木糖醇预防达12岁儿童急性中耳炎
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 663 healthy daycare children with normal ear status and without current ARI (normal tympanograms, A‐curve) recruited from 21 childcare centres in the city of Oulu, Finland between August 2001 and January 2002. Age: 7 months to 7 years Exclusion criteria: current antimicrobial prophylaxis, craniofacial malformations and structural middle ear abnormalities | |
| Interventions | Test group, unable to chew gum n = 60: 8 mL of a syrup containing 400 g/L xylitol 3 times a day (daily doses of 9.6 g of xylitol) Placebo group, able to chew gum n = 274: control chewing gum 3 times a day (daily dose 0.5 g of xylitol) also contained sucrose as a sweetener | |
| Outcomes | AOM based on a finding of middle ear effusion by pneumatic otoscopy and symptoms of acute respiratory infection | |
| Notes | The daily amount of xylitol was the same as other trials but it was administered only 3 times a day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed using tables of random numbers and a block approach with a block size of four.” |
| Allocation concealment (selection bias) | Low risk | A randomisation list was given to the product providers and the products were packed using this random allocation sequence |
| Blinding (performance bias and detection bias) | Low risk | Quote: "All study physicians, authors, and participating families were blinded to the group assignment until data entry and data checking were complete.” |
| Incomplete outcome data (attrition bias) | Low risk | By the end of trial, the dropout range was statistically higher in test group (17%) versus the control group (11%). The reasons for dropouts were: child refused to take the product; abdominal discomfort; chewing gum piece too big; duration of the trial too long; forgot to give the preparation when on holiday; illness; rash; parents tired of the trial; difficult to avoid additional xylitol products; other or unknown reasons |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry. Governmental source of funding. The study authors declared no conflict of interest but based on their previous paper, they have a US patent for the use of xylitol in treating respiratory infection |
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 1277 healthy daycare children the city of Oulu, Finland after screening with tympanometry during an ARI | |
| Interventions | Children unable to chew gum: Placebo group: n = 212: 5 mL of a syrup containing 20 g/L xylitol diluted in water without any other sweeteners (daily doses of 0.5 g of xylitol) Test group: n = 212: 5 mL of a syrup containing 400 g/L xylitol 5 times a day (daily doses of 10 g of xylitol) Syrups administered after meals with a syringe during a period of 5 minutes to maintain a high concentration of xylitol in the oral cavity for as long as practically possible Children able to chew gum: Placebo group: n = 280, control chewing gum with daily dose 0.5 g of xylitol Test group: n = 286: xylitol chewing gum with daily dose 8.4 g of xylitol Test group: n = 287: xylitol lozenges with daily dose 10 g of xylitol Two pieces of chewing gum or lozenges were chewed for at least 5 minutes 5 times a day after meals. 3 of the doses were given by the personnel at the childcare centres during the day and the rest were given by the parents at home. | |
| Outcomes | AOM based on a finding of middle ear effusion in tympanometry (B, C or positive pressure curve) and confirmed with pneumatic otoscopy; A total of 1253 of the 1277 randomised children were eligible for the analysis of the primary outcome | |
| Notes | The daily doses of control and xylitol products were equal to those used in earlier trials The parents began administering the products to their children at the onset of symptoms of ARI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 4 in the mixture groups and in blocks of 3 in chewing gum and lozenge groups, using a random number table to make the proportion of participants in each study group approximately the same at each child care centre." |
| Allocation concealment (selection bias) | Low risk | Each child was given a unique participation number at the time of the initial screening |
