Quimioterapia de primera línea para la neoplasia trofoblástica gestacional de bajo riesgo
Información
- DOI:
- https://doi.org/10.1002/14651858.CD007102.pub4Copiar DOI
- Base de datos:
-
- Cochrane Database of Systematic Reviews
- Versión publicada:
-
- 09 junio 2016see what's new
- Tipo:
-
- Intervention
- Etapa:
-
- Review
- Grupo Editorial Cochrane:
-
Grupo Cochrane de Cáncer ginecológico, neurooncología y otros cánceres
- Copyright:
-
- Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Cifras del artículo
Altmetric:
Citado por:
Autores
Contributions of authors
Mo'iad Alazzam: protocol development, methodological quality assessment, retrieval of papers, data extraction, data analysis and writing the review.
John Tidy: protocol development, methodological quality assessment, data extraction and revision of the review. Barry Hancock: content expert and revision of the review.
Ray Osborne: content expert, contributed to writing discussion and revision of the review.
Tess Lawrie: co‐ordination of the review updates, methodological quality assessment, retrieval of papers, data extraction, data analysis and updating the review.
Sources of support
Internal sources
-
Sheffield Teaching Hospitals NHS Trust, UK.
External sources
-
10/4001/12 National Institute for Health Research (NIHR) Cochrane Programme Grant Scheme, UK., UK.
This review received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme ‐ Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS.
Declarations of interest
Ray Osborne was an primary investigator of a study that has been included in this review (Osborne 2011). He was not involved in the consideration or assessment of this study for inclusion.
Acknowledgements
We thank the following people:
-
Jo Morrison, Clare Jess, Gail Quinn, and Jane Hayes at the Cochrane Gynaecological, Neuro, and Orphan Cancer Review Group in Bath for their advice and support throughout the review process;
-
Jo Platt for conducting the updated 2016 search and sifting the initial results;
-
Alison Little (Sheffield University) and Anne Oestmann (Cochrane Gynaecological Cancer Collaborative Review Group) for their assistance in developing the search strategy process and literature search for the original review; and
-
The authors of Lertkhachonsuk 2009 and Yarandi 2008 for providing additional data.
-
The 2012 review update received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme ‐ Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS.
-
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2016 Jun 09 | First‐line chemotherapy in low‐risk gestational trophoblastic neoplasia | Review | Theresa A Lawrie, Mo'iad Alazzam, John Tidy, Barry W Hancock, Raymond Osborne | |
| 2012 Jul 11 | First‐line chemotherapy in low‐risk gestational trophoblastic neoplasia | Review | Mo'iad Alazzam, John Tidy, Barry W Hancock, Raymond Osborne, Theresa A Lawrie | |
| 2009 Jan 21 | First line chemotherapy in low risk gestational trophoblastic neoplasia | Review | Mo'iad Alazzam, John Tidy, Barry W Hancock, Raymond Osborne | |
| 2008 Apr 23 | First line chemotherapy in low risk gestational trophoblastic neoplasia | Protocol | Mo'iad Alazzam, John Tidy, Barry W Hancock, Raymond Osborne | |
Differences between protocol and review
In the protocol and the original 2009 review, we included non‐randomised studies (NRS) as well as RCTs. For the updated and revised review, we included only RCTs.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Antineoplastic Agents [*administration & dosage, adverse effects];
- Case‐Control Studies;
- Cohort Studies;
- Dactinomycin [*administration & dosage, adverse effects];
- Drug Administration Schedule;
- Gestational Trophoblastic Disease [*drug therapy];
- Leucovorin [administration & dosage];
- Methotrexate [*administration & dosage, adverse effects];
- Randomized Controlled Trials as Topic;
- Risk;
- Vitamin B Complex [administration & dosage];
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICO
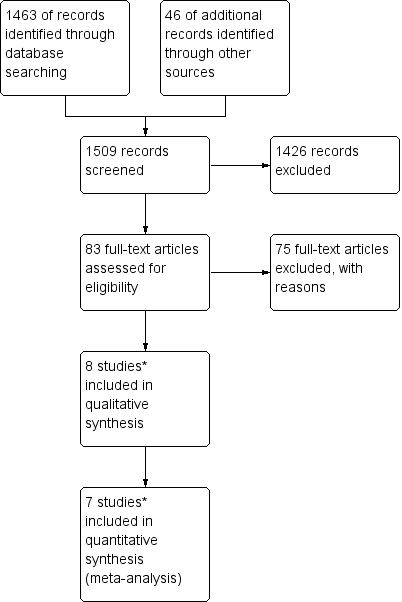
Study flow diagram of the original 2009 review
*The original 2009 review Included four non‐RCTs (Abrao 2008; Kohorn 1996; Smith 1982; Wong 1985) in the qualitative and three (Abrao 2008 not included) in the quantitative meta‐analysis). These non‐RCTs were excluded in the updated review.

