만 12세 이하 아동의 급성 중이염 매개체를 예방하는 자일리톨
초록
배경
급성 중이염 매개체(AOM)는 미국 어린이들 사이에서 가장 흔한 세균 감염이다. 항생제와 수술로 치료할 수 있는 한계와 우려가 있어 효과적인 예방책이 매력적이다. 잠재적인 예방책은 충치의 위험을 줄이는 천연 설탕 대체물인 자일리톨이다. 자일리톨은 시험관 내 비인두세포에 대한 스트렙토코쿠스 진폐증(S 진폐증 )과 해모필루스 인플루언제(H 인플루언제)의 결합을 줄일 수 있다. 이것은 2011년에 처음 출판된 문헌고찰의 업데이트다.
목적
자일리톨의 효능과 안전성을 평가하여 12세까지의 아동에게 AOM을 예방한다.
검색 전략
Central(2015년 이슈12), MEDLINE(1950~2016년 1월), Embase(1974~2016년 1월), CINAHL(1981~2016년 1월), LILACS(1982~2016년 1월), Web of Science(2011~2016년 1월), International Pharmetic Obracts(2000~2016년 1월)를 검색했다.
선정 기준
자일리톨 보충제를 위약과 비교하거나 AOM을 예방하기 위한 치료제가 없는 12세 이하 어린이의 무작위 제어 시험(RCT) 또는 준 RCT.
자료 수집 및 분석
두 명의 검토 저자는 검색 결과에서 독립적으로 시행을 선택하고, 연구 품질을 평가 및 평가했으며, 검토에 포함하기 위해 관련 데이터를 추출했다. 누락된 자료를 요청하기 위해 평가판 작성자들에게 연락했다. 자일리톨의 부작용에 대한 데이터를 주목했다. 관련 결과에 대한 데이터를 추출하고 위험비(RR), 위해성 차이(RD) 및 관련 95% 신뢰 구간(CI)을 계산하여 효과 크기를 추정했다.
주요 결과
3405명의 아이들이 포함된 5개의 임상실험을 확인했다. 이번 2016년 업데이트에 대해 포함을 위한 새로운 평가판을 하나 식별했다. 이 실험은 체계적으로 검토되었지만 이질성의 여러 원천 때문에 메타 분석에 포함되지 않았다. 나머지 4개의 실험은 적절한 방법론적 품질을 가지고 있었다. 총 1826명의 건강한 핀란드 어린이 탁아소가 참여한 3개의 RCT에서 자일리톨(어떤 형태든)이 대조군(RR 0.75, 95% CI 0.65~0.88)에 비해 AOM 위험을 30%에서 약 22%로 줄일 수 있다는 중간 품질의 근거가 있다. 자일리톨과 대조군 사이의 복부 불편과 발진에는 큰 차이가 없었다. 자일리톨은 호흡기 감염(RR 1.13, 95% CI 0.83~1.53, 중간 정도 품질의 근거)이나 중이염에 걸리기 쉬운 건강한 어린이(RR 0.90, 95% CI 0.67~1.21, 저품질 근거) 사이에서 AOM을 줄이는 데 효과적이지 않았다.
연구진 결론
어린이집에 다니는 건강한 어린이들 사이에서 자일리톨의 예방 투여가 AOM 발생을 줄일 수 있다는 것을 보여주는 적당한 품질의 근거가 있다. 호흡기 감염 아동이나 중이염에 걸리기 쉬운 아동들 사이에서 자일리톨이 AOM을 예방하는 효능에 대한 결론에 이르지 못한 근거가 있다. 메타분석은 데이터가 소수의 연구로부터 나왔고, 대부분이 같은 연구군에서 나왔기 때문에 제한적이었다.
PICO
쉬운 말 요약
만 12세 이하 아동의 중이염 예방을 위한 자일리톨 설탕 보충제
문헌고찰의 질문
만 12세 이하 아동의 급성 중이염(급성 중이염 매개체, AOM) 예방을 위한 자일리톨의 효과와 안전성에 대한 근거를 검토했다.
배경
AOM은 미국 어린이들 사이에서 가장 흔한 세균 감염이다. 비록 심각한 합병증은 드물지만, 이 흔한 소아 질환은 의료 시스템에 큰 영향을 미친다. 미국에서는 거의 2천만명의 사무실 방문자를 차지했다. AOM의 항생제 처리는 비용이 많이 들고 항생제에 내성이 있는 균주의 박테리아 개발에 대한 우려를 낳고 있다. 수술은 침습적이고 비용이 많이 들며, 이러한 요소들 때문에, AOM을 예방하기 위한 효과적인 조치를 모색한다. 대체 치료법은 자일리톨이나 자작나무 설탕이다. 자일리톨은 주로 씹는 잇몸, 과자, 치약, 약품 등에 쓰이는 천연 무당 감미료로 수십 년간 사용돼 충치의 위험을 줄일 수 있다.
검색 날짜
2016년 1월까지 문헌을 검색했다. 이것은 2011년에 마지막으로 출판된 문헌고찰의 업데이트다.
연구 특징
3405명의 아이들이 참여한 5개의 임상실험을 확인했는데, 대부분이 같은 연구그룹의 아이들이었다. 핀란드에서 4번의 임상시험이 진행되어 건강한 어린이(3회)나 급성 호흡기 감염 어린이(1회)를 등록하였다. 미국에서 5번째 임상시험이 진행됐으며 일반 의료진 참여로 모집된 중이염에 걸리기 쉬운 아이들을 등록했다.
연구 자금 지원
핀란드 연구진은 자일리톨을 호흡기 감염 치료에 사용할 수 있는 미국 특허를 보유하고 있다.
주요 결과
자일리톨은 껌, 로젠지, 시럽 등에 투여돼 급성 상부 호흡기 감염이 없는 건강한 어린이의 AOM 발생을 30%에서 22%로 줄일 수 있다. 부작용(명칭 복부 불편과 발진)에는 차이가 없다. 이러한 결과를 바탕으로 자일리톨 껌이 제공될 경우 AOM을 경험하게 될 194~263명의 어린이와 비교할 때, 최대 12세까지의 1000명의 어린이 중 299명이 AOM을 경험하게 될 것으로 예상한다. 호흡기 감염이 있는 건강한 어린이나 중이염에 걸리기 쉬운 어린이들 사이에서 예방 효과는 단정할 수 없다.
근거의 질
근거의 질은 건강한 어린이와 호흡기 감염이 있는 어린이에게는 적당했지만 중이염에 걸리기 쉬운 어린이에게는 낮았다.
Authors' conclusions
Summary of findings
| Xylitol versus control for prevention of AOM in healthy children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk with control in any form (gum, syrup) | Corresponding risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.75 | 1826 | ⊕⊕⊕⊝ | RR 0.74 (0.54, 1.01) with random‐effects meta‐analysis | |
| 299 per 1000 | 224 per 1000 | |||||
| Moderate | ||||||
| 296 per 1000 | 222 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.43 | 1826 | ⊕⊕⊕⊝ | RR 1.41 (0.60, 3.33) with random‐effects meta‐analysis | |
| 17 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 15 per 1000 | 21 per 1000 | |||||
| Antibiotic administration | Study population | RR 0.64 | 306 | ⊕⊕⊕⊝ | ||
| 289 per 1000 | 185 per 1000 | |||||
| Moderate | ||||||
| 289 per 1000 | 185 per 1000 | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ There is inconsistency in th findings of the first two studies as compared to the third study of the same group | ||||||
| Xylitol versus control for prevention of AOM in healthy children during a respiratory infection | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 1.13 | 1253 | ⊕⊕⊕⊝ | ||
| 115 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 130 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 2.82 | 1277 | ⊕⊕⊕⊝ | ||
| 4 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 12 per 1000 | |||||
| Antibiotic administration ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ 95% CIs are wide and imprecise. The evidence is based on one trial. Moreover, there are few events and the CI includes appreciable benefit and harm | ||||||
| Xylitol versus control for prevention of AOM in otitis‐prone children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.90 | 326 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 331 per 1000 | |||||
| Moderate | ||||||
| 368 per 1000 | 331 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.04 | 326 | ⊕⊕⊕⊝ | ||
| 765 per 1000 | 796 per 1000 | |||||
| Moderate | ||||||
| 765 per 1000 | 796 per 1000 | |||||
| Antibiotic administration | Study population | RR 0.90 | 326 | ⊕⊕⊕⊝ | ||
| 440 per 1000 | 396 per 1000 | |||||
| Moderate | ||||||
| 440 per 1000 | 396 per 1000 | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ High risk for attrition bias | ||||||
Background
Description of the condition
AOM is the most common infection for which antibacterial agents are prescribed for children in the United States (AAP 2013). According to the evidence‐based guidelines of the American Academy of Pediatrics and American Academy of Family Physicians, AOM is defined as the presence of middle ear effusion (thick or sticky fluid behind the eardrum in the middle ear) and a rapid onset of signs or symptoms of middle‐ear inflammation, such as ear pain, otorrhoea (discharge from the ear) or fever (AAP 2013).
