data set obtained for all participants. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from Methods section reported in Results section. Incidence of rescue medication reported in Results but not mentioned in Methods. |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo, 20 ketorolac) |
| Methods | Randomized, double‐blind, active‐controlled study Medications administered 15 min before discontinuation of anesthesia | |
| Participants | Type of surgery: functional endoscopic sinus Propacetamol group Entered/completing: 25/25 Age (mean, SD): 32 ± 10 Sex (male, %): 72 Parecoxib group Entered/completing: 25/25 Age (mean, SD): 34 ± 12 Sex (male, %): 76 | |
| Interventions | Intervention: 2 g propacetamol over 15 min Control: 40 mg IV parecoxib | |
| Outcomes | Pain intensity (VAS) and derived SPID Opioid consumption (morphine) Patient satisfaction (categorical) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics; type of procedure. Intraoperative and postoperative hemodynamic variables also reported to be similar, but no data shown. | |
| Details of preoperative pain | Participants with chronic pain requiring major analgesics, sedatives, or corticosteroids were excluded | |
| Notes | Participants had only mild pain at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Study drugs mixed by physician not involved in study. Double‐dummy technique employed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Analyses on ITT population, but no mention of imputation method |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (25 propacetamol, 25 parecoxib) |
| Methods | Randomized, double‐blinded, double‐dummy, active‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity | |
| Participants | Type of surgery: thoracic and abdominal elective Propacetamol group Entered/completing: 20/20 Age (mean, SD): unclear Sex (male, %): unclear Pethidine group Entered/completing: 20/20 Age (mean, SD): unclear Sex (male, %): unclear | |
| Interventions | Intervention: 2 g propacetamol in 100 ml saline Control: 50 mg pethidine IM | |
| Outcomes | Pain intensity (VAS, VRS) and derived SPID Pain relief (VAS, VRS) and derived TOTPAR Time to onset and duration of analgesia Global evaluation (categorical) | |
| Source of funding | Unclear ‐ one author was an employee of Squibb Pharmaceuticals | |
| Were treatment groups comparable at baseline? | Yes: demographics, disease categories, operation categories, anesthesia methods and duration, vital signs, hepatorenal function, and blood cell count | |
| Details of preoperative pain | Unclear | |
| Notes | Chinese language article with abstract and data in English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Allocation concealment (selection bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy technique |
| Incomplete outcome data (attrition bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Selective reporting (reporting bias) | Unclear risk | Chinese article ‐ unable to ascertain |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 pethidine) |
| Methods | Randomized, double‐blind, placebo‐controlled multiple dose, 24‐hour study Medication was administered 30 min after extubation | |
| Participants | Type of surgery: percutaneous nephrolithotomy Paracetamol group Entered/completing: 50/50 Age (mean, SD): 44.48 +/‐ 12.92 Sex (male, %): 34 (68%) Placebo group Entered/completing: 50/50 Age (mean, SD): 42.56 +/‐ 13.57 Sex (male, %): 40 (80%) | |
| Interventions | Paracetamol: 100 ml normal saline and 1 g paracetamol IV 30 min after extubation and every 8 h until 24 h (4 g total) 100 ml IV normal saline 30 min after extubation and every 8 h until 24 h | |
| Outcomes | Primary: pain intensity (VAS) over first 6 h and 24 h after extubation, demand for opioid analgesia (pethidine 25 to 50 mg IM up to 200 mg per day), total pethidine dose consumed Secondary: adverse effects | |
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes ‐ age, BMI, stone size, operative time, baseline VAS. No mention if # of males balanced – 40 versus 34. | |
| Details of preoperative pain | Participants were excluded if they reported use of a NSAID or other analgesic less than 12 h before prescribing the study medications. Also excluded if painful physical conditions that may affect pain assessment after percutaneous nephrolithotomy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced blocked randomization; randomization schedule was prepared by someone that was blinded to the study. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | “Serums containing placebo and paracetamol, identical in color and appearance were prepared by an assistant and administered by nursing personnel blinded to the study.” Other group blinded was not specifically stated but assumed to be participants. |
| Incomplete outcome data (attrition bias) | Unclear risk | Per Results, 2 participants did not complete the study but no additional information was provided. No deviations from the protocol was also noted. |
| Selective reporting (reporting bias) | Low risk | Frequency of VAS < or > 4 was not mentioned as an outcome in Methods (Table 2) |
| Size | Unclear risk | 50 to 199 participants per arm of the study (50 paracetamol, 50 placebo) |
| Methods | Randomized, single dose, double‐blind, active‐controlled parallel‐group Medication administered when baseline pain reached moderate‐to‐severe intensity | |
| Participants | Type of surgery: gynecological Paracetamol group Entered/completing: 80/80 Age (mean, SD): 38.3 ± 12.8 Sex (male, %): 0 Propacetamol group Entered/completing: 81/81 Age (mean, SD): 33.9 ± 12.0 Sex (male, %): 0 | |
| Interventions | Intervention: paracetamol 1g IV in 100 ml solution over 15 min Active control: propacetamol 2 g in 100 ml solution | |
| Outcomes | Primary outcome: tolerability, including pain at infusion site Pain intensity (VRS, VAS) Number of participants requesting rescue medication Patient satisfaction (categorical) | |
| Source of funding | Not mentioned, but senior author was employee of Bristol Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: weight, type of surgery, baseline pain intensity at surgical site. No ‐ age: 38.3 paracetamol versus 33.9 propacetamol. | |
| Details of preoperative pain | Patients with any painful physical condition (other than postoperative pain) were excluded. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 1:1 ratio according to a computer‐generated list of numbers to either group |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Study drugs mixed by pharmacist or nurse not involved in the study, were administered as a 100 ml solution infused over 15 min |
| Incomplete outcome data (attrition bias) | Unclear risk | "A total of 163 women were enrolled and 161 received the single infusion of study medication. All remaining 161 patients including 2 patients (1 in each group) who did not meet eligibility criteria, were included in the ITT population and analyses of demographic characteristics, tolerability, and efficacy". Not clear if data were imputed. |
| Selective reporting (reporting bias) | Low risk | Free of selective reporting. All outcomes from Methods section reported in Results section. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (80 paracetamol, 81 propacetamol) |
| Methods | Randomized, placebo‐ and active‐controlled Medications administered at completion of surgery | |
| Participants | Type of surgery: hepatic resection Propacetamol group Entered/completing: unclear/38 Age (median, range): 49 (28 to 75) Sex (male, %): 40 Nefopam group Entered/completing: unclear/36 Age (median, range): 57 (21 to 75) Sex (male, %): 53 Placebo group Entered/completing: unclear/38 Age (median, range): 57 (27 to 75) Sex (male, %): 58 | |
| Interventions | Intervention: 2 g propacetamol over 15 min Control: 20 mg nefopam over 60 min Placebo: no treatment | |
| Outcomes | Primary: opioid consumption (morphine via PCA) Pain intensity (VAS) Patient satisfaction (categorical) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: sex, weight, height, ASA physical status, duration of surgery and anesthesia, sufentanil and midazolam cumulative doses, VAS score at extubation, and morphine dose for titration Participants in the propacetamol group were younger versus other 2 groups. Compared with the propacetamol group, participants in the nefopam group had lower VAS scores at extubation and longer intervals between the completion of hepatic resection and extubation. | |
| Details of preoperative pain | Participants receiving chronic anaΙgesic or anti‐inflammatory treatment were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | Open study |
| Blinding (performance bias and detection bias) | High risk | Open study |
| Incomplete outcome data (attrition bias) | Low risk | From 120 participants 8 were withdrawn for various reasons. Data from all remaining participants were used in the analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (38 propacetamol, 36 nefopam, 38 placebo) |
| Methods | Randomized, double‐blind, parallel‐group controlled trial, multiple dose, active‐controlled. First dose after spinal block regression to T10. | |
| Participants | Type of surgery: cesarean section Paracetamol group Entered/completing: 101/101 Age (mean, SD): 25.92 ± 3.09 Sex (male, %): 0 Tramadol group Entered/completing: 103/103 Age (mean, SD): 26.04 ± 3.65 Sex (male, %): 0 | |
| Interventions | Diclofenac 100 mg suppository for all participants starting at the ‘end of surgery’ and every 8 h for 24 h Paracetamol IV in 10 cc of NS (no mention of injection time), 1 g every 6 h beginning at block regression to T10 Tramadol 75 mg IV in 10 cc NS per the above protocol | |
| Outcomes | Primary: summed pain intensities during the entire observation period, calculated as the sum of time‐weighted pain intensity scores as an area under the curve (AUC). NRS at rest and movement at 0, 1, 2, 4, 8, 12, 24 h Secondary: use of supplementary rescue analgesic (pethidine 30 mg IV, administered if the participant’s NRS scores > 4) | |
| Source of funding | Grant received from the Department of Science & Technology (DST), Ministry of Science & Technology, Government of India | |
| Were treatment groups comparable at baseline? | Yes ‐ age BMI, surgical duration, blood loss, NRS at rest and movement. No ‐ BP and HR lower in acetaminophen group | |
| Details of preoperative pain | Participants receiving long‐term analgesics were excluded | |
| Notes | Primary analysis plan was not pursued because of a non normal distribution of pain scores. Instead medians and interquartile ranges were reported and compared for each time point at rest and at movement. This includes time at 4 h. Use of rescue medications over the entire time period was summarized without specific information as to the time for request. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Coded, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Both the test drugs (tramadol and acetaminophen) were drawn up in similar (Dispovan, Faridabad, Haryana, India) 10 ml coded syringes and diluted with normal saline so as to make the final volume of injection to 10 ml. Participants and assessors were blinded to assignment, but blinding success not tested. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (101 paracetamol, 103 tramadol) |
| Methods | Randomized, double‐dummy, placebo and active‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 4 h after surgery | |
| Participants | Type of surgery: third molar extraction Paracetamol group Entered/completing: 51/51 Age (mean, SD): 24.5 ± 2.9 Sex (male, %): 69 Propacetamol group Entered/completing: 51/51 Age (mean, SD): 24.3 ± 3.6 Sex (male, %): 57 Placebo group Entered/completing: 50/50 Age (mean, SD): 24.5 ± 2.8 Sex (male, %): 68 | |
| Interventions | Intervention: IV paracetamol 1 g Control: propacetamol 2 g Placebo: 100 ml saline, or 100 ml solution (double‐dummy) | |
| Outcomes | Primary outcome: pain relief (VRS) Maximum pain relief, time of maximum pain relief, time to onset of pain relief, TOTPAR Pain intensity (VRS, VAS) and derived summary measures Time to rescue medication (oral ibuprofen 400 mg) Global evaluation (categorical) | |
| Source of funding | Supported by the Bristol‐Myers Squibb Company | |
| Were treatment groups comparable at baseline? | Yes: demographics and baseline postoperative pain | |
| Details of preoperative pain | Participants with other painful physical conditions were excluded | |
| Notes | Propacetamol and paracetamol were compared to placebo and then propacetamol was compared to paracetamol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatments were allocated according to block randomization (each block, n = 6) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy technique employed |
| Incomplete outcome data (attrition bias) | Low risk | "Treatments were randomized among 152 patients. No patients withdrew from the study and all patients were evaluated for efficacy and safety". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (51 paracetamol, 51 propacetamol, 50 placebo) |
| Methods | Randomized, double‐blinded, triple‐dummy, active‐ and placebo‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 4 h after surgery | |
| Participants | Type of surgery: third molar extraction Propacetamol group Entered/completing: 50/50 Age (mean, SD): 24.2 (range 18 to 39) Sex (male, %): 46 Placebo group Entered/completing: 25/25 Age (mean, SD): 23.4 (range 20 to 29) Sex (male, %): 44 | |
| Interventions | Intervention: 2 g propacetamol 15 min infusion Intervention: 2 g propacetamol 2 min bolus (not included in our analysis) Control: oral acetaminophen (not included in our analysis) Placebo: triple‐dummy, exact details not described | |
| Outcomes | Primary: time to analgesia onset (double‐click stopwatch method) Pain relief (categorical) and derived summary scores Pain intensity (VAS) and derived summary scores Global evaluation (categorical) Duration of analgesia (time when 50% of participants in a group requested rescue medication, oral ibuprofen 600 mg) | |
| Source of funding | The study was supported by a grant from Bristol–Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: sex, weight, baseline pain intensity. Age appears to be similar between the 2 groups included in our analysis. | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomization schedule assigned treatments to sequential patients |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | A triple‐dummy technique was employed |
| Incomplete outcome data (attrition bias) | Low risk | "Treatments were randomized between 175 patients. No patients withdrew from the study and all 175 patients were evaluated by the intent‐to‐treat analyses and for safety". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (50 propacetamol, 25 placebo) |
| Methods | Randomized, investigator‐blinded, parallel‐group study with active and placebo control, first dose at skin closure, outcomes X 24 h | |
| Participants | Type of surgery: variety of lower abdominal surgery (bowel resection, abdominal hysterectomy, abdominal myomectomy, radical prostatectomy) Paracetamol group Entered/completing: 20/20 Age (mean, SD): 49.4 ± 18.4 Sex (male, %): 20% Placebo group Entered/completing: 20/19 Age (mean, SD): 48.5 ± 14.4 Sex (male, %): 25% Lornoxicam group Entered/completing: 20/20 Age (mean, SD): 52.8 ± 16.1 Sex (male, %): 30% | |
| Interventions | Paracetamol: 1 g every 6 h in 100 cc IV x 24 h Placebo: 100 cc IV every 6 h x 24 h Lornoxicam: 16 mg in 100 cc saline at time 0 and 8 mg at time 12 h All: PCA pump containing morphine was attached to the participant in a separate IV cannula. The pumps were programmed to administer morphine 1 mg boluses at 10 min intervals and total of 20 mg through 4 h limits. | |
| Outcomes | Primary: pain score via VPS during rest and coughing at 1st, 2nd, 4th, 8th, 12th and 24th postoperative h Secondary: heart rate, blood pressure, respiratory rate, and morphine consumption of the participants were assessed at 1st, 2nd, 4th, 8th, 12th and 24th postoperative h Adverse effects, including nausea, vomiting, itching, sweating, urinary retention, sedation, respiratory depression, hypotension, tachycardia, bradycardia, gastric irritation, increased bleeding from the wound, hematemesis, and melena were recorded and managed accordingly. The Ramsay sedation score [16] was used to evaluate the level of sedation. | |
| Source of funding | Deanship of Scientific Research of Dammam University | |
| Were treatment groups comparable at baseline? | Reports no significant differences for study groups including operative time but states P value < 0.05 for all demographics, likely an error | |
| Details of preoperative pain | Patients who received pain medications on the day prior to surgery and chronic drug abusers were excluded | |
| Notes | Sample size based on internal pilot and VPS difference of 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online research randomizer (www.randomizer.org) |
| Allocation concealment (selection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) | Low risk | No mention of participants blinding. “The anesthetist who provided anesthesia and the on who followed up with the patients in the ward for assessment were blinded to the study drug given. Sealed and enclosed 100 ml bags containing either normal saline or the study drugs were used. The color of lornoxicam solution is yellow; to maintain blinding, the containers for all solutions were covered with aluminium foil during administration”. |
| Incomplete outcome data (attrition bias) | Low risk | Only one dropout in entire study |
| Selective reporting (reporting bias) | Unclear risk | Opioid consumption measured at 1, 2 and 4 h, but not reported No results available on clinicaltrials.gov |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo, 20 lornoxicam) |
| Methods | Randomized, double‐blind, placebo‐ and active‐controlled Medications administered 20 min before the end of surgery (and at 4, 10 and 16 h postoperatively) | |
| Participants | Type of surgery: breast cancer (segmental or mastectomy) Paracetamol group Entered/completing: 30/27 Age (mean, SD): 56 ± 13 Sex (male, %): 41 Metamizole group Entered/completing: 30/26 Age (mean, SD): 52 ± 12 Sex (male, %): 55 Placebo group Entered/completing: 30/26 Age (mean, SD): 58 ± 14 Sex (male, %): 55 | |
| Interventions | Intervention: IV paracetamol 1 g/100 ml normal saline over 10 to 15 min Control: metamizole 1 g IV Placebo: 100 ml normal saline | |
| Outcomes | Pain intensity (NRS) Cognitive function (TDT, DSST) All other outcomes assessed at 24 h | |
| Source of funding | Supported by Department of Anesthesiology and Bristol‐Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, nature of surgery, ASA risk category | |
| Details of preoperative pain | Not reported | |
| Notes | From 2 h postoperatively onwards, average pain ratings were below 2.5/10 in all groups. No data could be used in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Treatments appeared identical |
| Incomplete outcome data (attrition bias) | Unclear risk | 27/30 paracetamol and 26/30 in other groups completed study ‐ dropouts for various reasons specified. It appears that only data from those completing study were analyzed. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section. Minor omissions of complete data for some safety outcomes. |
| Size | High risk | Fewer than 50 participants per arm of the study (30 paracetamol, 30 metamizole, 30 placebo) |
| Methods | Blinded, randomized, controlled, parallel‐group, multidose trial. Intervention start at the ‘end of surgery’. | |
| Participants | Type of surgery: elective cesarean section Paracetamol group Entered/completing: 40/40 Age (mean, SD): 30.80 ± 4.79 Sex (male, %): 0 Normal saline group Entered/completing: 40/40 Age (mean, SD): 29.60 ± 5.20 Sex (male, %):0 | |
| Interventions | Paracetamol: 1 g IV in 100 cc start at end of surgery, then every 6 h x 24 h Control: 100 cc NS start at end of surgery, then every 6 h x 24 h All: every participant received 0.2 mg intrathecal morphine at the time of spinal placement. Clinically this intervention has an expected duration of action of up to 18 h. Pethidine for rescue analgesia | |
| Outcomes | Primary: number of participants requiring rescue analgesic drug use x 24 h Secondary: visual analog scale (VAS) was used to evaluate pain level (0 = no pain to 10 = worst pain) at 6, 12 and 24 h postoperatively by a resident and nurse who did not know about the treatment protocols. Satisfaction was evaluated at 12 and 24 h postoperatively (1 = very unsatisfied to 5 = very satisfied). | |
| Source of funding | None mentioned | |
| Were treatment groups comparable at baseline? | Yes, but no data on surgical duration or whether or not a participant had a repeat cesarean section | |
| Details of preoperative pain | Participants with history of chronic abdominal pain or treated with analgesics were excluded from the study | |
| Notes | No enrolment flow chart. When a spinal failed, a participant was dropped, likewise for participants with intraoperative complications for example, but unclear when participants were enrolled or randomized. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of randomization |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) | Unclear risk | Participant blinding not addressed. Personnel blinding per statement except that the chief resident was not blinded. |
| Incomplete outcome data (attrition bias) | Low risk | No missing data reported. Appears that all participants completed the study. |
