Partial breast irradiation for early breast cancer
Abstract
Background
Breast‐conserving therapy for women with breast cancer consists of local excision of the tumour (achieving clear margins) followed by radiotherapy (RT). RT is given to sterilize tumour cells that may remain after surgery to decrease the risk of local tumour recurrence. Most true recurrences occur in the same quadrant as the original tumour. Whole breast radiotherapy (WBRT) may not protect against the development of a new primary cancer developing in other quadrants of the breast. In this Cochrane review, we investigated the delivery of radiation to a limited volume of the breast around the tumour bed (partial breast irradiation (PBI)) sometimes with a shortened treatment duration (accelerated partial breast irradiation (APBI)).
Objectives
To determine whether PBI/APBI is equivalent to or better than conventional or hypo‐fractionated WBRT after breast‐conserving therapy for early‐stage breast cancer.
Search methods
We searched the Cochrane Breast Cancer Group Specialized Register (4 May 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 5), MEDLINE (January 1966 to 4 May 2015), EMBASE (1980 to 4 May 2015), CINAHL (4 May 2015) and Current Contents (4 May 2015). We searched the International Standard Randomised Controlled Trial Number Register (5 May 2015), the World Health Organization's International Clinical Trials Registry Platform (4 May 2015) and ClinicalTrials.gov (17 June 2015). We searched for grey literature: OpenGrey (17 June 2015), reference lists of articles, several conference proceedings and published abstracts, and applied no language restrictions.
Selection criteria
Randomized controlled trials (RCTs) without confounding, that evaluated conservative surgery plus PBI/APBI versus conservative surgery plus WBRT. Published and unpublished trials were eligible.
Data collection and analysis
Two review authors (BH and ML) performed data extraction and used Cochrane's 'Risk of bias' tool, and resolved any disagreements through discussion. We entered data into Review Manager 5 for analysis.
Main results
We included seven RCTs and studied 7586 women of the 8955 enrolled.
Local recurrence‐free survival appeared worse for women receiving PBI/APBI compared to WBRT (hazard ratio (HR) 1.62, 95% confidence interval (CI) 1.11 to 2.35; six studies, 6820 participants, low‐quality evidence). Cosmesis (physician‐reported) appeared worse with PBI/APBI (odds ratio (OR) 1.51, 95% CI 1.17 to 1.95, five studies, 1720 participants, low‐quality evidence). Overall survival did not differ with PBI/APBI (HR 0.90, 95% CI 0.74 to 1.09, five studies, 6718 participants, high‐quality evidence).
Late radiation toxicity (subcutaneous fibrosis) appeared worse with PBI/APBI (OR 6.58, 95% CI 3.08 to 14.06, one study, 766 participants, moderate‐quality evidence). Acute skin toxicity appeared reduced with PBI/APBI (OR 0.04, 95% CI 0.02 to 0.09, two studies, 608 participants). Telangiectasia (OR 26.56, 95% CI 3.59 to 196.51, 1 study, 766 participants) and radiological fat necrosis (OR 1.58, 95% CI 1.02 to 2.43, three studies, 1319 participants) appeared worse with PBI/APBI. Late skin toxicity (OR 0.21, 95% CI 0.01 to 4.39, two studies, 608 participants) and breast pain (OR 2.17, 95% CI 0.56 to 8.44, one study, 766 participants) appeared not to differ with PBI/APBI.
'Elsewhere primaries' (new primaries in the ipsilateral breast) appeared more frequent with PBI/APBI (OR 3.97, 95% CI 1.51 to 10.41, three studies, 3009 participants).
We found no clear evidence of a difference for the comparison of PBI/APBI with WBRT for the outcomes of: cause‐specific survival (HR 1.08, 95% CI 0.73 to 1.58, five studies, 6718 participants, moderate‐quality evidence), distant metastasis‐free survival (HR 0.94, 95% CI 0.65 to 1.37, four studies, 3267 participants, moderate‐quality evidence), relapse‐free survival (HR 1.36, 95% CI 0.88 to 2.09, three studies, 3811 participants), loco‐regional recurrence‐free survival (HR 1.80, 95% CI 1.00 to 3.25, two studies, 3553 participants) or mastectomy rates (OR 1.20, 95% CI 0.77 to 1.87, three studies, 4817 participants, low‐quality evidence). Compliance was met: more than 90% of the women in all studies received the RT they were assigned to receive. We found no data for the outcomes of costs, quality of life or consumer preference.
Authors' conclusions
It appeared that local recurrence and 'elsewhere primaries' (new primaries in the ipsilateral breast) are increased with PBI/APBI (the difference was small), but we found no evidence of detriment to other oncological outcomes. It appeared that cosmetic outcomes and some late effects were worse with PBI/APBI but its use was associated with less acute skin toxicity. The limitations of the data currently available mean that we cannot make definitive conclusions about the efficacy and safety or ways to deliver of PBI/APBI. We await completion of ongoing trials.
PICO
Plain language summary
Partial breast irradiation for early breast cancer
What is the issue?
Women with early breast cancer who choose to keep their breast need to have radiotherapy (RT) as well as surgery to remove the cancer to make sure it does not regrow in the breast. RT is treatment with high energy x‐rays. Having RT for breast cancer usually means 25 to 30 visits to the RT department, five times per week.
If breast cancer does regrow in the same breast (called local recurrence), it tends to come back in the area it was removed from. Women can also grow a new cancer (new 'elsewhere primary') in another part of the same breast. We are not sure if the RT given to stop cancer regrowth where the first cancer was does stop the growth of 'elsewhere primaries'.
Breast cancer is the most common cancer that women get. When women choose to keep their breast, it is important that they are happy with how it looks after treatment (cosmesis).
Why does it matter?
We always want to treat the smallest area we can with RT because this means fewer side effects. Treating only part of the breast could mean that RT might be able to be used again in another part of the same breast if needed. New ways of giving RT mean that treating part of the breast can be done with fewer treatments. This is likely to be easier for women and cost less money.
We asked if giving RT to part of the breast (called partial breast irradiation (PBI)) is as good as giving RT to the whole breast. It would need to control the cancer as well as giving RT to the whole breast does. It would also be important that the PBI gives about the same side effects and breast appearance as treating the whole breast.
We found seven studies, which involved 7586 women. Our evidence is current to May 2015. Local recurrence was rare, but more common with PBI (low‐quality evidence) and the breast appearance (scored by doctors) was worse with PBI (low‐quality evidence). Survival did not differ (high‐quality evidence). Scarring in the breast was worse with PBI (moderate‐quality evidence). The same number of women died of breast cancer with either treatment (moderate‐quality evidence). The same number of women developed spread of breast cancer around their body with either treatment (moderate‐quality evidence). There appeared to be the same number of women who eventually needed the breast removed (mastectomy) after both treatments. Mastectomy could happen because of cancer regrowth in the breast or bad side effects (low‐quality evidence).
This means that at the moment, PBI does not give the same cancer control in the breast as treating the whole breast, but the difference was small. It may cause worse side effects. There are five big ongoing studies that will be important to answer this question. We hope to have a clearer answer in the next update of this review.
Authors' conclusions
Summary of findings
| PBI/APBI for women with early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: radiotherapy centres Intervention: PBI/APBI Comparison: whole breast radiotherapy (WBRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival at 5 years | Study population | HR 1.62 | 6820 | ⊕⊕⊝⊝ | ‐ | |
| 10 per 10001 | 16 per 1000 | |||||
| Cosmesis assessed with 4‐point scale Follow‐up: range 29‐122 months | Study population | OR 1.51 | 1720 | ⊕⊕⊝⊝ | Cosmesis was assessed using a 4‐point scale. We reported those women with poor/fair cosmesis at final review | |
| 150 per 1000 | 218 per 1000 | |||||
| Late radiotherapy toxicity (subcutaneous fibrosis) Follow‐up: median 36 months | Study population | OR 6.58 | 766 | ⊕⊕⊕⊝ | Assessed using National Cancer Institute 3‐point scale, events were defined as: Grade II or higher toxicity Physician assessors, at 3 years' follow‐up | |
| 22 per 1000 | 128 per 1000 | |||||
| Cause‐specific survival at 5 years | Study population | HR 1.08 | 6718 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 10002 | 22 per 1000 | |||||
| Distant metastasis‐free survival at 5 years | Study population | HR 0.94 | 3267 | ⊕⊕⊕⊝ | ‐ | |
| 33 per 10002 | 31 per 1000 | |||||
| Mastectomy rate Follow‐up: range 29‐122 months | Study population | OR 1.20 | 4817 | ⊕⊕⊝⊝ | Mastectomy rate reflected both local recurrence and adverse cosmetic outcome | |
| 15 per 1000 | 18 per 1000 | |||||
| Mortality | Study population | HR 0.90 | 6718 | ⊕⊕⊕⊕ | Survival advantage from radiotherapy for breast cancer is not apparent before 15 years' follow‐up (EBCTCG 2011) | |
| 51 per 10002 | 46 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The baseline risk for the control group was calculated at the 5‐year time point from 5 studies. | ||||||
Background
Description of the condition
Breast cancer is the most common cancer occurring in women. One in eight women living in the US and the UK has a lifetime risk of being diagnosed with breast cancer; while one in nine women living in Australia are at risk (AIHW 2012; Howlader 2009; ONS 2010). Breast cancer is the second most common cause of cancer death in women.
Historically, mastectomy was the recommended therapeutic option for all stages of breast cancer. However, large randomised controlled trials (RCTs) demonstrated equivalent survival for women with early‐stage disease (Stages I, II) whether they were treated with breast‐conserving therapy or mastectomy (EBCTCG 1995; Fisher 1995; Fisher 2002; Jacobson 1995; Poggi 2003; van Dongen 2000; Veronesi 1995; Veronesi 2002). Consequently, breast conservation has become the preferred management option for these women.
Breast‐conserving therapy consists of local excision of the tumour (achieving clear margins) followed by radiotherapy (RT). RT is given to sterilize tumour cells that may remain after surgery. This practice is supported by data from detailed pathological examination of mastectomy specimens where residual tumour was found more than 2 cm from the original tumour in 41% of participants (Holland 1985). Conventional RT delivers 45 to 50 Gray (Gy) to the whole breast over five weeks frequently followed by a boost to the tumour bed (the most likely site of residual tumour cells) of 10 to 16 Gy over one to two weeks. This prolonged duration of treatment negatively impacts on quality of life (Whelan 2000), and contributes to the higher mastectomy rates observed in women residing in rural and remote areas who wish to avoid being away from home and family for extended periods (Schroen 2005).
Hypofractionated whole breast radiotherapy (WBRT) regimens, in which 40 to 42.5 Gy is delivered to the whole breast over three to four weeks using a larger radiation dose with each treatment have been investigated. Compared to conventional WBRT, hypofractionated WBRT results in no difference in breast recurrence rates at five and 10 years, no difference in overall survival (OS) and an improvement in cosmetic outcomes (START 2008; START B 2008; Whelan 2002).
RCTs have shown that the addition of conventional or hypofractionated WBRT to local excision decreases ipsilateral breast (same breast) recurrence rates from 30% to 40% (Fisher 1995; Fisher 2002; Freeman 1981; Lagios 1983; Montgomery 1978) to 10% to 20% with 10 to 15 years of follow‐up (Fisher 1995; Fisher 2002). Modern breast‐conserving therapy with postoperative WBRT achieves local control rates of 3.3% to 3.4% at five years and 5.2% to 6.7% at 10 years (Haviland 2013). With careful patient selection (aged 65 years or greater, T1‐2 tumours, tumour not greater than 3 cm, margin negative, oestrogen receptor positive and all receiving tamoxifen), surgery alone can achieve local recurrence rates of 4.1% at five years (Kunkler 2015). An ipsilateral recurrence can either be a true recurrence of the original cancer (typically arising in the same quadrant as the original tumour and known as local recurrence) or a second primary tumour developing elsewhere in that same breast. Studies evaluating ipsilateral breast tumour recurrence patterns showed that new primaries increasingly contribute to the rate of recurrence after five to eight years while true recurrence rates stabilize (Krauss 2004; Smith 2000). If WBRT was successful in preventing the recurrence of new primary cancers, the rate of such cancers in the treated breast should be lower than the rate of development of cancers in the other breast (contralateral breast cancer). Studies of ipsilateral breast tumour recurrence patterns have not found this (Krauss 2004; Smith 2000). Furthermore, studies examining primary and re‐excision pathological specimens removed at the time of breast‐conserving surgery revealed residual tumour 15 mm or less from the primary tumour in 91% of the specimens (Wallner 2004).