By the end of their first year of life, approximately 62% of children have experienced at least one episode of AOM, and by the age of three years, almost 83% of children have experienced at least one episode (Friedman 2006; Jansen 2009). The peak incidence occurs between six and 12 months of age (ACIP 2000). Although serious complications are rare, this common childhood ailment imposes a huge impact on healthcare systems. In the United States, AOM accounts for almost 20 million medical office visits annually (CDC 2015). Childhood AOM is a significant healthcare utilisation concern and accounts for approximately USD 2.88 billion in additional healthcare cost annually (Ahmed 2014)
The key step in the pathogenesis of AOM is colonisation of the upper airway with pathogenic bacteria which move from the nasopharynx to the middle ear by way of the eustachian tube (Murphy 2006). The aetiologic agents include bacteria, viruses and a combination of both (Guven 2006). S pneumoniae and H influenzae are the most common causes of bacterial otitis media (Murphy 2006). Studies using diagnostic tympanocentesis in children with AOM identified S pneumoniae in 28% to 55% of all middle ear aspirates (ACIP 2000).
Despite a large number of published RCTs, there is no consensus on therapy for AOM (Venekamp 2015). The rate of antibiotic use for AOM varies from 31% in the Netherlands (Akkerman 2005) to 95% in the USA and Canada (Froom 2001). AOM is the leading reason for antibiotic prescriptions in the United States (Venekamp 2015). This results in the widespread emergence of multidrug resistant pathogens (ACIP 2000; Friedman 2006). Treatment strategies for AOM include surgery, antibiotic prophylaxis and vaccination. Surgical procedures have limited and short‐term efficacy on the occurrence of persistent or recurrent otitis media (McDonald 2008; Paradise 1999; Wallace 2014). The effectiveness of antibiotic therapy for AOM appears to be limited. When potential adverse events such as vomiting, diarrhoea or rash were considered, one in every 14 children treated with antibiotics experienced an adverse event that would not have occurred if antibiotics were withheld (Venekamp 2015). Moreover, in high‐income countries most cases of AOM remit spontaneously with no complications (Venekamp 2015). Furthermore, pneumococcal vaccines make only a small difference (2% to 7% relative reduction) to numbers of AOM cases (Norhayati 2015).
Description of the intervention
Xylitol, or birch sugar, is chemically a pentitol or 5‐carbon polyol sugar alcohol, naturally found in plums, strawberries, raspberries and rowan berries. It does not cause dental caries because, although a sugar alcohol, it cannot be fermented by cariogenic bacteria in the oral cavity. It has the same relative sweetness as sucrose, and is accordingly an ideal non‐sugar sweetener approved in many countries, and principally used in chewing gums, confectionery, toothpaste and medicines (Maguire 2003; Uhari 2000). Xylitol consumption in the UK is about 1000 tonnes per year (Maguire 2003).
How the intervention might work
Xylitol inhibits the growth and acid production of certain strains of Streptococcus mutans (S mutans) (Uhari 2000). In an in vitro study, it was reported that 1% and 5% xylitol markedly reduced the growth of alpha‐haemolytic streptococci including S pneumoniae and Streptococcus mitis (S mitis) during their logarithmic phase of growth (Kontiokari 1995). Clues to the mechanism are suggested by the finding that 5% xylitol reduced adherence of both S pneumoniae and H influenzae to nasopharyngeal cells (Kontiokari 1998). Xylitol is potentially clinically useful in preventing pneumococcal diseases, including AOM.
Why it is important to do this review
Antibiotic treatment of AOM is costly and raises concerns about the development of antibiotic‐resistant strains of bacteria. Surgery is invasive and costly, and because of these factors, alternative, effective measures to prevent AOM are sought. An alternative treatment is xylitol. This review evaluated the efficacy of xylitol to prevent AOM in children.
Objectives
To assess the efficacy and safety of xylitol to prevent AOM in children aged up to 12 years.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and one quasi‐RCT of any type of xylitol intervention versus placebo or no intervention for the prevention of AOM in children aged up to 12 years. We did not include cluster trials due to potential differences in the ancillary treatments among centres. We included studies reporting on any primary or secondary outcomes.
Types of participants
Children up to 12 years of age without AOM.
Types of interventions
We included studies of xylitol in any form (e.g. syrup, chewing gum, lozenges or nasal spray) used to prevent AOM. We considered studies of any dose or duration of administration. Eligible control interventions included placebo or no treatment.
Types of outcome measures
Primary outcomes
-
Number of children (healthy children, children with a respiratory infection, and otitis‐prone children) with at least one AOM episode during the follow‐up period.
Secondary outcomes
-
Safety and adverse events.
-
Antibiotic administration.
-
Hospitalisation (and its length) secondary to AOM or its complications in studies where at least one hospitalisation was reported.
-
Mortality secondary to complications of AOM in studies where at least one death was reported.
-
Number of days missed at school or daycare centre.
-
Cost (direct such as emergency room or general practitioners visits; hospitalisations; medication and indirect costs such as time taken from work for parents or care givers; the opportunity cost of leisure time; informal nursing care; and out‐of‐pocket expenses for transportation).
Search methods for identification of studies
Electronic searches
For this 2016 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 12 December) (accessed 27 January 2016), which includes the Cochrane Acute Respiratory Infections Specialised Register, MEDLINE (June 2011 to January 2016), Embase (June 2011 to January 2016), CINAHL (June 2011 to January 2016), LILACS (2011 to January 2016), International Pharmaceutical Abstracts (June 2011 to Jan 2016), and Web of Science (2011 to January 2016). We did not apply language or publication restrictions. We used the terms in Appendix 1 to search MEDLINE (OVID) and CENTRAL. We did not combine the search with a strategy for identifying randomised trials in MEDLINE as there were too few results. We adapted this search strategy to search Embase (Appendix 2), CINAHL (Appendix 3), LILACS (Appendix 4) and Web of Science (Appendix 5). Previously we searched: CENTRAL 2011, Issue 3; MEDLINE (1950 to August week 1, 2011); Embase.com (1974 to August 2011); CINAHL (1981 to August 2011); Health and Psychosocial Instruments (1985 to August 2011); Healthstar (OVID) (1966 to August 2011); and the International Pharmaceutical Abstracts (2000 to August 2011).
Searching other resources
We searched the WHO ICTRP and ClinicalTrials.gov trials registries for completed and ongoing trials using the term 'xylitol' (latest search 27 January 2016). We attempted to search for unpublished studies (or grey literature) such as technical reports, dissertations, or studies published in languages other than English, which may not have been indexed to major databases, by contacting authors of identified trials. We also conducted Internet searches using Google Scholar™. We searched the first 100 hits that included xylitol and otitis media in the title. We searched dissertations and theses databases (from inception up to 2016) through ProQuest. We also searched in the Open Grey (System for Information on Grey Literature in Europe) database (from inception up to 2016) and the reference lists of identified articles. We also searched abstracts from the annual meetings of the American Association for Respiratory Care, European Respiratory Care Association, the Australian Asthma and Respiratory Educators Association, Society for Pediatric Research, American Pediatric Society and Pediatric Academic Societies published in Pediatric Research (2002 to 2016). There were no language or publication restrictions. We contacted trial authors if we needed to clarify or obtain relevant data on outcomes not reported in the study. We identified manufacturers and distributors of xylitol products based on information extracted mainly from included studies as well as an Internet search and contacted these agencies to locate unpublished trials. We contacted five xylitol distributors (Academy of Dental Resources, Xylarex, Oral Biotech, Xlear Inc. and Oxyfresh Worldwide, Inc.) and three researchers (October 2009) and requested information on any unpublished trials.
Data collection and analysis
Selection of studies
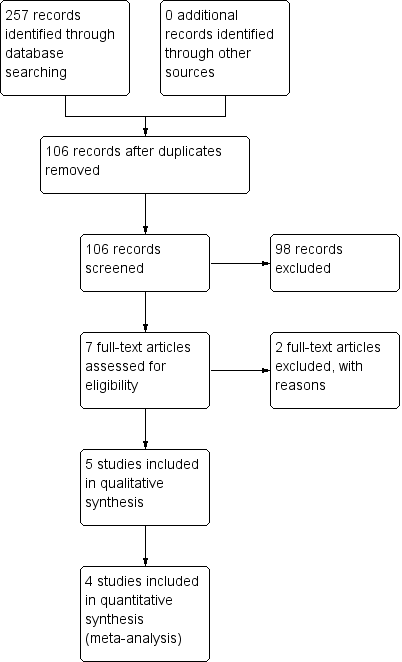
Two review authors (AA, PS) independently screened titles and abstracts for inclusion of all the potential studies we identified as a result of the search. We retrieved the full text study reports/publication and the same review authors independently screened full text and identified studies for inclusion, and recorded reasons for exclusions. We resolved disagreements through discussion and consensus, or by consulting a third review author (HPL). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) (Moher 2009) and Characteristics of excluded studies.