| Blinding (performance bias and detection bias) | Unclear risk | The study was blinded as far as the mixture and chewing gum groups were concerned but open as between the xylitol lozenge and control chewing gum groups |
| Incomplete outcome data (attrition bias) | Low risk | The children who dropped out (n = 24) were excluded from the statistical analysis, but those who prematurely stopped using the products but still visited the clinic (n = 35) were included. The children who dropped out contributed days at risk to the cumulative occurrence analysis for as long as they continued to participate |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry. Governmental source of funding. The study authors declared they have a US patent for the use of xylitol in treating respiratory infection |
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 306 children from 11 ordinary daycare nurseries for healthy, normal children from the city of Oulu, Finland were enrolled in March 1995 after parental consent. 30 dropouts, which left 276 children Xylitol group (n = 157), mean (SD) age = 5.0 (1.4); mean (SD) duration of daycare (months) = 26.5 (16.9) Sucrose group (n = 149), mean (SD) age = 4.9 (1.5) years; mean (SD) duration of daycare (months) = 25.2 (19.0) Exclusion criteria: children with dental caries | |
| Interventions | Xylitol chewing gum n = 157: 2 pieces five times (one box) a day after meals or snacks were chewed until there was no taste left or for at least 5 minutes, making a total dose of 8.4 g xylitol a day Sucrose (control) chewing gum n = 149 | |
| Outcomes | AOM based on symptoms and signs of ARI and simultaneous signs of middle ear effusion: a cloudy tympanic membrane or impaired tympanic membrane motility in pneumatic otoscopy Antimicrobial treatment received during the intervention and nasopharyngeal carriage of S pneumoniae | |
| Notes | Use of sucrose as the control group has been criticised as possibly increasing the dental caries experience. However, of the children who underwent a dental examination, there was no difference in the rate of dental decay (23/114 in sucrose group versus 21/111 in the xylitol group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was made by using random number tables which were also used to number the cartridges. Block randomisation with a block size of 4 in each daycare was used to ensure equal numbers of children in the 2 groups Quote: "A randomisation list was made by using random number tables.” To ensure equal numbers of children in the 2 groups in each of the nurseries we used block randomisation with a block size of 4 |
| Allocation concealment (selection bias) | Low risk | The list was sealed in an envelope. The observers were blinded to the randomisation scheme Quote: "The cartridges were numbered accordingly, and the list was sealed in an envelope" |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The observers in the trial were unaware of the randomisation scheme" |
| Incomplete outcome data (attrition bias) | Low risk | There was a dropout of 30 participants, 10 in xylitol group and 20 in control group representing a dropout rate of 6% and 13%, respectively. The reasons for dropouts were: child got tired of eating chewing gum, dental caries, moved from area, parents got tired, insufficient follow‐up data, loose stools |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry, no conflict of interest was reported. Governmental source of funding |
| Methods | Double‐blind, placebo‐controlled randomised study | |
| Participants | 857 children from 34 typical daycare centres for healthy children from the city of Oulu, Finland were recruited after parental consent between September and December 1996 | |
| Interventions | Test group, unable to chew gum n = 159: 5 mL of a syrup containing 400 g/L xylitol 5 times a day (daily doses of 10 g of xylitol) Test group, able to chew gum n = 179: 2 pieces of xylitol chewing gum 5 times a day (3 x in daycare and 2 x in home) after a meal for at least 5 minutes or as long as it tasted sweet (daily dose 8.4 g of xylitol), or 3 xylitol/maltitol lozenges (n = 176) lozenges 5 times a day after a meal for at least 5 minutes or as long as it tasted sweet (daily dose 10 g of xylitol) Control group, unable to chew gum n = 165: control syrup containing 20 g/L xylitol diluted in water without any other sweeteners with the same protocol as xylitol syrup group (daily doses of 0.5 g of xylitol) Control group, able to chew gum n = 178: chewing gum sweetened with sucrose and xylitol (daily dose 0.5 g of xylitol) with the same protocol as xylitol gum group | |
