低リスク妊娠性絨毛新生物に対する第1選択治療としての抗がん剤
Referencias
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre RCT (Iran). Study duration: 2001 to 2003. | |
| Participants | Low‐risk GTN. Number randomised: 46. Number evaluable:46. Randomisation ratio of 1.5 MTX:1 Act D applied due to economic limitations. | |
| Interventions | Group1: MTX, IM, 30 mg/m² repeated every week. Group2: ACT, IV, 1.25 mg/m² repeated every 2 weeks. | |
| Outcomes | Efficacy: remission rate, number of cycles to remission, duration of treatment, need for second‐line chemotherapy. Adverse effects: nausea. | |
| Notes | Risk scoring: WHO/FIGO 2000. Non‐response defined as < 10% decrease in BhCG over 3 weeks, more than 20% rise in BhCG over 2 consecutive weeks or the appearance of new metastatic disease. Remission defined as hCG < 5 IU/L. One additional cycle was given after remission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) | Low risk | 100% of participants analysed. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. Toxicity not reported in detail but said to be 'minimal' in both groups. |
| Other bias | Unclear risk | This trial has the same authors, location and protocol as Yarandi 2008, with consecutive enrolment dates, yet the later Yarandi 2008 study does not refer to Gilani 2005. It is not clear why. Attempts to clarify this with Dr Yarandi have been unsuccessful. |
| Methods | Single‐centre RCT (Thailand). Study duration: 1994 to 2005. Follow‐up: 1 year. | |
| Participants | Low‐risk GTN (FIGO stage 1). Number randomised: 49. Number evaluable: 45. | |
| Interventions | Group 1: Act D IV 10 µg/kg/day (D1 to D5) repeated every two weeks. Group 2: MTX‐FA: MTX IM 1 mg/kg/day (days 1, 3, 5, 7) and MTX‐FA IM 0.1 mg/kg/day (days 2, 4, 6, 8), repeated every two weeks. | |
| Outcomes | Efficacy: remission rate, number of cycles to remission, need for second‐line chemotherapy. Adverse effects: liver toxicity, neutropenia, skin pigmentation, alopecia and mucositis. | |
| Notes | Risk scoring: FIGO. Two participants in each arm of treatment were lost to follow‐up and were excluded from the analysis in the reporting article. Six participants in the MTX‐FA group were switched to Act D due to rising levels of liver enzymes. The investigators excluded these participants from analyses of remission rates (i.e. not ITT analyses), however we have added these data back. ITT analysis gives a remission rate of 14/25 in the MTX group, not 14/19 as reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) | Low risk | Four lost to follow‐up, two in each group. Therefore 92% analysed : 20/22 (91%) of 5‐day Act D arm and 25/27 (92.6%) of the MTX‐FA arm. |
| Selective reporting (reporting bias) | Unclear risk | Not ITT analysis. Six women in MTX‐FA who were switched to Act D due to hepatotoxicity were excluded from final analysis; therefore remission rate was reported as 14/19 instead of 14/25. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | RCT conducted in Iran; randomisation ratio not stated but appears to be 1 MTX:2 Act D Accrual dates: Jan 2008 to Dec 2010 Follow‐up: 1 year | |
| Participants | 75 women with FIGO stage I‐III; modified WHO risk scores ≤ 6; ; a rise in hCG of > 10% in the 3 weeks post‐termination of pregnancy or a hCG plateau of 4 weeks. Exclusion criteria were prior CT or hysterectomy. | |
| Interventions | Group 1: 5‐day IM MTX (0.4 mg/kg) repeated every two weeks vs. Group 2: bi‐weekly IV Act D (1.25 mg/m2 bolus) | |
| Outcomes | Primary remission; need for second‐line CT; duration of treatment; toxicity | |
| Notes | Non‐response defined as a BhCG plateau or rising BhCG titres for 2 consecutive weeks. No major adverse events were reported in either group (classified according to the GOG grading system). Baseline characteristic similar. Mucositis not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised'; no other details provided. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported. |
| Other bias | Low risk | None evident. Baseline characteristic similar. |
| Methods | Multicentre RCT (US, Japan, Canada and South Africa) with central randomisation through the GOG Centre. Clinical trial registration ID: NCT00003702 Study duration:1999 to 2007. Follow‐up: 1 year. | |
| Participants | Low‐risk GTN (see notes). Number randomised: 240. Number ineligible:24. Number evaluable: 216. | |
| Interventions | Group 1 = MTX, IM, 30 mg/m², weekly. Group 2 = ACT, IV, 1.25 mg/m², every two weeks. | |
| Outcomes | Primary: CR defined as a normal hCG sustained over four weekly measurements. Secondary: number of CT cycles to remission, treatment failure/need for second‐line CT. Adverse effects. | |
| Notes | Baseline characteristics were similar between the two groups. Risk scoring: WHO/FIGO 2000. WHO risk score 0 to 4 was used between June 1999 to June 2002, then modified score 0 to 6 was used from July 2002 to February 2007. Twenty‐seven women had WHO scores of 0 (balanced between groups) which may have inflated the CR rate. Two women did not receive their allocated treatment, therefore, they were included in ITT analysis but not in analysis of toxicity. Twenty‐four women deemed ineligible: 13 did not meet the entry criteria for persistent disease, 4 did not have GTN, 4 had inadequate documentation of the disease and 3 had a centrally re‐calculated risk WHO risk score > 6. Non‐response (NR) defined as any set of three consecutive assay results that declined by < 10%. Eleven NR women (5 MTX and 6 Act D) continued to receive their allocated treatment and went on to achieve CR. No women had to have allocated treatment terminated because of toxicity. Alopecia coded as dermatological toxicity. 11 women continued on the allocated regimen after being assessed as non‐responders and attained CR. If these women had been included in the analyses of CR the percentage of responders would have been 63% for MTX and 79% for Act D (compared with 53% and 70% respectively). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Central randomisation and allocation of treatment. |
| Blinding (performance bias and detection bias) | Unclear risk | Neither participants nor treatment providers were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | 90% of participants were analysed : 107/120 (89%) of weekly MTX arm and 108/120 (90%) of "pulsed" ACT D arm. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes reported. Analysis by ITT. |