Thus, as most true recurrences occur in the same quadrant as the original tumour and as WBRT does not appear to protect against the development of new primary cancer, investigators are examining the role of partial breast irradiation (PBI).
Description of the intervention
PBI (also known as less than WBRT) refers to irradiation of a limited volume of breast tissue around the tumour bed. It may be achieved by any of the following techniques.
-
Intracavitary brachytherapy or MammoSite® (applying radioactive sources directly into the cavity left after surgical removal of the tumour either at the time of surgery or at a later date, the latter requiring a second procedure).
-
Interstitial brachytherapy (inserting catheters into the surgical cavity and surrounding tissue to temporarily deliver radioactive sources).
-
Intra‐operative techniques using electrons or x‐rays at 50 kVp (using a dedicated machine to deliver a very localized radiation dose to the surgical cavity in the operating room or by moving the person with an open wound to the radiation machine, which may be in a different part of the hospital).
-
External beam radiotherapy (EBRT) using either three‐dimensional conformal radiotherapy (3D‐CRT) (EBRT delivered in the postoperative setting to a volume of breast tissue around the tumour cavity using a standard linear accelerator in a radiation oncology department) or other methods.
Conventional RT typically delivers a radiation dose of 2 Gy with each treatment. Some PBI techniques deliver a larger than standard dose of radiation with each treatment, allowing the overall duration of treatment to be shortened. This is termed accelerated partial breast irradiation (APBI).
How the intervention might work
PBI/APBI will only be of benefit if it confers the same local control benefit as standard WBRT with acceptable toxicity and cosmesis. Currently, some authorities consider PBI/APBI to be an experimental therapy (Clinical Evidence). The National Comprehensive Cancer Network (NCCN) state, "Preliminary studies of APBI suggest rates of local control in patients with early‐stage breast cancer may be comparable to those treated with WBRT. Follow‐up, however is limited and studies are ongoing" (NCCN). Because this technique has been widely adopted outside the context of clinical trials, published guidelines exist that identify women with early breast cancer for whom this technique may be safe. For those women ineligible for a trial, carefully selected women with early breast cancer may be offered PBI/APBI (Bellon 2011; Polgár 2010; Smith 2009).
PBI/APBI has a number of potential advantages including:
-
a reduction in treatment‐related toxicities, as a smaller volume of breast tissue is irradiated;
-
increased utilization of breast conservation;
-
a reduction in RT waiting times; a reduction in the overall treatment duration of a common malignancy has the potential to substantially impact on RT waiting times in countries with strained resources (including the UK, Canada, Australia and New Zealand);
-
a greater chance of preserving the breast should a recurrence occur elsewhere in the breast;
-
easier integration with chemotherapy schedules because RT time will be shorter, thus avoiding delays.
PBI/APBI has a number of potential disadvantages including:
-
an increased risk of local recurrence due to geographic miss. This is either because treatment was delivered before full pathological examination was obtained or because of difficulty in reproducing the target volume (the tissue that needed to be treated with RT) daily;
-
increased late toxicity. The late effects of radiation are dependent on the dose of radiation given at each treatment and, as PBI/APBI delivers a large radiation dose per fraction, late toxicity may be increased with resultant poor cosmetic outcome or breast appearance (cosmesis);
-
more patient inconvenience as some techniques may require a second anaesthetic or a further invasive procedure;
-
a number of techniques (e.g. interstitial and intracavitary brachytherapy) require operator expertise and specialized equipment that may not be available in all centres;
-
invasive techniques of delivering PBI/APBI (e.g. interstitial and intracavitary brachytherapy) may be associated with toxicity such as infection and delays in wound healing. Scarring post‐insertion of interstitial brachytherapy catheters can negatively impact on cosmetic appearance.
Why it is important to do this review
PBI/APBI has the potential to change the pattern of practice for a common malignancy and thereby impact on resource utilization, patient satisfaction and quality of life. However, as PBI/APBI is currently an experimental therapy, it must be thoroughly evaluated before being adopted as the new standard of care for early‐stage breast cancer. PBI/APBI can be recommended if it is as effective or better than conventional or hypofractionated WBRT for cancer‐related outcomes (local relapse‐free survival (LR‐FS), survival, breast cancer‐specific survival and metastasis‐free survival) as well as patient‐orientated outcomes (cosmesis, quality of life and consumer preference). We found one systematic review that concluded, "The data on PBI/APBI compared to whole‐breast irradiation are insufficient to draw any conclusions about the relative effectiveness of these modalities" (BlueCross BlueShield). The fact that the benefit versus risk profile of PBI/APBI is currently unknown makes it an ideal topic for a systematic review.
Objectives
To determine whether PBI/APBI is equivalent to or better than conventional or hypofractionated WBRT after breast‐conserving therapy for early‐stage breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) evaluating conservative surgery plus PBI/APBI versus conservative surgery plus WBRT. The comparisons had to be unconfounded (i.e. treatments given to the randomized groups had to differ only in relation to the volume of the breast irradiated). Trials incorporating adjuvant treatments, such as chemotherapy, monoclonal antibodies or hormonal therapy, were eligible if the RCT applied these other treatments in exactly the same way to both groups. Published and unpublished studies were eligible.
We did not consider studies in which PBI was used as a boost following conventional EBRT for inclusion.
Types of participants
Women with histologically confirmed early‐stage breast cancer who had conservative surgery. Early breast cancer included tumours classified as American Joint Committee on Cancer (AJCC) stage T1‐2N0‐1M0 (Fleming 1997). Surgery could include lumpectomy and wide local excision or quadrantectomy, with or without axillary dissection, axillary sampling or sentinel node biopsy. Women with a previous diagnosis of breast cancer were not eligible for inclusion.
Types of interventions
Radiation delivered to the partial breast (PBI) and PBI using larger than standard radiation dose per fraction such that the overall treatment time was reduced (APBI). We considered any method of PBI/APBI delivery including, but not limited to, intracavitary brachytherapy or MammoSite®, interstitial brachytherapy, intra‐operative techniques such as electrons or x‐rays at 50 kVp or EBRT using either 3D‐CRT or other methods. Conventional breast RT is delivered to the whole breast with or without the supra‐clavicular fossa and axilla, using standard fractionation (1.8 to 3.0 Gy per fraction) to deliver a total of 40 to 61 Gy at the reference point. Treatment could include a boost (using electrons, interstitial therapy, EBRT or new techniques).
Types of outcome measures
Primary outcomes
-
Local recurrence‐free survival (LR‐FS) in the ipsilateral breast. We defined local recurrence as a recurrence of the same histological type of cancer within the same quadrant of the breast as the primary cancer.
-
Cosmesis (cosmetic outcome or breast appearance).
Secondary outcomes
-
Overall survival (OS, time from date of randomization to death from any cause, or number of deaths from any cause).
-
Toxicity (including acute and late effects of RT, chemotherapy‐related toxicity and surgical toxicity; individual protocol‐based definitions).
-
New primary tumours in ipsilateral breast, 'elsewhere primary'. We defined a new primary as a lesion arising in a quadrant of the breast that was different from the original cancer or a tumour of a different histological subtype occurring anywhere within the breast.
-
Cause‐specific survival (C‐SS, deaths due to breast cancer at five years).
-
Distant metastasis‐free survival (DM‐FS, in isolation or at the same time as local recurrence (the occurrence of metastases at five years)).
-
Relapse‐free survival (R‐FS, length of time after treatment during which no recurrence was found). Recurrence referred to breast cancer in the ipsilateral breast or elsewhere in the body, excluding a new breast cancer in the contralateral breast.
-
Loco‐regional recurrence‐free survival (L‐RR‐FS, comprised local recurrence, "elsewhere" ipsilateral breast primaries (a new primary cancer in the same breast) and regional nodal relapse).
-
Subsequent mastectomy (ipsilateral partial mastectomy, modified radical mastectomy or radical mastectomy).
-
Compliance, defined as the number of women who commenced treatment with PBI/APBI or conventional external beam radiotherapy (EBRT) and completed the treatment course.
-
Costs (monetary costs of PBI versus EBRT) to women, government and insurance companies.
-
Quality of life (using trial‐specific instruments). The effects of PBI/APBI and EBRT on global quality of life and the physical, emotional and psychological domains.
-
Consumer preference, that is, did women prefer PBI/APBI or WBRT given the advantages and disadvantages of each approach.
Search methods for identification of studies
Electronic searches
Electronic databases
We searched the following databases:
-
the Cochrane Breast Cancer Group Specialized Register (4 May 2015). Details of search strategies used to identify studies and the procedure used to code references are outlined in the group's module (Cochrane Breast Cancer Group). We extracted studies on the specialized register with keywords 'early breast cancer', 'radiotherapy', 'partial breast irradiation', 'whole breast irradiation', 'whole breast radiotherapy', 'brachytherapy', 'high‐dose‐rate brachytherapy', 'accelerated partial breast irradiation', 'tumour bed boost', 'sole tumour bed irradiation', 'MammoSite', 'radiotherapy', 'PBI', 'APBI' and 'interstitial brachytherapy' for consideration;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 3; Appendix 1);
-
MEDLINE (January 1966 to 4 May 2015; Appendix 2);
-
EMBASE (1980 to 4 May 2015; Appendix 3);
-
CINAHL (1981 to 4 May 2015; Appendix 4);
-
Current Contents (1998 to 4 May 2015; Appendix 5).
We modified the MEDLINE search strategy (Appendix 2) to search the other databases, without language restrictions.
Unpublished literature
We searched following registers for ongoing clinical trials:
-
the International Standard Randomised Controlled Trial Number Register (www.controlled‐trials.com/isrctn) (5 May 2015);
-
the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search portal (apps.who.int/trialsearch/Default.aspx) (4 May, 2015; Appendix 6);
-
US clinical trials registry (www.clinicaltrials.gov) (17 June 2015; Appendix 7).
Grey literature
We checked OpenGrey (www.opengrey.eu/) (17 June 2015; Appendix 8).
Searching other resources
We contacted researchers located from the grey literature and unpublished literature to ask if they were aware of any other trials on this topic. We contacted the Barcelona authors on 15 October 2012 for more information. We contacted the IRMA authors on 2 October 2012 and they supplied us with the trial protocol. We contacted the authors of Polgár 2007 on 24 August 2013; we gratefully received and incorporated data from the authors of Polgár 2007 in November 2013.
Handsearching
We handsearched a number of conference proceedings and published abstracts including:
-
Adjuvant Therapy for Primary Breast Cancer International Conference (2001);
-
Primary Therapy of Early Breast Cancer (2001 and 2003);
-
6th and 7th Nottingham International Breast Cancer Meeting Conference Reports;
-
23rd and 24th Congress of the International Association for Breast Cancer Research;
-
3rd and 4th Perspectives in Breast Cancer Conference Reports;
-
26th and 27th Annual San Antonio Breast Cancer Symposium;
-
4th European Breast Cancer Conference;
-
94th and 95th American Association of Cancer Research;
-
American Society for Clinical Oncology (1995 to 2005);
-
European Society for Therapeutic and Radiation Oncology (1990, 1993, 2000 to 2010, 2012);
-
5th and 6th Milan Breast Cancer Conference;
-
Australian Breast Cancer Conference (2004);
-
27th and 28th Annual Symposium of the American Society of Breast Disease;
-
Centers for Disease Control and Prevention (CDC) Cancer Conference (2003);
-
International Journal of Radiation Oncology Biology Physics: proceedings of American Society for Radiation Oncology (ASTRO) 2011 to 2015;
-
Radiotherapy and Oncology: Proceedings of World Congress of Brachytherapy 2012;
-
American Society of Clinical Oncology (ASCO) from 1995 to 2010.