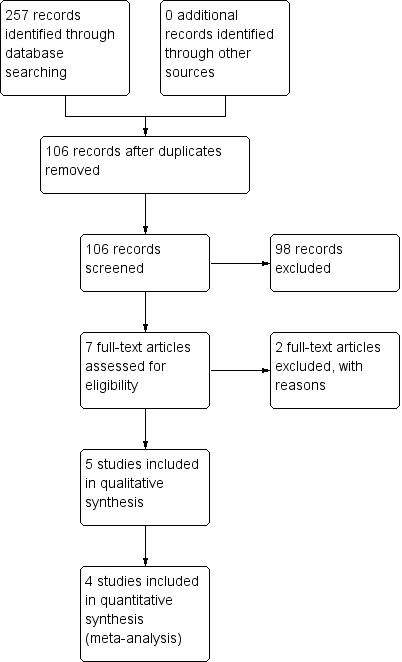
Study flow diagram.
Data extraction and management
We extracted data using a standardised data collection form. Disagreements were resolved by discussion with a third author (HPL). We extracted the following information:
-
participant characteristics (age, sex, race, representative of, history of recurrent AOM, attendance at daycare or school);
-
study setting;
-
type of intervention and control and number studied in each group;
-
adverse effects;
-
method for diagnosing AOM;
-
occurrence of AOM and its complications during follow‐up period;
-
percentages of children with at least one AOM episode during the follow‐up period;
-
use of antibiotics to treat AOM or its complications;
-
time lost from daycare/school (for children) or from work (for parents or care givers) due to AOM;
-
costs of managing AOM;
-
randomisation method;
-
masking method (in randomisation, intervention, outcome assessment); and
-
whether analysis was by intention‐to‐treat (ITT).
Assessment of risk of bias in included studies
We assessed methodological quality using the information in the original publications. Two review authors (AA, PS) independently evaluated study quality using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We evaluated six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues) based on what was reported in the study and assigned judgements relating to the risk of bias for each domain. Possible judgments for these domains could be high, low or unclear risk of bias.
Measures of treatment effect
We analysed the primary outcome of interest (number of children with at least one AOM episode) using RevMan 5.3 for statistical analysis (RevMan 2014). We used RR and RD and planned to use mean difference (MD) if appropriate. We reported 95% CI for estimates of treatment effects. We used data from all intervention arms in the analyses for studies with more than two arms, where the same xylitol forms were compared in two or more experimental groups (for example, xylitol in chewing gum, syrup or lozenges were compared to a common control group). We analysed the secondary outcomes of interest (adverse events, hospitalisation, mortality, percentage of children needing antibiotic therapy) when the data were available, using RR or RD. We planned to summarise length of hospitalisations, number of days missed at school and cost differences as MD or with 95% CI if data were available.
Unit of analysis issues
We included data from a participant once only, even if the participant was recruited more than once.
Dealing with missing data
We contacted trial authors to request missing data or clarify methods whenever possible, but we received few responses.
Assessment of heterogeneity
We considered a conservative P < 0.1 as significant. We calculated I² statistic values to quantify heterogeneity (Higgins 2003). In addition, we inspected the graphical display of trials estimated treatment effects (along with their 95% CIs) for assessing heterogeneity.
Assessment of reporting biases
We assessed publication bias by checking trials registries and contacting the authors of identified studies to ask if they had other publications or were aware of any other unpublished studies we had missed.
Data synthesis
We used a fixed‐effect model for meta‐analyses.
GRADE and 'Summary of findings' tables
We created three 'Summary of findings' tables for three populations of healthy children, children with a respiratory infection, and otitis‐prone children, in which the xylitol had been evaluated using the following outcomes: final diagnosis of at least one episode of AOM; adverse events; antibiotic administration; hospitalisation; mortality; number of days missed at school or daycare; and cost. We used the five GRADE (Atkins 2004) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence as it relates to the studies which contribute data to the meta‐analyses for the prespecified outcomes (GRADEpro 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEproGDT 2015). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed four subgroup analyses based on the methodology of the identified studies:
-
younger children unable to chew gum;
-
older children who were able to chew;
-
for those whom xylitol was administered during respiratory infection; and
-
comparison between different types of xylitol vehicles.
Sensitivity analysis
We also looked at the results in subsets of trials with different methods of xylitol administration (syrup, chewing gum or lozenges). We applied Cochrane’s Q test for between‐study heterogeneity (Deeks 2011). We also performed random‐effects meta‐analysis to compare the pooled results to those of the fixed‐effect meta‐analyses.
Results
Description of studies
Results of the search
This is an update of our previous review (Azarpazhooh 2011). For this 2016 update, we reviewed 33 potential citations. The study flow diagram is presented in Figure 1. We identified one new trial on ClinicalTrials.gov (www.clinicaltrials.gov/) completed and published (NCT01044030 as Vernacchio 2014) and a new trial that was not yet open for participant recruitment (NCT02592382). We saved this record for a future update (Characteristics of ongoing studies). Previously, our preliminary searches identified 84 potential citations. We read the titles and abstracts of these studies. We selected four studies for full text appraisal and these met the eligibility criteria for the first version of this review (Hautalahti 2007; Tapiainen 2002; Uhari 1996; Uhari 1998). This review included five clinical trials that involved 3405 children. Details of the participants enrolled in these studies are described in Characteristics of included studies.
Included studies
Vernacchio 2014 performed a pragmatic practice‐based RCT. Ninety‐seven practices of three paediatric practice–based networks (the Slone Center Office‐based Research Network at Boston University, the Pediatric Physicians’ Organization at Children’s (Boston), and the North Carolina Child Health Research Network at the University of North Carolina) referred 778 children, aged six to 71 months, to the study for eligibility review. These children were all otitis‐prone, that is, they had a history of at least three clinically diagnosed episodes of AOM in the previous 12 months with at least one in the previous six months and may have had middle ear effusions at the time of enrolment. Of these children, 326 were randomised to receive either a placebo syrup (n = 166, daily dose of 2.25 g sorbitol three times a day) or a viscous xylitol syrup (n = 160, daily dose of 5 g three times a day) for three months. All of the daily doses were administered by parents or other routine caregivers, not in a controlled daycare setting as in the previous trials. Compliance was monitored by asking parents, at each interview, how much of the recommended syrup the child had taken since the last interview. In total, about 28% of participants in the xylitol group and 24% of participants in the placebo group discontinued treatment before the end of the study period or the occurrence of the primary outcome. The most common reasons were gastrointestinal side effects (18 in the xylitol group and 11 in the placebo group), the participant refusing to take the solution (eight in each group), and the parent becoming too busy or finding the treatment too difficult to administer (eight xylitol, five placebo). At the end of the study, a blinded investigator reviewed medical records from each participant's primary care physician as well as any other healthcare provider whom the parent identified as having treated the participant during the study period. The clinical diagnoses of AOM was registered as reported in the medical records by a wide range of clinicians and were not otherwise verified. If medical records were not available, the diagnosis of AOM was registered as reported by the parents. By the end of the trial, two participants in the xylitol group and three in the placebo group declined further participation. Four participants in the xylitol group and three in the placebo group were lost to follow‐up without any outcome information. The details of the participants enrolled in this study are described in Characteristics of included studies.
Hautalahti 2007 randomised 663 healthy children enrolled in daycare in the city of Oulu, Finland to four groups to receive either a control product (n = 331, daily dose of 0.5 g in the control chewing gum group or in a syrup three times a day, pulled together as one control group) or xylitol (n = 332, daily dose of 9.6 g in chewing gum or in a syrup three times a day, pulled together as one test group) for three months. The total daily amount of received xylitol in this study was equal to that used in earlier trials but the size of a piece of chewing gum and the amount of syrup needed per dose were bigger and dosing was less frequent (three versus five daily doses). In working days, two daily doses were given at the daycare centre and one dose at home. At weekends and holidays all three doses were given at home. Compliance and the consumption of additional xylitol products were monitored by asking parents to record the doses actually given and any other xylitol products on a daily report sheet and also by counting and measuring the unused returned products at the end of the trial. The occurrence of the first AOM diagnosed during any period of respiratory symptoms during the follow‐up was the main outcome measure based on a finding of middle ear effusion in tympanometry (B, C or positive pressure curve) and confirmed with pneumatic otoscopy. Otorrhoea from a tympanostomy tube was counted as AOM. There was dropout of 96 participants (58 in the xylitol group and 38 in the control group). The baseline characteristics (demographic features, known AOM risk factors, or AOM history, earlier use of xylitol products) were similar in all four groups. By the end of trial, the dropouts range was statistically higher in the xylitol group (17%) versus the control group (11%). The reasons for dropouts and the related numbers per each group are as follows: child refused to take the product (control: 15 versus xylitol: 22); abdominal discomfort (control: seven versus xylitol: nine); chewing gum piece too big (control: one versus xylitol: five); duration of the trial too long (control: one versus xylitol: two); forgot to give the preparation when on holiday (control: three versus xylitol: three); illness (control: two versus xylitol: three); rash (control: three versus xylitol: three); parents tired of the trial (control: two versus xylitol: one); difficult to avoid additional xylitol products (control: zero versus xylitol: one); other (control: one versus xylitol: four) or unknown reasons (control: three versus xylitol: five). The details of the patients enrolled in this study are described in the Characteristics of included studies table.