| Other bias | Low risk | No evidence of other bias. |
| Methods | Randomised single‐blind trial conducted in Iran with recruitment between 2011 and 2013. Clinical trial registration ID: IRCT201105236563N1 | |
| Participants | Women with untreated low‐risk GTN (LRGTN) stage I and (WHO) prognostic scoring of 6 or less. Inclusion criteria: women with a plateau or increase in serum BhCG level or persistent elevation above the normal B‐hCG value 16 weeks after molar (complete or incomplete) evacuation . Plateau BhCG level is defined as 10% or less change in BhCG titre at least over 3 weeks (4 values in days 1, 7, 14, 21) and rising BhCG titre is defined as greater than 10% increase in BhCG titre at least over 2 weeks (3 values in days 1, 7, 14) . Exclusion criteria: histologically‐confirmed choriocarcinoma or placental site trophoblastic tumour (PSTT), did not agree to have effective contraception for the duration of the study, metastasis, FIGO prognostic score of 7 or more, willing to continue breast feeding, presence of other malignancies with disease free duration less than 5 years, contraindication for protocols of chemotherapy due to previous cancer treatment, prior chemotherapy or hysterectomy for GTN Number eligible: 64 Number evaluable: 64 | |
| Interventions | Group 1: Act D, IV, 1.25 mg/m² repeated every 2 weeks. Group 2: MTX 1 mg/kg per day on days 1, 3, 5,and 7 IM with IM Folinic Acid 0.1 mL/kg per day on days 2, 4, 6, and 8. | |
| Outcomes | Primary: CR Secondary: adverse events | |
| Notes | Info and data extracted from published English abstract only as the published paper could not be obtained for translation. Emailed for more details on the 28/3/16 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomly assigned' ‐ sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | Unclear risk | 'single‐blind' but no details provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Few data available in conference abstract only |
| Selective reporting (reporting bias) | Unclear risk | Published abstract gave data on response to treatment but was insufficient for other outcomes |
| Other bias | Unclear risk | Insufficient details in published abstract to make a judgement |
| Methods | Single‐centre RCT (Iran). Study duration: 09/2003 to 09/2006. Follow‐up: 1 year. | |
| Participants | Low‐risk GTN. Number eligible:131. Number evaluable:131. Participants randomised into two groups: group 1 = 81 and group 2 = 50 (randomisation ratio of 1.5 MTX:1 Act D applied). Reasons given for this were economic limitations. Excluded patients with choriocarcinoma. | |
| Interventions | Group 1: MTX, IM, 30 mg/m² repeated every week. Group 2: Act D, IV, 1.25 mg/m² repeated every 2 weeks. | |
| Outcomes | Efficacy: remission rate, number of cycles to remission, duration of treatment, need for second‐line chemotherapy. Adverse effects: nausea. | |
| Notes | Risk scoring: FIGO 2000. Six women ( 4 in group 1 and 2 in group 2) did not complete their first‐line chemotherapy, but were considered in the ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not described. |
| Incomplete outcome data (attrition bias) | Low risk | 100% of randomised participants were analysed. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified and expected outcomes were reported. Analysis was by ITT. |
| Other bias | Unclear risk | See 'Risk of bias' comment for Gilani 2005. |
| Methods | Single‐centre RCT (Iran) | |
| Participants | 62 women with LRGTN | |
| Interventions | Group 1: 5‐day MTX (0.4 mg/kg IM) (25 mg max) Group 2: bi‐weekly Act D (1.25 mg/m2 IV bolus) (2mg max) | |
| Outcomes | Primary: primary remission, resistance to chemotherapy | |
| Notes | Conference abstract only. Numbers randomised to each arm was not stated in the abstract, therefore data could not be extracted/included in the review. Results were reported as follows: "Complete remission after receiving first‐line chemotherapy was achieved in 79% of all cases, 80% of Act‐D and 78.1% of MTX group (P value = 0.86) 20% of Act‐D and 21.9% of MTX cases showed resistance to the first chemotherapy, of which 16.7% and 15.6% respectively responded completely to the second‐line mono therapy. 3.3% of Act‐D and 6.,3% of MTX group needed multiple drug therapy (P value = 0.86) Side‐effects were not significantly different in both groups." Baseline characteristics were reported to be similar viz. BhCG level, uterine mass size, lung metastasis, antecedent pregnancy and the duration from diagnosis to treatment. Authors were emailed for info but the email bounced back. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information in conference abstract |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; no study protocol identified |
| Other bias | Unclear risk | Insufficient information in conference abstract. Attempts to contact study investigators (F Yarandi) were unsuccessful. |
Abbreviations: Act D = Actinomycin D or Dactinomycin; BhCG = beta human chorionic gonadotrophin; CR = Complete response; CT = chemotherapy; FIGO = International Federation of Gynecology and Obstetrics; GOG = Gynecologic Oncology Group; GTN = Gestational trophoblastic neoplasia; IM = intramuscular; ITT = Intention‐to‐treat; IV = intravenous; LRGTN = low‐risk gestational trophoblastic neoplasia; MTX = Methotrexate; MTX‐FA = methotrexate‐folinic acid; RCT = randomised controlled trial; WHO = World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Not an RCT. In this case‐control study, 108 women were treated with 5‐day MTX (42 women) or 5‐day Act D (42 women) or MTX/Act D combined (24 women). The combined intervention was stopped due to high rates of toxicity. | |
| Not an RCT. In this prospective study conducted in Brazil, 60 women with LRGTN (risk score ≤ 6) were assigned to MTX (50 mg/day/IM) alternating with folinic acid rescue for 8 days (N = 20), 5‐day Act D (12 mcg/kg/IV) (n = 20) and etoposide (100 mg/m2/IV) (n = 20). Alopecia was the most common side‐effect caused by etoposide (occurred in 95% of women). | |
| Not an RCT. The study may have high‐risk patients. It was not clear if patients were balanced for demographic variables. It was not clear if all patients between 1976 to 1978 were treated with MTX @ 6 mg/kg or if the decision to use this dose was left to attending physician. | |