| Outcomes | AOM based on a finding of middle ear effusion in tympanometry (B‐ or C‐curve), verified otoscopically with signs of inflammation in the tympanic membrane, and the presence of symptoms of acute respiratory infection (rhinitis, cough, conjunctivitis, sore throat, earache) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed using tables of random numbers and using a block randomisation with a block size of six.” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned but probably done as per their earlier publication |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The study was double‐blind in the syrup and chewing gum groups and open between the chewing gum and lozenge groups. The xylitol syrup was sweeter than the control syrup, but the taste of the chewing gums was quite similar regardless of the sweeteners used” |
| Incomplete outcome data (attrition bias) | Low risk | By the end of trial, the dropouts range from 4.5% to 18.9% and were statistically higher in the xylitol syrups (18.9%) and xylitol lozenges (14.8%) as compared to their control groups of, respectively, control syrups (10.3%) and control chewing gum (4.5%). The reasons for dropouts were: unwilling to continue taking the product, left the area, abdominal discomfort, unknown reason |
| Selective reporting (reporting bias) | Low risk | None identified |
| Other bias | Unclear risk | The material was donated by industry, no conflict of interest was reported. Governmental source of funding |
| Methods | Double‐blind, pragmatic practice‐based RCT | |
| Participants | 326 otitis‐prone children ages 6 to 71 months with history of at least 3 clinically diagnosed episodes of acute otitis media (with/without middle ear effusions) in the previous 12 months with at least 1 in the previous 6 months, in good general health (see criteria for ‘good health’ on page 290) and English or Spanish speaking All children were identified and referred by participating physicians from 3 paediatric practice‐based networks (the Slone Center Office‐based Research Network at Boston University, the Pediatric Physicians’ Organization at Children’s (Boston), and the North Carolina Child Health Research Network at the University of North Carolina) | |
| Interventions | Active treatment: Xylitol oral solution (Xylarex; Arbor Pharmaceuticals, Atlanta, GA) consisting of a 66.7% aqueous xylitol solution with the addition of carboxymethylcellulose and potato starch as mucosal adherence agents and natural flavouring. The dose for the xylitol syrup was 7.5 mL 3 times daily for 12 weeks (i.e. 5 g xylitol per dose) Placebo medication: 30% sorbitol oral solution with the addition of carboxymethylcellulose and potato starch as mucosal adherence agents and natural flavouring at a dose of 2.25 g sorbitol 3 times per day | |
| Outcomes | Primary outcome: comparison of time to first clinically diagnosed AOM episode in the two study groups, using proportional hazards model. Secondary outcomes: 1. Proportion of participants in each group with no AOM episodes and no antibiotic use during the study period 2. Reduction in the 3‐month cumulative incidence of AOM episodes, as measured by the risk difference and the hazard rate for AOM (in Poisson regression) 3. Reduction in antibiotic use per 90 days, as measured by the risk difference and the hazard rate (in Poisson regression) Frequency of occurrence of adverse events in either group, as measured by the risk difference | |