Study flow diagram of the updated search conducted from January 2010 to February 2012.

Study flow diagram for the updated search conducted from Feb 2012 to January 2016.
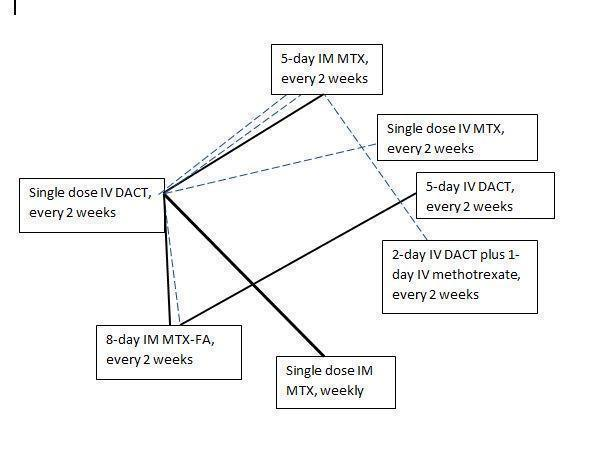
Chemotherapy treatment comparisons of included RCTs (solid lines) and ongoing RCTs (dotted lines)
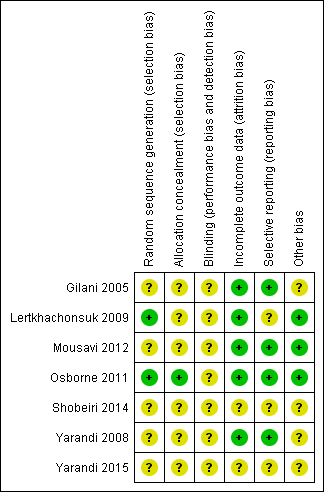
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
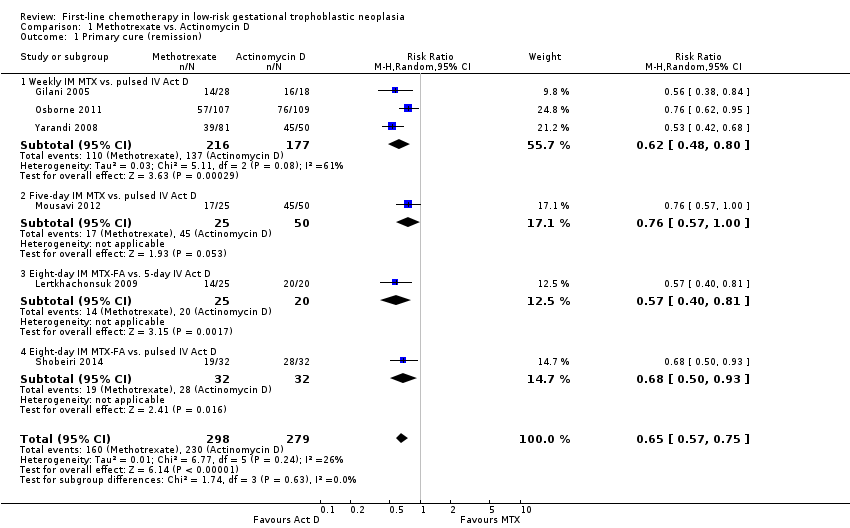
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 1 Primary cure (remission).