Data collection and analysis
Selection of studies
Three review authors (ML, BH and DF) checked the titles and abstracts retrieved by the searches. Each review author independently assessed the full text of the studies thought relevant to the review and we resolved any differences in assessment by discussion. We performed trial assessments with the results masked. In cases where data were limited or information on trial methods was limited, we requested further information from the trial authors.
Data extraction and management
Two review authors (ML and BH) performed data extraction and resolved disagreements through discussion. We entered data into Review Manager 5 for analysis (RevMan 2012). Where possible, we extracted data on tumour stage, nodal status, margin status, receptor status, hormonal manipulation, treatment allocation and surgery performed. The information extracted on RT included overall treatment time, radiation dose, dose per fraction and method of PBI. We extracted outcome data on local recurrence, deaths (all‐cause and breast cancer deaths), new ipsilateral primaries, mastectomy rate, distant metastases, treatment‐related toxicity (including that related to acute and late effects of RT and to surgery), cosmesis, costs of treatment, consumer preference and quality of life.
We derived data for OS, LR‐FS, DM‐FS, disease‐free survival and C‐SS from information in the text (ELIOT; Livi 2015; RAPID; Rodriguez; TARGIT) and data received from trial authors (Polgár 2007). Trial authors of Polgár 2007 provided the HR, 95% CI and P values, together with the number of events with further follow‐up. We used the spreadsheet developed by Matthew Sydes (Tierney 2007) to derive O‐E (observed minus expected events) and variance using the number of events, HRs, P values where available or calculated using the Review Manager 5 calculator (RevMan 2012). Although there were data for loco‐regional recurrence for ELIOT, we did not include them in the analysis, because some women were treated with regional nodal RT.
We converted the radiation doses to the equivalent dose in 2 Gy fractions (EQD2 ) (Maciejewski 1986; Withers 1983), using the formula: EQD2 = D [d + (alpha/beta/2 + alpha/beta)], where D = total dose, d = dose per fraction and alpha/beta = 4 Gy (Owen 2006). This was to facilitate comparison of radiation doses given at differing dose per fraction.
We plan to convert brachytherapy (radiation sources applied directly to the body) to the biological equivalent dose (BED) using the method of Stitt 1992 should it be necessary to pool brachytherapy data in future updates of this review.
Studies reported global cosmetic outcome using the Harvard Cosmetic Score (Polgár 2007; Livi 2015; Rodriguez), the European Organization for Research and Treatment of Cancer (EORTC) Cosmetic rating System for Breast Cancer) (Aaronson 1998; RAPID), and a software program (TARGIT). These were all four‐point scales; the results were dichotomized into good/excellent and fair/poor, with occurrence of fair/poor being counted as 'events'. See Table 1.
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
One study reported telangiectasia (RAPID; using NCI CTCAE Version 3.0; also a three‐point scale): we recorded any women with Grade 2 or higher toxicity as having events.
Three studies reported radiological or asymptomatic fat necrosis (ELIOT; Polgár 2007; RAPID); we were able to report Grade 1 fat necrosis, as this had the same definition in all three scales.
Assessment of risk of bias in included studies
BH and ML assessed trials to check that they met the inclusion criteria and independently assessed methodological quality. BH undertook 'Risk of bias' tables assessments, which were checked by ML, and reported them in the text and as a figure.
We described the risk of bias for each included trial. BH and ML judged risk of bias in eight specific domains, and resolved any differences through discussion. The eight domains were:
-
random sequence generation;
-
allocation concealment;
-
blinding of participants and personnel;
-
blinding of outcome assessors for objective outcomes;
-
blinding of outcome assessors for subjective outcomes;
-
incomplete outcome data;
-
selective outcome reporting;
-
other sources of bias (i.e. early stopping and differences in follow‐up examinations).
We will perform sensitivity analyses on the basis of trial quality when additional trials are available. We plan to perform the analysis both with and without trials at low risk of bias to assess the effect of bias on the results when more trials are available to examine.
Measures of treatment effect
We presented results as hazard ratios (HR) for time‐to‐event data and odds ratios (OR) with 95% confidence intervals (CI) where this was not possible (Deeks 2004).
For future updates, if we find results for continuous variables (such as quality of life), we will summarize them using the mean difference or the standardized mean difference when different measurement scales are used (Deeks 2004).
Unit of analysis issues
Because the unit of analysis was the individual participant, we did not anticipate any unit of analysis issues.
Dealing with missing data
If data are missing in future updates, we will contact the original investigators (by written correspondence).
We will specify what assumptions we make, for example, if we presume the missing data were missing at random, or that missing data were assumed to have a particular value such as a poor outcome. We will, if necessary, perform a sensitivity analysis to see how sensitive results are to the assumptions we have made. We will address the potential implications of this in the 'Discussion' section.
Assessment of heterogeneity
We assessed heterogeneity both visually and statistically using the Chi2 test of heterogeneity (Altman 1992; Walker 1988), and I2 statistic (Higgins 2002; Higgins 2003). The criterion for identification of heterogeneity is a P value less than 0.10 for the Chi2 test (acknowledging the limitations of this process) and an I2 statistic greater than 50%. Where we identified significant heterogeneity, we explored the reasons for it and made a cautious attempt to explain the heterogeneity.
Assessment of reporting biases
We acknowledge that there are multiple potential sources of reporting biases, including, but not limited to, publication bias, time‐lag bias, duplicate publication bias and selective outcome reporting. By searching multiple sources including trial registries, we hope to minimize publication bias. We noted the early reporting for the TARGIT was an example of time‐lag bias. We planned to use funnel plots to evaluate funnel plot asymmetry, but took into account that visual interpretation is subjective and that statistical methods to evaluate funnel plot asymmetry are unlikely to be valid if there are fewer than 10 included trials.
Data synthesis
We applied the intention‐to‐treat principle in analyzing data from the trials and determined a weighted mean treatment effect using the fixed‐effect model to combine results (Mantel 1959) with Review Manager 5 software (RevMan 2012).
We used the Mantel‐Haenszel methods to calculate pooled results when there was no significant heterogeneity (Greenland 1985; Mantel 1959), or if otherwise, the random‐effects model of Der Simonian and Laird (DerSimonian 1986).
We excluded two studies from the analysis (Dodwell 2005; Ribeiro 1993), because they used staging, surgery and RT techniques that do not reflect current practice (see Characteristics of excluded studies table).
We graded the quality of the evidence and created a 'Summary of findings' table using the following outcomes.
-
LR‐FS.
-
Cosmesis.
-
Mortality (follow‐up: 5‐year survival).
-
Late toxicity: late subcutaneous breast fibrosis.
-
C‐SS.
-
DM‐FS.
-
Subsequent mastectomy.
The population included women with early breast cancer and the intervention was PBI/APBI versus WBRT. We used GRADEpro and the GRADE approach to evaluate the strength of the evidence (GRADE Working Group 2004). To calculate the absolute risk for the control group for time‐to‐event outcomes, we estimated the event rate at a specific time point (five years for LR‐FS, BC‐SS, DM‐FS and OS) from the Kaplan‐Meier curves or reported event rates. We entered these estimated values in GRADEpro, which automatically calculated the corresponding absolute risks for the intervention group at five years.
Subgroup analysis and investigation of heterogeneity
In future updates if data are available, we may perform subgroup analyses to investigate whether the effects of using PBI/APBI or conventional breast RT differ depending on nodal status, margin status, receptor status, hormonal manipulation or tumour stage.
If heterogeneity is identified in future updates, we will assess it both statistically and visually using the Chi2 test of heterogeneity (Altman 1992; Walker 1988), and I2 statistic (Higgins 2002; Higgins 2003). If we do identify significant heterogeneity, we will explore the reasons for it and attempt to explain it.
Sensitivity analysis
We performed a sensitivity analysis by excluding the trials at high risk of bias for subjective outcomes (ELIOT; Livi 2015; Polgár 2007; Rodriguez). In future updates, if adequate data are available, we will perform a sensitivity analysis to assess the robustness of the results by excluding studies at high risk of bias for subjective outcomes and unpublished trials.
Results
Description of studies
See: Characteristics of included studies table.
Results of the search
For this review update, based on our search strategy, we identified and screened 3630 references from the major medical databases and identified 46 additional references from other sources. After exclusion of duplicates and screening of references (by title or abstract), we evaluated 46 full‐text references and excluded eight references (see Characteristics of excluded studies table). Of the remaining 38 references, 17 records related to five new included studies (ELIOT; GEC‐ESTRO; Livi 2015; RAPID; Rodriguez), six records provided data for two previously included studies (Polgár 2007; TARGIT), 14 records for four ongoing studies (IMPORT; IRMA; NSABP‐B39/RTOG; SHARE), and one record for one study awaiting classification (NCT02375048).
Overall, when combining the original and review update, there were 44 records relating to seven included studies, 14 records relating to four ongoing studies and one record awaiting classification (see Figure 1). Two studies that were included in the original review have now been excluded (Dodwell 2005; Ribeiro 1993). This is because the surgery and techniques used to define volume of breast treated and the technology used in these two studies do not reflect current RT practice. In sum, this review update included seven studies and the qualitative analysis included six studies.
Study flow diagram.
Included studies
Design
Seven RCTs enrolled 8955 women (ELIOT; GEC‐ESTRO; Livi 2015; Polgár 2007; RAPID; Rodriguez; TARGIT); we studied 7586 women included in these studies. These studies enrolled women from July 1998 to May 2004 (Polgár 2007), February 2006 to July 2011 (RAPID), November 2000 to December 2007 (ELIOT), March 2000 to June 2011 (TARGIT), March 2005 to June 2013 (Livi 2015), and April 2004 to July 2009 (GEC‐ESTRO); Rodriguez did not state accrual dates.
Sample size
Polgár 2007 enrolled 258 women of a planned sample size of 570 participants, RAPID enrolled 2135 women, Rodriguez enrolled 102 women, ELIOT enrolled 1305 women, TARGIT enrolled 3451 women, Livi 2015 enrolled 520 women and GEC‐ESTRO enrolled 1184 women.
Setting
Four studies were single institution trials from tertiary institutions: one in Hungary (Polgár 2007), two in Italy (ELIOT; Livi 2015), and one in Spain (Rodriguez). GEC‐ESTRO, TARGIT, and RAPID were multicentred, international studies.
Participants
Polgár 2007 included women with invasive breast cancer after wide local excision of tumour and negative pathological margins (unifocal tumours, tumour size less than 20 mm, clinically or pathologically N0, or single microscopic nodal metastasis (greater than 0.2 mm and less than 2.0 mm), that is, pT1N0‐1miM0, Grade I or II; T1N0‐N1miM0, Grade I or II. RAPID enrolled women with either invasive ductal carcinoma or ductal carcinoma in situ with tumours 3.3 cm or greater, with negative margins and no involved axillary nodes. Rodriguez included women with pT1‐2pN0M0 invasive ductal carcinoma, with tumour size 3 cm or less, with negative margins and Grade I or II histology. ELIOT enrolled women aged 48 to 75 years with early breast cancer, maximum tumour diameter 2.5 cm, "suitable for breast conservation". TARGIT enrolled women aged 45 years or over, with T1 and small T2N0‐1M0 invasive breast cancer, suitable for breast‐conserving surgery, available for 10 years' follow‐up. Livi 2015 included women aged over 40 years who had wide local excision or quadrantectomy for invasive breast cancer, negative margins and tumour size 2.5 cm or less. GEC‐ESTRO included women aged 40 years or more, small T1‐2N0‐miM0 (less than 3 cm) with negative margins and no lympho‐vascular invasion (LVI) and excluded women with multifocal tumours. GEC‐ESTRO included Tis. See Characteristics of included studies table.
