Консервативные вмешательства при лечении переломов средней трети ключицы у подростков и взрослых
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [author‐defined order]
| Methods | Study design: RCT Duration of the study: June 1981 to September 1982 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented Funding sources: not reported | |
| Participants | Location: Denmark Number of participants assigned: 79 (49 figure‐of‐eight bandage group; 34 arm sling group) Number of participants assessed: 61 (34 figure‐of‐eight bandage group; 27 arm sling group) Inclusion criteria:
Exclusion criteria:
Age (median; range):
Gender: not reported Side of injury: not reported Classification of injury: not reported, just fracture types (2 fragments, 1 intermediary fragment and 2 or more intermediary fragments) and fracture dislocations (undisplaced, minor displacement, major displacement) | |
| Interventions | Timing of intervention: after diagnosis Intervention 1 (figure‐of‐eight bandage):
Intervention 2 (arm sling):
Rehabilitation: not reported Any co‐interventions: not reported | |
| Outcomes | Length of follow‐up: The figure‐of‐eight group lasted a median interval of 12 weeks (10 to 16) and the sling group lasted a median interval of 13 weeks (10 to 17); the figure‐of‐eight group also was assessed at 2 days, 1 and 2 weeks Loss of follow‐up: 18 participants lost to follow‐up: Figure‐of‐eight bandage group ‐ 11 participants lost to follow‐up:
Arm sling group ‐ two seven participants lost to follow‐up:
Primary outcomes:
Secondary outcomes:
| |
| Notes | The outcomes were evaluated by a non‐validated scoring system | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table was used |
| Allocation concealment (selection bias) | Unclear risk | Details to ascertain that allocation was concealed were not provided |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) | High risk | Less than 80% of participants completed the follow‐up (23% of withdrawals) |
| Selective reporting (reporting bias) | High risk | The authors used non‐validated scores to assess function and pain; treatment failure was not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods | Study design: RCT Duration of the study: August 2012 and September 2013 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented Funding sources: the authors reported that no benefits in any form were received or will be received from a commercial party related directly or indirectly to the subject of this article | |
| Participants | Location: Istanbul, Turkey Number of participants assigned: 60 (30 figure‐of‐eight bandage group; 30 arm sling group) Number of participants assessed: 51 (23 figure‐of‐eight bandage group; 28 arm sling group) Inclusion criteria:
Exclusion criteria:
Age (mean; range):
Gender (male/female):
Side of injury (dominant/non‐dominant):
Classification of injury (displaced/not displaced):
| |
| Interventions | Timing of intervention: All participants were assessed on the day of injury Intervention 1 (figure‐of‐eight bandage):
Intervention 2 (arm sling):
Rehabilitation: not reported Any co‐interventions: not reported | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: nine participants lost to follow‐up: Figure‐of‐eight bandage group ‐ seven participants lost to follow‐up:
Arm sling group ‐ two participants lost to follow‐up:
Primary outcomes:
Secondary outcomes:
| |
| Notes | We found some data discrepancies between text and tables of paper – all data were checked by authors’ via email. This confirmed the denominators at follow‐up were 23 figure‐of‐eight bandages and 28 arm slings | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The authors employed non‐stratified randomisation in blocks of two using the sealed envelope method, so when one patient had chosen an envelope, the next patient would be allocated to a group according to the remaining envelope of the pair |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) | High risk | Only 82% of participants completed the follow‐up. Missing outcome data were not balanced in numbers across intervention groups; more participants in the figure‐of‐eight group were lost to follow‐up at 12 months; (2/30 (6.7%) in sling group vs. 7/30 (23.3%) in figure‐of‐eight group). This may have overestimated the benefits of sling |