Tapiainen 2002 randomised 1277 healthy children enrolled in daycare in the city of Oulu, Finland after screening with tympanometry to receive either the control mixture (n = 212), xylitol mixture (n = 212), control chewing gum (n = 280), xylitol chewing gum (n = 286) or xylitol lozenges (n = 287) during an acute respiratory infection. The parents began administering the products to their children at the onset of acute respiratory infection (ARI) symptoms, which was defined as the appearance of one or more of the following symptoms: clear or purulent discharge from the nose, congestive nose, cough, conjunctivitis, sore throat or earache. Compliance was monitored by asking the parents to list the doses actually given on the daily symptom sheets and by counting the unused pieces of chewing gum and lozenges returned at the last appointment and measuring the volume of the unused mixture. The follow‐up lasted until resolution of the symptoms or up to three weeks. AOM was diagnosed based on a finding of middle ear effusion in tympanometry (B, C or positive pressure curve) and confirmed with pneumatic otoscopy. A total of 1253 of the 1277 randomised children were eligible for the analysis of the primary outcome. The children who dropped out (n = 24) were excluded from the statistical analysis but those who prematurely stopped using the products but still visited the clinic (n = 35) were included. The reasons for dropouts and the related numbers per each group are as follows: parents got tired (only two in the xylitol syrup and four in the xylitol chewing gum); child disliked the product (two only in the xylitol lozenge); other antibiotics for ARI (only one in the xylitol syrup and one in the control chewing gum); left the area (only one in the control syrup, four in the xylitol chewing gum and two in the xylitol lozenge); and unknown (syrup: zero in the control versus two in the xylitol group; chewing gum: two in control versus one in xylitol; lozenge: two in xylitol). Moreover, the administration was discontinued due to abdominal discomfort (syrup: one in control versus three in xylitol; chewing gum: one in control versus one in xylitol; lozenge: five in xylitol); child disliked the product (one in xylitol chewing gum and 10 in xylitol lozenge); or unknown (syrup: two in control versus two in xylitol; chewing gum: three in control versus three in xylitol; five in xylitol lozenge). The details of the participants enrolled in this study are described in Characteristics of included studies.
Uhari 1996 randomised 306 children in the city of Oulu, Finland daycare (157 in the xylitol group and 149 children in the sucrose group). Each child was instructed to chew two pieces of gum five times a day after meals or snacks until there was no taste (or until five minutes), making a total dose of 8.4 g xylitol per day for two months. Compliance was assessed by asking the parents to report the actual number of pieces of chewing gum used at the end of each month of the trial and also by reporting on the use of additional xylitol products and possible medications. Nasopharyngeal samples were taken before the sugar challenge, at two weeks and at the end of the study. Pneumococci were identified by (alpha) haemolysis on sheep blood agar plates, colony morphology and optochin sensitivity. Parents completed symptom sheets, time and reasons for being absent from daycare, and the use of additional xylitol products and possible medications. At a physician appointment, clinical diagnosis and the drugs prescribed were registered. AOM was diagnosed based on symptoms and signs of ARI and simultaneous signs of middle ear effusion: a cloudy tympanic membrane or impaired tympanic membrane motility in pneumatic otoscopy. The baseline characteristics were similar in the two randomised groups. By the end of trial, there was a dropout of 30 participants; 10 in the xylitol group and 20 in the control group, representing a dropout rate of 6% and 13%, respectively. The reasons for dropouts and the related numbers per each group are as follows: child got tired of eating chewing gum (sucrose: eight versus xylitol one), dental caries (sucrose three versus xylitol four), moved from area (sucrose five versus xylitol one), parents got tired (sucrose two versus xylitol two), insufficient follow‐up data (sucrose: two versus xylitol zero) and loose stools (sucrose zero versus xylitol two). There were no differences in the duration of cough or other symptoms of infections between the groups as reported by parents. There was also no difference in the pneumococcal carriage rates during the study and between the groups as well as the numbers of upper respiratory tract infections without AOM, acute bronchitis, sinusitis and conjunctivitis. The details of the participants enrolled in this study are described in Characteristics of included studies.
Uhari 1998 randomised 857 healthy children enrolled in the city of Oulu, Finland daycare to one of five treatment groups. Participants received control syrup (n = 165), xylitol syrup (n = 159), control chewing gum (n = 178), xylitol gum (n = 179) or xylitol lozenge (n = 176) for three months. Two pieces of chewing gum or lozenges were chewed for at least five minutes five times a day after meals (three doses given by the personnel at the childcare centres during the day and the rest given by the parents at home). Compliance was monitored by asking the parents to list the doses actually given on the daily symptom sheets and also by counting and measuring the unused returned products at the end of the trial. AOM was diagnosed based on a finding of middle ear effusion in tympanometry (B‐ or C‐curve), verified otoscopically with signs of inflammation in the tympanic membrane and the presence of symptoms of ARI (rhinitis, cough, conjunctivitis, sore throat, earache). The baseline characteristics (demographic features, known AOM risk factors or AOM history) and the mean number of forgotten dosages were similar in all five groups. By the end of trial, the dropouts range from 4.5% to 18.9% and were statistically higher in the xylitol syrups (18.9%) and xylitol lozenges (14.8%) as compared to their control groups of respectively control syrups (10.3%) and control chewing gum (4.5%). The reasons for dropouts and the related numbers per each group are as follows: unwilling to continue taking the product (syrup: nine in control versus 14 in xylitol; chewing gum: seven in control versus eight in xylitol; lozenge: 14 in xylitol), left the area (syrup: zero in control versus two in xylitol; chewing gum: zero in control versus two in xylitol; lozenge: one in xylitol), abdominal discomfort (syrup: five in control versus eight in xylitol; chewing gum: zero in control versus one in xylitol; lozenge: seven in xylitol), and unknown reason (syrup: three in control versus six in xylitol; chewing gum: one in control versus one in xylitol; lozenge: four in xylitol). The details of the patients enrolled in this study are described in the Characteristics of included studies table. It should be noted that these trials were all conducted in similar populations in Finland. The methodology appears to be similar with the small change in the control group after the first study (change from sucrose to low‐dose xylitol in the control group), the population in the 2002 study (from healthy children to children with ARI) and the dosing in the 2007 study (five times per day to three times per day).
Excluded studies
We identified a recent trial that evaluated the effectiveness of xylitol paediatric topical oral syrup to reduce the incidence of dental caries among very young children (Milgrom 2009). In the published paper, it was mentioned that the effect of xylitol in reducing AOM will be published in a subsequent study. However, after contacting the primary investigator, it was understood that due to a lack of reliability of data regarding AOM, the plan to publish AOM results has been suspended.
Risk of bias in included studies
Figure 2 summarises the review authors' judgements about each risk of bias domain for each included study.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study
Allocation
With the exception of Vernacchio 2014 for which the sequence generation is unclear, the other four included trials had adequate sequence generation (low risk). For allocation concealment, three trials were considered to have low risk of bias (Hautalahti 2007; Tapiainen 2002; Uhari 1996) and the other two trials an unclear risk (Uhari 1998; Vernacchio 2014).
Blinding
Three out of five included studies had adequate blinding (Hautalahti 2007; Uhari 1996; Vernacchio 2014). The blinding procedure was not mentioned in one study (Uhari 1998) but it was judged to be 'probably done' as per the authors' earlier publication. Tapiainen 2002 was blinded as far as the mixture and chewing gum groups were concerned but open between the xylitol lozenge and control chewing gum groups.
Incomplete outcome data
In Vernacchio 2014, 62/160 allocated to xylitol (38.8%) declined participation, were lost to follow‐up or discontinued intervention versus 58/166 allocated to placebo (34.9%). Intention‐to‐treat (ITT) analysis was performed. The other four trials clarified the number of and the reasons for dropouts, but did not perform an ITT analysis.
Selective reporting
No selective reporting was identified in the five included trials.
Other potential sources of bias
In four Finnish trials the test material was donated by industry (Hautalahti 2007; Tapiainen 2002; Uhari 1996; Uhari 1998). All five trials had governmental funding. No conflict of interest was declared in any of the included trials; however, it should be noted that the study authors in Finnish trials have a US patent for the use of xylitol in treating respiratory infections.
