Contenido relacionado
Revisiones y protocolos relacionados
Jeremy Franklin, Dennis A. Eichenauer, Ingrid Becker, Ina Monsef, Andreas Engert | 13 septiembre 2017
Markus Schaaf, Marcel Reiser, Peter Borchmann, Andreas Engert, Nicole Skoetz | 18 enero 2012
Nicole Skoetz, Andrea Will, Ina Monsef, Corinne Brillant, Andreas Engert, Bastian von Tresckow | 25 mayo 2017
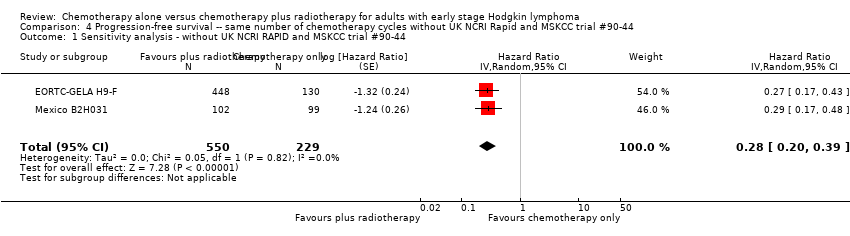
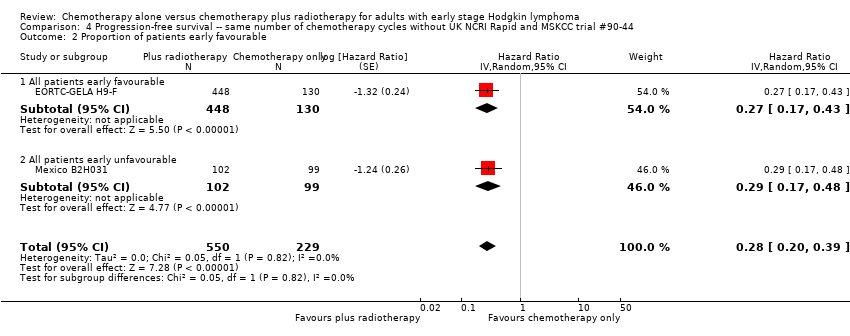
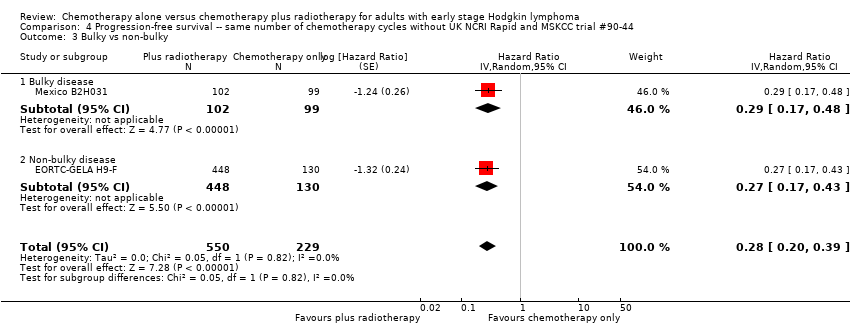
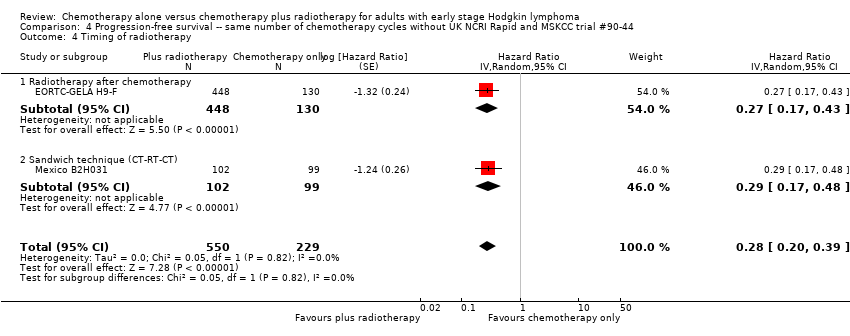
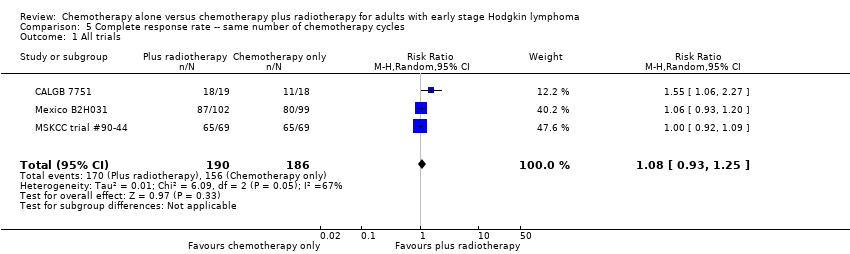
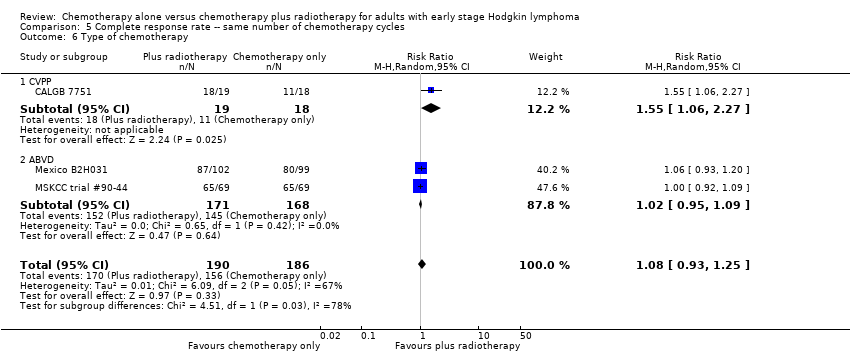
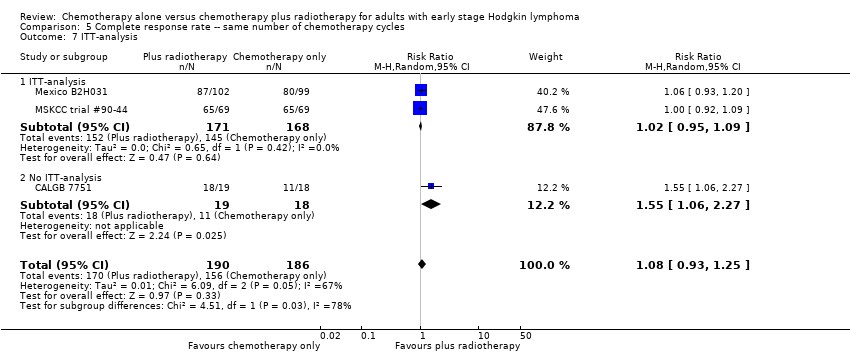
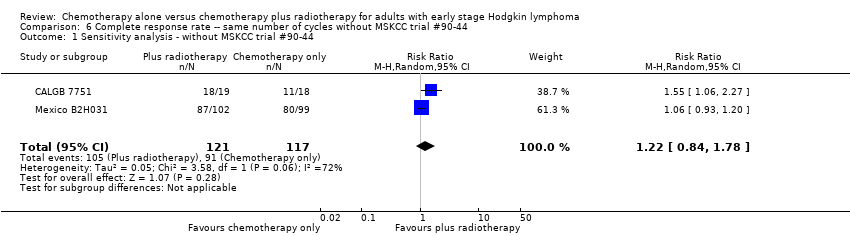
Jeremy Franklin, Marcus D Paus, Annette Pluetschow, Lena Specht | 19 octubre 2005
Holger Schulz, Julia Bohlius, Nicole Skoetz, Sven Trelle, Thilo Kober, Marcel Reiser, Martin Dreyling, Michael Herold, Guido Schwarzer, Michael Hallek, Andreas Engert | 17 octubre 2007
Gail G Louw, Charles Ross Pinkerton | 8 octubre 2008
Marie‐Therese Sickinger, Bastian von Tresckow, Carsten Kobe, Andreas Engert, Peter Borchmann, Nicole Skoetz | 9 enero 2015
Alexander Greb, Julia Bohlius, Daniel Schiefer, Guido Schwarzer, Holger Schulz, Andreas Engert | 23 enero 2008
Lise J Estcourt, Reem Malouf, Marialena Trivella, Dean A Fergusson, Sally Hopewell, Michael F Murphy | 27 enero 2017
Frauke Naumann‐Winter, Alexander Greb, Peter Borchmann, Julia Bohlius, Andreas Engert, Roland Schnell | 17 octubre 2012
Respuestas clínicas Cochrane
Simona Nistor‐Grahl | 3 agosto 2017