Interventions
Experimental arm
PBI using either:
-
brachytherapy:
-
high‐dose‐rate (HDR) brachytherapy: seven × 5.2 Gy HDR multi‐catheter brachytherapy for 88/128 women (Polgár 2007); 30.3 to 32.0 Gy/seven or eight fractions (GEC‐ESTRO);
-
pulsed‐dose‐rate (PDR) brachytherapy: 50 Gy at 0.6 to 0.8 Gy/hour in GEC‐ESTRO or
-
-
EBRT to partial breast. PBI delivered via EBRT was delivered:
-
via conventionally fractionated (2 Gy per fraction) in 50 Gy/25 fraction electron beam RT delivered using a linear accelerator to the partial breast for 40/128 women (Polgár 2007);
-
at greater than 2 Gy per fraction delivered using a linear accelerator. RAPID used 3D‐CRT in 38.5 Gy/10 fractions over five to eight days (with a minimum six hour gap between fractions given on the same day) and Rodriguez used 37.5 Gy/10 fractions twice daily over five days. Livi 2015 delivered 30 Gy in five fractions using intensity‐modulated radiotherapy (IMRT);
-
using electrons: intraoperative electron therapy to deliver 21 Gy in one fraction at the 90% isodose delivered at the time of surgery after tumour excision using 6 to 9 MeV (ELIOT);
-
using kilovoltage EBRT: TARGIT used a single fraction of RT given intraoperatively (using Intrabeam); 50 kV 20 Gy/one fraction at 2 mm beyond surface of 1.5 to 5.0 cm spherical applicator placed in excision cavity.
-
Control arm
WBRT using conventional fractionation:
-
ELIOT and Polgár 2007 used 50 Gy/25 fractions WBRT;
-
RAPID permitted two doses for WBRT: 50 Gy/25 fractions or 42.5 Gy/16 fractions;
-
Rodriguez gave 48 Gy in 24 fractions;
-
TARGIT gave "Standard post‐operative RT (40 to 56 Gy ± 10 to 16 Gy boost)";
-
Livi 2015 used 50 Gy/25 fractions plus 10 Gy boost;
-
GEC‐ESTRO used 50 to 50.4 Gy in 1.8 to 2.0 Gy/ fraction using 4 to 10 MV beams plus 10 Gy/5 fraction boost.
Co‐interventions
Chemotherapy and hormonal manipulation: in Livi 2015, Polgár 2007, GEC‐ESTRO, RAPID, and TARGIT, women received adjuvant systemic therapy according to institutional protocol, and Rodriguez treated oestrogen receptor positive women with hormonal manipulation. Systemic therapy in ELIOT was "administered according to the European Institute of Oncology policy" at the time (see Characteristics of included studies table for further details). In total, 6372/6820 (93%) women received hormonal manipulation and 977/6820 (14.3%) of the women received chemotherapy.
ELIOT treated any women with four or more involved nodes with regional nodal RT. No women in any of the other included studies received regional nodal RT.
Outcomes
Primary outcomes
Local recurrence‐free survival
Six trials reported local recurrence in the ipsilateral breast as a discrete outcome (GEC‐ESTRO; Rodriguez; ELIOT; Livi 2015; Polgár 2007; TARGIT).
Cosmesis
Six trials reported cosmesis (cosmetic outcome). Polgár 2007, Livi 2015, and Rodriguez reported a global cosmetic result (used the Harvard Cosmetic score (see Table 1)) and RAPID used the EORTC Cosmetic rating System for Breast Cancer (Aaronson 1998); both were four‐point scales. GEC‐ESTRO evaluated cosmesis using digital photos, with both participant‐reported outcomes and physician‐reported outcomes using a four‐point scale (Wazer 1992; see Table 2). Rodriguez and RAPID reported participant‐reported cosmetic outcomes. RAPID blinded assessment of cosmetic outcome. TARGIT assessed cosmetic outcome at a single centre in a sub‐study that included 105 women. A software program, blinded to treatment arm, assessed digital photos at a median 23 months using a four‐point scale.
| Score | Definition |
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
Secondary outcomes
Overall survival
Six trials reported OS (GEC‐ESTRO; Rodriguez; ELIOT; Livi 2015; TARGIT; Polgár 2007).
Toxicity
Four trials reported acute skin toxicity. Rodriguez, Livi 2015, and TARGIT used the Radiation Therapy Oncology Group Common Toxicity Criteria (RTOG CTC) (Cox 1995; Table 3) and ELIOT used a five‐point scale (which was not referenced).
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
| Description | Follicular, faint or dull erythema / epilation / dry desquamation / decreased sweating | Tender or bright erythema, patchy moist desquamation / moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria.
Five trials reported late toxicity (telangiectasia (small blood vessels visible on the treated skin), breast pain, fat necrosis and subcutaneous fibrosis). Polgár 2007 reported fat necrosis at four years according to a five‐point institutional scale (Table 4); RAPID reported late RT toxicity (telangiectasia, induration (subcutaneous fibrosis), breast pain and fat necrosis) using the National Cancer Institute Common Toxicity Criteria (NCI CTC 3.0; NCI; three‐point scales; Table 5). GEC‐ESTRO reported late toxicity (breast pain, late subcutaneous toxicity and late RT skin toxicity) using the RTOG CTC (Cox 1995). ELIOT reported fat necrosis and radiological radiation pneumonitis using late effects of normal tissue ‐ subjective objective management analytic criteria (LENT‐SOMA; Pavy 1995). Livi 2015 scored late RT toxicity using RTOG CTC (Cox 1995). TARGIT reported "complications arising six months after randomisation" and reported RTOG CTC (Cox 1995) Grade III or IV RT‐related skin complications, haematomas/seromas requiring greater than three aspirations or surgery, wound infections requiring intravenous antibiotics or surgery and skin breakdown/delayed wound healing.
| Grade | Findings |
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or |
| 2 | Symptomatic fat necrosis not requiring medication |
| 3 | Symptomatic fat necrosis requiring medication |
| 4 | Symptomatic fat necrosis requiring surgical |
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | ‐ |
| Telangiectasia | Few | Moderate | Many and confluent | ‐ |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria.
New primary tumours in ipsilateral breast ('elsewhere primaries')
Three trials reported new primary tumours in ipsilateral breast (ELIOT; GEC‐ESTRO; Livi 2015).
Cause‐specific survival
Five trials reported C‐SS (where an independent clinician, blinded to treatment arm, determined cause of death) (ELIOT; GEC‐ESTRO; Livi 2015; Polgár 2007; TARGIT).
Distant metastasis‐free survival
Five trials reported DM‐FS (ELIOT; GEC‐ESTRO; Livi 2015; Polgár 2007; Rodriguez).
Relapse‐free survival
One trial reported R‐FS (Polgár 2007).
Loco‐regional recurrence‐free survival
Subsequent mastectomy
Three trials reported subsequent mastectomy (GEC‐ESTRO; Polgár 2007; TARGIT).
Compliance
Three trials reported compliance (GEC‐ESTRO; Polgár 2007; TARGIT).
Costs
We found no trials reporting cost.
Quality of life
Two trials reported quality of life. RAPID used the Breast Cancer Questionnaire (a validated modification of the Breast Cancer Chemotherapy Quality of Life Questionnaire; Levine 1998) and GEC‐ESTRO used the EORTC QLQ‐C30 and QLQ‐BR23 (Levine 1998).
Consumer preference
We found no trials reporting consumer preference.
The included RCTs differed in several ways.
-
Population included: see above.
-
Surgery performed: margins were negative for women in GEC‐ESTRO (2 mm or greater); Rodriguez (greater than 3 mm); Polgár 2007 (less than 2 mm: 1/258, 2 mm or greater: 246/258 or had no tumour at ink: 11/258); RAPID ("microscopically clear") and TARGIT (1 mm or greater). ELIOT did not describe margin status. Surgery in Livi 2015 could be either wide local excision or quadrantectomy (margins 5 mm or greater); in TARGIT if margins were close/involved (invasive disease or in situ disease 1 mm or greater from margin) re‐excision was strongly advised.
-
Target volume definition for the treated PBI/APBI volume varied between the trials. Polgár 2007 clipped the cavity and the planning target volume (PTV) comprised the cavity plus a 1 to 2 cm margin isotropically (in all directions three dimensionally) for those women treated with interstitial therapy. If electrons were used, 6 to 15 MeV were used to treat the cavity with a 2 cm margin. In RAPID, the clinical target volume (CTV) was defined as the tumour bed seen on computer tomography (CT), including the surgical clips plus a 1 cm margin, with a 1 cm margin on CTV to derive PTV. In Rodriguez, the involved breast quadrant was contoured. In ELIOT, the "clinical target volume was decided according to the site and size of the tumour". In Livi 2015, a CTV was drawn on a planning CT with a uniform 1 cm margin around the surgical clips, then a 1 cm margin added to construct the PTV. The target volume was the tumour cavity for TARGIT. In GEC‐ESTRO, the target volume was defined individually for each woman, the CTV was the tumour bed plus a "safety margin" 2 cm or greater, this was planned using CT.
-
Radiation dose prescribed differed greatly between the trials. The PBI/APBI arm dose was higher than the WBRT dose for all studies except ELIOT (see Table 6 for dose, fraction size and dose prescription point, where it was specified).
-
The trials differed in fractionation used for APBI. In Polgár 2007, most women (88/128) received accelerated RT, RAPID used 3.85 Gy fractions, Rodriguez delivered 3.75 Gy fractions using daily RT and Livi 2015 used 6 Gy per fraction. GEC‐ESTRO used seven or eight fractions for HDR and 0.6 to 0.8 Gy pulses for PDR. ELIOT used 21 Gy at the 90% isodose delivered in a single fraction. TARGIT used 20 Gy to the cavity surface delivered in a single fraction (Table 6).
-
RT technique for PBI/APBI delivery differed. In Polgár 2007, most women (88/128) in the PBI/APBI arm received interstitial brachytherapy, but 40/128 women received EBRT, as they were not suitable for brachytherapy (Table 7). RAPID and Rodriguez used 3D‐CRT (using EBRT); Livi 2015 used IMRT to deliver EBRT; ELIOT delivered intraoperative electron therapy at the time of surgery after tumour excision using 6 to 9 MeV and TARGIT inserted a spherical applicator into the operative bed and sutured it in place to treat the tumour cavity (see Table 7).
-
Quality assurance differed. RAPID included an extensive quality assurance programme with credentialling, real‐time and post‐hoc review of radiation quality. GEC‐ESTRO involved both pre‐ and post‐implant assessment of geometry using CT, dose prescription and calculations were in accordance with International Commission of Radiation Units and Measurements (ICRU) 58 and strict dose volume histogram and dose maximums were mandated, the post‐hoc quality assurance requirements were clearly detailed in the study protocol. For the WBRT component of TARGIT, as long as treating centres conformed to a formal quality management system issued by the International Standards Organization, no additional quality assurance was required. For the APBI, quality assurance performed according to the manufacturer's instructions was to be performed and the resulting data to be made available to the trials centre. Data were submitted either annually or after every 50th participant treated with Intrabeam. Rodriguez, ELIOT, and Livi 2015 did not mention trial‐specific quality assurance to assess quality of the delivered RT. Polgár 2007 did not mention quality assurance for APBI using electrons or WBRT.
-
One study did not accrue the full sample size: Polgár 2007 stopped early because a competing multicentred RCT was started.