Effects of interventions
See: Summary of findings for the main comparison Xylitol versus control for prevention of AOM in healthy children; Summary of findings 2 Xylitol versus control for prevention of AOM in healthy children during a respiratory infection; Summary of findings 3 Xylitol versus control for prevention of AOM in otitis‐prone children
See summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3 for the main primary outcome 'Number of children (healthy children, children with a respiratory infection, and otitis‐prone children, respectively) with at least one AOM episode during the follow‐up period'. Overall, the quality of available evidence for this outcome was moderate for healthy children (due to inconsistency of the results) moderate for children with respiratory infections (due to imprecise estimates), and low for otitis‐prone children (due to imprecise estimates and high attrition).
Primary outcome
Number of children with at least one AOM episode during the follow‐up period
The included trials reported on the primary outcome of number of children with at least one AOM episode during the follow‐up period when xylitol was administered to healthy children (Hautalahti 2007; Uhari 1996; Uhari 1998), to healthy children with a respiratory infection (Tapiainen 2002), and to otitis‐prone healthy children (Vernacchio 2014). In particular, for the latter, the population was not homogenous, the total dose was increased by 50% (15 g daily in the form of viscous xylitol solution) and the outcome was measured as what had been documented in the patients' medical charts. Due to the heterogeneity among these three groups, the results are presented separately for each outcome.
Xylitol in any form versus control in any form
Figure 3 summarises available data on our primary outcome. We combined the result of three trials among 1826 healthy children to compare the effect of xylitol in any form (syrup, chewing gum or lozenges) versus control of any kind (syrup or gum) (Hautalahti 2007; Uhari 1996; Uhari 1998). There was a statistically significant 25% reduction in the risk of occurrence of AOM among healthy children at daycare centres in the xylitol group compared to the control group (RR 0.75, 95% CI) 0.65 to 0.88 and RD ‐0.07, 95% CI ‐0.12 to ‐0.03; quality of evidence: moderate ‐ downgraded for inconsistency). However, the effect of xylitol in reducing AOM among healthy children during a respiratory infection (Tapiainen 2002: 99 out of 765 children in the xylitol group versus 56 out of 488 children in the control group, RR 1.13, 95% CI 0.83 to 1.53; quality of evidence: moderate ‐ downgraded for imprecision), or among otitis‐prone healthy children (Vernacchio 2014: 53 out of 160 children in the xylitol group versus 61 out of 166 children in the control group, RR 0.90, 95% CI 0.67 to 1.21 and RD ‐0.07, 95% CI ‐0.14 to 0.07; quality of evidence: low ‐ downgraded for imprecision and high attrition) was inconclusive.

Forest plot of comparison: 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), outcome: 1.1 Final diagnosis of at least one episode of AOM.
Secondary outcomes
1. Safety and adverse events
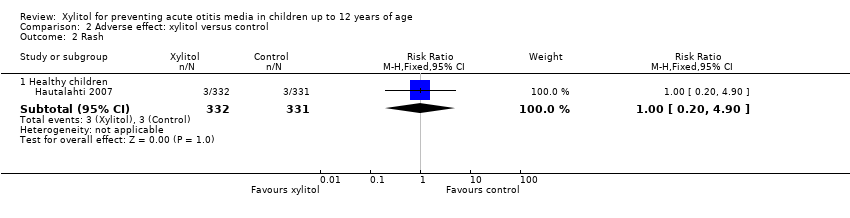
There were no significant differences in the number of children with gastrointestinal‐related adverse events during the follow‐up period between xylitol and control group among 1826 healthy children (Hautalahti 2007; Uhari 1996; Uhari 1998 combined: RR 1.43, 95% CI 0.74 to 2.75), among 1277 healthy children with a respiratory infection (Tapiainen 2002: RR 2.82, 95% CI 0.61 to 13.00), and among 326 otitis‐prone healthy children (Vernacchio 2014: RR 1.04, 95% CI 0.92 to 1.16). Similarly, there was no significant differences in the occurrence of rash during the follow‐up period between xylitol and control group (only reported in Hautalahti 2007: RR 1.00, 95% CI 0.20 to 4.90). The quality of evidence on this outcome was moderate, downgraded for imprecision.
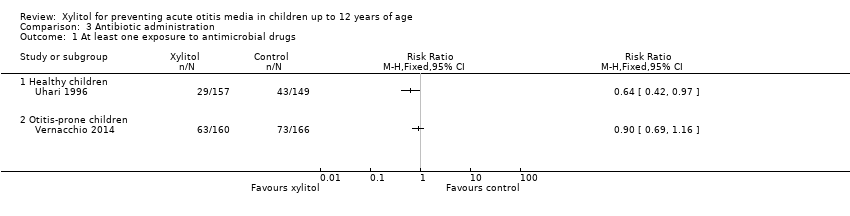
2. Antibiotic administration
Among healthy children: Uhari 1996 and Uhari 1998 reported on antibiotic administration. However, the reported data could not be pooled.
-
In Uhari 1996, the total number of antimicrobial drugs prescribed in the sucrose group was 60 compared with 34 in the xylitol group. There was a statistically significant 36% reduction in the risk of experiencing at least one exposure to antimicrobial drugs among children in the xylitol group than the children in the sucrose group (RR 0.64, 95% CI 0.42 to 0.97 and RD ‐0.10, 95% CI ‐0.20, ‐0.01; quality of evidence: moderate).
-
In Uhari 1998, for children unable to chew, during the three‐month follow‐up period, the incidence rate of antimicrobial prescriptions per person years at risk (PYR) was significantly lower in the xylitol syrup group versus the control syrup group (respectively 3.20 versus 4.33, P = 0.012). Similarly, the number of days on antimicrobials/PYR was significantly lower in the xylitol syrup group versus the control syrup group (respectively 25.0 versus 31.7, P < 0.0001). For children able to chew, during the three‐month follow‐up period, the incidence rate of antimicrobial prescriptions/PYR was significantly lower in the xylitol chewing gum group versus the control chewing gum group (respectively 1.66 versus 2.26, P = 0.046), but there was no difference between the xylitol lozenges group and control chewing gum (respectively 1.86 versus 2.26, P = 0.211). The number of days on antimicrobials/PYR was significantly lower (P < 0.0001) in both the xylitol chewing gum group (11.8) and xylitol lozenges (13.8) versus control chewing gum (17.8).
Antibiotic administration was not measured among healthy children with a respiratory infection.
Among otitis‐prone children, Vernacchio 2014 reported no significant differences in antibiotic administration between the xylitol and control groups (RR 0.90, 95% CI 0.69 to 1.16). The quality of evidence was moderate and was downgraded for imprecision and high attrition. Total antibiotic use was 6.8 days per 90 days in the xylitol group versus 6.4 in the placebo group (difference 0.4, 95% CI –1.8 to 2.7, P = 0.7). The incidence of AOM‐related antibiotic use was 5.2 days per participant per 90 days in the xylitol group versus 5.1 in the placebo group (absolute difference 0.1 days, 95% CI for difference –1.9 to 2.2 days. P = 0.9).
3. Hospitalisation (and its length) secondary to AOM or its complications
No case of hospitalisation was reported in the included studies.
4. Mortality secondary to complications of AOM in studies where at least one death was reported
No case of mortality was reported in the included studies.
5. Number of days missed at school or daycare centre
Identified studies did not report on this outcome.
6. Cost
Information on cost was not reported in the included studies.
Subgroup analysis
Based on the methodology of the identified studies, we defined the subgroup analyses of a) younger children unable to chew gum; b) older children who were able to chew and c) comparison between different types of xylitol vehicles. The subgroup analyses were tested among the population of healthy children, healthy children during respiratory infection, as well as otitis‐prone healthy children. It should be noted that although Hautalahti 2007 included the subgroups of chewing gum and syrup (for both xylitol group and control group), the data were presented collectively for control and xylitol group with no breakdown of the AOM in the subgroups. We contacted the trial authors several times but no further information was obtained and therefore the results of the subgroups of Hautalahti 2007 were not included in the subgroup analysis.