| A review of the management in GTN. | |
| A cohort study of 66 women treated with MTX. | |
| Not an RCT. High risk of selection bias " the choice of treatment was at the discretion of the attending oncologist". Follow‐up period not clear. | |
| A conference abstract of a cohort study of 50 women with GTD. | |
| Not an RCT. In this case‐control study, women were treated with a 5‐day Act D regimen (43 women) or a pulsed Act D regimen (18 women). | |
| High risk of selection bias; patients were not matched for the potential confounding variables in the different treatment groups. Included in subsequent publication. | |
| High risk of selection bias; study did not provide information about patient characteristics and if they were matched for the potential confounding variables in the different treatment groups. | |
| High risk of selection bias: study did not provide information about the characteristics of patients and if they were matched for potential confounding variables in the treatment groups. | |
| Case series rather than case‐control study; 61 patients received MTX, 4 ACT and 5 MACT. Risk of selection bias; patients were not matched for the potential confounding variables. | |
| Not an RCT. In this case‐control study, 39 women received MTX and 29 women received MTX‐FA. | |
| Not an RCT. In this case‐control study, 33 women received MTX and 68 women received MTX‐FA. |
Act D = actinomycin D; GTD = gestational trophoblastic disease; GTN = gestational trophoblastic neoplasia; IM = intramuscular; IV = intravenous; LRGTN = low‐risk gestational trophoblastic neoplasia; MTX = methotrexate; MTX‐FA = methotrexate‐folinic acid; RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Randomised trial of pulsed actinomycin D versus MTX infusion in low risk gestational trophoblastic neoplasia |
| Methods | Open‐label, randomised parallel arm trial in India, fixed block randomisation, sequentially numbered, sealed, opaque envelopes 5‐year follow‐up |
| Participants | 150 women with abnormal BhCG regression following any type of pregnancy (molar, term, abortion, ectopic), i.e.
FIGO score ≤ 6 |
| Interventions | Arm 1: MTX 300 mg/m² in 500 mL of normal saline given as an IV infusion over six hours followed by folinic acid 15 mg PO every 6 hours for four doses starting 24 hours after the start of MTX infusion.The same is repeated every two weeks until the hCG becomes normal. Arm 2: ACT D 1.25 mg/m2 slow IV push and the same repeated every two weeks until the hCG becomes normal |
| Outcomes | Primary: Primary remission rate, CR Secondary: Time to cure, number of cycles required for CR, toxicity and side‐effects, cost of treatment |
| Starting date | 19‐11‐2012. Estimated completion date 2017 |
| Contact information | Dr Anitha Thomas. Department of Obstetrics and Gynecology Unit 1 Christian Medical College Vellore, Tamil Nadu, India |
| Notes |
| Trial name or title | Methotrexate or actinomycin D in treating patients with low‐risk gestational trophoblastic neoplasia (NCT01535053) |
| Methods | Multicentre phase III RCT; open‐label |
| Participants | 384 women with LRGTN |
| Interventions | Arm I: MTX IM on days 1, 3, 5, and 7 and leucovorin calcium PO on days 2, 4, 6, and 8 OR MTX IV on days 1 to 5. Arm II: Act D IV over 15 minutes on day 1. Cycles repeated every 14 days for up to 13 courses in the absence of disease progression or unacceptable toxicity. Women receive 3 courses after hCG < (< 5 mIU/mL |
| Outcomes | Primary: CR rate Secondary: post‐protocol surgery; post‐protocol multi‐agent treatment; severe adverse events; QoL |
| Starting date | January 2012. Estimated completion August 2016 |
| Contact information | Dr Julian Schink Robert H. Lurie Cancer Center, Northwestern University, Chicago, Illinois, United States, 60611 Ph: 312‐472‐4684 |
| Notes | Investigators emailed 28/3/16 |
| Trial name or title | Comparison of pulsed actinomycin versus 5‐day methotrexate for the treatment of low‐risk gestational trophoblastic disease in patients |
| Methods | Randomised single‐blind trial in Iran |
| Participants | 44 women with WHO risk score 0‐6 |
| Interventions | Arm 1: Actinomycin 1.25 mg/m2 every 14 days IV (maximum dose 2 mg) Arm 2: MTX 5‐day (0.4 mg/kg IV) (25 mg max) |
| Outcomes | Primary: BhCG |
| Starting date | 21‐3‐2011. Estimated completion date 20‐3‐2015 |
| Contact information | Dr Abbaslou: [email protected]; [email protected] |
| Notes | Investigators emailed 28/3/16 to enquire about completion (no response received) |
| Trial name or title | Methotrexate single‐dose treatment and methotrexate/actinomycin‐D single‐dose treatment in low‐risk gestational trophoblastic neoplasia (GTN‐01) |
| Methods | Open‐label RCT in China; 3‐year follow‐up |
| Participants | 300 women with LRGTN |
| Interventions | Arm 1: MTX 0.4 mg/(kg·day) IM on days 1‐5. If BhCG level can not drop to 1/10 of the origin level during the following 3 weeks, additional course is administered at 2‐week intervals, after the first normal hCG value has been recorded. Arm 2: Act D 0.6 mg/m2, IV on day 1,2; MTX 100 mg/m2, IV, on day1 (after Act D); MTX 200 mg/m2, IV, on day 1 (after MTX, 500 mL NS > 4 hours) If BhCG level does not drop to 1/10 of the origin level during the following 3 weeks, additional courses are administered at 2‐week intervals, with one additional cycle after the first normal hCG value has been recorded. |
| Outcomes | Primary: adverse events; progression‐free survival, CR and treatment failure Secondary: resumption of menstruation, pregnancy rate, delivery rate of live‐born babies, abnormal pregnancy (stillbirth, neonatal deaths, miscarriage, terminations, ectopic and molar pregnancies) |
| Starting date | December 2012. Estimated completion date December 2017 |
| Contact information | Dr Danhui Weng Tongji Hospital, Wuhan, Hubei, China, 430030 |
| Notes |
Act D = Actinomycin D; BhCG = beta human chorionic gonadotrophin; CR = Complete response; CT = chemotherapy; FIGO = International Federation of Gynecology and Obstetrics; IM = intramuscular; IV = intravenous; LRGTN = low‐risk gestational trophoblastic neoplasia; MTX = Methotrexate; NS = normal saline; PO = by mouth; QoL = quality of life; RCT = randomised controlled trial; WHO = World Health Organization