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI | Control dose | Fraction size (Gy) | EQD2 Control |
| 20 Gy at surface of the applicator (attenuated to 5‐7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40‐56 Gy/20‐28 fractions ± 10‐16 Gy boost | 2 | 40‐56 Gy ± 10‐16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 75 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions bd (with 6 hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95‐107% of prescription dose | 3.85 | 74.1 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4‐5 fractions) based on criteria such as young age or close margins, pre‐specified by centre | 2 or 2.65 | 50 or 47.1 Gy | |
| 37.5 Gy/10 fractions bd (with 6 hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 71.22 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48‐58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI). | 5.2 or 2 | 53.6 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6‐0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7‐8 | 41.64‐42.67 Gy | 50.0‐50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8‐2.0 | 48.72‐50 + 10 = 58.72‐60 Gy | |
| 21 Gy/1 fraction at 90% using 6‐9 MeV | 21 | 131.2 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy |
3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; bd: twice daily; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose‐rate; PTV: planning target volume.
| Trial | RT technique |
| Interstitial brachytherapy (88/128) EBRT using photons (40/128) | |
| intra‐operative electrons | |
| EBRT (IMRT) | |
| intra‐operative kV RT | |
| EBRT | |
| EBRT (3D‐CRT) |
3D‐CRT: 3‐dimensional conformal radiotherapy; EBRT: external beam radiotherapy; IMRT: intensity‐modulated radiotherapy; RT: radiotherapy.
Excluded studies
In the 2015 review update, we excluded eight studies (see Characteristics of excluded studies table).
Risk of bias in included studies
Allocation
We judged the risk of bias for random sequence generation and allocation concealment as follows: ELIOT, GEC‐ESTRO, Livi 2015, Livi 2015, RAPID, Rodriguez, and TARGIT were at low risk of bias for sequence generation. Polgár 2007 was randomized, the method (sealed envelopes) described and allocation was done by the chief investigator. TARGIT, Livi 2015, and GEC‐ESTRO were at low risk of bias for allocation concealment. ELIOT, RAPID, Rodriguez, and Polgár 2007 did not clearly report allocation concealment, so were at unclear risk of bias.
Blinding
Objective outcomes
RAPID and Rodriguez did not mention blinding of participants. It would have been difficult to do so with this intervention, but the lack of blinding was unlikely to have introduced bias (Polgár 2007). Participants in GEC‐ESTRO, Livi 2015, and TARGIT were not blinded to treatment arm, but we considered this unlikely to introduce bias.
ELIOT did not blind participants; for objective outcomes, the clearly pre‐specified criteria for endpoints and the pre‐specified follow‐up protocol meant we deemed this domain at low risk of bias.
The trials did not mention blinding of physicians. It would have been difficult to do so with this intervention, but failure to do so is less likely to have introduced bias when the mammographic screening interval was pre‐specified as in GEC‐ESTRO, Livi 2015, RAPID, and Rodriguez. Polgár 2007 had a pre‐specified follow‐up protocol with pre‐determined interval for mammographic evaluation and Polgár 2007 and Rodriguez required biopsy confirmation for any local recurrence; therefore, this domain was at low risk of bias. This served to make the objective outcomes at low risk of bias. In TARGIT, physicians were not blinded to treatment allocation, which is unlikely to have been a source of bias.
ELIOT did not blind personnel; for objective outcomes, the clearly pre‐specified criteria for endpoints and the pre‐specified follow‐up protocol meant we deemed this domain at low risk of bias.
Polgár 2007 did not mention blinding of outcome assessors, but the pre‐determined mammographic follow‐up protocol and requirement for biopsy confirmation of local recurrence would have reduced the risk of bias. TARGIT did not blind outcome assessors, but the pre‐specified follow‐up protocol minimized the risk of bias. TARGIT reported that the analyses were done blinded, which reduced the risk of bias. ELIOT, GEC‐ESTRO, Livi 2015, RAPID, and Rodriguez were at low risk of bias for this outcome.
Subjective outcomes
Blinding of participants for subjective outcomes was not reported, but it is unlikely to have introduced bias because only two trials reported participant‐reported outcomes (RAPID; Rodriguez).
Polgár 2007 and Livi 2015 did not mention blinding of physicians and this may have introduced bias. In RAPID, the physician reviewers were blinded to treatment arm; this was not stated for the trained nurse observers, but we deemed the outcome at low risk of bias despite this.
As Polgár 2007 did not mention blinding of outcome assessors, it is unlikely to have been done and is potentially a source of bias and, therefore, at high risk of bias. The authors of TARGIT did not report blinding of outcome assessors, so it seems unlikely that they were. However, the use of a pre‐determined data collection form for toxicity would have reduced the risk of bias, because data were collected for all women studied. TARGIT performed a blinded assessment of cosmesis in a sub‐set of 105 women. The outcome assessors in ELIOT and GEC‐ESTRO were not blinded for assessment of subjective outcomes, so we deemed this domain at high risk of bias. In Rodriguez, although they noted a participant‐reported outcome, more data came from the physicians (not blinded to treatment arm), so this outcome of cosmesis was at high risk of bias.
Incomplete outcome data
ELIOT excluded no women, none were lost to follow‐up and there was no attrition reported, thus this study was at low risk of bias. Polgár 2007 excluded no women and they reported attrition by arm (without providing reasons), and the risk of bias was low. GEC‐ESTRO reported on all women randomized, and they reported post‐randomization exclusions by arm, with reasons, so we deemed this at low risk of bias. RAPID and Rodriguez were both interim reports of ongoing studies, so they were at unclear risk of bias for this domain. Livi 2015 reported no exclusion or attrition, so we deemed this at low risk of bias. TARGIT was at low risk of bias for this domain.
Selective reporting
For ELIOT, Livi 2015, and Polgár 2007, the trial protocols were not available for review and we judged them at unclear risk of bias. TARGIT did not report on all the outcomes pre‐specified in the protocol, but it is likely that these outcomes will form the basis of future publications so we judged this at low risk of bias. RAPID and Rodriguez were interim reports; therefore, we deemed them at unclear risk of bias. GEC‐ESTRO did not report on all their planned outcomes, but stated they will be the subject of a future paper, so we deemed this domain at low risk of bias.
Other potential sources of bias
Regarding other potential sources of bias: Polgár 2007 had low risk of bias. Polgár 2007 stopped the trial early because a competing trial started recruiting (GEC‐ESTRO). TARGIT had short median follow‐up, which put it at high risk of bias (see Figure 2). RAPID and Rodriguez were interim reports; therefore, we deemed them at unclear risk of bias. We identified no other sources of bias GEC‐ESTRO, Livi 2015, or ELIOT, so deemed them at low risk of bias (see Figure 2).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Primary outcomes
Local recurrence‐free survival in the ipsilateral breast
There were 89 local recurrences in the 6820 women studied in six studies.
LR‐FS appeared worse with PBI/APBI (HR 1.62, 95% CI 1.11 to 2.35; six studies, 6820 participants; Analysis 1.1; Figure 3). There was evidence of heterogeneity on visual inspection and statistical testing (P value = 0.008, I2 = 71%).

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.
We performed sensitivity analysis based on excluding studies deemed at high risk of bias (for the domains of objective outcomes and other bias; TARGIT). We found PBI/APBI versus WBRT was associated with worse LR‐FS (HR 1.48, 95% CI 0.95 to 2.29). There appeared to be some evidence of heterogeneity both on visual inspection and statistical testing (P value = 0.07, I2 = 68.6%).
Cosmesis
Cosmetic outcome appears worse with PBI/APBI when:
-
participant‐reported (OR 1.74, 95% CI 1.06 to 2.87; P value = 0.03, not tested for heterogeneity). We identified 89 events in 328 participants from one study (RAPID);
-
assessed by trained nurse observers (OR 3.14, 95% CI 1.81 to 5.45; P value < 0.0001). We did not test for heterogeneity, as we studied 78 events in 335 participants deriving from one study (RAPID);
-
physician‐reported (OR 1.51, 95% CI 1.17 to 1.95; P value = 0.002). There was considerable heterogeneity (I2 = 88%; P value = 0.00004; Analysis 1.2). We studied 315 events in 1720 participants in five studies.
We performed sensitivity analysis by excluding studies at high risk of bias for blinding of outcome assessors for subjective outcomes (ELIOT; Livi 2015; Polgár 2007; Rodriguez) for physician reported cosmesis. We found cosmesis was worse with PBI/APBI versus WBRT (OR 2.32, 95% CI 1.69 to 3.18; P value = 0.00001). Testing for heterogeneity revealed the presence of considerable heterogeneity (I2 = 84%; P value = 0.01).
Secondary outcomes
Overall survival
We found no difference in OS with PBI/APBI versus WBRT with data from 268 deaths in five studies with 6718 participants (HR 0.90, 95% CI 0.74 to 1.09; P value = 0.27; Analysis 1.3; Figure 4). There was no heterogeneity on either visual inspection or statistical testing (I2 = 40%; P value = 0.15).

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.
Toxicity
PBI/APBI reduced acute skin toxicity compared with WBRT (OR 0.04, 95% CI 0.02 to 0.09; P value < 0.00001; Analysis 1.4). We studied 150 events in 608 participants in two studies. We found little evidence of heterogeneity (I2 = 27%; P value = 0.24).
There was no different in haematomas needing surgical aspiration between APBI and WBRT (P value = 0.338; figure from text; TARGIT).
Seromas needing greater than three aspirations were more frequent with APBI compared with WBRT (P value = 0.012; figure from text; TARGIT).
There was no difference in infection requiring intravenous antibiotics or surgical intervention between APBI and WBRT (P value = 0.292; figure from text; TARGIT).
There was no difference in skin breakdown or delayed healing between APBI and WBRT (P value = 0.155; figure from text; TARGIT).
Regarding late toxicity:
-
there was no difference in late skin toxicity with PBI/APBI versus WBRT (OR 0.21, 95% CI 0.01 to 4.39; P value = 0.31; data from two events in 608 participants in two studies; Analysis 1.5). There was no difference in late RT skin toxicity between PBI and WBRT in GEC‐ESTRO (P value = 0.08; figure from text);
-
telangiectasia appeared worse with PBI/APBI compared with PBI (OR 26.56, 95% CI 3.59 to 196.51; P value = 0.001; data from one study with 28 events in 766 evaluable women; RAPID);
-
PBI/APBI increased radiological fat necrosis compared with WBRT (OR 1.58, 95% CI 1.02 to 2.43; P value = 0.04; three studies with 100 events in 1319 participants). We found little evidence of heterogeneity (I2 = 49%; P value = 0.14; Analysis 1.6);
-
PBI/APBI increased subcutaneous fibrosis compared with WBRT (OR 6.58, 95% CI 3.08 to 14.06; P value < 0.00001; data from one study with 59 events in 766 women). There was no difference in late subcutaneous toxicity between PBI and WBRT in GEC‐ESTRO (P value = 0.53; figures from text);
-
there was no difference in breast pain with PBI/APBI versus WBRT (OR 2.17, 95% CI 0.56 to 8.44; P value = 0.27; 10 events in one study with 766 evaluable women); ELIOT reported no difference in breast pain (data not shown). Breast pain was reduced with PBI compared with WBRT in GEC‐ESTRO (P value = 0.04; figures from text).
New primary tumours in ipsilateral breast 'elsewhere primaries'
New primaries in the treated breast appeared more frequent with PBI/APBI compared with WBRT (OR 3.97, 95% CI 1.51 to 10.41; Analysis 1.7). There were 24 events in 3009 participants in three studies. There was evidence of heterogeneity (I2 = 74%; P value = 0.02).
Cause‐specific survival
We found no clear evidence that C‐SS differed with PBI/APBI versus WBRT (HR 1.08, 95% CI 0.73 to 1.58, 107 breast cancer deaths in 6718 participants in five studies; Analysis 1.8; Figure 5). We found no evidence of heterogeneity on either visual inspection or statistical testing (I2 = 0%; P value = 0.81; Analysis 1.8).
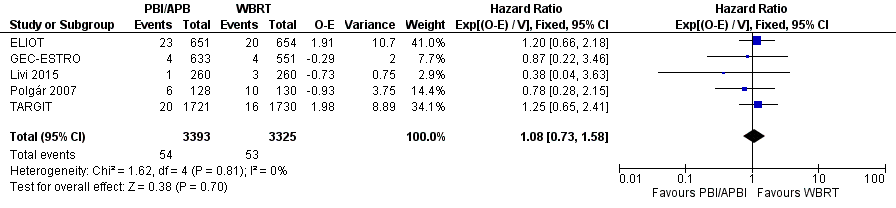
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.