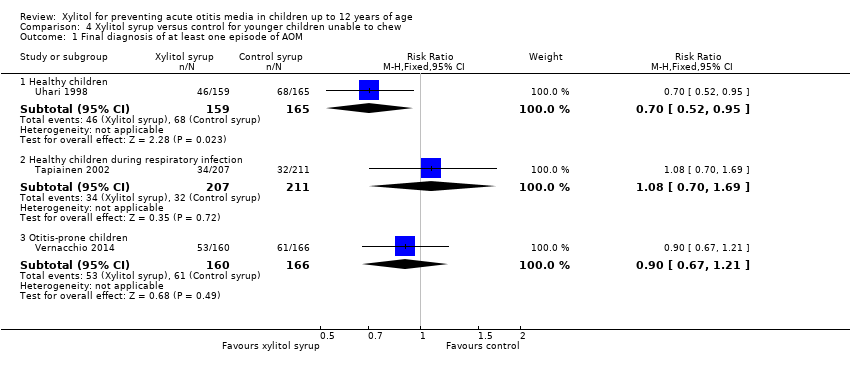
Younger children unable to chew gum
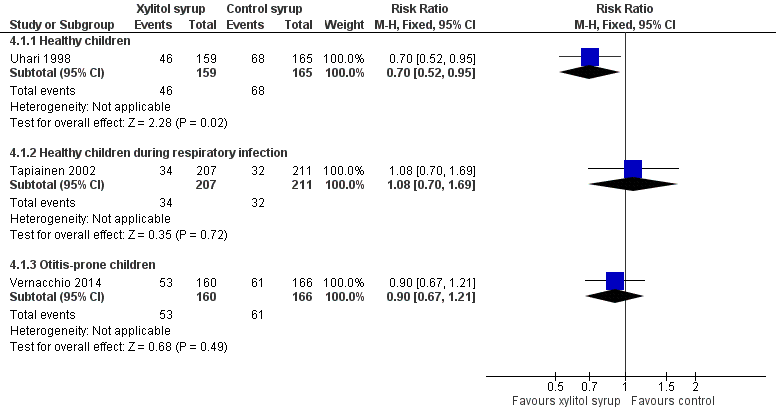
Figure 4 presents data from three trials that compared the effect of xylitol syrup versus control syrup in younger children unable to chew gum (Tapiainen 2002; Uhari 1998; Vernacchio 2014). Due to the heterogeneity in these studies, the results were not pooled. Among healthy children, Uhari 1998 showed that xylitol syrup resulted in a statistically significant 30% decrease in the occurrence of AOM among healthy younger children at daycare centres who were unable to chew gum (46 out of 159 children in the xylitol group versus 68 out of 165 children in the control group, RR 0.70, 95% CI 0.52 to 0.95; RD ‐0.12, 95% CI ‐0.23 to ‐0.02). However, the effect of xylitol syrup in reducing AOM among healthy children during a respiratory infection (Tapiainen 2002: 34 out of 207 children in xylitol group versus 32 out of 211 children in control group, RR 1.08, 95% CI 0.70 to 1.69), or among otitis‐prone healthy children (Vernacchio 2014: 53 out of 160 children in the xylitol group versus 61 out of 166 children in the control group, RR 0.90, 95% CI 0.77 to 1.21; RD ‐0.04, 95% CI ‐0.14 to 0.07) was inconclusive.

Forest plot of comparison: 3 Xylitol syrup versus control for younger children unable to chew, outcome: 3.1 Final diagnosis of at least one episode of AOM.
Older children who were able to chew
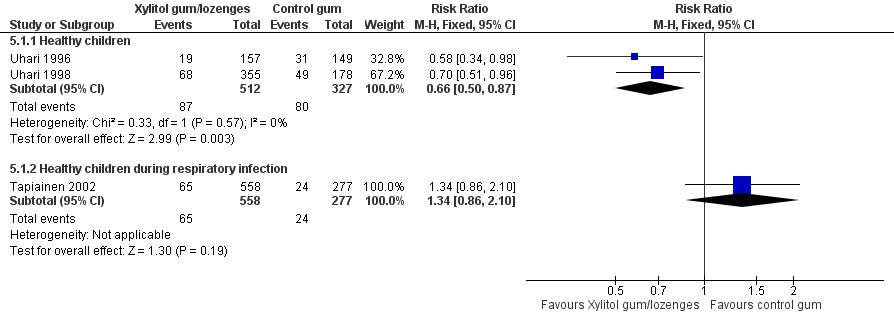
The results of Uhari 1996 and Uhari 1998 were combined to compare the effect of xylitol chewing gum or lozenges versus control chewing gum among the subgroup of older healthy children able to chew gum. Based on the pooled result of these two studies, compared to control chewing gum, xylitol resulted in a statistically significant 34% decrease in the occurrence of AOM if it was administered in chewing gum or lozenges (Figure 5 RR 0.66, 95% CI 0.50 to 0.87; RD ‐0.09, 95% CI ‐0.14 to ‐0.03), and in a statistically significant 41% decrease in the occurrence of AOM if it was administered in chewing gum only (Figure 6 RR 0.59, 95% CI 0.42 to 0.81; RD ‐0.10, 95% CI ‐0.16 to ‐0.04). The effect of xylitol lozenge in reducing AOM was inconclusive (only reported in Uhari 1998, RR 0.80, 95% CI 0.56 to 1.16; RD ‐0.05, 95% CI ‐0.14 to 0.04). Among the healthy children during a respiratory infection (only reported in Tapiainen 2002), there was no difference in the outcome whether xylitol was administered in chewing gum or lozenges (Figure 5 RR 1.34, 95% CI 0.86 to 2.10), in chewing gum only (Figure 6 RR 1.29, 95% CI 0.78 to 2.14), or in lozenges only (RR 1.40, 95% CI 0.85 to 2.29). Vernacchio 2014 did not report this subgroup.

Forest plot of comparison: 4 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, outcome: 4.1 Final diagnosis of at least one episode of AOM.
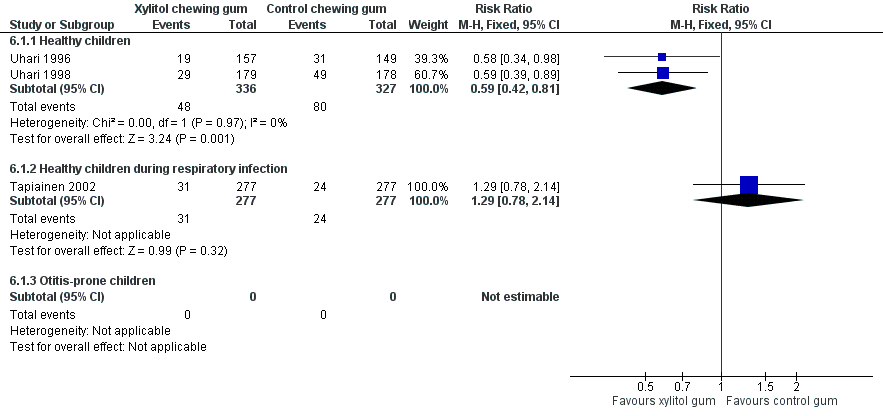
Forest plot of comparison: 5 Xylitol chewing gum versus control chewing gum for older children able to chew, outcome: 5.1 Final diagnosis of at least one episode of AOM.
Comparison of different types of xylitol vehicles
We also compared the outcome of the final diagnosis of at least one episode of AOM between the active ingredients groups to assess whether the mode of delivery for the ingredient makes any difference. We performed this analysis on healthy children (Uhari 1998), and also on children with a respiratory infection (Tapiainen 2002). It should be noted that this comparison is limited to one study in each population group: Uhari 1996 did not test different modes of delivery for xylitol (chewing gum only); and although Hautalahti 2007 used two modes of delivery for xylitol (chewing gum and syrup), the data were collectively presented as one test group. The analyses show that xylitol chewing gum is superior to xylitol syrup in preventing AOM among healthy children (RR 0.59, 95% CI 0.39 to 0.89), but not for children with a respiratory infection (RR 0.68, 95% CI 0.43 to 1.07). There was no difference between xylitol lozenges and xylitol syrup in preventing AOM among healthy children (RR 0.77, 95% CI 0.53 to 1.11), or among children with a respiratory infection (RR 0.74, 95% CI 0.47 to 1.14). Similarly, no difference was noted between xylitol chewing gum and xylitol lozenges in preventing AOM among healthy children (RR 0.73, 95% CI 0.47 to 1.13), or among children with a respiratory infection (RR 0.92, 95% CI 0.59 to 1.46). Vernacchio 2014 did not report this subgroup.
Discussion
Summary of main results
This review has attempted to find evidence for a preventive effect of xylitol in reducing the occurrence of acute otitis media (AOM). Safety and adverse events were not statistically significantly different. This finding is similar to Vernacchio 2007a, where oral xylitol solution at dosages of 5 g three times per day and 7.5 g once daily were found to be well‐tolerated by young children. However, the relative rarity of events and the few patients from whom data are available prevents us from arriving at firm conclusions on safety. Xylitol in connection with dental decay has been used for decades, since it has been shown to reduce mutans streptococci counts in plaque and saliva (Haresaku 2007) and reduce lactic acid production in dental plaque through interference with metabolic pathways (Twetman 2003). In a recent systematic review (Ly 2008) and meta‐analysis (Deshpande 2008), it is claimed that xylitol could benefit people at high risk of caries and should be considered for routine use in clinical dentistry. However, the use of xylitol in dental decay suggests that it may also be useful for preventing AOM, which is the focus of this review.