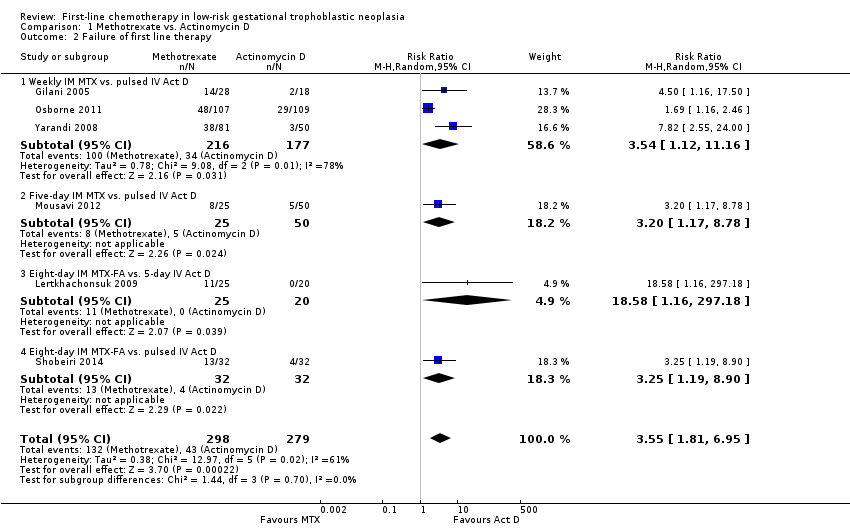
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 2 Failure of first line therapy.
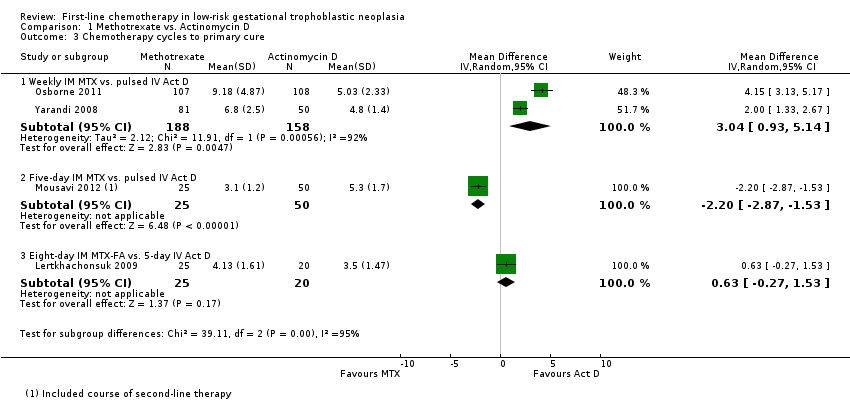
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 3 Chemotherapy cycles to primary cure.

Comparison 1 Methotrexate vs. Actinomycin D, Outcome 4 Adverse effects: Nausea.
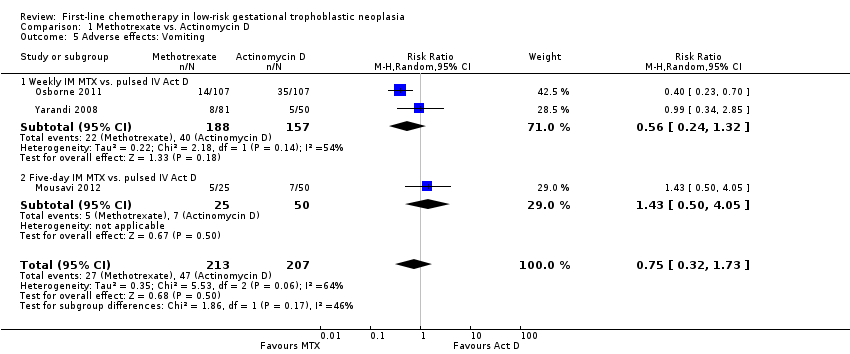
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 5 Adverse effects: Vomiting.

Comparison 1 Methotrexate vs. Actinomycin D, Outcome 6 Adverse effects: Diarrhoea.
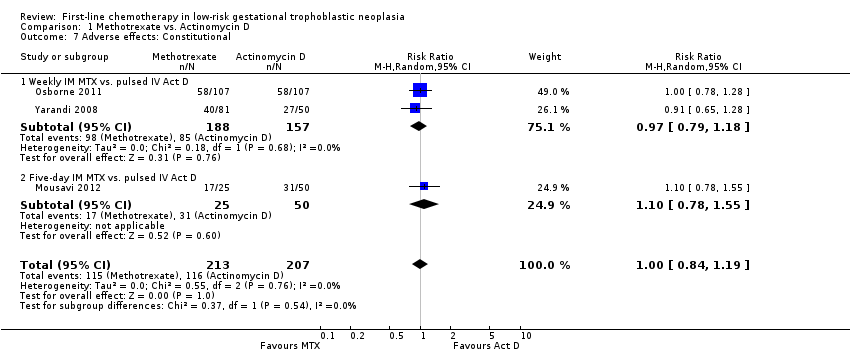
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 7 Adverse effects: Constitutional.