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary cure (remission) Show forest plot | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.75] |
| Analysis 1.1  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 1 Primary cure (remission). | ||||
| 1.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.80] |
| 1.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |
| 1.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| 1.4 Eight‐day IM MTX‐FA vs. pulsed IV Act D | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 2 Failure of first line therapy Show forest plot | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [1.81, 6.95] |
| Analysis 1.2  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 2 Failure of first line therapy. | ||||
| 2.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 3.54 [1.12, 11.16] |
| 2.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 3.2 [1.17, 8.78] |
| 2.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 18.58 [1.16, 297.18] |
| 2.4 Eight‐day IM MTX‐FA vs. pulsed IV Act D | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [1.19, 8.90] |
| 3 Chemotherapy cycles to primary cure Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 3 Chemotherapy cycles to primary cure. | ||||
| 3.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 346 | Mean Difference (IV, Random, 95% CI) | 3.04 [0.93, 5.14] |
| 3.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐2.87, ‐1.53] |
| 3.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.27, 1.53] |
| 4 Adverse effects: Nausea Show forest plot | 4 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.29, 1.26] |
| Analysis 1.4  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 4 Adverse effects: Nausea. | ||||
| 4.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.62] |
| 4.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.93] |
| 5 Adverse effects: Vomiting Show forest plot | 3 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.73] |
| Analysis 1.5  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 5 Adverse effects: Vomiting. | ||||
| 5.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.24, 1.32] |
| 5.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.50, 4.05] |
| 6 Adverse effects: Diarrhoea Show forest plot | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.85, 2.41] |
| Analysis 1.6  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 6 Adverse effects: Diarrhoea. | ||||
| 6.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.57, 3.16] |
| 6.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.58, 3.85] |
| 7 Adverse effects: Constitutional Show forest plot | 3 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| Analysis 1.7  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 7 Adverse effects: Constitutional. | ||||
| 7.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.18] |
| 7.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.55] |
| 8 Adverse effects: Alopecia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 8 Adverse effects: Alopecia. | ||||
| 8.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.27, 1.83] |
| 8.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.41, 4.30] |
| 8.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.53] |
| 9 Adverse effects: Mucositis/stomatitis Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 9 Adverse effects: Mucositis/stomatitis. | ||||
| 9.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.17] |
| 9.2 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.54] |
| 10 Adverse effects: Dermatological Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 10 Adverse effects: Dermatological. | ||||
| 10.1 Weekly IM MTX vs. pulsed IV Act D | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Adverse effects: Neutropenia Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.45] |
| Analysis 1.11  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 11 Adverse effects: Neutropenia. | ||||
| 11.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.15] |
| 11.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.43, 9.20] |
| 11.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.27, 21.89] |
| 12 Adverse effects: Thrombocytopenia Show forest plot | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.55] |
| Analysis 1.12  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 12 Adverse effects: Thrombocytopenia. | ||||
| 12.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 12.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.74, 8.50] |
| 12.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 6.41] |
| 13 Adverse effects: Anaemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 13 Adverse effects: Anaemia. | ||||
| 13.1 Weekly IM MTX vs. pulsed IV Act D | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Adverse effects: Hepatotoxicity Show forest plot | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.39, 16.88] |
| Analysis 1.14  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 14 Adverse effects: Hepatotoxicity. | ||||
| 14.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.56, 3.61] |
| 14.2 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 10.68 [0.63, 179.70] |
| 15 Adverse effects: Haemoptysis Show forest plot | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.30, 3.31] |
| Analysis 1.15  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 15 Adverse effects: Haemoptysis. | ||||
| 15.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.13, 2.94] |
| 15.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.30, 13.38] |
| 16 Severe adverse events (≥G3) Show forest plot | 5 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.08, 1.66] |
| Analysis 1.16  Comparison 1 Methotrexate vs. Actinomycin D, Outcome 16 Severe adverse events (≥G3). | ||||
| 16.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 16.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.88] |
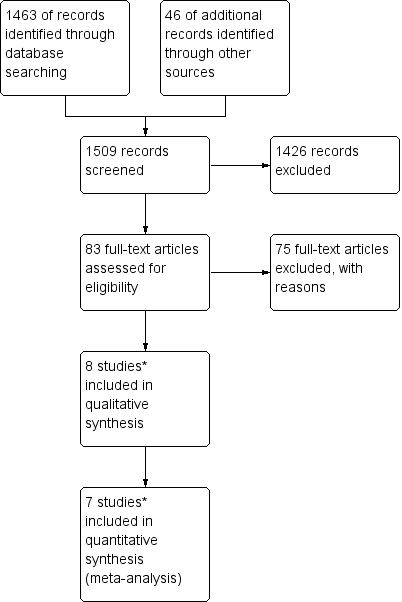
Study flow diagram of the original 2009 review
*The original 2009 review Included four non‐RCTs (Abrao 2008; Kohorn 1996; Smith 1982; Wong 1985) in the qualitative and three (Abrao 2008 not included) in the quantitative meta‐analysis). These non‐RCTs were excluded in the updated review.

Study flow diagram of the updated search conducted from January 2010 to February 2012.

Study flow diagram for the updated search conducted from Feb 2012 to January 2016.