| Notes | Analyses were based on the intention‐to‐treat principle. For the comparison of incidence of AOM episodes and antibiotic use per 90 days between the two groups, “rates were calculated individually (events divided by days of enrolment), assuming a zero rate for the 12 participants with no follow‐up.” Higher amount of xylitol per dose and per day over previous studies and the addition of mucosal adherence agents to the xylitol solution as compared to other studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No direct quote was found. There was an acknowledgement to Qiaoli (Lily) Chen who generated randomisation assignments |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Blinding (performance bias and detection bias) | Low risk | Blinded study syrups. Sorbitol is similar in appearance and taste to xylitol. “After randomisation, study materials, including the blinded study syrup, appropriate oral syringes, and a study calendar, were shipped to the subject’s home.” The principal investigator reviewed medical records in a blinded fashion. Quote: “At the end of the study, medical records from each subject’s primary care physician and from any other health care provider whom the parent identified as having treated the subject during the study period were obtained and reviewed by the principal investigator (LV) in a blinded fashion.” |
| Incomplete outcome data (attrition bias) | High risk | 62/160 allocated to xylitol (38.8%) declined participation, were lost to follow‐up or discontinued intervention versus 58/166 allocated to placebo (34.9%) |
| Selective reporting (reporting bias) | Low risk | As compared to protocol NCT01044030, 2 secondary outcomes were not reported, although they do not represent key outcomes: 1) To determine the effect of viscous‐adherent xylitol on nasopharyngeal and oropharyngeal colonisation with S pneumoniae and non‐typable H influenzae and 2) To compare the antimicrobial resistance patterns of isolates of S pneumoniae and non‐typable H influenzae cultured from the oropharynx and/or nasopharynx of subjects treated with viscous‐adherent xylitol compared to placebo |
| Other bias | Low risk | Potential bias for outcome assessment: The study did not have any protocol of assessment for healthcare providers. Quote: “For the primary outcome of clinical diagnoses of AOM, the medical record was considered the gold standard. For those cases in which the medical record was not available, the parent’s report of AOM diagnoses was used” The researchers assumed that inaccurate diagnoses would be equal in both groups. Quote: “AOM diagnoses were made by a wide range of clinicians and were not otherwise verified.” “… assuming that AOM diagnoses were equally inaccurate in both study groups, the overall effect would be to bias the study toward a null result.” The trial was performed under FDA New Drug application number 107246 and was registered at www.clinicaltrials.gov (NCT01044030) No financial incentives paid to parents/subjects or to enrolling practices, except a nominal reimbursement for staff time involved in referring potentially eligible parents. |
AOM: acute otitis media
ARI: acute respiratory infection
n: number
SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| No data on AOM. In the published paper, it was mentioned that the effect of xylitol in reducing AOM will be published in a subsequent study. However, after contacting the primary investigator, it was understood that due to lack of reliability of data regarding AOM, the plan to publish AOM results has been suspended |
AOM: acute otitis media
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled study |
| Participants | 82 male and 86 female outpatients from 1 to 15 years of age with recurrent AOM. Mean age was 6.14 years. The population is from Prague and Mid‐Bohemia region, Czech Republic |
| Interventions | Intervention: 3 daily doses of nasal wash, each consisting of 2 squirts per nostril, at regular assigned times daily (after waking up; after lunch; before going to bed). Patients received one of the following treatments as predetermined by their randomisation number: a solution of nasal xylitol (11% pure xylitol; XLEAR®) or distilled water |
| Outcomes | AOM recurrence; frequency of antibiotic therapy; frequency of upper airways infections; adverse events |
| Notes | Xlear Inc. has been contacted for further information about this unpublished report |
AOM: acute otitis media
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Nasal xylitol in the prevention of otitis media |
| Methods | Double‐blind, RCT |