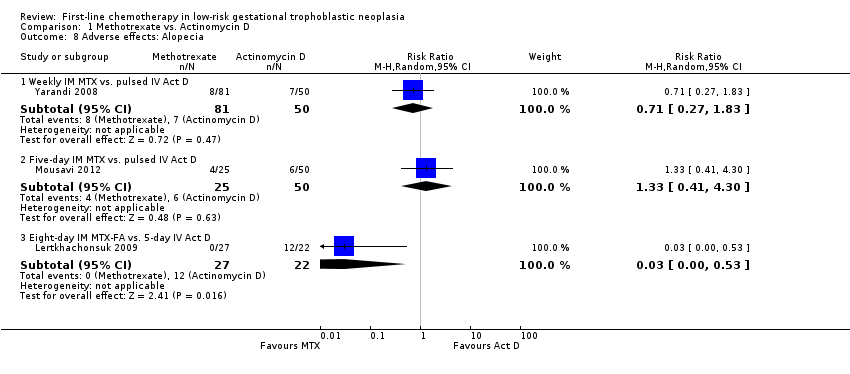
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 8 Adverse effects: Alopecia.
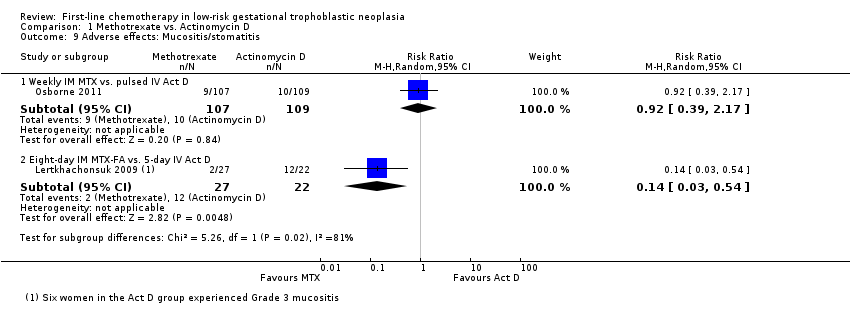
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 9 Adverse effects: Mucositis/stomatitis.
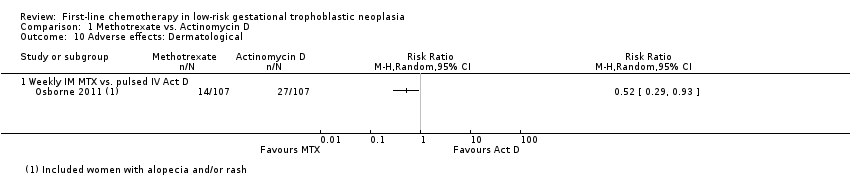
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 10 Adverse effects: Dermatological.

Comparison 1 Methotrexate vs. Actinomycin D, Outcome 11 Adverse effects: Neutropenia.
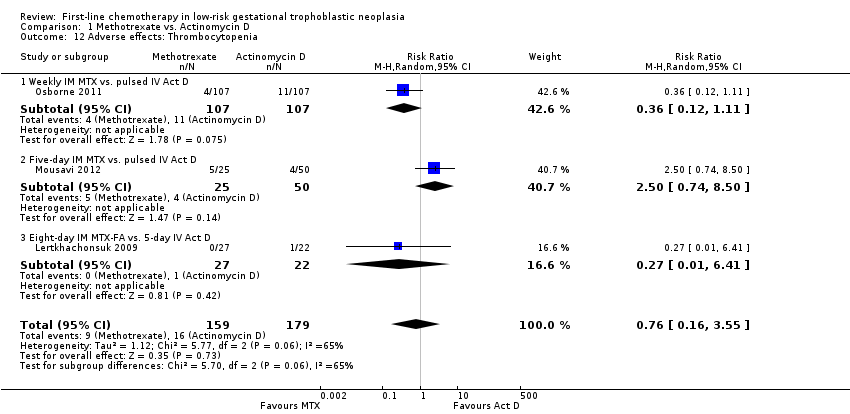
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 12 Adverse effects: Thrombocytopenia.