| Selective reporting (reporting bias) | High risk | Very limited outcomes (rescue analgesia, pain, and satisfaction scores). No side effects addressed at all. |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 placebo) |
| Methods | Double‐blind, double‐dummy, parallel‐group, active‐controlled randomized study | |
| Participants | Type of surgery: bimaxillary osteotomy Paracetamol group Entered/completing: 15/15 Age (median, range): 21 (16 to 38) Sex (male, %): 27 Diclofenac group Entered/completing: 15/15 Age (median, range): 24 (17 to 42) Sex (male, %): 27 | |
| Interventions | All: 160 mg of articaine LA with epinephrine to site of surgery at the start of case. General anesthesia, no opioids. Postoperative on demand rescue analgesic of 75 mg diclofenac IM. Observation period x 24 h. Paracetamol: intravenous solution of 1 g x 1 within 15 min of mucosal closure + IM placebo Diclofenac: IV solution of 1 g of placebo x 1 within 15 min of mucosal closure + IM 75 mg diclofenac | |
| Outcomes | Primary: the severity of postoperative pain was evaluated on the VAS after 30 min, and then at 1, 2, 4, 6, 8, 12, and 24 h Secondary: systolic blood pressures and heart rates were also recorded at the same times. The number and time of diclofenac rescue were also recorded. Early and late side effects during the first 30 min and after 24 h, such as nausea, vomiting, hypotension, sedation, cyanosis, hypertension, facial oedema, and urticaria, were also recorded. Patients’ satisfaction was assessed 24 h postoperatively using a 3‐point scale (1 = not satisfied, 2 = satisfied, 3 = very satisfied). | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: demographics, BMI, duration of surgery | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation to 2 groups, no further info |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | “a staff nurse premixed an intravenous solution containing either paracetamol or placebo 1 g. The same nurse gave an intramuscular injection of diclofenac 75 mg or placebo as assigned”. No mention of whether placebo and intervention appeared identical. |
| Incomplete outcome data (attrition bias) | Low risk | Appears all participants completed study and data were collected on all |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in Methods reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (15 paracetamol, 15 diclofenac) |
| Methods | Double‐blind, randomized, active‐controlled, parallel‐group study with intervention at end of surgery x 24 h for outcomes | |
| Participants | Type of surgery: total hip arthroplasty under spinal anesthesia Paracetamol group Entered/completing: 43/43 Age (mean, SD): 57.7 (13.8) Sex (male, %): 39.5 Metamizol group Entered/completing:51/51 Age (mean, SD): 62.2 (12.4) Sex (male, %):29.4 All: morphine PCA at baseline | |
| Interventions | Paracetamol: IV 1 g paracetamol every 8 h x 24 h, start at ICU admission Metamizol: IV 1.5 g every 8h x 24 h, start at ICU admission All: PCA morphine with continuous setting 1 to 2 mg/h (based on participant weight), 1 mg bolus with 15 min lockout x 24 h | |
| Outcomes | Primary: pain intensity in the first 24 h after surgery. Total pain over the study period was calculated as area under the pain/time curve. VAS 0 to 100 with a 10 cm ruler. Secondary: pain was assessed at time points of 1, 2, 3, 4, 6, 8, 10, 14, 18 and 22 h post‐baseline Amount of morphine consumption in 24 h | |
| Source of funding | No financial support | |
| Were treatment groups comparable at baseline? | Yes: demographics, estimated blood loss, duration of surgery | |
| Details of preoperative pain | None | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly generated list |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding (performance bias and detection bias) | Low risk | “Research team and the patients were not familiar with the information about products that patients received. Drugs were prescribed by an independent doctor and administered by nurses not involved in the research. Nurses not involved in the research recorded VAS scale results. Independent blinded researchers performed clinical observations”. |
| Incomplete outcome data (attrition bias) | Low risk | All randomized participants completed study. No mention of how missing data were imputed. |
| Selective reporting (reporting bias) | High risk | No reporting of adverse events |
| Size | High risk | Fewer than 50 participants per arm of the study (43 paracetamol, 51 metamizol) |
| Methods | Randomized, double‐blind, double‐dummy, parallel‐group, placebo‐controlled clinical trial x 24 h, starting with intervention immediately postoperatively | |
| Participants | Type of surgery: elective cesarean delivery under spinal anesthesia Paracetamol group Entered/completing: 32/32 Age (median, IQR): 31 (28 to 34) Sex (male, %): 0 Control group Entered/completing:23/23 Age (median, IQR): 30 (28 to 35) Sex (male, %): 0 Parecoxib group Entered/completing: 30/30 Age (median, IQR): 30 (26 to 35) Sex (male, %): 0 | |
| Interventions | IV solutions (200 ml for infusion over 15 min or 2 ml for bolus injection after delivery) and oral capsules of identical appearance (for administration after surgery). Study regimen started immediately after delivery. All: 1. Pethidine patient‐controlled epidural analgesia x at least 24 h, 20 mg on demand, 15 min lockout 2. If the verbal numerical rating score for pain was > 6 with movement or > 3 at rest at any time, supplementary analgesia was available in the form of immediate‐release tramadol 50 to 100 mg oral 2‐hourly on demand (maximum dose 600 mg across 24 h) 3. Thereafter, analgesia was at the discretion of the attending anesthetist or acute pain service 4. Postoperative pruritus was treated with IV ondansetron 4 mg 6‐hourly on demand or, if this was ineffective, IV naloxone 50 µg hourly on demand Paracetamol: 2 g IV x 1 after delivery, then oral 1 g at 6, 12 and 18 h and appropriate placebos Parecoxib: 40 mg IV x 1 after delivery, then oral celecoxib 400 mg at 12 h and placebos Parecoxib + paracetamol: 40 mg and 2 g IV after delivery, then paracetamol 1 g oral at 6, 12 and 18 h + celecoxib 400 mg oral at 12 h (not included in our analysis) Control: saline placebos and capsules after delivery and at 6, 12 and 18 h respectively as appropriate | |
| Outcomes | Primary: 24‐hour postoperative patient‐controlled epidural pethidine use Secondary: the main secondary outcomes were the 0 to 24 h AUC pain scores with movement, the quality of recovery score and the "SDQ" score 1. Postoperative pain measured as verbal numerical rating score (VRS) of 0 to 10 at rest and movement at 6, 12, 24 and 48 h 2. VRS sedation scores at the same times. Area under the curve (AUC) for rest and movement pain scores over 0 to 24 h 3. Presence of gastrointestinal upset, nausea or epigastric pain at 24 h 4. Satisfaction with analgesia (0 to 10 VRS and ratings of excellent, good, fair, or poor) at 24 h 5. Severity of overall nausea, sedation, and pruritus (VRS) at 24 h 6. OR score, the opioid related "SDQ" score and a Modified Brief Pain Inventory (short‐form) 7. At 48 h, pain and sedation scores were recorded and the presence of urinary retention post‐catheter removal or observed respiratory depression (respiratory rate < 8 breaths per minute or sedation score of 3 representing 'difficult to rouse') assessed | |
| Source of funding | Investigator‐initiated research grant from Pfizer Australia | |
| Were treatment groups comparable at baseline? | Yes: age, ASA II, BMI, gravidity, parity, previous cesarean section | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A randomization sequence for four groups in a 1:1:1:1 ratio was generated by the hospital Pharmacy Department using a computer‐generated random number sequence” |
| Allocation concealment (selection bias) | Low risk | “Allocation was by selection of the next sealed and coded study drug package and occurred intraoperatively”. |
| Blinding (performance bias and detection bias) | Low risk | All observers were blinded to study group allocation. Medications prepared to look identical. No further detail. |
| Incomplete outcome data (attrition bias) | Low risk | ITT analysis with LOCF, plus per‐protocol analysis and no dropouts |
| Selective reporting (reporting bias) | Unclear risk | Did not report all adverse event data |
| Size | High risk | Fewer than 50 participants per arm of the study (32 paracetamol, 23 placebo, 30 parecoxib) |
| Methods | Randomized, placebo‐controlled, double‐blind Medications administered after extubation | |
| Participants | Type of surgery: orthopedic Propacetamol group Entered/completing: 46/41 Age (mean, SD): 62.6 ± 8.3 Sex (male, %): 31 Placebo group Entered/completing: 51/45 Age (mean, SD): 60.5 ± 9.6 Sex (male, %): 32 | |
| Interventions | Intervention: propacetamol 2 g in 100 ml 5% dextrose over 15 min Placebo: 5% dextrose 100 ml | |
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VRS, VAS) Global efficacy (VRS) | |
| Source of funding | Financially supported by UΡSA MEDΙCA SPA | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, baseline pain intensity, pre‐intervention morphine use | |
| Details of preoperative pain | None | |
| Notes | Opioid consumption and global efficacy assessed at 24 h | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block 2:2 randomization |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Identical vials for further dilution |
| Incomplete outcome data (attrition bias) | Unclear risk | Inadequate intention‐to‐treat. "A total of 97 patients entered the study and 89 of them were evaluated". 8 were withdrawn from efficacy analyses due to malfunctioning of PCA. All of these 89 patients were used in the efficacy analyses including 3 cases of premature discontinuation of the study due to lack of efficacy and 1 withdrawal of consent. Time point of premature discontinuation is not defined |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (46 propacetamol, 51 placebo) |
| Methods | Prospective, randomized, double‐blinded and placebo‐controlled add‐on study with 3 parallel groups, paracetamol given 5 min after ketoprofen that was given after surgery, both likely within 1 hour of surgery end, but exact time not given | |
| Participants | Type of surgery: tonsillectomy Paracetamol 1 g group Entered/completing: 39/39 Age (mean, SD): 22 (6) Sex (male, %): 15, 40% Placebo group Entered/completing: 38/38 Age (mean, SD): 38 (10) Sex (male, %): 18, 47% | |
| Interventions | Paracetamol 1 g: 15 min infusion, 5 min after ketoprofen IV x 1 Paracetamol 2 g: 15 min infusion, 5 min after ketoprofen IV x 1 (not included in our analysis) Normal Saline: 15 min infusion 5 min after ketoprofen IV X 1 ALL: 2 µg/kg fentanyl intraoperatively, ketoprofen 1 mg/kg in 10 cc saline 5 min after surgery, oxycodone 2 mg IV was provided for rescue analgesia if VAS rest was > 30/100 mm or VAS swallowing > 50/100 mm. The oxycodone dose was repeated at 15‐min intervals until pain had diminished (VASr < 30mm and VASs < 50mm) x 6 h | |
| Outcomes | Primary: proportion of patients requiring oxycodone for rescue analgesia to maintain VASr (resting) < 30 mm and VASs (swallowing) < 50 mm over the first 6 h Secondary: VASr&s at 1, 2, 3, 4, and 6 h after the surgery and at discharge. Length of time until the first dose of rescue analgesic, the number of oxycodone doses during the first 6 h after surgery, the sedation score at discharge, and the incidence of adverse effects. | |
| Source of funding | Absence of external funding specifically mentioned | |
| Were treatment groups comparable at baseline? | Unclear: no statistical analysis was provided for the group characteristics | |
| Details of preoperative pain | Not reported | |
| Notes | 119 participants enrolled. 5 withdrew consent before randomization. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | “To ensure blinding, the syringes were prepared by a nurse otherwise not involved in the study, and the patients, surgeons and study nurses obtaining the outcome data were blinded to the treatment arms”. “Thus, we used an approach where all patients were provided similar infusions prepared by the study nurse. Because a paracetamol infusion is colorless, does not contain visible particles and does not irritate veins, we believe that the blinding should have performed sufficiently. Unfortunately, we did not test this in the present study.” |
| Incomplete outcome data (attrition bias) | Low risk | All randomized participants completed the study and appear to have contributed data for all outcomes |
| Selective reporting (reporting bias) | High risk | 6 h pain scores measured but not reported, patient characteristics analyzed but no statistical comparison provided, insufficient detail regarding adverse effects, including nausea. Adverse events were not reported group‐specific but in aggregate for the entire cohort. |
| Size | High risk | Fewer than 50 participants per arm of the study (39 paracetamol, 38 placebo) |
| Methods | Prospective, randomized, double‐blind study, parallel‐group, active control x 24 h | |
| Participants | Type of surgery: elective total abdominal hysterectomy with or without bilateral salpingo‐oophorectomy Paracetamol group Entered/completing: not stated Age (mean, SD): not stated Sex (male, %): 0 Diclofenac group Entered/completing: not stated Age (mean, SD): not stated Sex (male, %): 0 | |
| Interventions | Paracetamol: 1 g IV postoperatively every 8 h x 24 h Diclofenac: 75 mg IM every 8 h x 24 h | |
| Outcomes | Primary: requirement of rescue analgesic (time component not described) Secondary: VAS (scale not mentioned) for pain at least at 4 and 12 h postoperatively, time until first rescue analgesic administration, patient satisfaction score (scale not mentioned), nausea, vomiting, bronchospasm | |
| Source of funding | None mentioned | |
| Were treatment groups comparable at baseline? | Not described | |
| Details of preoperative pain | Not described | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | No information |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many participants completed the study or what method of analysis was used |
| Selective reporting (reporting bias) | High risk | No data reported |
| Size | High risk | Fewer than 50 participants per arm of the study |
| Methods | Double‐blind, randomized, placebo‐controlled. Single dose of intravenous paracetamol within the last 20 min of surgery or placebo. | |
| Participants | Type of surgery: lumbar discectomy Paracetamol group Entered/completing: dropouts not reported; presumably 24/24 Age (mean, SD): 46.50 ± 14.07 Sex (male, %): 46.2% for both groups Placebo group Entered/completing: dropouts not reported; presumably 28/28 Age (mean, SD): 52.25 ± 11.46 Sex (male, %): 46.2% for both groups | |
| Interventions | Paracetamol: 1 g in 100 ml normal saline. Duration of administration not stated. 100 ml normal saline. Duration of administration not stated. | |
| Outcomes | Primary: pain on 0 to 10 VAS at 1, 6, 12, 18, 24 h after surgery Morphine dosage for the first 24 h postop Secondary: Adverse effects | |
| Source of funding | Tabriz University of Medical Sciences | |
| Were treatment groups comparable at baseline? | Yes: age, sex | |
| Details of preoperative pain | Patients with preoperative treatment with narcotics, benzodiazepines, or clonidine were excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | All local anesthetic solutions and adjuvant drugs were prepared by an anesthesiologist who was not involved in the performance of the study agents, patient care, or data collection. No mention that interventions appeared identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if all participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects assessment mentioned in methods, but article states there were no side effects related to treatment (without any detail) |
| Size | High risk | Fewer than 50 participants per arm of the study (24 paracetamol, 28 placebo) |
| Methods | Randomized, double‐blind, double‐dummy, placebo‐ and active‐controlled Medications administered immediately after surgery | |
| Participants | Type of surgery: cesarean delivery Propacetamol group Entered/completing: 20/20 Age (mean, SD): 31 ± 4.6 Sex (male, %): 0 Diclofenac group Entered/completing: 20/20 Age (mean, SD): 31.4 ± 6 Sex (male, %): 0 Placebo group Entered/completing: 20/20 Age (mean, SD): 30.6 ± 5.1 Sex (male, %): 0 | |
| Interventions | Intervention: 2 g propacetamol Control: 100 mg rectal diclofenac Control: combination of 100 mg rectal diclofenac and 2 g propacetamol (not included in our analysis) Placebo: double‐dummy, exact details not described | |
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity at rest and on coughing (VAS 0 to 10) Global assessment (categorical, at 24 h) | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, weight, height, parity, and gestationaΙ age | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Patients and staff were unaware of the patients' group assignment |
| Blinding (performance bias and detection bias) | Low risk | "to ensure blinding of both the parturients and the anaesthesiologist, patients in all groups received both an IV injection and a suppository during the same period" |
| Incomplete outcome data (attrition bias) | Low risk | From 80 patients one was excluded due to technical problems of the PCA device. Data obtained from the 79 remaining patients were used for the analysis of all outcomes and time points. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (20 propacetamol, 20 diclofenac, 20 placebo) |
| Methods | Randomized, double‐blind, double‐dummy, placebo‐controlled Medications administered on postoperative day 1, when baseline pain reached moderate‐to‐severe intensity (after PCA disconnected) | |
| Participants | Type of surgery: total hip arthroplasty Paracetamol group Entered/completing: 49/46 Age (mean, SD): 61.7 ± 16.9 Sex (male, %): 57 Propacetamol group Entered/completing: 50/44 Age (mean, SD): 59.5 ± 14.2 Sex (male, %): 54 Placebo group Entered/completing: 52/47 Age (mean, SD): 59.2 ± 13.4 Sex (male, %): 42 | |
| Interventions | Intervention: 1 g IV paracetamol in 100 ml solution over 15 min Control: 2 g propacetamol Placebo: 100 ml solution | |
| Outcomes | Primary: pain relief (VRS) Pain intensity (VAS, VRS) Time to rescue medication (morphine) Opioid consumption (morphine) Patient global evaluation | |
| Source of funding | Supported by Bristol‐Myers Squibb Company, Rueil‐Malmaison, France | |
| Were treatment groups comparable at baseline? | Yes: demographics, anesthetic and surgical procedure and baseline pain intensity | |
| Details of preoperative pain | "The overwhelming majority of patients had symptoms of severe debilitating or painful osteoarthritis". Not mentioned if this was similar between groups. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Unblinded pharmacist was not involved in the study. All study medications were administered as a 100 ml solution infused over 15 min. |
| Incomplete outcome data (attrition bias) | Low risk | From a total of 156 randomized patients 151 included. "All of these 151 patients were included in the intent‐to‐treat population and analyzed for demographics, efficacy and safety". |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | Unclear risk | 50 to 199 participants per arm of the study (49 paracetamol, 50 propacetamol, 52 placebo) |
| Methods | Randomized, double‐blind, active‐controlled Medications administered at removal of gall bladder, around 30 min from end of surgery | |
| Participants | Type of surgery: laparoscopic cholecystectomy Paracetamol group Entered/completing: 40/39 Age (mean, SE): 38.9 ± 1.8 Sex (male, %): 18 Parecoxib group Entered/completing: 40/39 Age (mean, SE): 42.9 ± 1.7 Sex (male, %): 28 | |
| Interventions | Intervention: paracetamol 1 g IV Control: paracetamol 1 g IV with dexamethasone 10 mg IV (not included in our analysis) Control: parecoxib 40 mg IV Control: parecoxib 40 mg IV with dexamethasone 10 mg IV (not included in our analysis) | |
| Outcomes | Pain intensity at rest and with movement (VAS) Time to rescue medication (oxycodone) Opioid consumption (at 24 h onwards only) | |
| Source of funding | Pfizer Finland supplied parecoxib. No other details. | |
| Were treatment groups comparable at baseline? | Yes: age, weight, gender, ASA physical status, the duration of the operation, or the length of stay in hospital | |
| Details of preoperative pain | Patients regularly using analgesics were excluded | |
| Notes | Combination groups not analyzed. Contact author confirmed that doses of paracetamol/parecoxib were administered within half an hour of end of surgery. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Study drugs administered by nurse not otherwise involved in the study, but no further information given |
| Incomplete outcome data (attrition bias) | Low risk | 1/40 in each analyzed group excluded from analysis. It appears that all other participants contributed data for all time points of interest (more participants dropped out after day 1 of study, but were not part of our analysis). |
| Selective reporting (reporting bias) | Unclear risk | Pain at rest and with motion was recorded every 20 min in PACU 1 and every 30 min in PACU 2, but data not presented for time points 1.5 h, 2 h and 2.5 h |
| Size | High risk | Fewer than 50 participants per arm of the study (40 paracetamol, 40 parecoxib) |
| Methods | Double‐blind, random allocation to IV paracetamol 30 min before surgery versus IV tramadol 20 min before end of surgery | |
| Participants | Type of surgery: septo‐rhinoplasty Paracetamol group Entered/completing: 25/25 Age (mean, SD): 31.5 ± 11 Sex (male, %): 64 Tramadol group Entered/completing: 25/25 Age (mean, SD): 31.8 ± 10 Sex (male, %): 64 | |