| Participants | 50 children at the age of 1 to 5 years who had 3 episodes of recurrent otitis media in the last 6 months prior to entering the study. Those with immune deficiency, craniofacial malformations, chronic otitis media, or those who received prophylactic antibiotic treatment prior to entering the study (3 months) will be excluded |
| Interventions | Control: isotonic saline nasal spray; test: 5% xylitol spray (3 times daily for 2 months) |
| Outcomes | Primary outcome measures: prevalence of otitis media and the number of events of acute otitis media during the study period of 5 months |
| Starting date | January 2016 |
| Contact information | Arie Gordin, M, [email protected] and Shani [email protected] Sponsors and Collaborators: Rambam Health Care Campus, HaEmek Medical Center, Israel, Carmel Medical Center |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 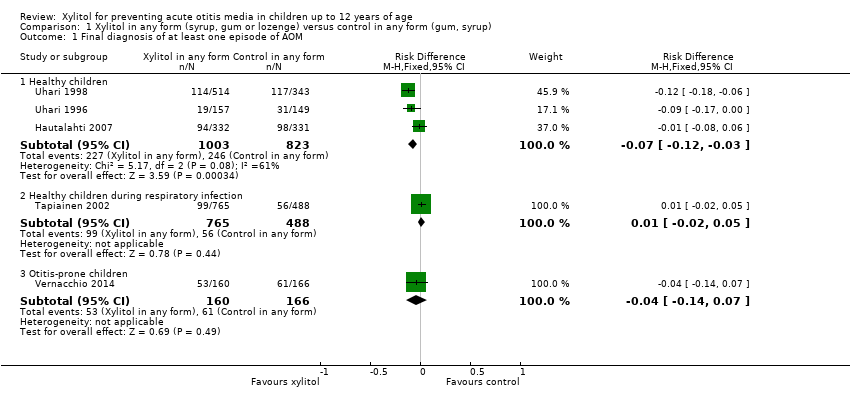 Comparison 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 3 | 1826 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.12, ‐0.03] |
| 1.2 Healthy children during respiratory infection | 1 | 1253 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gastrointestinal‐related adverse events Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Adverse effect: xylitol versus control, Outcome 1 Gastrointestinal‐related adverse events. | ||||
| 1.1 Healthy children | 3 | 1826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.74, 2.75] |
| 1.2 Healthy children during respiratory infection | 1 | 1277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.61, 13.00] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.16] |
| 2 Rash Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2 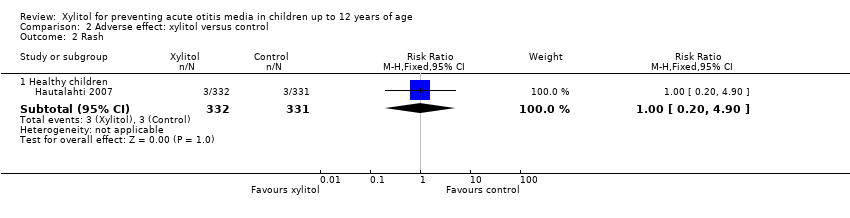 Comparison 2 Adverse effect: xylitol versus control, Outcome 2 Rash. | ||||
| 2.1 Healthy children | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one exposure to antimicrobial drugs Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 Antibiotic administration, Outcome 1 At least one exposure to antimicrobial drugs. | ||||
| 1.1 Healthy children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Otitis‐prone children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 4.1  Comparison 4 Xylitol syrup versus control for younger children unable to chew, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 1.2 Healthy children during respiratory infection | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.69] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 5.1 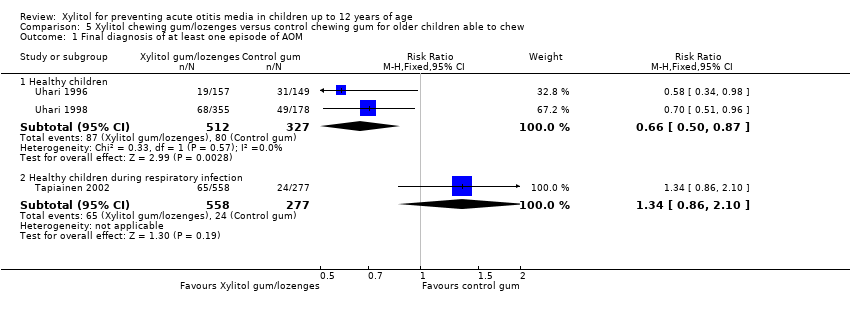 Comparison 5 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 2 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 1.2 Healthy children during respiratory infection | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.86, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1  Comparison 6 Xylitol chewing gum versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 2 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.81] |
| 1.2 Healthy children during respiratory infection | 1 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.78, 2.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 7.1 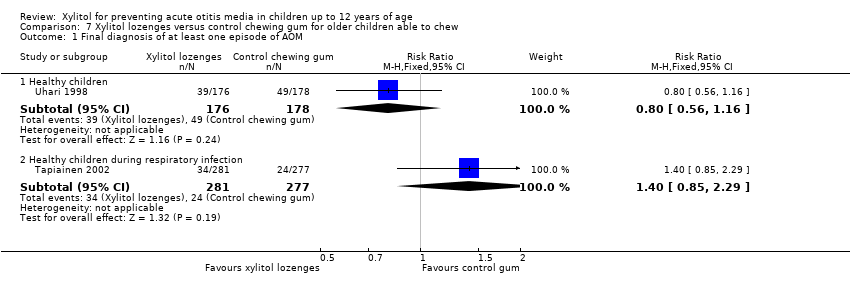 Comparison 7 Xylitol lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.16] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.85, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 8.1  Comparison 8 Comparison between active ingredients groups: xylitol chewing gum versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.89] |
| 1.2 Healthy children during respiratory infection | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 9.1  Comparison 9 Comparison between active ingredients groups: xylitol lozenges versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 1.2 Healthy children during respiratory infection | 1 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 10.1  Comparison 10 Comparison between active ingredients groups: xylitol chewing gum versus xylitol lozenges, Outcome 1 Final diagnosis of at least one episode of AOM. | ||||
| 1.1 Healthy children | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.46] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
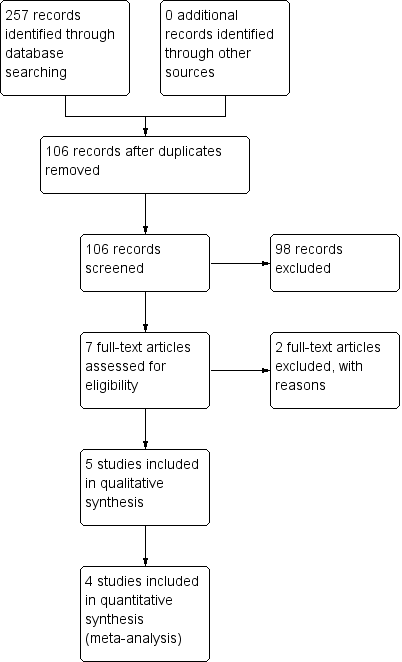
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), outcome: 1.1 Final diagnosis of at least one episode of AOM.