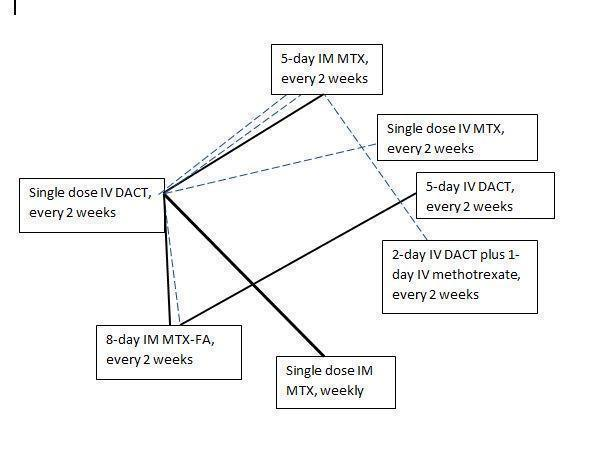
Chemotherapy treatment comparisons of included RCTs (solid lines) and ongoing RCTs (dotted lines)
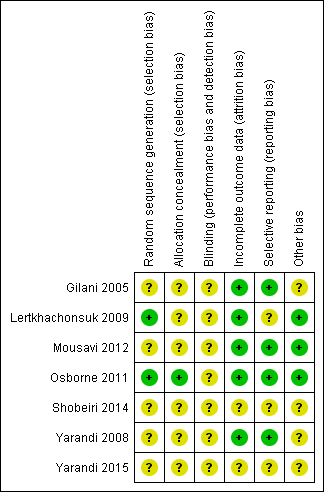
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
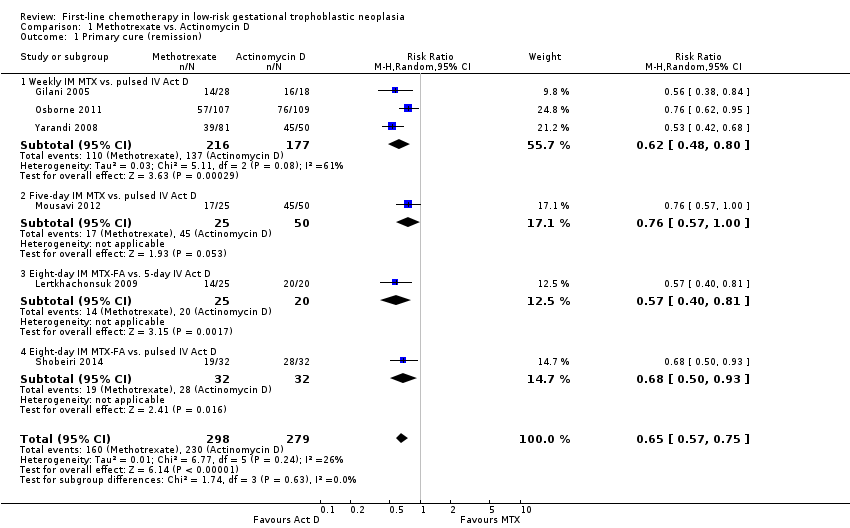
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 1 Primary cure (remission).
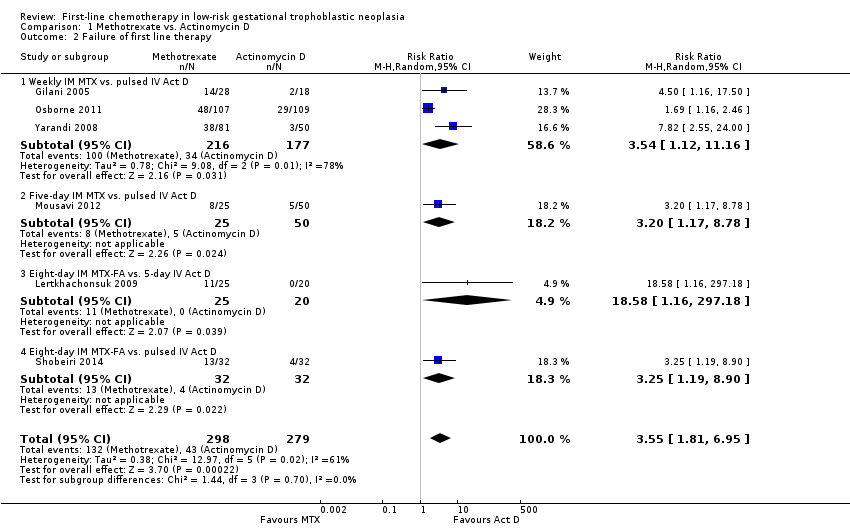
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 2 Failure of first line therapy.
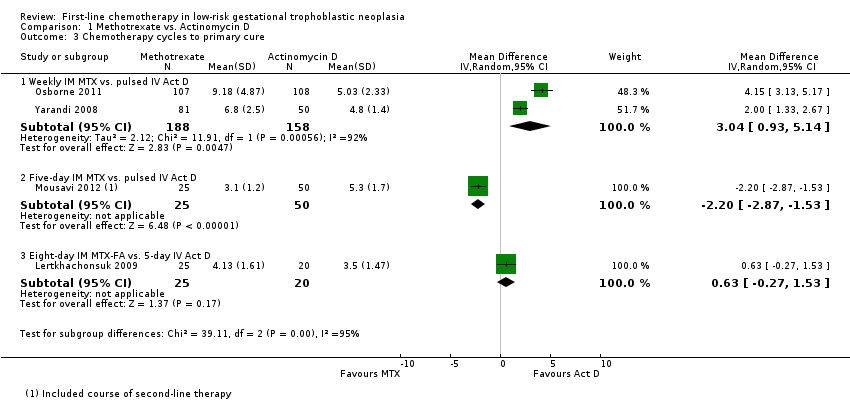
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 3 Chemotherapy cycles to primary cure.

Comparison 1 Methotrexate vs. Actinomycin D, Outcome 4 Adverse effects: Nausea.