| Interventions | Paracetamol infusion 1 g given 30 min before end of surgery IV tramadol given 30 min (in abstract written 20 min) before end of surgery, dose not stated. In abstract ‐ 1 mg/kg dose is mentioned. | |
| Outcomes | Primary: Pain intensity on 10 cm VAS Secondary: Patient satisfaction, drug side effects, analgesic need | |
| Source of funding | Not stated | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, duration of surgery, and anesthesia | |
| Details of preoperative pain | Not reported | |
| Notes | Postoperatively participants could take 500 mg oral paracetamol, up to 3 g/day, and if required, tramadol 0.5 mg/kg as IV bolus | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) | Unclear risk | Last paragraph of introduction states it was double‐blind but no additional details were provided |
| Incomplete outcome data (attrition bias) | Low risk | All participants have completed |
| Selective reporting (reporting bias) | High risk | Objective state adverse effect and patient satisfaction investigation. Adverse effect not reported. Only nausea % in one group is reported, stating it is higher than in the other group, but no quantitative data. Patient satisfaction not reported. |
| Size | High risk | Fewer than 50 participants per arm of the study (25 paracetamol, 25 tramadol) |
| Methods | Participants randomized to 3 groups to receive interventions at the time of wound closure (supposedly within 30 min from end of surgery) | |
| Participants | Type of surgery: microsurgical lumbar discectomy and/or laminectomy Paracetamol group Entered/completing: 20/18 Age (mean, SD): 46.39 ± 10.06 Sex (male, %): 33 Placebo group Entered/completing: 20/20 Age (mean, SD): 48.35 ± 9.93 Sex (male, %): 55 Dexketoprofen group Entered/completing: 20/18 Age (mean, SD): 39.17 ± 11.10 Sex (male, %): 38.9 | |
| Interventions | All administered IV in 100 ml infused over 15 min All participants used IV PCA with morphine Paracetamol: 1 g every 6 h for 24 h The study states paracetamol but in discussion says “Paracetamol is an IV formulation of a prodrug of acetaminophen and used as a supplemental analgesic to reduce postoperative pain” Dexketoprofen: 50 mg IV every 8 h for 24 h Placebo: 100 ml normal saline every 8 h for 24 h | |
| Outcomes | Primary: pain intensity on 0 to 10 VAS at 0, 1, 2, 6, 12, 24 h post‐op but primary outcome not defined Secondary: sedation on Ramsay score, cumulative PCA morphine consumption at the above time points, adverse effects | |
| Source of funding | No funding | |
| Were treatment groups comparable at baseline? | No: age in dexketoprofen group lower than in 2 other groups; pain at time 0 in dexketoprofen group substantially lower | |
| Details of preoperative pain | Not reported | |
| Notes | Frequency of administration different: every 6 h for paracetamol and every 8 h for dexketoprofen and saline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1 person prepared sealed envelopes and another person drew an envelope for each case |
| Allocation concealment (selection bias) | Unclear risk | Unclear if solutions looked any different |
| Blinding (performance bias and detection bias) | Unclear risk | The treatment frequency is different (3 times daily versus 4 times daily), true blinding not likely, although study data were collected by a blinded anesthesiologist |
| Incomplete outcome data (attrition bias) | Low risk | 4 participants were excluded, with reasons given. Outcomes seem to be reported in full. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in Methods reported in Results |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 placebo, 20 dexketoprofen) |
| Methods | Local wound infiltration versus IV paracetamol versus IV lornoxicam 30 min before extubation. Additional analgesia with tramadol and as required pethidine. | |
| Participants | Type of surgery: laparoscopic renal and adrenal surgery Paracetamol group Entered/completing: 20/not reported Age (mean, SD): not reported Sex (male, %): not reported Lornoxicam group Entered/completing: 20/not reported Age (mean, SD): not reported Sex (male, %): not reported | |
| Interventions | Paracetamol 1 g 30 min before extubation, then 5 g in 24 h postop, frequency/timing not reported 0.25% levobupivacaine infiltration to trocar incisions (not included in our analysis) Lornoxicam: 8 mg IV 30 min before extubation and another 8 mg during 24 h postop. Frequency not reported. | |
| Outcomes | Primary: pain VAS scores Secondary: cumulative tramadol and pethidine consumption | |
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Not reported | |
| Details of preoperative pain | Not reported | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | High risk | Not reported, and not stated to be unblinded |
| Blinding (performance bias and detection bias) | High risk | Not stated that study was blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear how many participants completed the study |
| Selective reporting (reporting bias) | High risk | Most of the outcomes not reported |
| Size | High risk | Fewer than 50 participants per arm of the study (20 paracetamol, 20 lornoxicam) |
| Methods | Administration of IV paracetamol versus IV dexketoprofen versus placebo during incision closure | |
| Participants | Type of surgery: total abdominal hysterectomy Paracetamol group Entered/completing: 21/20 Age (mean, SD): 48.1 ± 3.6 Sex (male, %): 0 Dexketoprofen group Entered/completing: 22/20 Age (mean, SD): 47.7 ± 5.9 Sex (male, %): 0 Placebo group Entered/completing: 21/20 Age (mean, SD): 48.1 ± 4.5 Sex (male, %): 0 | |
| Interventions | Paracetamol 1 g/100 ml in 15 min IV infusion, then every 6 h for 24 h Dexketoprofen: 50 mg in 100 ml 15 min IV infusion, then every 8 h for 24 h Placebo: normal saline 100 ml 15 min IV infusion, then every 6 h for 24 h | |
| Outcomes | Primary: differences in cumulative 24 h morphine consumption Secondary: VAS pain scores, adverse events, patient satisfaction | |
| Source of funding | None | |
| Were treatment groups comparable at baseline? | Yes: weight, height, BMI, ASA 1/2, anesthesia duration, surgery duration | |
| Details of preoperative pain | Participants with preoperative pain or regular analgesic use excluded | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block random allocation |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Unblinded person preparing drugs, blinded person assessed outcomes. Unclear if the solutions looked the same. Drug administration frequency different among groups (3 versus four times daily), so blinding might have been compromised |
| Incomplete outcome data (attrition bias) | Low risk | Number of dropouts low, evenly distributed among groups and reasons for dropout do not appear to be related to treatment |
| Selective reporting (reporting bias) | Unclear risk | Incomplete description of methodology for patient satisfaction assessment and incomplete presentation of data |
| Size | High risk | Fewer than 50 participants per arm of the study (21 paracetamol, 22 dexketoprofen, 21 placebo) |
| Methods | Randomized, double‐blinded, double‐dummy, placebo‐ and active‐controlled Medication administered when baseline pain reached moderate‐to‐severe intensity within 3 h after regaining consciousness | |
| Participants | Type of surgery: dental Propacetamol group Entered/completing: 31/31 Age (mean, SD): 20.0 ± 4.9 Sex (male, %): 25 Morphine group Entered/completing: 30/30 Age (mean, SD): 18.8 ± 4.3 Sex (male, %): 39 Placebo group Entered/completing: 34/34 Age (mean, SD): 20.9 ± 6.6 Sex (male, %): 29 | |
| Interventions | Intervention: 2 g propacetamol in 150 ml normal saline over 15 min Control: 10 mg morphine Placebo: 150 ml normal saline | |
| Outcomes | Pain intensity (VRS, VAS) and derived summary measures Pain relief (VRS) Proportion of patients requesting and time to rescue medication (morphine) Global assessment (at 10 h) | |
| Source of funding | Supported by a grant from Bristol‐Myers Squibb | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, baseline pain intensity No: the morphine group had a larger proportion of ASA II participants than the placebo group (P value < 0.01) | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | Double‐dummy technique employed |
| Incomplete outcome data (attrition bias) | Low risk | From 99 patients 4 were excluded due to protocol violations before any data had been collected. "All 95 remaining patients from whom efficacy data were obtained were included in the efficacy analysis". |
| Selective reporting (reporting bias) | Unclear risk | All outcome from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (31 propacetamol, 30 morphine, 34 placebo) |
| Methods | Randomized, double‐blinded, double‐dummy, active‐controlled Medications administered at time of extubation | |
| Participants | Type of surgery: elective hysterectomy Propacetamol group Entered/completing: 100/87 Age (mean, SD): 48.4 ± 6.7 Sex (male, %): 0 Ketorolac group Entered/completing: 100/89 Age (mean, SD): 49.8 ± 9.0 Sex (male, %): 0 | |
| Interventions | Intervention: 2 g propacetamol in 100 ml saline over 15 min Control: 30 mg IV ketorolac | |
| Outcomes | Opioid consumption (morphine via PCA) Pain intensity (VAS and VRS) Patient satisfaction (VRS at 12 h) | |
| Source of funding | Supported in part by UPSA MEDICA SPA | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of surgery, initial pain intensity | |
| Details of preoperative pain | Patients were excluded if they were receiving additional analgesic, antipyretic, or antiinflammatory treatment during the study | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced block (2:2) randomization, each study center receiving 4 case lots |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Propacetamol 2 g or ketorolac 30 mg was administered as an IV infusion (100 ml saline in 15 min). Unclear whether the 2 infusions appeared identical. |
| Incomplete outcome data (attrition bias) | Unclear risk | 24 patients were excluded before blinding and 2 patients, 1 from each treatment arm, were withdrawn from the study for reasons unrelated to treatment. Then 2 were withdrawn due to lack of efficacy. Remaining patients were 87 for propacetamol and 89 for ketorolac group and were all used in the outcome evaluating cumulative morphine consumption. Number of patients used for the outcomes "evaluation of pain intensity" and "percentage of patients rating pain as severe or very severe" differ. |
| Selective reporting (reporting bias) | Low risk | Free of selective reporting |
| Size | Unclear risk | 50 to 199 participants per arm of the study (100 propacetamol, 100 ketorolac) |
| Methods | Randomized, double‐blind, active‐controlled Medications administered at the end of anesthesia | |
| Participants | Type of surgery: various elective Propacetamol group Entered/completing: 40/38 Age (mean, SD): 39 ± 13 Sex (male, %): 47 Morphine group Entered/completing: 40/39 Age (mean, SD): 39 ± 14 Sex (male, %): 49 | |
| Interventions | Intervention: 30 mg/kg propacetamol in 150 ml dextrose 5% over 15 min Control: 0.2 mg/kg morphine IV | |
| Outcomes | Number of patients requiring rescue analgesia (repeat dose of intervention) Pain intensity (VAS) Vigilance (trailmaking test) | |
| Source of funding | UPSA Laboratories supplied drugs and covered logistical expenses of study | |
| Were treatment groups comparable at baseline? | Yes: demographics, duration of anesthesia, type of surgery | |
| Details of preoperative pain | Patients taking opioids were excluded | |
| Notes | French language paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | Solutions prepared by third party, but unclear if they appeared identical |
| Incomplete outcome data (attrition bias) | Low risk | From the 80 patients 3 were withdrawn. All the remaining 77 patients were included in efficacy analyses. |
| Selective reporting (reporting bias) | Low risk | All outcomes from Methods section reported in Results section |
| Size | High risk | Fewer than 50 participants per arm of the study (40 propacetamol, 40 morphine) |
| Methods | 2 regimens of IV paracetamol (every 4 h versus every 6 h) versus 2 same regimens of placebo. Participants after abdominal laparoscopy, who present with pain. 24‐h assessment. | |
| Participants | Type of surgery: abdominal laparoscopy Paracetamol 1000 mg group Entered/completing: 92/88 Age (mean, SD): 45.3 ± 12.26 Sex (male, %): 20 Placebo group 100 ml Entered/completing: 43/37 Age (mean, SD): 46.0 ± 11.70 Sex (male, %): 14 Placebo group 65 ml Entered/completing: 67/62 Age (mean, SD): 46.5 ± 13.08 Sex (male, %): 20 | |
| Interventions | All interventions administered on postoperative day 1 Paracetamol 1000 mg in 100 ml 15‐min IV infusion, every 6 h (total 4 doses over 24 h) Paracetamol 650 mg in 65 ml 15‐min IV infusion, every 4 h (total of 6 doses over 24 h, not included in our analysis) Placebo: 100 ml 15‐min IV infusion, every 6 h (total 4 doses over 24 h) Placebo: 65 ml 15‐min IV infusion, every 4 h (total of 6 doses over 24 h) | |
| Outcomes | Primary: SPID 0 to 24 h of acetaminophen 1000 mg versus combined placebo Secondary: SPID24 for acetaminophen 650 mg versus placebos (not included in our analysis) TOTPAR24 Pain intensity at baseline, and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 14, 16, 18, 20, 22, and 24 h Adverse events Vital signs CBC, Liver Function Tests | |
| Source of funding | Cadence Pharmaceuticals | |
| Were treatment groups comparable at baseline? | Yes: age, sex, race, height, weight. No clinically relevant differences observed between the placebo and intravenous acetaminophen groups with regard to type of primary abdominal laparoscopic surgery, additional procedures performed, the duration of surgery, or the time from end of surgery to T0. | |
| Details of preoperative pain | Exclusion: having a chronic pain condition, or use of opioids or tramadol daily for > 7 days immediately before study medication administration | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization by performed by kit randomization, but there was an error (all first 109 participants allocated to either 1000 mg paracetamol or 65 ml placebo group) |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Low risk | Blinding procedures seem adequate. Active drug and placebo solution, bottles, and labels were identical in appearance. |
| Incomplete outcome data (attrition bias) | Low risk | Outcomes seem complete. Dropouts completely described and similar between groups. WOCF imputation used for observations recorded after first request for analgesia. BOCF for observations before first request. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported Protocol published on clinicaltrials.gov. All outcomes from protocol reported as planned. SPID 4 & 6, TOTPAR 4 & 6, time to rescue, rescue requirements all reported despite not being mentioned on clinicaltrials.gov. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (92 paracetamol, 43 placebo, 67 placebo) |
| Methods | Randomized, double‐blinded, double‐dummy, placebo‐ and active‐controlled Medication administered on postoperative day 1 when baseline pain reached moderate‐to‐severe intensity | |
| Participants | Type of surgery: orthopedic Propacetamol group Entered/completing: 60/57 Age (mean, SD): 61.4 ± 12.0 Sex (male, %): 37 Ketorolac group Entered/completing: 28/27 Age (mean, SD): 60.6 ± 11.1 Sex (male, %): 22 Placebo group Entered/completing: 55/52 Age (mean, SD): 60.9 ± 12.4 Sex (male, %): 40 | |
| Interventions | Intervention: 2 g propacetamol over 15 min Control: 15 mg ketorolac (not included in our analysis) Control: 30 mg ketorolac Placebo: saline | |
| Outcomes | Time to onset of and number of patients experiencing analgesia (double‐stopwatch method) Pain intensity at rest and with activity (VRS, VAS) and derived summary measures Pain relief (categorical) and derived summary measures Time to, number of patients requesting, and consumption of rescue medication (morphine via PCA) Global evaluation (categorical) | |
| Source of funding | Supported in part by a grant from UPSA Inc., France, and in part by the White Mountain Institute (a non–for profit public charity) in Los Altos, CA | |
| Were treatment groups comparable at baseline? | Yes: demographic, anesthetic and surgical characteristics | |
| Details of preoperative pain | Not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Low risk | All study medication solutions prepared by a hospital pharmacist who was not involved in the data collection. Double‐dummy technique employed. |
| Incomplete outcome data (attrition bias) | Low risk | Although 172 patients were initially randomized into the study groups, 164 received the study medication and were included in the intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Free of selective reporting. All outcomes from Methods section reported in Results section. |
| Size | Unclear risk | 50 to 199 participants per arm of the study (60 propacetamol, 28 ketorolac, 55 placebo) |
AE = adverse event; ASA = American Society of Anesthesiologists physical status classification system; AUC = area under the curve; BMI: body mass index; BOCF = baseline observation carried forward; BP = blood pressure; DSST = digit symbol substitution test; h = hour; HR = heart rate; ICU = intensive care unit; IM = intramuscular; INR = international normalized ratio; IQR = interquartile range; ITT = intention‐to‐treat; IV = intravenous; LA = local anesthetic; LOCF = last observation carried forward; min = minutes; NRS = numerical rating scale; NS = normal saline; N/V = nausea/vomiting; OR = operating room; PACU = post anesthesia care unit; PCA = patient‐controlled analgesia; PI = pain intensity; PT = prothrombin time; SD = standard deviation; SPID = summed pain intensity difference; TDT = Trieger dot test; THA = total hip arthroplasty; TOTPAR = total pain relief; VAS = visual analog scale; VPS = verbal pain score; VRS = verbal rating scale; WOCF = worst observation carried forward.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Pain not patient‐reported | |
| Pain not patient‐reported | |
| Paracetamol administered via continuous infusion | |
| Outcomes assessed over 75 minutes only | |
| Propacetamol administered intramuscularly | |
| Available as abstract only with no usable data | |
| No pain or analgesic outcome | |
| Preoperative administration of interventions | |
| Not all participants had postoperative pain, efficacy outcomes were assessed at 24 hour intervals, not clear when drugs were administered | |
| Preemptive administration of interventions | |
| Appears that control group did not receive placebo. Pain data only presented up to 1 hour. | |
| First dose received after induction of anesthesia, no 4‐ or 6‐hour pain data | |
| Compares laparotomy to laparoscopy | |
| No data provided on 4‐ or 6‐hour intervals | |
| Participants received propacetamol 30 minutes before operation | |
| Paracetamol administered via continuous infusion and no data at 4 or 6 hours | |
| Available as abstract only with no usable data | |
| Interventions administered 10 minutes after induction of anesthesia. Operations were at least 1 hour long; therefore administration > 30 minutes before end of surgery. | |
| Multiple dose study without data for first dose | |
| Study drug administered more than 30 minutes before the end of surgery | |
| No data provided for either 4‐ or 6‐hour intervals | |
| All patients initially receive IV paracetamol | |
| Preoperative administration of interventions | |
| Pain not patient‐reported, unclear if interventions within 30 minutes of end of surgery | |
| Multiple dose study without data for first dose | |
| Some pain assessments investigator‐reported. Unable to ascertain numbers of participants self reporting pain. | |
| Pain not patient‐reported | |
| Pain not patient‐reported | |
| Not randomized | |
| No data beyond 3 h | |
| Received paracetamol in both arms to evaluate efficacy of drug metamizol | |
| No pain data until 8 h post interventions | |
| Study drug administered more than 30 minutes before the end of surgery | |
| Control group did not receive active control or placebo | |
| One control group had intervention administered before induction, and the other control group received no intervention (no placebo was administered) | |
| Interventions administered 30 minutes before surgical incision | |
| Control group received no intervention | |
| Paracetamol plus placebo versus paracetamol plus metamizole, versus no treatment | |
| Interventions administered 1 hour before the end of surgery | |
| Dose finding study with outcomes reported at 45 minutes | |
| All groups received paracetamol |
IV: intravenous
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Unclear |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Unable to obtain full article from any source |
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Not yet assessed |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 11 | 1149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [2.01, 3.19] |
| Analysis 1.1  Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 5 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.80 [2.30, 10.00] |
| 1.2 Propacetamol vs placebo | 8 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.77] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 5 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.18] |
| Analysis 1.2  Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 2.2 Propacetamol vs NSAIDs | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.34] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.59] |