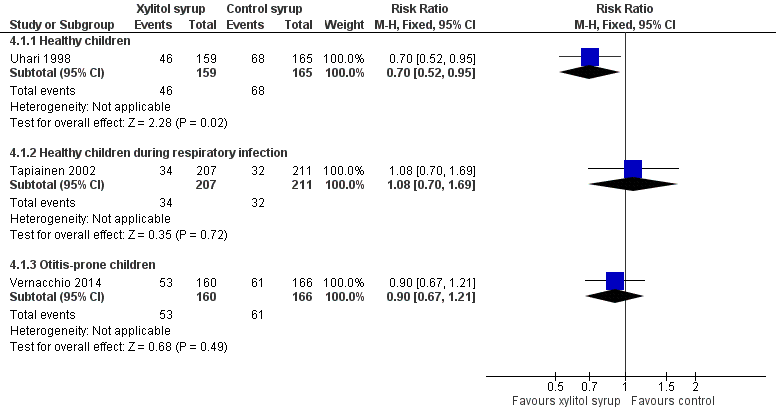
Forest plot of comparison: 3 Xylitol syrup versus control for younger children unable to chew, outcome: 3.1 Final diagnosis of at least one episode of AOM.
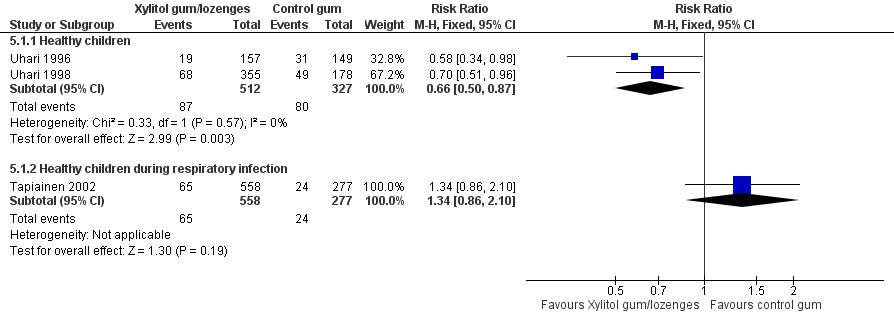
Forest plot of comparison: 4 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, outcome: 4.1 Final diagnosis of at least one episode of AOM.

Forest plot of comparison: 5 Xylitol chewing gum versus control chewing gum for older children able to chew, outcome: 5.1 Final diagnosis of at least one episode of AOM.

Comparison 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 2 Adverse effect: xylitol versus control, Outcome 1 Gastrointestinal‐related adverse events.
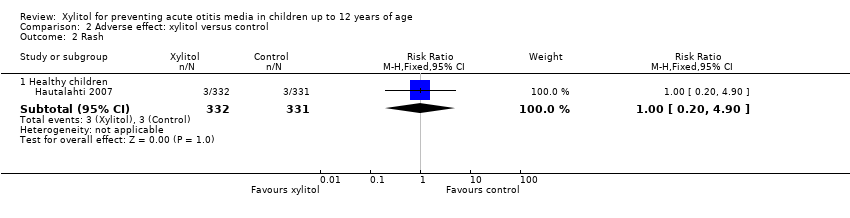
Comparison 2 Adverse effect: xylitol versus control, Outcome 2 Rash.