Distant metastasis‐free survival
We found no clear evidence that DM‐FS differed with PBI/APBI versus WBRT (HR 0.94, 95% CI 0.65 to 1.37, 110 events in 3267 participants in four studies; Analysis 1.9; Figure 6). We found no heterogeneity with either visual inspection or statistical testing (I2 = 0%; P value = 1.0).
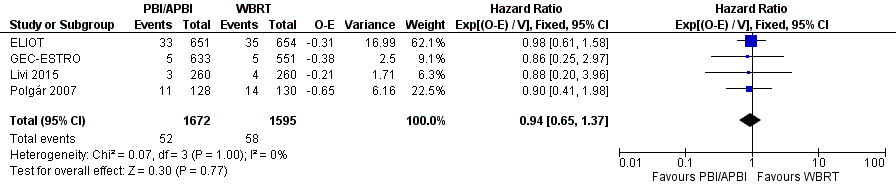
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.
Relapse‐free survival
We found no clear evidence that R‐FS differed with PBI/APBI versus WBRT (HR 1.36, 95% CI 0.88 to 2.09; P value = 0.16; 87 relapses in 3811 participants in three studies; Analysis 1.10). We found little evidence of heterogeneity (I2 = 46%; P value = 0.17). R‐FS did not differ in GEC‐ESTRO (P value = 0.79; figure from text).
Loco‐regional recurrence‐free survival
We found that L‐RR‐FS was worse with PBI/APBI versus WBRT (HR 1.80, 95% CI 1.00 to 3.25; Analysis 1.11). We studied 48 events in 3553 participants in two studies.
Subsequent mastectomy
Subsequent mastectomy rate did not appear to differ between PBI/APBI and WBRT (OR 1.20, 95% CI 0.77 to 1.87; Analysis 1.12). There were 79 events in 4817 participants in three studies. There was no evidence of heterogeneity (I2 = 0%; P value = 0.45).
Compliance
Greater than 90% of the women randomized to PBI/APBI received treatment in all of the included studies. For those women randomized to receive WBRT, greater than 90% of the women received the planned RT in all of the included studies.
Costs
There were no data on costs.
Quality of life
There were no data on quality of life, although it was assessed in RAPID.
Consumer preference
There were no data on consumer preference.
Discussion
Summary of main results
LR‐FS appeared worse with PBI/APBI compared with WBRT (Analysis 1.1). Cosmetic outcome appeared worse with PBI/APBI compared with WBRT when participant‐reported (OR 1.74, 95% CI 1.06 to 2.87; P value = 0.03), assessed by trained nurse observers (OR 3.14, 95% CI 1.81 to 5.45; P value = 0.00001) or physician‐reported (OR 1.51, 95% CI 1.17 to 1.95; P value = 0.002) (Analysis 1.2).
We found no evidence of a difference in OS when PBI/APBI was used compared with WBRT (HR 0.90, 95% CI 0.74 to 1.09; P value = 0.27; Analysis 1.3).
We found PBI/APBI versus WBRT was associated with decreased acute skin toxicity (OR 0.04, 95% CI 0.02 to 0.09; P value < 0.00001; Analysis 1.4). Late skin toxicity did not differ with PBI/APBI versus WBRT, neither did breast pain, but we found it was associated with more telangiectasia, radiologically evident fat necrosis and more subcutaneous fibrosis. We found PBI/APBI versus WBRT was associated with more 'elsewhere primaries' (OR 3.97, 95% CI 1.51 to 10.41; Analysis 1.7).
We found no clear evidence of a difference in C‐SS (Analysis 1.8), DM‐FS (Analysis 1.9) or R‐FS (Analysis 1.10) with PBI/APBI compared with WBRT but we could not exclude potentially important differences between the treatment groups. We found no difference with PBI/APBI versus WBRT for L‐RR‐FS (HR 1.80, 95% CI 1.00 to 3.25; P value = 0.05; Analysis 1.11). The mastectomy rate did not appear affected with PBI/APBI compared with WBRT (Analysis 1.12). Compliance with allocated treatment was high at greater than 90% for all women studied. We found no data for the effects of PBI/APBI and WBRT on costs, quality of life and consumer preference.
Based on these findings, PBI/APBI appeared to be associated with worse LR‐FS in comparison to WBRT. Local relapse was rare in the carefully selected participant population in this review. Local relapse was increased by 5 per 1000 women (range 1/1000 to 11/1000) with PBI/APBI. The use of PBI/APBI was associated with decreased acute toxicity. There was no strong evidence that PBI/APBI was associated with increased breast pain or late skin toxicity. We found that PBI/APBI caused increased telangiectasia, radiologically evident fat necrosis (Table 4) and subcutaneous fibrosis (see Results, Description of studies, included studies for definitions).
Overall completeness and applicability of evidence
There were limitations in completeness and applicability of evidence due to clinical heterogeneity between the trials, duration of follow‐up and little or no useful information available for some outcome measures.
Clinical heterogeneity between the trials
There was evident clinical heterogeneity in both the study participants and the interventions.
The PBI/APBI delivered was heterogeneous in dose, technique, fractionation and target delineation.
-
The RT doses varied between the trials. In all studies, the EQD2 delivered in the APBI arm was higher than the WBRT dose (with the exception of ELIOT; see Table 6). Despite the dose escalation in the APBI arm, we found that LR‐FS was worse with APBI, which suggests there may be technical issues with RT delivery (e.g. target volume definition or target coverage).
-
The RT techniques used to deliver PBI/APBI varied (see Table 7).
-
The trials differed in fractionation used; all the studies used greater than 2 Gy per fraction in the experimental arm, with the exception of Polgár 2007, where some of the women in the PBI/APBI arm received 2 Gy per fraction (see Table 6).
-
The techniques used to define the target volume in GEC‐ESTRO, Livi 2015, Polgár 2007, RAPID, and TARGIT were consistent with current practice, but ELIOT provided inadequate details for the technique used in to be certain of this.
Avoiding geographic miss is imperative in RT. Delivery of RT is a technical exercise. Peters 2010 demonstrated the extremely important role that quality assurance plays in the delivery of high‐quality RT. Poor‐quality RT (a non‐compliant plan) for locally advanced head and neck cancer was associated with a 20% detriment in survival and a 29% detriment to loco‐regional control (Peters 2010). Three studies did not mention quality assurance (ELIOT; Livi 2015; Rodriguez). Although Polgár 2007 assessed implant quality, less than 20% of the implants had post‐implant CT to document PTV coverage and quality assurance for APBI using electrons or WBRT was not mentioned (see Characteristics of included studies table). RAPID included an extensive quality assurance programme with credentialling, real‐time and post‐hoc review of radiation quality. GEC‐ESTRO had rigorous pre‐ and post‐implant quality assurance procedures.
The lack of precision in target definition, the variety of methods used to deliver PBI/APBI (even within the experimental arm of the trials; see Table 7) and the omission of robust quality assurance mean that the RT delivered in some of the trials was not reproducible. We cannot be certain of the dose delivered and the volume of breast treated.
We found there was heterogeneity for the outcome of cosmesis (physician‐reported). There were some possible sources of clinical heterogeneity that may have contributed to this: in Polgár 2007, 40/128 (30%) women in the PBI/APBI arm received 50 Gy in 25 fractions to the tumour bed using EBRT; these women who had conventionally fractionated RT at the same dose as the WBRT arm were likely to have very similar cosmetic outcome. Livi 2015 delivered PBI/APBI using IMRT, with careful dose constraints applied: we know that IMRT is associated with improvement in cosmetic outcome in the setting of WBRT (Donovan 2007; Pignol 2008).
Modern breast‐conserving therapy with postoperative WBRT achieves local control rates of 3.3% to 3.4% at five years and 5.2% to 6.7% at 10 years (Haviland 2013). With careful participant selection (aged 65 years or greater, T1‐2 tumours, not greater than 3 cm, margin negative, oestrogen receptor positive and all receiving tamoxifen), surgery alone can achieve local recurrence rates of 4.1% at five years (Kunkler 2015). The risk of local recurrence continues with longer follow‐up, in modern series where women with good prognosis were treated with surgery alone, the 10‐year risk of loco‐regional or distant recurrence was 19.9% (reduced to 6.3% with RT; Blamey 2013; Fisher 2002; Fyles 2004; Hughes 2004; Potter 2007; Prescott 2007; Winzer 2010). Even if PBI/APBI is poorly targeted, in a low‐risk population being treated with systemic therapy, low local recurrence rates can be expected.
The paradigm for PBI/APBI relies on the basis that WBRT does not reduce the number of 'elsewhere primaries' within the treated breast, but we found that PBI/APBI appeared to be associated with an increase in 'elsewhere primaries' (OR 3.97, 95% CI 1.51 to 10.41; Analysis 1.7).
Duration of follow‐up
The length of follow‐up in five of the included trials was adequate to detect local recurrences (10 years (Polgár 2007), 69 months (ELIOT), 65 months (Livi 2015), 60 months (Rodriguez), 79.2 months (GEC‐ESTRO)). The median follow‐up for TARGIT, of 29 months, made it inadequate for the outcome of local control. Local recurrences do continue to increase over time (at about 1% per year), as can be seen in the START data: 3.3% to 3.4% at five years and 5.2% to 6.7% at 10 years (Haviland 2013). Any effect on breast C‐SS related to local control requires much longer follow‐up (EBCTCG 2011). None of the included trials had long enough follow‐up to report OS results.
We know that RT reduces the risk of any first recurrence, and although the proportional reduction is most evident in the first year post‐treatment, it is still present in years five to nine (RR 0.59, 95% CI 0.5 to 0.7; EBCTCG 2011). Even in modern studies that included low‐risk women treated with lumpectomy (enrolled after 1989), the 10‐year risk of loco‐regional and distant recurrence was 19.9% (reduced to 6.3% with RT; Blamey 2013; Fisher 2002; Fyles 2004; Hughes 2004; Potter 2007; Prescott 2007; Winzer 2010). Even women at low risk of recurrence (if they are at risk for another 30 years) are likely to have increases in recurrence with longer follow‐up. The use of PBI/APBI may mean they are at risk of 'elsewhere primaries' for this time period.
Little or no useful information available for some outcome measures
The authors of TARGIT (completed, but with short follow‐up) indicated that cosmesis, participant satisfaction, health economics and participant preference will be the subject of a sub‐protocol and information with respect to these outcomes will be available in the future. The five ongoing trials that we found are likely to address these outcomes (IMPORT; IRMA; NSABP‐B39/RTOG; SHARE; see Characteristics of ongoing studies table).
Quality of the evidence
The available evidence does allow for the drawing of robust conclusions in respect of the objective of the review: to determine whether PBI/APBI is equivalent to or better than conventional or hypo‐fractionated WBRT after breast‐conserving therapy for early‐stage breast cancer. We found seven studies with 8895 participants.
LR‐FS: we downgraded for risk of bias because 3451/6820 (38%) women contributing to this outcome came from a study deemed at high risk of bias because of short follow‐up. We did not downgrade for indirectness because the included studies used contemporary RT techniques. With respect to inconsistency (I2 = 71%; P value = 0.008), there was considerable clinical heterogeneity with respect to RT dose, technique and use of quality assurance procedures. However, the RT techniques employed all delivered a dose that was the same or higher in the PBI/APBI arm than the WBRT arm, which should mean the LR‐FS is better or the same (see Table 6). We downgraded for imprecision; there were fewer than 300 events, and although the optimum information size (OIS) had been met, the CIs did not exclude either clinically unimportant harms or clinically important harms. We did not downgrade for publication bias, given the systematic literature search we had performed. The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcomes was low.