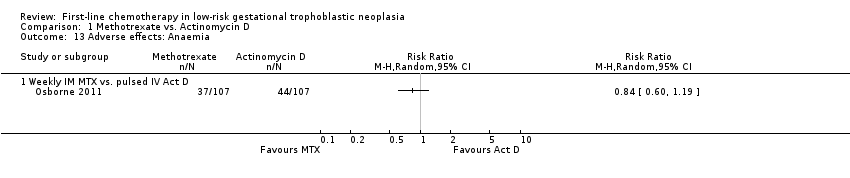
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 13 Adverse effects: Anaemia.
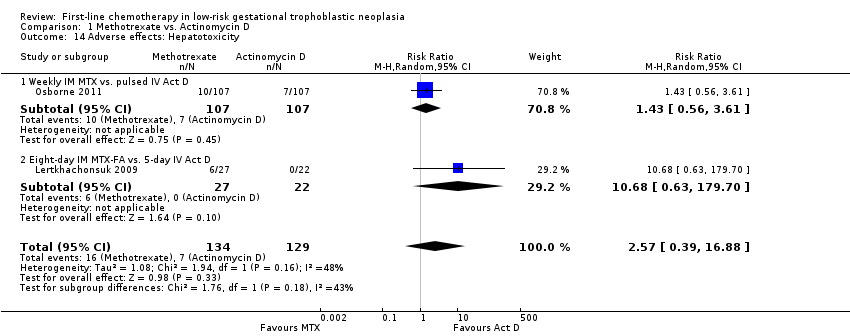
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 14 Adverse effects: Hepatotoxicity.
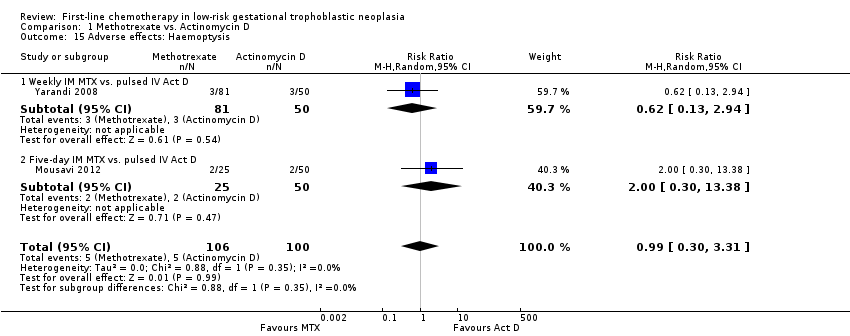
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 15 Adverse effects: Haemoptysis.
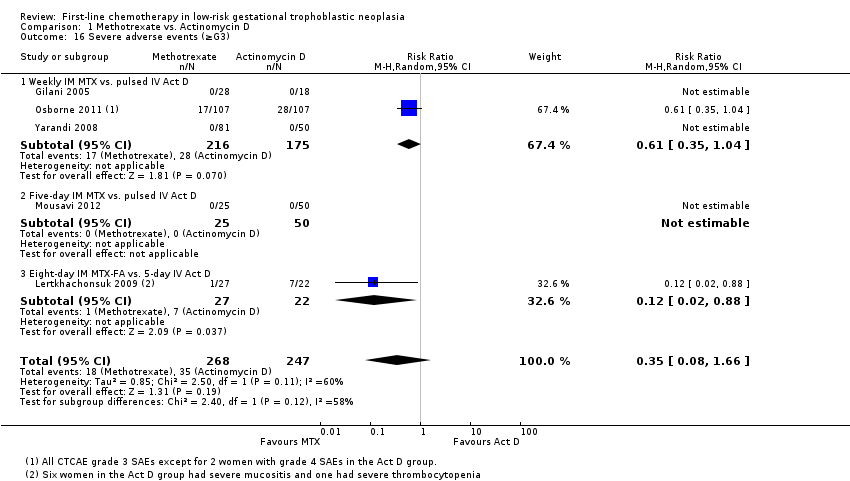
Comparison 1 Methotrexate vs. Actinomycin D, Outcome 16 Severe adverse events (≥G3).