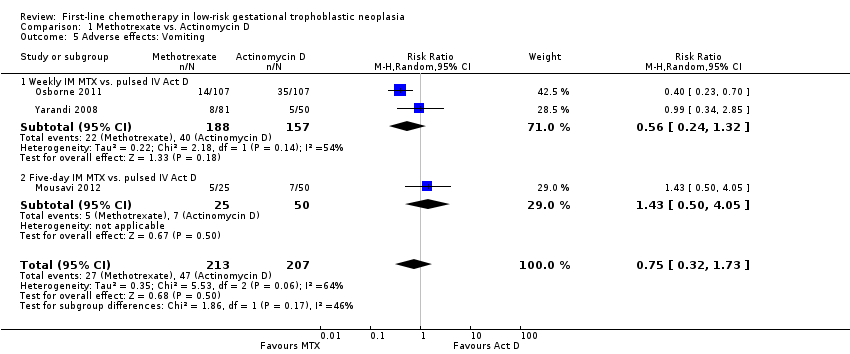
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 5 Adverse effects: Vomiting.

Comparison 1 Methotrexate vs. Actinomycin D, Outcome 6 Adverse effects: Diarrhoea.
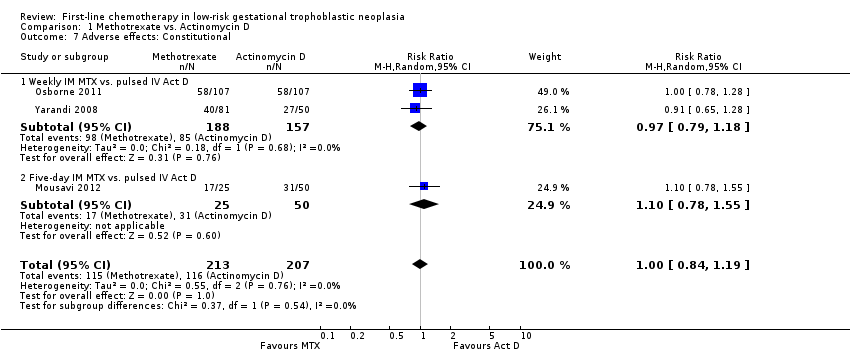
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 7 Adverse effects: Constitutional.
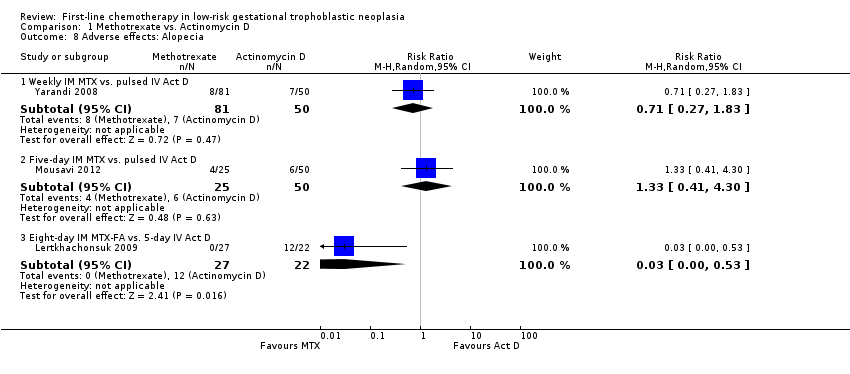
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 8 Adverse effects: Alopecia.
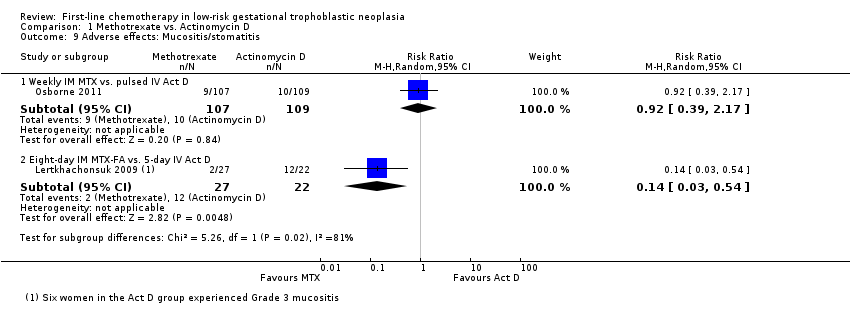
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 9 Adverse effects: Mucositis/stomatitis.
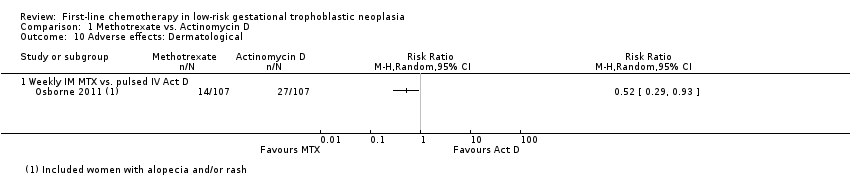
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 10 Adverse effects: Dermatological.

Comparison 1 Methotrexate vs. Actinomycin D, Outcome 11 Adverse effects: Neutropenia.
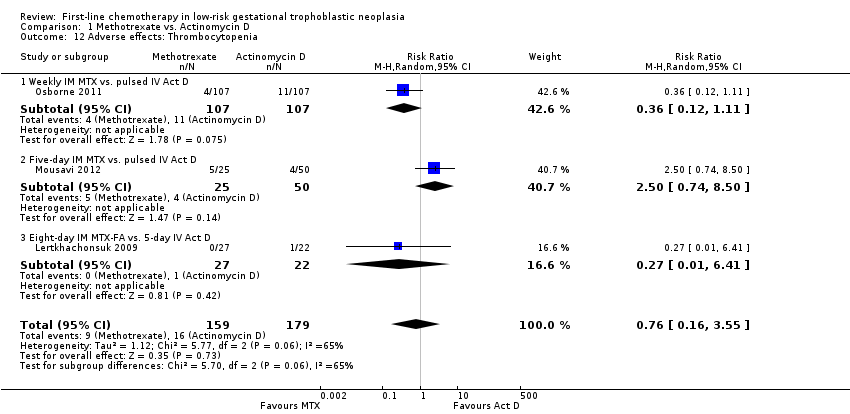
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 12 Adverse effects: Thrombocytopenia.