| Selective reporting (reporting bias) | High risk | The study protocol was not available. Function or disability was measured by validated scores, however, only at the end of follow‐up that raged by 6 to 12 months ‐ the authors did not report functional outcomes at each time point |
| Other bias | High risk | The results were published in imprecise format. We found some data discrepancies between text and tables of paper |
| Methods | Study design: RCT Duration of the study: December 1983 to May 1987 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented Funding sources: not reported | |
| Participants | Location: Department of Surgery, Saint Elisabeth Hospital, Tilburg, The Netherlands Number of participants assigned: 157 (78 figure‐of‐eight bandage group; 79 arm sling group) Number of participants assessed: 152 (74 figure‐of‐eight bandage group; 78 arm sling group) Inclusion criteria:
Exclusion criteria:
Age (mean; SD):
Gender (male/female):
Side (left/right): 85/72 Classification of injury: not specified, just fracture displacement (undisplaced and displacement) and multiple fragment fractures (with or without shortening) | |
| Interventions | Timing of intervention: after diagnosis Intervention 1 (figure‐of‐eight bandage): details not reported Intervention 2 (arm sling): details not reported Rehabilitation: not reported Any co‐interventions: not reported | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: five participants lost to follow‐up: Figure‐of‐eight bandage group ‐ four participants lost to follow‐up:
Arm sling group ‐ one participant lost to follow‐up:
Primary outcomes:
Secondary outcomes:
| |
| Notes | Nine participants ‐ all without complications ‐ refused x‐rays at final follow‐up. Participant numbers for each intervention were not known despite contacting the authors; thus we have used the numbers available at follow‐up for denominators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomised to the 2 treatment groups by opening of pre‐numbered envelopes; however, details to ascertain that allocation was concealed were not provided |
| Blinding of participants and personnel (performance bias) | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) | Low risk | More than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups and an intention‐to‐treat analysis was likely, but outcome data for participants who had withdrawn from the trial or were lost to follow‐up were not presented |
| Selective reporting (reporting bias) | High risk | The authors used non‐validated scores to assess function and treatment failure was not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
| Methods | Study design: multicentre, double‐blind RCT Duration of the study: March 2001 and December 2003 Protocol was published before recruitment of patients: not reported Details of trial registration: not reported Intention‐to‐treat analysis: Likely, but data of those patients who withdrew could not be collected Funding sources: data collection and data analysis were supported by a financial grant from Smith and Nephew Inc, Memphis, USA. Transducers (placebo and active) were provided free of cost. No author had any financial or personal relationships with people or organisations that could inappropriately influence their work | |
| Participants | Location: 6 hospitals in the Netherlands participated in the study (Meander Medical Centre, Amersfoort; Onze Lieve Vrouwen Gasthuis Hospital, Amsterdam; Reinier de Graaf Hospital, Delft; Saint Antonius Hospital, Nieuwegein; Diakonessen Hospital, Utrecht; University Medical Centre Utrecht, Utrecht) Number of participants assigned: 120 (61 LIPUS; 59 control (placebo)) Number of participants assessed: 101 (52 LIPUS; 49 control (placebo)) Inclusion criteria:
Exclusion criteria:
*Age (mean/SD):
Gender (male/female):
Side (left/right):
Classification of injury: AO system (A1, A2, A3, B1, B2, B3, C1, C2, C3) | |
| Interventions | Timing of intervention: up to 5 days after the diagnosis Intervention 1 (LIPUS: low‐intensity pulsed ultrasound):
Intervention 2 (placebo):
Duration of treatment (mean): LIPUS = 25.38 days; control (placebo) = 24.43 days (mean difference 0.95, 95% CI ‐3.72 to +1.81, P = 0.49) Rehabilitation: it was not done All participants were treated with passive support for their own convenience. Free arm movements within pain range were allowed from day 1 Any co‐interventions: not reported | |
| Outcomes | Length of follow‐up:
Loss of follow‐up: 9 participants lost to follow‐up: LIPUS group ‐ nine participants lost to follow‐up:
Control (placebo) group ‐ 10 participant lost to follow‐up:
Primary outcomes:
Secondary outcomes:
| |
| Notes | *Data assessed by personal contact with the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used |
| Allocation concealment (selection bias) | Low risk | Double‐blind, randomised, placebo‐controlled trial Each participating hospital was delivered consecutive numbered transducers in packs of 4 (2 LIPUS and 2 placebos) |
| Blinding of participants and personnel (performance bias) | Low risk | Participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) | Low risk | More than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups, and an intention‐to‐treat analysis was reported for the primary outcomes; however, data for those patients who withdrew were not reported |
| Selective reporting (reporting bias) | High risk | The study protocol is not available and function and/or disability were not evaluated using a validated score |
| Other bias | Low risk | The study appears to be free of other sources of bias |
<: less than
>: more than
≥: more or equal to
AO: Arbeitsgemeinschaft für Osteosynthesefragen
CI: confidence interval
DVT: deep‐venous thrombosis
ITT: intention‐to‐treat
LIPUS: low‐intensity pulsed ultrasound
kHz: kilohertz
MHz: megahertz
mW/cm²: milliWatt per square centimetre
µs: microsecond
RCT: randomised controlled trial
SATA: spatial average, temporal average
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| This was a prospective cohort study | |
| This study, which was registered in the Netherlands trial register, was listed as an ongoing trial in the first version of the review. It planned to compare Kinesio® tape plus sling versus sling alone, with a start date of October 2008 and end date October 2010. However, the contact author reported that for a variety of reasons the trial was ended and no data are available | |
| This study, logged in the National Research Register (UK), was intended to be a randomised trial of shoulder brace versus arm sling in 100 adults with isolated closed middle third clavicle fractures. It was planned to start in April 2002; however, the contact author indicated that for a variety of reasons this study never took place | |
| Not RCT or quasi‐RCT |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Figure‐of‐eight bandage versus arm sling for treating middle third clavicle fractures in adults: a randomised controlled trial |
| Methods | Study design: parallel randomised controlled trial Random sequence generation: participants will be randomised according to computer generated randomisation Allocation concealment: by sealed opaque envelope Masking: open label |
| Participants | Location: São Paulo, Brazil Target sample size: 110 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: figure‐of‐eight bandage Intervention 2: arm sling |
| Outcomes | Outcomes: function or disability measured by: DASH questionnaire and UCLA score; pain measured by VAS; failure of treatment; adverse events measured by: a) cosmetic results: perception of deformity or asymmetry (dichotomous data); b) asymptomatic nonunion (i.e. the fracture has not radiographically healed, although pain is absent); c) stiffness/restriction of the shoulder movement (compared with contralateral side); and numbers returning to previous activities Timing of outcomes measurement: 12 months |
| Starting date | Main ID: NCT02398006 Date of registration: 12 March 2015 Last refreshed on: 19 March 2015 Date of first enrolment: January 2016 Status: recruiting |
| Contact information | Name: Dr Mario Lenza Address: Hospital Israelita Albert Einstein – Avenida Albert Einstein, 627/701 – Jardim Leonor – CEP: 05652‐900 – São Paulo, SP, Brazil Telephone: 55 11 21511444 Email: [email protected] Affiliation: Hospital Israelita Albert Einstein |
| Notes | A published protocol for this trial is available (Lenza 2016) |
DASH: Disability of the Arm, Shoulder and Hand questionnaire
UCLA: University of California, Los Angeles
VAS: visual analog score
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Shoulder function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1 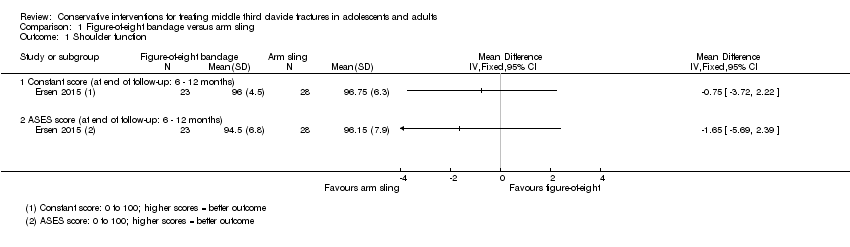 Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 1 Shoulder function. | ||||