| Actinomycin D compared with methotrexate (MTX) for low‐risk gestational trophoblastic neoplasia (GTN) | ||||||
| Patient or population: women withe low‐risk GTN Settings: outpatient or hospital Intervention: actinomycin D (Act D) Comparison: MTX | ||||||
| Outcomes | Illustrative Assumed risk* (Act D) | Illustrative Corresponding risk (MTX) | Relative effect | No of Participants | Quality of the evidence | Comments |
| Primary cure (remission) | 824 per 1000 | 536 per 1000 (470 to 618) | RR 0.65 (0.57 to 0.75) | 577 women (6 studies) | ⊕⊕⊕⊝ | Act D is probably more likely to achieve a primary cure than MTX. 55% of the data came from trials of weekly IM MTX, which may be less effective than the 5‐ or 8‐day MTX regimens. |
| Failure of first‐line therapy | 154 per 1000 | 547 per 1000 (279 to 1000) | RR 3.55 (1.81 to 6.95) | 577 women (6 studies) | ⊕⊕⊕⊝ | Act D as a first‐line treatment is probably less likely to fail than MTX. 59% of the data came from trials of weekly IM MTX, which may be less effective than the 5‐ or 8‐day MTX regimens. |
| Severe adverse events (≥ grade 3) | 142 per 1000 | 50 per 1000 (11 to 235) | RR 0.35 (0.08 to 1.66) | 515 women (5 studies) | ⊕⊕⊝⊝ low1,2 | There may be little or no difference between interventions overall. However, the point estimate and subgroup analyses favoured MTX. SAEs occurred in 3 out of 6 studies, but one study did not contribute to the meta‐analysis due to insufficient data. |
| Nausea | 462 per 1000 | 282 per 1000 (134 to 582) | RR 0.61 (0.29 to 1.26) | 466 women (4 studies) | ⊕⊕⊕⊝ | There is probably little or no difference between MTX and Act D for nausea. |
| Alopecia | Subtotals only | ⊕⊕⊝⊝ low1,2 | Data on alopecia were not pooled due to substantial subgroup differences. However, in general the evidence suggested that there may be little or no difference between MTX and Act D regimens with regard to alopecia, except for the five‐day Act D regimen, which may be more frequently associated with alopecia than the 8‐day MTX regimen. | |||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence IM = intramuscular; SAE = severe adverse effects | ||||||
| 1 Downgraded for clinical or statistical inconsistency 2 Downgraded for imprecision | ||||||
| Stage I | Disease confined to the uterus |
| Stage II | GTN extends outside of the uterus, but is limited to the genital structures (adnexae, vagina, broad ligament) |
| Stage III | GTN extends to the lungs with or without known genital tract involvement |
| Stage IV | All other metastatic sites |
| *FIGO 2009 | |
| Scores | 0 | 1 | 2 | 4 |
| Age (years) | < 40 | ≥ 40 | – | – |
| Antecedent pregnancy | mole | abortion | term | – |
| Interval months from index pregnancy | < 4 | 4–6 | 7–12 | > 12 |
| Pretreatment serum hCG (IU/L) | < 103 | 103 to 104 | 104 to 105 | > 105 |
| Largest tumour size (including uterus) | < 3 | 3cm to 4 cm | ≥ 5 cm | – |
| Site of metastases | lung | spleen, kidney | gastrointestinal | liver, brain |
| Number of metastases | – | 1to 4 | 5 to 8 | > 8 |
| Previous failed chemotherapy | – | – | single drug | ≥ 2 drugs |
| To stage and allot a risk factor score, a patient's diagnosis is allocated to a stage as represented by a Roman numeral I, II, III, and IV. This is then separated by a colon from the sum of all the actual risk factor scores expressed in Arabic numerals, i.e., stage II:4, stage IV:9. This stage and score will be allotted for each patient.(FIGO 2009). A score ≤ 6 indicates low‐risk; > 6 indicates high‐risk. | ||||
| hCG = human chorionic gonadotrophin; IU = Internationa Units | ||||
| Drug | Study | Comment |
| Intravenous (IV) methotrexate (100, 150, or 300 mg/m²) with folinic acid rescue 24 hours later, repeated weekly | The original Bagshawe regimen. | |
| Bolus (100 mg/m² IV or IM) and 12‐hour continuous methotrexate infusion (200 mg/m²) with folinic acid rescue 24 hours later, repeated fortnightly | ||
| Combined 5‐day methotrexate (day 1 to 5) and 5‐day actinomycin D (day 15 to 19), repeated every 28 days | Associated with a high incidence of toxicity. | |
| High‐dose methotrexate (600 mg/m²) | Did not effect a higher cure than other methotrexate regimens. | |
| Etoposide (oral and parenteral) | Reported to be highly effective but not widely used for low‐risk GTN due to the high risk of side‐effects, particularly alopecia. | |
| Fluorouracil | Used in China for several decades, mainly because of its low cost, but is not favoured elsewhere. | |
| Intra‐lesional methotrexate infusion | Not favoured in Europe or North America. | |
| Chinese preparations | Not favoured in Europe or North America. | |
| GTN = gestational trophoblastic neoplasia | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary cure (remission) Show forest plot | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.57, 0.75] |