| Analysis 1.3  Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 3 Propacetamol vs opioids. | ||||
| 4 Paracetamol vs propacetamol Show forest plot | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| Analysis 1.4  Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 4 Paracetamol vs propacetamol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 10 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [2.10, 3.91] |
| Analysis 2.1  Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 6 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.65 [2.15, 6.21] |
| 1.2 Propacetamol vs placebo | 6 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.64, 3.50] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 5 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.66, 0.95] |
| Analysis 2.2  Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.02] |
| 2.2 Propacetamol vs NSAIDs | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.02] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| Analysis 2.3  Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 3 Propacetamol vs opioids. | ||||
| 4 Paracetamol vs propacetamol Show forest plot | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| Analysis 2.4  Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 4 Paracetamol vs propacetamol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol vs placebo Show forest plot | 6 | 485 | Mean Difference (IV, Fixed, 95% CI) | ‐1.21 [‐3.73, 1.31] |
| Analysis 3.1  Comparison 3 Pain intensity at 4 h, Outcome 1 Paracetamol vs placebo. | ||||
| 2 Paracetamol vs NSAIDs Show forest plot | 6 | 350 | Mean Difference (IV, Fixed, 95% CI) | 5.02 [3.18, 6.86] |
| Analysis 3.2  Comparison 3 Pain intensity at 4 h, Outcome 2 Paracetamol vs NSAIDs. | ||||
| 3 Paracetamol vs opioids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐19.23, ‐4.77] |
| Analysis 3.4  Comparison 3 Pain intensity at 4 h, Outcome 4 Paracetamol vs ketamine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol vs placebo Show forest plot | 12 | 837 | Mean Difference (IV, Fixed, 95% CI) | ‐7.48 [‐8.98, ‐5.97] |
| Analysis 4.1  Comparison 4 Pain intensity at 6 h, Outcome 1 Paracetamol vs placebo. | ||||
| 2 Paracetamol vs NSAIDs Show forest plot | 9 | 524 | Mean Difference (IV, Fixed, 95% CI) | 2.95 [1.18, 4.72] |
| Analysis 4.2  Comparison 4 Pain intensity at 6 h, Outcome 2 Paracetamol vs NSAIDs. | ||||
| 3 Paracetamol vs opioids Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐1.57, 7.57] |
| Analysis 4.3  Comparison 4 Pain intensity at 6 h, Outcome 3 Paracetamol vs opioids. | ||||
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐18.28, ‐7.72] |
| Analysis 4.4  Comparison 4 Pain intensity at 6 h, Outcome 4 Paracetamol vs ketamine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 9 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.64, 0.77] |
| Analysis 5.1  Comparison 5 Number of participants requiring rescue medication, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 6 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.82] |
| 1.2 Propacetamol vs placebo | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.35, 0.69] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 5 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.63] |
| Analysis 5.2  Comparison 5 Number of participants requiring rescue medication, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.98] |
| 2.2 Propacetamol vs NSAIDs | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.77] |
| 3 Propacetamol vs opioids Show forest plot | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.72, 4.64] |
| Analysis 5.3  Comparison 5 Number of participants requiring rescue medication, Outcome 3 Propacetamol vs opioids. | ||||
| 4 Paracetamol vs propacetamol Show forest plot | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.82, 2.17] |
| Analysis 5.4  Comparison 5 Number of participants requiring rescue medication, Outcome 4 Paracetamol vs propacetamol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 9 | 839 | Mean Difference (IV, Fixed, 95% CI) | 6.43 [4.54, 8.32] |
| Analysis 6.1  Comparison 6 Time to rescue medication (minutes), Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 6 | 523 | Mean Difference (IV, Fixed, 95% CI) | 5.78 [3.86, 7.71] |
| 1.2 Propacetamol vs placebo | 3 | 316 | Mean Difference (IV, Fixed, 95% CI) | 23.72 [13.79, 33.65] |
| 2 Paracetamol vs NSAIDs Show forest plot | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐36.21, 33.92] |
| Analysis 6.2  Comparison 6 Time to rescue medication (minutes), Outcome 2 Paracetamol vs NSAIDs. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 6 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.81, ‐1.03] |
| Analysis 7.1  Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 4 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.75, ‐0.91] |
| 1.2 Propacetamol vs placebo | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.15, ‐0.95] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.37, ‐0.02] |
| Analysis 7.2  Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐1.04, 1.59] |
| 2.2 Propacetamol vs NSAIDs | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.09, 1.09] |
| Analysis 7.3  Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 3 Propacetamol vs opioids. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 13 | 777 | Mean Difference (IV, Fixed, 95% CI) | ‐1.92 [‐2.41, ‐1.42] |
| Analysis 8.1  Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 8 | 404 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐2.35, ‐1.31] |
| 1.2 Propacetamol vs placebo | 6 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐2.67 [‐4.21, ‐1.13] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 8 | 540 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.37, 0.12] |
| Analysis 8.2  Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐0.87, 2.49] |
| 2.2 Propacetamol vs NSAIDs | 5 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.39, 0.11] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.01, 2.01] |
| Analysis 8.3  Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 3 Propacetamol vs opioids. | ||||
| 4 Paracetamol vs propacetamol Show forest plot | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.15, 3.35] |
| Analysis 8.4  Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 4 Paracetamol vs propacetamol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 16 | 2015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.25, 1.43] |
| Analysis 9.1  Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 9 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.31, 1.60] |
| 1.2 Propacetamol vs placebo | 9 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.37] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 11 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| Analysis 9.2  Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 6 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 2.2 Propacetamol vs NSAIDs | 5 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| Analysis 9.3  Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 3 Propacetamol vs opioids. | ||||
| 3.1 Propacetamol | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 4 Paracetamol vs propacetamol Show forest plot | 2 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.15] |
| Analysis 9.4  Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 4 Paracetamol vs propacetamol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 3 | 342 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.04, 0.66] |
| Analysis 10.1  Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 1.2 Propacetamol vs placebo | 2 | 282 | Mean Difference (IV, Fixed, 95% CI) | 1.64 [1.04, 2.25] |
| 2 Paracetamol vs NSAIDs Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.63, 0.03] |
| Analysis 10.2  Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 2 Paracetamol vs NSAIDs. | ||||
| 3 Propacetamol vs opioids Show forest plot | 2 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.18, 0.98] |
| Analysis 10.3  Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 3 Propacetamol vs opioids. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 20 | 2359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| Analysis 11.1  Comparison 11 Number of participants with adverse events, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 12 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.19] |
| 1.2 Propacetamol vs placebo | 10 | 1409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 6 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.32] |
| Analysis 11.2  Comparison 11 Number of participants with adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.68] |
| 2.2 Propacetamol vs NSAIDs | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.37] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.23] |
| Analysis 11.3  Comparison 11 Number of participants with adverse events, Outcome 3 Propacetamol vs opioids. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 10 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.19, 6.59] |
| Analysis 12.1  Comparison 12 Number of participants with serious adverse events, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 6 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.19, 6.59] |
| 1.2 Propacetamol vs placebo | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 9 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.65] |
| Analysis 12.2  Comparison 12 Number of participants with serious adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 4 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 2.2 Propacetamol vs NSAIDs | 5 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.65] |
| 3 Paracetamol or propacetamol vs opioids Show forest plot | 3 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| Analysis 12.3  Comparison 12 Number of participants with serious adverse events, Outcome 3 Paracetamol or propacetamol vs opioids. | ||||
| 3.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 37 | 2654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.56, 2.84] |
| Analysis 13.1  Comparison 13 Number of participants withdrawing due to adverse events, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 27 | 1912 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.17] |
| 1.2 Propacetamol vs placebo | 12 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.25, 6.68] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 24 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.42, 3.12] |
| Analysis 13.2  Comparison 13 Number of participants withdrawing due to adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 19 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.22] |
| 2.2 Propacetamol vs NSAIDs | 5 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.22, 12.37] |
| 3 Paracetamol or propacetamol vs opioids Show forest plot | 6 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| Analysis 13.3  Comparison 13 Number of participants withdrawing due to adverse events, Outcome 3 Paracetamol or propacetamol vs opioids. | ||||
| 3.1 Paracetamol vs opioids | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 13.4  Comparison 13 Number of participants withdrawing due to adverse events, Outcome 4 Paracetamol vs ketamine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 38 | 2600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.79] |
| Analysis 14.1  Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 1 Paracetamol or propacetamol vs placebo. | ||||
| 1.1 Paracetamol vs placebo | 26 | 1711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.78] |
| 1.2 Propacetamol vs placebo | 14 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.80] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 24 | 1393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.81, 2.08] |
| Analysis 14.2  Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 2 Paracetamol or propacetamol vs NSAIDs. | ||||
| 2.1 Paracetamol vs NSAIDs | 18 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.89] |
| 2.2 Propacetamol vs NSAIDs | 6 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.78, 2.03] |
| 3 Paracetamol or propacetamol vs opioids Show forest plot | 6 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| Analysis 14.3  Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 3 Paracetamol or propacetamol vs opioids. | ||||
| 3.1 Paracetamol vs opioids | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 14.4  Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 4 Paracetamol vs ketamine. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol vs placebo Show forest plot | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.80, 11.54] |
| Analysis 15.1  Comparison 15 Number of participants with pain on infusion, Outcome 1 Paracetamol vs placebo. | ||||
| 2 Propacetamol vs placebo Show forest plot | 6 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.07 [5.35, 31.98] |
| Analysis 15.2  Comparison 15 Number of participants with pain on infusion, Outcome 2 Propacetamol vs placebo. | ||||
| 3 Propacetamol vs paracetamol Show forest plot | 3 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.31 [4.20, 16.46] |
| Analysis 15.3  Comparison 15 Number of participants with pain on infusion, Outcome 3 Propacetamol vs paracetamol. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 15 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.98] |
| Analysis 16.1  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 1 Nausea. | ||||
| 1.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.66, 0.90] |
| 1.2 Propacetamol vs placebo | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.98, 2.69] |
| 2 Vomiting Show forest plot | 15 | 1414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.87] |
| Analysis 16.2  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 2 Vomiting. | ||||
| 2.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.80] |
| 2.2 Propacetamol vs placebo | 3 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.75, 3.48] |
| 3 Nausea/vomiting Show forest plot | 10 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.08] |
| Analysis 16.3  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 3 Nausea/vomiting. | ||||
| 3.1 Paracetamol vs placebo | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.57, 1.70] |
| 3.2 Propacetamol vs placebo | 6 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 4 Pruritus Show forest plot | 7 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.60, 1.40] |
| Analysis 16.4  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 4 Pruritus. | ||||
| 4.1 Paracetamol vs placebo | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.72] |
| 4.2 Propacetamol vs placebo | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.51] |
| 5 Respiratory depression Show forest plot | 11 | 1082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.31, 1.92] |
| Analysis 16.5  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 5 Respiratory depression. | ||||
| 5.1 Paracetamol vs placebo | 6 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.65] |
| 5.2 Propacetamol vs placebo | 5 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.35, 2.80] |
| 6 Sedation Show forest plot | 10 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.66, 1.51] |
| Analysis 16.6  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 6 Sedation. | ||||
| 6.1 Paracetamol vs placebo | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.42, 2.01] |
| 6.2 Propacetamol vs placebo | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.69] |
| 7 Urinary retention Show forest plot | 8 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| Analysis 16.7  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 7 Urinary retention. | ||||
| 7.1 Paracetamol vs placebo | 5 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.28, 6.66] |
| 7.2 Propacetamol vs placebo | 3 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.65, 1.66] |
| 8 Allergy/skin rash/local reaction Show forest plot | 7 | 1131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.61, 3.91] |
| Analysis 16.8  Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 8 Allergy/skin rash/local reaction. | ||||
| 8.1 Paracetamol vs placebo | 4 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.24, 4.34] |
| 8.2 Propacetamol vs placebo | 4 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.57, 6.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 11 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.28] |
| Analysis 17.1  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 1 Nausea. | ||||
| 1.1 Paracetamol vs NSAIDs | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.31] |
| 1.2 Propacetamol vs NSAIDs | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.44] |
| 2 Vomiting Show forest plot | 11 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.89, 1.55] |
| Analysis 17.2  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 2 Vomiting. | ||||
| 2.1 Paracetamol vs NSAIDs | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.68] |
| 2.2 Propacetamol vs NSAIDs | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.81] |
| 3 Nausea/vomiting Show forest plot | 8 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.62, 1.97] |
| Analysis 17.3  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 3 Nausea/vomiting. | ||||
| 3.1 Paracetamol vs NSAIDs | 4 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.42, 2.39] |
| 3.2 Propacetamol vs NSAIDs | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.60] |
| 4 Pruritus Show forest plot | 8 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.34] |
| Analysis 17.4  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 4 Pruritus. | ||||
| 4.1 Paracetamol vs NSAIDs | 5 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.51] |
| 4.2 Propacetamol vs NSAIDs | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.37, 1.60] |
| 5 Respiratory depression Show forest plot | 9 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.26] |
| Analysis 17.5  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 5 Respiratory depression. | ||||
| 5.1 Paracetamol vs NSAIDs | 8 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.26] |
| 5.2 Propacetamol vs NSAIDs | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sedation Show forest plot | 6 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.63, 10.75] |
| Analysis 17.6  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 6 Sedation. | ||||
| 6.1 Paracetamol vs NSAIDs | 4 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 6.2 Propacetamol vs NSAIDs | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.63, 21.22] |
| 7 Urinary retention Show forest plot | 6 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.51, 2.32] |
| Analysis 17.7  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 7 Urinary retention. | ||||
| 7.1 Paracetamol vs NSAIDs | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 4.02] |
| 7.2 Propacetamol vs NSAIDs | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.47, 2.78] |
| 8 Allergy/skin rash/local reaction Show forest plot | 8 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.26] |
| Analysis 17.8  Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 8 Allergy/skin rash/local reaction. | ||||
| 8.1 Paracetamol vs NSAIDs | 6 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.02] |
| 8.2 Propacetamol vs NSAIDs | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 6 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.65] |
| Analysis 18.1  Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 1 Nausea. | ||||
| 1.1 Paracetamol vs opioids | 4 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.56] |
| 1.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.91] |
| 2 Vomiting Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.72] |
| Analysis 18.2  Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 2 Vomiting. | ||||
| 2.1 Paracetamol vs opioids | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.71] |
| 2.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.62] |
| 3 Nausea/vomiting Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.64] |
| Analysis 18.3  Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 3 Nausea/vomiting. | ||||
| 3.1 Propacetamol vs opioids | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.64] |
| 4 Pruritus Show forest plot | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.43] |
| Analysis 18.4  Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 4 Pruritus. | ||||
| 4.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| 4.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.10, 1.19] |
| 5 Respiratory depression Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Analysis 18.5  Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 5 Respiratory depression. | ||||
| 5.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sedation Show forest plot | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| Analysis 18.6  Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 6 Sedation. | ||||
| 6.1 Paracetamol vs opioids | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.30] |
| Analysis 19.1  Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 1 Nausea. | ||||
| 2 Vomiting Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.22, 1.33] |
| Analysis 19.2  Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 2 Vomiting. | ||||
| 3 Sedation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| Analysis 19.3  Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 3 Sedation. | ||||
2016 Study flow diagram.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Number of participants with > 50% pain relief over 4 hours, outcome: 1.1 Propacetamol or paracetamol versus placebo.