Cosmesis: we did not downgrade for risk of bias because less than 30% of events came from studies at high risk of bias for lack of blinding of outcome assessors, which is particularly important for a subjective outcome such as cosmesis. We did not downgrade for indirectness, but did so for inconsistency (I2 = 88%; P value < 0.00001) even though all assessments used the same four‐point scale. We did downgrade for imprecision (there were greater than 300 events (315 events)) and the CIs did not exclude the possibility of clinically unimportant harms. We did not downgrade for publication bias given the systematic literature search we had performed. The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcomes was low.
OS: we did not downgrade for risk of bias, indirectness, inconsistency (I2 = 40%; P value = 0.15) or imprecision (the CIs crossed one, but excluded clinically significant harms or benefits). We did not downgrade for publication bias (based on examination of a Funnel plot). The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcome was high.
Late RT toxicity (subcutaneous fibrosis): we did not downgrade for risk of bias, indirectness or inconsistency (heterogeneity testing was not appropriate as only one study contributed data for this outcome). We downgraded for imprecision, as there were fewer than 300 events and the OIS was not met (the studies in PBI/APBI were powered for other endpoints than this one) and CIs did not exclude clinically important harms. We did not downgrade for publication bias. The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcomes was moderate.
C‐SS: we did not downgrade for risk of bias, indirectness or inconsistency (I2 = 0%; P value = 0.81). We did downgrade for imprecision, given there were fewer than 300 events (99 events) and the CIs did not include the possibility of clinically significant benefits or harms from PBI/APBI. We did not downgrade for publication bias for this outcome. The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcomes was moderate.
DM‐FS: we did not downgrade for risk of bias, indirectness or inconsistency (I2 = 0%; P value = 1.0). We did downgrade for imprecision as there were fewer than 300 events (100 events) and the CIs did not exclude clinical meaningful benefits or harms. We did not downgrade for publication bias. The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcomes was moderate.
Subsequent mastectomy: we downgraded for risk of bias, because one of the studies contributing data had follow‐up too short for assessment of this outcome. We did not downgrade for indirectness or inconsistency (I2 = 0%; P value = 0.45). We did downgrade for imprecision as there were fewer than 300 events (78 events) and the CIs did not exclude clinically meaningful benefits or harms. The overall GRADE (GRADE Working Group 2004) quality of evidence for this outcomes was low.
Refer to the summary of findings Table for the main comparison.
Potential biases in the review process
We consider that we have identified all the relevant completed RCTs. For reporting of adverse effects, the precision was low because the OIS was not met. This may improve in future updates, as more information with respect to these outcomes is reported.
Agreements and disagreements with other studies or reviews
We found five systematic reviews examining PBI/APBI compared with WBRT.
We found one meta‐analysis (Valachis 2010), which included one study (Polgár 2007) in common with our systematic review and recruited 1140 women (Dodwell 2005; Polgár 2007; Ribeiro 1993). They found no difference for mortality (OR 0.91, 95% CI 0.67 to 1.23; P value = 0.55) and distant metastasis (OR 0.74, 95% CI 0.51 to 1.08; P value = 0.12) for the comparison of PBI/APBI versus WBRT. PBI/APBI was associated with increased local recurrence (OR 2.15, 95% CI 1.40 to 3.31; P value = 0.001) and axillary recurrence (OR 3.43, 95% CI 2.06 to 5.71; P value < 0.0001) when compared with WBRT. They concluded that PBI does not seem to jeopardize survival and may be used as an alternative to WBRT (Valachis 2010). The authors pooled the data from the trials (see Data synthesis section).
We found one systematic review with a search date in 2010 (BlueCross BlueShield). They included two RCTs (Polgár 2007; Ribeiro 1993 (which we excluded)) and seven non‐RCTs. They concluded that "the body of evidence on interstitial PBI/APBI compared to conventional whole‐breast irradiation is weak, and it is extremely weak (i.e. no comparative studies) for balloon brachytherapy, intraoperative PBI/APBI, and external‐beam PBI/APBI. The data on PBI/APBI compared to whole‐breast irradiation are insufficient to draw any conclusions about the relative effectiveness of these modalities. Furthermore, it is becoming increasingly clear that each type of PBI/APBI should be judged on its own merits, and studies comparing different PBI/APBI techniques to each other as well as to whole‐breast irradiation are needed" (BlueCross BlueShield).
We found one review that used a systematic search (search date February 2014) (Marta 2015). Our review differed in that we included updated data for TARGIT and they included Dodwell 2005 and Ribeiro 1993 (which we excluded). They reported decreased local control with APBI versus WBRT (HR 4.54, 95% CI 1.78 to 11.61; P value = 0.002). They concluded that APBI was associated with an increase in local recurrence, but found no difference in other outcomes.
Kong 2014 did a systematic search (search date June 2012). They included 10 studies, and included two retrospective cohorts, two matched pair analyses and three prospective cohort studies. They found that PBI/APBI was associated with increased local recurrence (OR 1.54, 95% CI 1.15 to 2.06; P value = 0.004). They found no difference for the comparison of PBI/APBI for the outcomes of distant metastases, OS and disease‐free survival.
One review by Ye 2013 studied 919 women in four studies; their search was not systematic (search date June 2013). They included a matched pair analysis, and the fourth study was not referenced. They reported no difference in local control, but appeared to have double counted the data for Polgár 2007 at two time points. They concluded that other outcomes did not differ, although they reported more women who were treated with APBI had good/excellent cosmesis.
The review in Clinical Evidence (search date April 2009) compared intraoperative RT versus standard postoperative RT in women receiving breast‐conserving surgery, and found no fully published RCTs (see comment, ASERNIP‐S 2002). The review concluded, "Studies are currently attempting to address the question as to whether partial breast irradiation may be sufficient treatment for some sub‐groups of breast cancer. Until those studies are complete and the follow‐up data mature, it is not possible to recommend partial breast irradiation as an appropriate treatment for breast cancer outside of a properly conducted trial" (Clinical Evidence).
Several guidelines have been published on PBI/APBI. The American Society of Therapeutic Radiology and Oncology (ASTRO) consensus evolved because it was clear that many women are treated in the US with PBI/APBI outside clinical trials (over 32,000 women have had treatment using MammoSite®; Cytec). They indicated a patient population "suitable" for PBI/APBI outside the context of a clinical trial: women aged 60 years and over, with pT1N0(i‐, i+)M0, margins greater than 2 mm, unifocal disease, oestrogen receptor positive, who have had no neoadjuvant therapy. They also described women deemed unsuitable for PBI/APBI. They state that, "patients who choose treatment with PBI/APBI should be informed that whole‐breast irradiation (WBI) is an established treatment with a much longer track record that has documented long‐term effectiveness and safety" (Smith 2009). This statement provides guidance in selecting participants who may be appropriate for PBI/APBI outside the context of a clinical trial, but the Task Force strongly endorsed enrolment of all eligible participants considering PBI/APBI onto NSABP‐B39/RTOG and encouraged enrolment of other participants considering PBI/APBI, particularly those not in the "suitable" group, into prospective clinical studies to address many of the unanswered questions in PBI/APBI (Smith 2009). NCCN states, "patients are encouraged to enter clinical trials, and endorses the ASTRO guidelines".
In the absence of an available trial, the American College of Radiology (ACR) Appropriateness Criteria® panel recommends following the consensus guidelines of the ASTRO (Bellon 2011).
The GEC‐ESTRO Breast Cancer Working Group recommends three categories guiding patient selection for PBI/APBI:
-
a low‐risk group for whom PBI/APBI outside the context of a clinical trial is an acceptable treatment option; including participants aged at least 50 years with unicentric, unifocal, pT1‐2 (less than 30 mm) pN0, non‐lobular invasive breast cancer without the presence of an extensive intraductal component (EIC) and LVI and with negative surgical margins of at least 2 mm;
-
a high‐risk group, for whom PBI/APBI is considered contraindicated; including participants aged 40 years or less; having positive margins, with or without any of the following pathological features (multicentric or large (greater than 30 mm) tumours, EIC positive or LVI positive tumours, four or more positive lymph nodes, unknown axillary status (pNx)); and
-
an intermediate‐risk group, for whom PBI/APBI is considered acceptable only in the context of prospective clinical trials (Polgár 2010).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.1 Local recurrence‐free survival.
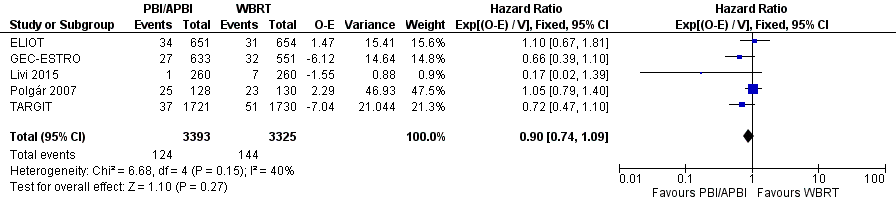
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.3 Overall survival.

Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.8 Cause‐specific survival.
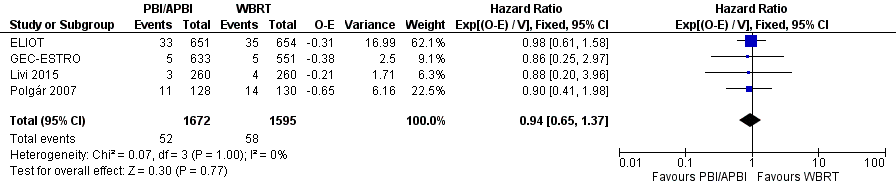
Forest plot of comparison: 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), outcome: 1.9 Distant metastasis‐free survival.
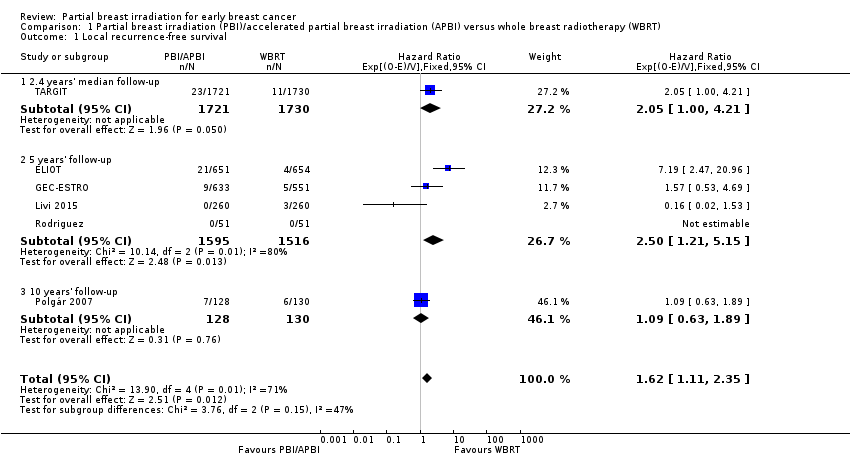
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 1 Local recurrence‐free survival.
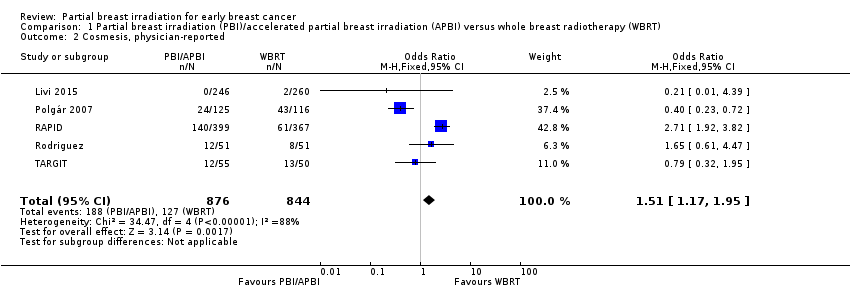
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 2 Cosmesis, physician‐reported.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 3 Overall survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 4 Acute radiotherapy (RT) skin toxicity.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 5 Late RT skin toxicity.