| 1.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.48, 0.80] |
| 1.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.57, 1.00] |
| 1.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.40, 0.81] |
| 1.4 Eight‐day IM MTX‐FA vs. pulsed IV Act D | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.50, 0.93] |
| 2 Failure of first line therapy Show forest plot | 6 | 577 | Risk Ratio (M‐H, Random, 95% CI) | 3.55 [1.81, 6.95] |
| 2.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 393 | Risk Ratio (M‐H, Random, 95% CI) | 3.54 [1.12, 11.16] |
| 2.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 3.2 [1.17, 8.78] |
| 2.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 18.58 [1.16, 297.18] |
| 2.4 Eight‐day IM MTX‐FA vs. pulsed IV Act D | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 3.25 [1.19, 8.90] |
| 3 Chemotherapy cycles to primary cure Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 346 | Mean Difference (IV, Random, 95% CI) | 3.04 [0.93, 5.14] |
| 3.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Mean Difference (IV, Random, 95% CI) | ‐2.20 [‐2.87, ‐1.53] |
| 3.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.63 [‐0.27, 1.53] |
| 4 Adverse effects: Nausea Show forest plot | 4 | 466 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.29, 1.26] |
| 4.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.62] |
| 4.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.93] |
| 5 Adverse effects: Vomiting Show forest plot | 3 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.73] |
| 5.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.24, 1.32] |
| 5.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.50, 4.05] |
| 6 Adverse effects: Diarrhoea Show forest plot | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.85, 2.41] |
| 6.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.57, 3.16] |
| 6.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.58, 3.85] |
| 7 Adverse effects: Constitutional Show forest plot | 3 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.84, 1.19] |
| 7.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.18] |
| 7.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.78, 1.55] |
| 8 Adverse effects: Alopecia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.27, 1.83] |
| 8.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.41, 4.30] |
| 8.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.03 [0.00, 0.53] |
| 9 Adverse effects: Mucositis/stomatitis Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.39, 2.17] |
| 9.2 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.54] |
| 10 Adverse effects: Dermatological Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.1 Weekly IM MTX vs. pulsed IV Act D | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Adverse effects: Neutropenia Show forest plot | 4 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.45] |
| 11.1 Weekly IM MTX vs. pulsed IV Act D | 2 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.38, 1.15] |
| 11.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.43, 9.20] |
| 11.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.27, 21.89] |
| 12 Adverse effects: Thrombocytopenia Show forest plot | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.55] |
| 12.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 12.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.74, 8.50] |
| 12.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.01, 6.41] |
| 13 Adverse effects: Anaemia Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.1 Weekly IM MTX vs. pulsed IV Act D | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Adverse effects: Hepatotoxicity Show forest plot | 2 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.39, 16.88] |
| 14.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.56, 3.61] |
| 14.2 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 10.68 [0.63, 179.70] |
| 15 Adverse effects: Haemoptysis Show forest plot | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.30, 3.31] |
| 15.1 Weekly IM MTX vs. pulsed IV Act D | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.13, 2.94] |
| 15.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.30, 13.38] |
| 16 Severe adverse events (≥G3) Show forest plot | 5 | 515 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.08, 1.66] |
| 16.1 Weekly IM MTX vs. pulsed IV Act D | 3 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.35, 1.04] |
| 16.2 Five‐day IM MTX vs. pulsed IV Act D | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Eight‐day IM MTX‐FA vs. 5‐day IV Act D | 1 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.88] |