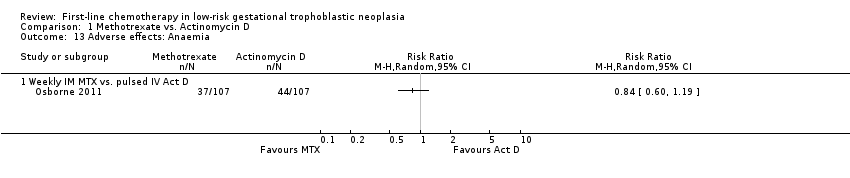
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 13 Adverse effects: Anaemia.
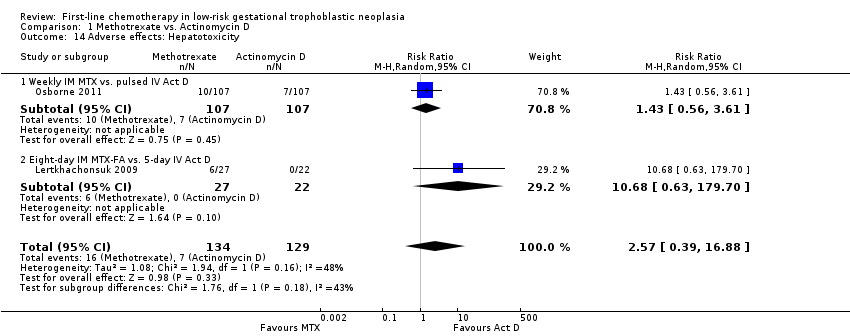
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 14 Adverse effects: Hepatotoxicity.
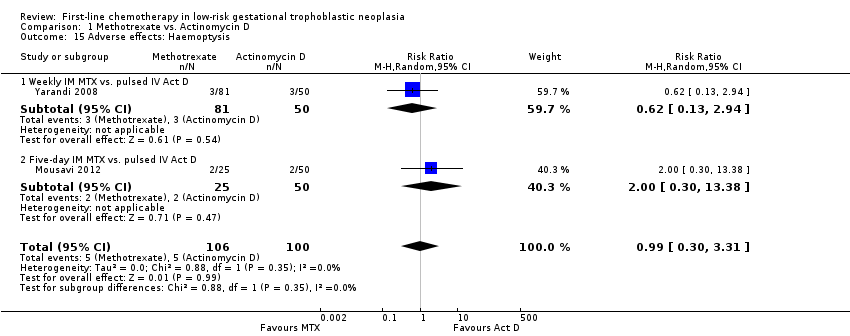
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 15 Adverse effects: Haemoptysis.
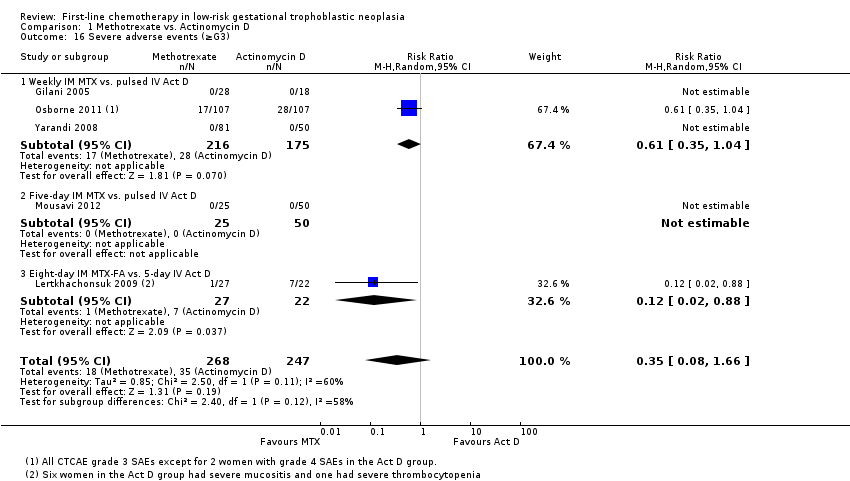
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 16 Severe adverse events (≥G3).