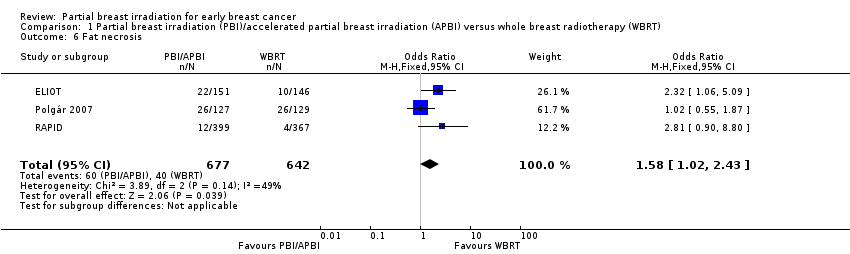
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 6 Fat necrosis.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 7 'Elsewhere primary'.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 8 Cause‐specific survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 9 Distant metastasis‐free survival.
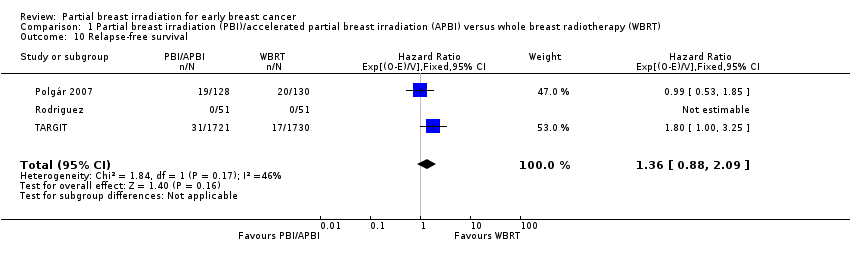
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 10 Relapse‐free survival.
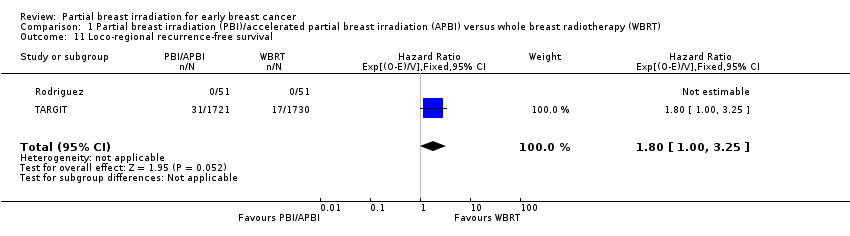
Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 11 Loco‐regional recurrence‐free survival.

Comparison 1 Partial breast irradiation (PBI)/accelerated partial breast irradiation (APBI) versus whole breast radiotherapy (WBRT), Outcome 12 Mastectomy.
| PBI/APBI for women with early breast cancer | ||||||
| Patient or population: women with early breast cancer Setting: radiotherapy centres Intervention: PBI/APBI Comparison: whole breast radiotherapy (WBRT) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Risk with WBRT | Risk with PBI/APBI | |||||
| Local recurrence‐free survival at 5 years | Study population | HR 1.62 | 6820 | ⊕⊕⊝⊝ | ‐ | |
| 10 per 10001 | 16 per 1000 | |||||
| Cosmesis assessed with 4‐point scale Follow‐up: range 29‐122 months | Study population | OR 1.51 | 1720 | ⊕⊕⊝⊝ | Cosmesis was assessed using a 4‐point scale. We reported those women with poor/fair cosmesis at final review | |
| 150 per 1000 | 218 per 1000 | |||||
| Late radiotherapy toxicity (subcutaneous fibrosis) Follow‐up: median 36 months | Study population | OR 6.58 | 766 | ⊕⊕⊕⊝ | Assessed using National Cancer Institute 3‐point scale, events were defined as: Grade II or higher toxicity Physician assessors, at 3 years' follow‐up | |
| 22 per 1000 | 128 per 1000 | |||||
| Cause‐specific survival at 5 years | Study population | HR 1.08 | 6718 | ⊕⊕⊕⊝ | ‐ | |
| 20 per 10002 | 22 per 1000 | |||||
| Distant metastasis‐free survival at 5 years | Study population | HR 0.94 | 3267 | ⊕⊕⊕⊝ | ‐ | |
| 33 per 10002 | 31 per 1000 | |||||
| Mastectomy rate Follow‐up: range 29‐122 months | Study population | OR 1.20 | 4817 | ⊕⊕⊝⊝ | Mastectomy rate reflected both local recurrence and adverse cosmetic outcome | |
| 15 per 1000 | 18 per 1000 | |||||
| Mortality | Study population | HR 0.90 | 6718 | ⊕⊕⊕⊕ | Survival advantage from radiotherapy for breast cancer is not apparent before 15 years' follow‐up (EBCTCG 2011) | |
| 51 per 10002 | 46 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 The baseline risk for the control group was calculated at the 5‐year time point from 5 studies. | ||||||
| Cosmetic score |
| Excellent |
| Good |
| Fair |
| Poor |
| Score | Definition |
| Excellent | Perfect symmetry, no visible distortion or skin changes and no visible catheter entry/exit sequelae |
| Good | Slight skin distortion, retraction or oedema, any visible telangiectasia, any visible catheter entry/exit scar or mild hyperpigmentation |
| Fair | Moderate distortion of the nipple or breast symmetry, moderate hyperpigmentation, or prominent skin retraction, oedema or telangiectasia |
| Poor | Marked distortion, oedema, fibrosis or severe hyperpigmentation |
| RTOG CTC | Grade I | Grade II | Grade III | Grade IV |
| Description | Follicular, faint or dull erythema / epilation / dry desquamation / decreased sweating | Tender or bright erythema, patchy moist desquamation / moderate oedema | Confluent, moist desquamation other than skin folds, pitting oedema | Ulceration, haemorrhage, necrosis |
| RTOG CTC: Radiation Therapy Oncology Group Common Toxicity Criteria. | ||||
| Grade | Findings |
| 0 | No fat necrosis |
| 1 | Asymptomatic fat necrosis (only radiological or |
| 2 | Symptomatic fat necrosis not requiring medication |
| 3 | Symptomatic fat necrosis requiring medication |
| 4 | Symptomatic fat necrosis requiring surgical |
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Induration (subcutaneous fibrosis) | Increased density on palpation | Moderate increase in density, not interfering with ADL; marked increase in density and firmness on palpation with or without minimal retraction | Dysfunction interfering with ADL; very marked density, retraction or fixation | ‐ |
| Telangiectasia | Few | Moderate | Many and confluent | ‐ |
| Pain | Pain mild, not interfering with function | Moderate pain; pain or analgesics interfering with function, but not with ADL | Severe pain; pain or analgesics interfering with ADL | Disability |
| ADL: activities of daily living; NCI CTC: National Cancer Institute Common Toxicity Criteria. | ||||
| Trial | PBI/APBI dose | Fraction size (Gy) | EQD2 PBI/APBI | Control dose | Fraction size (Gy) | EQD2 Control |
| 20 Gy at surface of the applicator (attenuated to 5‐7 Gy at 1 cm) (APBI) | 80 at cavity surface 12.8 at 1 cm | 80 Gy at cavity surface 12.8 Gy at 1 cm | 40‐56 Gy/20‐28 fractions ± 10‐16 Gy boost | 2 | 40‐56 Gy ± 10‐16 Gy | |
| 30 Gy/5 daily fractions EBRT IMRT. 100% of the PTV was covered by 95% of the prescribed dose | 6 | 75 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost | 2 | 50 + 10 = 60 Gy | |
| 38.5 Gy/10 fractions bd (with 6 hour gap) Dose‐evaluation volume (that part of PTV within the breast) received 95‐107% of prescription dose | 3.85 | 74.1 Gy | 50 Gy/25 fractions or 42.5 Gy/16 fractions ± boost (10 Gy/4‐5 fractions) based on criteria such as young age or close margins, pre‐specified by centre | 2 or 2.65 | 50 or 47.1 Gy | |
| 37.5 Gy/10 fractions bd (with 6 hour gap) (APBI). PTV covered by ≥ 95% of prescribed dose, with < 105% hot spot | 3.75 | 71.22 Gy | 48 Gy/24 fractions ± 10 Gy/5 fractions boost | 2 | 48 ± 10 = 48‐58 Gy | |
| 7 × 5.2 Gy HDR (APBI) or 50 Gy/25 fractions (PBI). | 5.2 or 2 | 53.6 Gy or 50 Gy | 50 Gy/25 fractions (3D‐CRT was not used) | 2 | 50 Gy | |
| 30.3 Gy/7 fractions or 32 Gy/8 fractions HDR twice daily or 50 Gy at 0.6‐0.8 Gy/hour pulses (1 pulse per hour, 24 hours per day) PDR | 7‐8 | 41.64‐42.67 Gy | 50.0‐50.4 Gy to a reference point + 10 Gy/5 fractions boost. Electron dose was prescribed to the point of maximum dose on the beam axis (Dmax), ensuring the 85% isodose encompassed the tumour bed | 1.8‐2.0 | 48.72‐50 + 10 = 58.72‐60 Gy | |
| 21 Gy/1 fraction at 90% using 6‐9 MeV | 21 | 131.2 Gy | 50 Gy/25 fractions + 10 Gy/5 fractions boost (using electrons) | 2.0 | 50 + 10 Gy | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; APBI: accelerated partial breast irradiation; bd: twice daily; CT: computer tomography; EBRT: external beam radiotherapy; EQD2: equivalent dose in 2 Gy fractions; Gy: Gray; HDR: high‐dose‐rate; IMRT: intensity‐modulated radiotherapy; MeV: mega electron volt; PBI: partial breast irradiation; PDR: pulsed‐dose‐rate; PTV: planning target volume. | ||||||
| Trial | RT technique |
| Interstitial brachytherapy (88/128) EBRT using photons (40/128) | |
| intra‐operative electrons | |
| EBRT (IMRT) | |
| intra‐operative kV RT | |
| EBRT | |
| EBRT (3D‐CRT) | |
| 3D‐CRT: 3‐dimensional conformal radiotherapy; EBRT: external beam radiotherapy; IMRT: intensity‐modulated radiotherapy; RT: radiotherapy. | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Local recurrence‐free survival Show forest plot | 6 | 6820 | Hazard Ratio (95% CI) | 1.62 [1.11, 2.35] |
| 1.1 2.4 years' median follow‐up | 1 | 3451 | Hazard Ratio (95% CI) | 2.05 [1.00, 4.21] |
| 1.2 5 years' follow‐up | 4 | 3111 | Hazard Ratio (95% CI) | 2.50 [1.21, 5.15] |
| 1.3 10 years' follow‐up | 1 | 258 | Hazard Ratio (95% CI) | 1.09 [0.63, 1.89] |
| 2 Cosmesis, physician‐reported Show forest plot | 5 | 1720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.17, 1.95] |
| 3 Overall survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 0.90 [0.74, 1.09] |
| 4 Acute radiotherapy (RT) skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.09] |
| 5 Late RT skin toxicity Show forest plot | 2 | 608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.39] |
| 6 Fat necrosis Show forest plot | 3 | 1319 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.02, 2.43] |
| 7 'Elsewhere primary' Show forest plot | 3 | 3009 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.97 [1.51, 10.41] |
| 8 Cause‐specific survival Show forest plot | 5 | 6718 | Hazard Ratio (95% CI) | 1.08 [0.73, 1.58] |
| 9 Distant metastasis‐free survival Show forest plot | 4 | 3267 | Hazard Ratio (95% CI) | 0.94 [0.65, 1.37] |
| 10 Relapse‐free survival Show forest plot | 3 | 3811 | Hazard Ratio (95% CI) | 1.36 [0.88, 2.09] |
| 11 Loco‐regional recurrence‐free survival Show forest plot | 2 | 3553 | Hazard Ratio (95% CI) | 1.80 [1.00, 3.25] |
| 12 Mastectomy Show forest plot | 3 | 4817 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.77, 1.87] |