Forest plot of comparison: 2 Number of participants with > 50% pain relief over 6 hours, outcome: 2.1 Propacetamol or paracetamol versus placebo.
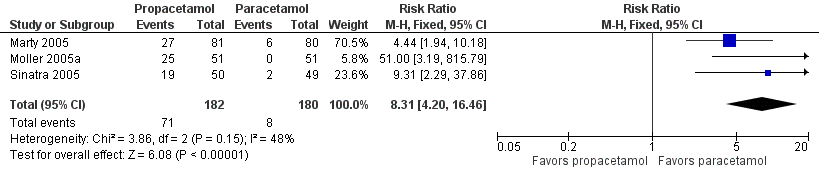
Forest plot of comparison: 15 Pain on infusion, outcome: 15.3 Propacetamol versus paracetamol.

Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.

Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 3 Propacetamol vs opioids.

Comparison 1 Number of participants with > 50% pain relief over 4 hours, Outcome 4 Paracetamol vs propacetamol.

Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
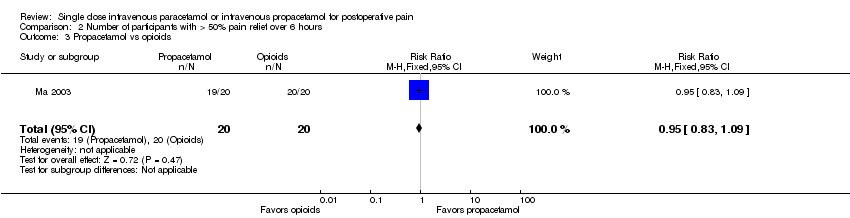
Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 3 Propacetamol vs opioids.

Comparison 2 Number of participants with > 50% pain relief over 6 hours, Outcome 4 Paracetamol vs propacetamol.
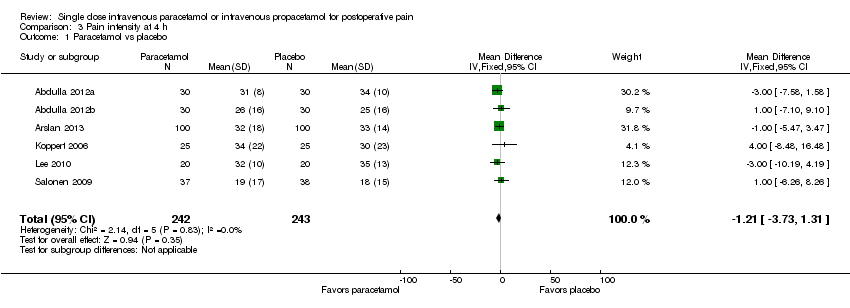
Comparison 3 Pain intensity at 4 h, Outcome 1 Paracetamol vs placebo.
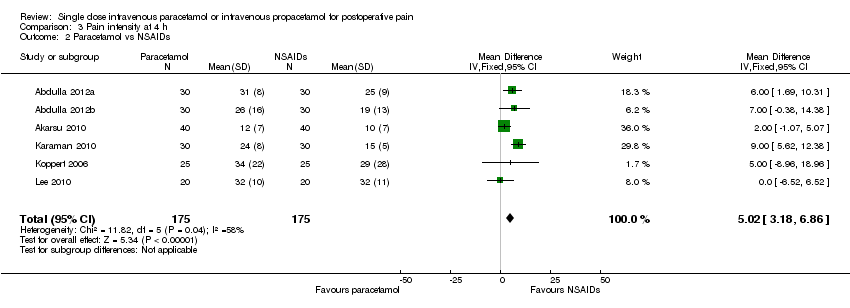
Comparison 3 Pain intensity at 4 h, Outcome 2 Paracetamol vs NSAIDs.

Comparison 3 Pain intensity at 4 h, Outcome 4 Paracetamol vs ketamine.
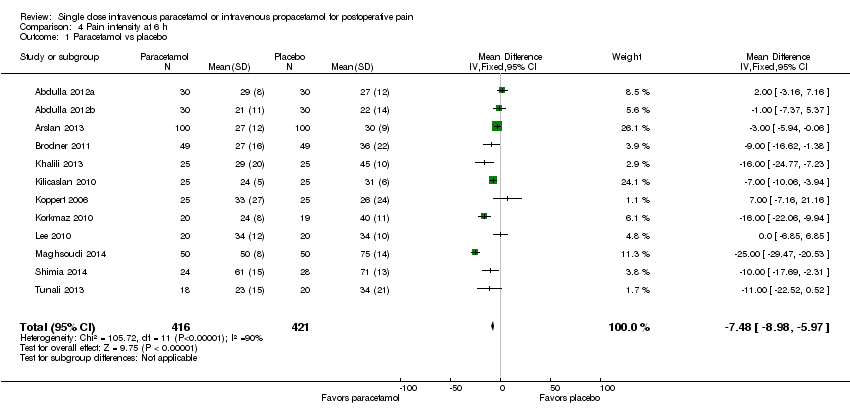
Comparison 4 Pain intensity at 6 h, Outcome 1 Paracetamol vs placebo.

Comparison 4 Pain intensity at 6 h, Outcome 2 Paracetamol vs NSAIDs.

Comparison 4 Pain intensity at 6 h, Outcome 3 Paracetamol vs opioids.

Comparison 4 Pain intensity at 6 h, Outcome 4 Paracetamol vs ketamine.

Comparison 5 Number of participants requiring rescue medication, Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 5 Number of participants requiring rescue medication, Outcome 2 Paracetamol or propacetamol vs NSAIDs.

Comparison 5 Number of participants requiring rescue medication, Outcome 3 Propacetamol vs opioids.
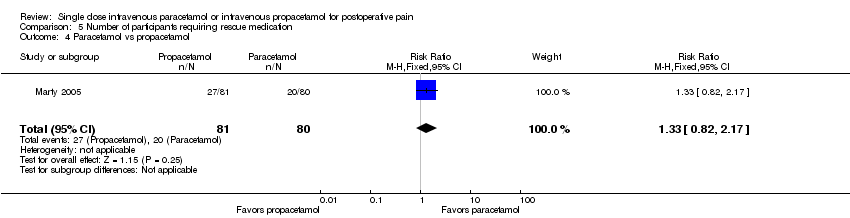
Comparison 5 Number of participants requiring rescue medication, Outcome 4 Paracetamol vs propacetamol.

Comparison 6 Time to rescue medication (minutes), Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 6 Time to rescue medication (minutes), Outcome 2 Paracetamol vs NSAIDs.
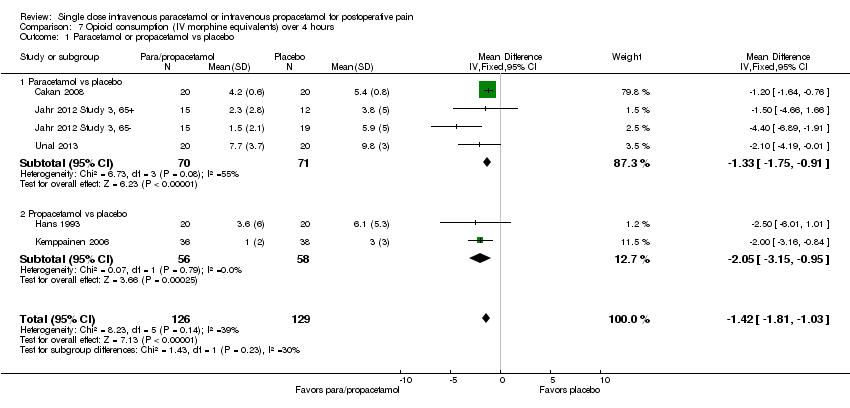
Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 1 Paracetamol or propacetamol vs placebo.
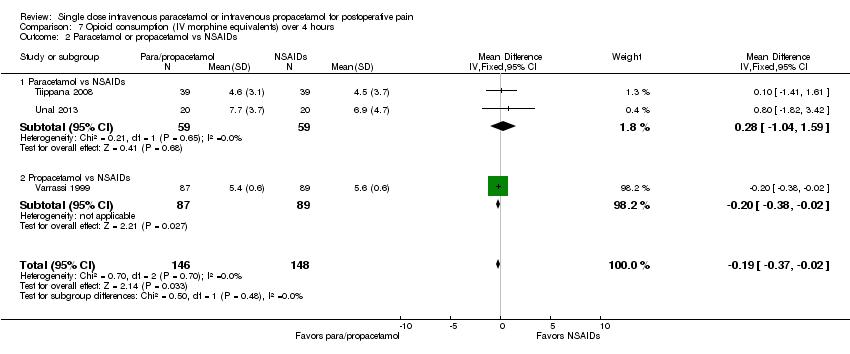
Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.