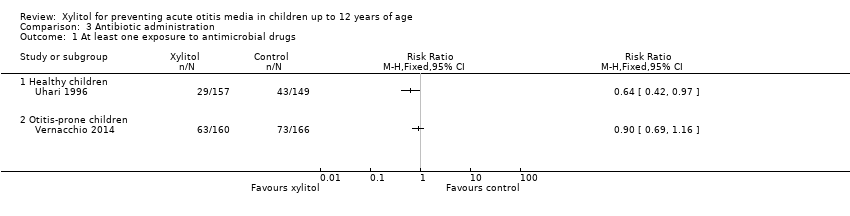
Comparison 3 Antibiotic administration, Outcome 1 At least one exposure to antimicrobial drugs.
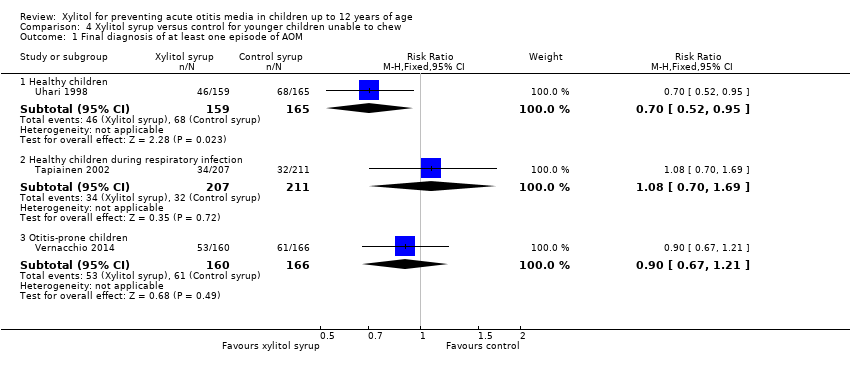
Comparison 4 Xylitol syrup versus control for younger children unable to chew, Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 5 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 6 Xylitol chewing gum versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 7 Xylitol lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.
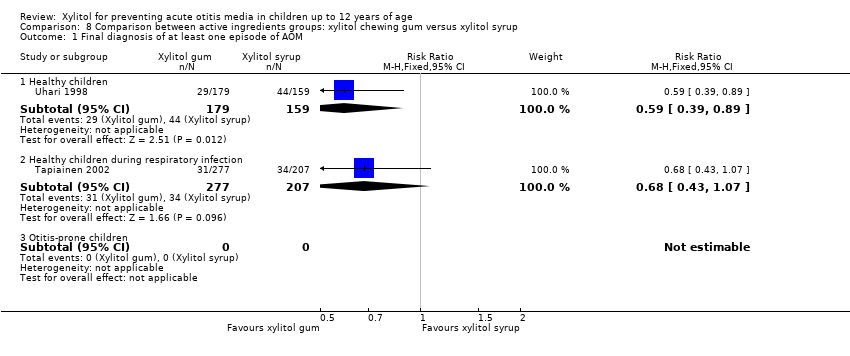
Comparison 8 Comparison between active ingredients groups: xylitol chewing gum versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM.
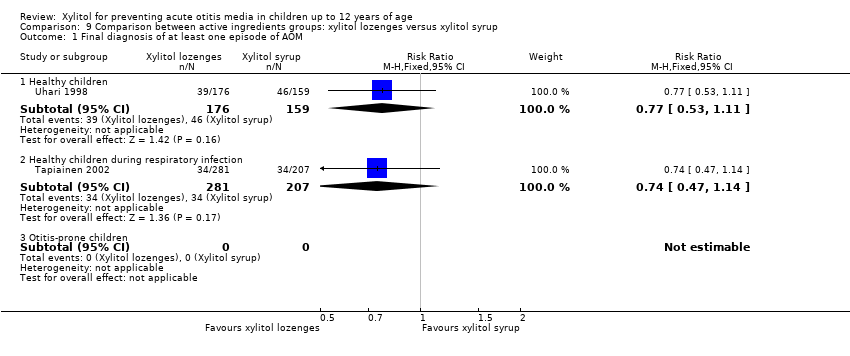
Comparison 9 Comparison between active ingredients groups: xylitol lozenges versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM.