Overall completeness and applicability of evidence
Considering the concerns over the treatment of AOM using antibiotics and surgery, we wanted to explore the literature for alternative preventive strategies. In the earlier version of this review (Azarpazhooh 2011), following an exhaustive search strategy, we found three clinical trials that investigated the efficacy of xylitol administered prophylactically to children in daycare centres (Hautalahti 2007; Uhari 1996; Uhari 1998). All these trials were double‐blinded, randomised and placebo‐controlled, with appropriate randomisation and masking procedures. While the trials stemmed from the same group of trial authors, the design of the trials was improved following the first one (Uhari 1996), which utilised sucrose in the control group. This study was later criticised due to the possibility of risking dental caries in the participants. Therefore, in the following studies, xylitol was used as a sweetener in the control groups as well, to avoid the increased risk for caries. While the chewing gums were similar in taste, the xylitol mixture tasted sweeter than the control syrup (Hautalahti 2007; Uhari 1998). These studies did not discuss why the low dose of xylitol (0.5 g/day) in the control group can be considered as equivalent to placebo. In this update, we identified a pragmatic practice‐based RCT (Vernacchio 2014), which determined if viscous xylitol solution versus sorbitol solution could reduce the occurrence of clinically diagnosed AOM among otitis‐prone children six months through to five years of age. We elected not to pool the result of this trial with those we identified in 2011 (Azarpazhooh 2011), because as compared to trials included in our 2011 review, the population was not homogenous, the total dose was increased by 50% (15 g daily) and the outcome was measured as what had been documented in the patients' medical charts.
Based on the studies we reviewed, xylitol seems to be a promising alternative to conventional therapies to prevent AOM among healthy children. Even in one study (having controlled for parental education and smoking, breast feeding, use of pacifier, sibling prone to otitis, previous history of AOM, previous use of xylitol and nasopharyngeal carriage of pneumococci) children receiving xylitol had fewer AOM episodes (P = 0.045) (Uhari 1996). The overall results from combining the data from the preventive clinical trials among healthy children (comparing xylitol to a control group of sucrose or minimum non‐therapeutic dose of xylitol plus sorbitol) are encouraging. The magnitude of the reduction in the occurrence of AOM was an overall 25% (1‐ RR 0.75) when the results of all types of administration are considered (i.e. syrup, lozenge or chewing gum) and was 30% (1‐ RR 0.70) if it is administered in a syrup for younger children unable to chew. Among those older children who were able to chew, xylitol in chewing gum form was effective in the prevention of AOM by 41% (1‐ RR 0.59); in contrast, xylitol lozenges were not preventive. Also, it was found that chewing gum is a better vehicle for xylitol administration with an extra 41% preventive effect when compared to xylitol syrup (1‐ RR of 0.59). That said, in this update, the result of the identified pragmatic practice‐based RCT (Vernacchio 2014) showed that 15 g daily dose of a viscous xylitol solution was ineffective in reducing clinically diagnosed AOM among otitis‐prone children. The observed effect in this trial could be the result of lack of adherence to treatment since almost 28% in the xylitol group and 24% in the placebo group discontinued treatment before the end of the study period or the occurrence of the primary outcome. Furthermore, xylitol in reducing AOM among healthy children during a respiratory infection (Tapiainen 2002) was ineffective. The trial authors speculated their findings to the increased bacterial adherence to pharyngeal cells during viral infection, and the increased incidence of otitis media pathogens in the nasopharynx at such times.
Through the literature search and as part of the peer refereeing process, we came across some important papers that can shed more light on the applicability of the results of this review and the potential limitations and obstacles to the applicability of these findings, in particular those of chewing gum. The first obstacle is the knowledge of medical practitioners about medical uses for xylitol. A recent national survey of paediatricians in the USA regarding their knowledge and opinions about xylitol use as a prophylaxis for AOM found that only about half of the participants knew about medical uses for xylitol; of those, most were aware of its use in chewing gum to prevent AOM but had not used it with patients (Danhauer 2010b). Perhaps the most encouraging result of this survey, and a follow‐up focus group survey by the same research group on 10 US paediatricians known to have more experience with xylitol for AOM (Stockwell 2010), was that the majority of the US paediatricians would use xylitol if evidence supported it and wanted information about it via reprints or electronically.
It should also be noted that xylitol chewing gums are commercially available in Finland (the country where four out of five included studies took place) and their use is recommended by the dental organisations. However, in the USA, some school districts prohibit gum chewing altogether, while others leave it up to the discretion of individual teachers. For example, a recent survey of all the Kindergarten through 3rd Grade teachers in Santa Barbara, CA School Districts (Danhauer 2011a), regarding their knowledge about AOM and their willingness to participate in AOM prevention programmes, found that all of the schools and almost all of the teachers did not permit chewing gum on campus or in their classrooms. Other obstacles identified in this pilot survey were supervision, liability, school regulation, compliance issues, potential distractions to classes and risk of choking. These obstacles point to the importance of educational and informational outreach programmes to educate practitioners, parents and school teachers about the medical use of xylitol, in particular when added to chewing gum for both the reduction of dental caries and the occurrence of AOM.
Given the evidence that parental reporting of children's recent AOM history correlates well with medical records (Vernacchio 2007b), in our previous review, we proposed that considering the potential obstacles to AOM prevention programme in daycare and school settings, in‐home use of xylitol needs to be investigated. Fortunately, one such study became available for this update (Vernacchio 2014); however, this study did not find a significant effect for a viscous xylitol solution at a dose of 5 g three times per day in reducing clinically diagnosed AOM among otitis‐prone children. There is some evidence to show that mothers' chewing xylitol gum was a prophylaxis against bacteria and caries in their children. The use of such mother‐child transmission model as a possible vehicle for preventing AOM would be worth further investigations (Danhauer 2011b).
Although the results of this present meta‐analysis show that chewing gum is a better vehicle for xylitol administration, the present obstacles could preclude the use of xylitol chewing gum in school settings for small children who are unable to chew gum. This happens to be the group most susceptible to AOM and therefore, for such children, vehicles other than chewing gum need to be pursued (Danhauer 2011a). For example, we found unpublished evidence internal to Xlear Inc. Orem (Utah, USA) that a xylitol nasal wash three times daily during a three‐month period lowered the recurrence of AOM and the frequency of antibiotic therapy and upper airways infections (Kalanin 2005). Moreover, in our 2016 updated search, we identified a randomised, double‐blinded clinical trial registered with Clinicaltrial.gov that is not yet open for participant recruitment (NCT02592382). This trial, based in Rambam Health Care Campus, Haifa, Israel, is designed to enrol 50 children aged from one to five years who have had three episodes of recurrent otitis media in the last six months prior to entering the study. They will randomly receive isotonic saline nasal spray or 5% xylitol spray (three times daily for two months) and the occurrence of AOM will be measured for five months. We will monitor the progress of this trial for future updates of this review.
Xylitol syrup can also be administered with pacifiers that have reservoirs used to supply medicine to the infant at bed time. That said, a recent web‐based survey of 136 parents of young children in preschool and kindergarten settings (Danhauer 2015), found that most parents were unaware of xylitol's use for AOM and would be unwilling to use xylitol products or comply with a required regimen to prevent AOM in their children. That said, those who knew about xylitol and had children with a history of AOM would be more likely to do so, most would prefer chewing gum for older children and pacifiers for younger children (Danhauer 2015). There remain to be a need for more educational public campaign on the potential benefits of xylitol and the importance of parental and school compliance for successful AOM prevention regimens. Finally, in our protocol, we decided not to include cluster‐design RCTs due to potential differences in ancillary treatments between centres. Although we did not find any cluster‐RCTs in our search, we will look for them in the future updates to this review.
Quality of the evidence
The quality of evidence for the number of children with at least one AOM episode during the follow‐up period was moderate for healthy children due to the inconsistency of the results among trials, moderate for children with respiratory infections due to imprecise estimates, and low for otitis‐prone children due to imprecise estimates and high attrition. Hence, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Potential biases in the review process
In our previous review, we decided to use fixed‐effect analysis as there was sufficient homogeneity among the three Finnish studies. These studies were all from the same group of researchers in Finland; the studied population was homogenous (healthy children attending daycare centres in Oulu, Finland); the total dose of xylitol was similar (8 to 10 g/day); the control groups include 0 to 0.5 g of xylitol per day plus other ineffective sweetener in preventing AOM; the outcome measurement was similar. Althoug we had calculated I² statistic values which ranged from 0% to 69% in different analyses, the number of trials included in meta‐analyses remain low and test for statistical heterogeneity tend to be underpowered.
Agreements and disagreements with other studies or reviews
Since publication of the protocol of this review in 2008, two systematic reviews have been published (Danhauer 2010a; Danhauer 2011b). Our results are in agreement with these systematic reviews that also concluded that xylitol can be a prophylaxis for AOM, but warrants further study, especially of vehicles other than chewing gum for young children.
Study flow diagram.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study

Forest plot of comparison: 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), outcome: 1.1 Final diagnosis of at least one episode of AOM.