| Actinomycin D compared with methotrexate (MTX) for low‐risk gestational trophoblastic neoplasia (GTN) | ||||||
| Patient or population: women withe low‐risk GTN Settings: outpatient or hospital Intervention: actinomycin D (Act D) Comparison: MTX | ||||||
| Outcomes | Illustrative Assumed risk* (Act D) | Illustrative Corresponding risk (MTX) | Relative effect | No of Participants | Quality of the evidence | Comments |
| Primary cure (remission) | 824 per 1000 | 536 per 1000 (470 to 618) | RR 0.65 (0.57 to 0.75) | 577 women (6 studies) | ⊕⊕⊕⊝ | Act D is probably more likely to achieve a primary cure than MTX. 55% of the data came from trials of weekly IM MTX, which may be less effective than the 5‐ or 8‐day MTX regimens. |
| Failure of first‐line therapy | 154 per 1000 | 547 per 1000 (279 to 1000) | RR 3.55 (1.81 to 6.95) | 577 women (6 studies) | ⊕⊕⊕⊝ | Act D as a first‐line treatment is probably less likely to fail than MTX. 59% of the data came from trials of weekly IM MTX, which may be less effective than the 5‐ or 8‐day MTX regimens. |
| Severe adverse events (≥ grade 3) | 142 per 1000 | 50 per 1000 (11 to 235) | RR 0.35 (0.08 to 1.66) | 515 women (5 studies) | ⊕⊕⊝⊝ low1,2 | There may be little or no difference between interventions overall. However, the point estimate and subgroup analyses favoured MTX. SAEs occurred in 3 out of 6 studies, but one study did not contribute to the meta‐analysis due to insufficient data. |
| Nausea | 462 per 1000 | 282 per 1000 (134 to 582) | RR 0.61 (0.29 to 1.26) | 466 women (4 studies) | ⊕⊕⊕⊝ | There is probably little or no difference between MTX and Act D for nausea. |
| Alopecia | Subtotals only | ⊕⊕⊝⊝ low1,2 | Data on alopecia were not pooled due to substantial subgroup differences. However, in general the evidence suggested that there may be little or no difference between MTX and Act D regimens with regard to alopecia, except for the five‐day Act D regimen, which may be more frequently associated with alopecia than the 8‐day MTX regimen. | |||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence IM = intramuscular; SAE = severe adverse effects | ||||||
| 1 Downgraded for clinical or statistical inconsistency 2 Downgraded for imprecision | ||||||
| Stage I | Disease confined to the uterus |
| Stage II | GTN extends outside of the uterus, but is limited to the genital structures (adnexae, vagina, broad ligament) |
| Stage III | GTN extends to the lungs with or without known genital tract involvement |
| Stage IV | All other metastatic sites |
| *FIGO 2009 | |
| Scores | 0 | 1 | 2 | 4 |
| Age (years) | < 40 | ≥ 40 | – | – |
| Antecedent pregnancy | mole | abortion | term | – |
| Interval months from index pregnancy | < 4 | 4–6 | 7–12 | > 12 |
| Pretreatment serum hCG (IU/L) | < 103 | 103 to 104 | 104 to 105 | > 105 |
| Largest tumour size (including uterus) | < 3 | 3cm to 4 cm | ≥ 5 cm | – |
| Site of metastases | lung | spleen, kidney | gastrointestinal | liver, brain |
| Number of metastases | – | 1to 4 | 5 to 8 | > 8 |
| Previous failed chemotherapy | – | – | single drug | ≥ 2 drugs |
| To stage and allot a risk factor score, a patient's diagnosis is allocated to a stage as represented by a Roman numeral I, II, III, and IV. This is then separated by a colon from the sum of all the actual risk factor scores expressed in Arabic numerals, i.e., stage II:4, stage IV:9. This stage and score will be allotted for each patient.(FIGO 2009). A score ≤ 6 indicates low‐risk; > 6 indicates high‐risk. | ||||
| hCG = human chorionic gonadotrophin; IU = Internationa Units | ||||
| Drug | Study | Comment |
| Intravenous (IV) methotrexate (100, 150, or 300 mg/m²) with folinic acid rescue 24 hours later, repeated weekly | The original Bagshawe regimen. | |
| Bolus (100 mg/m² IV or IM) and 12‐hour continuous methotrexate infusion (200 mg/m²) with folinic acid rescue 24 hours later, repeated fortnightly | ||
| Combined 5‐day methotrexate (day 1 to 5) and 5‐day actinomycin D (day 15 to 19), repeated every 28 days | Associated with a high incidence of toxicity. | |
| High‐dose methotrexate (600 mg/m²) | Did not effect a higher cure than other methotrexate regimens. | |
| Etoposide (oral and parenteral) | Reported to be highly effective but not widely used for low‐risk GTN due to the high risk of side‐effects, particularly alopecia. | |
| Fluorouracil | Used in China for several decades, mainly because of its low cost, but is not favoured elsewhere. | |
| Intra‐lesional methotrexate infusion | Not favoured in Europe or North America. | |
| Chinese preparations | Not favoured in Europe or North America. | |
| GTN = gestational trophoblastic neoplasia | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary cure (remission) Show forest plot | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.75] |
| 1.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.80] |
| 1.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |
| 1.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| 1.4 Eight‐day IM MTX‐FA vs. pulsed IV Act D | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 2 Failure of first line therapy Show forest plot | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [1.81, 6.95] |
| 2.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 3.54 [1.12, 11.16] |
| 2.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 3.2 [1.17, 8.78] |
| 2.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 18.58 [1.16, 297.18] |
| 2.4 Eight‐day IM MTX‐FA vs. pulsed IV Act D | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [1.19, 8.90] |
| 3 Chemotherapy cycles to primary cure Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 346 | Mean Difference (IV, Random, 95% CI) | 3.04 [0.93, 5.14] |
| 3.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐2.87, ‐1.53] |
| 3.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.27, 1.53] |
| 4 Adverse effects: Nausea Show forest plot | 4 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.29, 1.26] |
| 4.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.62] |
| 4.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.93] |
| 5 Adverse effects: Vomiting Show forest plot | 3 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.73] |
| 5.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.24, 1.32] |
| 5.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.50, 4.05] |
| 6 Adverse effects: Diarrhoea Show forest plot | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.85, 2.41] |
| 6.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.57, 3.16] |
| 6.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.58, 3.85] |
| 7 Adverse effects: Constitutional Show forest plot | 3 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 7.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.18] |
| 7.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.55] |
| 8 Adverse effects: Alopecia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.27, 1.83] |
| 8.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.41, 4.30] |
| 8.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.53] |
| 9 Adverse effects: Mucositis/stomatitis Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.17] |
| 9.2 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.54] |
| 10 Adverse effects: Dermatological Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Weekly IM MTX vs. pulsed IV Act D | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Adverse effects: Neutropenia Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.45] |
| 11.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.15] |
| 11.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.43, 9.20] |
| 11.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.27, 21.89] |
| 12 Adverse effects: Thrombocytopenia Show forest plot | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.55] |
| 12.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 12.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.74, 8.50] |
| 12.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 6.41] |
| 13 Adverse effects: Anaemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.1 Weekly IM MTX vs. pulsed IV Act D | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Adverse effects: Hepatotoxicity Show forest plot | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.39, 16.88] |
| 14.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.56, 3.61] |
| 14.2 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 10.68 [0.63, 179.70] |
| 15 Adverse effects: Haemoptysis Show forest plot | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.30, 3.31] |
| 15.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.13, 2.94] |
| 15.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.30, 13.38] |
| 16 Severe adverse events (≥G3) Show forest plot | 5 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.08, 1.66] |
| 16.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 16.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.88] |