| 1.1 Constant score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ASES score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Shoulder function: number of participants with 'good function' Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2  Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 2 Shoulder function: number of participants with 'good function'. | ||||
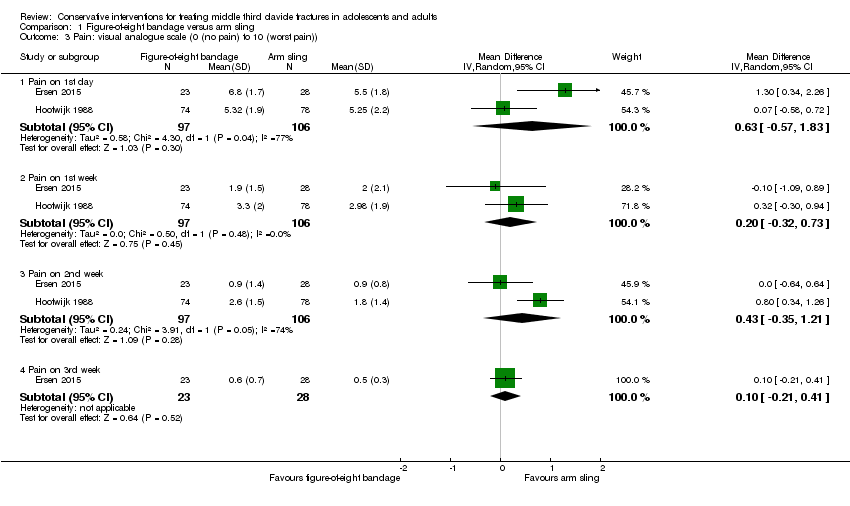
| 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)). | ||||
| 3.1 Pain on 1st day | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.57, 1.83] |
| 3.2 Pain on 1st week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.32, 0.73] |
| 3.3 Pain on 2nd week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.35, 1.21] |
| 3.4 Pain on 3rd week | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.21, 0.41] |
| 4 Pain: duration of painkiller consumption (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 4 Pain: duration of painkiller consumption (days). | ||||
| 5 Clinical healing: time to clinical fracture consolidation (weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5  Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 5 Clinical healing: time to clinical fracture consolidation (weeks). | ||||
| 6 Adverse event Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 6 Adverse event. | ||||
| 6.1 Poor cosmetic appearance post fracture healing | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.55, 3.16] |
| 6.2 Change in allocated treatment due to pain and discomfort | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.35, 25.83] |
| 6.3 Worsened fracture position on healing | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.44] |
| 6.4 Shortening > 15 mm | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.35, 2.90] |
| 6.5 Non‐union | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 6.6 Permanent pain at mean 10 months | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 7 Time to return to previous activities (weeks) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7 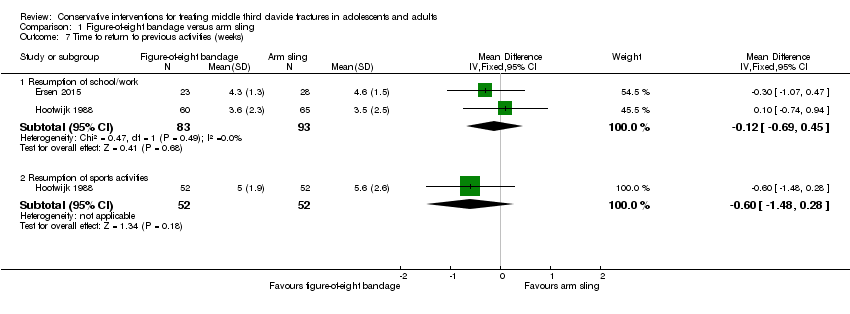 Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 7 Time to return to previous activities (weeks). | ||||
| 7.1 Resumption of school/work | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.69, 0.45] |
| 7.2 Resumption of sports activities | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.48, 0.28] |
| 8 Patient dissatisfaction with course of treatment Show forest plot | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.03, 7.23] |
| Analysis 1.8  Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 8 Patient dissatisfaction with course of treatment. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)). | ||||
| 2 Pain: number of painkillers (tablets/28 days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 2 Pain: number of painkillers (tablets/28 days). | ||||
| 3 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3  Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 3 Treatment failure. | ||||
| 3.1 Number who had surgical procedure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Clinical healing: time to clinical fracture consolidation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.4 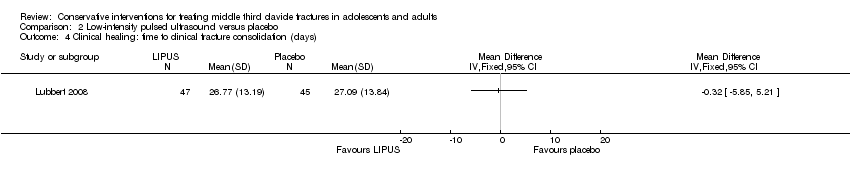 Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 4 Clinical healing: time to clinical fracture consolidation (days). | ||||
| 5 Adverse events: skin irritation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5  Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 5 Adverse events: skin irritation. | ||||
| 6 Time to return to previous activities (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 6 Time to return to previous activities (days). | ||||
| 6.1 Resumption of household activities | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Resumption of professional work | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Resumption of sport | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Study flow diagram

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 1 Shoulder function.
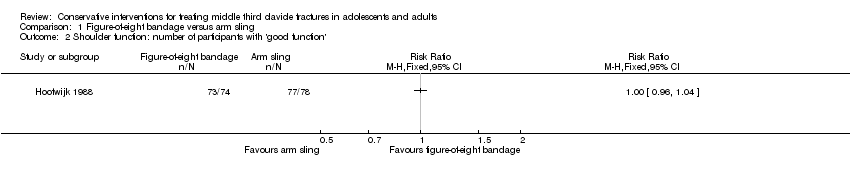
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 2 Shoulder function: number of participants with 'good function'.
Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 4 Pain: duration of painkiller consumption (days).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 5 Clinical healing: time to clinical fracture consolidation (weeks).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 6 Adverse event.