Comparison 7 Opioid consumption (IV morphine equivalents) over 4 hours, Outcome 3 Propacetamol vs opioids.

Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 1 Paracetamol or propacetamol vs placebo.
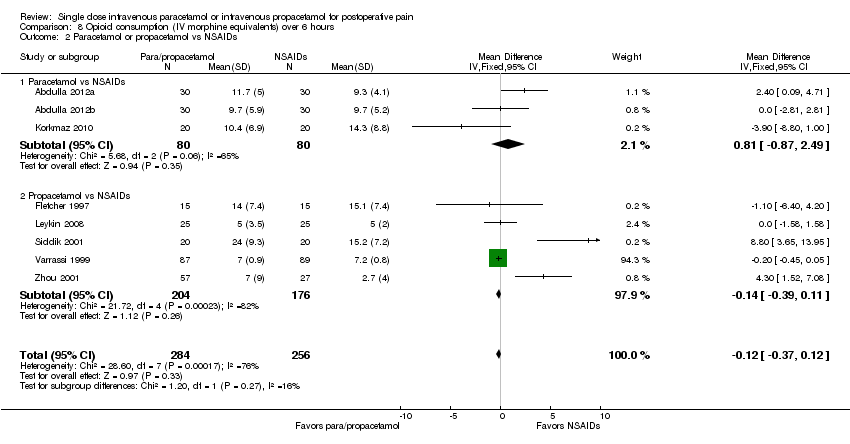
Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 2 Paracetamol or propacetamol vs NSAIDs.

Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 3 Propacetamol vs opioids.

Comparison 8 Opioid consumption (IV morphine equivalents) over 6 hours, Outcome 4 Paracetamol vs propacetamol.

Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 1 Paracetamol or propacetamol vs placebo.
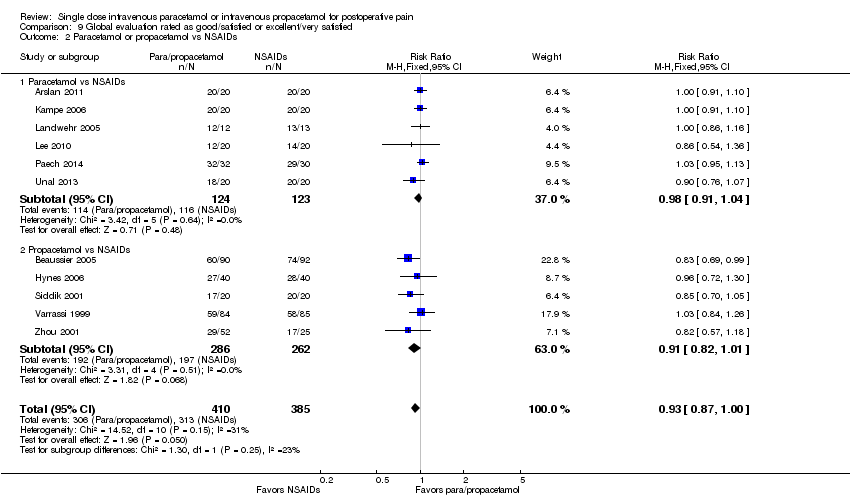
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
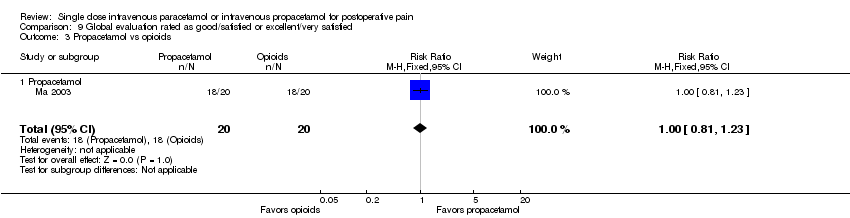
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 3 Propacetamol vs opioids.
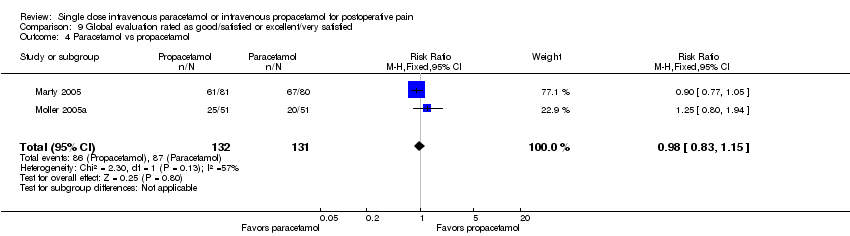
Comparison 9 Global evaluation rated as good/satisfied or excellent/very satisfied, Outcome 4 Paracetamol vs propacetamol.

Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 1 Paracetamol or propacetamol vs placebo.
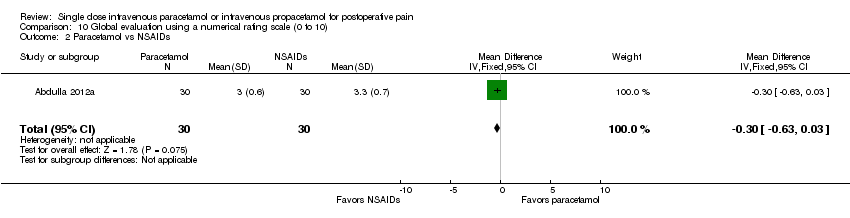
Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 2 Paracetamol vs NSAIDs.

Comparison 10 Global evaluation using a numerical rating scale (0 to 10), Outcome 3 Propacetamol vs opioids.

Comparison 11 Number of participants with adverse events, Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 11 Number of participants with adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
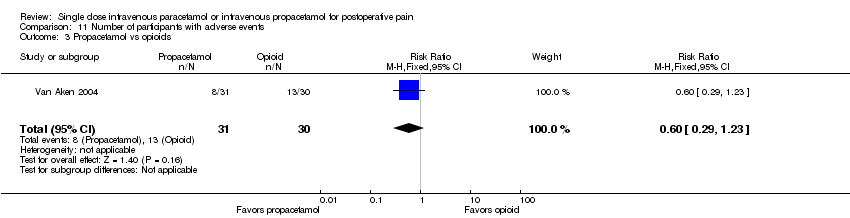
Comparison 11 Number of participants with adverse events, Outcome 3 Propacetamol vs opioids.

Comparison 12 Number of participants with serious adverse events, Outcome 1 Paracetamol or propacetamol vs placebo.
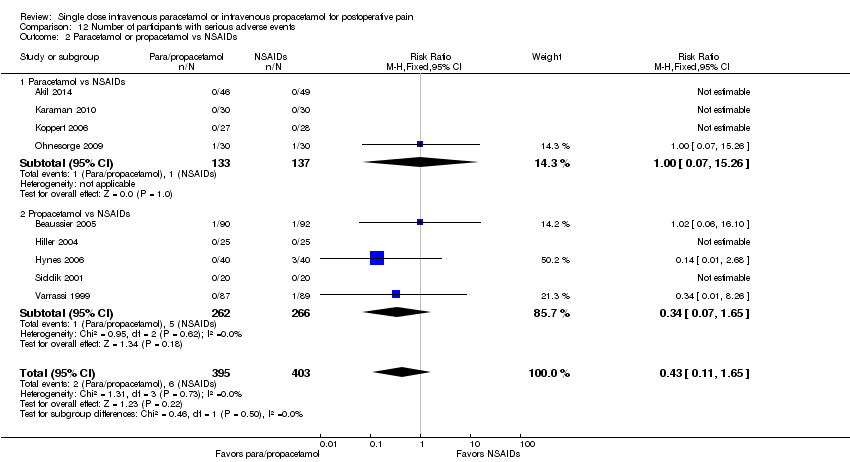
Comparison 12 Number of participants with serious adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs.

Comparison 12 Number of participants with serious adverse events, Outcome 3 Paracetamol or propacetamol vs opioids.

Comparison 13 Number of participants withdrawing due to adverse events, Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 13 Number of participants withdrawing due to adverse events, Outcome 2 Paracetamol or propacetamol vs NSAIDs.
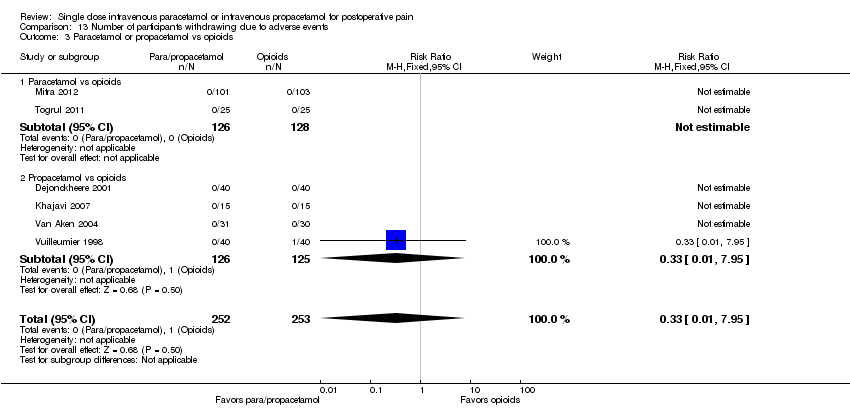
Comparison 13 Number of participants withdrawing due to adverse events, Outcome 3 Paracetamol or propacetamol vs opioids.

Comparison 13 Number of participants withdrawing due to adverse events, Outcome 4 Paracetamol vs ketamine.

Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 1 Paracetamol or propacetamol vs placebo.

Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 2 Paracetamol or propacetamol vs NSAIDs.

Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 3 Paracetamol or propacetamol vs opioids.

Comparison 14 Number of participants withdrawing due to lack of efficacy, Outcome 4 Paracetamol vs ketamine.

Comparison 15 Number of participants with pain on infusion, Outcome 1 Paracetamol vs placebo.

Comparison 15 Number of participants with pain on infusion, Outcome 2 Propacetamol vs placebo.
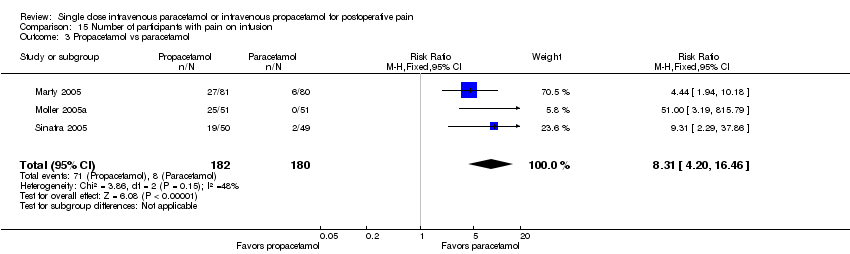
Comparison 15 Number of participants with pain on infusion, Outcome 3 Propacetamol vs paracetamol.

Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 1 Nausea.

Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 2 Vomiting.
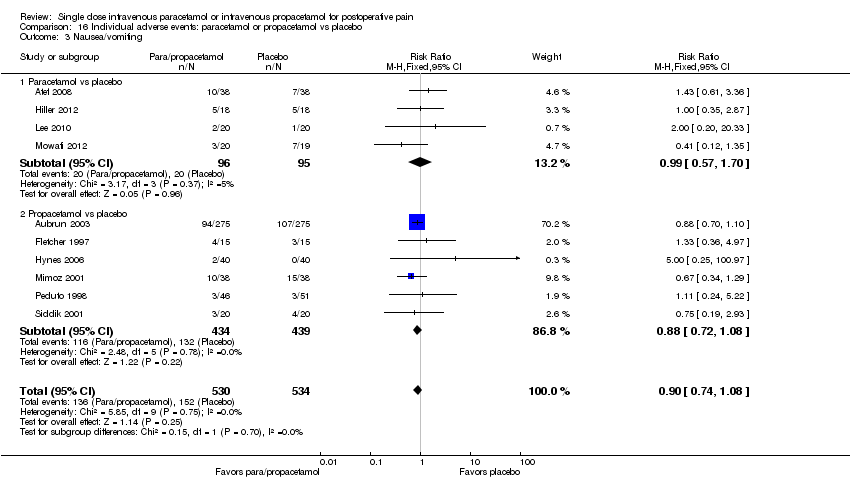
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 3 Nausea/vomiting.

Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 4 Pruritus.

Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 5 Respiratory depression.
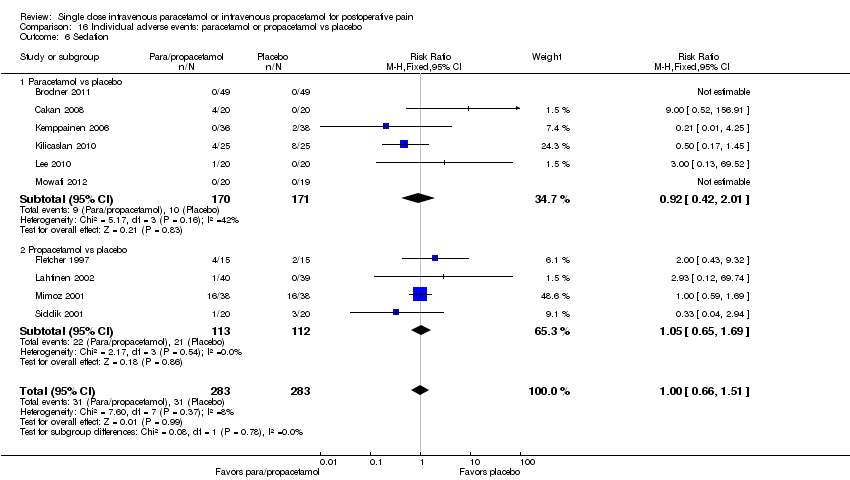
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 6 Sedation.
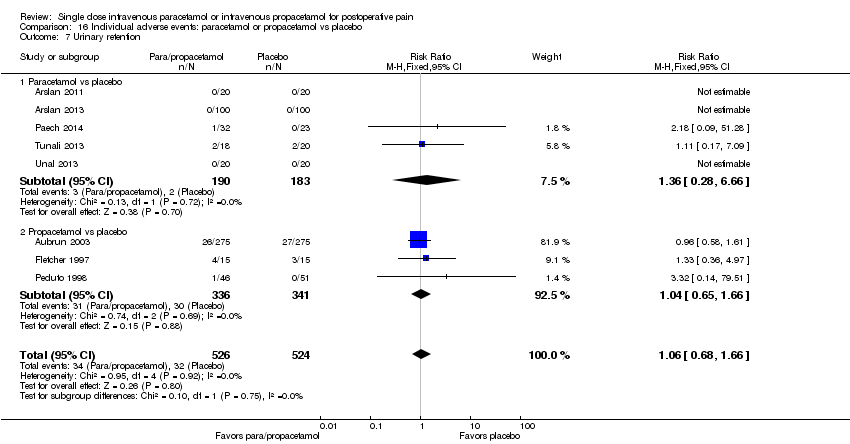
Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 7 Urinary retention.

Comparison 16 Individual adverse events: paracetamol or propacetamol vs placebo, Outcome 8 Allergy/skin rash/local reaction.

Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 1 Nausea.

Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 2 Vomiting.
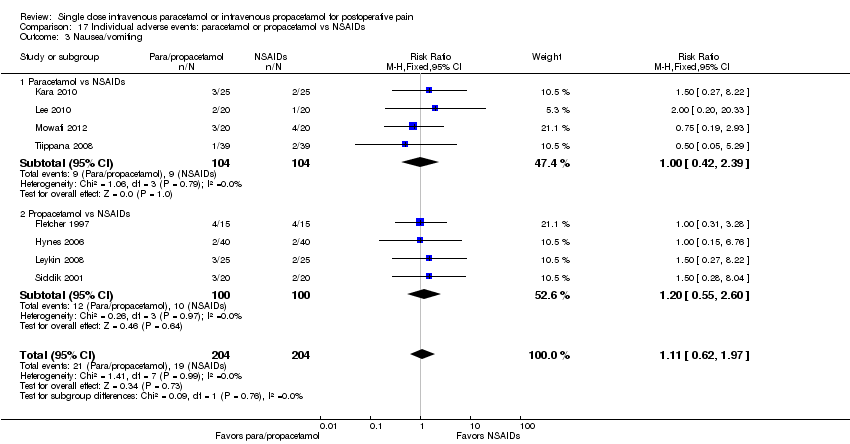
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 3 Nausea/vomiting.
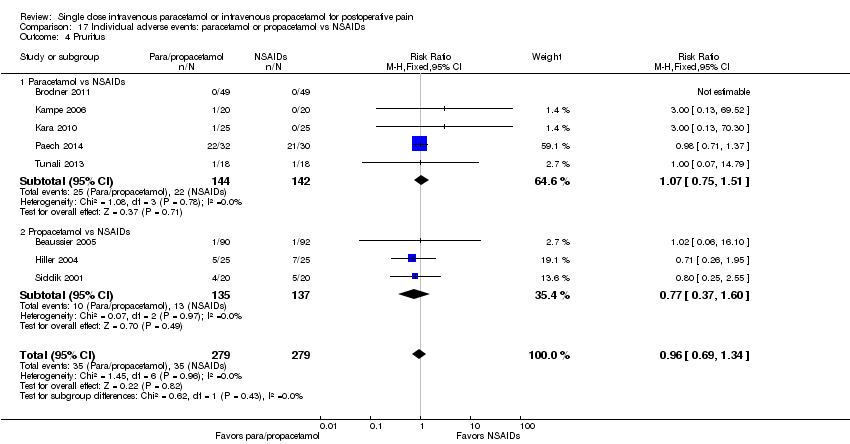
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 4 Pruritus.
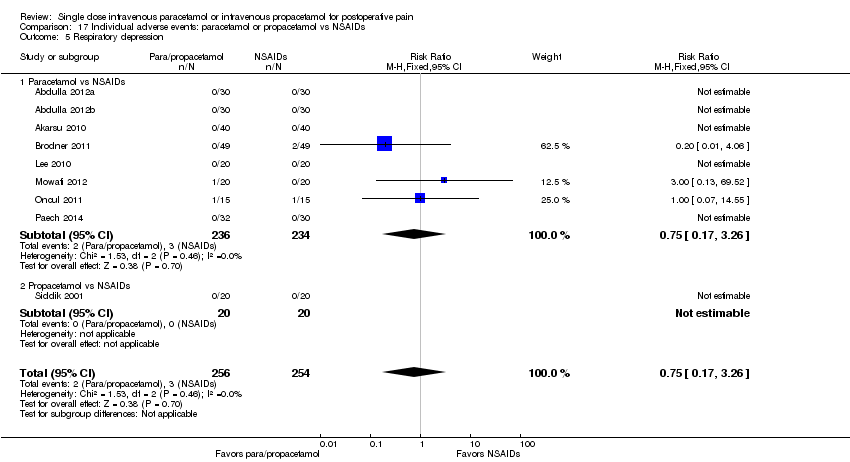
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 5 Respiratory depression.
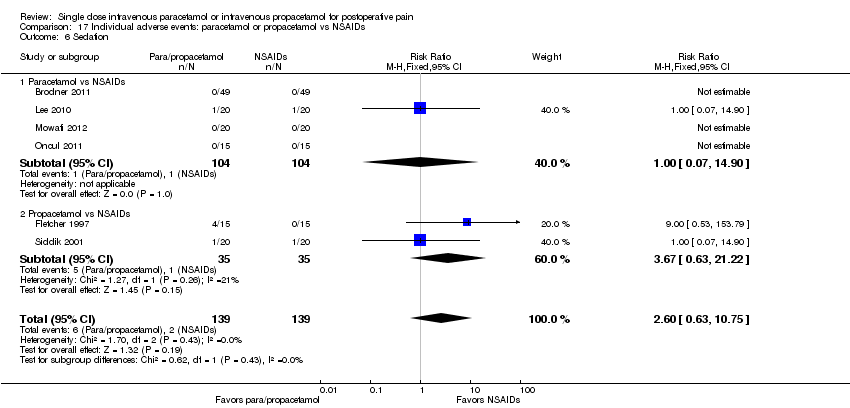
Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 6 Sedation.

Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 7 Urinary retention.

Comparison 17 Individual adverse events: paracetamol or propacetamol vs NSAIDs, Outcome 8 Allergy/skin rash/local reaction.

Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 1 Nausea.

Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 2 Vomiting.

Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 3 Nausea/vomiting.
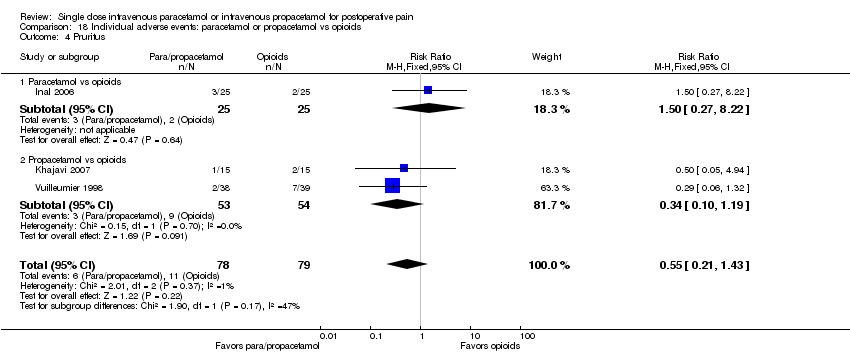
Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 4 Pruritus.

Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 5 Respiratory depression.

Comparison 18 Individual adverse events: paracetamol or propacetamol vs opioids, Outcome 6 Sedation.
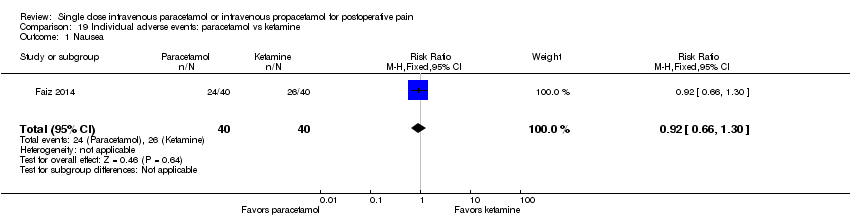
Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 1 Nausea.

Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 2 Vomiting.
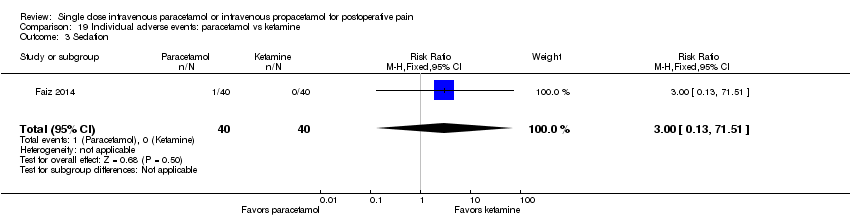
Comparison 19 Individual adverse events: paracetamol vs ketamine, Outcome 3 Sedation.
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain | |||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo | 156 per 1000 | 394 per 1000 | RR 2.53 | 1149 | ⊕⊕⊕⊕ |
| Paracetamol vs placebo | 66 per 1000 | 317 per 1000 | RR 4.8 | 393 | ⊕⊕⊕⊝ |
| Propacetamol vs placebo | 188 per 1000 | 411 per 1000 | RR 2.19 | 756 | ⊕⊕⊕⊝ |
| Para/propacetamol vs NSAIDs | 599 per 1000 | 605 per 1000 | RR 1.01 | 353 | ⊕⊕⊝⊝ |
| Paracetamol vs NSAIDs | 631 per 1000 | 568 per 1000 | RR 0.9 | 130 | ⊕⊝⊝⊝ |
| Propacetamol vs NSAIDs | 577 per 1000 | 624 per 1000 | RR 1.08 | 223 | ⊕⊝⊝⊝ |
| Paracetamol vs propacetamol | 428 per 1000 | 419 per 1000 | RR 0.98 | 361 | ⊕⊕⊕⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1TOTPAR or SPID using either VAS or categorical data, and calculating their corresponding percentage of theoretical maximum TOTPAR and SPID. | |||||
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain | |||||
| Comparison | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo | 97 per 1000 | 276 per 1000 | RR 2.86 | 1143 | ⊕⊕⊕⊝ |
| Paracetamol vs placebo | 83 per 1000 | 304 per 1000 | RR 3.65 | 532 | ⊕⊕⊕⊝ |
| Propacetamol vs placebo | 105 per 1000 | 252 per 1000 | RR 2.4 | 611 | ⊕⊕⊝⊝ |
| Para/propacetamol vs NSAIDs | 632 per 1000 | 499 per 1000 | RR 0.79 | 355 | ⊕⊝⊝⊝ |
| Paracetamol vs NSAIDs | 623 per 1000 | 511 per 1000 | RR 0.82 | 212 | ⊕⊝⊝⊝ |
| Propacetamol vs NSAIDs | 649 per 1000 | 487 per 1000 | RR 0.75 | 143 | ⊕⊝⊝⊝ |
| Paracetamol vs propacetamol | 411 per 1000 | 386 per 1000 | RR 0.94 | 361 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1TOTPAR or SPID using either VAS or categorical data, and calculating their corresponding percentage of theoretical maximum TOTPAR and SPID. | |||||
| IV paracetamol compared to placebo or other analgesics for postoperative pain | |||
| Patient or population: patients with postoperative pain | |||
| Comparison | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence |
| Paracetamol vs placebo | The mean pain intensity over a 4‐hour period was: | 485 | ⊕⊕⊝⊝ |
| Paracetamol vs NSAIDs | The mean pain intensity over a 4‐hour period was: | 350 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1See 'Risk of bias' tables; several unclear assessments related to randomization; unclear to high risk for selective reporting. | |||
| IV paracetamol compared to placebo or other analgesics for postoperative pain | |||
| Patient or population: patients with postoperative pain | |||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence |
| Paracetamol vs placebo | The mean pain intensity over a 6‐hour period was: | 837 | ⊕⊕⊝⊝ |
| Paracetamol vs NSAIDs | The mean pain intensity over a 6‐hour period was: | 524 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1Majority of all individual studies had < 100 total participants. | |||
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo | 820 per 1000 | 574 per 1000 | RR 0.7 | 859 | ⊕⊕⊝⊝ |
| Paracetamol vs placebo | 892 per 1000 | 669 per 1000 | RR 0.75 | 655 | ⊕⊕⊝⊝ |
| Propacetamol vs placebo | 625 per 1000 | 306 per 1000 | RR 0.49 | 204 | ⊕⊕⊝⊝ |
| Para/propacetamol vs NSAIDs | 284 per 1000 | 338 per 1000 | RR 1.19 | 309 | ⊕⊝⊝⊝ |
| Paracetamol vs NSAIDs | 200 per 1000 | 216 per 1000 | RR 1.08 | 120 | ⊕⊝⊝⊝ |
| Propacetamol vs NSAIDs | 337 per 1000 | 414 per 1000 | RR 1.23 | 189 | ⊕⊝⊝⊝ |
| Propacetamol vs opioids | 86 per 1000 | 157 per 1000 | RR 1.83 | 139 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1See 'Risk of bias' tables: several unclear assessments related to randomization and attrition bias; unclear to high risk for selective outcome reporting. | |||||
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||
| Patient or population: patients with postoperative pain | |||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants | Quality of the evidence |
| Para/propacetamol vs placebo | The mean opioid consumption (IV morphine equivalents) over 6 hours was: | 777 | ⊕⊕⊕⊝ |
| Paracetamol vs placebo | The mean opioid consumption (IV morphine equivalents) over 6 hours was: | 404 | ⊕⊕⊝⊝ |
| Propacetamol vs placebo | The mean opioid consumption (IV morphine equivalents) over 6 hours was: | 373 | ⊕⊕⊝⊝ |
| Para/propacetamol vs NSAIDs | The mean opioid consumption (IV morphine equivalents) over 6 hours was: | 540 | ⊕⊝⊝⊝ |
| Paracetamol vs NSAIDs | The mean opioid consumption (IV morphine equivalents) over 6 hours was: | 160 | ⊕⊝⊝⊝ |
| Propacetamol vs NSAIDs | The mean opioid consumption (IV morphine equivalents) over 6 hours was: | 380 | ⊕⊝⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||
| GRADE Working Group grades of evidence | |||
| 1Mean opioid consumption (in mg) over 6 hours in each treatment arm converted into IV morphine‐equivalents, using commonly used and widely accepted opioid conversion tables. | |||
| IV paracetamol/propacetamol compared to placebo or other analgesics for postoperative pain | |||||
| Patient or population: patients with postoperative pain | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Placebo or other analgesics | IV paracetamol/propacetamol | ||||
| Para/propacetamol vs placebo | 208 per 1000 | 145 per 1000 | RR 0.7 | 1414 | ⊕⊝⊝⊝ |
| Paracetamol vs placebo | 263 per 1000 | 168 per 1000 | RR 0.64 | 1037 | ⊕⊝⊝⊝ |
| Propacetamol vs placebo | 45 per 1000 | 74 per 1000 | RR 1.62 | 377 | ⊕⊕⊝⊝ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1Majority of all individual studies had < 100 participants. | |||||
| Comparison/outcome | Number of studies | Number of participants | n with outcome/total | % with outcome | Risk difference | NNT | Susceptibility to publication bias | ||
| Active | Control | Active | Control | ||||||
| Comparison 1. Number of participants with > 50% pain relief over 4 hours | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 11 | 1149 | 250/687 | 72/462 | 36 | 16 | 0.23 (0.18 to 0.27) | 5 | 1492 |
| 1.1 Paracetamol vs placebo | 5 | 393 | 84/272 | 8/121 | 31 | 7 | 0.24 (0.17 to 0.31) | 5 | 549 |
| 1.2 Propacetamol vs placebo | 8 | 756 | 166/415 | 64/341 | 40 | 19 | 0.22 (0.17 to 0.27) | 5 | 906 |
| Comparison 2. Number of participants with > 50% pain relief over 6 hours | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 10 | 1143 | 200/708 | 42/435 | 28 | 10 | 0.18 (0.14 to 0.22) | 6 | 913 |
| 1.1 Paracetamol vs placebo | 6 | 532 | 109/364 | 14/168 | 30 | 8 | 0.22 (0.16 to 0.29) | 5 | 637 |
| 1.2 Propacetamol vs placebo | 6 | 611 | 91/344 | 28/267 | 26 | 10 | 0.15 (0.10 0.20) | 7 | 305 |
| 2. Paracetamol or propacetamol vs NSAIDs | 5 | 355 | 95/192 | 103/163 | 50 | 63 | ‐0.13 (‐0.23 to ‐0.03) | 8* | 107 |
| Comparison 5. Number of participants requiring rescue medication | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 9 | 859 | 295/476 | 314/383 | 62 | 82 | ‐0.25 (‐0.30 to ‐0.19) | 4 | 1289 |
| 1.1 Paracetamol vs placebo | 6 | 655 | 267/376 | 249/279 | 71 | 89 | ‐0.22 (‐0.28 to ‐0.17) | 5 | 785 |
| 1.2 Propacetamol vs placebo | 3 | 204 | 31/100 | 65/104 | 31 | 62 | ‐0.32 (‐0.44 to ‐0.19) | 4 | 449 |
| Comparison 9. Global evaluation rated as good/satisfied or excellent/very satisfied | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 16 | 2015 | 787/1100 | 529/915 | 72 | 58 | 0.19 (0.15 to 0.23) | 6 | 1816 |
| 1.1 Paracetamol vs placebo | 9 | 876 | 355/508 | 207/368 | 70 | 56 | 0.24 (0.19 to 0.29) | 5 | 1225 |
| 1.2 Propacetamol vs placebo | 9 | 1139 | 432/592 | 322/547 | 73 | 59 | 0.15 (0.10 to 0.21) | 7 | 569 |
| 2. Paracetamol or propacetamol vs NSAIDs | 11 | 795 | 306/410 | 313/385 | 75 | 81 | ‐0.06 (‐0.11 to ‐4.81) | 17* | NNT > 10 |
| *NSAID superior | |||||||||
| Comparison/outcome | Number of studies | Number of participants | n with outcome/total | % with outcome | Risk difference | NNH | Susceptibility to publication bias | ||
| Active | Control | Active | Control | ||||||
| Comparison 11. Number of participants with adverse events | |||||||||
| 1 Paracetamol or propacetamol vs placebo | 20 | 2359 | 557/1278 | 400/1081 | 44 | 37 | 0.04 (0.01 to 0.08) | 25 | NNH > 10 |
| 1.2 Propacetamol vs placebo | 10 | 1409 | 278/740 | 197/669 | 38 | 29 | 0.05 (0.01 to 0.10) | 20 | NNH > 10 |
| Comparison 14. Number of participants withdrawing due to lack of efficacy | |||||||||
| 1.2 Propacetamol vs placebo | 14 | 889 | 25/477 | 47/412 | 5 | 11 | ‐0.05 (‐0.08 to ‐0.02) | 20* | NNH > 10 |
| Comparison 15. Number of participants with pain on infusion | |||||||||
| 2. Propacetamol vs placebo | 6 | 645 | 75/333 | 4/312 | 23 | 1 | 0.20 (0.16 to 0.24) | 5 | 645 |
| 3. Propacetamol vs paracetamol | 3 | 362 | 71/182 | 8/180 | 39 | 4 | 0.35 (0.27 to 0.42) | 3 | 904 |
| Comparison 16. Individual adverse events: paracetamol or propacetamol vs placebo | |||||||||
| 1. Nausea: Paracetamol or propacetamol vs placebo | 15 | 1267 | 189/660 | 213/607 | 29 | 35 | ‐0.05 (‐0.10 to ‐0.01) | 20* | NNH > 10 |
| 1.1. Nausea: Paracetamol vs placebo | 13 | 1037 | 153/520 | 196/517 | 29 | 38 | ‐0.09 (‐0.14 to ‐0.04) | 11* | NNH > 10 |
| 2. Vomiting: Paracetamol or propacetamol vs placebo | 15 | 1414 | 103/721 | 144/693 | 14 | 21 | ‐0.06 (‐0.10 to ‐0.03) | 17* | NNH > 10 |
| 2.1. Vomiting: Paracetamol vs placebo | 13 | 1037 | 88/520 | 136/517 | 17 | 26 | ‐0.10 (‐0.14 to ‐0.05) | 10* | NNH > 10 |
| Comparison 18. Individual adverse events: paracetamol or propacetamol vs opioids | |||||||||
| 1. Nausea: Paracetamol or propacetamol vs opioids | 6 | 545 | 19/272 | 48/273 | 7 | 18 | ‐0.11 (‐0.16 to ‐0.05) | 10* | 55 |
| 1.1 Nausea: Paracetamol vs opioids | 4 | 438 | 12/219 | 39/219 | 5 | 18 | ‐0.12 (‐0.18 to ‐0.07) | 9* | 88 |
| 2. Vomiting: Paracetamol or propacetamol vs opioids | 5 | 495 | 6/247 | 20/248 | 2 | 8 | ‐0.06 (‐0.09 to ‐0.02) | 17* | NNH > 10 |
| 2.1. Vomiting: Paracetamol vs opioids | 3 | 388 | 4/194 | 16/194 | 2 | 8 | ‐0.06 (‐0.10 to ‐0.02) | 17* | NNH > 10 |
| 6.1. Sedation: Paracetamol vs opioids | 3 | 354 | 2/176 | 26/178 | 1 | 15 | ‐0.14 (‐0.18 to ‐0.09) | 8* | 142 |
| *lower occurrence of adverse event in paracetamol and/or propacetamol arm | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 11 | 1149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [2.01, 3.19] |
| 1.1 Paracetamol vs placebo | 5 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.80 [2.30, 10.00] |
| 1.2 Propacetamol vs placebo | 8 | 756 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.77] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 5 | 353 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.86, 1.18] |
| 2.1 Paracetamol vs NSAIDs | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 2.2 Propacetamol vs NSAIDs | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.86, 1.34] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.85, 1.59] |
| 4 Paracetamol vs propacetamol Show forest plot | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.77, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 10 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.86 [2.10, 3.91] |
| 1.1 Paracetamol vs placebo | 6 | 532 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.65 [2.15, 6.21] |
| 1.2 Propacetamol vs placebo | 6 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.64, 3.50] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 5 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.66, 0.95] |
| 2.1 Paracetamol vs NSAIDs | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.66, 1.02] |
| 2.2 Propacetamol vs NSAIDs | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.56, 1.02] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.83, 1.09] |
| 4 Paracetamol vs propacetamol Show forest plot | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.73, 1.20] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol vs placebo Show forest plot | 6 | 485 | Mean Difference (IV, Fixed, 95% CI) | ‐1.21 [‐3.73, 1.31] |
| 2 Paracetamol vs NSAIDs Show forest plot | 6 | 350 | Mean Difference (IV, Fixed, 95% CI) | 5.02 [3.18, 6.86] |
| 3 Paracetamol vs opioids | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐19.23, ‐4.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol vs placebo Show forest plot | 12 | 837 | Mean Difference (IV, Fixed, 95% CI) | ‐7.48 [‐8.98, ‐5.97] |
| 2 Paracetamol vs NSAIDs Show forest plot | 9 | 524 | Mean Difference (IV, Fixed, 95% CI) | 2.95 [1.18, 4.72] |
| 3 Paracetamol vs opioids Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐1.57, 7.57] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐13.0 [‐18.28, ‐7.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 9 | 859 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.64, 0.77] |
| 1.1 Paracetamol vs placebo | 6 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.82] |
| 1.2 Propacetamol vs placebo | 3 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.35, 0.69] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 5 | 309 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.87, 1.63] |
| 2.1 Paracetamol vs NSAIDs | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.98] |
| 2.2 Propacetamol vs NSAIDs | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.77] |
| 3 Propacetamol vs opioids Show forest plot | 2 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.72, 4.64] |
| 4 Paracetamol vs propacetamol Show forest plot | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.82, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 9 | 839 | Mean Difference (IV, Fixed, 95% CI) | 6.43 [4.54, 8.32] |
| 1.1 Paracetamol vs placebo | 6 | 523 | Mean Difference (IV, Fixed, 95% CI) | 5.78 [3.86, 7.71] |
| 1.2 Propacetamol vs placebo | 3 | 316 | Mean Difference (IV, Fixed, 95% CI) | 23.72 [13.79, 33.65] |
| 2 Paracetamol vs NSAIDs Show forest plot | 2 | 138 | Mean Difference (IV, Fixed, 95% CI) | ‐1.14 [‐36.21, 33.92] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 6 | 255 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐1.81, ‐1.03] |
| 1.1 Paracetamol vs placebo | 4 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐1.33 [‐1.75, ‐0.91] |
| 1.2 Propacetamol vs placebo | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.05 [‐3.15, ‐0.95] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 3 | 294 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.37, ‐0.02] |
| 2.1 Paracetamol vs NSAIDs | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐1.04, 1.59] |
| 2.2 Propacetamol vs NSAIDs | 1 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.09, 1.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 13 | 777 | Mean Difference (IV, Fixed, 95% CI) | ‐1.92 [‐2.41, ‐1.42] |
| 1.1 Paracetamol vs placebo | 8 | 404 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐2.35, ‐1.31] |
| 1.2 Propacetamol vs placebo | 6 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐2.67 [‐4.21, ‐1.13] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 8 | 540 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.37, 0.12] |
| 2.1 Paracetamol vs NSAIDs | 3 | 160 | Mean Difference (IV, Fixed, 95% CI) | 0.81 [‐0.87, 2.49] |
| 2.2 Propacetamol vs NSAIDs | 5 | 380 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.39, 0.11] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.01, 2.01] |
| 4 Paracetamol vs propacetamol Show forest plot | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐4.15, 3.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 16 | 2015 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.25, 1.43] |
| 1.1 Paracetamol vs placebo | 9 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.31, 1.60] |
| 1.2 Propacetamol vs placebo | 9 | 1139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.16, 1.37] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 11 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.87, 1.00] |
| 2.1 Paracetamol vs NSAIDs | 6 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.91, 1.04] |
| 2.2 Propacetamol vs NSAIDs | 5 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 3.1 Propacetamol | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.81, 1.23] |
| 4 Paracetamol vs propacetamol Show forest plot | 2 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 3 | 342 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.04, 0.66] |
| 1.1 Paracetamol vs placebo | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.46, 0.26] |
| 1.2 Propacetamol vs placebo | 2 | 282 | Mean Difference (IV, Fixed, 95% CI) | 1.64 [1.04, 2.25] |
| 2 Paracetamol vs NSAIDs Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.63, 0.03] |
| 3 Propacetamol vs opioids Show forest plot | 2 | 141 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐0.18, 0.98] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 20 | 2359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [1.01, 1.22] |
| 1.1 Paracetamol vs placebo | 12 | 950 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.19] |
| 1.2 Propacetamol vs placebo | 10 | 1409 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.35] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 6 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.72, 1.32] |
| 2.1 Paracetamol vs NSAIDs | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.66, 1.68] |
| 2.2 Propacetamol vs NSAIDs | 3 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.62, 1.37] |
| 3 Propacetamol vs opioids Show forest plot | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 10 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.19, 6.59] |
| 1.1 Paracetamol vs placebo | 6 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.19, 6.59] |
| 1.2 Propacetamol vs placebo | 5 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 9 | 798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.65] |
| 2.1 Paracetamol vs NSAIDs | 4 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.26] |
| 2.2 Propacetamol vs NSAIDs | 5 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.65] |
| 3 Paracetamol or propacetamol vs opioids Show forest plot | 3 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| 3.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 37 | 2654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.56, 2.84] |
| 1.1 Paracetamol vs placebo | 27 | 1912 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.17] |
| 1.2 Propacetamol vs placebo | 12 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.25, 6.68] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 24 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.42, 3.12] |
| 2.1 Paracetamol vs NSAIDs | 19 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.31, 3.22] |
| 2.2 Propacetamol vs NSAIDs | 5 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.22, 12.37] |
| 3 Paracetamol or propacetamol vs opioids Show forest plot | 6 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 3.1 Paracetamol vs opioids | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol or propacetamol vs placebo Show forest plot | 38 | 2600 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.79] |
| 1.1 Paracetamol vs placebo | 26 | 1711 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.78] |
| 1.2 Propacetamol vs placebo | 14 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.80] |
| 2 Paracetamol or propacetamol vs NSAIDs Show forest plot | 24 | 1393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.81, 2.08] |
| 2.1 Paracetamol vs NSAIDs | 18 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.89] |
| 2.2 Propacetamol vs NSAIDs | 6 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.78, 2.03] |
| 3 Paracetamol or propacetamol vs opioids Show forest plot | 6 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| 3.1 Paracetamol vs opioids | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Propacetamol vs opioids | 4 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.97] |
| 4 Paracetamol vs ketamine Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Paracetamol vs placebo Show forest plot | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.80, 11.54] |
| 2 Propacetamol vs placebo Show forest plot | 6 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.07 [5.35, 31.98] |
| 3 Propacetamol vs paracetamol Show forest plot | 3 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.31 [4.20, 16.46] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 15 | 1267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.98] |
| 1.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.66, 0.90] |
| 1.2 Propacetamol vs placebo | 3 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.98, 2.69] |
| 2 Vomiting Show forest plot | 15 | 1414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.57, 0.87] |
| 2.1 Paracetamol vs placebo | 13 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.51, 0.80] |
| 2.2 Propacetamol vs placebo | 3 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.75, 3.48] |
| 3 Nausea/vomiting Show forest plot | 10 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.08] |
| 3.1 Paracetamol vs placebo | 4 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.57, 1.70] |
| 3.2 Propacetamol vs placebo | 6 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 4 Pruritus Show forest plot | 7 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.60, 1.40] |
| 4.1 Paracetamol vs placebo | 5 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.64, 1.72] |
| 4.2 Propacetamol vs placebo | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.51] |
| 5 Respiratory depression Show forest plot | 11 | 1082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.31, 1.92] |
| 5.1 Paracetamol vs placebo | 6 | 363 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.03, 2.65] |
| 5.2 Propacetamol vs placebo | 5 | 719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.35, 2.80] |
| 6 Sedation Show forest plot | 10 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.66, 1.51] |
| 6.1 Paracetamol vs placebo | 6 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.42, 2.01] |
| 6.2 Propacetamol vs placebo | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.69] |
| 7 Urinary retention Show forest plot | 8 | 1050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.68, 1.66] |
| 7.1 Paracetamol vs placebo | 5 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.28, 6.66] |
| 7.2 Propacetamol vs placebo | 3 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.65, 1.66] |
| 8 Allergy/skin rash/local reaction Show forest plot | 7 | 1131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.61, 3.91] |
| 8.1 Paracetamol vs placebo | 4 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.24, 4.34] |
| 8.2 Propacetamol vs placebo | 4 | 761 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.57, 6.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 11 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.90, 1.28] |
| 1.1 Paracetamol vs NSAIDs | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.31] |
| 1.2 Propacetamol vs NSAIDs | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.44] |
| 2 Vomiting Show forest plot | 11 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.89, 1.55] |
| 2.1 Paracetamol vs NSAIDs | 8 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.77, 1.68] |
| 2.2 Propacetamol vs NSAIDs | 3 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.81] |
| 3 Nausea/vomiting Show forest plot | 8 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.62, 1.97] |
| 3.1 Paracetamol vs NSAIDs | 4 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.42, 2.39] |
| 3.2 Propacetamol vs NSAIDs | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.55, 2.60] |
| 4 Pruritus Show forest plot | 8 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.34] |
| 4.1 Paracetamol vs NSAIDs | 5 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.75, 1.51] |
| 4.2 Propacetamol vs NSAIDs | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.37, 1.60] |
| 5 Respiratory depression Show forest plot | 9 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.26] |
| 5.1 Paracetamol vs NSAIDs | 8 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.26] |
| 5.2 Propacetamol vs NSAIDs | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sedation Show forest plot | 6 | 278 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [0.63, 10.75] |
| 6.1 Paracetamol vs NSAIDs | 4 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.90] |
| 6.2 Propacetamol vs NSAIDs | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.63, 21.22] |
| 7 Urinary retention Show forest plot | 6 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.51, 2.32] |
| 7.1 Paracetamol vs NSAIDs | 4 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.24, 4.02] |
| 7.2 Propacetamol vs NSAIDs | 2 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.47, 2.78] |
| 8 Allergy/skin rash/local reaction Show forest plot | 8 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.26] |
| 8.1 Paracetamol vs NSAIDs | 6 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.26, 4.02] |
| 8.2 Propacetamol vs NSAIDs | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.16] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 6 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.65] |
| 1.1 Paracetamol vs opioids | 4 | 438 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.56] |
| 1.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.91] |
| 2 Vomiting Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.12, 0.72] |
| 2.1 Paracetamol vs opioids | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.71] |
| 2.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.10, 2.62] |
| 3 Nausea/vomiting Show forest plot | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.64] |
| 3.1 Propacetamol vs opioids | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.15, 1.64] |
| 4 Pruritus Show forest plot | 3 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.21, 1.43] |
| 4.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.22] |
| 4.2 Propacetamol vs opioids | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.10, 1.19] |
| 5 Respiratory depression Show forest plot | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Paracetamol vs opioids | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sedation Show forest plot | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| 6.1 Paracetamol vs opioids | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nausea Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.30] |
| 2 Vomiting Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.22, 1.33] |
| 3 Sedation Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.51] |