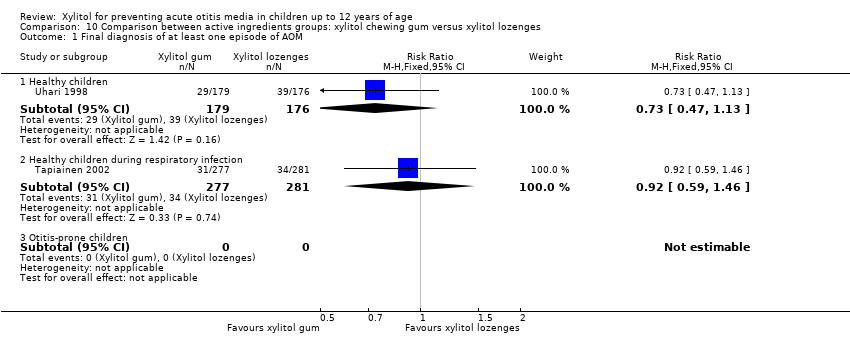
Comparison 10 Comparison between active ingredients groups: xylitol chewing gum versus xylitol lozenges, Outcome 1 Final diagnosis of at least one episode of AOM.
| Xylitol versus control for prevention of AOM in healthy children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk with control in any form (gum, syrup) | Corresponding risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.75 | 1826 | ⊕⊕⊕⊝ | RR 0.74 (0.54, 1.01) with random‐effects meta‐analysis | |
| 299 per 1000 | 224 per 1000 | |||||
| Moderate | ||||||
| 296 per 1000 | 222 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.43 | 1826 | ⊕⊕⊕⊝ | RR 1.41 (0.60, 3.33) with random‐effects meta‐analysis | |
| 17 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 15 per 1000 | 21 per 1000 | |||||
| Antibiotic administration | Study population | RR 0.64 | 306 | ⊕⊕⊕⊝ | ||
| 289 per 1000 | 185 per 1000 | |||||
| Moderate | ||||||
| 289 per 1000 | 185 per 1000 | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ There is inconsistency in th findings of the first two studies as compared to the third study of the same group | ||||||
| Xylitol versus control for prevention of AOM in healthy children during a respiratory infection | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 1.13 | 1253 | ⊕⊕⊕⊝ | ||
| 115 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 130 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 2.82 | 1277 | ⊕⊕⊕⊝ | ||
| 4 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 12 per 1000 | |||||
| Antibiotic administration ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ 95% CIs are wide and imprecise. The evidence is based on one trial. Moreover, there are few events and the CI includes appreciable benefit and harm | ||||||
| Xylitol versus control for prevention of AOM in otitis‐prone children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.90 | 326 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 331 per 1000 | |||||
| Moderate | ||||||
| 368 per 1000 | 331 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.04 | 326 | ⊕⊕⊕⊝ | ||
| 765 per 1000 | 796 per 1000 | |||||
| Moderate | ||||||
| 765 per 1000 | 796 per 1000 | |||||
| Antibiotic administration | Study population | RR 0.90 | 326 | ⊕⊕⊕⊝ | ||
| 440 per 1000 | 396 per 1000 | |||||
| Moderate | ||||||
| 440 per 1000 | 396 per 1000 | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ High risk for attrition bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 3 | 1826 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.12, ‐0.03] |
| 1.2 Healthy children during respiratory infection | 1 | 1253 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gastrointestinal‐related adverse events Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 3 | 1826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.74, 2.75] |
| 1.2 Healthy children during respiratory infection | 1 | 1277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.61, 13.00] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.16] |
| 2 Rash Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Healthy children | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one exposure to antimicrobial drugs Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Healthy children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Otitis‐prone children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 1.2 Healthy children during respiratory infection | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.69] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 2 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 1.2 Healthy children during respiratory infection | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.86, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 2 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.81] |
| 1.2 Healthy children during respiratory infection | 1 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.78, 2.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.16] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.85, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.89] |
| 1.2 Healthy children during respiratory infection | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 1.2 Healthy children during respiratory infection | 1 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.46] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