Forest plot of comparison: 3 Xylitol syrup versus control for younger children unable to chew, outcome: 3.1 Final diagnosis of at least one episode of AOM.
Forest plot of comparison: 4 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, outcome: 4.1 Final diagnosis of at least one episode of AOM.

Forest plot of comparison: 5 Xylitol chewing gum versus control chewing gum for older children able to chew, outcome: 5.1 Final diagnosis of at least one episode of AOM.

Comparison 1 Xylitol in any form (syrup, gum or lozenge) versus control in any form (gum, syrup), Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 2 Adverse effect: xylitol versus control, Outcome 1 Gastrointestinal‐related adverse events.
Comparison 2 Adverse effect: xylitol versus control, Outcome 2 Rash.
Comparison 3 Antibiotic administration, Outcome 1 At least one exposure to antimicrobial drugs.
Comparison 4 Xylitol syrup versus control for younger children unable to chew, Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 5 Xylitol chewing gum/lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 6 Xylitol chewing gum versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.

Comparison 7 Xylitol lozenges versus control chewing gum for older children able to chew, Outcome 1 Final diagnosis of at least one episode of AOM.
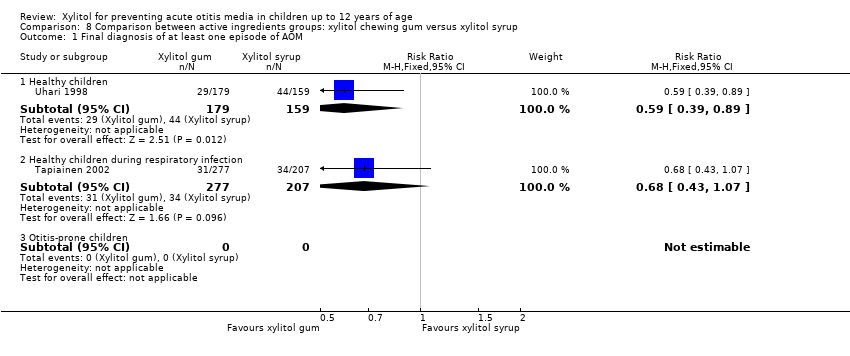
Comparison 8 Comparison between active ingredients groups: xylitol chewing gum versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM.
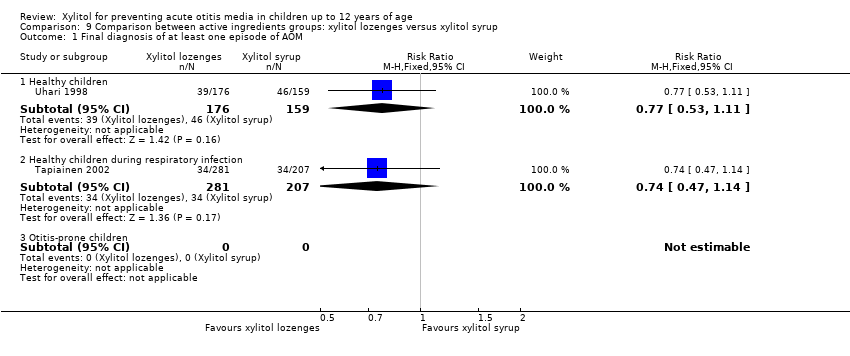
Comparison 9 Comparison between active ingredients groups: xylitol lozenges versus xylitol syrup, Outcome 1 Final diagnosis of at least one episode of AOM.
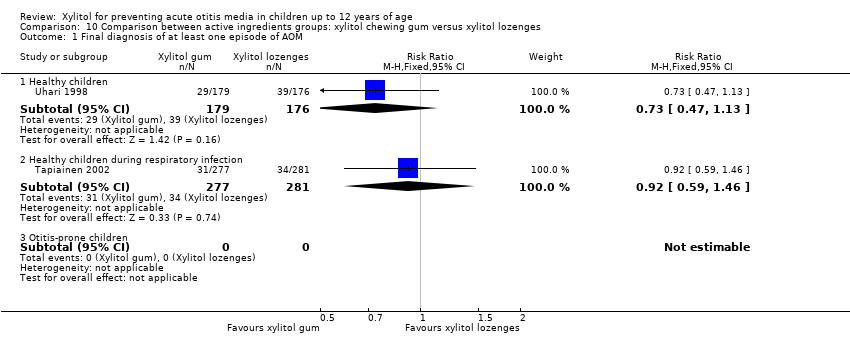
Comparison 10 Comparison between active ingredients groups: xylitol chewing gum versus xylitol lozenges, Outcome 1 Final diagnosis of at least one episode of AOM.
| Xylitol versus control for prevention of AOM in healthy children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk with control in any form (gum, syrup) | Corresponding risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.75 | 1826 | ⊕⊕⊕⊝ | RR 0.74 (0.54, 1.01) with random‐effects meta‐analysis | |
| 299 per 1000 | 224 per 1000 | |||||
| Moderate | ||||||
| 296 per 1000 | 222 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.43 | 1826 | ⊕⊕⊕⊝ | RR 1.41 (0.60, 3.33) with random‐effects meta‐analysis | |
| 17 per 1000 | 24 per 1000 | |||||
| Moderate | ||||||
| 15 per 1000 | 21 per 1000 | |||||
| Antibiotic administration | Study population | RR 0.64 | 306 | ⊕⊕⊕⊝ | ||
| 289 per 1000 | 185 per 1000 | |||||
| Moderate | ||||||
| 289 per 1000 | 185 per 1000 | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ There is inconsistency in th findings of the first two studies as compared to the third study of the same group | ||||||
| Xylitol versus control for prevention of AOM in healthy children during a respiratory infection | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 1.13 | 1253 | ⊕⊕⊕⊝ | ||
| 115 per 1000 | 130 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 130 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 2.82 | 1277 | ⊕⊕⊕⊝ | ||
| 4 per 1000 | 11 per 1000 | |||||
| Moderate | ||||||
| 4 per 1000 | 12 per 1000 | |||||
| Antibiotic administration ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ 95% CIs are wide and imprecise. The evidence is based on one trial. Moreover, there are few events and the CI includes appreciable benefit and harm | ||||||
| Xylitol versus control for prevention of AOM in otitis‐prone children | ||||||
| Patient or population: preventing acute otitis media in children up to 12 years of age | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with control in any form (gum, syrup) | Risk with xylitol in any form (syrup, gum or lozenge) | |||||
| Final diagnosis of at least one episode of AOM | Study population | RR 0.90 | 326 | ⊕⊕⊝⊝ | ||
| 367 per 1000 | 331 per 1000 | |||||
| Moderate | ||||||
| 368 per 1000 | 331 per 1000 | |||||
| Gastrointestinal‐related adverse events | Study population | RR 1.04 | 326 | ⊕⊕⊕⊝ | ||
| 765 per 1000 | 796 per 1000 | |||||
| Moderate | ||||||
| 765 per 1000 | 796 per 1000 | |||||
| Antibiotic administration | Study population | RR 0.90 | 326 | ⊕⊕⊕⊝ | ||
| 440 per 1000 | 396 per 1000 | |||||
| Moderate | ||||||
| 440 per 1000 | 396 per 1000 | |||||
| Hospitalisation ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Mortality ‐ not reported | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Number of days missed at school or daycare ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| Cost ‐ not measured | See comment | See comment | Not estimable | (0 studies) | ‐ | Not reported in included studies |
| CI: Confidence interval; RR: Risk ratio *The basis for the assumed risk for ‘Study population’ was the average risk in the control groups (i.e. total number of participants with events divided by total number of participants included in the meta‐analysis). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| ¹ High risk for attrition bias | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 5 | Risk Difference (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 3 | 1826 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.12, ‐0.03] |
| 1.2 Healthy children during respiratory infection | 1 | 1253 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.02, 0.05] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.07] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Gastrointestinal‐related adverse events Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 3 | 1826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.74, 2.75] |
| 1.2 Healthy children during respiratory infection | 1 | 1277 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.61, 13.00] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.16] |
| 2 Rash Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Healthy children | 1 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.20, 4.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 At least one exposure to antimicrobial drugs Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Healthy children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Otitis‐prone children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 1.2 Healthy children during respiratory infection | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.70, 1.69] |
| 1.3 Otitis‐prone children | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.67, 1.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 2 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.50, 0.87] |
| 1.2 Healthy children during respiratory infection | 1 | 835 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.86, 2.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 2 | 663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.81] |
| 1.2 Healthy children during respiratory infection | 1 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.78, 2.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.16] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.85, 2.29] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.89] |
| 1.2 Healthy children during respiratory infection | 1 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.43, 1.07] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.53, 1.11] |
| 1.2 Healthy children during respiratory infection | 1 | 488 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.14] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Final diagnosis of at least one episode of AOM Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Healthy children | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.13] |
| 1.2 Healthy children during respiratory infection | 1 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.46] |
| 1.3 Otitis‐prone children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |