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 7 Time to return to previous activities (weeks).

Comparison 1 Figure‐of‐eight bandage versus arm sling, Outcome 8 Patient dissatisfaction with course of treatment.

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)).

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 2 Pain: number of painkillers (tablets/28 days).

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 3 Treatment failure.
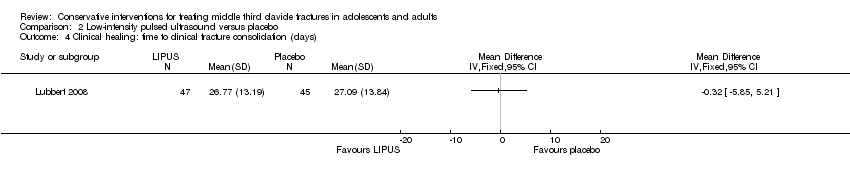
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 4 Clinical healing: time to clinical fracture consolidation (days).
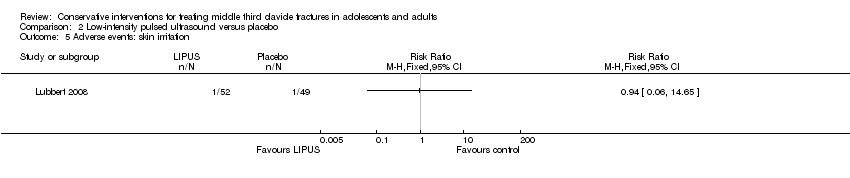
Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 5 Adverse events: skin irritation.

Comparison 2 Low‐intensity pulsed ultrasound versus placebo, Outcome 6 Time to return to previous activities (days).
| Figure‐of‐eight bandage compared with arm sling for treating fractures of the middle third of the clavicle | ||||||
| Patient or population: patients (mainly young male adults) with fractures of the middle third of the clavicle Settings: hospital (initially) Intervention: figure‐of‐eight bandage Comparison: arm sling | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Arm sling | Figure‐of‐eight bandage | |||||
| Shoulder function Constant score (0 to 100 points: higher = better) Follow‐up: 6 to 12 months | Mean (SD) population Constant score 89 (7)¹ | Mean function in the figure‐of‐eight bandage groups was 0.75 points lower (3.72 lower to 2.39 higher) | MD ‐0.75 points (‐3.72 to 2.39) | 51 | ⊕⊝⊝⊝ | The 95% CI does not include a clinically important difference.³ Shoulder function was measured using non‐validated measures in two other trials (61 and 152 participants). Both trials found evidence of similar shoulder function in the two groups |
| Pain (early) Visual Analogue Scale ‐ VAS (0 (no pain) to 10 (worst pain)) Follow‐up: 2 weeks | Mean pain in the arm sling groups ranged from | Mean pain in the figure‐of‐eight bandage groups was 0.43 points higher (0.35 lower to 1.21 higher) | MD 0.43 points (‐0.35 to 1.21) | 203 (2 studies) | ⊕⊝⊝⊝ | The 95% CI do not include a clinically important difference. A third trial (data for 61 participants) provided very low quality evidence based on a non‐validated scoring system of more pain and discomfort during the course of treatment in the figure‐of‐eight group |
| Treatment failure (Number of participants who have undergone or are being considered for a surgical intervention) | See comment | See comment | Not estimable | ‐ | See comment | Poorly reported outcome. One trial (152 participants) reported that one participant in the arm sling group had successful surgery for a secondary plexus nerve palsy |
| Clinical healing ‐ time to clinical fracture consolidation (weeks) | Mean clinical healing in the arm sling group was 3.6 weeks | Mean clinical healing in the figure‐of‐eight bandage group was 0.20 weeks longer (0.11 week shorter to 0.51 week longer) | MD 0.20 weeks (‐0.11 to 0.51) | 148 | ⊕⊝⊝⊝ | In addition, there were four non‐unions in the figure‐of‐eight group; none were problematic |
| Adverse events ‐ total participants with adverse events | See comment | See comment | Not estimable | ‐ | See comment | The very low evidence quality data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) did not confirm a difference between the two groups⁵ |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial |
| Return to previous activities ‐ Resumption of school/work (weeks) | Mean time to return to previous activities ranged across control groups from | Mean time to return to previous activities (weeks) ‐ resumption of school/work in the intervention groups was 0.12 weeks lower (0.69 lower to 0.45 higher) | MD ‐0.12 weeks (‐0.69 to 0.45) | 176 (2 studies) | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ These are based on the Constant score in healthy people as reported in Yian 2005. ² We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of blinding. We downgraded the evidence one further level for imprecision given the wide confidence interval and that the available data were from only one trial. ³ For the purposes of this review, the minimally clinical important difference was considered to be 10 points for the Constant score (Kukkonen 2013). ⁴ We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence one further level for inconsistency given the considerable heterogeneity between the findings of the two groups (I² = 74%). ⁵ Data for individual adverse outcomes (poor cosmetic appearance; change in allocated treatment due to pain and discomfort, worsened fracture position on healing; shortening > 15 mm; non‐symptomatic non‐union and permanent pain) confirmed a difference between the two groups. ⁶ We downgraded the evidence for this outcome two levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence one further level for imprecision given the low numbers of participants contributing data to this outcome. | ||||||
| Low‐intensity pulsed ultrasound compared with placebo for treating fractures of the middle third of the clavicle | ||||||
| Patient or population: patients with fractures of the middle third of the clavicle Settings: hospital Intervention: low‐intensity pulsed ultrasound Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Low‐intensity pulsed ultrasound | |||||
| Shoulder function | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the trial |
| Pain Visual analogue scale ‐ VAS (0 (no pain) to 10 (worst pain)) Follow‐up: in the 28‐day treatment period | Mean pain in the control group was | Mean pain in the intervention group was 0.04 points lower (0.61 lower to 0.53 higher) | MD ‐0.04 (95% CI ‐0.61 to 0.53) | 101 (1 study) | ⊕⊕⊕⊝ | |
| Treatment failure ‐ Number who had surgical procedure | See comment | See comment | RR 1.13 (0.37 to 3.47) | 101 (1 study) | ⊕⊕⊕⊝ | Only one trial assessed this comparison |
| Clinical healing ‐ Time to clinical fracture consolidation (days) | Mean clinical healing in the control group was 27.09 days | Mean clinical healing: time to clinical/radiographic fracture consolidation (days) in the intervention group was 0.32 days lower (5.85 lower to 5.21 higher) | MD ‐0.32 weeks (‐5.85 to 5.21) | 101 (1 study) | ⊕⊕⊕⊝ | |
| Adverse events ‐ total of adverse events (Skin irritation) Follow‐up: during the intervention | See comment | See comment | RR 0.94 (0.06 to 14.65) | 101 (1 study) | ⊕⊕⊕⊝ | Only one trial assessed this comparison |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured in the trial |
| Return to previous activities ‐ Resumption of work (days) | Mean time to return to previous activities in the control group was | Mean time to return to previous activities (days) ‐ resumption of work in the intervention group was 1.95 weeks higher (2.18 lower to 6.08 higher) | MD 1.95 days (‐2.18 to 6.08) | 101 (1 study) | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ We downgraded the evidence one level for imprecision given the wide confidence interval and that the available data were from only one trial. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Shoulder function Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Constant score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 ASES score (at end of follow‐up: 6 ‐ 12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Shoulder function: number of participants with 'good function' Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Pain on 1st day | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.57, 1.83] |
| 3.2 Pain on 1st week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.32, 0.73] |
| 3.3 Pain on 2nd week | 2 | 203 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.35, 1.21] |
| 3.4 Pain on 3rd week | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.21, 0.41] |
| 4 Pain: duration of painkiller consumption (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Clinical healing: time to clinical fracture consolidation (weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse event Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Poor cosmetic appearance post fracture healing | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.55, 3.16] |
| 6.2 Change in allocated treatment due to pain and discomfort | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.35, 25.83] |
| 6.3 Worsened fracture position on healing | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.15, 2.44] |
| 6.4 Shortening > 15 mm | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.35, 2.90] |
| 6.5 Non‐union | 3 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 6.6 Permanent pain at mean 10 months | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.48 [0.52, 173.09] |
| 7 Time to return to previous activities (weeks) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Resumption of school/work | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.69, 0.45] |
| 7.2 Resumption of sports activities | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.48, 0.28] |
| 8 Patient dissatisfaction with course of treatment Show forest plot | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [1.03, 7.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain: visual analogue scale (0 (no pain) to 10 (worst pain)) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain: number of painkillers (tablets/28 days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Treatment failure Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number who had surgical procedure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Clinical healing: time to clinical fracture consolidation (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events: skin irritation Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Time to return to previous activities (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Resumption of household activities | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Resumption of professional work | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Resumption of sport | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

